Impact of PM2.5 Emitted by Wood Smoke on the Expression of Glucose Transporter 1 (GLUT1) and Sodium-Dependent Vitamin C Transporter 2 (SVCT2) in the Rat Placenta: A Pregestational and Gestational Exposure Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure Site
2.2. Exposure Conditions
2.3. Air Analysis
2.4. Study Design
2.5. Groups
2.6. Outcome Measures
2.7. Immunofluorescence and Image Acquisition
2.8. Cell Analysis and Classification
2.9. Spectral Confocal Microscopy
2.10. Statistical Analysis
3. Results
3.1. Air Pollution Exposure
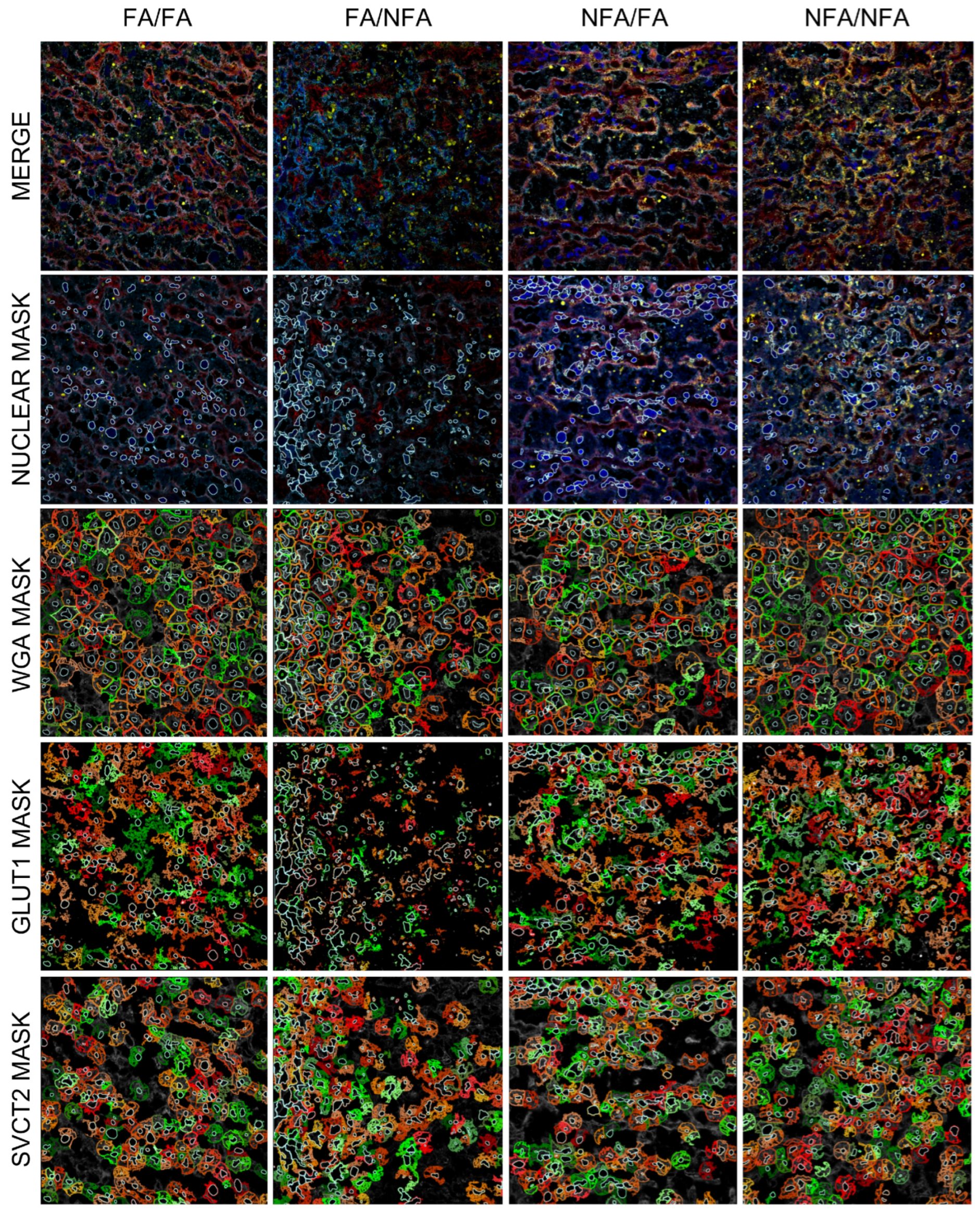
3.2. Nuclear Area and WGA Expression
3.3. Expression of GLUT1 and SVCT2 Transporters in the Placental Labyrinth Zone
3.4. GLUT1 Transporter Expression by Cell Type
3.5. SVCT2 Transporter Expression by Cell Type
3.6. Distribution and Colocalization of GLUT1 and SVCT2 Transporters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM2.5 | Particulate Matter 2.5 |
| GLUT1 | Glucose Transporter 1 |
| SVCT2 | Sodium-Dependent Vitamin C Transporter 2 |
| FA | Filtered Air |
| NFA | Non-Filtered Air |
| ROS | Reactive Oxygen Species |
| OS | Oxidative Stress |
| RNS | Reactive Nitrogen Species |
| ATP | Adenosine Triphosphate |
| AA | Ascorbic Acid |
| DHA | Dehydroascorbic Acid |
| WHO | World Health Organization |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| CT | Cytotrophoblast |
| ST | Syncytiotrophoblast |
| ECs | Endothelial Cells |
| WGA | Wheat Germ Agglutinin |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| FOV | Field of View |
| MERV8 | Minimum Efficiency Reporting Value 8 |
| HEPA | High-Efficiency Particulate Air |
| CO | Carbon Monoxide |
| NO2 | Nitrogen Dioxide |
| dpf | Days Post-Fertilization |
| G1 | First Generation |
| G2 | Second Generation |
| G3 | Third Generation |
| IUGR | Intrauterine Growth Restriction |
| MDA | Malondialdehyde |
References
- Villarroel, F.; Ponce, N.; Gómez, F.A.; Muñoz, C.; Ramírez, E.; Nualart, F.; Salinas, P. Exposure to fine particulate matter 2.5 from wood combustion smoke causes vascular changes in placenta and reduce fetal size. Reprod. Toxicol. 2024, 127, 108610. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef]
- Duan, R.R.; Hao, K.; Yang, T. Air pollution and chronic obstructive pulmonary disease. Chronic Dis. Transl. Med. 2020, 6, 260–269. [Google Scholar] [CrossRef]
- Sinzato, Y.K.; Bevilacqua, E.M.; Volpato, G.T.; Hernandez-Pando, R.E.; Rudge, M.V.C.; Damasceno, D.C. Maternal oxidative stress, placental morphometry, and fetal growth in diabetic rats exposed to cigarette smoke. Reprod. Sci. 2019, 26, 1287–1293. [Google Scholar] [CrossRef]
- Biondi, C.; Pavan, B.; Lunghi, L.; Fiorini, S.; Vesce, F. The role and modulation of the oxidative balance in pregnancy. Curr. Pharm. Des. 2005, 11, 2075–2089. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress: A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef]
- Katirci, E.; Kendirci-Katirci, R.; Korgun, E.T. Effects of stevioside on the expressions of GLUT1, GLUT3, and GLUT4 proteins in diabetic rat placenta. Planta Med. 2023, 89, 735–745. [Google Scholar]
- Mamun, A.A.; Hayashi, H.; Yamamura, A.; Nayeem, M.J.; Sato, M. Hypoxia induces the translocation of glucose transporter 1 to the plasma membrane in vascular endothelial cells. J. Physiol. Sci. 2020, 70, 44. [Google Scholar] [CrossRef]
- Stanirowski, P.J.; Szukiewicz, D.; Majewska, A.; Watroba, M.; Pyzlak, M.; Bomba-Opoń, D.; Wielgoś, M. Differential expression of glucose transporter proteins GLUT1, GLUT3, GLUT8, and GLUT12 in the placenta of macrosomic, small-for-gestational-age, and growth-restricted fetuses. J. Clin. Med. 2021, 10, 5833. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative stress in pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Schjoldager, J.G.; Paidi, M.D.; Lindblad, M.M.; Birck, M.M.; Kjærgaard, A.B.; Dantzer, V.; Lykkesfeldt, J.; Tveden-Nyborg, P. Maternal vitamin C deficiency during pregnancy results in transient fetal and placental growth retardation in guinea pigs. Eur. J. Nutr. 2015, 54, 667–676. [Google Scholar] [CrossRef]
- Zein, S.; Rachidi, S.; Hininger-Favier, I. Is oxidative stress induced by iron status associated with gestational diabetes mellitus? J. Trace Elem. Med. Biol. 2014, 28, 65–69. [Google Scholar] [CrossRef]
- Chappell, L.C.; Seed, P.T.; Briley, A.L.; Kelly, F.J.; Lee, R.; Hunt, B.J.; Parmar, K.; Bewley, S.J.; Shennan, A.H.; Steer, P.J.; et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: A randomised trial. Lancet 1999, 354, 810–816. [Google Scholar] [CrossRef]
- Davidge, S.T. Oxidative stress and altered endothelial cell function in preeclampsia. Semin. Reprod. Endocrinol. 1998, 16, 65–73. [Google Scholar] [CrossRef]
- Chatterjee, P.; Holody, C.D.; Kirschenman, R.; Graton, M.E.; Spaans, F.; Phillips, T.J.; Case, C.P.; Bourque, S.L.; Lemieux, H.; Davidge, S.T. Sex-specific effects of prenatal hypoxia and a placental antioxidant treatment on cardiac mitochondrial function in the young adult offspring. Int. J. Mol. Sci. 2023, 24, 13624. [Google Scholar] [CrossRef]
- Fischer, A.P.; Miles, S.L. Ascorbic acid, but not dehydroascorbic acid, increases intracellular vitamin C content to decrease hypoxia-inducible factor-1 alpha activity and reduce malignant potential in human melanoma. Biomed. Pharmacother. 2017, 86, 502–513. [Google Scholar] [CrossRef]
- Richter, H.G.; Camm, E.J.; Modi, B.N.; Naeem, F.; Cross, C.M.; Cindrova-Davies, T.; Spasic-Boskovic, O.; Dunster, C.; Mudway, I.S.; Kelly, F.J.; et al. Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J. Physiol. 2012, 590, 1377–1387. [Google Scholar] [CrossRef]
- Ornoy, A.; Avgil Tsadok, M.; Yaffe, P.; Zangen, S.W. The Cohen diabetic rat as a model for fetal growth restriction: Vitamins C and E reduce fetal oxidative stress but do not restore normal growth. Reprod. Toxicol. 2009, 28, 521–529. [Google Scholar] [CrossRef]
- Li, Y.; Fang, J.; Zhou, K.; Wang, C.; Mu, D.; Hua, Y. Evaluation of oxidative stress in placenta of fetal cardiac dysfunction rat model and antioxidant defenses of maternal vitamin C supplementation with the impacts on P-glycoprotein. J. Obstet. Gynaecol. Res. 2014, 40, 1632–1642. [Google Scholar] [CrossRef]
- Harrison, F.E.; Dawes, S.M.; Meredith, M.E.; Babaev, V.R.; Li, L.; May, J.M. Low vitamin C and increased oxidative stress and cell death in mice that lack the sodium-dependent vitamin C transporter SVCT2. Free Radic. Biol. Med. 2010, 49, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, N.; Yoshiba, K.; Yoshiba, N.; Edanami, N.; Ohshima, H.; Takenaka, S.; Noiri, Y. SVCT2-GLUT1-mediated ascorbic acid transport pathway in rat dental pulp and its effects during wound healing. Sci. Rep. 2023, 13, 1251. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Aigaki, T.; Maruyama, N.; Ishigami, A. Ascorbic acid depletion enhances expression of the sodium-dependent vitamin C transporters, SVCT1 and SVCT2, and uptake of ascorbic acid in livers of SMP30/GNL knockout mice. Arch. Biochem. Biophys. 2010, 496, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Johnson, D.; Jarvis, S.M. Vitamin C transport systems of mammalian cells. Mol. Membr. Biol. 2001, 18, 87–95. [Google Scholar] [CrossRef]
- Sultana, Z.; Maiti, K.; Aitken, J.; Morris, J.; Dedman, L.; Smith, R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am. J. Reprod. Immunol. 2017, 77, e12653. [Google Scholar] [CrossRef]
- Blanco, E.; Rubilar, F.; Quinteros, M.E.; Cayupi, K.; Ayala, S.; Lu, S.; Jimenez, R.B.; Cárdenas, J.P.; Blazquez, C.A.; Delgado-Saborit, J.M.; et al. Spatial distribution of particulate matter on winter nights in Temuco, Chile: Studying the impact of residential wood-burning using mobile monitoring. Atmos. Environ. 2022, 286, 119255. [Google Scholar] [CrossRef]
- Villalobos, A.M.; Barraza, F.; Jorquera, H.; Schauer, J. Wood burning pollution in southern Chile: PM2.5 source apportionment using CMB and molecular markers. Environ. Pollut. 2017, 225, 514–523. [Google Scholar] [CrossRef]
- Álamos, N.; Huneeus, N.; Opazo, M.; Osses, M.; Puja, S.; Pantoja, N.; Denier van der Gon, H.; Schueftan, A.; Reyes, R.; Calvo, R. High-resolution inventory of atmospheric emissions from transport, industrial, energy, mining, and residential activities in Chile. Earth Syst. Sci. Data 2022, 14, 361–379. [Google Scholar] [CrossRef]
- Salinas, P.; Ponce, N.; del Sol, M.; Vásquez, B. Impact of PM2.5 exposure from wood combustion on reproductive health: Implications for fertility, ovarian function, and fetal development. Toxics 2025, 13, 238. [Google Scholar] [CrossRef]
- IQAir. First in Air Quality. 2022. Available online: https://www.iqair.com/world-air-quality-report (accessed on 2 June 2023).
- Boso, A.; Álvarez, B.; Oltra, C.; Hofflinger, Á.; Vallejos-Romero, A.; Garrido, J. Examining patterns of air quality perception: A cluster analysis for Southern Chilean cities. SAGE Open 2019, 9, 2158244019863563. [Google Scholar] [CrossRef]
- Díaz-Robles, L.A.; Fu, J.S.; Vergara-Fernández, A.; Etcharren, P.; Schiappacasse, L.N.; Reed, G.D.; Silva, M.P. Health risks caused by short-term exposure to ultrafine particles generated by residential wood combustion: A case study of Temuco, Chile. Environ. Int. 2014, 66, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, H.; Barraza, F.; Heyer, J.; Valdivia, G.; Schiappacasse, L.N.; Montoya, L.D. Indoor PM2.5 in an urban zone with heavy wood smoke pollution: The case of Temuco, Chile. Environ. Pollut. 2018, 236, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, P.A.; Torreblanca, M.A.; Díaz-Robles, L.A.; Schiappacasse, L.N.; Silva, M.P.; Astete, T.D. Particulate air pollution and health effects for cardiovascular and respiratory causes in Temuco, Chile: A wood-smoke-polluted urban area. J. Air Waste Manag. Assoc. 2009, 59, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.M.; Damaceno-Rodrigues, N.R.; Guimarães Silva, R.M.; Scoriza, J.N.; Saldiva, P.H.; Caldini, E.G.; Dolhnikoff, M. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ. Res. 2009, 109, 536–543. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, C.; Cai, B.; Qiu, W.; Zhai, J.; Zeng, Y.; Zhang, A.; Shi, S.; Zhang, Y.; Yang, X.; et al. Impact of antioxidants on PM2.5 oxidative potential, radical level, and cytotoxicity. Sci. Total Environ. 2024, 912, 169555. [Google Scholar] [CrossRef]
- Wu, L.; Xu, W.; Li, H.; Dong, B.; Geng, H.; Jin, J.; Xie, S. Vitamin C attenuates oxidative stress, inflammation, and apoptosis induced by acute hypoxia through the Nrf2/Keap1 signaling pathway in gibel carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef]
- Tannetta, D.S.; Sargent, I.L.; Linton, E.A.; Redman, C.W.G. Vitamins C and E inhibit apoptosis of cultured human term placenta trophoblast. Placenta 2008, 29, 680–690. [Google Scholar] [CrossRef]
- Jansson, T.; Wennergren, M.; Illsley, N.P. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J. Clin. Endocrinol. Metab. 1993, 77, 1554–1562. [Google Scholar]
- Lüscher, B.P.; Marini, C.; Joerger-Messerli, M.S.; Huang, X.; Hediger, M.A.; Albrecht, C.; Baumann, M.U.; Surbek, D.V. Placental glucose transporter (GLUT)-1 is down-regulated in preeclampsia. Placenta 2017, 55, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.C.; Heazell, A.E.P.; Sibley, C.; Wright, R.; Bischof, H.; Beards, F.; Guevara, T.; Girard, S.; Jones, R.L. Hypoxia and oxidative stress induce sterile placental inflammation in vitro. Sci. Rep. 2021, 11, 7281. [Google Scholar] [CrossRef]
- Mukherjee, I.; Dhar, R.; Singh, S.; Sharma, J.B.; Nag, T.C.; Mridha, A.R.; Jaiswal, P.; Biswas, S.; Karmakar, S. Oxidative stress-induced impairment of trophoblast function causes preeclampsia through the unfolded protein response pathway. Sci. Rep. 2021, 11, 18415. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Hempstock, J.; Greenwold, N.; Burton, G.J. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am. J. Pathol. 2003, 162, 115–125. [Google Scholar] [CrossRef]
- Joshi, N.P.; Mane, A.R.; Sahay, A.S.; Sundrani, D.P.; Joshi, S.R.; Yajnik, C.S. Role of placental glucose transporters in determining fetal growth. Reprod. Sci. 2022, 29, 2744–2759. [Google Scholar] [CrossRef]
- Chandrasiri, U.P.; Chua, C.L.; Umbers, A.J.; Chaluluka, E.; Glazier, J.D.; Rogerson, S.J.; Boeuf, P. Insight into the pathogenesis of fetal growth restriction in placental malaria: Decreased placental glucose transporter isoform 1 expression. J. Infect. Dis. 2014, 209, 1663–1667. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Y.; Xu, Y.; Guo, X.; Wang, B.; Sun, L.; Liu, L.; Cui, F.; Zhuang, Q.; Bao, X.; et al. The Hypoxia-inducible factor renders cancer cells more sensitive to Vitamin C-induced toxicity. J. Biol. Chem. 2014, 289, 3339–3351. [Google Scholar] [CrossRef]
- de Rijk, E.P.; van Esch, E.; Flik, G. Pregnancy dating in the rat: Placental morphology and maternal blood parameters. Toxicol. Pathol. 2002, 30, 271–282. [Google Scholar] [CrossRef]
- Furukawa, S.; Tsuji, N.; Sugiyama, A. Morphology and physiology of rat placenta for toxicological evaluation. J. Toxicol. Pathol. 2019, 32, 1–17. [Google Scholar] [CrossRef]
- Pereira, A.G.A.; Molino, G.O.G.; Santos, A.C.F.F.; Dias, M.M.F.; Pimenta, N.D.S.; da Silva, P.H.C.M. Efficacy of vitamin C supplementation during pregnancy in the prevention of preterm birth: A systematic review and meta-analysis. Rev. Bras. Ginecol. Obstet. 2025, 47, e-rbgo1. [Google Scholar] [CrossRef]







| FA/FA | FA/NFA | NFA/FA | NFA/NFA | p-Value | ||
|---|---|---|---|---|---|---|
| Area (um2) | WGA | 521.2 ± 242.3 abc | 377.8 ± 221.1 a | 457.5 ± 266.9 b | 434.3 ± 248.6 c | <0.0001 |
| DAPI | 53.75 ± 39.88 a | 59.91 ± 48.17 | 63.97 ± 51.45 a | 59.09 ± 48.48 | 0.0016 | |
| Intensity | WGA | 10,455 ± 2606 ab | 8562 ± 2304 a | 9948 ± 2660 b | 10,245 ± 3081 | <0.0001 |
| FA/FA | FA/NFA | NFA/FA | NFA/NFA | p-Value | ||
|---|---|---|---|---|---|---|
| Area (um2) | GLUT1 | 269 ± 138 abc | 209 ± 129 a | 242 ± 136 b | 252 ± 155 c | <0.0001 |
| SVCT2 | 216 ± 151 a | 130 ± 112 a | 234 ± 178 | 214 ± 160.4 | <0.0001 | |
| Intensity | GLUT1 | 6519 ± 2278 abc | 5493 ± 2407 a | 7532 ± 2860 b | 7738 ± 3542 c | <0.0001 |
| SVCT2 | 12,352 ± 2284 abc | 13,345 ± 2835 a | 21,572 ± 3144 b | 26,621 ± 4871 c | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarroel, F.; Ramírez, E.; Ponce, N.; Nualart, F.; Salinas, P. Impact of PM2.5 Emitted by Wood Smoke on the Expression of Glucose Transporter 1 (GLUT1) and Sodium-Dependent Vitamin C Transporter 2 (SVCT2) in the Rat Placenta: A Pregestational and Gestational Exposure Study. Antioxidants 2025, 14, 1050. https://doi.org/10.3390/antiox14091050
Villarroel F, Ramírez E, Ponce N, Nualart F, Salinas P. Impact of PM2.5 Emitted by Wood Smoke on the Expression of Glucose Transporter 1 (GLUT1) and Sodium-Dependent Vitamin C Transporter 2 (SVCT2) in the Rat Placenta: A Pregestational and Gestational Exposure Study. Antioxidants. 2025; 14(9):1050. https://doi.org/10.3390/antiox14091050
Chicago/Turabian StyleVillarroel, Francisca, Eder Ramírez, Nikol Ponce, Francisco Nualart, and Paulo Salinas. 2025. "Impact of PM2.5 Emitted by Wood Smoke on the Expression of Glucose Transporter 1 (GLUT1) and Sodium-Dependent Vitamin C Transporter 2 (SVCT2) in the Rat Placenta: A Pregestational and Gestational Exposure Study" Antioxidants 14, no. 9: 1050. https://doi.org/10.3390/antiox14091050
APA StyleVillarroel, F., Ramírez, E., Ponce, N., Nualart, F., & Salinas, P. (2025). Impact of PM2.5 Emitted by Wood Smoke on the Expression of Glucose Transporter 1 (GLUT1) and Sodium-Dependent Vitamin C Transporter 2 (SVCT2) in the Rat Placenta: A Pregestational and Gestational Exposure Study. Antioxidants, 14(9), 1050. https://doi.org/10.3390/antiox14091050






