Electrochemical Measures for Determining the Total Antioxidant Capacity of Açaí Pulp (Euterpe oleracea) at a Glassy Carbon Electrode
Abstract
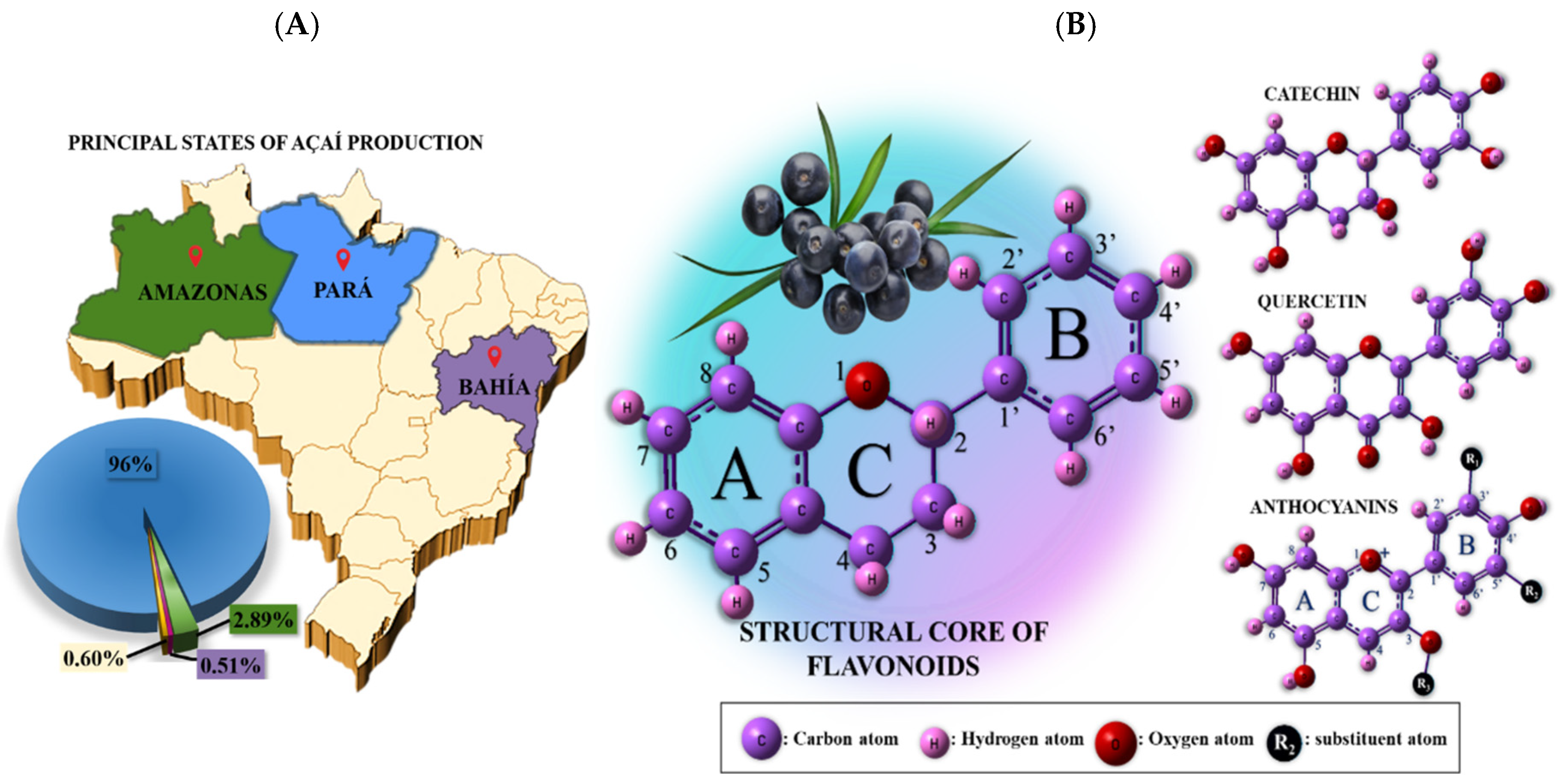
1. Introduction
2. Materials
Chemical Reagents
3. Methodology
3.1. Extraction and Sample Preparation
3.2. Total Phenolic Content (TPC)
3.3. DPPH Assay
3.4. Electrochemical Assays
4. Results and Discussions
4.1. Concentration Test
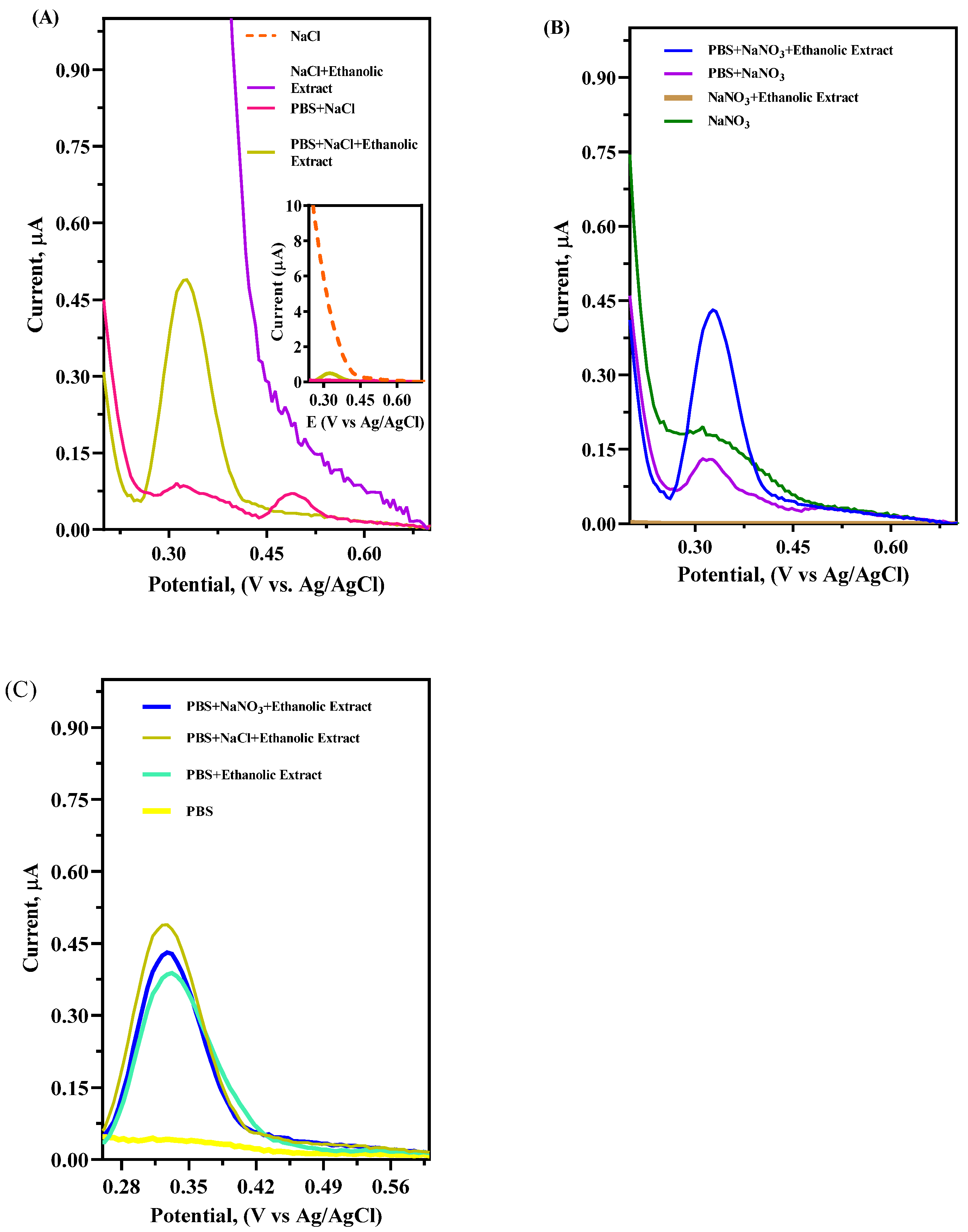
4.2. Supporting Electrolyte Analysis
4.3. Scan Rate Test
4.4. Antioxidant Power
4.5. Total Phenolic Content (TPC)
4.6. DPPH Test
4.7. Assessment of Antioxidant Activity: Methods, Merits, and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avila-Sosa, R.; Montero-Rodríguez, A.F.; Aguilar-Alonso, P.; Vera-López, O.; Lazcano-Hernández, M.; Morales-Medina, J.C.; Navarro-Cruz, A.R. Antioxidant Properties of Amazonian Fruits: A Mini Review of In Vivo and In Vitro Studies. Oxid. Med. Cell. Longev. 2019, 2019, 8204129. [Google Scholar] [CrossRef]
- Silva Cedrim, P.C.A.; Barros, E.M.A.; Do Nascimento, T.G. Antioxidant Properties of Acai (Euterpe oleracea) in the Metabolic Syndrome. Braz. J. Food Technol. 2018, 21, e2017092. [Google Scholar] [CrossRef]
- Silveira, J.T.d.; Rosa, A.P.C.d.; Morais, M.G.d.; Victoria, F.N.; Costa, J.A.V. An Integrative Review of Açaí (Euterpe oleracea and Euterpe precatoria): Traditional Uses, Phytochemical Composition, Market Trends, and Emerging Applications. Food Res. Int. 2023, 173, 113304. [Google Scholar] [CrossRef] [PubMed]
- IBGE Produção Agrícola Municipal. 2022. Available online: https://sidra.ibge.gov.br/tabela/1613#resultado (accessed on 23 October 2023).
- Jobim, M.L.; Barbisan, F.; Fortuna, M.; Teixeira, C.F.; Boligon, A.A.; Ribeiro, E.E.; da Cruz, I.B.M. Açai (Euterpe oleracea, Mart.), an Amazonian fruit has antitumor effects on prostate cancer cells. Arch. Biosci. Health 2019, 1, 61–76. [Google Scholar] [CrossRef]
- Yildirim, C.; Aydin, S.; Donertas, B.; Oner, S.; Kilic, F.S. Effects of Euterpe oleracea to Enhance Learning and Memory in a Conditioned Nicotinic and Muscarinic Receptor Response Paradigm by Modulation of Cholinergic Mechanisms in Rats. J. Med. Food 2020, 23, 388–394. [Google Scholar] [CrossRef]
- Souza-Monteiro, J.R.; Arrifano, G.P.F.; Queiroz, A.I.D.G.; Mello, B.S.F.; Custodio, C.S.; Macedo, D.S.; Hamoy, M.; Paraense, R.S.O.; Bittencourt, L.O.; Lima, R.R.; et al. Antidepressant and Antiaging Effects of Açaí (Euterpe oleracea Mart.) in Mice. Oxid. Med. Cell. Longev. 2019, 2019, 3614960. [Google Scholar] [CrossRef]
- Martínez-Flórez, S.; González-Gallego, J.; Culebras, J.M.; Tuñón, M.J. Los Flavonoides: Propiedades y Acciones Antioxidantes. Nutr. Hosp. 2002, 17, 271–278. [Google Scholar]
- George, S.A.; Rajeev, R.; Thadathil, D.A.; Varghese, A. A Comprehensive Review on the Electrochemical Sensing of Flavonoids. Crit. Rev. Anal. Chem. 2023, 53, 1133–1173. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M. Electrochemistry of Flavonoids: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 15667. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Kim, J.S. Flavonoids, an Overview: Chemical Structures, Dietary Sources, and Biological Properties. Food Eng. Prog. 2020, 24, 151–163. [Google Scholar] [CrossRef]
- Gil, E.S.; Couto, R.O. Flavonoid Electrochemistry: A Review on the Electroanalytical Applications. Rev. Bras. Farmacogn. 2013, 23, 542–558. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural Phenolic Antioxidants Electrochemistry: Towards a New Food Science Methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Amidi, S.; Mojab, F.; Moghaddam, A.B.; Tabib, K.; Kobarfard, F. A Simple Electrochemical Method for the Rapid Estimation of Antioxidant Potentials of Some Selected Medicinal Plants. Iran. J. Pharm. Res. 2012, 11, 117–121. [Google Scholar]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic Voltammetry Method Suitable for Characterizing Antioxidant Properties of Wine and Wine Phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Diniz, G.C.; Pinheiro Gomes, V.T.R.; de Assis, M.; Alejandro Figueroa, S.J.; Ferreira Torquato, I.; de Freitas Borges, L.G.; Aguilar Vitorino, H.; de Lima, R.B.; Suller Garcia, M.A.; de Araujo Rodrigues, I. Electrochemical Sensitivity Improvement by the Cooperation between Pt and Ru for Total Antioxidant Evaluation in Natural Extracts. Chemosensors 2023, 11, 314. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M.; Chrzescijanska, E. Electrooxidation of Flavonoids at Platinum Electrode Studied by Cyclic Voltammetry. Food Chem. 2011, 127, 699–704. [Google Scholar] [CrossRef]
- Osorio-Valencia, A.I.; de Jesús Franco-Mejía, J.; Hoyos-Arbeláez, J.A.; Blandón-Naranjo, L.; Vega-Castro, O.A.; del Carmen Contreras-Calderón, J. Evaluation of Antioxidant Capacity in Different Food Matrices through Differential Pulse Voltammetry and Its Correlation with Spectrophotometric Methods. J. Appl. Electrochem. 2023, 53, 2495–2505. [Google Scholar] [CrossRef]
- Gomes, S.M.C.; Ghica, M.E.; Rodrigues, I.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Flavonoids Electrochemical Detection in Fruit Extracts and Total Antioxidant Capacity Evaluation. Talanta 2016, 154, 284–291. [Google Scholar] [CrossRef]
- Méndez-Durazno, C.; Cisneros-Perez, P.A.; Loja-Ojeda, B.A.; Monge-Sevilla, R.; Romero-Estévez, D.; Fernández, L.; Espinoza-Montero, P.J. Antioxidant Capacity through Electrochemical Methods and Chemical Composition of Oenocarpus Bataua and Gustavia Macarenensis from the Ecuadorian Amazon. Antioxidants 2023, 12, 318. [Google Scholar] [CrossRef]
- de Macêdo, I.Y.L.; Garcia, L.F.; Oliveira Neto, J.R.; de Siqueira Leite, K.C.; Ferreira, V.S.; Ghedini, P.C.; de Souza Gil, E. Electroanalytical Tools for Antioxidant Evaluation of Red Fruits Dry Extracts. Food Chem. 2017, 217, 326–331. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Valek, L.; Stipčević, T.; Martinez, S. Electrochemical Determination of Antioxidant Capacity of Fruit Tea Infusions. Food Chem. 2010, 121, 820–825. [Google Scholar] [CrossRef]
- Demir, E. Sensitive and Selective Pathway of Total Antioxidant Capacity in Commercially Lemon, Watermelon and Mango-Pineapple Cold Teas by Square Wave Adsorptive Stripping Voltammetry. Gazi Univ. J. Sci. 2019, 32, 1123–1136. [Google Scholar] [CrossRef]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Nawaz, A. Electrochemical Methodologies for Investigating the Antioxidant Potential of Plant and Fruit Extracts: A Review. Antioxidants 2022, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical Methods as a Tool for Determining the Antioxidant Capacity of Food and Beverages: A Review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- da Costa, D.S.; Bragotto, A.P.A.; de Carvalho, L.M.; Amado, L.L.; Lima, R.R.; Rogez, H. Analysis of Polyphenols, Anthocyanins and Toxic Elements in Açaí Juice (Euterpe oleracea Mart.): Quantification and in Vivo Assessment of the Antioxidant Capacity of Clarified Açaí Juice. Meas. Food 2024, 14, 100149. [Google Scholar] [CrossRef]
- Gomes, S.M.C.; Fernandes, I.P.G.; Shekhawat, N.S.; Kumbhat, S.; Oliveira-Brett, A.M. Calligonum Polygonoides Linnaeus Extract: HPLC-EC and Total Antioxidant Capacity Evaluation. Electroanalysis 2015, 27, 293–301. [Google Scholar] [CrossRef]
- Campanella, L.; Martini, E.; Rita, G.; Tomassetti, M. Antioxidant Capacity of Dry Vegetal Extracts Checked by Voltammetric Method. J. Food Agric. Environ. 2006, 4, 135–144. [Google Scholar]
- Muhammad, H.; Tahiri, I.A.; Qasim, M.; Versiani, M.A.; Hanif, M.; Gul, B.; Ali, S.T.; Ahmed, S. Electrochemical Determination of Antioxidant Activity and HPLC Profiling of Some Dry Fruits. Monatsh. Chem. 2019, 150, 1195–1203. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Paiva, S.S.M.; da Silva, D.R.; Vilar, V.J.P.; Martínez-Huitle, C.A.; dos Santos, E.V. Novel Cork-Graphite Electrochemical Sensor for Voltammetric Determination of Caffeine. J. Electroanal. Chem. 2019, 839, 283–289. [Google Scholar] [CrossRef]
- Ahmadpour, S.; Tashkhourian, J.; Hemmateenejad, B. A Chemometric Investigation on the Influence of the Nature and Concentration of Supporting Electrolyte on Charging Currents in Electrochemistry. J. Electroanal. Chem. 2020, 871, 114296. [Google Scholar] [CrossRef]
- de Mello e Silva, G.N.; Batista Rodrigues, E.S.; Lopes de Macêdo, I.Y.; Vicente Gil, H.P.; Campos, H.M.; Ghedini, P.C.; Cardozo da Silva, L.; Batista, E.A.; Lopes de Araújo, G.; Vaz, B.G.; et al. Blackberry Jam Fruit (Randia formosa (Jacq.) K. Schum): An Amazon Superfruit with in Vitro Neuroprotective Properties. Food Biosci. 2022, 50, 102084. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Da Silva, D.R.; Quiroz, M.A.; Vilar, V.J.P.; Martínez-Huitle, C.A.; Dos Santos, E.V. Applicability of Cork as Novel Modifiers to Develop Electrochemical Sensor for Caffeine Determination. Materials 2020, 14, 37. [Google Scholar] [CrossRef]
- Monge-Sevilla, R.D.; Fernández, L.; Espinoza-Montero, P.J.; Méndez-Durazno, C.; Cisneros-Pérez, P.A.; Romero-Estévez, D.; Bolaños-Méndez, D.; Alvarez-Paguay, J.; Jadán, M. Chemical Composition and Antioxidant Properties of Native Ecuadorian Fruits: Rubus glabratus Kunth, Vaccinium floribundum Kunth, and Opuntia soederstromiana. Heliyon 2024, 10, e30593. [Google Scholar] [CrossRef]
- Belda-Galbis, C.M.; Jiménez-Carretón, A.; Pina-Pérez, M.C.; Martínez, A.; Rodrigo, D. Antimicrobial Activity of Açaí against Listeria innocua. Food Control 2015, 53, 212–216. [Google Scholar] [CrossRef]
- Junior, C.; Maria Martins Siqueira, L.; Luiza de Barros Souza Campos, A.; Cristina Seabra Pires, F.; Caroline Rodrigues Ferreira, M.; Paula de Souza Silva, A.; Gama Ortiz Menezes, E.; Nayara de Farias Ramos, I.; Salim Khayat, A.; de Arimateia Rodrigues do Rêgo, J.; et al. Evaluation of Bioactive Compounds and Antioxidant and Cytotoxic Effects of Oil and Pulp without Açaí Fat (Euterpe oleracea) Obtained by Supercritical Extraction. Foods 2024, 13, 2819. [Google Scholar] [CrossRef]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian Fruit Pulps as Functional Foods and Additives: Evaluation of Bioactive Compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef]
- Gordon, A.; Cruz, A.P.G.; Cabral, L.M.C.; De Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; De Andrade Mattietto, R.; Friedrich, M.; Da Matta, V.M.; Marx, F. Chemical Characterization and Evaluation of Antioxidant Properties of Açaí Fruits (Euterpe oleraceae Mart.) during Ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef]
- Carvalho, A.V.; Ferreira Ferreira da Silveira, T.; Mattietto, R.D.A.; Padilha de Oliveira, M.D.S.; Godoy, H.T. Chemical Composition and Antioxidant Capacity of Açaí (Euterpe oleracea) Genotypes and Commercial Pulps. J. Sci. Food Agric. 2017, 97, 1467–1474. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic Composition and Antioxidant Activity of Açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef]
- Luz, J.R.D.D.; Barbosa, E.A.; Sousa, R.M.D.; Oliveira, M.L.D.A.; Dias, M.F.S.; Alves, I.R.; Souza, G.C.D.; Ferreira, E.F.B.; Guzmán-Pincheira, C.; Almeida, M.D.G.; et al. Phytochemical Profiling of Processed Açaí Pulp (Euterpe oleracea) Through Mass Spectrometry and Its Protective Effects Against Oxidative Stress in Cardiomyocytes and Rats. Antioxidants 2025, 14, 642. [Google Scholar] [CrossRef]
- de Souza Silva, A.P.; de Camargo, A.C.; Lazarini, J.G.; Carvalho, G.R.; de Alencar, S.M. How Does in Vitro Gastrointestinal Digestion Affect the Biological Activities and Phenolic Profile of Açaí (Euterpe oleracea) and Inajá (Maximiliana maripa) by-Products? Food Chem. 2025, 484, 144364. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A Comprehensive Review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef]


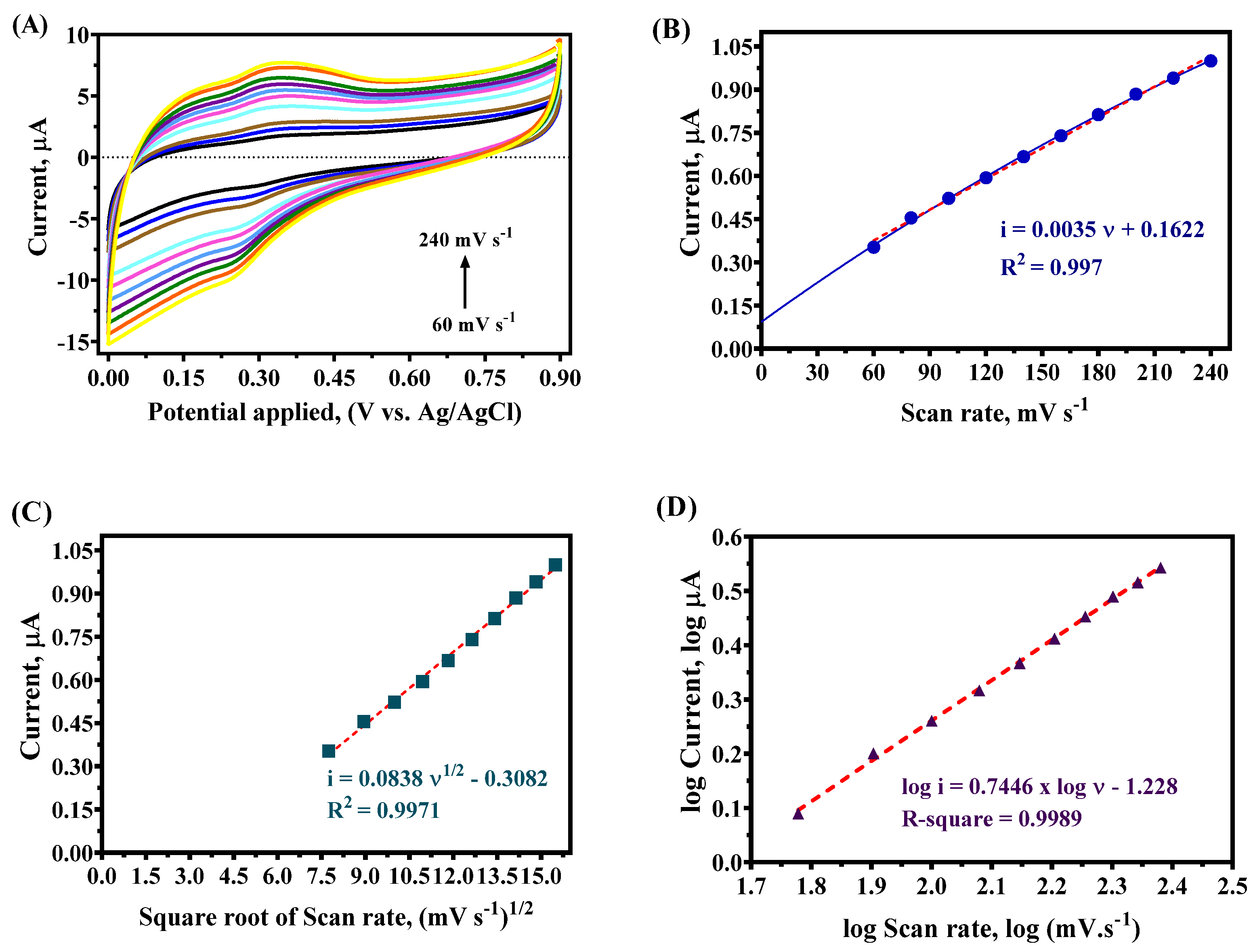

| ѵ, mV s−1 | Eap, V | Ecp, V | ∆E = |Ecp − Eap| | iap, µA | icp, µA | ∆i = |icp − iap| |
|---|---|---|---|---|---|---|
| 60 | 0.361 | 0.302 | 0.059 | 0.957 | −1.131 | 2.088 |
| 80 | 0.368 | 0.292 | 0.076 | 1.301 | −1.498 | 2.799 |
| 100 | 0.370 | 0.287 | 0.083 | 1.586 | −1.837 | 3.423 |
| 120 | 0.373 | 0.283 | 0.090 | 1.976 | −2.202 | 4.178 |
| 140 | 0.380 | 0.261 | 0.120 | 2.291 | −2.743 | 5.033 |
| 160 | 0.383 | 0.258 | 0.125 | 2.572 | −3.014 | 5.586 |
| 180 | 0.385 | 0.248 | 0.137 | 2.825 | −3.472 | 6.298 |
| 200 | 0.392 | 0.246 | 0.146 | 3.119 | −3.781 | 6.901 |
| 220 | 0.400 | 0.246 | 0.154 | 3.405 | −4.056 | 7.461 |
| 240 | 0.402 | 0.239 | 0.164 | 3.647 | −4.474 | 8.122 |
| Fruit | Electrochemical Assay | EQI, µA/V | Reference |
|---|---|---|---|
| Blackberry jam fruit (Randia formosa (Jacq.) K. Shum) | SWV | 6.78 | [34] |
| Acerola | CV | 5.80 | [23] |
| Blackberry (Rubus spp.) | DPV | 0.738 | [21] |
| Mora de monte (R. glabratus Kunth) | DPV | 2.26 × 10−5 | [36] |
| Açaí | CV | 6.9 | [23] |
| Spray-dried açaí powder | DPV | 0.168 | [21] |
| Açaí | DPV | 1.08 | In this work |
| CV | 2.30 |
| Method | Mechanism | Sample Suitability | Advantages | Disadvantages | Limitations | Usefulness |
|---|---|---|---|---|---|---|
| DPPH | Electron or H-donation to DPPH radical | Lipophilic | Simple, fast, cost-effective | Not suitable for hydrophilic antioxidants; sensitive to light/pH | Poor correlation to biological systems | Good for rapid screening |
| ABTS | Electron donation to ABTS•+ radical | Hydrophilic and lipophilic | Broad-range applicability, stable | Requires pre-oxidation; possible overestimation | Non-physiological radicals | Excellent for general antioxidant profiling |
| FRAP | Reduction of Fe3+ to Fe2+ | Hydrophilic | Reproducible, easy | Only reducing power; acidic pH required | Not suitable for all antioxidants | Useful for total reducing capacity |
| ORAC | Scavenging of peroxyl radicals | Biological fluids, extracts | Physiologically relevant radicals, kinetic data | Time-consuming, costly, fluorescent probe needed | Sensitive to conditions, reproducibility issues | Ideal for biological antioxidant capacity |
| CUPRAC | Reduction of Cu2+ to Cu+ | Hydrophilic and lipophilic | Stable reagents, broad compatibility | Can have interferences | Not widely used, Cu not biologically relevant | Effective for mixed antioxidant systems |
| TPC | Reaction with Folin–Ciocalteu reagent | Phenolic compounds | Quick, correlates with phenolic content | Non-specific, including non-phenolics | Not a true antioxidant assay | Useful for phenolic content estimation |
| Feature | EQI | DPPH | ABTS | FRAP | ORAC | CUPRAC | TPC |
|---|---|---|---|---|---|---|---|
| Principle | Electron transfer (redox potential and current) | Radical scavenging | Radical scavenging | Reducing power | Peroxyl radical scavenging | Cu2+ reducing power | Total reducing compounds |
| Measurement Type | Electrochemical | Spectrophotometric | Spectrophotometric | Spectrophotometric | Fluorescence | Spectrophotometric | Spectrophotometric |
| Kinetics | Fast; real-time response | End-point | End-point | End-point | Kinetic | End-point | End-point |
| Sensitivity | High for electroactive compounds | Moderate | Moderate | Moderate | High | Moderate | Moderate |
| Selectivity | Selective for electroactive antioxidants | Non-selective | Non-selective | Non-selective | More selective | Non-selective | Poor selectivity |
| Biological Relevance | Moderate; mimics oxidative mechanisms | Low | Low–Moderate | Low | High | Moderate | Indirect |
| Cost and Simplicity | Requires potentiostat; moderate training | Simple and cheap | Moderate | Simple | Expensive instrumentation | Moderate | Very simple |
| Solubility Range | Hydrophilic and lipophilic (depends on electrode material) | Mostly lipophilic | Both | Hydrophilic | Hydrophilic | Both | Hydrophilic |
| Reproducibility | High, if properly standardized | Variable | Variable | Good | Variable | Good | Variable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feijoó, T.N.; Loor-Urgilés, L.D.; de Araújo, D.M.; Santos, E.V.d.; Goulart, M.O.F.; Martínez-Huitle, C.A. Electrochemical Measures for Determining the Total Antioxidant Capacity of Açaí Pulp (Euterpe oleracea) at a Glassy Carbon Electrode. Antioxidants 2025, 14, 1082. https://doi.org/10.3390/antiox14091082
Feijoó TN, Loor-Urgilés LD, de Araújo DM, Santos EVd, Goulart MOF, Martínez-Huitle CA. Electrochemical Measures for Determining the Total Antioxidant Capacity of Açaí Pulp (Euterpe oleracea) at a Glassy Carbon Electrode. Antioxidants. 2025; 14(9):1082. https://doi.org/10.3390/antiox14091082
Chicago/Turabian StyleFeijoó, Tabata N., Luis D. Loor-Urgilés, Danyelle M. de Araújo, Elisama V. dos Santos, Marília Oliveira Fonseca Goulart, and Carlos A. Martínez-Huitle. 2025. "Electrochemical Measures for Determining the Total Antioxidant Capacity of Açaí Pulp (Euterpe oleracea) at a Glassy Carbon Electrode" Antioxidants 14, no. 9: 1082. https://doi.org/10.3390/antiox14091082
APA StyleFeijoó, T. N., Loor-Urgilés, L. D., de Araújo, D. M., Santos, E. V. d., Goulart, M. O. F., & Martínez-Huitle, C. A. (2025). Electrochemical Measures for Determining the Total Antioxidant Capacity of Açaí Pulp (Euterpe oleracea) at a Glassy Carbon Electrode. Antioxidants, 14(9), 1082. https://doi.org/10.3390/antiox14091082









