The Addition of Marigold Extract to the Diet Improved the Performance of Laying Hens in the Late Laying Period by Increasing Their Antioxidant Capacity, Lipid Metabolism, and Microbial Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Experimental Design
2.2. Laying Performance and Egg Quality
2.3. Serum Biochemical Parameters
2.4. Liver Lipid Metabolism Parameters
2.5. Antioxidants of the Body
2.6. RNA Isolation and Real-Time Quantitative PCR
2.7. Cecal Content
2.8. Statistical Analysis
3. Results
3.1. Production Performance
3.2. Egg Quality
3.3. Serum Biochemistry
3.4. Antioxidants of Eggs
3.5. Antioxidants in the Serum and Liver
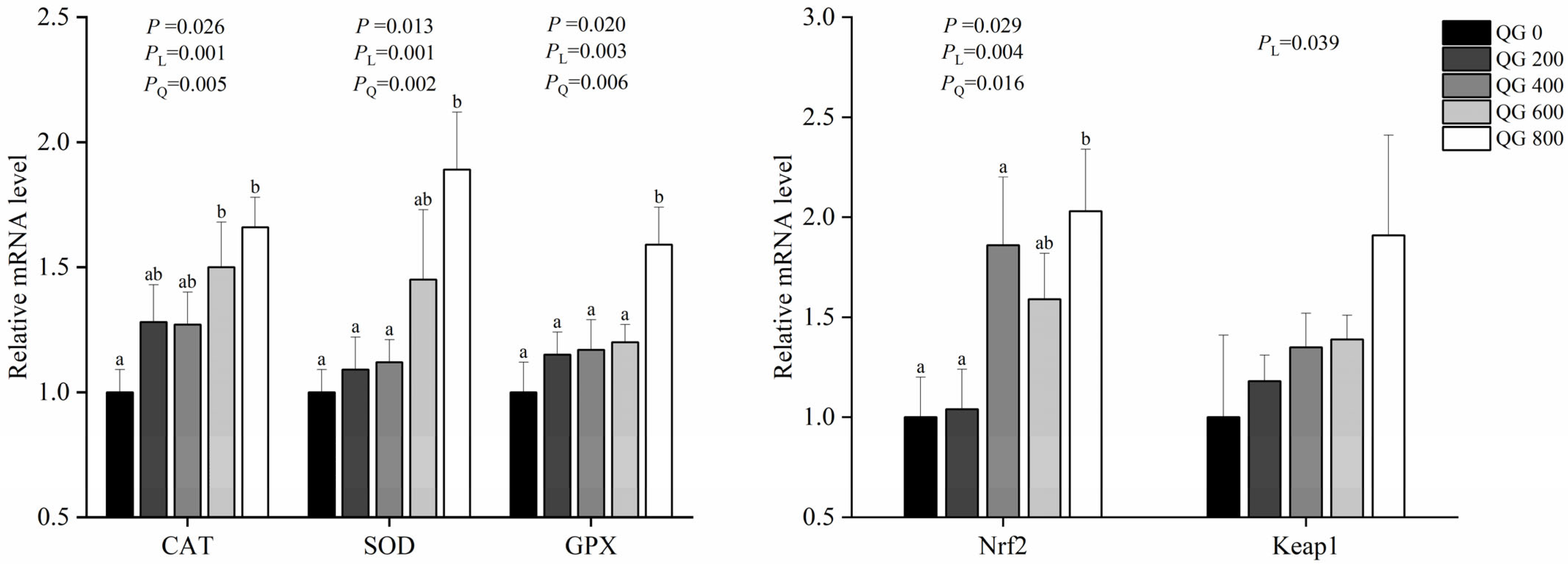
3.6. Antioxidant Gene Expression
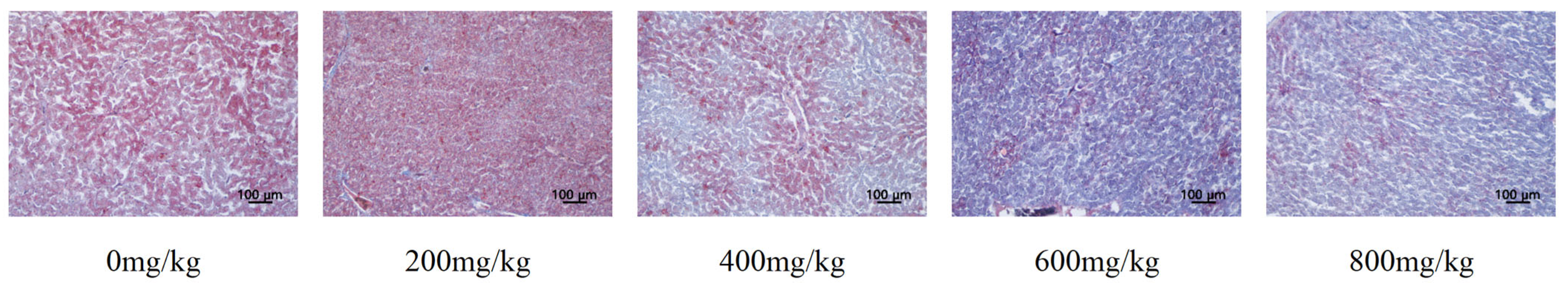
3.7. Liver Lipid Metabolism
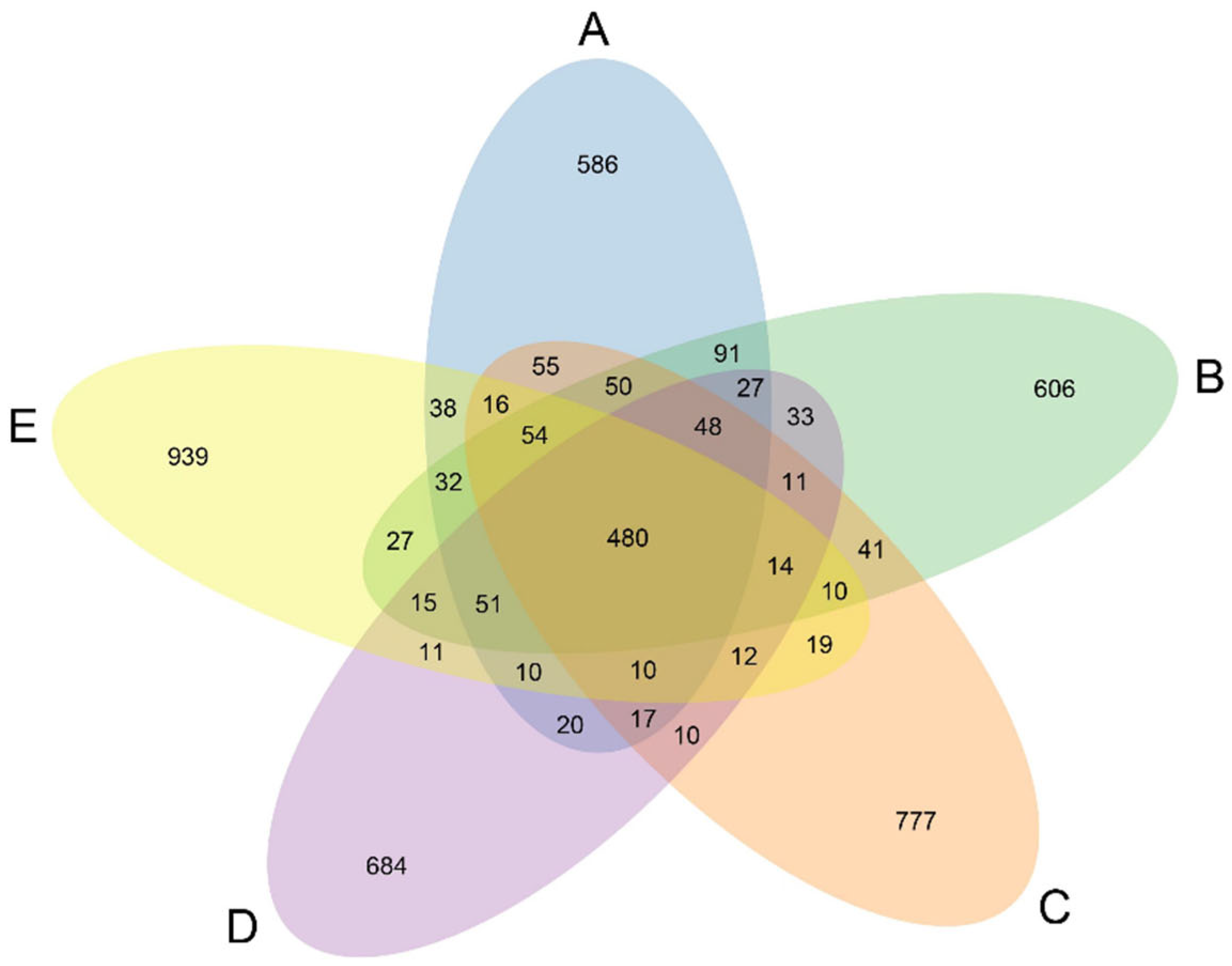
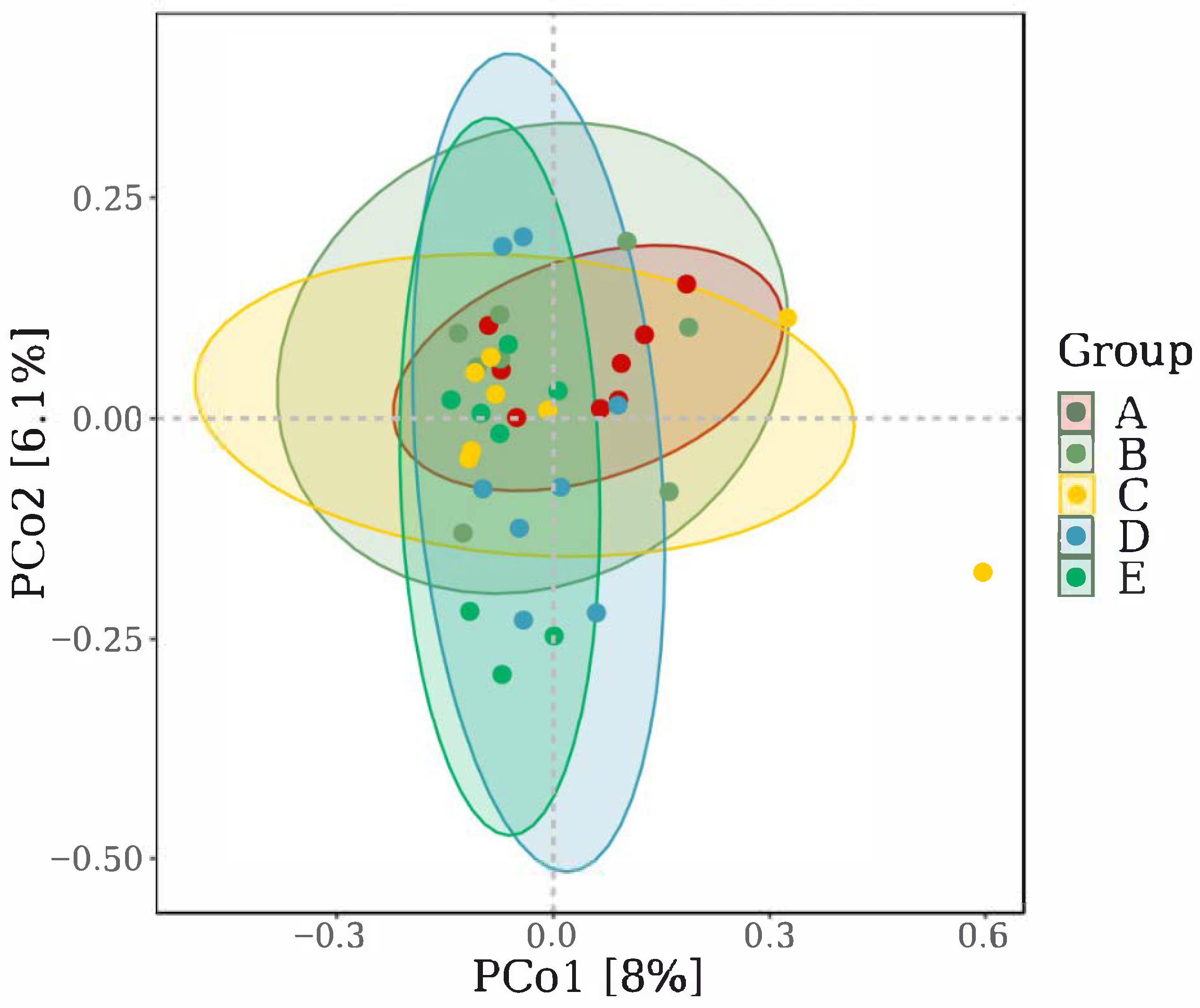
3.8. Microbial Composition and Function
4. Discussion
4.1. Dietary Supplementation with Marigold Extract Improves the Performance of Laying Hens in the Late-Laying Period
4.2. Addition of Marigold Extract to Feeding Rations Increases Antioxidant Levels in Eggs
4.3. Dietary Supplementation with Marigold Extract Improves Antioxidant Capacity in Late-Laying Hens
4.4. Dietary Supplementation with Marigold Extract Improves Lipid Metabolism in Late-Laying Hens
4.5. Addition of Marigold Extract to the Ration Modulates the Intestinal Flora Structure of Laying Hens in the Late-Laying Period
4.6. Determination of Optimal Dose by Quadratic Regression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TC | Total cholesterol |
| VLDL | Very-low-density lipoproteins |
| ADFI | Average daily feed intake |
| TP | Total protein |
| ALB | Albumin |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| UA | Uric acid |
| TG | Triglyceride |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| QG | Quercetagetin |
| OUT | Operational Taxonomic Unit |
| MDA | Malondialdehyde |
| T-AOC | Total antioxidant capacity |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPX | Glutathione peroxidase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| ROS | Reactive oxygen species |
| VLDLy | Very-low-density lipoprotein yolk |
References
- Tůmová, E.; Uhlířová, L.; Tůma, R.; Chodová, D.; Máchal, L. Age related changes in laying pattern and egg weight of different laying hen genotypes. Anim. Reprod. Sci. 2017, 183, 21–26. [Google Scholar] [CrossRef]
- Lin, H.; De Vos, D.; Decuypere, E.; Buyse, J. Dynamic changes in parameters of redox balance after mild heat stress in aged laying hens (Gallus gallus domesticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 30–35. [Google Scholar] [CrossRef]
- Gu, Y.F.; Chen, Y.P.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y.M. Age-related changes in liver metabolism and antioxidant capacity of laying hens. Poult. Sci. 2021, 100, 101478. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xu, L.P.; Sun, X.L.; Wan, X.H.; Sun, G.R.; Jiang, R.R.; Li, W.T.; Tian, Y.D.; Liu, X.J.; Kang, X.T. Characteristics of the fecal microbiota of high-and low-yield hens and effects of fecal microbiota transplantation on egg production performance. Res. Vet. Sci. 2020, 129, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Peebles, E.; Basenko, E.; Branton, S.; Whitmarsh, S.; Gerard, P. Effects of S6-strain Mycoplasma gallisepticum inoculation at 10, 22, or 45 weeks of age on the digestive and reproductive organ characteristics of commercial egg-laying hens. Poult. Sci. 2006, 85, 825–830. [Google Scholar] [CrossRef]
- Gu, Y.F.; Chen, Y.P.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y.M. A comparison of intestinal integrity, digestive function, and egg quality in laying hens with different ages. Poult. Sci. 2021, 100, 100949. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef]
- Fellenberg, M.; Speisky, H. Antioxidants: Their effects on broiler oxidative stress and its meat oxidative stability. World’s Poult. Sci. J. 2006, 62, 53–70. [Google Scholar] [CrossRef]
- Sihvo, H.-K.; Immonen, K.; Puolanne, E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Vercellotti, J.; St. Angelo, A.J.; Spanier, A.M. Lipid oxidation in foods: An overview. In Lipid Oxidation in Foods; American Chemical Society: Washington, DC, USA, 1992; pp. 1–11. [Google Scholar]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Ismail, I.; Al-Busadah, K.; El-Bahr, S. Oxidative stress biomarkers and biochemical profile in broilers chicken fed zinc bacitracin and ascorbic acid under hot climate. Am. J. Biochem. Mol. Biol. 2013, 2, 202–214. [Google Scholar] [CrossRef]
- Xing, J.Y.; Kang, L.; Hu, Y.; Xu, Q.Y.; Zhang, N.B.; Jiang, Y.L. Effect of dietary betaine supplementation on mRNA expression and promoter CpG methylation of lipoprotein lipase gene in laying hens. J. Poult. Sci. 2009, 46, 224–228. [Google Scholar] [CrossRef]
- Shini, A.; Shini, S.; Bryden, W. Fatty liver haemorrhagic syndrome occurrence in laying hens: Impact of production system. Avian Pathol. 2019, 48, 25–34. [Google Scholar] [CrossRef]
- Ding, H.R.; Wang, J.L.; Ren, H.Z.; Shi, X.L. Lipometabolism and glycometabolism in liver diseases. Biomed. Res. Int. 2018, 2018, 1287127. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Freimer, D.; Yang, T.T.; Ho, T.C.; Tymofiyeva, O.; Leung, C. The gut microbiota, HPA axis, and brain in adolescent-onset depression: Probiotics as a novel treatment. Brain Behav. Immun. Health 2022, 26, 100541. [Google Scholar] [CrossRef] [PubMed]
- Senchukova, M.A. Microbiota of the gastrointestinal tract: Friend or foe? World J. Gastroenterol. 2023, 29, 19. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Agbai Kalu, N.; Wang, J.; Zhang, H.J.; Qi, G.H.; Qiu, K.; Wu, S.G. Recent trends on mitigative effect of probiotics on oxidative-stress-induced gut dysfunction in broilers under necrotic enteritis challenge: A review. Antioxidants 2023, 12, 911. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, Z.Y.; Wang, G.Q.; Song, X.; Qian, Y.Y.; Liao, Z.; Sui, L.; Ai, L.Z.; Xia, Y.J. Understanding the connection between gut homeostasis and psychological stress. J. Nutr. 2023, 153, 924–939. [Google Scholar] [CrossRef]
- Wu, G.P.; Li, Z.H.; Zheng, Y.; Zhang, Y.H.; Liu, L.; Gong, D.Q.; Geng, T.Y. Supplementing cholamine to diet lowers laying rate by promoting liver fat deposition and altering intestinal microflora in laying hens. Poult. Sci. 2022, 101, 102084. [Google Scholar] [CrossRef]
- Liu, M.; Kang, Z.Y.; Cao, X.K.; Jiao, H.C.; Wang, X.J.; Zhao, J.P.; Lin, H. Prevotella and succinate treatments altered gut microbiota, increased laying performance, and suppressed hepatic lipid accumulation in laying hens. J. Anim. Sci. Biotechnol. 2024, 15, 26. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.Y.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- Sakuma, S.; Sumida, M.; Endoh, Y.; Kurita, A.; Yamaguchi, A.; Watanabe, T.; Kohda, T.; Tsukiyama, Y.; Fujimoto, Y. Curcumin inhibits adipogenesis induced by benzyl butyl phthalate in 3T3-L1 cells. Toxicol. Appl. Pharmacol. 2017, 329, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, S.S.; Oh, M.Y.; Lee, H.H.; Han, D.J. Quercetin Enhanced the Function of Mouse Islets. Transplantation 2018, 102, S744. [Google Scholar] [CrossRef]
- Yang, S.; Huo, M.; Su, Z.; Wang, F.; Zhang, Y.; Zhong, C.; Shi, Y. The impact of dietary supplementation of Quercetagetin on growth, antioxidant capacity, and gut microbiota of diquat-challenged broilers. Front. Microbiol. 2024, 15, 1453145. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Tian, H.-L.; Zhu, X.-P.; Yin, B.-Y.; Li, Y.-Z. Effects of quercetagetin on growth performance, diarrhea rate, and immunity of piglets. Feed. Res. 2024, 47, 25. [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994.
- NY/T 33-2004; Feeding Standard of Chicken. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2004.
- Zhang, T.; Li, Y.H.; Miao, M.; Jiang, B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. 2011, 128, 28–33. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Julian, R.J. Production and growth related disorders and other metabolic diseases of poultry—A review. Vet. J. 2005, 169, 350–369. [Google Scholar] [CrossRef]
- Jiang, J.L.; Qi, L.N.; Dai, H.J.; Hu, C.H.; Lv, Z.P.; Wei, Q.W.; Shi, F.X. Dietary stevioside supplementation improves laying performance and eggshell quality through increasing estrogen synthesis, calcium level and antioxidant capacity of reproductive organs in aged breeder hens. Anim. Feed. Sci. Technol. 2020, 269, 114682. [Google Scholar] [CrossRef]
- Liang, X.X.; Fu, Y.W.; Niu, K.M.; Zhai, Z.Y.; Shi, H.X.; Wang, R.X.; Yin, Y.L. Dietary Eucommia ulmoides leaf extract improves laying performance by altering serum metabolic profiles and gut bacteria in aged laying hens. Anim. Nutr. 2023, 15, 307–319. [Google Scholar] [CrossRef]
- Amevor, F.K.; Cui, Z.F.; Ning, Z.F.; Du, X.X.; Jin, N.N.; Shu, G.; Deng, X.; Zhu, Q.; Tian, Y.F.; Li, D.Y. Synergistic effects of quercetin and vitamin E on egg production, egg quality, and immunity in aging breeder hens. Poult. Sci. 2021, 100, 101481. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Wang, Y.J.; Zhou, Y.C.; Ni, Y.D.; Lu, L.Z.; Grossmann, R.; Chen, J. Dietary daidzein influences laying performance of ducks (Anas platyrhynchos) and early post-hatch growth of their hatchlings by modulating gene expression. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 138, 459–466. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, J.; Hou, J.F. Effects of ipriflavone on caged layer bone metabolism in vitro and in vivo. Poult. Sci. 2007, 86, 503–507. [Google Scholar] [CrossRef]
- Van Eerden, E.; Van Den Brand, H.; Heetkamp, M.; Decuypere, E.; Kemp, B. Energy partitioning and thyroid hormone levels during salmonella enteritidis infections in pullets with high or low residual feed intake. Poult. Sci. 2006, 85, 1775–1783. [Google Scholar] [CrossRef]
- Koenig, M.; Hahn, G.; Damme, K.; Schmutz, M. Utilization of laying-type cockerels as “coquelets”: Influence of genotype and diet characteristics on growth performance and carcass composition. Eur. Poult. Sci. 2012, 76, 197–202. [Google Scholar] [CrossRef]
- Simitzis, P.; Spanou, D.; Glastra, N.; Goliomytis, M. Impact of dietary quercetin on laying hen performance, egg quality and yolk oxidative stability. Anim. Feed. Sci. Technol. 2018, 239, 27–32. [Google Scholar] [CrossRef]
- Reis, J.H.; Gebert, R.R.; Barreta, M.; Boiago, M.M.; Souza, C.F.; Baldissera, M.D.; Santos, I.D.; Wagner, R.; Laporta, L.V.; Stefani, L.M. Addition of grape pomace flour in the diet on laying hens in heat stress: Impacts on health and performance as well as the fatty acid profile and total antioxidant capacity in the egg. J. Therm. Biol. 2019, 80, 141–149. [Google Scholar] [CrossRef]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef]
- Shang, H.M.; Zhang, H.X.; Guo, Y.; Wu, H.X.; Zhang, N.Y. Effects of inulin supplementation in laying hens diet on the antioxidant capacity of refrigerated stored eggs. Int. J. Biol. Macromol. 2020, 153, 1047–1057. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I. Nutritional modulation of the antioxidant capacities in poultry: The case of selenium. Poult. Sci. 2019, 98, 4231–4239. [Google Scholar] [CrossRef]
- Kishawy, A.T.; Ibrahim, D.; Roushdy, E.M.; Moustafa, A.; Eldemery, F.; Hussein, E.M.; Hassan, F.A.; Elazab, S.T.; Elabbasy, M.T.; Kanwal, R. Impact of resveratrol-loaded liposomal nanocarriers on heat-stressed broiler chickens: Effects on performance, sirtuin expression, oxidative stress regulators, and muscle building factors. Front. Vet. Sci. 2023, 10, 1137896. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.B.; Liu, T.Y.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Hu, L.F.; Wang, Y.; Ren, R.J.; Huo, H.R.; Sun, J.H.; Li, H.M.; Zhu, Y.Y.; Tan, Y.Q. Anti-oxidative stress actions and regulation mechanisms of Keap1-Nrf2/ARE signal pathway. J. Int. Pharm. Res. 2016, 146–152, 166. [Google Scholar]
- Qi, M.; Liu, K.; He, J. Alleviation effect of ginsenoside Rg1 in rats with cholestasis by sirt5 pathway. China Pharm. 2022, 25, 1718–1723. [Google Scholar]
- Chen, X.C.; Zhang, Y.W.; Ma, W.F.; Wang, Z.B. Effects of Ligustrum lucidum on egg production, egg quality, and caecal microbiota of hens during the late laying period. Ital. J. Anim. Sci. 2020, 19, 687–696. [Google Scholar] [CrossRef]
- Du, J.H.; Xu, M.Y.; Wang, Y.; Lei, Z.; Yu, Z.; Li, M.Y. Evaluation of Taraxacum mongolicum flavonoids in diets for Channa argus based on growth performance, immune responses, apoptosis and antioxidant defense system under lipopolysaccharide stress. Fish. Shellfish Immunol. 2022, 131, 1224–1233. [Google Scholar] [CrossRef]
- Yang, S.; Jin, X.; Xu, Y.Q.; Shi, B.L. Immunomodulatory effect of flavonoids on broilers and its mechanism. Chin. J. Anim. Nutr. 2020, 32, 4003–4009. [Google Scholar]
- Lu, C.C.; Fan, G.X.; Wang, D.Y. Akebia Saponin D ameliorated kidney injury and exerted anti-inflammatory and anti-apoptotic effects in diabetic nephropathy by activation of NRF2/HO-1 and inhibition of NF-KB pathway. Int. Immunopharmacol. 2020, 84, 106467. [Google Scholar] [CrossRef]
- Gao, X.N.; Liu, P.; Wu, C.; Wang, T.C.; Liu, G.H.; Cao, H.B.; Zhang, C.Y.; Hu, G.L.; Guo, X.Q. Effects of fatty liver hemorrhagic syndrome on the AMP-activated protein kinase signaling pathway in laying hens. Poult. Sci. 2019, 98, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Trott, K.; Giannitti, F.; Rimoldi, G.; Hill, A.; Woods, L.; Barr, B.; Anderson, M.; Mete, A. Fatty liver hemorrhagic syndrome in the backyard chicken: A retrospective histopathologic case series. Vet. Pathol. 2014, 51, 787–795. [Google Scholar] [CrossRef]
- Hamid, H.; Zhang, J.Y.; Li, W.X.; Liu, C.; Li, M.L.; Zhao, L.H.; Ji, C.; Ma, Q.G. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult. Sci. 2019, 98, 2509–2521. [Google Scholar] [CrossRef]
- Jian, H.F.; Xu, Q.Q.; Wang, X.M.; Liu, Y.T.; Miao, S.S.; Li, Y.; Mou, T.M.; Dong, X.Y.; Zou, X.T. Amino acid and fatty acid metabolism disorders trigger oxidative stress and inflammatory response in excessive dietary valine-induced NAFLD of laying hens. Front. Nutr. 2022, 9, 849767. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Arain, M.; Arif, M.; Abd El-Hack, M.; Alagawany, M.; Siyal, F.; Soomro, R.; Sun, C. Quercetin: Nutritional and beneficial effects in poultry. World’s Poult. Sci. J. 2017, 73, 355–364. [Google Scholar] [CrossRef]
- Sohaib, M.; Butt, M.S.; Shabbir, M.A.; Shahid, M. Lipid stability, antioxidant potential and fatty acid composition of broilers breast meat as influenced by quercetin in combination with α-tocopherol enriched diets. Lipids Health Dis. 2015, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Xu, Y.D.; Wang, D.R.; Yang, S.H.; Song, Z.H.; Li, R.; He, X. Dietary silymarin improves performance by altering hepatic lipid metabolism and cecal microbiota function and its metabolites in late laying hens. J. Anim. Sci. Biotechnol. 2024, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Videnska, P.; Sedlar, K.; Lukac, M.; Faldynova, M.; Gerzova, L.; Cejkova, D.; Sisak, F.; Rychlik, I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS ONE 2014, 9, e115142. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Qi, G.H.; Wang, J.; Zhang, H.J.; Qiu, K.; Wu, S.G. Intestinal microbiota of layer hens and its association with egg quality and safety. Poult. Sci. 2022, 101, 102008. [Google Scholar] [CrossRef] [PubMed]
- Panasevich, M.R.; Meers, G.M.; Linden, M.A.; Booth, F.W.; Perfield, J.; Fritsche, K.L.; Wankhade, U.D.; Chintapalli, S.V.; Shankar, K.; Ibdah, J. High-fat, high-fructose, high-cholesterol feeding causes severe NASH and cecal microbiota dysbiosis in juvenile Ossabaw swine. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E78–E92. [Google Scholar] [CrossRef]
- Wang, Y.M.; Chen, B.; Cao, J.M.; Huang, Y.H.; Wang, G.X.; Peng, K.; Mo, W.Y.; Zhao, H.X. Effects of mulberry leaf flavonoids on intestinal mucosal morphology and intestinal flora of Litopenaeus vannamei. Chin. J. Anim. Nutr. 2020, 32, 1817–1825. [Google Scholar]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]



| Ingredients | (%) |
|---|---|
| Corn | 57.82 |
| Soybean meal (CP, 43%) | 23.80 |
| Wheat bran | 5.00 |
| Soybean oil | 1.75 |
| Grainy limestone | 6.90 |
| Powdery limestone | 2.30 |
| CaHPO4 | 1.40 |
| DL-methionine | 0.10 |
| NaCl | 0.30 |
| Choline chloride | 0.10 |
| Vitamin premix 1 | 0.03 |
| Mineral premix 1 | 0.50 |
| Total | 100.00 |
| Nutrient levels | |
| Metabolizable energy (Kcal/Kg) | 2639.25 |
| Crude protein | 15.48 |
| Calcium | 3.95 |
| Total phosphorus | 0.60 |
| Available phosphorus | 0.34 |
| Digestible lysine | 0.80 |
| Digestible methionine | 0.34 |
| Digestible threonine | 0.59 |
| Digestible tryptophan | 0.17 |
| Digestible methionine + cystine | 0.62 |
| Gene Name | Primer Sequence (5′-3′) | Gene Bank ID |
|---|---|---|
| β-Actin | Forward: CCA GCC ATG TAT GTA GCC ATC CAG Reverse: ACG GCC AGC CAG ATC CAG AC | NM_205518 |
| CAT | Forward: TAC TGC AAG GCG AAA GTG TT Reverse: GGA AAC AAC ATT GCA TCC CG | NM_001031215 |
| SOD | Forward: GCA GGT GCT CAC TTT AAT CCT Reverse: CCA CAA GCT AAA CGA GGT CC | GGU28407 |
| GPX | Forward: AAC GGC TTC AAA CCC AAC TT Reverse: GAC CAG ATG ATG TAC TGC GG | HM590226 |
| KEAP1 | Forward: CAT CGA CTG TTA CAA CCC CAT Reverse: CGG CGT ACA GCA GTA TGT T | KU321503.1 |
| NRF2 | Forward: CCC AAA ACT GCC GTA AGA GA Reverse: TGC CAT CTC TAG TTT GCT GC | NM_205117.1 |
| Items | Marigold Extract Supplemental Levels (mg/kg) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| ADFI (g/day) | 108.70 | 109.12 | 109.51 | 108.27 | 109.19 | 0.15 | 0.076 | 0.907 | 0.882 |
| Egg production rate (%) | 87.09 a | 88.52 ab | 89.87 bc | 89.77 bc | 90.39 c | 0.31 | 0.002 | <0.001 | <0.001 |
| Qualified egg rate (%) | 86.17 a | 87.54 ab | 88.71 b | 88.62 b | 89.45 b | 0.33 | 0.009 | <0.001 | <0.001 |
| Average egg weight (g) | 61.18 | 61.52 | 61.44 | 61.28 | 61.06 | 0.11 | 0.732 | 0.557 | 0.397 |
| Feed to egg rate (g/g) | 2.04 a | 2.01 ab | 1.98 b | 1.97 b | 1.98 b | 0.01 | 0.012 | <0.001 | <0.001 |
| Items | Marigold Extract Supplemental Levels (mg/kg) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| Week 12 | |||||||||
| Albumen height (mm) | 7.15 | 6.97 | 6.64 | 6.44 | 6.85 | 0.09 | 0.086 | 0.072 | 0.038 |
| Haugh unit | 83.04 | 82.34 | 79.55 | 78.72 | 81.32 | 0.59 | 0.098 | 0.092 | 0.050 |
| Egg yolk color | 5.38 | 5.80 | 5.31 | 5.58 | 5.90 | 0.08 | 0.109 | 0.174 | 0.291 |
| Eggshell strength (kg/cm2) | 4.58 | 4.67 | 4.88 | 4.62 | 4.65 | 0.06 | 0.575 | 0.838 | 0.497 |
| Week 24 | |||||||||
| Albumen height (mm) | 6.90 | 7.07 | 7.06 | 6.94 | 7.11 | 0.08 | 0.927 | 0.621 | 0.875 |
| Haugh unit | 81.33 | 82.73 | 82.74 | 81.68 | 82.72 | 0.60 | 0.912 | 0.685 | 0.866 |
| Egg yolk color | 5.44 | 5.21 | 4.97 | 5.06 | 4.88 | 0.08 | 0.241 | 0.031 | 0.082 |
| Eggshell strength (kg/cm2) | 4.13 | 4.37 | 4.40 | 4.30 | 4.06 | 0.07 | 0.495 | 0.688 | 0.178 |
| Items | Marigold Extract Supplemental Levels (mg/kg) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| ALT (U/L) | 5.68 | 6.52 | 6.05 | 5.11 | 5.60 | 0.31 | 0.763 | 0.575 | 0.760 |
| AST (U/L) | 163.99 | 157.74 | 163.54 | 161.53 | 156.79 | 1.63 | 0.603 | 0.469 | 0.722 |
| TP (g/L) | 52.08 | 54.39 | 55.63 | 52.64 | 53.60 | 1.07 | 0.864 | 0.872 | 0.735 |
| ALB (g/L) | 17.95 | 19.22 | 17.56 | 17.82 | 17.53 | 0.26 | 0.346 | 0.302 | 0.555 |
| GLB (g/L) | 32.14 | 35.86 | 36.67 | 36.34 | 33.71 | 1.10 | 0.644 | 0.619 | 0.278 |
| AKP U/L) | 802.71 | 783.00 | 754.33 | 730.50 | 689.67 | 70.56 | 0.989 | 0.571 | 0.853 |
| UA (μmol/L) | 145.06 | 149.88 | 143.31 | 142.39 | 145.23 | 5.13 | 0.996 | 0.957 | 0.983 |
| TC (mmol/L) | 3.03 | 3.52 | 3.22 | 2.72 | 2.98 | 0.12 | 0.335 | 0.379 | 0.503 |
| TG (mmol/L) | 17.27 | 15.70 | 15.89 | 15.02 | 16.67 | 0.63 | 0.833 | 0.567 | 0.551 |
| LDL (mmol/L) | 1.49 | 1.64 | 1.42 | 1.38 | 1.50 | 0.05 | 0.526 | 0.513 | 0.774 |
| HDL (mmol/L) | 1.02 | 1.04 | 0.94 | 0.92 | 0.84 | 0.04 | 0.447 | 0.064 | 0.167 |
| VLDL (mmol/L) | 7.12 | 6.58 | 7.70 | 6.98 | 7.21 | 0.16 | 0.258 | 0.600 | 0.845 |
| Items | Marigold Extract Supplemental Levels (mg/kg) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| Week 12 | |||||||||
| Triglyceride (mmol/g) | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.00 | 0.189 | 0.017 | 0.053 |
| Yolks MDA (nmol/mg) | 4.71 | 3.75 | 3.71 | 3.82 | 3.98 | 0.12 | 0.067 | 0.181 | 0.022 |
| Yolks DPPH (%) | 42.40 a | 42.34 a | 45.07 ab | 47.09 b | 44.26 ab | 0.60 | 0.050 | 0.051 | 0.059 |
| Yolks restoring power (A700nm) | 0.35 | 0.37 | 0.36 | 0.37 | 0.38 | 0.01 | 0.695 | 0.293 | 0.576 |
| Protein carbonyls | 0.18 | 0.16 | 0.16 | 0.18 | 0.14 | 0.01 | 0.447 | 0.273 | 0.518 |
| Egg white MDA (nmol/mg) | 1.50 | 1.53 | 1.55 | 1.66 | 1.76 | 0.06 | 0.641 | 0.123 | 0.280 |
| Egg white DPPH (%) | 22.77 a | 25.49 a | 28.77 b | 28.43 b | 28.3 b | 0.63 | 0.004 | <0.001 | <0.001 |
| Egg white restoring power (A700nm) | 0.14 | 0.14 | 0.14 | 0.14 | 0.15 | 0.00 | 0.924 | 0.928 | 0.776 |
| Week 24 | |||||||||
| Triglyceride (mmol/g) | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.00 | 0.391 | 0.045 | 0.138 |
| Yolks MDA (nmol/mg) | 4.53 a | 3.64 b | 3.75 b | 3.59 b | 3.42 b | 0.12 | 0.032 | 0.008 | 0.014 |
| Yolks DPPH (%) | 37.39 a | 42.49 b | 42.45 b | 44.05 b | 42.40 b | 0.75 | 0.043 | 0.025 | 0.010 |
| Yolks restoring power (A700nm) | 0.32 | 0.34 | 0.36 | 0.35 | 0.34 | 0.01 | 0.519 | 0.293 | 0.191 |
| Protein carbonyls | 0.17 a | 0.13 b | 0.13 b | 0.13 b | 0.12 b | 0.01 | 0.041 | 0.009 | 0.016 |
| Egg white MDA (nmol/mg) | 1.58 | 1.67 | 1.71 | 1.76 | 1.78 | 0.05 | 0.758 | 0.174 | 0.384 |
| Egg white DPPH (%) | 24.13 a | 27.26 b | 27.65 b | 27.71 b | 28.86 b | 0.51 | 0.036 | 0.005 | 0.010 |
| Egg white restoring power (A700nm) | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.00 | 0.971 | 0.664 | 0.911 |
| Items | Marigold Extract Supplemental Levels (mg/kg) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| 12-week serum | |||||||||
| T-AOC (UmL) | 0.66 | 0.72 | 0.70 | 0.79 | 0.66 | 0.02 | 0.235 | 0.594 | 0.299 |
| MDA (nmol/mL) | 8.54 a | 7.62 ab | 7.26 ab | 6.87 ab | 5.94 b | 0.28 | 0.047 | 0.002 | 0.008 |
| SOD (U/mL) | 84.69 | 86.76 | 86.48 | 92.16 | 87.19 | 1.14 | 0.312 | 0.201 | 0.283 |
| 24-week serum | |||||||||
| T-AOC (U/mL) | 0.79 | 0.87 | 0.89 | 0.82 | 0.86 | 0.02 | 0.561 | 0.502 | 0.468 |
| MDA (nmol/mL) | 8.12 | 8.11 | 7.98 | 8.00 | 7.41 | 0.17 | 0.707 | 0.215 | 0.373 |
| SOD (U/mL) | 77.76 | 81.58 | 86.74 | 83.11 | 89.05 | 1.65 | 0.219 | 0.036 | 0.109 |
| 24-week liver | |||||||||
| T-AOC (U/mgprot) | 0.10 | 0.11 | 0.11 | 0.11 | 0.11 | 0.00 | 0.993 | 0.887 | 0.949 |
| MDA (nmol/mgprot) | 0.49 | 0.44 | 0.54 | 0.52 | 0.52 | 0.02 | 0.661 | 0.378 | 0.664 |
| SOD (U/mgprot) | 3.61 | 3.85 | 4.53 | 4.82 | 4.83 | 0.21 | 0.242 | 0.024 | 0.069 |
| DPPH (%) | 93.32 a | 94.98 b | 94.79 b | 94.66 b | 95.04 b | 0.15 | <0.001 | 0.003 | <0.001 |
| Restoring power (A700nm) | 0.40 | 0.42 | 0.43 | 0.45 | 0.43 | 0.01 | 0.401 | 0.110 | 0.199 |
| Protein carbonyls | 0.26 a | 0.22 ab | 0.20 b | 0.18 b | 0.25 a | 0.01 | 0.005 | 0.341 | <0.001 |
| Items | Marigold Extract Supplemental Levels (mg/kg) | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| TG (mmol/L) | 7.25 | 6.94 | 6.97 | 6.61 | 6.55 | 0.19 | 0.797 | 0.206 | 0.455 |
| TC (mmol/L) | 7.27 | 7.79 | 7.40 | 6.78 | 6.44 | 0.18 | 0.195 | 0.042 | 0.052 |
| LDL (mmol/L) | 6.52 | 5.92 | 6.16 | 5.84 | 5.15 | 0.18 | 0.175 | 0.024 | 0.071 |
| HDL (mmol/L) | 19.20 ab | 19.70 abc | 18.52 a | 22.72 c | 22.15 bc | 0.54 | 0.036 | 0.018 | 0.044 |
| VLDL (mmol/L) | 2.38 a | 2.74 ab | 3.21 b | 3.02 b | 3.10 b | 0.09 | 0.037 | 0.011 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zhang, K.; Wang, J.; Bai, S.; Zeng, Q.; Peng, H.; Mu, Y.; Xuan, Y.; Li, S.; Ding, X. The Addition of Marigold Extract to the Diet Improved the Performance of Laying Hens in the Late Laying Period by Increasing Their Antioxidant Capacity, Lipid Metabolism, and Microbial Composition. Antioxidants 2025, 14, 1126. https://doi.org/10.3390/antiox14091126
Yang Q, Zhang K, Wang J, Bai S, Zeng Q, Peng H, Mu Y, Xuan Y, Li S, Ding X. The Addition of Marigold Extract to the Diet Improved the Performance of Laying Hens in the Late Laying Period by Increasing Their Antioxidant Capacity, Lipid Metabolism, and Microbial Composition. Antioxidants. 2025; 14(9):1126. https://doi.org/10.3390/antiox14091126
Chicago/Turabian StyleYang, Qiyue, Keying Zhang, Jianping Wang, Shiping Bai, Qiufeng Zeng, Huanwei Peng, Yadong Mu, Yue Xuan, Shanshan Li, and Xuemei Ding. 2025. "The Addition of Marigold Extract to the Diet Improved the Performance of Laying Hens in the Late Laying Period by Increasing Their Antioxidant Capacity, Lipid Metabolism, and Microbial Composition" Antioxidants 14, no. 9: 1126. https://doi.org/10.3390/antiox14091126
APA StyleYang, Q., Zhang, K., Wang, J., Bai, S., Zeng, Q., Peng, H., Mu, Y., Xuan, Y., Li, S., & Ding, X. (2025). The Addition of Marigold Extract to the Diet Improved the Performance of Laying Hens in the Late Laying Period by Increasing Their Antioxidant Capacity, Lipid Metabolism, and Microbial Composition. Antioxidants, 14(9), 1126. https://doi.org/10.3390/antiox14091126








