Harnessing Mitochondrial Function for Post-Stroke Rehabilitation: Unlocking Antioxidant Power
Abstract
1. Introduction
1.1. The Brain-Muscle Axis and Its Susceptibility to Oxidative Stress—Barriers to Functional Recovery
1.2. Ferroptosis-Mediated Oxidative Damage of the Brain Tissue
1.3. Iron Dysregulation in the Neuromuscular Unit (NMU) After Stroke
1.4. Mitochondrial Dysfunction and Lack of Energy After Ischemic Injury
1.5. Mitochondrial Dysfunction and Lack of Energy in Muscles After Stroke
1.6. Antioxidative Strategies with an Impact on Functional Recovery Targeting NMU
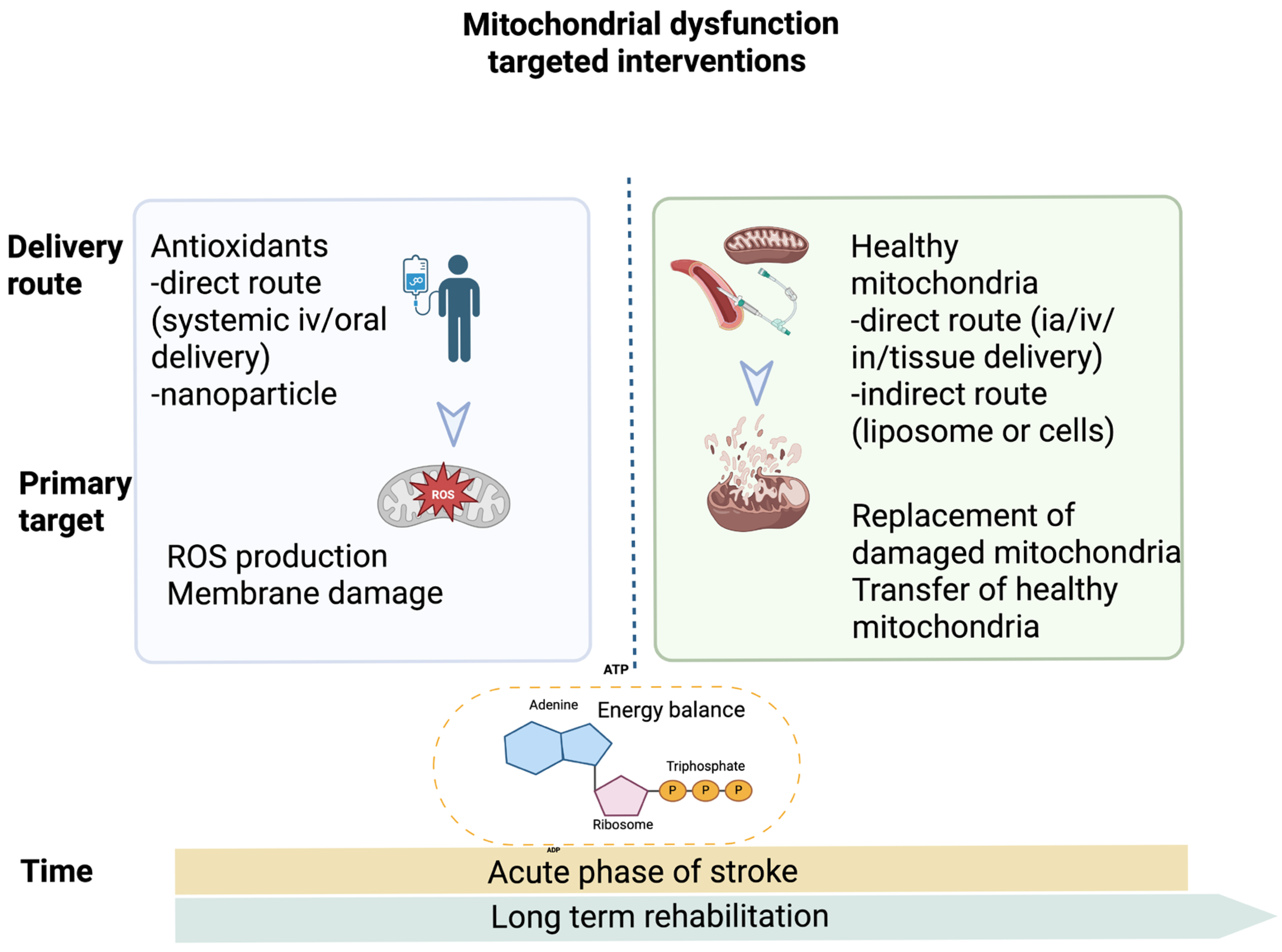
1.7. Antioxidant Versus Mitochondrial Transplantation in Post-Stroke Rehabilitation
2. Clinical Impact and Future Perspective
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | Alpha-lipoic acid |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| ATP | Adenosine triphosphate |
| BBB | Brain blood barrier |
| BDNF | Brain derived neurotrophic factor |
| CoQ: | Coenzyme Q |
| CoQ10 | Coenzyme Q10 |
| CRP | C Reactive Protein |
| Cy c | Cytochrome c |
| DNA | Deoxynucleic acid |
| Drp1 | Dynamin-related protein 1 |
| ER | Endoplasmic reticulum |
| ETC | Electron transport chain |
| EVs | Extracellular vesicles |
| Fe2+ | Ferrous form of iron |
| Fe3+ | Ferric form of iron |
| FES | Functional electrical stimulation |
| GFAP | Glial fibrillary acidic protein |
| GPX4 | Glutathione peroxidase 4 |
| GSH | Reduced form of glutathione |
| HIF | Hypoxia-inducible factor |
| HO | Hydroxyl radical |
| HREs | Hypoxia response element |
| ICT | Interventional clinical trial |
| IL6 | Interleukin 6 |
| IV | Intravenous |
| MCU | Mitochondrial calcium uniporter |
| MDA | Malondialdehyde |
| MitoQ | Mitoquinone |
| MMPs | Matrix metalloproteases |
| MMSE | Mini-Mental State Examination |
| mPTP | Mitochondrial permeability transition pore |
| MRI | Magnetic resonance imaging |
| mRS | Modified Rankin Scale |
| mtDNA | Mitochondrial DNA |
| NAC | N-acetylcysteine |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NF-κB: | Nuclear factor κB |
| NIHSS | National Institutes of Health Stroke Scale |
| NMDA | N-methyl-D-aspartate receptor |
| NMJ | Neuromuscular junction |
| NMU | Neuromotor unit |
| NRF2 | Nuclear receptor factor 2 |
| NVU | Neurovascular unit |
| Opa 1 | OPA1 mitochondrial dynamin-like GTPase |
| PGC-1α | Proliferator-activated receptor-gamma coactivator 1-alpha |
| PHDs | HIF prolyl hydroxylases |
| RCT | Randomized controlled trial |
| RET | Reverse electron transport |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| rtPA | Recombined tissue plasminogen activator |
| SOD | Superoxide dismutase |
| TAC | Total Antioxidant Capacity |
| TNF alpha | Tumour necrosis factor alpha |
| VHL | von Hippel Lindau |
| vit C | Ascorbic acid |
| vit E | Alpha-tocopherol |
References
- Holland, H.D. Volcanic gases, black smokers, and the Great Oxidation Event. Geochim. Cosmochim. Acta 2002, 66, 3811–3826. [Google Scholar] [CrossRef]
- Thiemens, M.H. Oxygen origins. Nat. Chem. 2012, 4, 66. [Google Scholar] [CrossRef]
- Cardona, T.; Murray, J.W.; Rutherford, A.W. Origin and Evolution of Water Oxidation before the Last Common Ancestor of the Cyanobacteria. Mol. Biol. Evol. 2015, 32, 1310–1328. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, B.E.; de Vos, J.M.; Antonelli, A.; Bagheri, H.C. Evolution of multicellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event. Proc. Natl. Acad. Sci. USA 2013, 110, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Steiner, P. Brain fuel utilization in the developing brain. Ann. Nutr. Metab. 2019, 75, 8–18. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, U.J.; Lee, B.H.; Cha, M. Safeguarding the brain from oxidative damage. Free Radic. Biol. Med. 2025, 226, 143–157. [Google Scholar] [CrossRef]
- Mira, R.G.; Cerpa, W. Building a Bridge Between NMDAR-Mediated Excitotoxicity and Mitochondrial Dysfunction in Chronic and Acute Diseases. Cell. Mol. Neurobiol. 2021, 41, 1413–1430. [Google Scholar] [CrossRef]
- Dupuis, J.P.; Nicole, O.; Groc, L. NMDA receptor functions in health and disease: Old actor, new dimensions. Neuron 2023, 111, 2312–2328. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2021, 41, 770–784. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxid. Med. Cell. Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2024, 15, 15–34. [Google Scholar] [CrossRef]
- Sultana, R.; Butterfield, D.A. Protein Oxidation in Aging and Alzheimer’s Disease Brain. Antioxidants 2024, 13, 574. [Google Scholar] [CrossRef]
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron imbalance in neurodegeneration. Mol. Psychiatry 2024, 29, 1139–1152. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- McCarthy, R.C.; Kosman, D.J. Iron transport across the blood-brain barrier: Development, neurovascular regulation and cerebral amyloid angiopathy. Cell. Mol. Life Sci. 2015, 72, 709–727. [Google Scholar] [CrossRef]
- Dixon, S.J. Ferroptosis: Bug or feature? Immunol. Rev. 2017, 277, 150–157. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Halliwell, B. Mini-Review: Oxidative stress, redox stress or redox success? Biochem. Biophys. Res. Commun. 2018, 502, 183–186. [Google Scholar] [CrossRef]
- Costa, I.; Barbosa, D.J.; Benfeito, S.; Silva, V.; Chavarria, D.; Borges, F.; Remião, F.; Silva, R. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Ther. 2023, 244, 108373. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Zhang, X.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in muscle diseases and disorders: Mechanisms and therapeutic prospects. Bone Res. 2025, 13, 27. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Yuan, X.; Wang, S.; Ge, A.; Xu, H.; Zeng, J.; Ge, J. The mechanism of ferroptosis regulating oxidative stress in ischemic stroke and the regulation mechanism of natural pharmacological active components. Biomed. Pharmacother. 2022, 154, 113611. [Google Scholar] [CrossRef]
- Wei, C. The role of glutathione peroxidase 4 in neuronal ferroptosis and its therapeutic potential in ischemic and hemorrhagic stroke. Brain Res. Bull. 2024, 217, 111065. [Google Scholar] [CrossRef] [PubMed]
- DeGregorio-Rocasolano, N.; Martí-Sistac, O.; Ponce, J.; Castelló-Ruiz, M.; Millán, M.; Guirao, V.; García-Yébenes, I.; Salom, J.B.; Ramos-Cabrer, P.; Alborch, E.; et al. Iron-loaded transferrin (Tf) is detrimental whereas iron-free Tf confers protection against brain ischemia by modifying blood Tf saturation and subsequent neuronal damage. Redox. Biol. 2018, 15, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.; Robinson, L.; Yeatts, S.D.; Conwit, R.A.; Shehadah, A.; Lioutas, V.; Selim, M. Effect of Deferoxamine on Trajectory of Recovery After Intracerebral Hemorrhage: A Post Hoc Analysis of the i-DEF Trial. Stroke 2022, 53, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Tan, C.O.; LaRose, S.; Monk, A.D.; Fichorova, R.; Ryan, S.; Lipsitz, L.A. Deferoxamine, Cerebrovascular Hemodynamics, and Vascular Aging: Potential Role for Hypoxia-Inducible Transcription Factor-1-Regulated Pathways. Stroke 2015, 46, 2576–2583. [Google Scholar] [CrossRef][Green Version]
- Millán, M.; DeGregorio-Rocasolano, N.; Pérez de la Ossa, N.; Reverté, S.; Costa, J.; Giner, P.; Silva, Y.; Sobrino, T.; Rodríguez-Yáñez, M.; Nombela, F.; et al. Targeting Pro-Oxidant Iron with Deferoxamine as a Treatment for Ischemic Stroke: Safety and Optimal Dose Selection in a Randomized Clinical Trial. Antioxidants 2021, 10, 1270. [Google Scholar] [CrossRef]
- Van der Loo, L.E.; Aquarius, R.; Teernstra, O.; Klijn, K.; Menovsky, T.; van Dijk, J.M.C.; Bartels, R.; Boogaarts, H.D. Iron chelators for acute stroke. Cochrane Database Syst. Rev. 2020, 11, Cd009280. [Google Scholar] [CrossRef]
- Scherbakov, N.; Sandek, A.; Valentova, M.; Mayer, A.; von Haehling, S.; Jankowska, E.; Anker, S.D.; Doehner, W. Iron Deficiency and Reduced Muscle Strength in Patients with Acute and Chronic Ischemic Stroke. J. Clin. Med. 2022, 11, 595. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ji, M.; Wu, C.; Zhang, Y.; Ji, S. Targeting ferroptosis in neuroimmune and neurodegenerative disorders for the development of novel therapeutics. Biomed. Pharmacother. 2024, 176, 116777. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Donato, L.; Alibrandi, S.; Scimone, C.; D’Angelo, R.; Sidoti, A. Oxidative Stress and the Neurovascular Unit. Life 2021, 11, 767. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Z.; Ren, X.; Gao, X.; Chen, X.; Zhou, P. Complex Neuromuscular Changes Post-Stroke Revealed by Clustering Index Analysis of Surface Electromyogram. IEEE Trans. Neural. Syst. Rehabil. Eng. 2017, 25, 2105–2112. [Google Scholar] [CrossRef]
- Long, H.; Zhu, W.; Wei, L.; Zhao, J. Iron homeostasis imbalance and ferroptosis in brain diseases. MedComm 2023, 4, e298. [Google Scholar] [CrossRef]
- Powers, S.K.; Wiggs, M.P.; Duarte, J.A.; Zergeroglu, A.M.; Demirel, H.A. Mitochondrial signaling contributes to disuse muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E31–E39. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Marzetti, E.; Coelho-Junior, H.J.; Calvani, R.; Pesce, V.; Landi, F.; Leeuwenburgh, C.; Picca, A. Iron Metabolism and Muscle Aging: Where Ferritinophagy Meets Mitochondrial Quality Control. Cells 2025, 14, 672. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Bellanti, F.; Coda, A.R.D.; Trecca, M.I.; Lo Buglio, A.; Serviddio, G.; Vendemiale, G. Redox Imbalance in Inflammation: The Interplay of Oxidative and Reductive Stress. Antioxidants 2025, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Buga, A.M.; Ciobanu, O.; Bădescu, G.M.; Bogdan, C.; Weston, R.; Slevin, M.; Di Napoli, M.; Popa-Wagner, A. Up-regulation of serotonin receptor 2B mRNA and protein in the peri-infarcted area of aged rats and stroke patients. Oncotarget 2016, 7, 17415–17430. [Google Scholar] [CrossRef] [PubMed]
- Buga, A.-M.; Oancea, C.-N. Oxidative Stress-Induced Neurodegeneration and Antioxidative Strategies: Current Stage and Future Perspectives. Antioxidants 2023, 12, 1762. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Bakleh, M.Z.; Al Haj Zen, A. The Distinct Role of HIF-1α and HIF-2α in Hypoxia and Angiogenesis. Cells 2025, 14, 673. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Chavda, V.; Lu, B. Reverse Electron Transport at Mitochondrial Complex I in Ischemic Stroke, Aging, and Age-Related Diseases. Antioxidants 2023, 12, 895. [Google Scholar] [CrossRef]
- Liu, F.; Lu, J.; Manaenko, A.; Tang, J.; Hu, Q. Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis. 2018, 9, 924–937. [Google Scholar] [CrossRef]
- Shademan, B.; Avci, C.B.; Karamad, V.; Soureh, G.J.; Olia, J.B.H.; Esmaily, F.; Nourazarian, A.; Nikanfar, M. The Role of Mitochondrial Biogenesis in Ischemic Stroke. J. Integr. Neurosci. 2023, 22, 88. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Craven, L.; Russell, O.M.; Turnbull, D.M.; Vincent, A.E. Diagnosis and Treatment of Mitochondrial Myopathies. Neurotherapeutics 2018, 15, 943–953. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Ischemic stroke and mitochondria: Mechanisms and targets. Protoplasma 2020, 257, 335–343. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lu, M.H.; Yuan, D.J.; Xu, D.E.; Yao, P.P.; Ji, W.L.; Chen, H.; Liu, W.L.; Yan, C.X.; Xia, Y.Y.; et al. Mitochondrial Dysfunction in Neural Injury. Front. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef]
- Aderinto, N.; Olatunji, G.; Kokori, E.; Agbo, C.E.; Babalola, A.E.; Aboje, J.E.; Tolulope, E.M.; Oyelude, A.O.; Adejumo, F.A. Clinical Applications of Non-Invasive Brain Stimulation in Stroke Recovery: A Review of Current Evidence and Therapeutic Strategies. Curr. Treat. Options Neurol. 2025, 27, 8. [Google Scholar] [CrossRef]
- Li, K.P.; Wu, J.J.; Zhou, Z.L.; Xu, D.S.; Zheng, M.X.; Hua, X.Y.; Xu, J.G. Noninvasive Brain Stimulation for Neurorehabilitation in Post-Stroke Patients. Brain Sci. 2023, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, K.T. Functional electrical stimulation after spinal cord injury: Current use, therapeutic effects and future directions. Spinal Cord 2008, 46, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, H.; Wang, L.; Lenahan, C.; Lian, L.; Ou, Y.; He, Y. Mitochondrial Dynamics: A Potential Therapeutic Target for Ischemic Stroke. Front. Aging Neurosci. 2021, 13, 721428. [Google Scholar] [CrossRef] [PubMed]
- Mojaver, A.; Khazaei, M.; Ahmadpanah, M.; Zarei, M.; Soleimani Asl, S.; Habibi, P.; Shahidi, S. Dietary intake of coenzyme Q10 reduces oxidative stress in patients with acute ischemic stroke: A double-blind, randomized placebo-controlled study. Neurol. Res. 2025, 47, 232–241. [Google Scholar] [CrossRef]
- Ramezani, M.; Sahraei, Z.; Simani, L.; Heydari, K.; Shahidi, F. Coenzyme Q10 supplementation in acute ischemic stroke: Is it beneficial in short-term administration? Nutr. Neurosci. 2020, 23, 640–645. [Google Scholar] [CrossRef]
- Ullegaddi, R.; Powers, H.J.; Gariballa, S.E. Antioxidant supplementation enhances antioxidant capacity and mitigates oxidative damage following acute ischaemic stroke. Eur. J. Clin. Nutr. 2005, 59, 1367–1373. [Google Scholar] [CrossRef]
- Komakula, S.; Bhatia, R.; Sahib, A.; Upadhyay, A.; Joseph, S.L.; Garg, A.; Vishnu, V.Y.; Pandit, A.K.; Vibha, D.; Singh, M.B.; et al. Safety and efficacy of N-acetylcysteine (NAC) as an adjunct to standard treatment in patients with acute ischemic stroke: A randomized controlled pilot trial (NACTLYS). Sci. Rep. 2024, 14, 1103. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Mazdeh, M.; Rahmani, E.; Khazaie, M.; Ahmadimoghaddam, D. Melatonin supplementation may benefit patients with acute ischemic stroke not eligible for reperfusion therapies: Results of a pilot study. J. Clin. Neurosci. 2022, 106, 66–75. [Google Scholar] [CrossRef]
- Zhao, K.; Li, G.Z.; Nie, L.Y.; Ye, X.M.; Zhu, G.Y. Edaravone for Acute Ischemic Stroke: A Systematic Review and Meta-analysis. Clin. Ther. 2022, 44, e29–e38. [Google Scholar] [CrossRef]
- Mohammadi, V.; Khorvash, F.; Feizi, A.; Askari, G. Does Alpha-lipoic Acid Supplementation Modulate Cardiovascular Risk Factors in Patients with Stroke? A Randomized, Double-blind Clinical Trial. Int. J. Prev. Med. 2018, 9, 34. [Google Scholar] [CrossRef]
- Maeneja, R.; Silva, C.R.; Ferreira, I.S.; Abreu, A.M. Aerobic physical exercise versus dual-task cognitive walking in cognitive rehabilitation of people with stroke: A randomized clinical trial. Front. Psychol. 2023, 14, 1258262. [Google Scholar] [CrossRef]
- Nighoghossian, N.; Berthezène, Y.; Mechtouff, L.; Derex, L.; Cho, T.H.; Ritzenthaler, T.; Rheims, S.; Chauveau, F.; Béjot, Y.; Jacquin, A.; et al. Cyclosporine in acute ischemic stroke. Neurology 2015, 84, 2216–2223. [Google Scholar] [CrossRef]
- Mantle, D.; Dewsbury, M.; Hargreaves, I.P. The Ubiquinone-Ubiquinol Redox Cycle and Its Clinical Consequences: An Overview. Int. J. Mol. Sci. 2024, 25, 6765. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim. Biophys. Acta 2014, 1842, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef]
- Solmonson, A.; DeBerardinis, R.J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem. 2018, 293, 7522–7530. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.A.; Sayeed, I.; Siemen, D.; Wolf, G.; Horn, T.F. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. Faseb J. 2004, 18, 869–871. [Google Scholar] [CrossRef]
- Slone, J.; Huang, T. The special considerations of gene therapy for mitochondrial diseases. NPJ Genom. Med. 2020, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Kashbour, M.; Shata, A.; Wagdy, M.; Alnajjar, A.Z.; Aldemerdash, M.A.; Tarakhan, H.; Abouelmagd, M.E. Efficacy and safety of edaravone dexborneol in acute ischemic stroke: Systematic review and meta-analysis of randomized controlled trials. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 9569–9582. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhang, S.; Ge, H.; Liu, L.; Liu, L.; Feng, H.; Chen, L. The effect of cyclosporin a on ischemia-reperfusion damage in a mouse model of ischemic stroke. Neurol. Res. 2020, 42, 721–729. [Google Scholar] [CrossRef]
- Nusrat, L.; Livingston-Thomas, J.M.; Raguthevan, V.; Adams, K.; Vonderwalde, I.; Corbett, D.; Morshead, C.M. Cyclosporin A-Mediated Activation of Endogenous Neural Precursor Cells Promotes Cognitive Recovery in a Mouse Model of Stroke. Front. Aging Neurosci. 2018, 10, 93. [Google Scholar] [CrossRef]
- Skulachev, V.P. How to clean the dirtiest place in the cell: Cationic antioxidants as intramitochondrial ROS scavengers. IUBMB Life 2005, 57, 305–310. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Abdel Mageed, S.S.; Safar, M.M.; El-Yamany, M.F.; Oraby, M.A. MitoQ alleviates hippocampal damage after cerebral ischemia: The potential role of SIRT6 in regulating mitochondrial dysfunction and neuroinflammation. Life Sci. 2023, 328, 121895. [Google Scholar] [CrossRef]
- Russo, E.; Nguyen, H.; Lippert, T.; Tuazon, J.; Borlongan, C.V.; Napoli, E. Mitochondrial targeting as a novel therapy for stroke. Brain Circ. 2018, 4, 84–94. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Z.; Guo, J.; Qin, Y.; Yu, Q.; Shi, X.; Guo, F.; Zhang, H.; Sun, X.; Gao, C.; et al. Mitochondrial transplantation confers protection against the effects of ischemic stroke by repressing microglial pyroptosis and promoting neurogenesis. Neural Regen. Res. 2024, 19, 1325–1335. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Singh, K.K.; Aquilano, K.; Becker, L.B.; Berridge, M.V.; Boilard, E.; Caicedo, A.; Crewe, C.; Enríquez, J.A.; Gao, J.; et al. Recommendations for mitochondria transfer and transplantation nomenclature and characterization. Nat. Metab. 2025, 7, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lai, Y.; Tang, W.; Wang, G.; Shen, J.; Liu, H. Mitochondrial transplantation: A promising strategy for treating degenerative joint diseases. J. Transl. Med. 2024, 22, 941. [Google Scholar] [CrossRef]
- Kubat, G.B.; Picone, P.; Tuncay, E.; Aryan, L.; Girgenti, A.; Palumbo, L.; Turkel, I.; Akat, F.; Singh, K.K.; Nuzzo, D. Biotechnological approaches and therapeutic potential of mitochondria transfer and transplantation. Nat. Commun. 2025, 16, 5709. [Google Scholar] [CrossRef]
- Kidwell, C.U.; Casalini, J.R.; Pradeep, S.; Scherer, S.D.; Greiner, D.; Bayik, D.; Watson, D.C.; Olson, G.S.; Lathia, J.D.; Johnson, J.S.; et al. Transferred mitochondria accumulate reactive oxygen species, promoting proliferation. Elife 2023, 12, e85494. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Q.; Ji, J.; Lu, W.; Liu, Z.; Tian, H.; Guo, L.; Wang, Y. Mitochondrial transplantation ameliorates hippocampal damage following status epilepticus. Anim. Model. Exp. Med. 2023, 6, 41–50. [Google Scholar] [CrossRef]
- Neikirk, K.; Stephens, D.C.; Beasley, H.K.; Marshall, A.G.; Gaddy, J.A.; Damo, S.M.; Hinton, A.O., Jr. Considerations for Developing Mitochondrial Transplantation Techniques for Individualized Medicine. BioTechniques 2024, 76, 125–134. [Google Scholar] [CrossRef] [PubMed]

| Intervention Type | Compounds/Approach | Mechanism/Target | Study Design | Key outcome | Statistical Significance | Clinical Relevance |
|---|---|---|---|---|---|---|
| General antioxidant | CoQ10 [56] | Support ETC function | Double-blind RCT, n = 50, 600 mg/day × 1 week, acute stroke (<24 h), 1-week follow-up | ↓ serum MDA, ↓ IL6, ↑ BDNF and SOD level | Yes (p < 0.05) | Potential neuroprotective benefits |
| Vit. E + Vit. C [57] | ROS neutralization, lipid peroxidation prevention | Double-blind, RCT, n = 60, 300 mg/day x 4 weeks, acute stroke (<24 h), 4-week follow-up | improved NIHSS and MMSE score; no significant changes in MDA, SOD, GFAP | Yes (p = 0.05 for NIHSS; p = 0.03 for MMSE) | Functional benefit, unclear mechanistic impact | |
| NAC [58,59] | Glutathione precursor | Double-blind RCT, n = 24, 14 days in acute phase (<12 h) | ↓ plasma MDA and CRP; ↑ TAC | Yes (p < 0.003) for TAC and p < 0.002 for MDA | Possible adjuvant benefit | |
| RCT, n = 40, NAC + rtPA within 4.5 h after stroke, | Ongoing Phase II (NCT04918719) | - | No results | |||
| Melatonin [60]. | Free radical scavenger; antioxidant targeted at the mitochondria | Double-blind RCT, n = 65, 20 mg/day × 5 days, <24 h post-stroke, 90 d follow-up | improved NIHSS and MMSE score | Yes | Potential neuroprotective benefits | |
| Endaravone [61] | Free radical scavenger | 9 RCT + 4 cohort studies, n = 2102, Endaravone + rtPA, <24 h post-stroke; short-term follow-up | improved Barthel index, NIHSS | Yes (p < 0.001 for Barthel index; p = 0.003 for NIHSS | Neuroprotective benefits | |
| Mitochondria-targeted antioxidants | ||||||
| MitoQ | Scavenging mitochondrial ROS | 1 × 80 mg of Mito Q, 45 min follow-up | Ongoing Phase III stroke trials NCT06930638 | No available data | Clinical data pending | |
| ALA [62] | Scavenging mitochondrial ROS, enhancing endogenous antioxidant capacity | RCT, n = 67, 1200 mg/day × 3 weeks | ↓ TNF-α, ↓ IL6 No significant for antioxidant markers, | Mixed | Anti-inflammatory effect, unclear functional relevance | |
| ALA + rtPA within 6 h | Ongoing Phase III (NCT04041167) | No available data | Clinical data pending | |||
| Exercise-targeted mitochondrial biogenesis | Aerobic Exercise [63] | Activates PGC-1α → ↑ mitochondrial biogenesis, ↑ antioxidant capacity | Blinded RCT, 36 sessions/12 weeks, subacute/chronic stroke | Improved cognitive function | yes | Relevant cognitive benefit |
| mPTP-targeted antioxidants | ||||||
| Cyclosporin A [64] | Inhibits cyclophilin D, prevents mPTP opening | Phase II, ICT, n = 126, 2 mg/kg bolus within 15 min of thrombolysis | Did not significantly reduce infarct size (NCT01527240) | No | No evidence-based clinical benefit up to date |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaru, G.; Buga, A.-M.; Sandu, R.E.; Padureanu, V.; Popa, D.G.; Calina, D. Harnessing Mitochondrial Function for Post-Stroke Rehabilitation: Unlocking Antioxidant Power. Antioxidants 2025, 14, 1080. https://doi.org/10.3390/antiox14091080
Olaru G, Buga A-M, Sandu RE, Padureanu V, Popa DG, Calina D. Harnessing Mitochondrial Function for Post-Stroke Rehabilitation: Unlocking Antioxidant Power. Antioxidants. 2025; 14(9):1080. https://doi.org/10.3390/antiox14091080
Chicago/Turabian StyleOlaru, Gabriela, Ana-Maria Buga, Raluca Elena Sandu, Vlad Padureanu, Dragos George Popa, and Daniela Calina. 2025. "Harnessing Mitochondrial Function for Post-Stroke Rehabilitation: Unlocking Antioxidant Power" Antioxidants 14, no. 9: 1080. https://doi.org/10.3390/antiox14091080
APA StyleOlaru, G., Buga, A.-M., Sandu, R. E., Padureanu, V., Popa, D. G., & Calina, D. (2025). Harnessing Mitochondrial Function for Post-Stroke Rehabilitation: Unlocking Antioxidant Power. Antioxidants, 14(9), 1080. https://doi.org/10.3390/antiox14091080










