Maternal Hydroxytyrosol Supplementation Enhances Antioxidant Capacity and Immunometabolic Adaptations in Nutrient-Restricted Beef Cows and Their Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animals and Husbandry
2.3. Dietary Treatments and Performance Recordings
2.4. Blood and Colostrum Sample Collection
2.5. Sample Analysis
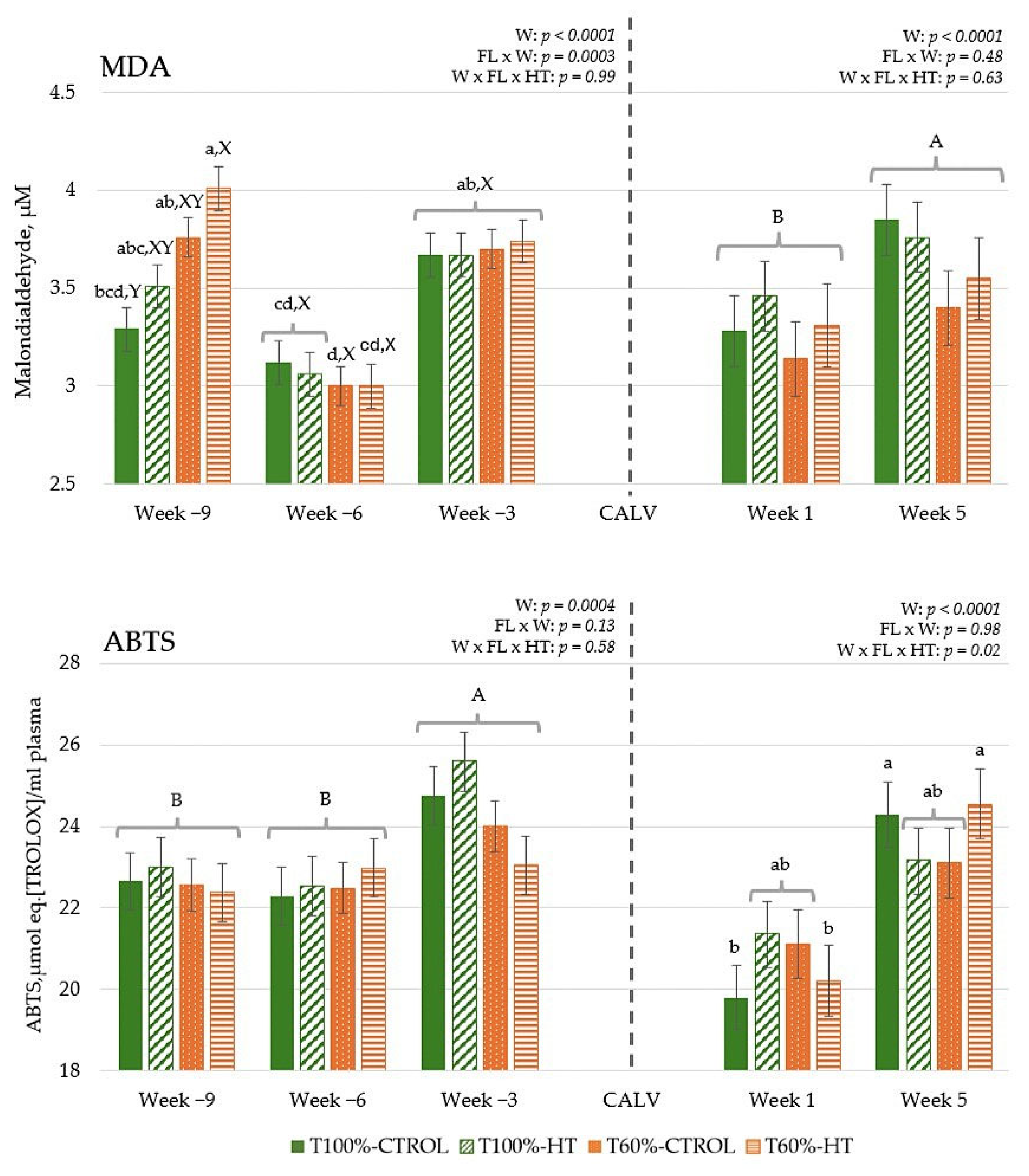
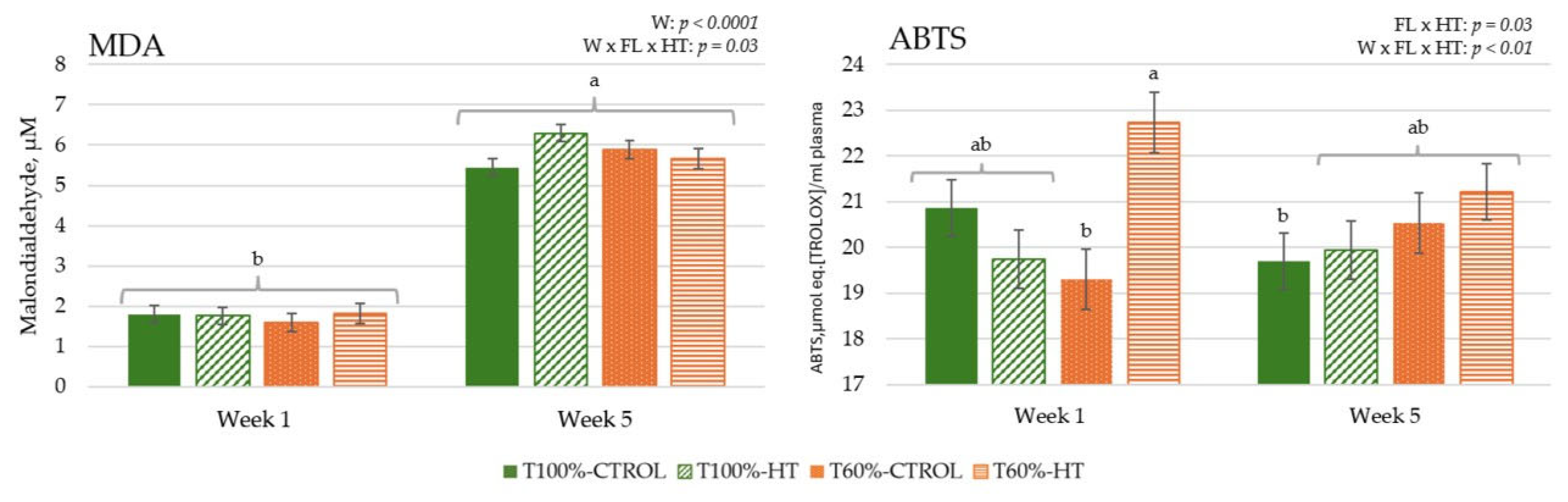
2.5.1. Determination of Malondialdehyde and Total Antioxidant Activity (ABTS Assay) in Plasma
2.5.2. HT Metabolite Analysis
2.5.3. RNA Extraction, cDNA Synthesis, and qPCR Analysis
2.6. Data Analysis
3. Results
3.1. Cow Performance and Circulating HT Metabolites
3.2. MDA Concentration and Total Antioxidant Capacity in Plasma
3.3. Gene Expression
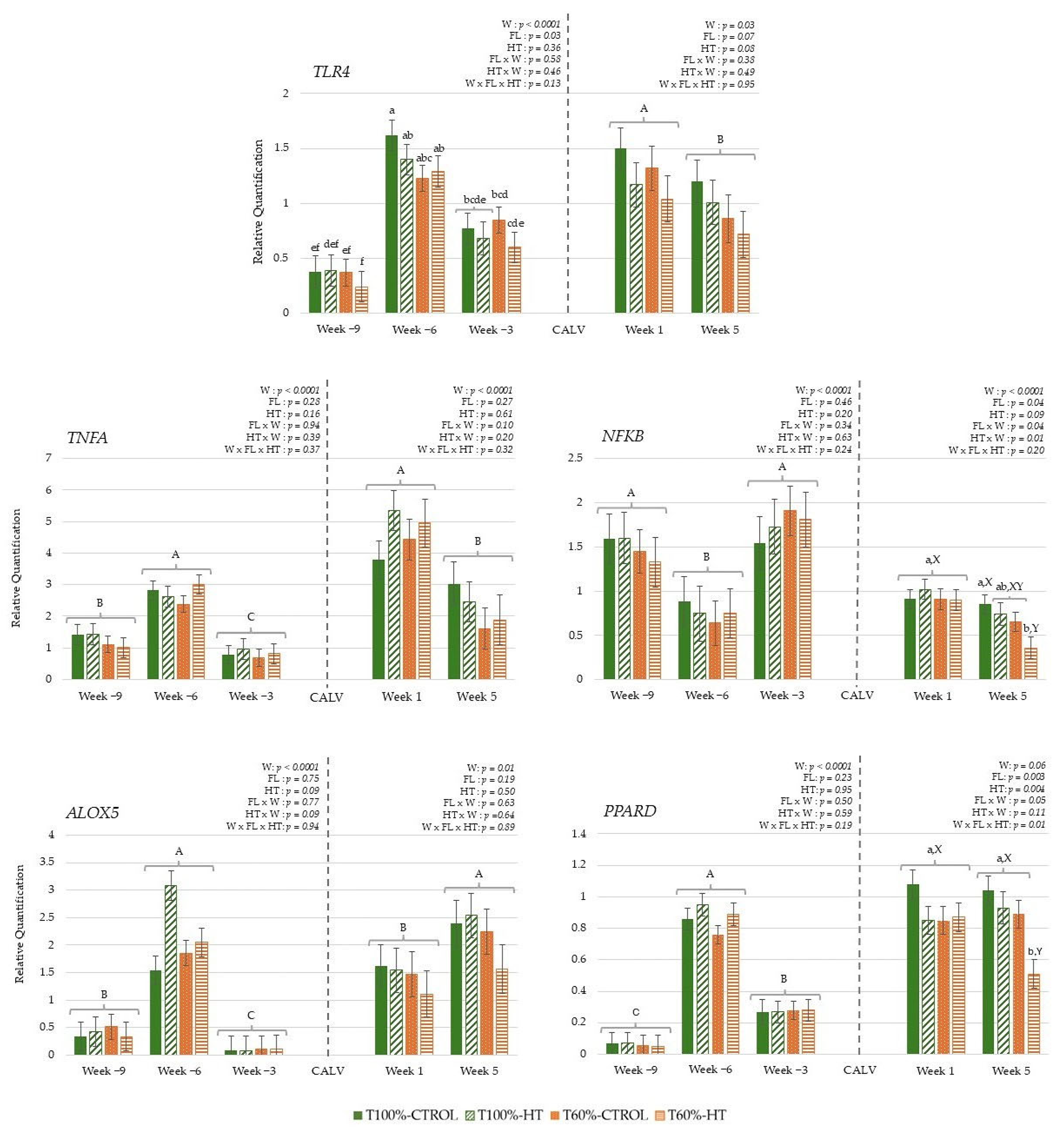
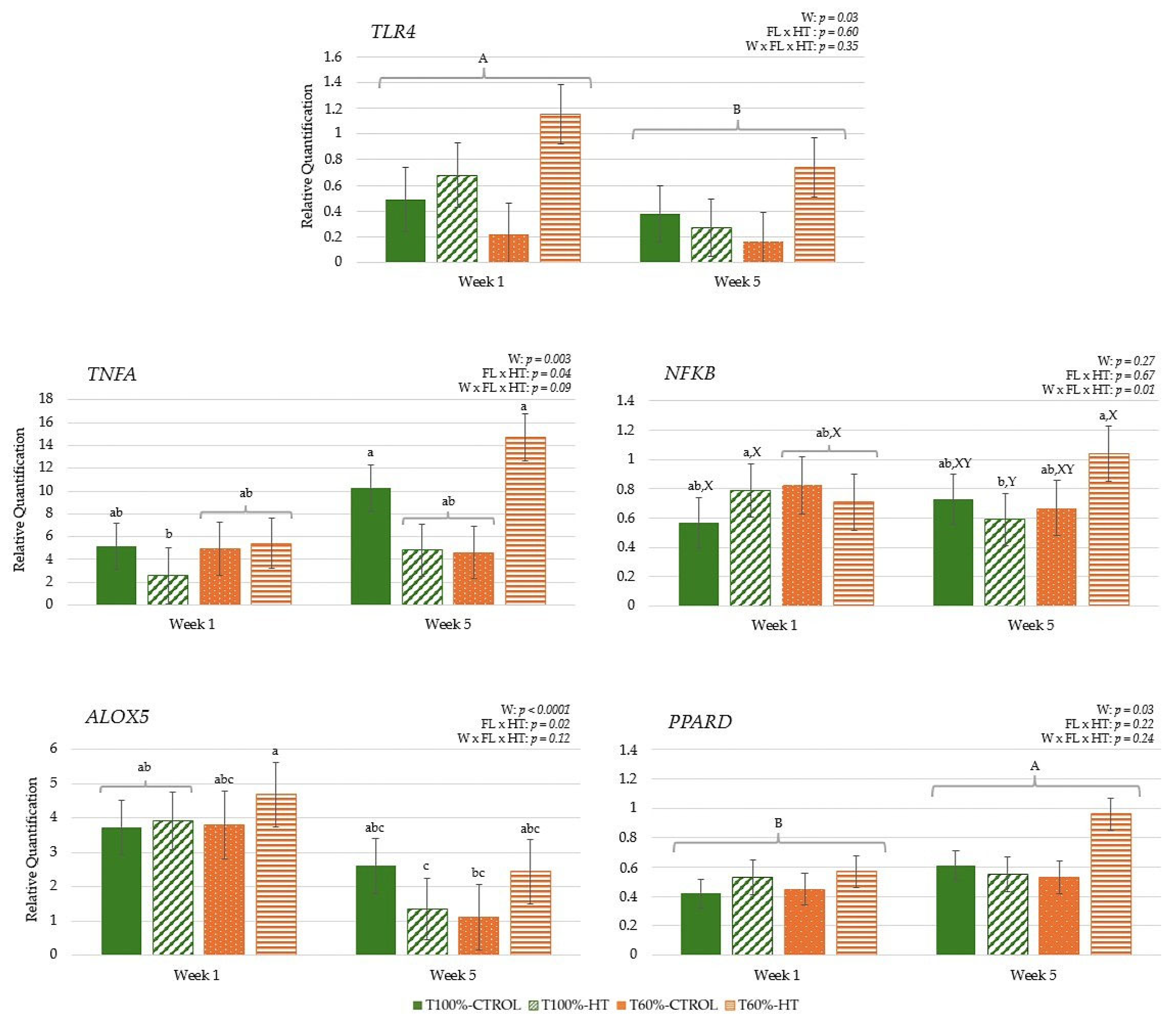
3.3.1. Genes Related to Antioxidant Response
3.3.2. Genes Related to Immune Response
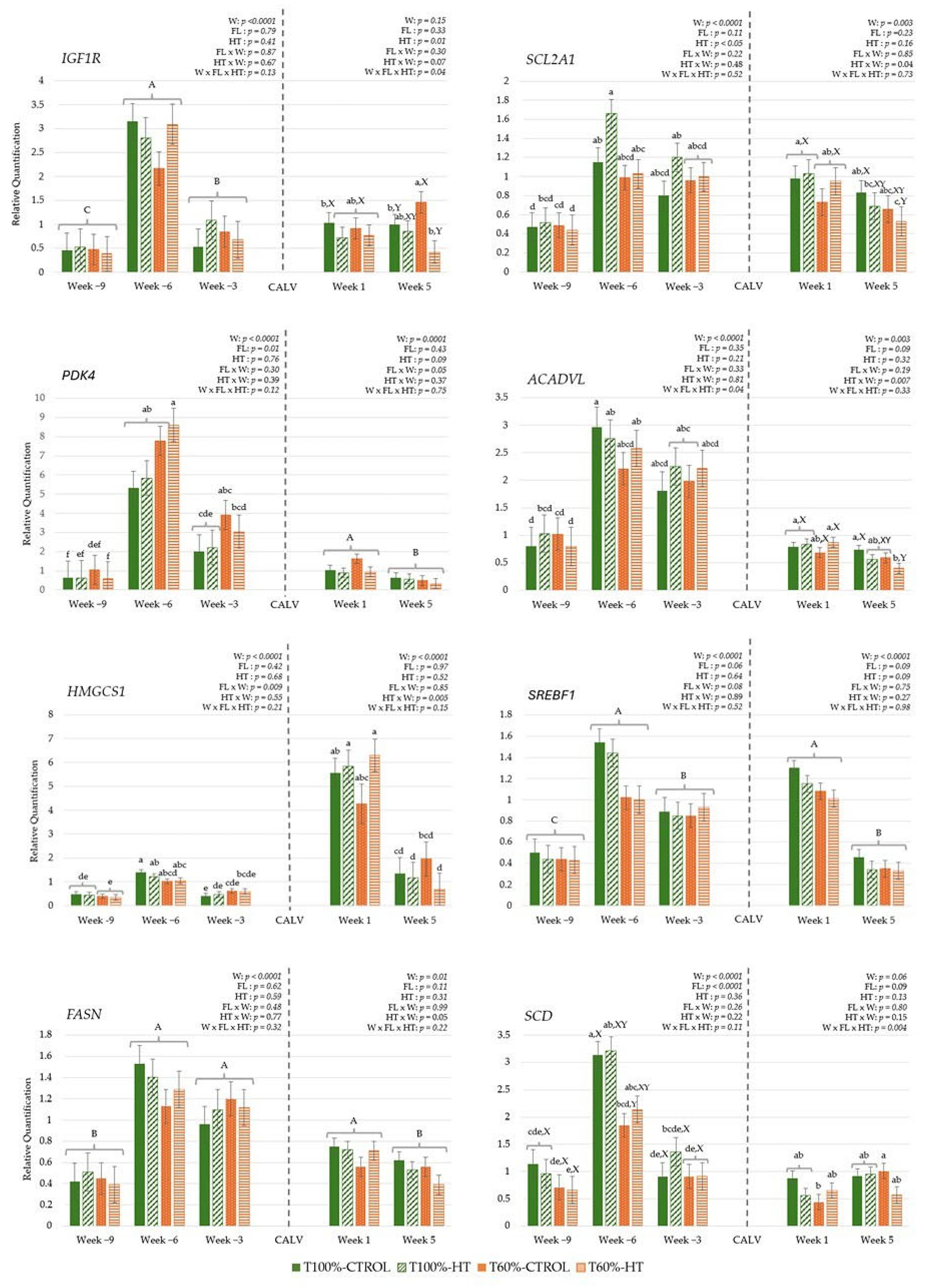
3.3.3. Genes Related to Energy Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, A.; Bernués, A.; Villalba, D.; Casasús, I.; Revilla, R. Influence of management and nutrition on postpartum interval in Brown Swiss and Pirenaica cows. Livest. Prod. Sci. 2004, 86, 179–191. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bouvier-Muller, J.; Allain, C.; Tabouret, G.; Enjalbert, F.; Portes, D.; Noirot, C.; Rupp, R.; Foucras, G. Whole blood transcriptome analysis reveals potential competition in metabolic pathways between negative energy balance and response to inflammatory challenge. Sci. Rep. 2017, 7, 2379. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Gao, D.; Pararasa, C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017, 12, 50–57. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Bidault, G.; Virtue, S.; Petkevicius, K.; Jolin, H.E.; Dugourd, A.; Guénantin, A.-C.; Leggat, J.; Mahler-Araujo, B.; Lam, B.Y.H.; Ma, M.K.; et al. SREBP1-induced fatty acid synthesis depletes macrophages antioxidant defences to promote their alternative activation. Nat. Metab. 2021, 3, 1150–1162. [Google Scholar] [CrossRef]
- Kabat, A.M.; Pearce, E.L.; Pearce, E.J. Metabolism in type 2 immune responses. Immunity 2023, 56, 723–741. [Google Scholar] [CrossRef]
- Celi, P.; Gabai, G. Oxidant/antioxidant balance in animal nutrition and health: The role of protein oxidation. Front. Vet. Sci. 2015, 2, 48. [Google Scholar] [CrossRef]
- Larson, D.M.; Martin, J.L.; Adams, D.C.; Funston, R.N. Winter grazing system and supplementation during late gestation influence performance of beef cows and steer progeny. J. Anim. Sci. 2009, 87, 1147–1155. [Google Scholar] [CrossRef]
- LeMaster, C.T.; Taylor, R.K.; Ricks, R.E.; Long, N.M. The effects of late gestation maternal nutrient restriction with or without protein supplementation on endocrine regulation of newborn and postnatal beef calves. Theriogenology 2017, 87, 64–71. [Google Scholar] [CrossRef]
- Maresca, S.; Lopez Valiente, S.; Rodriguez, A.M.; Long, N.M.; Pavan, E.; Quintans, G. Effect of protein restriction of bovine dams during late gestation on offspring postnatal growth, glucose-insulin metabolism and IGF-1 concentration. Livest. Sci. 2018, 212, 120–126. [Google Scholar] [CrossRef]
- Noya, A.; Serrano-Pérez, B.; Villalba, D.; Casasús, I.; Molina, E.; López-Helguera, I.; Sanz, A. Effects of maternal subnutrition during early pregnancy on cow hematological profiles and offspring physiology and vitality in two beef breeds. Anim. Sci. J. 2019, 90, 857–869. [Google Scholar] [CrossRef]
- Noya, A.; Casasús, I.; Ferrer, J.; Sanz, A. Long-term effects of maternal subnutrition in early pregnancy on cow-calf performance, immunological and physiological profiles during the next lactation. Animals 2019, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Weaver, J.A.; Cao, Y.Z.; Corl, C.; Sylte, M.J.; Mullarky, I.K. Enhanced 15-HPETE production during oxidant stress induces apoptosis of endothelial cells. Prostaglandins Other Lipid Mediat. 2005, 76, 19–34. [Google Scholar] [CrossRef]
- Godden, S.M.; Haines, D.M.; Konkol, K.; Peterson, J. Improving passive transfer of immunoglobulins in calves. II: Interaction between feeding method and volume of colostrum fed. J. Dairy Sci. 2009, 92, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.C.; Hurley, D.J.; Reber, A.J. Neonatal immune development in the calf and its impact on vaccine response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef]
- Loor, J.J.; Everts, R.E.; Bionaz, M.; Dann, H.M.; Morin, D.E.; Oliveira, R.; Lewin, H.A. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol. Genom. 2007, 32, 105–116. [Google Scholar] [CrossRef]
- Naeem, A.; Drackley, J.K.; Stamey, J.; Loor, J.J. Role of metabolic and cellular proliferation genes in ruminal development in response to enhanced plane of nutrition in neonatal Holstein calves. J. Dairy Sci. 2012, 95, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Parraguez, V.H.; Sales, F.; Peralta, O.; De Los Reyes, M.; Gonzalez-Bulnes, A. Oxidative stress and fetal growth restriction set up earlier in undernourished sheep twin pregnancies: Prevention with antioxidant and nutritional supplementation. Antioxidants 2022, 11, 1287. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Shao, Q.; Meng, S.; Wang, X.; Kong, T.; Li, Y. Antioxidant monoammonium glycyrrhizinate alleviates damage from oxidative stress in perinatal cows. J. Anim. Physiol. Anim. Nutr. 2023, 107, 475–484. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceutical properties of olive oil polyphenols. An itinerary from cultured cells through animal models to humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Britton, J.; Davis, R.; O’Connor, K.E. Chemical, physical and biotechnological approaches to the production of the potent antioxidant hydroxytyrosol. Appl. Microbiol. Biotechnol. 2019, 103, 5957–5974. [Google Scholar] [CrossRef] [PubMed]
- Chimi, H.; Ciliard, J.; Ciliard, P.; Rahmani, M. Peroxyl and hydroxyl radical scavenging activity of some natural phenolic antioxidants. J. Am. Oil Chem. Soc. 1991, 68, 307–312. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef]
- Vázquez-Gómez, M.; Garcia-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; Gonzalez-Añover, P.; Gomez-Fidalgo, E.; Sánchez-Sánchez, R.; Óvilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, e0177593. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Pardo, Z.; Heras-Molina, A.; Encinas, T.; Torres-Rovira, L.; Astiz, S.; Nieto, R.; Ovilo, C.; Gonzalez-Bulnes, A.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on hepatic fat accretion and energy and fatty acids profile of fetal tissues. Nutrients 2019, 11, 1534. [Google Scholar] [CrossRef] [PubMed]
- López de Armentia, L.; Noya, A.; Álvarez-Rodríguez, J.; Villalba, D.; Serrano-Pérez, B.; Casasús, I.; Alabart, J.L.; Sanz, A. Effects of undernutrition and hydroxytyrosol supplementation in late pregnancy on cow-calf performance, metabolic and immune status, and newborn vitality in beef herds. Animal, 2025; submitted. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific opinion on safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 4728. [Google Scholar] [CrossRef]
- INRA. INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- BioRender. BioRender Scientific Illustration Software. 2025. Available online: https://biorender.com/ (accessed on 16 July 2025).
- Orquera-Arguero, K.G.; Casasús, I.; Ferrer, J.; Blanco, M. Beef cows’ performance and metabolic response to short nutritional challenges in different months of lactation. Res. Vet. Sci. 2023, 159, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Small Rumin. Res. 2010, 89, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Dragsted, L.O.; Daneshvar, B.; Pulido, R.; Saura-Calixto, F. In vitro antioxidant activities of edible artichoke (Cynara scolymus L.) and effect on biomarkers of antioxidants in rats. J. Agric. Food Chem. 2003, 51, 5540–5545. [Google Scholar] [CrossRef]
- Rubió, L.; Farràs, M.; de La Torre, R.; Macià, A.; Romero, M.-P.; Valls, R.M.; Solà, R.; Farré, M.; Fitó, M.; Motilva, M.-J. Metabolite profiling of olive oil and thyme phenols after a sustained intake of two phenol-enriched olive oils by humans: Identification of compliance markers. Food Res. Int. 2014, 65, 59–68. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Serrano-Pérez, B.; Hansen, P.J.; Mur-Novales, R.; García-Ispierto, I.; de Sousa, N.M.; Beckers, J.F.; Almería, S.; López-Gatius, F. Crosstalk between uterine serpin (SERPINA14) and pregnancy-associated glycoproteins at the fetal–maternal interface in pregnant dairy heifers experimentally infected with Neospora caninum. Theriogenology 2016, 86, 824–830. [Google Scholar] [CrossRef]
- Monteiro, P.L.J.; Ribeiro, E.S.; Maciel, R.P.; Dias, A.L.G.; Sole, E.; Lima, F.S.; Bisinotto, R.; Thatcher, W.; Sartori, R.; Santos, J. Effects of supplemental progesterone after artificial insemination on expression of interferon-stimulated genes and fertility in dairy cows. J. Dairy Sci. 2014, 97, 4907–4921. [Google Scholar] [CrossRef]
- Lesage-Padilla, A.; Forde, N.; Poirée, M.; Healey, G.D.; Giraud-Delville, C.; Reinaud, P.; Eozenou, C.; Vitorino Carvalho, A.; Galio, L.; Raliou, M.; et al. Maternal metabolism affects endometrial expression of oxidative stress and FOXL2 genes in cattle. PLoS ONE 2017, 12, e0189942. [Google Scholar] [CrossRef]
- Bühler, S.; Frahm, J.; Tienken, R.; Kersten, S.; Meyer, U.; Huber, K.; Dänicke, S. Effects of energy supply and nicotinic acid supplementation on serum anti-oxidative capacity and on expression of oxidative stress-related genes in blood leucocytes of periparturient primi- and pluriparous dairy cows. J. Anim. Physiol. Anim. Nutr. 2018, 102, e87–e98. [Google Scholar] [CrossRef]
- Sassu, E.L.; Kangethe, R.T.; Settypalli, T.B.K.; Chibssa, T.R.; Cattoli, G.; Wijewardana, V. Development and evaluation of a real-time PCR panel for the detection of 20 immune markers in cattle and sheep. Vet. Immunol. Immunopathol. 2020, 227, 110092. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.F.; Jiang, L.Y.; Wang, D.M.; Zhao, F.Q.; Liu, J.X. Supplementation with N-carbamoylglutamate during the transition period improves the function of neutrophils and reduces inflammation and oxidative stress in dairy cows. J. Dairy Sci. 2022, 105, 5786–5795. [Google Scholar] [CrossRef]
- Moyes, K.M.; Graugnard, D.E.; Khan, M.J.; Mukesh, M.; Loor, J.J. Postpartal immunometabolic gene network expression and function in blood neutrophils are altered in response to prepartal energy intake and postpartal intramammary inflammatory challenge. J. Dairy Sci. 2014, 97, 2165–2177. [Google Scholar] [CrossRef]
- Gu, M.; Cosenza, G.; Iannaccone, M.; Guo, Y.; Di Stasio, L.; Pauciullo, A. The single nucleotide polymorphism g.133A>C in the stearoyl CoA desaturase gene (SCD) promoter affects gene expression and quali-quantitative properties of river buffalo milk. J. Dairy Sci. 2019, 102, 442–451. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Georgieva, T.M.; Georgiev, I.P.; Ontsouka, E.; Hageleit, M.; Blum, J.W. Real-time RT-PCR quantification of insulin-like growth factor (IGF)-1, IGF-1 receptor, IGF-2, IGF-2 receptor, insulin receptor, growth hormone receptor, IGF-binding proteins 1, 2 and 3 in the bovine species. Domest. Anim. Endocrinol. 2002, 22, 91–102. [Google Scholar] [CrossRef]
- Graber, M.; Kohler, S.; Kaufmann, T.; Doherr, M.G.; Bruckmaier, R.M.; van Dorland, H.A. A field study on characteristics and diversity of gene expression in the liver of dairy cows during the transition period. J. Dairy Sci. 2010, 93, 5200–5215. [Google Scholar] [CrossRef]
- Jermann, P.M.; Fritsche, D.; Wagner, L.A.; Wellnitz, O.; Bruckmaier, R.M.; Gross, J.J. Effect of Different Dietary Regimens at Dry-Off on Performance, Metabolism, and Immune System in Dairy Cows. J. Dairy Sci. 2022, 105, 4624–4642. [Google Scholar] [CrossRef]
- Bauman, D.E.; Currie, W.B. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Hong, L.; Diao, L.; Cai, S.; Yin, T.; Zeng, Y. Characterization of progesterone-induced dendritic cells in metabolic and immunologic reprogramming. J. Reprod. Immunol. 2023, 159, 104128. [Google Scholar] [CrossRef] [PubMed]
- Minuti, A.; Jahan, N.; Lopreiato, V.; Piccioli-Cappelli, F.; Bomba, L.; Capomaccio, S.; Loor, J.J.; Ajmone-Marsan, P.; Trevisi, E. Evaluation of circulating leukocyte transcriptome and its relationship with immune function and blood markers in dairy cows during the transition period. Funct. Integr. Genom. 2020, 20, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Horst, E.A.; Kvidera, S.K.; Baumgard, L.H. Invited review: The influence of immune activation on transition cow health and performance—A critical evaluation of traditional dogmas. J. Dairy Sci. 2021, 104, 8380–8410. [Google Scholar] [CrossRef]
- Habel, J.; Sundrum, A. Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows. Animals 2020, 10, 1028. [Google Scholar] [CrossRef]
- Eger, M.; Hussen, J.; Koy, M.; Danicke, S.; Schuberth, H.-J.; Breves, G. Glucose transporter expression differs between bovine monocyte and macrophage subsets and is influenced by milk production. J. Dairy Sci. 2016, 99, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Everts, B.; Amiel, E.; Huang, S.C.; Smith, A.M.; Chang, C.H.; Lam, W.Y.; Redmann, V.; Freitas, T.C.; Blagih, J.; van der Windt, G.J.; et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014, 15, 323–332. [Google Scholar] [CrossRef]
- Carroll, R.G.; Zasłona, Z.; Galván-Peña, S.; Koppe, E.L.; Sévin, D.C.; Angiari, S.; Triantafilou, M.; Triantafilou, K.; Modis, L.K.; O’Neill, L.A. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 2018, 293, 5509–5521. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Wang, C.; Yosef, N.; Gaublomme, J.; Wu, C.; Lee, Y.; Clish, C.B.; Kaminski, J.; Xiao, S.; Meyer Zu Horste, G.; Pawlak, M.; et al. CD5L/AIM Regulates Lipid Biosynthesis and Restrains Th17 Cell Pathogenicity. Cell 2015, 163, 1413–1427. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid–Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Vailati-Riboni, M.; Kanwal, M.; Bulgari, O.; Meier, S.; Priest, N.V.; Burke, C.R.; Trevisi, E.; Loor, J.J. Body Condition Score and Plane of Nutrition Prepartum Affect Adipose Tissue Transcriptome Regulators of Metabolism and Inflammation in Grazing Dairy Cows during the Transition Period. J. Dairy Sci. 2016, 99, 758–770. [Google Scholar] [CrossRef]
- Van Dorland, H.A.; Richter, S.; Morel, I.; Doherr, M.G.; Castro, N.; Bruckmaier, R.M. Variation in Hepatic Regulation of Metabolism during the Dry Period and in Early Lactation in Dairy Cows. J. Dairy Sci. 2009, 92, 1924–1940. [Google Scholar] [CrossRef]
- Faulconnier, Y.; Chilliard, Y.; Torbati, M.B.; Leroux, C. The Transcriptomic Profiles of Adipose Tissues Are Modified by Feed Deprivation in Lactating Goats. Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 139–149. [Google Scholar] [CrossRef]
- Loor, J.J. Genomics of Metabolic Adaptations in the Peripartal Cow. Animal 2010, 4, 1110–1139. [Google Scholar] [CrossRef]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward Clinical Application of the Keap1-Nrf2 Pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- Schogor, A.L.B.; Palin, M.F.; dos Santos, G.T.; Benchaar, C.; Lacasse, P.; Petit, H.V. Mammary Gene Expression and Activity of Antioxidant Enzymes and Oxidative Indicators in the Blood, Milk, Mammary Tissue and Ruminal Fluid of Dairy Cows Fed Flax Meal. Br. J. Nutr. 2013, 110, 1743–1750. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Valverde, I.; Pereira, V.; Sotillo, J.; Alonso, M.L.; Benedito, J.L. Plasma Malonaldehyde (MDA) and Total Antioxidant Status (TAS) during Lactation in Dairy Cows. Res. Vet. Sci. 2006, 80, 133–139. [Google Scholar] [CrossRef]
- Bell, A.W. Regulation of Organic Nutrient Metabolism during Transition from Late Pregnancy to Early Lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 2002, 85, 2173–2179. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Li, L.; Li, M.; Zhang, C.; Ao, C.; Hou, X. Effects of maternal undernutrition during late pregnancy on the development and function of ovine fetal liver. Anim. Reprod. Sci. 2014, 147, 99–105. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, C.; Hu, F.; Zhu, W.; Mao, S. Undernutrition-induced lipid metabolism disorder triggers oxidative stress in maternal and fetal livers using a model of pregnant sheep. FASEB J. 2020, 34, 6508–6520. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Abdullah, M.A.M.; Mavrommatis, A.; Chatzikonstantinou, M.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Labrou, N.E.; Zervas, G. The effect of dietary Chlorella vulgaris inclusion on goat’s milk chemical composition, fatty acids profile and enzymes activities related to oxidation. J. Anim. Physiol. Anim. Nutr. 2018, 102, 142–151. [Google Scholar] [CrossRef]
- Leduc, A.; Souchet, S.; Gelé, M.; Le Provost, F.; Boutinaud, M. Effect of feed restriction on dairy cow milk production: A review. J. Anim. Sci. 2021, 99, skab130. [Google Scholar] [CrossRef]
- Barrio, E.; Hervás, G.; Gindri, M.; Friggens, N.C.; Toral, P.G.; Frutos, P. Relationship between feed efficiency and resilience in dairy ewes subjected to acute underfeeding. J. Dairy Sci. 2023, 106, 6028–6040. [Google Scholar] [CrossRef]
- Cattaneo, L.; Mezzetti, M.; Lopreiato, V.; Piccioli-Cappelli, F.; Trevisi, E.; Minuti, A. Gene network expression of whole blood leukocytes in dairy cows with different milk yield at dry-off. PLoS ONE 2021, 16, e0260745. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P.; Horst, R.L. Physiological Changes at Parturition and Their Relationship to Metabolic Disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Contreras, G.A.; Aitken, S.L. Metabolic Factors Affecting the Inflammatory Response of Periparturient Dairy Cows. Anim. Health Res. Rev. 2009, 10, 53–63. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M.C. Hydroxytyrosol: Bioavailability, Toxicity, and Clinical Applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Scheibl, P.; Zerbe, H. Einfluss von Progesteron auf das Immunsystem mit Berücksichtigung der bovinen Retentio secundinarum (Effect of progesterone on the immune system in consideration of bovine placental retention). Dtsch. Tierarztl. Wochenschr. 2000, 107, 221–227. [Google Scholar]
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Tang, S.; Wan, F.; Zhong, R.; Chen, L.; Zhang, H. The PI3K/Akt-Nrf2 Signaling Pathway and Mitophagy Synergistically Mediate Hydroxytyrosol to Alleviate Intestinal Oxidative Damage. Int. J. Biol. Sci. 2024, 20, 4258–4276. [Google Scholar] [CrossRef]
- Lee, S.; Hu, L. Nrf2 activation through the inhibition of Keap1-Nrf2 protein-protein interaction. Med. Chem. Res. 2020, 29, 846–867. [Google Scholar] [CrossRef]
- Casuso, R.A.; Al Fazazi, S.; Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Rueda-Robles, A.; Aragón-Vela, J.; Huertas, J.R. Hydroxytyrosol modifies skeletal muscle GLUT4/AKT/Rac1 axis in trained rats. J. Cell. Physiol. 2021, 236, 489–494. [Google Scholar] [CrossRef]
- Casuso, R.A.; Al Fazazi, S.; Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Rueda-Robles, A.; Aragón-Vela, J.; Huertas, J.R. Physiological Doses of Hydroxytyrosol Modulate Gene Expression in Skeletal Muscle of Exercised Rats. Life 2021, 11, 1393. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Sheikhahmadi, A.; Wang, Y.; Jiao, H.; Lin, H.; Song, Z. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus). Int. J. Biometeorol. 2015, 59, 127–135. [Google Scholar] [CrossRef]
- Abbas, Z.; Sammad, A.; Hu, L.; Fang, H.; Xu, Q.; Wang, Y. Glucose metabolism and dynamics of facilitative glucose transporters (GLUTs) under the influence of heat stress in dairy cattle. Metabolites 2020, 10, 312. [Google Scholar] [CrossRef]
- Loor, J.J.; Dann, H.M.; Guretzky, N.A.J.; Everts, R.E.; Oliveira, R.; Green, C.A.; Litherland, N.B.; Rodriguez-Zas, S.L.; Lewin, H.A.; Drackley, J.K. Plane of nutrition prepartum alters hepatic gene expression and function in dairy cows as assessed by longitudinal transcript and metabolic profiling. Physiol. Genom. 2006, 27, 29–41. [Google Scholar] [CrossRef]
- Raulien, N.; Friedrich, K.; Strobel, S.; Rubner, S.; Baumann, S.; von Bergen, M.; Körner, A.; Krueger, M.; Rossol, M.; Wagner, U. Fatty Acid Oxidation Compensates for Lipopolysaccharide-Induced Warburg Effect in Glucose-Deprived Monocytes. Front. Immunol. 2017, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Eder, J.M.; Sacco, R.E. Ex vivo activated CD4+ T cells from young calves exhibit Th2-biased effector function with distinct metabolic reprogramming compared to adult cows. Vet. Immunol. Immunopathol. 2022, 248, 110418. [Google Scholar] [CrossRef] [PubMed]
- Bühler, C.; Hammon, H.; Rossi, G.L.; Blum, J.W. Small intestinal morphology in eight-day-old calves fed colostrum for different durations or only milk replacer and treated with long-R3-insulin-like growth factor I and growth hormone. J. Anim. Sci. 1998, 76, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.W. Nutritional Physiology of Neonatal Calves. J. Anim. Physiol. Anim. Nutr. 2006, 90, 1–11. [Google Scholar] [CrossRef]
- Von Knethen, A.; Brüne, B. PPARgamma—An Important Regulator of Monocyte/Macrophage Function. Arch. Immunol. Ther. Exp. 2003, 51, 219–226. [Google Scholar] [PubMed]
- Sumner-Thomson, J.M.; Vierck, J.L.; McNamara, J.P. Differential Expression of Genes in Adipose Tissue of First-Lactation Dairy Cattle. J. Dairy Sci. 2011, 94, 361–369. [Google Scholar] [CrossRef]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Supplemental Smartamine M or MetaSmart during the Transition Period Benefits Postpartal Cow Performance and Blood Neutrophil Function. J. Dairy Sci. 2013, 96, 6248–6263. [Google Scholar] [CrossRef]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.M.; Tyler, J.W.; VanMetre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive Transfer of Colostral Immunoglobulins in Calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.; Cullens-Nobis, F.M.; Owczarzak, E.J.; Abuelo, A. Effect of Parenteral Micronutrient Supplementation at Birth on Immunity, Growth, and Health in Preweaning Dairy Heifers. J. Dairy Sci. 2024, 107, 4926–4941. [Google Scholar] [CrossRef]
- Osorio, J.S.; Jacometo, C.B.; Zhou, Z.; Luchini, D.; Cardoso, F.C.; Loor, J.J. Hepatic Global DNA and Peroxisome Proliferator-Activated Receptor Alpha Promoter Methylation Are Altered in Peripartal Dairy Cows Fed Rumen-Protected Methionine. J. Dairy Sci. 2016, 99, 234–244. [Google Scholar] [CrossRef]
- Bender, C.; Straßmann, S.; Heidrich, P. Cellular Antioxidant Effects and Bioavailability of Food Supplements Rich in Hydroxytyrosol. Appl. Sci. 2021, 11, 4763. [Google Scholar] [CrossRef]
- Castillo, C.; Pereira, V.; Abuelo, Á.; Hernández, J. Effect of Supplementation with Antioxidants on the Quality of Bovine Milk and Meat Production. Sci. World J. 2013, 2013, 616098. [Google Scholar] [CrossRef]
- Novoselec, J.; Klir Šalavardić, Ž.; Đidara, M.; Novoselec, M.; Vuković, R.; Ćavar, S.; Antunović, Z. The Effect of Maternal Dietary Selenium Supplementation on Blood Antioxidant and Metabolic Status of Ewes and Their Lambs. Antioxidants 2022, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Lin, T.; Yue, X.; Zhang, S.; Liu, X.; Chen, F.; Guan, W. Maternal Selenium-Enriched Yeast Supplementation in Sows Enhances Offspring Growth and Antioxidant Status through the Nrf2/Keap1 Pathway. Antioxidants 2023, 12, 2064. [Google Scholar] [CrossRef]
- Komatsu, T.; Itoh, F.; Kushibiki, S.; Hodate, K. Changes in Gene Expression of Glucose Transporters in Lactating and Nonlactating Cows. J. Anim. Sci. 2005, 83, 557–564. [Google Scholar] [CrossRef]
- Wachter, C.M.; McDaniel, B.T.; Whitlow, L.W.; Pettyjohn, S. Genetics of antioxidant activity in Holsteins and Jerseys: Associations with various traits. J. Dairy Sci. 1999, 82, 31. [Google Scholar]
- Rtibi, K.; Jabri, M.A.; Selmi, S.; Souli, A.; Sebai, H.; El-Benna, J.; Marzouki, L. Carob Pods (Ceratonia siliqua L.) Inhibit Human Neutrophils Myeloperoxidase and In Vitro ROS-Scavenging Activity. RSC Adv. 2015, 5, 84207–84215. [Google Scholar] [CrossRef]
- Pelegrin-Valls, J.; Álvarez-Rodríguez, J.; Martín-Alonso, M.J.; Aquilué, B.; Serrano-Pérez, B. Impact of Carob (Ceratonia siliqua L.) Pulp Inclusion and Warm Season on Gastrointestinal Morphological Parameters, Immune-Redox Defences and Coccidiosis in Concentrate-Fed Light Lambs. Res. Vet. Sci. 2023, 163, 104969. [Google Scholar] [CrossRef] [PubMed]




| Gene | General Gene Role | Forward and Reverse Primer (5′–3′) | Base Pair | Access. No. | Efficiency (%) | nM | Source |
|---|---|---|---|---|---|---|---|
| RPL19 | Housekeeping | F: GATCCGGAAGCTGATCAAAG R: ATTCGAGCATTGGCAGTACC | 147 | NM_001040516.1 | 97.5 | 200 | [38] |
| ACTB | Housekeeping | F: CTGGACTTCGAGCAGGAGAT R: GATGTCGACGTCACACTTC | 207 | AY141970 | 105 | 200 | [39] |
| SOD1 | Antioxidant enzyme | F: CACCATCCACTTCGAGGCAA R: GCACTGGTACAGCCTTGTGT | 126 | NM_174615.2 | 95.1 | 300 | [40] |
| SOD2 | Antioxidant enzyme | F: GGATCCCCTGCAAGGAACAA R: TGGCCTTCAGATAATCGGGC | 110 | NM_201527.2 | 98.6 | 300 | [40] |
| CAT | Antioxidant enzyme | F: TCACTCAGGTGCGGACTTTC R: GGATGCGGGAGCCATATTCA | 162 | NM_001035386.2 | 111.1 | 300 | [40] |
| GPX1 | Antioxidant enzyme | F: GAGCCCTTCAACCTGTCCTC R: GCGTTTTCCTGATGCCCAAAC | 179 | NM_174076.3 | 95.5 | 250 | [41] |
| NRF2 | Transcription factor | F: AGCTCAGCATGATGGACTTGGA R: CAGCTCATGCTCCTTCTGTCG | 152 | NM_001011678.2 | 101.1 | 333 | [41] |
| TLR4 | Immune response | F: TCCCCGACAACATCCCCATA R: AAAGGCTCCCCAGGCTAAAC | 224 | NM_174198 | 103.2 | 125 | [42] |
| NFKB | Transcription factor | F: CGGGGACTACGACCTGAATG R: GCCTGGTCCCGTGAAATACA | 250 | NM_001080242 | 95.5 | 250 | [42] |
| TNFA | Immune response | F: CCAGAGGGAAGAGCAGTCC R: GGCTACAACGTGGGCTACC | 112 | NM_173966.3 | 89.9 | 125 | [42] |
| ALOX5 | Inflammation regulation | F: GCAGGAAGACCGCATGTTTG R: GTTCCCTTGCTCGATCTCCT | 163 | NM_001192792 | 107.2 | 200 | [43] |
| PPARD | Transcription factor | F: TGTGGCAGCCTCAATATGGA R: GACGGAAGAAGCCCTTGCA | 100 | NM_001083636.1 | 106.2 | 400 | [44] |
| PDK4 | Energy metabolism | F: TGTATCCCAAGCAAGGAACC R: TTTGATCCCTTAGCGTGTCC | 86 | NM_001101883.1 | 99.9 | 400 | [5] |
| SCD | Energy metabolism | F: CAGCGGAAGGTCCCGA R: CAAGTGGGCCGGCATC | 157 | NM_173959.4 | 90.5 | 400 | [45] |
| IGF1R | Energy metabolism | F: TTAAAATGGCCAGAACCTGAG R: ATTATAACCAAGCCTCCCAC | 314 | NM_001244612.1 | 104 | 400 | [46] |
| SREBF1 | Energy metabolism | F: CCAGCTGACAGCTCCATTGA R: TGCGCGCCACAAGGA | 67 | NM_001113302 | 95.7 | 400 | [47] |
| FASN | Energy metabolism | F: CTGAGTCGGAGAACCTGGAG R: ACAATGGCCTCGTAGGTGAC | 232 | NM_001012669 | 90.3 | 400 | [47] |
| HMGCS1 | Energy metabolism | F: TGTACGGCTCCCTGGCTTCTG R: CATGTTCCTTCGAAGAGGGAATC | 313 | BC_102850 | 93.5 | 200 | [47] |
| SLC2A1/GLUT1 | Energy metabolism | F: GCTTCTCCAACTGGACTTCG R: ACAGCTCCTCAGGTGTCTTG | 225 | NM_174602 | 99.8 | 250 | [48] |
| ACADVL | Energy metabolism | F: TCCCCAAACTGGCATCTGGG R: ATGGGTGACGCCGCCAAAGC | 275 | BC_103104 | 93.2 | 400 | [47] |
| p-Values | |||||||
|---|---|---|---|---|---|---|---|
| T100%-CTROL | T100%-HT | T60%-CTROL | T60%-HT | FL | HT | FL × HT | |
| Gestation (n) | 11 | 10 | 14 | 11 | |||
| BW at −12 weeks before calving (kg) | 672 ± 15.3 | 683 ± 16.4 | 669 ± 13.5 | 668 ± 15.3 | 0.56 | 0.76 | 0.69 |
| ADG 2 during 12 weeks prepartum (kg/day) | 0.62 ± 0.08 a | 0.43 ± 0.08 a | 0.03 ± 0.07 b | 0.15 ± 0.08 b | <0.001 | 0.66 | 0.05 |
| BW after calving (kg) | 679 ± 6.4 a | 666 ± 6.5 a | 625 ± 5.3 b | 624 ± 6.2 b | <0.001 | 0.24 | 0.34 |
| Postpartum (n) | 10 | 9 | 9 | 9 | |||
| ADG (5 weeks postpartum (kg/day)) | −0.71 ± 0.23 a | −0.66 ± 0.24 a | −0.06 ± 0.25 b | −0.30 ± 0.25 b | 0.04 | 0.69 | 0.56 |
| Cows with key HT metabolites detected in serum at week −3 before calving 3 (n) | 6 | 6 | 6 | 6 | |||
| Hydroxytyrosol sulphate (HTS) | 0/6 a | 5/6 b | 0/6 a | 5/6 b | 1.00 | <0.001 | 1.00 |
| Alcohol homovanillic sulphate (AHVS) | 0/6 a | 5/6 b | 0/6 a | 5/6 b | 1.00 | <0.001 | 1.00 |
| Both AHVS and HTS | 0/6 a | 4/6 b | 0/6 a | 4/6 b | 1.00 | <0.001 | 1.00 |
| Cows with key HT metabolites detected in colostrum (<12 h postpartum) (n) | 6 | 6 | 6 | 6 | |||
| Hydroxytyrosol sulphate (HTS) | 0/6 a | 5/6 b | 0/6 a | 3/6 b | 1.00 | <0.001 | 0.39 |
| Alcohol homovanillic sulphate (AHVS) | 0/6 a | 4/6 b | 0/6 a | 4/6 b | 1.00 | <0.001 | 1.00 |
| Both AHVS and HTS | 0/6 a | 4/6 b | 0/6 a | 2/6 b | 1.00 | <0.001 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalera-Moreno, N.; Álvarez-Rodríguez, J.; López de Armentia, L.; Macià, A.; Martín-Alonso, M.J.; Molina, E.; Villalba, D.; Sanz, A.; Serrano-Pérez, B. Maternal Hydroxytyrosol Supplementation Enhances Antioxidant Capacity and Immunometabolic Adaptations in Nutrient-Restricted Beef Cows and Their Offspring. Antioxidants 2025, 14, 1097. https://doi.org/10.3390/antiox14091097
Escalera-Moreno N, Álvarez-Rodríguez J, López de Armentia L, Macià A, Martín-Alonso MJ, Molina E, Villalba D, Sanz A, Serrano-Pérez B. Maternal Hydroxytyrosol Supplementation Enhances Antioxidant Capacity and Immunometabolic Adaptations in Nutrient-Restricted Beef Cows and Their Offspring. Antioxidants. 2025; 14(9):1097. https://doi.org/10.3390/antiox14091097
Chicago/Turabian StyleEscalera-Moreno, Nieves, Javier Álvarez-Rodríguez, Leire López de Armentia, Alba Macià, Maria José Martín-Alonso, Ester Molina, Daniel Villalba, Albina Sanz, and Beatriz Serrano-Pérez. 2025. "Maternal Hydroxytyrosol Supplementation Enhances Antioxidant Capacity and Immunometabolic Adaptations in Nutrient-Restricted Beef Cows and Their Offspring" Antioxidants 14, no. 9: 1097. https://doi.org/10.3390/antiox14091097
APA StyleEscalera-Moreno, N., Álvarez-Rodríguez, J., López de Armentia, L., Macià, A., Martín-Alonso, M. J., Molina, E., Villalba, D., Sanz, A., & Serrano-Pérez, B. (2025). Maternal Hydroxytyrosol Supplementation Enhances Antioxidant Capacity and Immunometabolic Adaptations in Nutrient-Restricted Beef Cows and Their Offspring. Antioxidants, 14(9), 1097. https://doi.org/10.3390/antiox14091097









