Multielemental Profile for Seminal Plasma Through Inductively Coupled Plasma–Tandem Mass Spectrometry and Its Relationship with Seminal Parameters, Spermatic Biomarkers, and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Sample Analysis
2.2. Sperm Capacitation by Swim-Up
2.3. Induction of Acrosomal Reaction
2.4. Fixation
2.5. Evaluation of Acrosomal Reaction
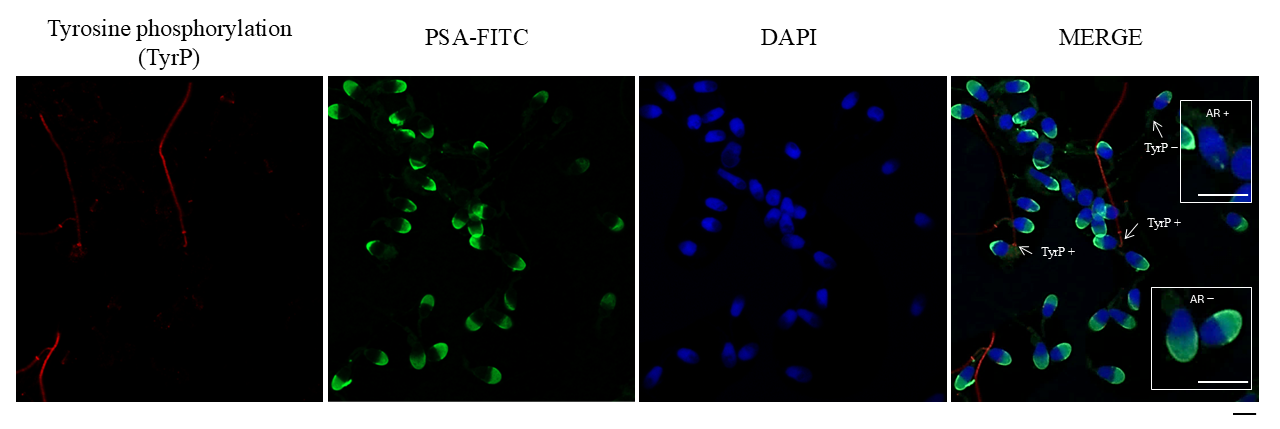
2.6. Immunofluorescence of Tyrosine Phosphorylation
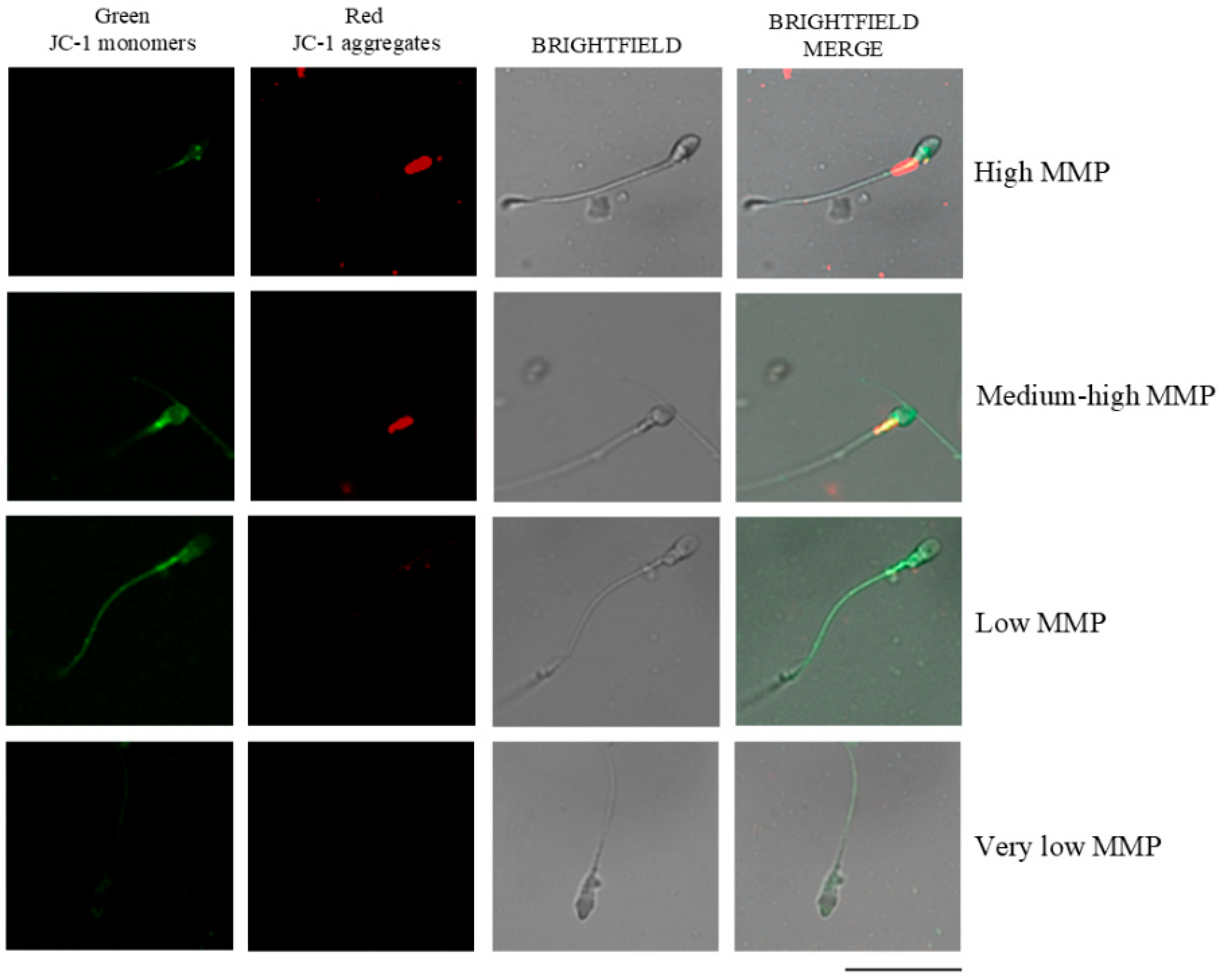
2.7. Mitochondrial Membrane Potential Assessment (MMP)
2.8. Seminal Plasma Samples’ Treatment and Standards
2.9. Instrumentation
2.10. Statistical Analysis
3. Results
3.1. Identification of the Conditions Resulting in Optimum Sensitivities and Accurate Results
3.1.1. Optimization of the Operating Conditions from the Point of View of Sensitivity
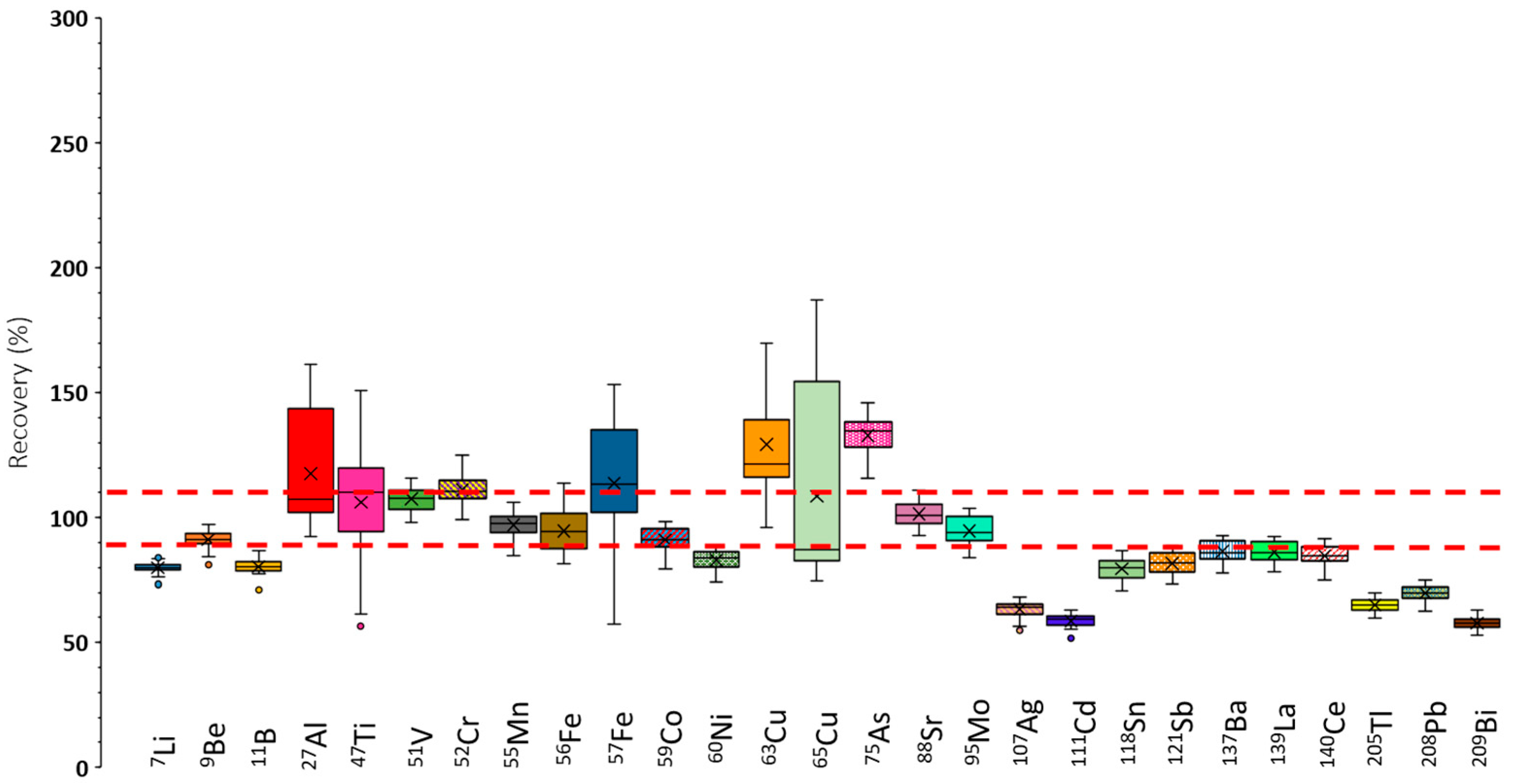
3.1.2. Recovery Tests Under Sensitivity Optimum Conditions
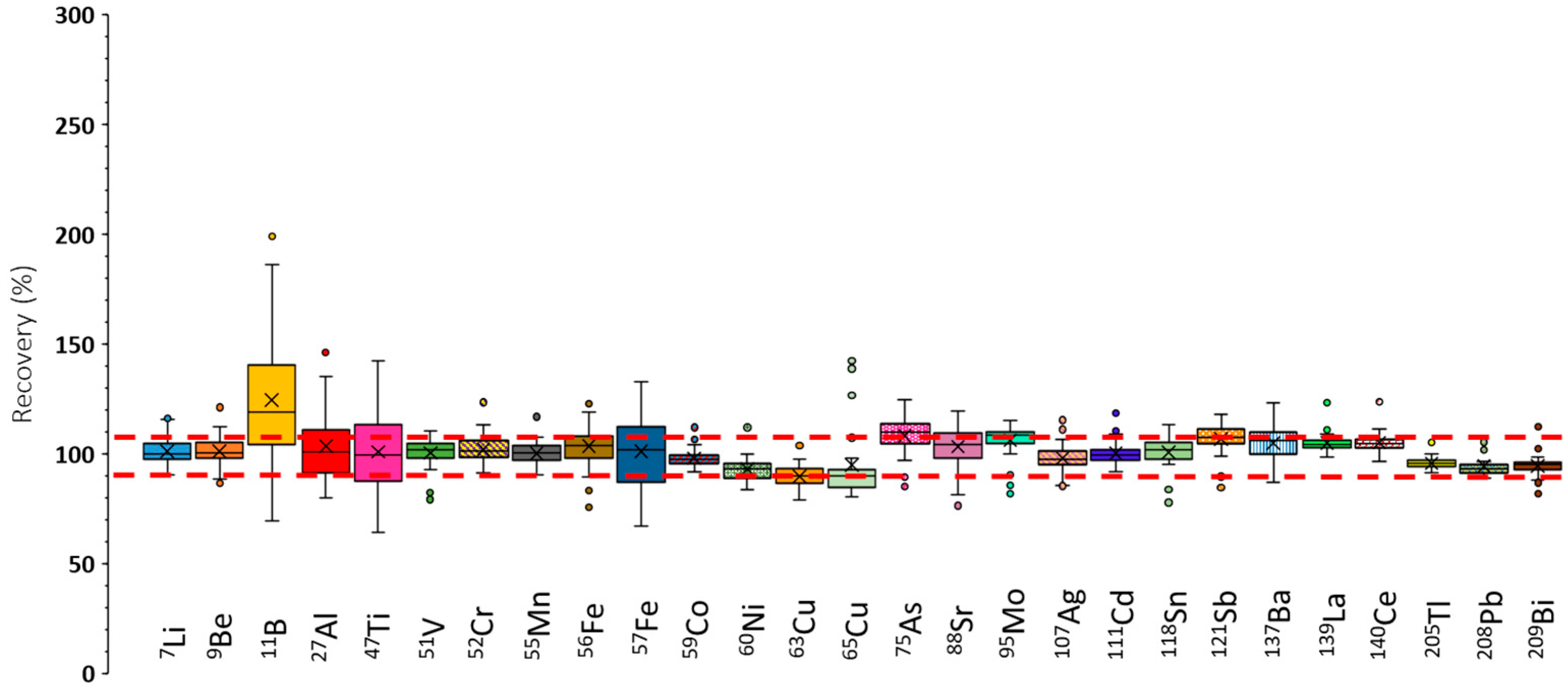
3.1.3. Recovery Tests Under Robust ICP-MS/MS Conditions
3.1.4. Limits of Detection and Quantification Under Robust ICP-MS/MS Conditions
3.2. Andrology Results
3.2.1. Characteristics of This Study’s Population, Semen Analysis Results, and Sperm Biomarkers
3.2.2. Concentrations of Elements in Seminal Plasma
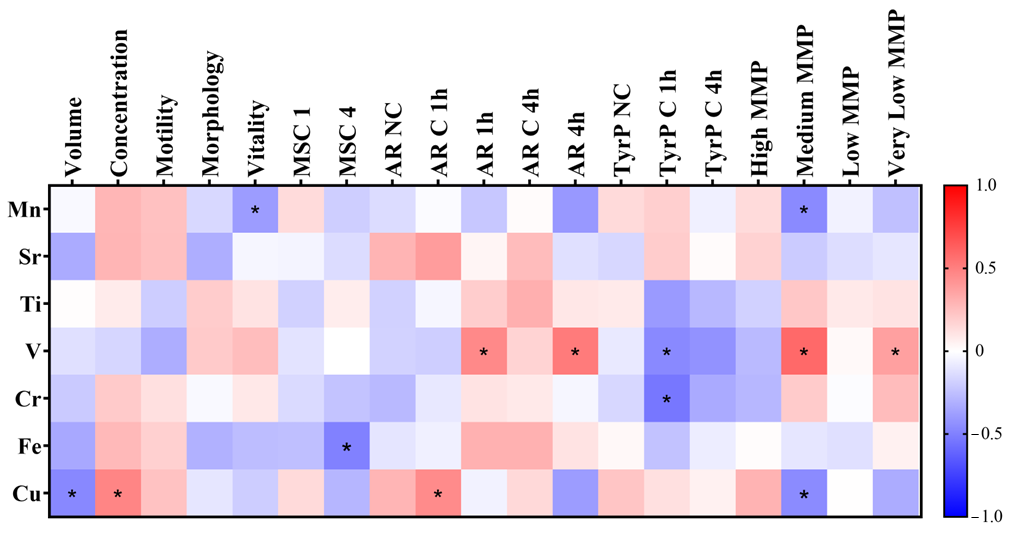
3.2.3. Correlations of Heavy Metals and Metalloids Concentrations with Seminal Parameters and Spermatic Biomarkers
3.2.4. Comparison of Sperm Parameters Between High and Low Element Levels in Seminal Plasma
4. Discussion
4.1. Novel ICP-MS/MS Analysis Method
4.2. Relationship of Heavy Metals and Metalloids in Seminal Plasma with Seminal Parameters and Sperm Biomarkers
4.2.1. Impact of Manganese
4.2.2. Impact of Strontium
4.2.3. Impact of Titanium
4.2.4. Impact of Iron
4.2.5. Impact of Copper
4.2.6. Impact of Chromium
4.2.7. Impact of Arsenic, Molybdenum, and Lead
4.2.8. Biological Implications of the Results
4.3. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- López-Botella, A.; Velasco, I.; Acién, M.; Sáez-Espinosa, P.; Todolí-Torró, J.-L.; Sánchez-Romero, R.; Gómez-Torres, M.J. Impact of Heavy Metals on Human Male Fertility—An Overview. Antioxidants 2021, 10, 1473. [Google Scholar] [CrossRef]
- Figa-Talamanca, I. Occupational Exposures to Metals, Solvents and Pesticides: Recent Evidence on Male Reproductive Effects and Biological Markers. Occup. Med. (Chic. Ill.) 2001, 51, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E. What Are Heavy Metals? Long-Standing Controversy over the Scientific Use of the Term ‘Heavy Metals’—Proposal of a Comprehensive Definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Balabanič, D.; Rupnik, M.; Klemenčič, A.K. Negative Impact of Endocrine-Disrupting Compounds on Human Reproductive Health. Reprod. Fertil. Dev. 2011, 23, 403. [Google Scholar] [CrossRef]
- Oladosu, W.O.; Lawani, O.A.; Oyewo, R.A.; Oderinu, K.A.; Jimoh, O.S.; Motayo, B.O.; AbdulGhaffaar, O.H.J. Evaluating the Effects of Heavy Metals on Seminal Fluid Anti Oxidants Status and Semen Parameters among Males with Infertility at Tertiary Health Centre in Nigeria. Adv. Hum. Biol. 2025, 15, 81–87. [Google Scholar] [CrossRef]
- Dorostghoal, M.; Izadi, F.; Gholami, M.; Zahraei, S. Seminal Plasma Levels of Heavy Metals (Lead, Cadmium and Arsenic) and Oxidative Status in Asthnozoospermic Men. Gene Cell Tissue 2025, 12, e155559. [Google Scholar] [CrossRef]
- Kasperczyk, A.; Dobrakowski, M.; Czuba, Z.; Kapka-Skrzypczak, L.; Kasperczyk, S. Environmental Exposure to Zinc and Copper Influences Sperm Quality in Fertile Males. Ann. Agric. Environ. Med. 2015, 23, 138–143. [Google Scholar] [CrossRef]
- Nenkova, G.; Petrov, L.; Alexandrova, A. Role of Trace Elements for Oxidative Status and Quality of Human Sperm. Balkan Med. J. 2017, 34, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Droller, M.J. Environment and The Genitourinary Tract. Otolaryngol.–Head Neck Surg. 1996, 114, 248–252. [Google Scholar] [CrossRef]
- Ilieva, I.; Sainova, I.; Yosifcheva, K. Toxic Effects of Heavy Metals (Lead and Cadmium) on Sperm Quality and Male Fertility. Acta Morphol. Anthropol. 2020, 27, 3–4. [Google Scholar]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires. A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar] [CrossRef]
- Ashiru, O.A.; Odusanya, O.O. Fertility and Occupational Hazards: Review of the Literature. Afr. J. Reprod. Health 2009, 13, 159–165. [Google Scholar] [PubMed]
- López-Botella, A.; Sánchez, R.; Paul, R.; Aizpurua, J.; Gómez-Torres, M.J.; Todolí-Torró, J.L. Analytical Determination of Heavy Metals in Human Seminal Plasma—A Systematic Review. Life 2023, 13, 925. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Varghese, A.C.; Mandal, S.; Bhattacharyya, S.; Nandi, P.; Rahman, S.M.; Kar, K.K.; Saha, R.; Roychoudhury, S.; Murmu, N. Lead and Cadmium Exposure Induces Male Reproductive Dysfunction by Modulating the Expression Profiles of Apoptotic and Survival Signal Proteins in Tea-Garden Workers. Reprod. Toxicol. 2020, 98, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, G.U.S.; Fernando, D.M.S.; Wijerathna, S.; Bandara, N. Environmental and Occupational Exposures as a Cause of Male Infertility: A Caveat. Ceylon Med. J. 2015, 60, 52. [Google Scholar] [CrossRef]
- Živković, T.; Tariba, B.; Pizent, A. Multielement Analysis of Human Seminal Plasma by Octopole Reaction Cell ICP-MS. J. Anal. At. Spectrom. 2014, 29, 2114–2126. [Google Scholar] [CrossRef]
- Balcaen, L.; Bolea-Fernandez, E.; Resano, M.; Vanhaecke, F. Inductively Coupled Plasma—Tandem Mass Spectrometry (ICP-MS/MS): A Powerful and Universal Tool for the Interference-Free Determination of (Ultra)Trace Elements—A Tutorial Review. Anal. Chim. Acta 2015, 894, 7–19. [Google Scholar] [CrossRef]
- Meyer, S.; Markova, M.; Pohl, G.; Marschall, T.A.; Pivovarova, O.; Pfeiffer, A.F.H.; Schwerdtle, T. Development, Validation and Application of an ICP-MS/MS Method to Quantify Minerals and (Ultra-)Trace Elements in Human Serum. J. Trace Elem. Med. Biol. 2018, 49, 157–163. [Google Scholar] [CrossRef]
- Galusha, A.L.; Haig, A.C.; Bloom, M.S.; Kruger, P.C.; McGough, A.; Lenhart, N.; Wong, R.; Fujimoto, V.Y.; Mok-Lin, E.; Parsons, P.J. Ultra-Trace Element Analysis of Human Follicular Fluid by ICP-MS/MS: Pre-Analytical Challenges, Contamination Control, and Matrix Effects. J. Anal. At. Spectrom. 2019, 34, 741–752. [Google Scholar] [CrossRef]
- Gómez-Torres, M.J.; Hernández-Falcó, M.; López-Botella, A.; Huerta-Retamal, N.; Sáez-Espinosa, P. IZUMO1 Receptor Localization during Hyaluronic Acid Selection in Human Spermatozoa. Biomedicines 2023, 11, 2872. [Google Scholar] [CrossRef]
- Sáez-Espinosa, P.; Torrijo-Boix, S.; Huerta-Retamal, N.; Avilés, M.; Aizpurua, J.; Romero, A.; Gómez-Torres, M.J. La Capacitación y La Reacción Acrosómica Se Asocian Con Cambios En La Localización Del Ácido Siálico y En La Morfometría de La Región Cefálica Del Espermatozoide Humano. Rev. Int. Androl. 2018, 16, 20–27. [Google Scholar] [CrossRef]
- Sáez-Espinosa, P.; Huerta-Retamal, N.; Robles-Gómez, L.; Avilés, M.; Aizpurua, J.; Velasco, I.; Romero, A.; Gómez-Torres, M. Influence of in Vitro Capacitation Time on Structural and Functional Human Sperm Parameters. Asian J. Androl. 2020, 22, 447–453. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Freire-Brito, L.; Oliveira, P.F.; Alves, M.G. Evaluation of Human Spermatozoa Mitochondrial Membrane Potential Using the JC-1 Dye. Curr. Protoc. 2022, 2, e531. [Google Scholar] [CrossRef]
- López-Botella, A.; Hernández-Falcó, M.; Sáez-Espinosa, P.; Robles-Gómez, P.; Gómez-Torres, M.J. Sperm Mitochondrial Membrane Potential: Relationship with Seminal Parameters and Lifestyle Habits. Rev. Int. Androl. 2025, 23, 57. [Google Scholar] [CrossRef]
- Huggins, C.; Scott, W.W.; Heinen, J.H. Chemical composition of human semen and of the secretions of the prostate and seminal vesicles. Am. J. Physiol.-Leg. Content 1942, 136, 467–473. [Google Scholar] [CrossRef]
- May, T.W.; Wiedmeyer, R.H. S A Atomic Spectroscopy A Table of Polyatomic Interferences in ICP-MS Isotope Abundance Interference Reference A Table of Polyatomic Interferences in ICP-MS. At. Spectrosc. 1998, 19, 150–155. [Google Scholar]
- Wang, Y.-X.; Wang, P.; Feng, W.; Liu, C.; Yang, P.; Chen, Y.-J.; Sun, L.; Sun, Y.; Yue, J.; Gu, L.-J.; et al. Relationships between Seminal Plasma Metals/Metalloids and Semen Quality, Sperm Apoptosis and DNA Integrity. Environ. Pollut. 2017, 224, 224–234. [Google Scholar] [CrossRef]
- Bakulski, K.M.; Seo, Y.A.; Hickman, R.C.; Brandt, D.; Vadari, H.S.; Hu, H.; Park, S.K. Heavy Metals Exposure and Alzheimer’s Disease and Related Dementias. J. Alzheimer’s Dis. 2020, 76, 1215–1242. [Google Scholar] [CrossRef]
- Li, P.; Zhong, Y.; Jiang, X.; Wang, C.; Zuo, Z.; Sha, A. Seminal Plasma Metals Concentration with Respect to Semen Quality. Biol. Trace Elem. Res. 2012, 148, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Zhou, W.; Gao, E. Effects of Manganese on Routine Semen Quality Parameters: Results from a Population-Based Study in China. BMC Public Health 2012, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, X.-M.; He, D.-L.; Zou, X.-M.; Wu, C.-Q.; Guo, W.-Z.; Feng, W. Evaluation of Urinary Metal Concentrations and Sperm DNA Damage in Infertile Men from an Infertility Clinic. Environ. Toxicol. Pharmacol. 2016, 45, 68–73. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional Aspects of Manganese Homeostasis. Mol. Aspects Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St. Clair, D.K. Manganese Superoxide Dismutase: Guardian of the Powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Chaspoul, F.; Anderson, L.; Bergé-Lefranc, D.; Achard, V.; Perrin, J.; Gallice, P.; Guichaoua, M. Mapping Fifteen Trace Elements in Human Seminal Plasma and Sperm DNA. Biol. Trace Elem. Res. 2017, 175, 244–253. [Google Scholar] [CrossRef]
- Miao, Y.; Liu, L.; Liu, C.; Deng, Y.-L.; Chen, P.-P.; Luo, Q.; Cui, F.-P.; Zhang, M.; Lu, W.-Q.; Zeng, Q. Urinary Biomarker of Strontium Exposure Is Positively Associated with Semen Quality among Men from an Infertility Clinic. Ecotoxicol. Environ. Saf. 2021, 208, 111694. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Akça, H.; Turna, F.; Aksakal, S.; Burgucu, D.; Kaya, B.; Tokgün, O.; Vales, G.; Creus, A.; Marcos, R. Genotoxic and Cell-Transforming Effects of Titanium Dioxide Nanoparticles. Environ. Res. 2015, 136, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Nigro, M.; Bernardeschi, M.; Costagliola, D.; Della Torre, C.; Frenzilli, G.; Guidi, P.; Lucchesi, P.; Mottola, F.; Santonastaso, M.; Scarcelli, V.; et al. N-TiO2 and CdCl2 Co-Exposure to Titanium Dioxide Nanoparticles and Cadmium: Genomic, DNA and Chromosomal Damage Evaluation in the Marine Fish European Sea Bass (Dicentrarchus Labrax). Aquat. Toxicol. 2015, 168, 72–77. [Google Scholar] [CrossRef]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of Titanium Dioxide Nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Colacurci, N.; Iovine, C.; Pacifico, S.; Cammarota, M.; Cesaroni, F.; Rocco, L. In Vitro Genotoxic Effects of Titanium Dioxide Nanoparticles (N-TiO2) in Human Sperm Cells. Mol. Reprod. Dev. 2019, 86, 1369–1377. [Google Scholar] [CrossRef]
- Ammar, O.; Houas, Z.; Mehdi, M. The Association between Iron, Calcium, and Oxidative Stress in Seminal Plasma and Sperm Quality. Environ. Sci. Pollut. Res. 2019, 26, 14097–14105. [Google Scholar] [CrossRef]
- Aydemir, B.; Kiziler, A.R.; Onaran, I.; Alici, B.; Ozkara, H.; Akyolcu, M.C. Impact of Cu and Fe Concentrations on Oxidative Damage in Male Infertility. Biol. Trace Elem. Res. 2006, 112, 193–204. [Google Scholar] [CrossRef]
- Marzec-Wróblewska, U.; Kamiński, P.; Łakota, P.; Szymański, M.; Wasilow, K.; Ludwikowski, G.; Kuligowska-Prusińska, M.; Odrowąż-Sypniewska, G.; Stuczyński, T.; Michałkiewicz, J. Zinc and Iron Concentration and SOD Activity in Human Semen and Seminal Plasma. Biol. Trace Elem. Res. 2011, 143, 167–177. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Tseng, W.-C.; Lin, T.-H. In Vitro Effects of Metal Ions (Fe2+, Mn2+, Pb2+) on Sperm Motility and Lipid Peroxidation in Human Semen. J. Toxicol. Environ. Health A 2001, 62, 259–267. [Google Scholar] [CrossRef]
- Yuyan, L.; Junqing, W.; Wei, Y.; Weijin, Z.; Ersheng, G. Are Serum Zinc and Copper Levels Related to Semen Quality? Fertil. Steril. 2008, 89, 1008–1011. [Google Scholar] [CrossRef]
- Van Niekerk, F.E.; Van Niekerk, C.H. The Influence of Experimentally Induced Copper Deficiency on the Fertility of Rams. I. Semen Parameters and Peripheral Plasma Androgen Concentration. J. S. Afr. Vet. Assoc. 1989, 60, 28–31. [Google Scholar]
- Telisman, S.; Cvitković, P.; Jurasović, J.; Pizent, A.; Gavella, M.; Rocić, B. Semen Quality and Reproductive Endocrine Function in Relation to Biomarkers of Lead, Cadmium, Zinc, and Copper in Men. Environ. Health Perspect. 2000, 108, 45–53. [Google Scholar] [CrossRef]
- Chang, S.I.; Jin, B.; Youn, P.; Park, C.; Park, J.-D.; Ryu, D.-Y. Arsenic-Induced Toxicity and the Protective Role of Ascorbic Acid in Mouse Testis. Toxicol. Appl. Pharmacol. 2007, 218, 196–203. [Google Scholar] [CrossRef]
- Adeogun, A.E.; Ogunleye, O.D.; Akhigbe, T.M.; Oyedokun, P.A.; Adegbola, C.A.; Saka, W.A.; Afolabi, O.A.; Akhigbe, R.E. Impact of Arsenic on Male and Female Reproductive Function: A Review of the Pathophysiology and Potential Therapeutic Strategies. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 398, 1283–1297. [Google Scholar] [CrossRef]
- Wu, S.; Wang, M.; Deng, Y.; Qiu, J.; Zhang, X.; Tan, J. Associations of Toxic and Essential Trace Elements in Serum, Follicular Fluid, and Seminal Plasma with In Vitro Fertilization Outcomes. Ecotoxicol. Environ. Saf. 2020, 204, 110965. [Google Scholar] [CrossRef]
- Sukhn, C.; Awwad, J.; Ghantous, A.; Zaatari, G. Associations of Semen Quality with Non-Essential Heavy Metals in Blood and Seminal Fluid: Data from the Environment and Male Infertility (EMI) Study in Lebanon. J. Assist. Reprod. Genet. 2018, 35, 1691–1701. [Google Scholar] [CrossRef]
- Zhai, X.-W.; Zhang, Y.-L.; Qi, Q.; Bai, Y.; Chen, X.-L.; Jin, L.-J.; Ma, X.-G.; Shu, R.-Z.; Yang, Z.-J.; Liu, F.-J. Effects of Molybdenum on Sperm Quality and Testis Oxidative Stress. Syst. Biol. Reprod. Med. 2013, 59, 251–255. [Google Scholar] [CrossRef]
- Bersényi, A.; Berta, E.; Kádár, I.; Glávits, R.; Szilágyi, M.; Fekete, S. Effects of High Dietary Molybdenum in Rabbits. Acta Vet. Hung. 2008, 56, 41–55. [Google Scholar] [CrossRef][Green Version]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Cadmium, Lead, and Other Metals in Relation to Semen Quality: Human Evidence for Molybdenum as a Male Reproductive Toxicant. Environ. Health Perspect. 2008, 116, 1473–1479. [Google Scholar] [CrossRef]
- Javorac, D.; Baralić, K.; Marić, Đ.; Mandić-Rajčević, S.; Đukić-Ćosić, D.; Bulat, Z.; Djordjevic, A.B. Exploring the Endocrine Disrupting Potential of Lead through Benchmark Modelling—Study in Humans. Environ. Pollut. 2023, 316, 120428. [Google Scholar] [CrossRef]
- Wu, H.-M.; Lin-Tan, D.-T.; Wang, M.-L.; Huang, H.-Y.; Lee, C.-L.; Wang, H.-S.; Soong, Y.-K.; Lin, J.-L. Lead Level in Seminal Plasma May Affect Semen Quality for Men without Occupational Exposure to Lead. Reprod. Biol. Endocrinol. 2012, 10, 91. [Google Scholar] [CrossRef]
- Taha, E.A.; Sayed, S.K.; Ghandour, N.M.; Mahran, A.M.; Saleh, M.A.; Amin, M.M.; Shamloul, R. Correlation between Seminal Lead and Cadmium and Seminal Parameters in Idiopathic Oligoasthenozoospermic Males. Cent. Eur. J. Urol. 2013, 65, 84–92. [Google Scholar] [CrossRef]
- Kasperczyk, A.; Dobrakowski, M.; Czuba, Z.P.; Horak, S.; Kasperczyk, S. Environmental Exposure to Lead Induces Oxidative Stress and Modulates the Function of the Antioxidant Defense System and the Immune System in the Semen of Males with Normal Semen Profile. Toxicol. Appl. Pharmacol. 2015, 284, 339–344. [Google Scholar] [CrossRef]
- Kasperczyk, A.; Machnik, G.; Dobrakowski, M.; Sypniewski, D.; Birkner, E.; Kasperczyk, S. Gene Expression and Activity of Antioxidant Enzymes in the Blood Cells of Workers Who Were Occupationally Exposed to Lead. Toxicology 2012, 301, 79–84. [Google Scholar] [CrossRef]
- Nunzio, A.D.; Giarra, A.; Toscanesi, M.; Amoresano, A.; Piscopo, M.; Ceretti, E.; Zani, C.; Lorenzetti, S.; Trifuoggi, M.; Montano, L. Comparison between Macro and Trace Element Concentrations in Human Semen and Blood Serum in Highly Polluted Areas in Italy. Int. J. Environ. Res. Public Health 2022, 19, 11635. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| RF power (kW) | 1.6 |
| Plasma flow (L/min) | 15 |
| Auxiliary flow (L/min) | 1 |
| Nebulizer flow (L/min) | 0.4 |
| Makeup gas (L/min) | 0.3 |
| Spray chamber temperature (°C) | −5 |
| Sampling depth (mm) | 8 |
| Liquid flow (μL/min) | 100 |
| Scan type | MS/MS |
| Replicates | 6 |
| Collision reaction gas/flow rate (mL/min) | He/3.0 |
| Stabilization time (s) | No gas: 10 He: 20 |
| Sweeps | 10 |
| Internal standards | Analytes |
| 45Sc | 7Li, 9Be, 11B, 27Al, 47Ti, 51V, 52Cr, 55Mn, 56Fe, 57Fe |
| 72Ge | 59Co, 60Ni, 63Cu, 65Cu, 75As |
| 103Rh | 88Sr, 95Mo, 107Ag, 111Cd, 118Sn, 121Sb, 137Ba, 139La, 140Ce |
| 185Re | 205Tl, 208Pb, 209Bi |
| Nuclide | Abundance | Interfering Polyatomic Ion |
|---|---|---|
| 27Al | 100 | 12C15N+, 13C14N+, 14N2+, n1H12C14N+ |
| 47Ti | 7.32 | 32S14N1H+, 32S15N+, 33N14N+, 33S14N+, 15N16O2+, 14N16O21H+, 12C35Cl+, 31P16O+ |
| 52Cr | 83.76 | 35Cl16O1H+, 40Ar12C+, 36Ar16O+, 37Cl15N+, 34S18O+, 36S16O+, 38Ar14N+, 36Ar15N1H+, 35Cl17O+ |
| 56Fe | 91.66 | 40Ar16O+, 40Ca16O+, 40Ar15N1H+, 38Ar18O+, 38Ar17O1H+, 37Cl18O1H+ |
| 57Fe | 2.19 | 40Ar16O1H+, 40Ca16O1H+, 40Ar17O+, 38Ar18O1H+ |
| 60Ni | 26.16 | 44Ca16O+, 23Na37Cl+, 43Ca16O1H+ |
| 63Cu | 69.1 | 31P16O2+, 40Ar23Na+, 47Ti16O+, 23Na40Ca+, 46Ca16O1H+, 36Ar12C14N1H+, 14N12C37Cl+, 16O12C35Cl+ |
| 65Cu | 30.9 | 32S16O21H+, 40Ar25Mg+, 40Ca16O1H+, 36Ar14N21H+, 32S33S+, 32S16O17O+, 33S16O2+, 12C16O37Cl+, 12C18O35Cl+, 31P16O18O+ |
| 75As | 100 | 40Ar35Cl+, 36Ar38Ar1H+, 38Ar37Cl+, 36Ar39K, 43Ca16O2, 23Na12C40Ar, 12C31P16O2+ |
| LOD (μg/L) | LOQ (μg/L) | |||
|---|---|---|---|---|
| Analyte | He Gas * | No Gas | He Gas * | No Gas |
| 47Ti | 2.36 | 2.98 | 7.85 | 9.92 |
| 51V | 0.16 | 0.09 | 0.54 | 0.32 |
| 52Cr | 0.52 | 1.05 | 1.73 | 3.49 |
| 55Mn | 0.63 | 0.41 | 2.1 | 1.36 |
| 56Fe | 1.64 | 63.43 | 5.48 | 211.45 |
| 63Cu | 9.51 | 6.63 | 31.70 | 26.09 |
| 75As | 1.71 | 0.66 | 5.69 | 2.21 |
| 88Sr | 0.28 | 0.26 | 0.94 | 0.87 |
| 95Mo | 1.70 | 1.07 | 5.67 | 3.58 |
| 208Pb | 0.20 | 0.29 | 0.67 | 0.96 |
| Mean ± SEM | Min | Max | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 25.28 ± 6.60 | 18.00 | 49.00 |
| BMI (kg/m2) | 23.90 ± 2.85 | 18.51 | 29.98 |
| Expositional data | |||
| Occupationally exposed | 5 (17.24%) | ||
| Environmentally exposed | 0 | ||
| Smoker | 8 (27.59%) | ||
| Seminal data | |||
| Volume (mL) | 3.54 ± 1.20 | 1.00 | 7.00 |
| Sperm concentration (mill/mL) | 103.93 ± 67.12 | 19.50 | 327.67 |
| Sperm motility (P +NP) (%) | 67.51 ± 18.02 | 18.90 | 96.00 |
| Sperm morphology (%) | 8.29 ± 2.37 | 4.00 | 13.21 |
| Sperm viability (%) | 86.35 ± 8.16 | 55.00 | 99.07 |
| MSC 1 h (mill/mL) | 19.36 ± 20.07 | 0.40 | 74 |
| MSC 4 h (mill/mL) | 10.15 ± 6.45 | 2.7 | 22.6 |
| Sperm biomarkers | |||
| Acrosome reaction | |||
| AR NC | 17.53 ± 11.82 | 2.00 | 51.00 |
| AR C 1 h | 13.41 ± 8.86 | 3.00 | 30.00 |
| AR 1 h | 52.90 ± 25.11 | 16.00 | 95.20 |
| AR C 4 h | 11.28 ± 8.13 | 2.00 | 26.87 |
| AR 4 h | 62.33 ± 21.95 | 19.12 | 95.00 |
| Tyrosine phosphorylation | |||
| TyrP NC | 5.85 ± 7.35 | 0.00 | 28.00 |
| TyrP C 1 h | 16.20 ± 13.17 | 2.00 | 41.10 |
| TyrP C 4 h | 18.37 ± 12.91 | 1.94 | 41.00 |
| Mitochondrial activity | |||
| High MMP (%) | 57.01 ± 15.13 | 26.79 | 90.90 |
| Medium MMP (%) | 3.00 ± 3.79 | 0 | 13.46 |
| Low MMP (%) | 22.16 ± 17.49 | 4.00 | 59.80 |
| Very low MMP (%) | 17.83 ± 13.96 | 0 | 48.15 |
| Analyte | Mean ± SEM | Min | Max | Samples Above LOD (%) | Samples Above LOQ (%) |
|---|---|---|---|---|---|
| 55Mn | 5.23 ± 2.5 | 1.41 | 11.76 | 100 | 100 |
| 88Sr | 74.29 ± 31.93 | 28.09 | 166.36 | 100 | 100 |
| 47Ti | 567.42 ± 2474.52 | 5.55 | 13,156.53 | 100 | 100 |
| 51V | 1.13 ± 0.45 | <LOQ | 2.52 | 100 | 96.55 |
| 75As | 10.84 ± 11.22 | <LOD | 30.90 | 58.62 | 17.24 |
| 52Cr | 5.55 ± 15.34 | <LOQ | 84.99 | 100 | 93.10 |
| 56Fe | 126.93 ± 86.13 | 47.53 | 497.89 | 100 | 100 |
| 63Cu | 88.58 ± 41.18 | 31.09 | 206.09 | 100 | 100 |
| 95Mo | 1.78 ± 0.96 | <LOD | 3.34 | 55.17 | 10.34 |
| 208Pb | 0.37 ± 0.88 | <LOD | 4.92 | 6.90 | 3.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Botella, A.; Cenitagoya-Alonso, N.; Sánchez-Romero, R.; Sáez-Espinosa, P.; Hernández-Falcó, M.; Gómez-Torres, M.J.; Todolí-Torró, J.L. Multielemental Profile for Seminal Plasma Through Inductively Coupled Plasma–Tandem Mass Spectrometry and Its Relationship with Seminal Parameters, Spermatic Biomarkers, and Oxidative Stress. Antioxidants 2025, 14, 1118. https://doi.org/10.3390/antiox14091118
López-Botella A, Cenitagoya-Alonso N, Sánchez-Romero R, Sáez-Espinosa P, Hernández-Falcó M, Gómez-Torres MJ, Todolí-Torró JL. Multielemental Profile for Seminal Plasma Through Inductively Coupled Plasma–Tandem Mass Spectrometry and Its Relationship with Seminal Parameters, Spermatic Biomarkers, and Oxidative Stress. Antioxidants. 2025; 14(9):1118. https://doi.org/10.3390/antiox14091118
Chicago/Turabian StyleLópez-Botella, Andrea, Natalia Cenitagoya-Alonso, Raquel Sánchez-Romero, Paula Sáez-Espinosa, Miranda Hernández-Falcó, María José Gómez-Torres, and José Luis Todolí-Torró. 2025. "Multielemental Profile for Seminal Plasma Through Inductively Coupled Plasma–Tandem Mass Spectrometry and Its Relationship with Seminal Parameters, Spermatic Biomarkers, and Oxidative Stress" Antioxidants 14, no. 9: 1118. https://doi.org/10.3390/antiox14091118
APA StyleLópez-Botella, A., Cenitagoya-Alonso, N., Sánchez-Romero, R., Sáez-Espinosa, P., Hernández-Falcó, M., Gómez-Torres, M. J., & Todolí-Torró, J. L. (2025). Multielemental Profile for Seminal Plasma Through Inductively Coupled Plasma–Tandem Mass Spectrometry and Its Relationship with Seminal Parameters, Spermatic Biomarkers, and Oxidative Stress. Antioxidants, 14(9), 1118. https://doi.org/10.3390/antiox14091118







