Castalin Induces ROS Production, Leading to DNA Damage and Increasing the Activity of CHK1 Inhibitor in Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blotting
2.3. Immunofluorescence Analysis
2.4. Cell Proliferation Assay
2.5. Natural Comet Assay
2.6. Detection of Radical Oxygene Species
2.7. Mitotic Catastrophe
2.8. Reagents for NMR
2.9. Chestnut Shell Collection, Extraction, and Chromatographic Separation
2.10. NMR and LC-MS/MS Analyses of the Ethyl Acetate Chestnut Shell Extract
2.11. NMR Data of Ellagic Acid
2.12. RNA-Seq and Analysis
2.13. Statistical Analysis
3. Results
3.1. Castalin Induces Cell Death in Cancer Cell Models
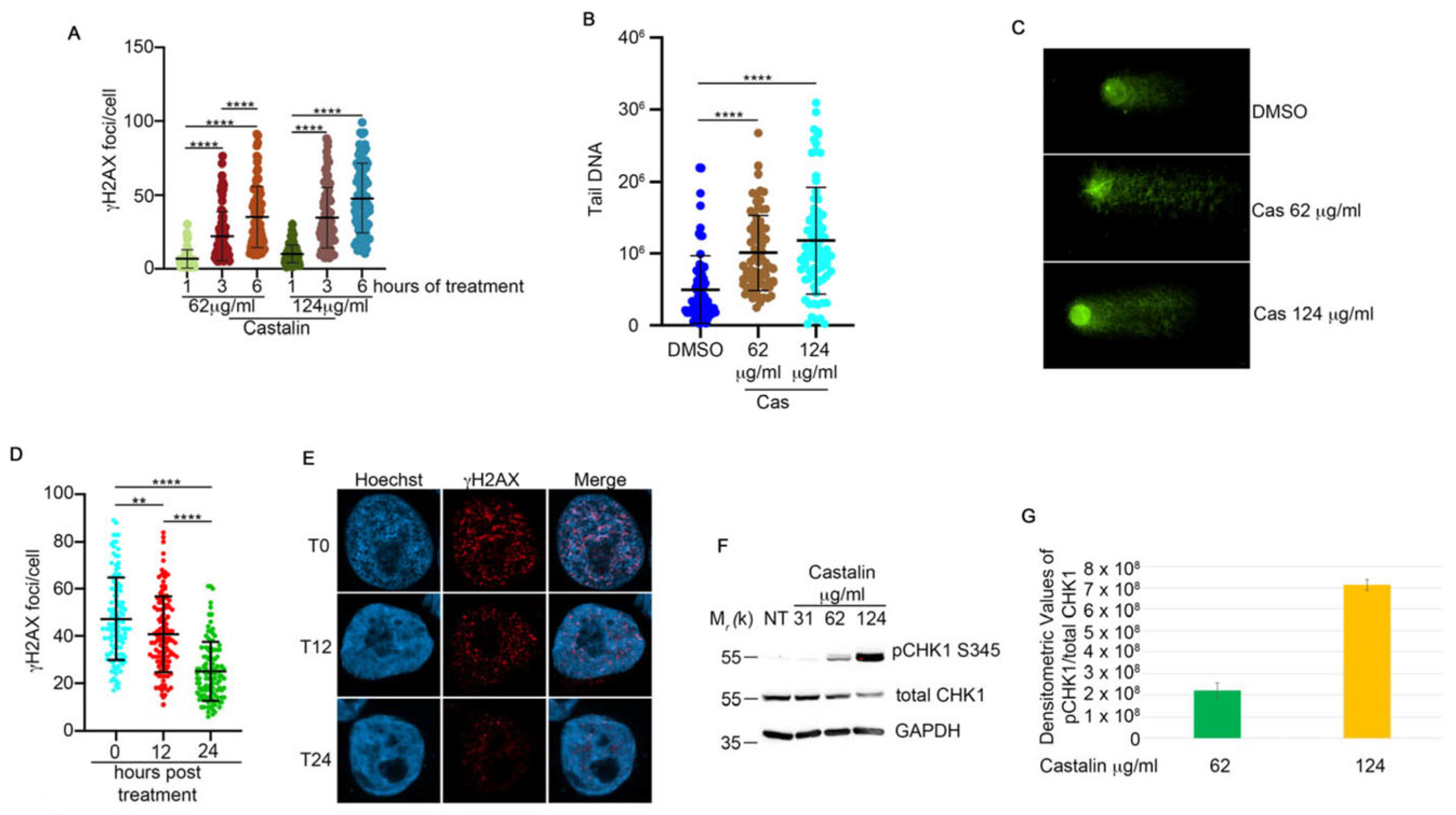
3.2. Castalin Activates the DNA Damage Response
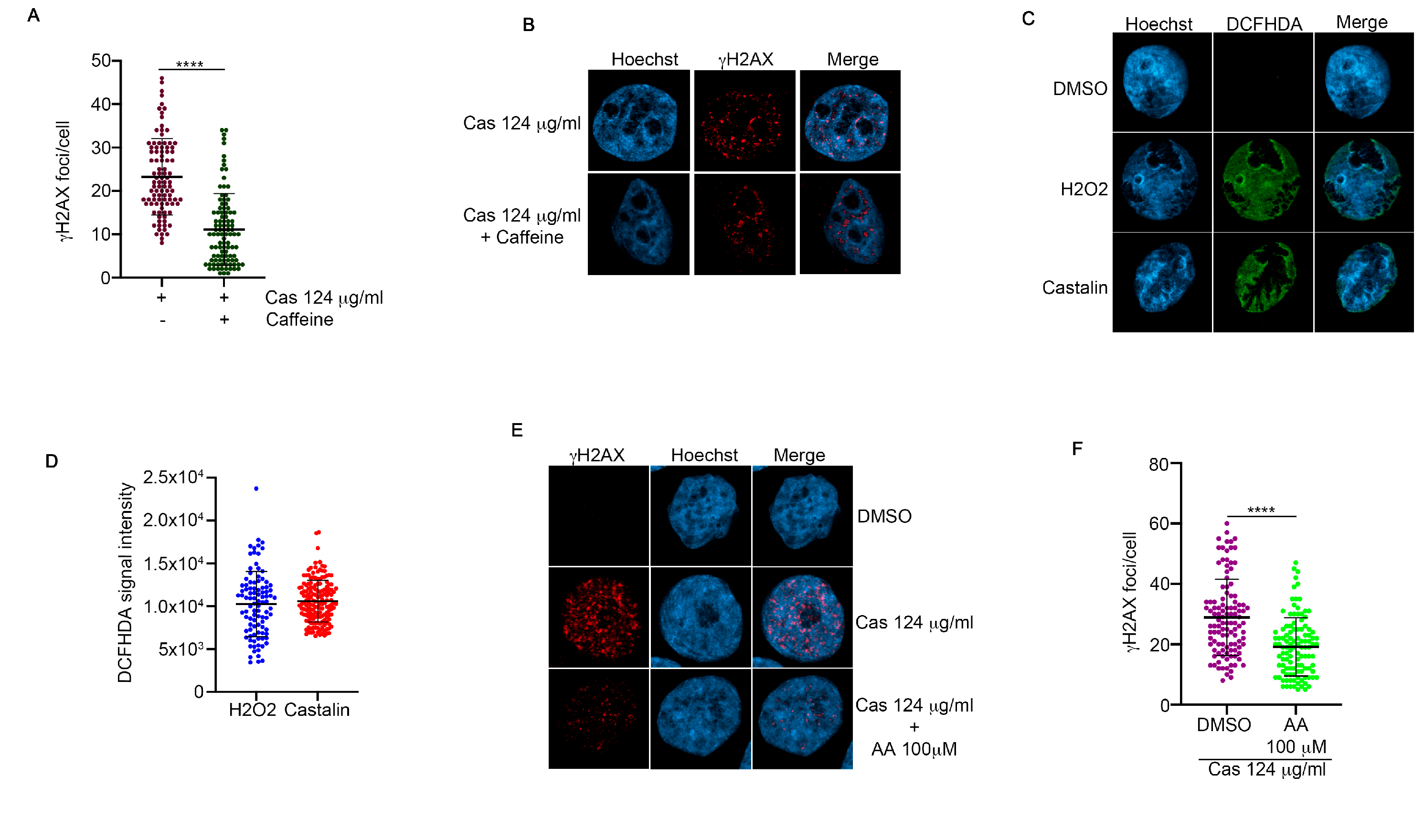
3.3. Castalin Was Able to Produce ROS Inducing the Activation of NHEJ
3.4. Castalin Potentiates the Effect of a CHK1 Inhibitor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Jeggo, P.; Lavin, M.F. Cellular Radiosensitivity: How Much Better Do We Understand It? Int. J. Radiat. Biol. 2009, 85, 1061–1081. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2018, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Az-Zahra, F.; Wongso, H.; Setyawati, L.U.; Novitasari, D.; Ikram, E.H.K. Molecular Mechanism of Natural Food Antioxidants to Regulate ROS in Treating Cancer: A Review. Antioxidants 2024, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Mazzarotti, G.; Cuomo, M.; Ragosta, M.C.; Russo, A.; D’Angelo, M.; Medugno, A.; Napolitano, G.M.; Iannuzzi, C.A.; Forte, I.M.; Camerlingo, R.; et al. Oleanolic Acid Modulates DNA Damage Response to Camptothecin Increasing Cancer Cell Death. Int. J. Mol. Sci. 2024, 25, 13475. [Google Scholar] [CrossRef]
- Barone, D.; Iannuzzi, C.A.; Forte, I.M.; Ragosta, M.C.; Cuomo, M.; Dell’Aquila, M.; Altieri, A.; Caporaso, A.; Camerlingo, R.; Rigano, M.M.; et al. The Hydrophilic Extract from a New Tomato Genotype (Named DHO) Kills Cancer Cell Lines through the Modulation of the DNA Damage Response Induced by Campthotecin Treatment. Front. Oncol. 2023, 13, 1117262. [Google Scholar] [CrossRef]
- Willis, J.; Patel, Y.; Lentz, B.L.; Yan, S. APE2 Is Required for ATR-Chk1 Checkpoint Activation in Response to Oxidative Stress. Proc. Natl. Acad. Sci. USA 2013, 110, 10592–10597. [Google Scholar] [CrossRef]
- Chang, M.C.; Lin, L.D.; Wu, M.T.; Chan, C.P.; Chang, H.H.; Lee, M.S.; Sun, T.Y.; Jeng, P.Y.; Yeung, S.Y.; Lin, H.J.; et al. Effects of Camphorquinone on Cytotoxicity, Cell Cycle Regulation and Prostaglandin E2 Production of Dental Pulp Cells: Role of ROS, ATM/Chk2, MEK/ERK and Hemeoxygenase-1. PLoS ONE 2015, 10, e0143663. [Google Scholar] [CrossRef]
- Poetsch, A.R. The Genomics of Oxidative DNA Damage, Repair, and Resulting Mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Krokan, H.E.; Bjørås, M. Base Excision Repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H.J. Understanding Nucleotide Excision Repair and Its Roles in Cancer and Ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Vilenchik, M.M.; Knudson, A.G. Endogenous DNA Double-Strand Breaks: Production, Fidelity of Repair, and Induction of Cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 12871–12876. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of Reactive Oxygen Species Levels and Radioresistance in Cancer Stem Cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Kirtonia, A.; Sethi, G.; Garg, M. The Multifaceted Role of Reactive Oxygen Species in Tumorigenesis. Cell Mol. Life Sci. 2020, 77, 4459. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Castalin|C27H20O18|CID 99973—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Castalin (accessed on 11 March 2025).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Li, X.-C.; Elsohly, H.N.; Hufford, C.D.; Clark, A.M. NMR Assignments of Ellagic Acid Derivatives. Magn. Reson. Chem. 1999, 37, 856–859. [Google Scholar] [CrossRef]
- Mullen, W.; Yokota, T.; Lean, M.E.J.; Crozier, A. Analysis of Ellagitannins and Conjugates of Ellagic Acid and Quercetin in Raspberry Fruits by LC–MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- Venter, P.; Causon, T.; Pasch, H.; de Villiers, A. Comprehensive Analysis of Chestnut Tannins by Reversed Phase and Hydrophilic Interaction Chromatography Coupled to Ion Mobility and High Resolution Mass Spectrometry. Anal. Chim. Acta 2019, 1088, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Corilagin|C27H22O18|CID 73568—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/73568 (accessed on 20 March 2025).
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010, 920161. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J.; Lukas, J. Chk1 and Chk2 Kinases in Checkpoint Control and Cancer. Cancer Cell 2003, 3, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Stracker, T.H.; Usui, T.; Petrini, J.H.J. Taking the Time to Make Important Decisions: The Checkpoint Effector Kinases Chk1 and Chk2 and the DNA Damage Response. DNA Repair. 2009, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Inhibition of ATM and ATR Kinase Activities by the Radiosensitizing Agent, Caffeine—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10485486/ (accessed on 21 March 2025).
- de Melo, L.F.M.; de Queiroz Aquino-Martins, V.G.; da Silva, A.P.; Oliveira Rocha, H.A.; Scortecci, K.C. Biological and Pharmacological Aspects of Tannins and Potential Biotechnological Applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef]
- Afri, M.; Frimer, A.A.; Cohen, Y. Active Oxygen Chemistry within the Liposomal Bilayer: Part IV: Locating 2′,7′-Dichlorofluorescein (DCF), 2′,7′-Dichlorodihydrofluorescein (DCFH) and 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFH-DA) in the Lipid Bilayer. Chem. Phys. Lipids 2004, 131, 123–133. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen Peroxide—Production, Fate and Role in Redox Signaling of Tumor Cells. Cell Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Jackson, S.P. Sensing and Repairing DNA Double-Strand Breaks. Carcinogenesis 2002, 23, 687–696. [Google Scholar] [CrossRef]
- Chapman, J.R.; Barral, P.; Vannier, J.B.; Borel, V.; Steger, M.; Tomas-Loba, A.; Sartori, A.A.; Adams, I.R.; Batista, F.D.; Boulton, S.J. RIF1 Is Essential for 53BP1-Dependent Nonhomologous End Joining and Suppression of DNA Double-Strand Break Resection. Mol. Cell 2013, 49, 858–871. [Google Scholar] [CrossRef]
- Jiang, W.; Crowe, J.L.; Liu, X.; Nakajima, S.; Wang, Y.; Li, C.; Lee, B.J.; Dubois, R.L.; Liu, C.; Yu, X.; et al. Differential Phosphorylation of DNA-PKcs Regulates the Interplay between End-Processing and End-Ligation during Nonhomologous End-Joining. Mol. Cell 2015, 58, 172–185. [Google Scholar] [CrossRef]
- San Filippo, J.; Sung, P.; Klein, H. Mechanism of Eukaryotic Homologous Recombination. Annu. Rev. Biochem. 2008, 77, 229–257. [Google Scholar] [CrossRef]
- Sartori, A.A.; Lukas, C.; Coates, J.; Mistrik, M.; Fu, S.; Bartek, J.; Baer, R.; Lukas, J.; Jackson, S.P. Human CtIP Promotes DNA End Resection. Nature 2007, 450, 509–514. [Google Scholar] [CrossRef]
- Liu, L.F.; Desai, S.D.; Li, T.K.; Mao, Y.; Sun, M.; Sim, S.P. Mechanism of Action of Camptothecin. In Proceedings of the Annals of the New York Academy of Sciences; Wiley: Hoboken, NJ, USA, 2000; Volume 922. [Google Scholar]
- Qiu, Z.; Oleinick, N.L.; Zhang, J. ATR/CHK1 Inhibitors and Cancer Therapy. Radiother. Oncol. 2017, 126, 450. [Google Scholar] [CrossRef]
- Kristeleit, R.; Plummer, R.; Jones, R.; Carter, L.; Blagden, S.; Sarker, D.; Arkenau, T.; Evans, T.R.J.; Danson, S.; Symeonides, S.N.; et al. A Phase 1/2 Trial of SRA737 (a Chk1 Inhibitor) Administered Orally in Patients with Advanced Cancer. Br. J. Cancer 2023, 129, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu, H.; Olsson, M.; Zhivotovsky, B. Death through a Tragedy: Mitotic Catastrophe. Cell Death Differ. 2008, 15, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.L.; Cho, H.M.; Ceppi, I.; Gao, B.; Yadav, T.; Silveira, G.G.; Boon, R.; Martinez-Pastor, B.; Amoh, N.Y.A.; Machin, B.; et al. ZNF280A Links DNA Double-Strand Break Repair to Human 22q11.2 Distal Deletion Syndrome. Nat. Cell Biol. 2025, 27, 1006–1020. [Google Scholar] [CrossRef]
- Xu, X.Q.; Huang, C.M.; Zhang, Y.F.; Chen, L.; Cheng, H.; Wang, J.M. S1PR1 Mediates Anti-apoptotic/Pro-proliferative Processes in Human Acute Myeloid Leukemia Cells. Mol. Med. Rep. 2016, 14, 3369–3375. [Google Scholar] [CrossRef][Green Version]
- Cheng, M.B.; Zhang, Y.; Cao, C.Y.; Zhang, W.L.; Zhang, Y.; Shen, Y.F. Specific Phosphorylation of Histone Demethylase KDM3A Determines Target Gene Expression in Response to Heat Shock. PLoS Biol. 2014, 12, e1002026. [Google Scholar] [CrossRef]
- Baker, M.; Petasny, M.; Taqatqa, N.; Bentata, M.; Kay, G.; Engal, E.; Nevo, Y.; Siam, A.; Dahan, S.; Salton, M. KDM3A Regulates Alternative Splicing of Cell-Cycle Genes Following DNA Damage. RNA 2021, 27, 1353–1362. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Errichiello, F.; D’Amato, M.; Gambuti, A.; Moio, L.; Pastore, A.; AL-Hmadi, H.; Stornaiuolo, M.; Serino, E.; Taglialatela-Scafati, O.; Forino, M. Oleanolic Acid: A Promising Antidiabetic Metabolite Detected in Aglianico Grape Pomace. J. Funct. Foods 2023, 104, 105548. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Ganguly, R.; Rana, H.K.; Pandey, P.K.; Sethi, G.; Bishayee, A.; Pandey, A.K. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules 2019, 24, 3399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Y.; Wang, X.; Qi, C.; Tian, J.; Zou, Z. Synergistic Lethality between Auranofin-Induced Oxidative DNA Damage and ATR Inhibition in Cancer Cells. Life Sci. 2023, 332, 122131. [Google Scholar] [CrossRef]
- Patil, M.; Pabla, N.; Dong, Z. Checkpoint Kinase 1 in DNA Damage Response and Cell Cycle Regulation. Cell Mol. Life Sci. 2013, 70, 4009. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of Hypoxia-Inducible Factor-1a by Reactive Oxygen Species: New Developments in an Old Debate. J. Cell Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2020, 221, 107753. [Google Scholar] [CrossRef]
- Ragu, S.; Dardillac, E.; Caillat, S.; Ravanat, J.-L.; Lopez, B.S. Dysregulation of Replication Stress-Induced ROS in Transformed Cell Lines: A Vicious Circle at Cancer Initiation. bioRxiv 2025. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, M.; Medugno, A.; Cuomo, M.; Ragosta, M.C.; Russo, A.; Mazzarotti, G.; Napolitano, G.M.; Iannuzzi, C.A.; Errichiello, F.; Frusciante, L.; et al. Castalin Induces ROS Production, Leading to DNA Damage and Increasing the Activity of CHK1 Inhibitor in Cancer Cell Lines. Antioxidants 2025, 14, 1096. https://doi.org/10.3390/antiox14091096
D’Angelo M, Medugno A, Cuomo M, Ragosta MC, Russo A, Mazzarotti G, Napolitano GM, Iannuzzi CA, Errichiello F, Frusciante L, et al. Castalin Induces ROS Production, Leading to DNA Damage and Increasing the Activity of CHK1 Inhibitor in Cancer Cell Lines. Antioxidants. 2025; 14(9):1096. https://doi.org/10.3390/antiox14091096
Chicago/Turabian StyleD’Angelo, Margherita, Annamaria Medugno, Maria Cuomo, Maria Carmen Ragosta, Andrea Russo, Giulio Mazzarotti, Giuseppe Maria Napolitano, Carmelina Antonella Iannuzzi, Francesco Errichiello, Luigi Frusciante, and et al. 2025. "Castalin Induces ROS Production, Leading to DNA Damage and Increasing the Activity of CHK1 Inhibitor in Cancer Cell Lines" Antioxidants 14, no. 9: 1096. https://doi.org/10.3390/antiox14091096
APA StyleD’Angelo, M., Medugno, A., Cuomo, M., Ragosta, M. C., Russo, A., Mazzarotti, G., Napolitano, G. M., Iannuzzi, C. A., Errichiello, F., Frusciante, L., Forino, M., Cucciniello, R., Martinelli, C., Salvati, A., Memoli, D., Nassa, G., Bucci, E., De Laurentiis, M., Giordano, A., & Alfano, L. (2025). Castalin Induces ROS Production, Leading to DNA Damage and Increasing the Activity of CHK1 Inhibitor in Cancer Cell Lines. Antioxidants, 14(9), 1096. https://doi.org/10.3390/antiox14091096











