Molecular Hydrogen as an Antioxidant and Radioprotector: Mechanistic Insights from Monte Carlo Radiation-Chemical Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Low-LET Radiolysis of Pure Deaerated and Aerated Water: Time Scale of Events, Formation of Radical and Molecular Products, and Monte Carlo Track Chemistry Modeling
2.2. Effect of Dissolved Oxygen in Water Radiolysis
2.3. Modeling Water Radiolysis in the Presence of Cystamine: Reaction Scheme
3. Results
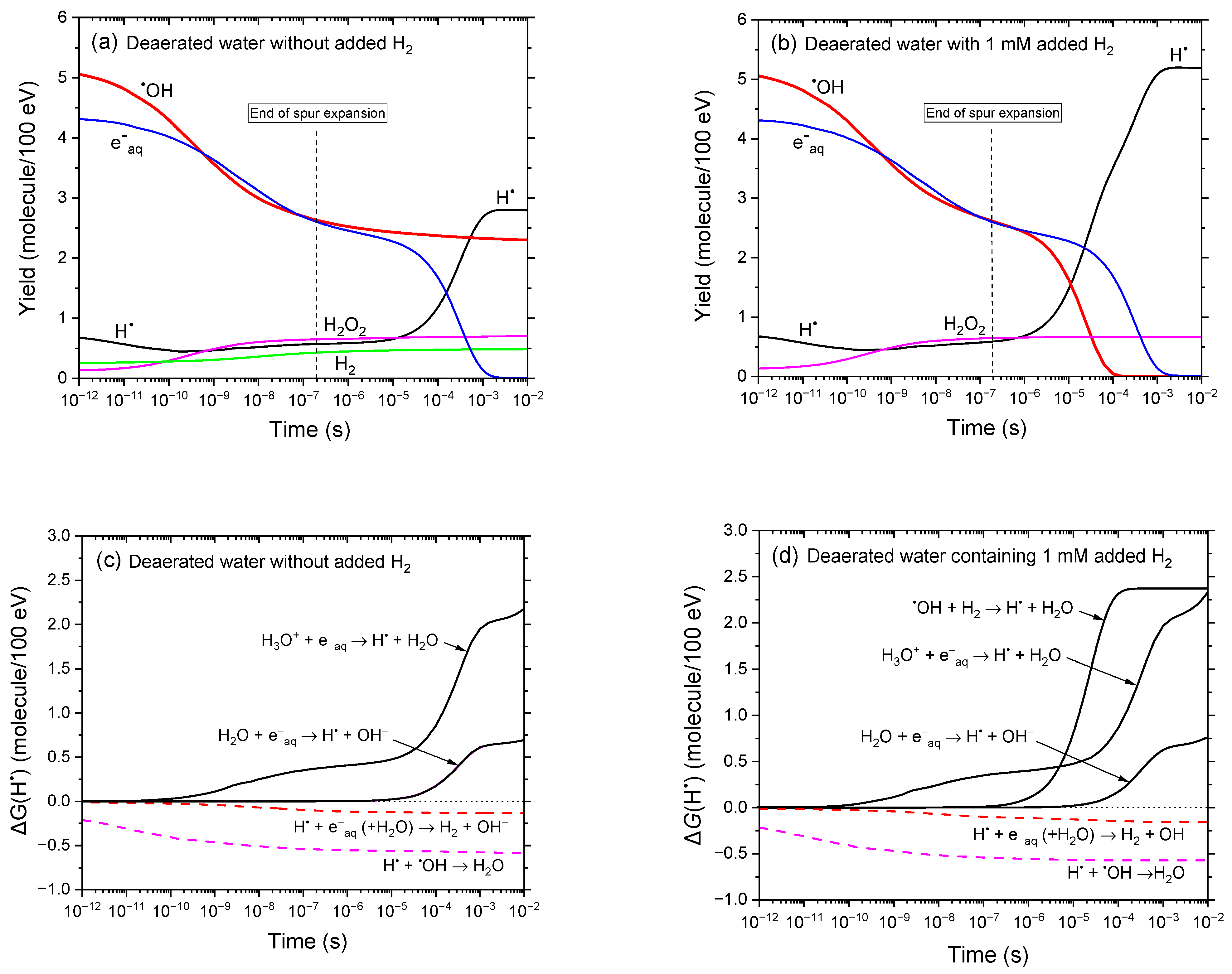
3.1. Yields of Reactive Species in the Radiolysis of Aqueous Solutions with and Without Added H2 Under Deaerated Conditions
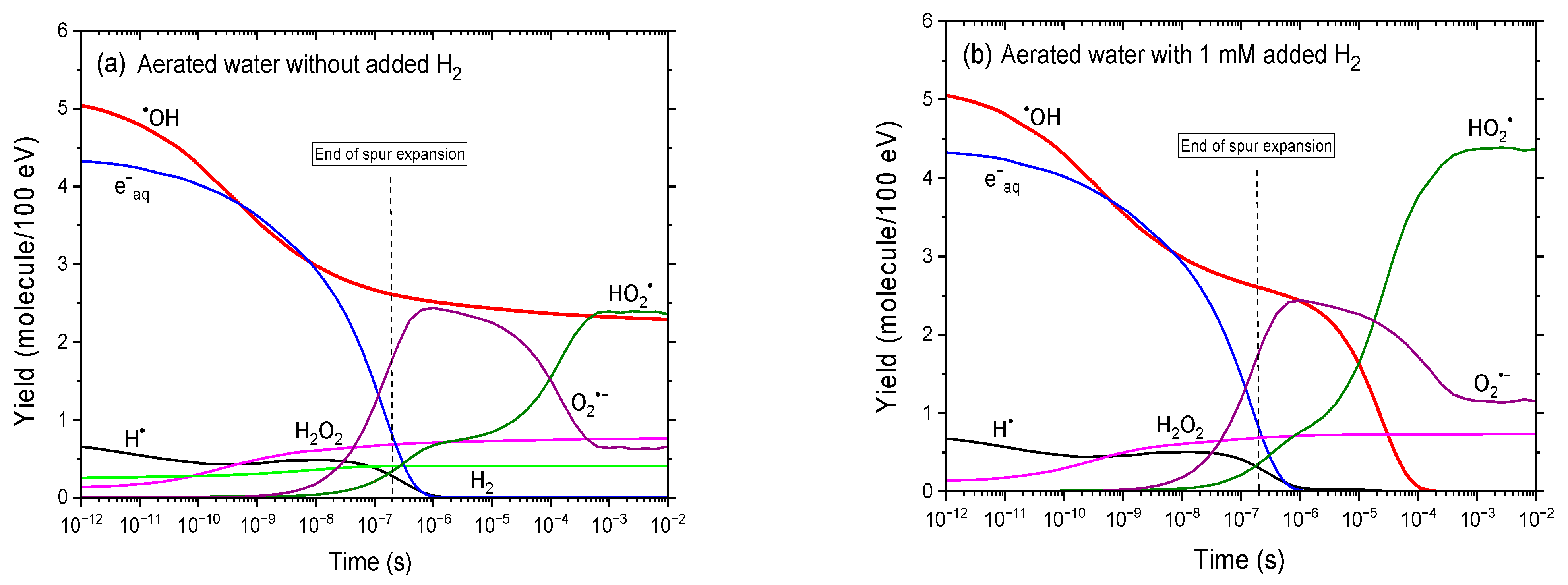
3.2. Yields of Reactive Species in the Radiolysis of Aqueous Solutions with and Without Added H2 Under Aerated Conditions
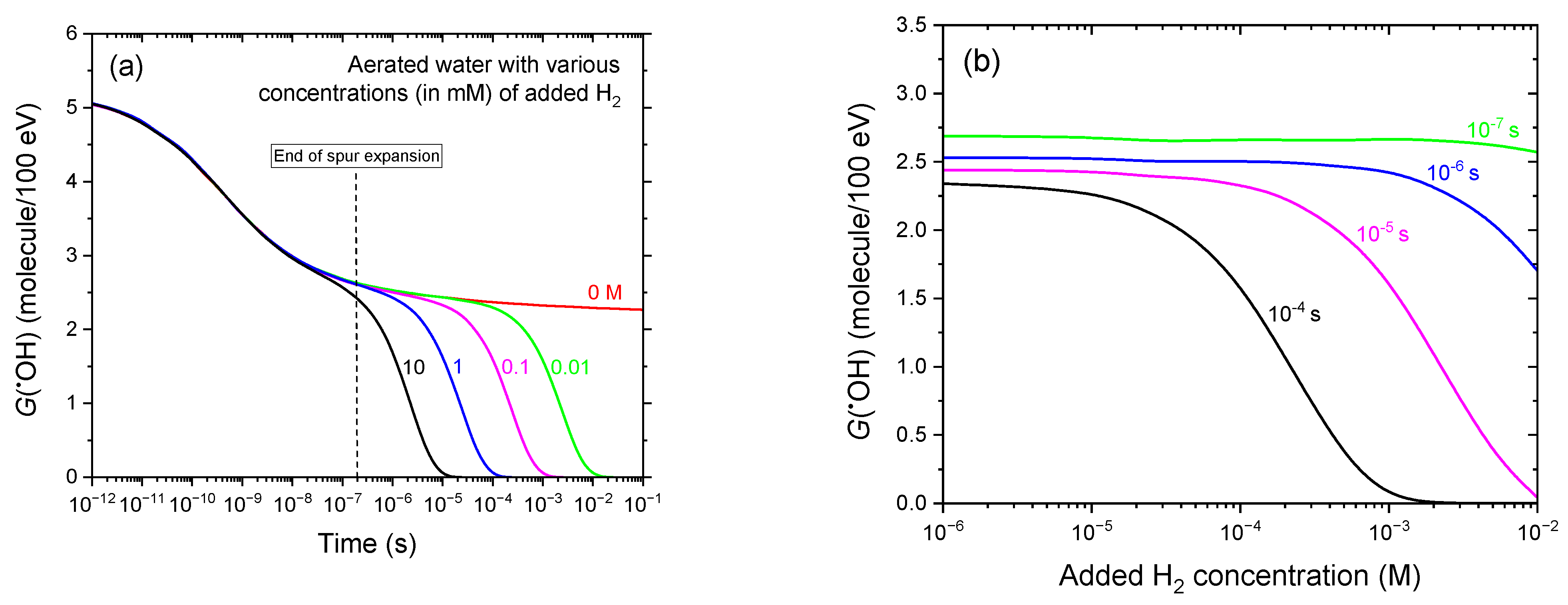
3.3. Time Profiles of G(•OH) in the Radiolysis of Aerated Water Containing Various H2 Concentrations
3.4. Comparison of the Antioxidant and Radioprotective Efficiency of H2 and Cystamine
4. Discussion, Conclusions, and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jay-Gerin, J.-P. Fundamentals of water radiolysis. Encyclopedia 2025, 5, 38. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Loh, Z.-H.; Doumy, G.; Arnold, C.; Kjellsson, L.; Southworth, S.H.; Al Haddad, A.; Kumagai, Y.; Tu, M.-F.; Ho, P.J.; March, A.M.; et al. Observation of the fastest chemical processes in the radiolysis of water. Science 2020, 367, 179–182. [Google Scholar] [CrossRef]
- Meesungnoen, J.; Jay-Gerin, J.-P. Radiation chemistry of liquid water with heavy ions: Monte Carlo simulation studies. In Charged Particle and Photon Interactions with Matter. Recent Advances, Applications, and Interfaces; Hatano, Y., Katsumura, Y., Mozumder, A., Eds.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2011; Chapter 14; pp. 355–400. [Google Scholar]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. In Progress in Nucleic Acid Research and Molecular Biology; Cohn, W.E., Moldave, K., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume 35, pp. 95–125. [Google Scholar] [CrossRef]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair. A Chemical Perspective; Springer: Berlin, Germany, 2006. [Google Scholar]
- Baatout, S. (Ed.) Radiobiology Textbook; Belgian Nuclear Research Centre (SCK CEN): Mol, Belgium; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015; pp. 199–283. [Google Scholar]
- Robert, G.; Wagner, J.R.; Cadet, J. Oxidatively generated tandem DNA modifications by pyrimidinyl and 2-deoxyribosyl peroxyl radicals. Free Radic. Biol. Med. 2023, 196, 22–36. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Gutiérrez Coronado, O.; Sandoval Salazar, C.; Muñoz Carrillo, J.L.; Gutiérrez Villalobos, O.A.; Miranda Beltrán, M.d.l.L.; Soriano Hernández, A.D.; Beltrán Campos, V.; Villalobos Gutiérrez, P.T. Functionalized nanomaterials in cancer treatment: A review. Int. J. Mol. Sci. 2025, 26, 2633. [Google Scholar] [CrossRef]
- Apak, R.; Capanoglu, E.; Shahidi, F. (Eds.) Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Rabeya, I.; Meesungnoen, J.; Jay-Gerin, J.-P. Oxygen depletion and the role of cellular antioxidants in FLASH radiotherapy: Mechanistic insights from Monte Carlo radiation-chemical modeling. Antioxidants 2025, 14, 406. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O2− radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Ward, J.F. Chemical aspects of DNA radioprotection. In Radioprotectors and Anticarcinogens; Nygaard, O.F., Simić, M.G., Eds.; Academic Press: New York, NY, USA, 1983; pp. 73–85. [Google Scholar]
- Bump, E.A.; Malaker, K. (Eds.) Radioprotectors: Chemical, Biological, and Clinical Perspectives, 1st ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncol. 2010, 15, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubert, J.; Agrawal, D.K. Radioprotective agents to prevent cellular damage due to ionizing radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Li, Z.; Wu, H.; Zou, B.; Xu, Y. Exploring natural products as radioprotective agents for cancer therapy: Mechanisms, challenges, and opportunities. Cancers 2023, 15, 3585. [Google Scholar] [CrossRef] [PubMed]
- Penabeï, S.; Meesungnoen, J.; Jay-Gerin, J.-P. Comparative analysis of cystamine and cysteamine as radioprotectors and antioxidants: Insights from Monte Carlo chemical modeling under high linear energy transfer radiation and high dose rates. Int. J. Mol. Sci. 2024, 25, 10490. [Google Scholar] [CrossRef]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Yanagihara, T.; Arai, K.; Miyamae, K.; Sato, B.; Shudo, T.; Yamada, M.; Aoyama, M. Electrolyzed hydrogen-saturated water for drinking use elicits an antioxidative effect: A feeding test with rats. Biosci. Biotechnol. Biochem. 2005, 69, 1985–1987. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Fukuda, K.-I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007, 361, 670–674. [Google Scholar] [CrossRef]
- Qian, L.; Cao, F.; Cui, J.; Huang, Y.; Zhou, X.; Liu, S.; Cai, J. Radioprotective effect of hydrogen in cultured cells and mice. Free Radic. Res. 2010, 44, 275–282. [Google Scholar] [CrossRef]
- Qian, L.; Shen, J.; Chuai, Y.; Cai, J. Hydrogen as a new class of radioprotective agent. Int. J. Biol. Sci. 2013, 9, 887–894. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- Hirano, S.-I.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Molecular hydrogen as a potential clinically applicable radioprotective agent. Int. J. Mol. Sci. 2021, 22, 4566. [Google Scholar] [CrossRef] [PubMed]
- Slezák, J.; Kura, B. Molecular Hydrogen in Health and Disease; Springer Nature: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Jin, J.; Yue, L.; Du, M.; Geng, F.; Gao, X.; Zhou, Y.; Lu, Q.; Pan, X. Molecular hydrogen therapy: Mechanisms, delivery methods, preventive, and therapeutic application. MedComm 2025, 6, e70194. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Elliot, A.J.; Bartels, D.M. The Reaction Set, Rate Constants and g-Values for the Simulation of the Radiolysis of Light Water over the Range 20 to 350 °C Based on Information Available in 2008; Report No. 153-127160-450-001; Atomic Energy of Canada Limited: Mississauga, ON, Canada, 2009. [Google Scholar]
- Jay-Gerin, J.-P.; Ferradini, C. Are there protective enzymatic pathways to regulate high local nitrite oxide (·NO) concentrations in cells under stress conditions? Biochimie 2000, 82, 161–166. [Google Scholar] [CrossRef]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. Lung Cell. Mol. Physiol. 1995, 268, L699–L722. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am. J. Physiol. Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Huie, R.E.; Padmaja, S. The reaction of NO with superoxide. Free Radic. Res. Commun. 1993, 18, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Czapski, G. The reaction of ·NO with O2·− and HO2·: A pulse radiolysis study. Free Radic. Biol. Med. 1995, 19, 505–510. [Google Scholar] [CrossRef]
- Meesat, R.; Sanguanmith, S.; Meesungnoen, J.; Lepage, M.; Khalil, A.; Jay-Gerin, J.-P. Utilization of the ferrous sulfate (Fricke) dosimeter for evaluating the radioprotective potential of cystamine: Experiment and Monte Carlo simulation. Radiat. Res. 2012, 177, 813–826. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a novel therapeutic molecule, regulates oxidative stress, inflammation, and apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Artamonov, M.Y.; Martusevich, A.K.; Pyatakovich, F.A.; Minenko, I.A.; Dlin, S.V.; LeBaron, T.W. Molecular hydrogen: From molecular effects to stem cells management and tissue regeneration. Antioxidants 2023, 12, 636. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.-N.; Yin, X.-X.; Song, W.-G. Molecular hydrogen; A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.-T.; Qin, S.-C. Neuroprotective effects of molecular hydrogen: A critical review. Neurosci. Bull. 2021, 37, 389–404. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Molecular hydrogen neuroprotection in post-ischemic neurodegeneration in the form of Alzheimer’s disease proteinopathy: Underlying mechanisms and potential for clinical implementation—Fantasy or reality? Int. J. Mol. Sci. 2022, 23, 6591. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, E.; Sanguanmith, S.; Meesungnoen, J.; Jay-Gerin, J.-P. Evaluation of the radioprotective ability of cystamine for 150 keV–500 MeV proton irradiation: A Monte Carlo track chemistry simulation study. Can. J. Chem. 2019, 97, 100–111. [Google Scholar] [CrossRef]
- Yildiz, F.; LeBaron, T.W.; Alwazeer, D. A comprehensive review of molecular hydrogen as a novel nutrition therapy in relieving oxidative stress and diseases: Mechanisms and perspectives. Biochem. Biophys. Rep. 2025, 41, 101933. [Google Scholar] [CrossRef]
- Johnsen, H.M.; Hiorth, M.; Klaveness, J. Molecular hydrogen therapy—A review on clinical studies and outcomes. Molecules 2023, 28, 7785. [Google Scholar] [CrossRef]
- Magee, J.L. Radiation chemistry. Annu. Rev. Nucl. Sci. 1953, 3, 171–192. [Google Scholar] [CrossRef]
- Freeman, G.R. Basics of radiation chemistry. In The Study of Fast Processes and Transient Species by Electron Pulse Radiolysis; Baxendale, J.H., Busi, F., Eds.; Reidel Publishing: Dordrecht, The Netherlands, 1982; pp. 19–34. [Google Scholar]
- Sanguanmith, S.; Meesungnoen, J.; Muroya, Y.; Lin, M.; Katsumura, Y.; Jay-Gerin, J.-P. On the spur lifetime and its temperature dependence in the low linear energy transfer radiolysis of water. Phys. Chem. Chem. Phys. 2012, 14, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Pastina, B.; LaVerne, J.A.; Pimblott, S.M. Dependence of molecular hydrogen formation in water on scavengers of the precursor to the hydrated electron. J. Phys. Chem. A 1999, 103, 5841–5846. [Google Scholar] [CrossRef]
- Meesungnoen, J.; Sanguanmith, S.; Jay-Gerin, J.-P. Yields of H2 and hydrated electrons in low-LET radiolysis of water determined by Monte Carlo track chemistry simulations using phenol/N2O aqueous solutions up to 350 °C. RSC Adv. 2015, 5, 76813–76824. [Google Scholar] [CrossRef]
- Sterniczuk, M.; Bartels, D.M. Source of molecular hydrogen in high-temperature water radiolysis. J. Phys. Chem. A 2016, 120, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Cobut, V.; Frongillo, Y.; Patau, J.P.; Goulet, T.; Fraser, M.-J.; Jay-Gerin, J.-P. Monte Carlo simulation of fast electron and proton tracks in liquid water. I. Physical and physicochemical aspects. Radiat. Phys. Chem. 1998, 51, 229–243. [Google Scholar] [CrossRef]
- Frongillo, Y.; Goulet, T.; Fraser, M.-J.; Cobut, V.; Patau, J.P.; Jay-Gerin, J.-P. Monte Carlo simulation of fast electron and proton tracks in liquid water. II. Nonhomogeneous chemistry. Radiat. Phys. Chem. 1998, 51, 245–254. [Google Scholar] [CrossRef]
- Pimblott, S.M.; Pilling, M.J.; Green, N.J.B. Stochastic models of spur kinetics in water. Radiat. Phys. Chem. 1991, 37, 377–388. [Google Scholar] [CrossRef]
- Pimblott, S.M.; Green, N.J.B. Recent advances in the kinetics of radiolytic processes. Res. Chem. Kinet. 1995, 3, 117–174. [Google Scholar] [CrossRef]
- Tachiya, M. Theory of diffusion-controlled reactions: Formulation of the bulk reaction rate in terms of the pair probability. Radiat. Phys. Chem. 1983, 21, 167–175. [Google Scholar] [CrossRef]
- Goulet, T.; Fraser, M.-J.; Frongillo, Y.; Jay-Gerin, J.-P. On the validity of the independent reaction times approximation for the description of the nonhomogeneous kinetics of liquid water radiolysis. Radiat. Phys. Chem. 1998, 51, 85–91. [Google Scholar] [CrossRef]
- Plante, I. Développement de Codes de Simulation Monte Carlo de la Radiolyse de l’Eau par des Électrons, Ions Lourds, Photons et Neutrons. Applications à Divers Sujets d’Intérêt Expérimental. Ph.D. Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 2009. [Google Scholar]
- Kuppermann, A. Diffusion kinetics in radiation chemistry. In Actions Chimiques et Biologiques des Radiations; Haïssinsky, M., Ed.; Masson: Paris, France, 1961; Volume 5, pp. 85–166. [Google Scholar]
- Schmidt, K.H.; Bartels, D.M. Lack of ionic strength effect in the recombination of hydrated electrons: (e−)aq + (e−)aq → 2(OH−) + H2. Chem. Phys. 1995, 190, 145–152. [Google Scholar] [CrossRef]
- Weston, R.E., Jr.; Schwarz, H.A. Chemical Kinetics; Prentice-Hall: Englewood Cliffs, NJ, USA, 1972. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 6–201. [Google Scholar]
- Watt, D.E. Quantities for Dosimetry of Ionizing Radiations in Liquid Water; Taylor & Francis: London, UK, 1996. [Google Scholar]
- International Commission on Radiation Units and Measurements. Stopping Powers and Ranges for Protons and Alpha Particles; ICRU Report No. 49; International Commission on Radiation Units and Measurements: Bethesda, DC, USA, 1993. [Google Scholar]
- McCracken, D.R.; Tsang, K.T.; Laughton, P.J. Aspects of the Physics and Chemistry of Water Radiolysis by Fast Neutrons and Fast Electrons in Nuclear Reactors; Report AECL No. 11895; Atomic Energy of Canada Limited: Chalk River, ON, Canada, 1998. [Google Scholar]
- Bacq, Z.-M.; Alexander, P. Principes de Radiobiologie; Masson: Paris, France, 1955; pp. 361–407. [Google Scholar]
- Bacq, Z.-M.; Beaumariage, M.L. Action radioprotectrice de la cystéamine et de la cystamine chez la souris en fonction du temps séparant l’injection du protecteur du début de l’irradiation par rayons X. Arch. Int. Pharmacodyn. Ther. 1965, 153, 457–459. [Google Scholar]
- Jayson, G.G.; Owen, T.C.; Wilbraham, A.C. The radiation chemistry of cystamine sulphate. J. Chem. Soc. B 1967, 944–949. [Google Scholar] [CrossRef]
- Bidzilya, V.A.; Golovkova, L.P.; Beregovskaya, N.N.; Basyuk, V.V.; Korol’, É.N.; Chuiko, A.A.; Znamenskii, V.V.; Barkaya, V.S. Radioprotective effect of immobilized cystamine. Pharm. Chem. J. 1991, 25, 782–786. [Google Scholar] [CrossRef]
- Jayson, G.G.; Wilbraham, A.C. The utilization of the Fricke dosimeter for evaluating the biological radiation-protective potential of water-soluble organic compounds. Chem. Commun. 1968, 641–642. [Google Scholar] [CrossRef]
- Lalitha, B.; Mittal, J.P. Electron transfer reaction in the radiation chemistry of some biologically important disulphide compounds. Radiat. Eff. 1971, 7, 159–162. [Google Scholar] [CrossRef]
- Rahman, M.H.; Jeong, E.-S.; You, H.S.; Kim, C.-S.; Lee, K.-J. Redox-mechanisms of molecular hydrogen promote health longevity. Antioxidants 2023, 12, 988. [Google Scholar] [CrossRef]


| Reactions | k (M−1 s−1) | Reaction No. |
|---|---|---|
| RSSR + e−aq → (RSSR)•− | 4.1 × 1010 | (11) |
| RSSR + H• → RS• + RSH | 8 × 109 | (12) |
| RSSR + •OH → (RSSR)•+ + OH− | 1.7 × 1010 | (13) |
| (RSSR)•− + H+ → RS• + RSH | 4.2 × 109 | (14) |
| 2(RSSR)•+ → (RSSR)2+ + RSSR | 2.5 × 109 | (15) |
| RS• + RSSR → RSSSR + R• | 106 | (16) |
| RSH + e−aq → R• + HS− | 3 × 1010 | (17) |
| RSH + H• → RS• + H2 | 1.8 × 109 | (18) |
| RSH + •OH → RS• + H2O | 1.7 × 1010 | (19) |
| RS• + RSH → (RSSR)•− + H+ | 3.5 × 108 | (20) |
| R• + RSH → RH + RS• | 1.1 × 108 | (21) |
| RS• + RS• → RSSR | 1.5 × 109 | (22) |
| RS• + O2 → RSOO• | 2 × 109 | (23) |
| RSOO• + RSH → RSO• + RSOH | 2 × 106 | (24) |
| RH + •OH → R• + H2O | 5 × 108 | (2) |
| R• + O2 → ROO• | 2 × 109 | (3) |
| (RSSR)•− + O2 → RSSR + O2•− | 5.1 × 108 | (25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ria, S.A.; Meesungnoen, J.; Jay-Gerin, J.-P. Molecular Hydrogen as an Antioxidant and Radioprotector: Mechanistic Insights from Monte Carlo Radiation-Chemical Simulations. Antioxidants 2025, 14, 1054. https://doi.org/10.3390/antiox14091054
Ria SA, Meesungnoen J, Jay-Gerin J-P. Molecular Hydrogen as an Antioxidant and Radioprotector: Mechanistic Insights from Monte Carlo Radiation-Chemical Simulations. Antioxidants. 2025; 14(9):1054. https://doi.org/10.3390/antiox14091054
Chicago/Turabian StyleRia, Sumaiya Akhter, Jintana Meesungnoen, and Jean-Paul Jay-Gerin. 2025. "Molecular Hydrogen as an Antioxidant and Radioprotector: Mechanistic Insights from Monte Carlo Radiation-Chemical Simulations" Antioxidants 14, no. 9: 1054. https://doi.org/10.3390/antiox14091054
APA StyleRia, S. A., Meesungnoen, J., & Jay-Gerin, J.-P. (2025). Molecular Hydrogen as an Antioxidant and Radioprotector: Mechanistic Insights from Monte Carlo Radiation-Chemical Simulations. Antioxidants, 14(9), 1054. https://doi.org/10.3390/antiox14091054







