Abstract
Cardiovascular disease (CVD) remains the leading cause of global mortality, despite advances in adult-focused prevention and therapy. Mounting evidence supports the Developmental Origins of Health and Disease (DOHaD) paradigm, which identifies early-life exposures as critical determinants of long-term cardiovascular health. Among the key mechanistic pathways, oxidative stress and gut microbiota dysbiosis have emerged as central, interrelated contributors to cardiovascular programming. Prenatal and postnatal insults can induce sustained redox imbalance and disrupt microbial homeostasis. This disruption creates a feed-forward loop that predisposes offspring to CVD later in life. Antioxidants offer a promising reprogramming strategy by targeting both oxidative stress and gut microbiota composition. Preclinical studies demonstrate that maternal antioxidant interventions—such as vitamins, amino acids, melatonin, polyphenols, N-acetylcysteine, and synthetic agents—can restore redox homeostasis, modulate gut microbial communities, and attenuate cardiovascular risk in offspring. This review synthesizes current evidence on how oxidative stress and gut microbiota act together to shape cardiovascular trajectories. It also examines how antioxidant-based therapies may disrupt this pathological axis during critical developmental windows. Although human data remain limited due to ethical and practical constraints, advancing microbiota-targeted antioxidant interventions may offer a transformative approach to prevent CVD at its origins.
1. Introduction
Despite decades of progress in adult-focused management and therapeutic strategies [1,2], cardiovascular disease (CVD) continues to be the foremost cause of mortality globally [3]. Evidence is accumulating that events and exposures during fetal and early postnatal life can shape lifelong cardiovascular risk, a phenomenon described by the Developmental Origins of Health and Disease (DOHaD) hypothesis [4,5]. The DOHaD framework addresses the root causes of CVD by targeting critical early-life windows [6], offering a proactive, preventive, and intergenerational approach to reduce cardiovascular risk more effectively and equitably across populations. Understanding how prenatal and postnatal environments influence cardiovascular development—via factors such as maternal nutrition, stress, toxin exposure, disease, and early-life microbiota composition [7,8,9,10]—enables a shift from reactive adult care to a life-course strategy that prioritizes early prevention.
The fetal and early postnatal periods are especially vulnerable, representing stages of rapid growth and organ maturation during which adverse environmental exposures can lead to permanent alterations in cardiovascular structure and function—commonly referred to as cardiovascular programming [10,11]. This process involves a multifaceted interplay of mechanisms, including oxidative stress [12], hormonal imbalances [13], inflammation [14], epigenetic modifications [15], and gut microbiota dysbiosis [10]. These early-life disturbances reshape the developmental trajectory of the cardiovascular system, predisposing individuals to hypertension, vascular dysfunction, and heart disease in later life. Among these mechanisms, gut microbiota dysbiosis has emerged as a particularly central and modifiable contributor to CVD risk [11].
Evidence increasingly implicates the gut microbiota in CVD, primarily through its effects on metabolic balance, immune signaling, and inflammation [16,17,18]. Protective metabolites like short-chain fatty acids (SCFAs) enhance vascular integrity and regulate blood pressure (BP), while harmful products such as trimethylamine-N-oxide (TMAO) drive atherosclerosis and endothelial dysfunction [19,20]. Dysbiosis—an imbalance in microbial composition—can disrupt gut barrier integrity, leading to systemic inflammation and endothelial injury, both key processes in CVD pathogenesis. Furthermore, early-life disruptions in gut microbiota, as emphasized in the DOHaD framework, may program long-term cardiovascular risk [21,22]. Given its modifiable nature, the gut microbiota represents a promising target for early-life interventions and precision therapies aimed at reducing CVD risk across the lifespan [11].
Given that oxidative stress is a central pathogenic mechanism underlying CVD, antioxidants have been widely studied in both human and animal models as potential therapeutic agents [23]. While some interventions have shown promise in reducing oxidative biomarkers and improving endothelial function, large clinical trials have not consistently demonstrated significant reductions in major cardiovascular events [24]. The role of antioxidants in CVD and cardiovascular programming is related but distinct as they act at different stages of disease development—namely, adult treatment versus early-life prevention of future cardiovascular risk. Antioxidants have gained attention as a reprogramming strategy, which involves shifting the focus of intervention from disease management in adulthood to early-life prevention. This approach aims to halt or reverse adverse developmental programming processes, thereby reducing disease susceptibility later in life [25,26]. Emerging evidence from animal studies suggests that antioxidant supplementation during pregnancy and lactation may prevent cardiovascular dysfunction in offspring through developmental reprogramming, underscoring its potential as an early-life intervention within the DOHaD framework [27,28,29].
A bidirectional relationship exists between antioxidants and the gut microbiota: antioxidants can modulate microbial composition [30,31], while gut microbes enhance antioxidant bioavailability and activity [32,33]. Together, they synergistically regulate oxidative stress, inflammation, and metabolic pathways—key processes in CVD pathogenesis. Thus, targeting the antioxidant–microbiota axis may provide novel strategies for CVD prevention or mitigation, particularly through antioxidants rich diets and microbiota-directed therapies. This narrative review explores the role of antioxidants in the interplay between gut microbiota and cardiovascular programming, emphasizing their potential as a reprogramming approach to prevent CVD originating in early life.
2. Materials and Methods
This review aims to integrate current evidence on how antioxidants influence the gut microbiome and the potential consequences of these interactions for cardiovascular development and long-term disease risk, framed within the context of the DOHaD. The focus was on early-life mechanisms linking oxidative stress, gut dysbiosis, and CVD risk, with particular attention to antioxidant–microbiota interactions as potential reprogramming strategies. While oxidative stress and gut microbiota dysbiosis have each been implicated in CVD, their mechanistic interconnection—particularly in shaping long-term cardiovascular outcomes from early life—remains underexplored. By integrating evidence on how oxidative stress influences microbial composition and how microbiota-derived metabolites, in turn, modulate host redox balance, this review offers a novel perspective on a bidirectional oxidative stress–microbiota axis in CVD development. In addition, one of the objectives is to assess the translational potential of microbiota-targeted antioxidant interventions as future strategies for CVD prevention and therapy.
A comprehensive literature search was performed using PubMed, Scopus, and Web of Science databases to identify relevant peer-reviewed articles published up to June 2025. Search terms included combinations of the following keywords: “antioxidants”, “oxidative stress”, “gut microbiota”, “dysbiosis”, “cardiovascular disease”, “hypertension’, “developmental programming”, “DOHaD”, “reprogramming”, “early-life intervention”, “pregnancy”, “lactation”, “offspring”, “short-chain fatty acids”, “TMAO”, and “endothelial function”. Boolean operators (AND, OR) were applied to refine the search. Reference lists of key articles were manually screened to identify additional relevant studies.
Both preclinical (animal) and clinical (human) studies were included if they examined the role of antioxidants in modulating oxidative stress or cardiovascular outcomes, their effects on gut microbiota composition or function, or early-life exposures relevant to cardiovascular programming. Eligible publications consisted of original research articles, systematic reviews, and meta-analyses published in English. Studies were excluded if they were non-peer-reviewed (e.g., editorials or conference abstracts) or lacked relevance to early-life developmental programming or antioxidant–microbiota interactions. Given the interdisciplinary scope and heterogeneity of the available literature, a narrative review approach was chosen over a systematic or scoping format to facilitate a comprehensive and integrative exploration of emerging concepts and mechanisms spanning developmental biology, microbiome research, cardiovascular science, and nutrition. We used Napkin AI for generating the figures.
3. Distinct Roles of Gut Microbiota in Overt CVD and Cardiovascular Programming
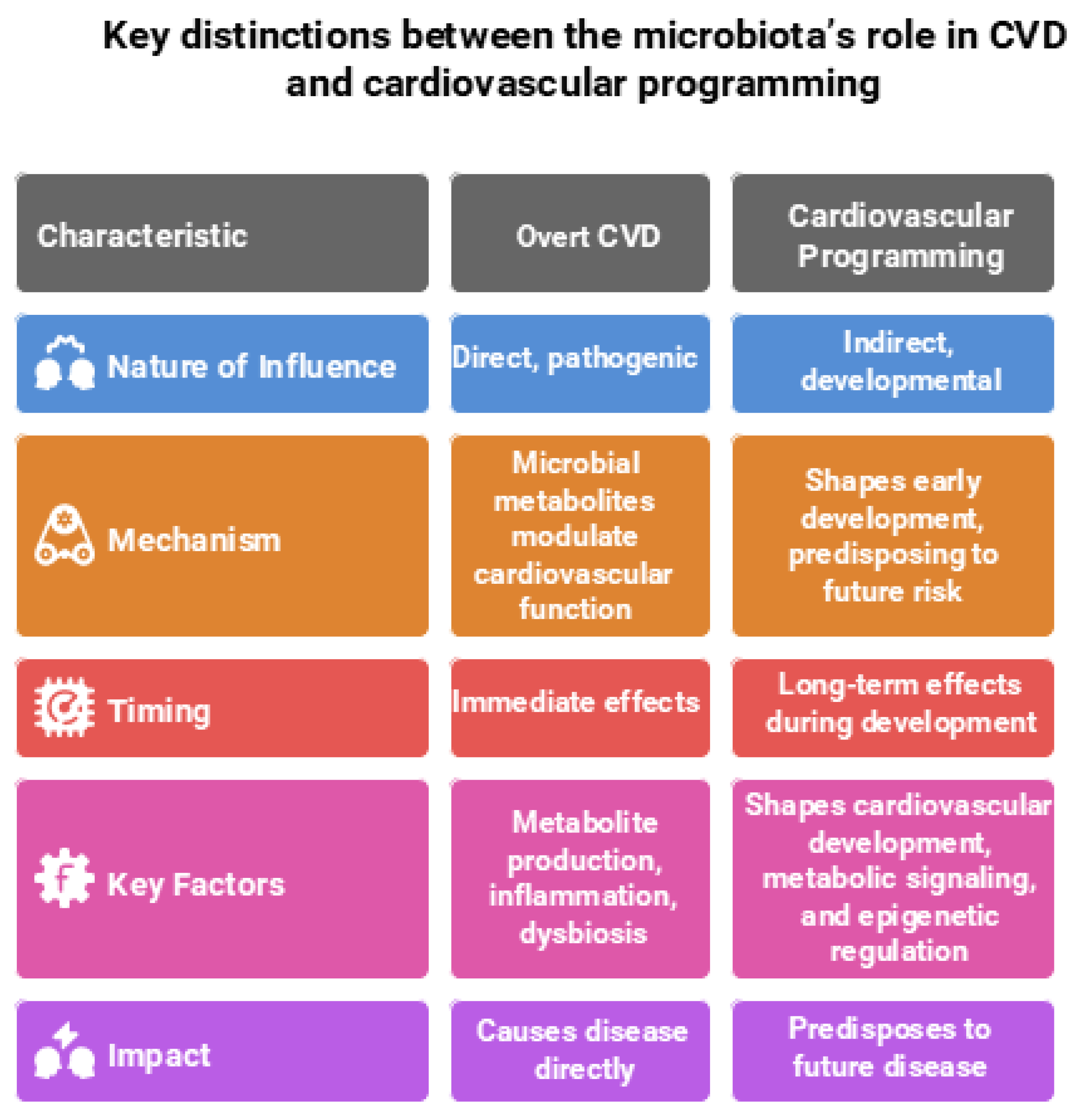
The gut microbiota plays a critical yet distinct role in both CVD and cardiovascular programming, reflecting its influence across different life stages and disease trajectories. In the context of overt CVD, the gut microbiota plays a direct, pathogenic role. In CVD, microbial metabolites—such as TMAO, SCFAs, and tryptophan derivatives (e.g., indoxyl sulfate and indole-3-propionic acid)—exert direct effects by modulating inflammation, oxidative stress, endothelial function, and BP regulation [16,17,18].
In contrast, during cardiovascular programming—which encompasses long-term alterations in cardiovascular structure and function originating from early-life exposures—the gut microbiota plays a more indirect, developmental role. At this stage, the microbiota does not directly cause disease but instead shapes cardiovascular development, metabolic signaling, and epigenetic regulation during critical windows of growth. These early-life microbial influences establish physiological trajectories that predispose individuals to increased cardiovascular risk later in life [10,15]. Understanding these divergent roles is crucial for developing targeted interventions—whether for managing overt CVD or preventing cardiovascular disease through early-life microbiome modulation. Key distinctions between the microbiota’s role in CVD and cardiovascular programming are illustrated in Figure 1 and elaborated upon in the following sections.
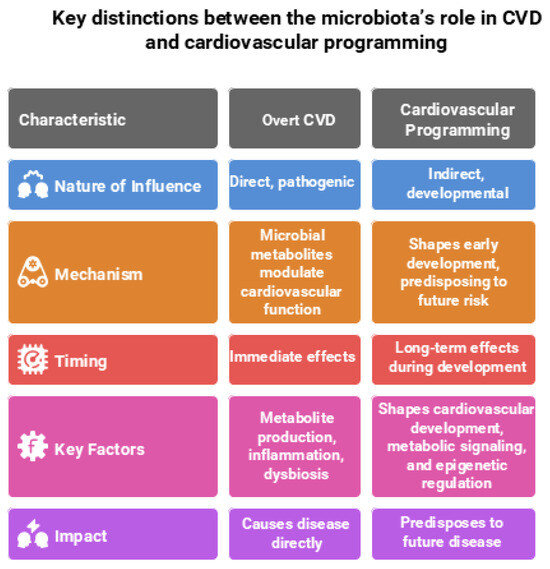
Figure 1.
Comparison of the gut microbiota’s role in overt cardiovascular disease (CVD) and cardiovascular programming. Figure created using Napkin AI Image Generator [https://www.napkin.ai/ (accessed on 20 July 2025)].
3.1. The Role of Gut Microbiota in CVD
The gut microbiota contributes to atherosclerosis by promoting lipid accumulation, vascular inflammation, and immune activation while also driving endothelial dysfunction through impaired nitric oxide (NO) signaling, increased oxidative stress, and compromised vascular integrity [34]. These alterations create a pro-atherogenic environment that predisposes to CVD. Gut dysbiosis can worsen this process by increasing intestinal permeability, enabling bacterial components like lipopolysaccharides (LPS) to enter the bloodstream and provoke systemic inflammation and atherosclerotic plaque development [35,36]. Key microbial metabolites—including TMAO, SCFAs, and tryptophan derivatives—modulate vascular function, oxidative balance, and lipid and glucose metabolism. Collectively, these mechanisms contribute to endothelial injury, atherogenesis, thrombosis, and BP dysregulation [19,20].
3.1.1. Gut Microbiota and Hypertension
Hypertension, a major modifiable risk factor for CVD, is closely linked to gut microbiota and derived metabolites [37,38]. A meta-analysis of 19 studies showed that hypertensive individuals tend to have lower microbial diversity, reduced levels of the SCFA-producing genus Faecalibacterium, and increased abundances of Streptococcus and Enterococcus [39]. Animal models, such as spontaneously hypertensive rats (SHRs), consistently exhibit gut dysbiosis and early intestinal pathologies—including reduced goblet cells, shortened villi, and loss of tight junction proteins—prior to the onset of hypertension [40]. Studies in which microbiota from hypertensive individuals were introduced into germ-free or normotensive animals show a pronounced rise in blood pressure, highlighting the causal role of gut microbial imbalance in hypertension [41]. Conversely, modifying gut microbiota through dietary interventions—such as the Mediterranean diet—and supplementation with probiotics, prebiotics, postbiotics, or FMT has been shown to lower BP and improve cardiovascular health in both humans and animal models [42,43,44,45]. These findings underscore a mechanistic link between gut microbiota and hypertension.
3.1.2. TMAO and CVD
TMAO, a small amine oxide produced by gut microbial metabolism of dietary choline and L-carnitine, has been closely linked to atherosclerosis, thrombosis, and adverse cardiovascular outcomes [46]. In humans, elevated plasma TMAO levels are associated with an increased risk of myocardial infarction, heart failure, stroke, and all-cause mortality and have been proposed as a predictive biomarker for cardiovascular risk [47]. Mechanistically, TMAO promotes foam cell formation and cholesterol accumulation in macrophages, impairs endothelial function by increasing oxidative stress and reducing NO bioavailability, enhances platelet hyperreactivity, and triggers vascular inflammation [48,49,50]. In ApoE−/− and LDLr−/− mouse models, dietary supplementation with TMAO or its precursors accelerates atherosclerotic plaque development [51], while in hypertensive and atherosclerotic models, TMAO elevates reactive oxygen species (ROS) and disrupts endothelial integrity [52,53]. In chronic kidney disease (CKD) rat models, TMAO also contributes to endothelial dysfunction, inflammation, and elevated BP [54]. Notably, inhibition of trimethylamine (TMA), the microbial precursor of TMAO, has been shown to prevent these adverse effects, highlighting the TMA/TMAO metabolic pathway as a potential therapeutic target for CVD [54].
3.1.3. Impact of Short-Chain Fatty Acids on CVD
Acetate, propionate, and butyrate, the main SCFAs, arise from microbial fermentation of fibers present in fruits, legumes, vegetables, and whole grains [55]. Key SCFA-producing bacteria include members of the Ruminococcaceae and Lachnospiraceae families, such as Faecalibacterium prausnitzii, Akkermansia muciniphila, etc., which are commonly associated with a healthy gut microbiome [56]. In both humans and animal models, SCFAs exert cardioprotective effects by regulating BP, reducing systemic inflammation, and improving endothelial function [55]. These actions are mediated in part through activation of SCFA-sensing G-protein-coupled receptors—GPR41, GPR43, and GPR109A—expressed in vascular, immune, and renal tissues [57,58]. Butyrate enhances gut barrier integrity and has anti-inflammatory properties, while propionate and acetate influence sympathetic nervous system activity, renin–angiotensin signaling, and vascular tone [59]. Reduced SCFA production—due to gut dysbiosis or low fiber intake—is linked to hypertension, atherosclerosis, and endothelial dysfunction [59]. Experimental studies demonstrate that enhancing SCFA availability through high-fiber diets or SCFA supplementation can lower BP and reduce cardiovascular risk [60], underscoring their therapeutic potential in CVD prevention.
3.1.4. Inflammation and Immune Dysregulation
Inflammation and immune dysregulation are key pathways linking gut microbiota-derived metabolites to CVD, particularly in the context of CKD. Uremic toxins such as TMAO and several tryptophan metabolites—including indoxyl sulfate (IS), indoleacetic acid, and indoxyl-D-glucuronide—exert proinflammatory, prooxidant, and prothrombotic effects that contribute to CVD pathogenesis [61,62]. IS, for example, activates monocytes, enhances inflammatory cytokine release, promotes oxidative stress, and disrupts hemostasis, all of which drive vascular injury and atherosclerosis [63]. These microbial metabolites also interact with the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor involved in immune modulation [64]. Activation of AhR by tryptophan-derived catabolites promotes differentiation of pro-inflammatory helper T (Th)17 cells and suppresses regulatory T cells (Tregs), leading to a Th17/Treg imbalance implicated in hypertension and cardiovascular complications in CKD [65,66]. Additionally, IS and AhR signaling have been associated with thrombosis, a key mechanism in plaque rupture and acute CVD events. Other uremic toxins, such as p-cresyl sulfate, further exacerbate vascular inflammation by upregulating proinflammatory cytokines and adhesion molecules, thereby promoting atherogenesis [67]. Collectively, these findings highlight the central role of inflammation and immune imbalance in mediating the cardiovascular effects of gut-derived uremic toxins.
3.1.5. Bile Acid Metabolism
Bile acid metabolism, shaped by both host physiology and gut microbiota activity, has gained attention for its role in CVD through its influence on lipid regulation, immune responses, and vascular homeostasis [68]. Evidence from both human and animal studies indicates that disruptions in bile acid composition—particularly elevated microbial-derived secondary bile acids—are linked to atherosclerosis, abnormal lipid profiles, and elevated BP. Bile acids exert systemic effects through receptors such as farnesoid X receptor (FXR) and TGR5, which regulate cholesterol transport, glucose metabolism, and inflammatory signaling pathways [69]. Gut dysbiosis can alter bile acid signaling, contributing to cardiometabolic dysfunction and vascular injury [68]. On the other hand, pharmacologic activation of FXR has been shown to lower plasma triglyceride levels and attenuate vascular inflammation in animal models [70], while TGR5 stimulation enhances endothelial function and reduces BP [71]. These findings highlight the importance of the gut microbiota–bile acid axis in CVD pathogenesis and its potential as a target for therapeutic intervention.
3.2. Impact of Early-Life Gut Microbiota on Cardiovascular Programming
Cardiovascular programming refers to the long-term alterations in cardiovascular structure and function that originate during early life, often triggered by adverse early-life exposures [10,72]. Maternal factors—including nutritional imbalance, toxins, stress, or illness—can disrupt the gut microbiota composition of both the mother and her offspring [21,73]. These early microbial disturbances exert indirect effects by modulating developmental pathways that govern cardiovascular maturation, thereby increasing the risk of hypertension, endothelial dysfunction, and CVD in later life [10].
Increasing data indicate that disturbances in the gut microbiota during early life may shape cardiovascular development indirectly by modulating oxidative stress [12], inflammatory and immune pathways [74], epigenetic modifications [15], and the renin–angiotensin–aldosterone system (RAAS) [75]. Microbial metabolites—such as SCFAs, TMAO, and secondary bile acids—do not act as immediate effectors but rather shape the developmental microenvironment. For instance, SCFAs can modulate gene expression via histone deacetylase (HDAC) inhibition, regulate immune maturation, and influence host receptor signaling. These microbiota-derived cues may gradually establish a pro-inflammatory and pro-hypertensive milieu, indirectly predisposing the offspring to cardiovascular dysfunction over time.
3.2.1. Early-Life Gut Microbiota
Although microbial colonization of the neonatal gut begins immediately after birth, the gut microbiota continues to mature and diversify until it reaches an adult-like composition around 2–3 years of age [76]. The establishment of this early-life microbiome is shaped by a variety of maternal and perinatal factors, including gestational age, mode of delivery, maternal health status, feeding practices, antibiotic exposure, and broader ecological influences [77].
During pregnancy and lactation, maternal gut microbiota can influence the offspring’s microbial composition through vertical transmission and breast milk components, underscoring the pivotal role of maternal factors in shaping the early-life microbiome [73]. Notably, several conditions associated with the developmental origins of CVD—such as maternal obesity [78], diabetes [79], preterm birth [80], low birth weight [81], and maternal malnutrition [82]—are linked to alterations in early gut microbiota composition.
The early-life microbiome does not directly cause CVD but rather contributes indirectly by programming key physiological systems during critical developmental windows. These include immune system maturation and metabolic regulation—both of which are foundational to long-term cardiovascular health [83,84]. Disruptions in microbial development during this sensitive period may promote inflammation, oxidative stress, endothelial dysfunction, aberrant activation of the RAAS, and impaired metabolic signaling—features that gradually increase susceptibility to CVD later in life.
Additionally, prenatal exposure to environmental toxicants such as heavy metals, dioxins, and polycyclic aromatic hydrocarbons not only perturbs cardiovascular development but also alters the gut microbiota in ways that may further predispose the individual to hypertension and atherosclerosis in adulthood [85,86].
Collectively, these findings underscore that adverse maternal and environmental exposures shape the early-life gut microbiota, which in turn exerts indirect, developmental influences on the long-term risk of CVD through the programming of immune, metabolic, and vascular systems.
3.2.2. Human and Animal Evidence of Cardiovascular Programming
Both epidemiological and experimental studies support the concept that adverse early-life exposures contribute to cardiovascular programming and elevate long-term risk for CVD. Human data have consistently linked early-life events—such as famine, maternal illness, pregnancy complications, medication use, environmental pollutants, and suboptimal nutrition—with increased risk of hypertension, obesity, dyslipidemia, type 2 diabetes, and CVD in adulthood [10,79,87,88,89]. For instance, offspring of diabetic pregnancies or those prenatally exposed to glucocorticoids or NSAIDs show higher susceptibility to cardiometabolic disorders [90,91]. Other early-life risk factors include low birth weight, gestational hypertension, short breastfeeding duration, rapid postnatal weight gain, vitamin D deficiency, and exposure to endocrine-disrupting chemicals [10,92,93].
While human studies establish strong associations, animal models provide critical causal insights into the timing, mechanisms, and potential for reprogramming. A growing body of animal research demonstrates that various maternal and perinatal exposures can induce cardiovascular programming, frequently accompanied by alterations in gut microbiota. These exposures include maternal high-fat [94] or high-fructose diets [95], uremia [96], antibiotic treatment [97], TMAO or other metabolite exposure [98], hypertension [99], Western-style diets [100], androgen excess [101], and dyslipidemia [102].
Animal studies indicate that gut microbial dysregulation can promote hypertension, vascular dysfunction, cardiac hypertrophy, and metabolic abnormalities such as obesity, insulin resistance, and fatty liver. Although such dysbiosis is implicated in a range of CVD—including coronary artery disease, cardiomyopathy, and heart failure [103]—its role in developmental programming of these conditions is not yet fully understood.
3.2.3. Mechanisms Underlying Cardiovascular Programming
The fact that diverse early-life insults can result in similar cardiovascular outcomes—such as hypertension—in adulthood suggests the involvement of converging mechanistic pathways in the developmental origins of CVD. Several interrelated mechanisms have been implicated, including oxidative stress, NO deficiency, aberrant activation of the RAAS, chronic inflammation, gut microbiota dysbiosis, and epigenetic modifications [6,10,104,105]. Among these, gut microbiota dysbiosis appears to function as a central mediator, interacting with and modulating other key pathways.
Recent findings underscore the mechanistic contributions of the gut microbiota to cardiovascular programming. Microbial-derived metabolites such as SCFAs, TMAO, and uremic toxins can influence vascular function, immune regulation, and metabolic homeostasis [10]. SCFAs—particularly acetate, propionate, and butyrate—regulate BP via activation of specific receptors (e.g., GPR41, GPR43, and Olfr78) [56], affecting vasodilation and renin secretion [106]. Reduced SCFA levels during early life—often resulting from maternal high-fat diets or antibiotic exposure—have been linked to programmed hypertension [97], while SCFA supplementation during pregnancy and lactation has demonstrated protective effects [107]. TMAO has been shown in animal models to induce hypertension when administered during gestation, whereas inhibition of its formation prevents this outcome [95]. Likewise, in maternal CKD models, offspring hypertension was mitigated by maternal tryptophan supplementation, implicating modulation of the AhR signaling pathway [108]. Moreover, gut dysbiosis influences the RAAS, a critical regulator of cardiovascular homeostasis. Gut microbiota have been shown to affect ACE2 expression and intestinal amino acid transport, indicating a bidirectional relationship between microbial composition and RAAS function [109]. Therapeutic interventions targeting either gut microbiota (e.g., probiotics) or the RAAS have demonstrated reprogramming potential in animal models of developmental hypertension.
Taken together, these findings support a multifactorial model in which gut microbiota act not only as a mediator but also as an integrator of key molecular pathways—indirectly shaping cardiovascular development and increasing disease susceptibility later in life.
3.2.4. Oxidative Stress and Its Link to Gut Microbiota in Cardiovascular Programming
Oxidative stress is a key mechanism underlying the developmental origins of hypertension and CVD. During pregnancy, suboptimal intrauterine conditions can lead to excessive production of reactive oxygen species (ROS), overwhelming the fetal antioxidant defense system and impairing cardiovascular development [110]. Various prenatal insults—including maternal illness, malnutrition, and environmental toxins—have been shown to induce oxidative stress and program hypertension in offspring [111]. This process involves increased activity of ROS-generating enzymes [112,113], reduced antioxidant capacity [114], impaired NO signaling [115], lipid peroxidation [116], DNA damage [117], and peroxynitrite formation [118].
Emerging evidence suggests that oxidative stress and gut microbiota dysbiosis are closely interconnected in cardiovascular programming. Animal models of maternal CKD [43], high-fructose [95], and high-fat diets [110] reveal that increased oxidative stress frequently coexists with altered gut microbiota in offspring predisposed to CVD. The gut microbiota contributes to redox homeostasis by modulating ROS signaling and maintaining gut–vascular integrity; disruption of this balance can amplify inflammation, compromise the intestinal barrier, and perpetuate microbial imbalance—a vicious cycle that enhances cardiovascular risk [119].
In a maternal CKD model, for instance, offspring hypertension was associated with both oxidative stress and gut dysbiosis, along with impaired NO signaling [117]. Perinatal treatment with resveratrol—an antioxidant with prebiotic properties—conferred protection by reducing oxidative stress and reshaping gut microbial composition, supporting a dual mechanism of reprogramming [96].
Together, these findings underscore oxidative stress and gut dysbiosis as interdependent and modifiable contributors to cardiovascular programming. This mechanistic insight supports the rationale for antioxidant-based interventions during pregnancy and lactation as a promising strategy to restore oxidative balance, reshape the gut microbiota, and prevent CVD of developmental origins.
4. Antioxidants as a Reprogramming Strategy
Antioxidants—whether dietary or synthetic—have emerged as promising reprogramming agents that target the intricate interplay between gut microbiota, oxidative stress, and cardiovascular programming. When administered during critical developmental windows, antioxidants can neutralize ROS, restore redox homeostasis, and modulate key signaling pathways that influence both host physiology and the gut microbial ecosystem [30,31,120,121]. Preclinical studies indicate that antioxidant-driven reprogramming occurs not only through ROS scavenging and enhancement of endogenous antioxidant defenses but also via modulation of gut microbiota. Antioxidants have been shown to shift microbial composition toward health-promoting profiles—reducing the production of harmful metabolites such as TMAO and indoxyl sulfate, while enhancing beneficial metabolites SCFAs. Through these integrated actions, antioxidants may interrupt the feed-forward loop linking gut dysbiosis, oxidative stress, and early-life programming of cardiovascular disease (CVD) [122].
While antioxidant interventions in adult CVD populations have produced inconsistent results—likely due to late-stage treatment, suboptimal dosing, or interindividual variability [23,24]—evidence from DOHaD models points to a more effective application: early-life antioxidant therapy as a reprogramming strategy. Targeting oxidative stress during fetal or early postnatal development may reset disease trajectories by preserving vascular integrity, shaping a balanced gut microbiome, and preventing maladaptive cardiovascular remodeling.
Dietary antioxidants are defined as naturally occurring food compounds that exhibit measurable antioxidant effects in vivo [123]. These encompass water-soluble molecules (e.g., vitamin C, glutathione, and lipoic acid) and lipid-soluble compounds (e.g., vitamins A and E, carotenoids, polyphenols, and coenzyme Q), many of which can additionally modulate gut microbial communities and their metabolites [30,31].
In addition to dietary sources, synthetic antioxidants such as N-acetylcysteine (NAC) and mitochondria-targeted agents like MitoQ have demonstrated efficacy in animal models of DOHaD [111,124]. Similarly, specific amino acids (e.g., L-arginine and L-citrulline) and hormones (e.g., melatonin) exhibit both antioxidant and microbiota-modulating properties, making them attractive dual-action reprogramming agents when used during pregnancy or early life [125,126].
The following section explores selected antioxidants investigated in the context of gut microbiota–cardiovascular interactions, highlighting their mechanisms of action and potential for translation into early-life therapeutic strategies to prevent CVD of developmental origin.
4.1. Vitamins and Micronutrients
Vitamins C and E, along with selenium and other micronutrients, possess antioxidant properties and have demonstrated beneficial effects on cardiovascular health [127]. Among these, vitamins C and E are the most extensively studied. Vitamin C, a water-soluble antioxidant, acts as a free radical scavenger and maintains redox balance [128], while vitamin E, a lipid-soluble antioxidant, inhibits oxidative enzymes and limits ROS generation [129]. Prenatal supplementation with either vitamin C or E has been shown to prevent hypertension in offspring subjected to maternal inflammatory stress, such as lipopolysaccharide (LPS) exposure [130,131]. Moreover, combined supplementation with vitamins C and E, selenium, and folic acid has conferred protection against hypertension and cardiovascular dysfunction in offspring from nutrient-restricted dams [132]. In a rabbit model, prenatal vitamin E administration attenuated maternal hypercholesterolemia-induced atherosclerosis in offspring [133].
Folic acid—a key B vitamin involved in one-carbon metabolism and DNA methylation—has demonstrated reprogramming effects by lowering hypertension and cardiovascular risk in offspring of protein-restricted pregnancies [134]. Similarly, maternal choline or betaine supplementation has mitigated cardiometabolic risks in offspring exposed to prenatal insults [135,136]. While these compounds act as methyl donors for epigenetic regulation [137,138], their role in preventing CKM syndrome via targeted epigenetic modulation remains incompletely understood.
Although these vitamins show protective effects on cardiovascular programming and are known to influence gut microbiota composition [30], their capacity to directly reprogram early-life microbiota and modulate gut–vascular signaling in developmental contexts has received limited investigation. Future studies are needed to clarify how micronutrient antioxidants might shape early microbiome development and contribute to long-term cardiovascular health.
4.2. Amino Acids
Perinatal amino acid supplementation has been explored in both clinical and experimental studies to improve pregnancy outcomes and fetal growth [139]. Several amino acids—such as arginine, citrulline, taurine, cysteine, glycine, tryptophan, and branched-chain amino acids (BCAAs)—possess antioxidant properties and play roles in cardiovascular and metabolic programming [140]. Arginine and citrulline are key precursors for NO production [141,142]. Arginine administration during lactation improved hepatic insulin signaling and suppressed gluconeogenesis in offspring [143]. Citrulline supplementation during pregnancy or lactation prevented offspring hypertension in models of antenatal dexamethasone exposure, maternal diabetes, and maternal CKD [144,145,146]. Its protective effect may involve modulation of hypertension-associated gut microbes such as Monoglobus and Streptococcus [147].
Taurine, a sulfur-containing amino acid, has shown consistent protective effects against hypertension and CVD programmed by various maternal insults [148,149,150,151]. In maternal CKD, perinatal taurine treatment reshaped the offspring gut microbiota—restoring Bifidobacterium and increasing Asteroleplasma and Dehalobacterium, while reducing Erisipelatoclostridium—with Bifidobacterium restoration contributing to its antihypertensive effect [151]. Cysteine, another sulfur-containing amino acid, protected offspring from hypertension by boosting hydrogen sulfide (H2S) production and promoting beneficial microbes (Oscillibacter and Butyricicoccus) while suppressing indole-producing bacteria (Akkermansia and Alistipes) and their metabolites [152].
Other amino acids, including glycine [153], BCAAs [154], and tryptophan [155], have also demonstrated reprogramming potential. In maternal CKD, tryptophan supplementation altered tryptophan-metabolizing microbes and modulated AhR signaling, contributing to protection against programmed hypertension [155]. Given the critical role of maternal–fetal amino acid metabolism in development, it is essential to clarify the specific functions and interactions of individual amino acids in cardiovascular programming and microbiota modulation to ensure safe and effective reprogramming strategies.
4.3. Melatonin
As a key hormone in pregnancy, melatonin (N-acetyl-5-methoxytryptamine) supports fetal development [156]. Beyond its physiological roles, it functions as a potent antioxidant, with both melatonin and its metabolites reducing oxidative stress by scavenging reactive oxygen species, promoting antioxidant enzyme expression, and increasing nitric oxide levels [157,158]. It also interacts bidirectionally with the gut microbiota—modulating microbial composition, alleviating dysbiosis, and serving as a signaling mediator between gut health and host circadian and physiological regulation [159]. Given these properties, perinatal melatonin supplementation has emerged as a promising reprogramming strategy to prevent adult-onset diseases linked to DOHaD [160].
The beneficial effects of maternal melatonin therapy have been supported be different models against cardiovascular programming-induced offspring hypertension [161,162,163] and CVD [164]. It is noteworthy that melatonin’s cardioprotective attributes are mainly ascribed to its various antioxidant actions, including the inhibition of mitochondrial respiratory chain complex, the activation of nuclear factor erythroid 2-related factor 2 (Nrf2), and the suppression of inflammatory cytokine release [165,166,167,168].
Previous studies show that melatonin modulates early-life gut microbiota and metabolism, enhancing resistance to inflammation. Maternal melatonin supplementation increased Firmicutes abundance, elevated gut melatonin and butyrate levels, and reduced intestinal inflammation in neonatal mice [169]. In young CKD rats, melatonin therapy restored microbial α-diversity, increased Proteobacteria and Roseburia abundance, and reversed CKD-related TMAO dysregulation, linking its protective effects to gut microbiota modulation [170].
However, clinical use of melatonin during pregnancy remains uncertain [171,172], despite its use in some neonatal conditions [173]. Further translational research is urgently needed to clarify its long-term effects on cardiovascular programming.
4.4. Polyphenols
Polyphenols, a diverse group of phytochemicals abundant in plant-based foods, are recognized for their antioxidant capacity and emerging cardiovascular benefits [174,175]. These compounds counteract oxidative stress via multiple pathways, such as scavenging reactive oxygen species, enhancing nitric oxide bioavailability through nitric oxide synthase activation, chelating pro-oxidant metal ions, and stimulating endogenous antioxidant defenses [176,177]. Beyond redox regulation, polyphenols also interact with the gut microbiota, acting as microbiota-modulating agents that promote a healthier microbial balance [178,179].
Although numerous meta-analyses have linked polyphenol-rich diets to reduced CVD risk [180,181,182,183], only a fraction of individual polyphenols has been examined in developmental models of cardiovascular programming. Polyphenols are broadly categorized into flavonoids and non-flavonoids [184]. Among the flavonoids, quercetin and epigallocatechin gallate (EGCG) have shown promise in mitigating hypertension in adult offspring exposed to prenatal insults, such as maternal high-fat intake [185] or antenatal glucocorticoid exposure [186].
Resveratrol, a well-studied non-flavonoid polyphenol, is notable for its dual antioxidant and prebiotic functions [187]. Resveratrol exerts cardioprotective effects primarily through mechanisms including antioxidant activity, anti-inflammatory signaling, activation of MAPK1 pathway, and modulation of endothelial function [188]. Evidence from experimental models suggests that early-life resveratrol exposure confers long-term protection against hypertension and cardiovascular dysfunction [189,190,191]. In maternal models of CKD, high-fructose diets, and metabolic overload, perinatal resveratrol supplementation improved gut microbial composition in offspring—marked by elevated Bifidobacterium and Lactobacillus levels, increased microbial diversity, and normalization of the Firmicutes-to-Bacteroidetes ratio [96,192,193]. These microbial shifts are linked to reduced risk of hypertension and suggest that resveratrol may act as a microbiota-targeted intervention to counter CKD programming.
Despite their therapeutic potential, clinical application of polyphenols is hindered by poor bioavailability and inter-individual differences in absorption and metabolism [194]. This complexity underscores the need for further investigation into the specific roles of individual polyphenols and their gut microbiota interactions in cardiovascular programming. Optimizing delivery strategies and identifying responsive phenotypes may enhance translational outcomes in future preventive medicine.
4.5. SCFAs
Produced primarily by gut microbes, SCFAs influence oxidative stress and support fetal growth [195,196]. Perinatal administration of acetate, butyrate, or propionate has been found to mitigate programmed hypertension in offspring subjected to maternal high-fructose intake [96], minocycline [197], or tryptophan-deficient [198] conditions. In the high-fructose model, acetate lowered plasma TMA levels, reduced the TMA/TMAO ratio, and upregulated renal SCFA receptors [196]. In the minocycline model, acetate reduced hypertension and increased probiotic genera such as Roseburia and Bifidobacterium [197]. Butyrate was protective in the tryptophan-deficient model, associated with gut microbiota modulation, enhanced SCFA receptor expression, and restoration of RAS balance [198]. A comparative study in high-fructose-fed dams found that while both butyrate and propionate raised circulating SCFA levels, butyrate more strongly impacted TMAO metabolism and NO signaling, whereas propionate primarily influenced gut microbiota composition [199]. These findings suggest SCFAs function not only as antioxidants but also as key microbiota-mediated modulators in hypertension reprogramming.
4.6. N-Acetylcysteine
NAC, a naturally occurring antioxidant in Allium plants, serves as a precursor to glutathione [200] and a stable L-cysteine analogue involved in hydrogen sulfide (H2S) synthesis [201]. Perinatal NAC therapy has been shown to prevent offspring hypertension in various early-life insult models [202,203,204,205]. Reprogramming effects of NAC have been linked to reduced lipid peroxidation, increased glutathione levels, decreased renal oxidative stress, and enhanced NO bioavailability [202,203,204,205]. In spontaneously hypertensive rats (SHRs), maternal NAC treatment was linked to increased abundance of Bifidobacterium and its parent phylum Actinobacteria [202], known sulfur-oxidizing bacteria (SOB) [206]. As NAC increased both Actinobacteria and fecal thiosulfate—an H2S oxidation product—its protective effects may involve enhanced SOB activity and thiosulfate production. Additionally, in a maternal suramin exposure model, NAC administered during gestation and lactation reprogrammed hypertension and reduced renal oxidative stress [204]. These findings highlight NAC’s dual function as an antioxidant and microbiota modulator in developmental hypertension prevention.
4.7. Synthetic Antioxidants
In addition to natural antioxidants, a variety of synthetic antioxidants have been investigated in animal models for their potential to reprogram cardiovascular risks, particularly hypertension of developmental origins. Among these, lipid peroxidation inhibitors such as lazaroids have shown promising results—maternal lazaroid therapy effectively prevented elevated BP in adult offspring exposed to maternal protein restriction [207].
Another notable class is NRF2 (nuclear factor erythroid 2-related factor 2) activators, such as dimethyl fumarate (DMF). NRF2 is a redox-sensitive transcription factor that, upon release from its inhibitor KEAP1, translocates to the nucleus and activates the expression of antioxidant genes via the antioxidant response element (ARE) [208,209]. In a rat model involving maternal dexamethasone exposure and postnatal high-fat diet, DMF treatment prevented the development of offspring hypertension [210], highlighting NRF2 activators as potential therapeutic agents against oxidative-stress-driven cardiovascular programming.
Although synthetic antioxidants like tempol and MitoQ have been extensively studied in oxidative-stress-related models [211,212], their efficacy in reprogramming cardiovascular outcomes remains largely unexplored.
Beyond classical antioxidants, several pharmacological agents have demonstrated antioxidant-like effects by modulating the ROS–NO balance through regulation of asymmetric dimethylarginine (ADMA) metabolism. Agents such as rosuvastatin [213], metformin [214], salvianolic acid A [215], oxymatrine [216], telmisartan [217], and GLP-1 receptor agonists [218] can reduce ADMA levels either by inhibiting its synthesis or enhancing its degradation. Among these, metformin has been shown to prevent offspring hypertension in a maternal high-fructose and post-weaning high-fat diet model in parallel with its ADMA-lowering effect [219]. While metformin is also known to modulate gut microbiota [220], it remains unclear whether this contributes to its reprogramming effects.
Collectively, these findings underscore the therapeutic promise of synthetic antioxidants and related agents as reprogramming strategies to mitigate cardiovascular risks originating from early-life oxidative stress. However, further studies are warranted to clarify their mechanisms—particularly interactions with gut microbiota—and to confirm their long-term efficacy and safety in offspring.
4.8. Others
Recent evidence indicates that probiotics and prebiotics exhibit antioxidant and anti-inflammatory properties, helping to counteract oxidative stress [221,222]. Consequently, interventions aimed at modulating the gut microbiota—including the use of probiotics, prebiotics, postbiotics, and microbial-metabolite-targeted strategies—hold potential as approaches to prevent cardiovascular programming driven by oxidative stress, though further research is needed [10].
While animal models have supported the role of oxidative stress as a viable target for developmental reprogramming, translation into human studies remains limited. A key barrier is the ethical and logistical challenge of conducting interventional trials involving pregnant or lactating women. In this context, breast milk emerges as a practical and biologically potent reprogramming tool, owing to its rich antioxidant profile [223] and distinct microbiome, which plays a pivotal role in shaping the infant gut microbiota [224,225]. Universally acknowledged as the optimal source of infant nutrition, breast milk contains an array of nutrients and bioactive compounds—including hormones, growth factors, cytokines, microRNAs, metabolites, prebiotics, probiotics, and oligosaccharides—that collectively contribute to infant development and immune programming [226,227].
Importantly, breastfeeding has been associated with reduced long-term cardiovascular risk, particularly in preterm infants [228,229]. Given current global recommendations for exclusive breastfeeding during the first six months of life [230], its dual capacity to deliver antioxidants and beneficial microbes positions breast milk as a compelling, naturally integrated strategy for preventing early-life cardiovascular programming. A concise overview of the key findings is presented in Table 1.

Table 1.
Summary of antioxidant-based reprogramming strategies targeting gut microbiota, oxidative stress, and cardiovascular programming.
5. Conclusions and Future Perspective
This narrative review highlights current evidence on the interplay between oxidative stress and gut microbiota during early life, emphasizing the potential of antioxidants as a reprogramming strategy to prevent cardiovascular programming and reduce the risk of CVD later in life. While antioxidant-based interventions are promising, most current studies are associative in nature and often constrained by small sample sizes, heterogeneous methodologies for microbiome profiling, and inadequate control for key confounders such as maternal diet, socioeconomic status, and antibiotic exposure. These limitations underscore the need for more targeted and translational research. Key knowledge gaps include identifying the optimal antioxidant mechanisms, timing, and formulations [120]; elucidating the bidirectional interactions between oxidative stress and gut microbiota within the DOHaD framework [10]; and bridging preclinical findings to clinical application [231].
Targeting oxidative stress in early life is an attractive reprogramming strategy, but several important limitations must be considered. Antioxidant therapies—whether oral or intravenous—circulate systemically and may reduce ROS levels below the physiological range in healthy tissues, potentially disrupting redox-sensitive signaling pathways essential for normal pregnancy and fetal development [232,233]. Moreover, the oxidant–antioxidant system is closely interconnected with inflammatory and immune networks, and perturbations in this balance could lead to immediate or long-term adverse outcomes for both the mother and fetus. Therefore, antioxidant supplementation during gestation or lactation should be approached cautiously and reserved for cases with clear evidence of oxidative imbalance, rather than used as routine prophylaxis.
Moreover, excessive antioxidant exposure may paradoxically induce “antioxidant stress”, disrupting redox homeostasis, and potentially contributing to adverse developmental outcomes [234]. While antioxidant interventions are biologically plausible as reprogramming agents, excessive or indiscriminate use—particularly at higher doses of vitamins and micronutrients—may disrupt physiological redox signaling, interfere with inflammatory and immune networks, and pose risks to both mother and fetus. Clinical trials have yielded inconsistent results, possibly due to suboptimal timing, limited bioavailability, or failure to target oxidative pathways most relevant to developmental programming. The optimal dose, timing, and therapeutic window for antioxidant-based reprogramming remain undefined, and comprehensive clinical and experimental studies elucidating the mechanisms linking early-life antioxidant exposure to adult cardiovascular outcomes are lacking. Future research should identify precise developmental windows (e.g., prenatal vs. pre-weaning), delineate organ-specific redox-sensitive pathways involved in cardiovascular programming, and match antioxidant strategies to the right dose and timing for safe and effective outcomes. These efforts should be coupled with longitudinal birth cohort studies integrated with mechanistic experimentation and multi-omics approaches to disentangle the interplay between microbiota-derived metabolites, redox balance, and cardiovascular phenotypes.
In parallel, significant gaps exist in our understanding of how oxidative stress and gut microbiota interact within the DOHaD paradigm. Although both are known contributors to early-life CVD risk, their dynamic interplay is seldom investigated as an integrated system. The mechanisms by which oxidative stress influences microbial composition and, conversely, how microbiota-derived metabolites modulate host redox balance are still poorly understood.
A longitudinal birth cohort should be established to profile newborn gut microbiota at birth using metagenomic sequencing, with participants followed into adulthood through periodic cardiovascular assessments. This design would enable identification of early-life microbial signatures predictive of later CVD, integrating environmental, dietary, genetic, and maternal factors—including oxidative status and microbiota composition during pregnancy—to generate critical evidence linking perinatal microbiota to long-term cardiovascular risk and to pinpoint modifiable early-life targets for prevention.
Therapeutic strategies that concurrently target oxidative stress and gut dysbiosis—such as probiotics, prebiotics, synbiotics, and postbiotics—offer promising yet underexplored avenues. Addressing these research gaps will pave the way for the development of precision-based, integrative interventions aimed at preventing CVD from its origins in early life.
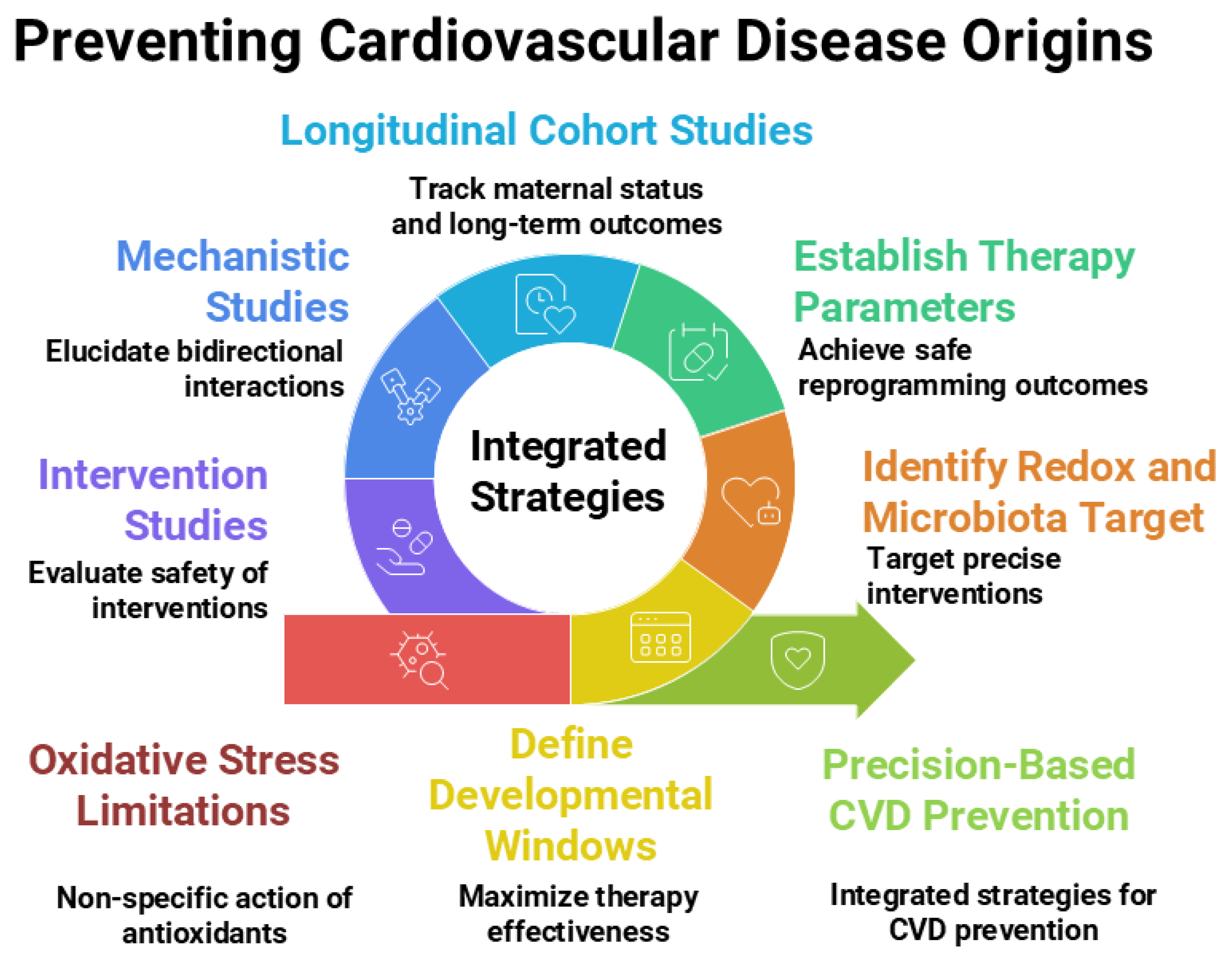
Moving forward, a multidisciplinary approach will be essential—engaging experts from developmental biology, free radical medicine, cardiovascular physiology, nutrition, and microbiology—to unravel these complex interconnections and translate them into safe, effective strategies for lifelong cardiovascular health. Figure 2 illustrates the current research gaps and outlines future research directions.
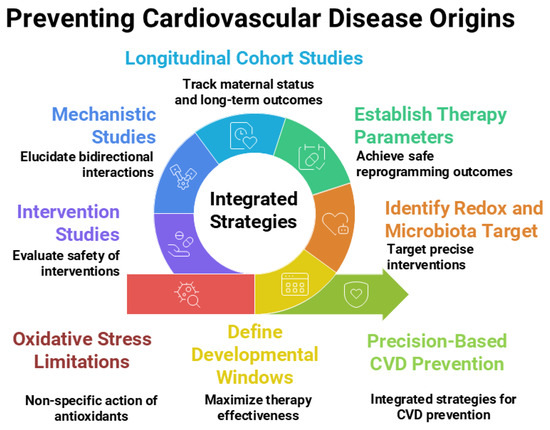
Figure 2.
Integrated strategies, current research gaps, and future directions related to antioxidants and gut microbiota within the Developmental Origins of Health and Disease (DOHaD) framework for preventing the developmental origins of cardiovascular disease. Figure created using Napkin AI Image Generator [https://www.napkin.ai/ (accessed on 20 July 2025)].
Author Contributions
Conceptualization and writing—original draft, Y.-L.T. and C.-N.H.; funding acquisition, Y.-L.T. and C.-N.H.; data curation, C.-Y.H., Y.-W.C., Y.-L.T., Y.-J.L., G.-P.C.-C., S.-F.L. and C.-N.H.; writing—review and editing, Y.-J.L., C.-Y.H., Y.-W.C., Y.-L.T., G.-P.C.-C., S.-F.L. and C.-N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by financial assistance from the National Science and Technology Council, Taiwan, under grant numbers 114-2314-B-182A-036-MY3, 114-2314-B-182A-048, and CMRPG8Q0261 from Kaohsiung Chang Gung Memorial Hospital, Taiwan, and Industry-Academia Grant No. 26, Year 114 (2025), from Cheng Shiu University, Taiwan.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the Super Micro Mass Research and Technology Center, the Institute of Environmental Toxin and Emerging-Contaminant, and the Center for Environmental Toxin and Emerging Contaminant Research, Cheng Shiu University, Kaohsiung, for technical support. During the preparation of this manuscript, the authors used Napkin AI Image Generator [https://www.napkin.ai/ (accessed on 20 July)] for the purpose of generating figures. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest with regard to the contents of this manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| ROS | Reactive Oxygen Species |
| BP | Blood Pressure |
| CVD | Cardiovascular Disease |
| CKD | Chronic Kidney Disease |
| NO | Nitric Oxide |
| H2S | Hydrogen Sulfide |
| BCAAs | Branched-Chain Amino Acids |
| AhR | Aryl Hydrocarbon Receptor |
| DOHaD | Developmental Origins of Health and Disease |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| SCFAs | Short-Chain Fatty Acids |
| NAC | N-acetylcysteine |
| SHR | Spontaneously Hypertensive Rat |
| DMF | Dimethyl Fumarate |
| ADMA | Asymmetric Dimethylarginine |
| EGCG | Epigallocatechin Gallate |
| RAAS | Renin–Angiotensin–Aldosterone System |
| LPS | Lipopolysaccharide |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| FXR | Farnesoid X Receptor |
| TGR5 | G protein–coupled Bile Acid Receptor 1 |
| GPR41 | G-protein-coupled Receptor 41 |
| GPR43 | G-protein-coupled Receptor 43 |
| GPR109A | G-protein-coupled Receptor 109A |
References
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Thomas, H.; Diamond, J.; Vieco, A.; Chaudhuri, S.; Shinnar, E.; Cromer, S.; Perel, P.; Mensah, G.A.; Narula, J.; Johnson, C.O.; et al. Global Atlas of Cardiovascular Disease 2000-2016: The Path to Prevention and Control. Glob. Heart 2018, 13, 143–163. [Google Scholar] [CrossRef]
- Huang, M.; Li, J.; Zhao, X.; Fu, R.; Li, X.; Jiang, W. Global and regional prevalence and cardiovascular risk of primary aldosteronism: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102791. [Google Scholar] [CrossRef]
- Barker, D.J.; Eriksson, J.G.; Forsén, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1239. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T. A Conceptual Framework for the Developmental Origins of Health and Disease. J. Dev. Orig. Health Dis. 2010, 1, 6–18. [Google Scholar] [CrossRef]
- Santos, K.L. The programming of cardiovascular disease. J. Dev. Orig. Health Dis. 2015, 6, 366–376. [Google Scholar]
- Loche, E.; Ozanne, S.E. Early nutrition, epigenetics, and cardiovascular disease. Curr. Opin. Lipidol. 2016, 27, 449–458. [Google Scholar] [CrossRef]
- LaKind, J.S.; Lehmann, G.M.; Davis, M.H.; Hines, E.P.; Marchitti, S.A.; Alcala, C.; Lorber, M. Infant Dietary Exposures to Environmental Chemicals and Infant/Child Health: A Critical Assessment of the Literature. Environ. Health Perspect. 2018, 126, 96002. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Seckl, J.R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Cardiovascular Diseases of Developmental Origins: Preventive Aspects of Gut Microbiota-Targeted Therapy. Nutrients 2021, 13, 2290. [Google Scholar] [CrossRef]
- Bourque, S.L.; Davidge, S.T. Developmental programming of cardiovascular function: A translational perspective. Clin. Sci. 2020, 134, 3023–3046. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; González, M.C.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, L.; Li, L.; Deng, F.; Zhang, M.; Wang, C.; Qiu, J.; Gao, Q. Prenatal glucocorticoids exposure and adverse cardiovascular effects in offspring. Front. Endocrinol. 2024, 15, 1430334. [Google Scholar] [CrossRef]
- Rogers, L.K.; Velten, M. Maternal inflammation, growth retardation, and preterm birth: Insights into adult cardiovascular disease. Life Sci. 2011, 89, 417–421. [Google Scholar] [CrossRef]
- Benincasa, G.; Napoli, C.; DeMeo, D.L. Transgenerational Epigenetic Inheritance of Cardiovascular Diseases: A Network Medicine Perspective. Matern. Child Health J. 2024, 28, 617–630. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Scarmozzino, F.; Poli, A.; Visiolim, F. Microbiota and cardiovascular disease risk: A scoping review. Pharmacol. Res. 2020, 159, 104952. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; De La Cochetiere, M.F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef]
- Młynarska, E.; Hajdys, J.; Czarnik, W.; Fularski, P.; Leszto, K.; Majchrowicz, G.; Lisińska, W.; Rysz, J.; Franczyk, B. The Role of Antioxidants in the Therapy of Cardiovascular Diseases-A Literature Review. Nutrients 2024, 16, 2587. [Google Scholar] [CrossRef]
- Manson, J.E.; Gaziano, J.M.; Jonas, M.A.; Hennekens, C.H. Antioxidants and cardiovascular disease: A review. J. Am. Coll. Nutr. 1993, 12, 426–432. [Google Scholar] [CrossRef]
- Tain, Y.L.; Joles, J.A. Reprogramming: A Preventive Strategy in Hypertension Focusing on the Kidney. Int. J. Mol. Sci. 2015, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Paauw, N.D.; van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2017, 219, 241–259. [Google Scholar] [CrossRef]
- Aljunaidy, M.M.; Morton, J.S.; Cooke, C.M.; Davidge, S.T. Prenatal hypoxia and placental oxidative stress: Linkages to developmental origins of cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R395–R399. [Google Scholar] [CrossRef]
- Diniz, M.S.; Magalhães, C.C.; Tocantins, C.; Grilo, L.F.; Teixeira, J.; Pereira, S.P. Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases. Nutrients 2023, 15, 4623. [Google Scholar] [CrossRef]
- Rock, C.R.; Miller, S.L.; Allison, B.J. The Use of Antioxidants for Cardiovascular Protection in Fetal Growth Restriction: A Systematic Review. Antioxidants 2024, 13, 1400. [Google Scholar] [CrossRef]
- Li, X.Y.; Meng, L.; Shen, L.; Ji, H.F. Regulation of gut microbiota by vitamin C, vitamin E and β-carotene. Food Res. Int. 2023, 169, 112749. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Xu, W.; Chen, D.; Zhou, H.L. S-Nitrosylation: Mechanistic Links between Nitric Oxide Signaling and Atherosclerosis. Curr. Atheroscler. Rep. 2025, 27, 78. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, Z.; Liu, H.; Lim, J.Y.; Li, D.W.H.; Zhang, S.; Luo, F.; Wang, X.; Sun, C.; Tang, R.; et al. Research development on gut microbiota and vulnerable atherosclerotic plaque. Heliyon 2024, 10, e25186. [Google Scholar] [CrossRef]
- Ni, M.; Wang, Y.; Zhang, M.; Zhang, P.F.; Ding, S.F.; Liu, C.X.; Liu, X.L.; Zhao, Y.X.; Zhang, Y. Atherosclerotic plaque disruption induced by stress and lipopolysaccharide in apolipoprotein E knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1598–H1606. [Google Scholar] [CrossRef] [PubMed]
- Khodor, S.A.; Reichert, B.; Shatat, I.F. The microbiome and blood pressure: Can microbes regulate our blood pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Cai, M.; Lin, L.; Jiang, F.; Peng, Y.; Li, S.; Chen, L.; Lin, Y. Gut microbiota changes in patients with hypertension: A systematic review and meta-analysis. J. Clin. Hypertens. 2023, 25, 1053–1068. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.O.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Thushara, R.M.; Gangadaran, S.; Solati, Z.; Moghadasian, M.H. Cardiovascular benefits of probiotics: A review of experimental and clinical studies. Food Funct. 2016, 7, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics-A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Leshem, A.; Horesh, N.; Elinav, E. Fecal microbial transplantation and its potential application in cardiometabolic syndrome. Front. Immunol. 2019, 10, 1341. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Yang, Y.; Karampoor, S.; Mirzaei, R.; Borozdkin, L.; Zhu, P. The interplay between microbial metabolites and macrophages in cardiovascular diseases: A comprehensive review. Int. Immunopharmacol. 2023, 121, 110546. [Google Scholar] [CrossRef]
- Querio, G.; Antoniotti, S.; Geddo, F.; Levi, R.; Gallo, M.P. Modulation of Endothelial Function by TMAO, a Gut Microbiota-Derived Metabolite. Int. J. Mol. Sci. 2023, 24, 5806. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Hernández, P.; Leonard, K.A.; Zhao, Y.Y.; Curtis, J.M.; Field, C.J.; Jacobs, R.L. Dietary Choline or Trimethylamine N-oxide Supplementation Does Not Influence Atherosclerosis Development in Ldlr-/- and Apoe-/- Male Mice. J. Nutr. 2020, 150, 249–255. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Li, N.; Liu, H.; Tang, J. Increased circulating trimethylamine N-oxide plays a contributory role in the development of endothelial dysfunction and hypertension in the RUPP rat model of preeclampsia. Hypertens. Pregnancy 2019, 38, 96–104. [Google Scholar] [CrossRef]
- Hamad, A.; Ozkan, M.H.; Uma, S. Trimethylamine-N-oxide (TMAO) Selectively Disrupts Endothelium-Dependent Hyperpolarization-Type Relaxations in a Time-Dependent Manner in Rat Superior Mesenteric Artery. Biol. Pharm. Bull. 2021, 44, 1220–1229. [Google Scholar] [CrossRef]
- Li, T.; Gua, C.; Wu, B.; Chen, Y. Increased circulating trimethylamine N-oxide contributes to endothelial dysfunction in a rat model of chronic kidney disease. Biochem. Biophys. Res. Commun. 2018, 495, 2071–2077. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Madella, A.M.; Van Bergenhenegouwen, J.; Garssen, J.; Masereeuw, R.; Overbeek, S.A. Microbial-Derived Tryptophan Catabolites, Kidney Disease and Gut Inflammation. Toxins 2022, 14, 645. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Centron, P.; Barrows, I.; Dwivedi, R.; Raj, D.S. Gut Microbiota and cardiovascular uremic toxicities. Toxins 2018, 10, 287. [Google Scholar] [CrossRef]
- Sallée, M.; Dou, L.; Cerini, C.; Poitevin, S.; Brunet, P.; Burtey, S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins 2014, 6, 934–949. [Google Scholar] [CrossRef]
- Hung, S.C.; Kuo, K.L.; Wu, C.C.; Tarng, D.C. Indoxyl sulfate: A novel cardiovascular risk factor in chronic kidney disease. J. Am. Heart Assoc. 2017, 6, e005022. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and tryptophan metabolism: Endogenous and dietary routes to ah receptor activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Ren, J.; Crowley, S.D. Role of T-cell activation in salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1345–H1353. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, T.; Wang, X.; Wu, M.; Zhang, R.; Yang, X.; Fu, Y.; Liu, Z. Role of the microbiota-gut-heart axis between bile acids and cardiovascular disease. Biomed. Pharmacother. 2024, 174, 116567. [Google Scholar] [CrossRef]
- Hanafi, N.I.; Mohamed, A.S.; Sheikh Abdul Kadir, S.H.; Othman, M.H.D. Overview of bile acids signaling and perspective on the signal of ursodeoxycholic acid, the most hydrophilic bile acid, in the heart. Biomolecules 2018, 8, 159. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E. Linking liver metabolic and vascular disease via bile acid signaling. Trends Mol. Med. 2022, 28, 51–66. [Google Scholar] [CrossRef]
- Miyazaki-Anzai, S.; Masuda, M.; Levi, N.; Keenan, A.L.; Miyazaki, M. Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS ONE 2014, 9, e108270. [Google Scholar] [CrossRef]
- Crispi, F.; Sepúlveda-Martínez, Á.; Crovetto, F.; Gómez, O.; Bijnens, B.; Gratacós, E. Main Patterns of Fetal Cardiac Remodeling. Fetal Diagn. Ther. 2020, 47, 337–344. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Nagareddy, P.; Smyth, S.S. Inflammation and thrombosis in cardiovascular disease. Curr. Opin. Hematol. 2013, 20, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; South, A.M.; August, P.; Bertagnolli, M.; Ferranti, E.P.; Grobe, J.L.; Jones, E.J.; Loria, A.S.; Safdar, B.; Sequeira-Lopez, M.L.S.; et al. Appraising the Preclinical Evidence of the Role of the Renin-Angiotensin-Aldosterone System in Antenatal Programming of Maternal and Offspring Cardiovascular Health Across the Life Course: Moving the Field Forward: A Scientific Statement From the American Heart Association. Hypertension 2023, 80, e75–e89. [Google Scholar] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, F. Microbial transmission, colonisation and succession: From pregnancy to infancy. Gut 2023, 72, 772–786. [Google Scholar] [CrossRef]
- Zhou, L.; Xiao, X. The role of gut microbiota in the effects of maternal obesity during pregnancy on offspring metabolism. Biosci. Rep. 2018, 38, BSR20171234. [Google Scholar] [CrossRef]
- Dabelea, D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 2007, 30 (Suppl. S2), S169–S174. [Google Scholar] [CrossRef]
- Groer, M.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.M.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef]
- Unger, S.; Stintzi, A.; Shah, P.; Mack, D.; O’Connor, D.L. Gut microbiota of the very- low-birth-weight infant. Pediatr. Res. 2015, 77, 205–213. [Google Scholar] [CrossRef]
- Mischke, M.; Plösch, T. More than just a gut instinct–the potential interplay between a baby’s nutrition, its gut microbiome, and the epigenome. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R1065–R1069. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: Impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, W.; Zeng, M.; Hu, X.; Su, Z.; Liu, Y.; Liu, Z.; Yuan, J.; Li, L.; Zhang, X.; et al. Mapping the early life gut microbiome in neonates with critical congenital heart disease: Multiomics insights and implications for host metabolic and immunological health. Microbiome 2022, 10, 245. [Google Scholar] [CrossRef]
- Tsiaoussis, J.; Antoniou, M.N.; Koliarakis, I.; Mesnage, R.; Vardavas, C.I.; Izotov, B.N.; Psaroulaki, A.; Tsatsakis, A. Effects of single and combined toxic exposures on the gut microbiome: Current knowledge and future directions. Toxicol. Lett. 2019, 312, 72–97. [Google Scholar] [CrossRef]
- Singh, R.D.; Koshta, K.; Tiwari, R.; Khan, H.; Sharma, V.; Srivastava, V. Developmental Exposure to Endocrine Disrupting Chemicals and Its Impact on Cardio-Metabolic-Renal Health. Front. Toxicol. 2021, 3, 663372. [Google Scholar] [CrossRef]
- Roseboom, T.; de Rooij, S.; Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006, 82, 485–491. [Google Scholar] [CrossRef]
- Santos, M.S.; Joles, J.A. Early determinants of cardiovascular disease. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 581–597. [Google Scholar]
- Hult, M.; Tornhammar, P.; Ueda, P.; Chima, C.; Bonamy, A.-K.E.; Ozumba, B.; Norman, M. Hypertension, diabetes and overweight: Looming legacies of the biafran famine. PLoS ONE 2010, 5, e13582. [Google Scholar] [CrossRef]
- Dalziel, S.R.; Walker, N.K.; Parag, V.; Mantell, C.; Rea, H.H.; Rodgers, A.; Harding, J.E. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 2005, 365, 1856–1862. [Google Scholar] [CrossRef]
- Antonucci, R.; Zaffanello, M.; Puxeddu, E.; Porcella, A.; Cuzzolin, L.; Pilloni, M.D.; Fanos, V. Use of non-steroidal anti-inflammatory drugs in pregnancy: Impact on the fetus and newborn. Curr. Drug Metab. 2012, 13, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Halvorsen, C.P.; Andolf, E.; Hu, J.; Pilo, C.; Winbladh, B.; Norman, M. Discordant twin growth in utero and differences in blood pressure and endothelial function at 8 years of age. J. Intern. Med. 2006, 259, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.S.L.; Braga, V.A.; Noronha, S.I.S.R.; Costa, W.K.A.D.; Makki, K.; Cruz, J.C.; Brandão, L.R.; Chianca Junior, D.A.; Meugnier, E.; Leulier, F.; et al. Lactiplantibacillus plantarum WJL administration during pregnancy and lactation improves lipid profile, insulin sensitivity and gut microbiota diversity in dyslipidemic dams and protects male offspring against cardiovascular dysfunction in later life. Food Funct. 2020, 11, 8939–8950. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Tain, Y.L. Targeting on gut microbial metabolite trimethylamine-N-Oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Perinatal resveratrol therapy prevents hypertension programmed by maternal chronic kidney disease in adult male offspring: Implications of the gut microbiome and their metabolites. Biomedicines 2020, 8, 567. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chan, J.Y.H.; Wu, K.L.H.; Yu, H.R.; Lee, W.C.; Hou, C.Y.; Tain, Y.L. Altered gut microbiota and its metabolites in hypertension of developmental origins: Exploring differences between fructose and antibiotics exposure. Int. J. Mol. Sci. 2021, 22, 2674. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Maternal resveratrol therapy protected adult rat offspring against hypertension programmed by combined exposures to asymmetric dimethylarginine and trimethylamine-Noxide. J. Nutr. Biochem. 2021, 93, 108630. [Google Scholar] [CrossRef]
- Li, H.B.; Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. Maternal Treatment with captopril persistently alters gut-brain communication and attenuates hypertension of male offspring. Hypertension 2020, 75, 1315–1324. [Google Scholar] [CrossRef]
- Friedman, J.E.; Dobrinskikh, E.; Alfonso-Garcia, A.; Fast, A.; Janssen, R.C.; Soderborg, T.K.; Anderson, A.L.; Reisz, J.A.; D’Alessandro, A.; Frank, D.N.; et al. Pyrroloquinoline quinone prevents developmental programming of microbial dysbiosis and macrophage polarization to attenuate liver fibrosis in offspring of obese mice. Hepatol. Commun. 2018, 2, 313–328. [Google Scholar] [CrossRef]
- Sherman, S.B.; Sarsour, N.; Salehi, M.; Schroering, A.; Mell, B.; Joe, B.; Hill, J.W. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes 2018, 9, 400–421. [Google Scholar] [CrossRef]
- de Oliveira, Y.; Cavalcante, R.G.S.; Cavalcanti Neto, M.P.; Magnani, M.; Braga, V.A.; de Souza, E.L.; de Brito Alves, J.L. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020, 11, 5581–5594. [Google Scholar] [CrossRef]
- Marzullo, P.; Di Renzo, L.; Pugliese, G.; De Siena, M.; Barrea, L.; Muscogiuri, G.; Colao, A.; Savastano, S. Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. From obesity through gut microbiota to cardiovascular diseases: A dangerous journey. Int. J. Obes. Suppl. 2020, 10, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, H.L.; Ozanne, S.E. Programming of cardiovascular disease across the life-course. J. Mol. Cell. Cardiol. 2015, 83, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Interplay between maternal nutrition and epigenetic programming on offspring hypertension. J. Nutr. Biochem. 2024, 127, 109604. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Tiao, M.M.; Tain, Y.L. Maternal tryptophan supplementation protects adult rat offspring against hypertension programmed by maternal chronic kidney disease: Implication of tryptophan-metabolizing microbiome and aryl hydrocarbon receptor. Int. J. Mol. Sci. 2020, 21, 4552. [Google Scholar] [CrossRef]
- Oliveira Andrade, J.M.; de Farias Lelis, D.; Mafra, V.; Cota, J. The angiotensin converting enzyme 2 (ACE2), gut microbiota, and cardiovascular health. Protein Pept. Lett. 2017, 24, 827–832. [Google Scholar] [CrossRef]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010, 49, 1147–1151. [Google Scholar]
- Paixão, A.D.; Alexander, B.T. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol. Reprod. 2013, 89, 144. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, L.; Wu, J.; Xu, T.; Yang, C.; Chen, X.; Lv, J.; Xu, Z. Prenatal high-salt diet impaired vasodilatation with reprogrammed renin-angiotensin system in offspring rats. J. Hypertens. 2018, 36, 2369–2379. [Google Scholar] [CrossRef]
- Svitok, P.; Okuliarova, M.; Varga, I.; Zeman, M. Renal impairment induced by prenatal exposure to angiotensin II in male rat offspring. Exp. Biol. Med. 2019, 244, 923–931. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, L.C.P.; Neto, J.P.R.C.; de Andrade Braga, V.; Lagranha, C.J.; de Brito Alves, J.L. Maternal exposure to high-fat and high-cholesterol diet induces arterial hypertension and oxidative stress along the gut-kidney axis in rat offspring. Life Sci. 2020, 261, 118367. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zadi, Z.H.; Zhang, J.; Scharf, S.M.; Pae, E.K. Intermittent hypoxia in utero damages postnatal growth and cardiovascular function in rats. J. Appl. Physiol. 2018, 124, 821–830. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef]
- Piecha, G.; Koleganova, N.; Ritz, E.; Müller, A.; Fedorova, O.V.; Bagrov, A.Y.; Lutz, D.; Schirmacher, P.; Gross-Weissmann, M.L. High salt intake causes adverse fetal programming–vascular effects beyond blood pressure. Nephrol. Dial. Transplant. 2012, 27, 3464–3476. [Google Scholar] [CrossRef]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin. Fetal Neonatal Med. 2007, 12, 287–295. [Google Scholar] [CrossRef]
- Barteková, M.; Adameová, A.; Görbe, A.; Ferenczyová, K.; Pecháňová, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in cardiometabolic diseases. Free Radic. Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and on Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board; Institute of Medicine, 17 National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Tain, Y.L.; Hsu, C.N. Metabolic Syndrome Programming and Reprogramming: Mechanistic Aspects of Oxidative Stress. Antioxidants 2022, 11, 2108. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Amino Acids during Pregnancy and Offspring Cardiovascular-Kidney-Metabolic Health. Nutrients 2024, 16, 1263. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Melatonin Use during Pregnancy and Lactation Complicated by Oxidative Stress: Focus on Offspring’s Cardiovascular-Kidney-Metabolic Health in Animal Models. Antioxidants 2024, 13, 226. [Google Scholar] [CrossRef]
- Zhang, F.F.; Barr, S.I.; McNulty, H.; Li, D.; Blumberg, J.B. Health effects of vitamin and mineral supplements. BMJ 2020, 369, 2511. [Google Scholar] [CrossRef]
- Said, H.M.; Nexo, E. Gastrointestinal Handling of Water-Soluble Vitamins. Compr. Physiol. 2018, 8, 1291–1311. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Wang, J.; Yin, N.; Deng, Y.; Wei, Y.; Huang, Y.; Pu, X.; Li, L.; Zheng, Y.; Guo, J.; Yu, J.; et al. Ascorbic Acid Protects against Hypertension through Downregulation of ACE1 Gene Expression Mediated by Histone Deacetylation in Prenatal InflammationInduced Offspring. Sci. Rep. 2016, 6, 39469. [Google Scholar] [CrossRef]
- Farias, J.S.; Santos, K.M.; Lima, N.K.S.; Cabral, E.V.; Aires, R.S.; Veras, A.C.; Paixão, A.D.; Vieira, L.D. Maternal endotoxemia induces renal collagen deposition in adult offspring: Role of NADPH oxidase/TGF-β1/MMP-2signalingpathway. Arch. Biochem. Biophys. 2020, 684, 108306. [Google Scholar] [CrossRef]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef]
- Palinski, W.; D’Armiento, F.P.; Witztum, J.L.; de Nigris, F.; Casanada, F.; Condorelli, M.; Silvestre, M.; Napoli, C. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ. Res. 2001, 89, 991–996. [Google Scholar] [CrossRef]
- Torrens, C.; Brawley, L.; Anthony, F.W.; Dance, C.S.; Dunn, R.; Jackson, A.A.; Poston, L.; Hanson, M.A. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension 2006, 47, 982–987. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Edwards, K.; Dave, B.; Jack-Roberts, C.; Yu, H.; Saxena, A.; Salvador, M.; Dembitzer, M.; Phagoora, J.; Jiang, X. Prenatal Choline Supplementation during High-Fat Feeding Improves Long-Term Blood Glucose Control in Male Mouse Offspring. Nutrients 2020, 12, 144. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Kadam, I.; Reaz, A.; Bretter, R.; Saxena, A.; Johnson, C.H.; Caviglia, J.M.; Jiang, X. Prenatal Choline Supplement in a Maternal Obesity Model Modulates Offspring Hepatic Lipidomes. Nutrients 2023, 15, 965. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Li, D.; Li, N.; Dindot, S.V.; Satterfield, M.C.; Bazer, F.W.; Wu, G. Nutrition, epigenetics, and metabolic syndrome. Antioxid. Redox Signal. 2012, 17, 282–301. [Google Scholar] [CrossRef]
- Li, K.; Wahlqvist, M.L.; Li, D. Nutrition, One-Carbon Metabolism and Neural Tube Defects: A Review. Nutrients 2016, 8, 741. [Google Scholar] [CrossRef]
- Terstappen, F.; Tol, A.J.C.; Gremmels, H.; Wever, K.E.; Paauw, N.D.; Joles, J.A.; Beek, E.M.V.; Lely, A.T. Prenatal Amino Acid Supplementation to Improve Fetal Growth: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2535. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Ten Have, G.A.M.; Wolfe, R.R.; Deutz, N.E.P. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Boger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Luo, K.; Chen, P.; Li, S.; Li, W.; He, M.; Wang, T.; Chen, J. Effect of L-arginine supplementation on the hepatic phosphatidylinositol 3-kinase signaling pathway and gluconeogenic enzymes in early intrauterine growth-restricted rats. Exp. Ther. Med. 2017, 14, 2355–2360. [Google Scholar] [CrossRef]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Perinatal Use of Citrulline Rescues Hypertension in Adult Male Offspring Born to Pregnant Uremic Rats. Int. J. Mol. Sci. 2024, 25, 1612. [Google Scholar] [CrossRef]
- Boucknooghe, T.; Remacle, C.; Reusens, B. Is taurine a functional nutrient? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 728–733. [Google Scholar] [CrossRef]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar]
- Thaeomor, A.; Tangnoi, C.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents the Adverse Effects of Maternal Dyslipidemia on Growth and Cardiovascular Control in Adult Rat Offspring. Adv. Exp. Med. Biol. 2019, 1155, 415–427. [Google Scholar]
- Mensegue, M.F.; Burgueño, A.L.; Tellechea, M.L. Perinatal taurine exerts a hypotensive effect in male spontaneously hypertensive rats and down-regulates endothelial oxide nitric synthase in the aortic arch. Clin. Exp. Pharmacol. Physiol. 2020, 47, 780–789. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Protective Role of Taurine on Rat Offspring Hypertension in the Setting of Maternal Chronic Kidney Disease. Antioxidants 2023, 12, 2059. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants 2022, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.A.; Dunn, R.L.; Marchand, M.C.; Langley-Evans, S.C. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin. Sci. 2002, 103, 633–639. [Google Scholar] [CrossRef]
- Fujii, T.; Yura, S.; Tatsumi, K.; Kondoh, E.; Mogami, H.; Fujita, K.; Kakui, K.; Aoe, S.; Itoh, H.; Sagawa, N.; et al. Branched-chain amino acid supplemented diet during maternal food restriction prevents developmental hypertension in adult rat offspring. J. Dev. Orig. Health Dis. 2011, 2, 176–183. [Google Scholar] [CrossRef]
- Fortunato, M.C.; Neves, J.; Pais, M.L.; Fonseca, C.; Silva, D.; Martins, J.; Castelo-Branco, M.; Fortuna, A.; Gonçalves, J. Maternal Tryptophan Supplementation Alters Offspring Gut-Brain Axis and Behavior in a Sex-Specific Manner. J. Neurochem. 2025, 169, e70161. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [CrossRef]
- Bonmatí-Carrión, M.Á.; Rol, M.A. Melatonin as a Mediator of the Gut Microbiota-Host Interaction: Implications for Health and Disease. Antioxidants 2023, 13, 34. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N. Developmental Programming of Adult Disease: Reprogramming by Melatonin? Int. J. Mol. Sci. 2017, 18, 426. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lin, Y.J.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal melatonin or agomelatine therapy prevents programmed hypertension in male offspring of mother exposed to continuous light. Biol. Reprod. 2017, 97, 636–643. [Google Scholar] [CrossRef]
- Lee, S.K.; Sirajudeen, K.N.; Sundaram, A.; Zakaria, R.; Singh, H.J. Effects of antenatal, postpartum and post-weaning melatonin supplementation on blood pressure and renal antioxidant enzyme activities in spontaneously hypertensive rats. J. Physiol. Biochem. 2011, 67, 249–257. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, B.N.; Kim, J.S. Effects of maternal-melatonin treatment on open-field behaviors and hypertensive phenotype in spontaneously hypertensive rats’ (SHR) offspring. Exp. Anim. 2002, 51, 69–74. [Google Scholar] [CrossRef]
- Hansell, J.A.; Richter, H.G.; Camm, E.J.; Herrera, E.A.; Blanco, C.E.; Villamor, E.; Patey, O.V.; Lock, M.C.; Trafford, A.W.; Galli, G.L.J.; et al. Maternal melatonin: Effective intervention against developmental programming of cardiovascular dysfunction in adult offspring of complicated pregnancy. J. Pineal Res. 2022, 72, e12766. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Moro, N.; Ruggiero, F.M.; Paradies, G. Melatonin Inhibits Cardiolipin Peroxidation in Mitochondria and Prevents the Mitochondrial Permeability Transition and Cytochrome c Release. Free Radic. Biol. Med. 2009, 47, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.N.; Guo, S.; Zhao, X.Y.; Liu, Y.Z.; Liang, C.; Tu, S.; Wang, D.; Li, L.; Dong, J.Z.; et al. Melatonin Improves Cardiac Function in a Mouse Model of Heart Failure with Preserved Ejection Fraction. Redox Biol. 2018, 18, 211–221. [Google Scholar] [CrossRef]
- Veneroso, C.; Tuñón, M.J.; González-Gallego, J.; Collado, P.S. Melatonin Reduces Cardiac Inflammatory Injury Induced by Acute Exercise. J. Pineal Res. 2009, 47, 184–191. [Google Scholar] [CrossRef]
- Li, F.; Lai, J.; Ma, F.; Cai, Y.; Li, S.; Feng, Z.; Lu, Z.; Liu, X.; Ke, Q.; Hao, H.; et al. Maternal melatonin supplementation shapes gut microbiota and protects against inflammation in early life. Int. Immunopharmacol. 2023, 120, 110359. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Melatonin Prevents Chronic Kidney Disease-Induced Hypertension in Young Rat Treated with Adenine: Implications of Gut Microbiota-Derived Metabolites. Antioxidants 2021, 10, 1211. [Google Scholar] [CrossRef]
- Vine, T.; Brown, G.M.; Frey, B.N. Melatonin use during pregnancy and lactation: A scoping review of human studies. Braz. J. Psychiatry 2022, 44, 342–348. [Google Scholar] [CrossRef]
- Boutin, J.A.; Kennaway, D.J.; Jockers, R. Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules 2023, 13, 943. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tain, Y.L.; Sheen, J.M.; Huang, L.T. Melatonin utility in neonates and children. J. Formos. Med. Assoc. 2012, 111, 57–66. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Zhou, Y.; Tan, Y.; Feng, W.; Peng, C. Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef]
- Wang, Z.M.; Zhou, B.; Wang, Y.S.; Gong, Q.Y.; Wang, Q.M.; Yan, J.J.; Gao, W.; Wang, L.S. Black and green tea consumption and the risk of coronary artery disease: A meta-analysis. Am. J. Clin. Nutr. 2011, 93, 506–515. [Google Scholar] [CrossRef]
- Hartley, L.; Flowers, N.; Holmes, J.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 2013, CD009934. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxidative Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, L.; Torun, G.; Bahar, Z.; Fernandez, R. Effects of flavonoid-rich fruits on hypertension in adults: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 2075–2105. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Oest, M.E.; Prater, M.R. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 377–384. [Google Scholar] [CrossRef]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef]
- Kursvietiene, L.; Staneviciene, I.; Mongirdiene, A.; Bernatoniene, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, J.; Jian, W. Unlocking Resveratrol’s Potential: Targeting Ferroptosis in Atherosclerosis Through MAPK1. Food Sci. Nutr. 2025, 13, e70466. [Google Scholar] [CrossRef]
- Ros, P.; Díaz, F.; Freire-Regatillo, A.; Argente-Arizón, P.; Barrios, V.; Argente, J.; Chowen, J.A. Resveratrol Intake during Pregnancy and Lactation Modulates the Early Metabolic Effects of Maternal Nutrition Differently in Male and Female Offspring. Endocrinology 2018, 159, 810–825. [Google Scholar] [CrossRef]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef]
- Liu, T.Y.; Yu, H.R.; Tsai, C.C.; Huang, L.T.; Chen, C.C.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Lai, Y.J.; et al. Resveratrol intake during pregnancy and lactation re-programs adiposity and ameliorates leptin resistance in male progeny induced by maternal high-fat/high sucrose plus postnatal high-fat/high sucrose diets via fat metabolism regulation. Lipids Health Dis. 2020, 19, 174. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 30, e1800066. [Google Scholar] [CrossRef]
- Chen, H.E.; Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Tsai, C.C.; Huang, L.T.; Tain, Y.L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Ferrer, M.; Buey, B.; Grasa, L.; Mesonero, J.E.; Latorre, E. Protective role of short-chain fatty acids on intestinal oxidative stress induced by TNF-α. Cell Stress Chaperones 2024, 29, 769–776. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, M.; Chen, S.; Tang, Y.; Cui, J.; Ding, G. Short-chain fatty acids in fetal development and metabolism. Trends Mol. Med. 2024, 31, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Yu, H.R.; Chan, J.Y.H.; Lee, W.C.; Lin, I.C.; Wu, K.L.H.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Acetate Supplementation Reverses Blood Pressure Increase in Male Offspring Induced by Exposure to Minocycline during Pregnancy and Lactation. Int. J. Mol. Sci. 2022, 23, 7924. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Yu, H.R.; Lin, I.C.; Tiao, M.M.; Huang, L.T.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. J. Nutr. Biochem. 2022, 108, 109090. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tzeng, H.T.; Lee, W.C.; Wu, K.L.H.; Yu, H.R.; Chan, J.Y.H.; Hsu, C.N. Reprogramming Effects of Postbiotic Butyrate and Propionate on Maternal High-Fructose Diet-Induced Offspring Hypertension. Nutrients 2023, 15, 1682. [Google Scholar] [CrossRef]
- Šalamon, Š.; Kramar, B.; Marolt, T.P.; Poljšak, B.; Milisav, I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef]
- Van Goor, H.; van den Born, J.C.; Hillebrands, J.L.; Joles, J.A. Hydrogen sulfide in hypertension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 107–113. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal N-Acetylcysteine Therapy Prevents Hypertension in Spontaneously Hypertensive Rat Offspring: Implications of Hydrogen Sulfide-Generating Pathway and Gut Microbiota. Antioxidants 2020, 9, 856. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal NG-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636.e1–636.e72. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Lee, C.T.; Lin, Y.J.; Tsai, C.C. N-Acetylcysteine prevents programmed hypertension in male rat offspring born to suramin-treated mothers. Biol. Reprod. 2016, 95, 8. [Google Scholar] [CrossRef] [PubMed]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide 2016, 53, 6–12. [Google Scholar] [CrossRef]
- Di Masi, A.; Ascenzi, P. H2S: A “double face” molecule in health and disease. Biofactors 2013, 39, 186–196. [Google Scholar] [CrossRef]
- Huang, S.H.; Leonard, S.; Shi, X.; Goins, M.R.; Vallyathan, V. Antioxidant activity of lazaroid (U-75412E) and its protective effects against crystalline silica-induced cytotoxicity. Free Radic. Biol. Med. 1998, 24, 529–536. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Yu, H.R.; Lin, I.C.; Sheen, J.M.; Huang, L.T.; Tain, Y.L. Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy. Int. J. Mol. Sci. 2019, 20, 3957. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef]
- Pang, M.; Wang, S.; Shi, T.; Chen, J. Overview of MitoQ on prevention and management of cardiometabolic diseases: A scoping review. Front. Cardiovasc. Med. 2025, 12, 1506460. [Google Scholar] [CrossRef]
- Zhou, R.; Ma, P.; Xiong, A.; Xu, Y.; Wang, Y.; Xu, Q. Protective effects of low dose rosuvastatin on isoproterenol-induced chronic heart failure in rats by regulation of DDAH-ADMA-NO pathway. Cardiovasc. Ther. 2017, 35, e12241. [Google Scholar] [CrossRef] [PubMed]
- Bal, F.; Bekpinar, S.; Unlucerci, Y.; Kusku-Kiraz, Z.; Önder, S.; Uysal, M.; Gurdol, F. Antidiabetic drug metformin is effective on the metabolism of asymmetric dimethylarginine in experimental liver injury. Diabetes Res. Clin. Pract. 2014, 106, 295–302. [Google Scholar] [CrossRef]
- He, H.; Li, X.; Wang, H.; Zhang, W.; Jiang, H.; Wang, S.; Yuan, L.; Liu, Y.; Liu, X. Effects of salvianolic acid A on plasma and tissue dimethylarginine levels in a rat model of myocardial infarction. J. Cardiovasc. Pharmacol. 2013, 61, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Liu, Y.K.; Liu, J.; Wang, X.; Xu, Q.; Wang, Y.; Xu, X.; Dai, G. Cardioprotective effects of oxymatrine on isoproterenol-induced heart failure via regulation of DDAH/ADMA metabolism pathway in rats. Eur. J. Pharmacol. 2014, 745, 29–35. [Google Scholar] [CrossRef]
- Onozato, M.L.; Tojo, A.; Leiper, J.; Fujita, T.; Palm, F.; Wilcox, C.S. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: Effects of angiotensin II receptor blockers. Diabetes 2008, 57, 172–180. [Google Scholar] [CrossRef]
- Ojima, A.; Ishibashi, Y.; Matsui, T.; Maeda, S.; Nishino, Y.; Takeuchi, M.; Fukami, K.; Yamagishi, S. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am. J. Pathol. 2013, 182, 132–141. [Google Scholar] [CrossRef]
- Tain, Y.L.; Wu, K.L.H.; Lee, W.C.; Leu, S.; Chan, J.Y.H. Prenatal Metformin Therapy Attenuates Hypertension of Developmental Origin in Male Adult Offspring Exposed to Maternal High-Fructose and Post-Weaning High-Fat Diets. Int. J. Mol. Sci. 2018, 19, 1066. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Kamyshnyi, A. Effects of metformin on the gut microbiota: A systematic review. Mol. Metab. 2023, 77, 101805. [Google Scholar] [CrossRef]
- Kleniewska, P.; Pawliczak, R. The Link Between Dysbiosis, Inflammation, Oxidative Stress, and Asthma-The Role of Probiotics, Prebiotics, and Antioxidants. Nutrients 2024, 17, 16. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Yuksel, S.; Yigit, A.A.; Cinar, M.; Atmaca, N.; Onaran, Y. Oxidant and Antioxidant Status of Human Breast Milk during Lactation Period. Dairy Sci. Technol. 2015, 95, 295–302. [Google Scholar] [CrossRef]
- Hinde, K.; Lewis, Z.T. MICROBIOTA. Mother’s littlest helpers. Science 2015, 348, 1427–1428. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.; Low, W.Y. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar]
- Singhal, A.; Cole, T.J.; Lucas, A. Early Nutrition in Preterm Infants and Later Blood Pressure: Two Cohorts after Randomised Trials. Lancet 2001, 357, 413–419. [Google Scholar] [CrossRef]
- l-Khuffash, A.; Jain, A.; Lewandowski, A.J.; Levy, P.T. Preventing disease in the 21st century: Early breast milk exposure and later cardiovascular health in premature infants. Pediatr. Res. 2020, 87, 385–390. [Google Scholar] [CrossRef]
- Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Larson, K.; Russ, S.A.; Kahn, R.S.; Flores, G.; Goodman, E.; Cheng, T.L.; Halfon, N. Health Disparities: A Life Course Health Development Perspective and Future Research Directions. In Handbook of Life Course Health Development [Internet]; Halfon, N., Forrest, C.B., Lerner, R.M., Faustman, E.M., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Halliwell, B. The antioxidant paradox. Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Villanueva, C.; Kross, R.D. Antioxidant-induced stress. Int. J. Mol. Sci. 2012, 13, 2091–2109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).