Non-Electrophilic Activation of NRF2 in Neurological Disorders: Therapeutic Promise of Non-Pharmacological Strategies
Abstract
1. Introduction
2. Mechanistic Characterization of Non-Pharmacological NRF2 Activation: Strategic Implementation of Non-Electrophilic Pathways
2.1. Electrophilic NRF2 Activation: Mechanistic Paradoxes and Therapeutic Limitations
2.2. Non-Pharmacological NRF2 Activation Under Physiological Conditions: Mechanistic Insights in the Absence of Oxidative Stress
2.3. Non-Pharmacological NRF2 Activation Under Conditions of Progressive Oxidative Stress
| Model | Intervention Type | Species | Intervention Parameters | Focal Organ/Cell | NRF2 Measurement Timing | NRF2 Modulation | Other Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Healthy | Physical exercise | Mice | Treadmill running; 2 consecutive days; 60 min per day; 14 m/min; 10% slope | Heart | Directly after AES | AES: ↑ NRF2 | NRF2−/− mice: ↓ GSH | ROS production | [63] |
| Physical exercise | Mice | Treadmill running; 20 m/min; 5% slope; one or six hours | Hind limb | Directly after PE | 6H PE: ↑ NRF2/ARE binding activity 1H PE: ↔ NRF2/ARE binding activity | 6H: ↑ GCLm, GCLc, SOD1, SOD2, CAT, HO-1 | ROS → KEAP1 | [64] | |
| Physical exercise | Young human males | Cycling; 30 min at 70% VO2max OR 7 cycles at 90% VO2max | Peripheral blood mononuclear cell | Directly after PE | ↑ NRF2 Intensity had no effect | Cycling for 7 cycles ↑ 8-isoprostanes and glutathione reductase in comparison to cycling for 30 min | Not specified | [65] | |
| Physical exercise | Mice | Muscle stimulation on the right leg, high intensity OR low intensity | Gastrocnemius and soleus muscles | 30 min after muscle stimulation | High intensity: local and systemic ↑NRF2 Low intensity: systemic ↓ NRF2 local ↑ NRF2 | ↔ glutathione ↑ NQO1 | KEAP1 | [105] | |
| Physical exercise | Mice | Endurance exercise (EES): 90 min/per day for 2 days OR moderate exercise training (MET): 50 min/day for 6 weeks | Heart | Directly after PE | EES: ↑ NRF2 in young mice Prolonged MET: ↑ NRF2 in aging mice | MET: NQO1, HO-1, and GSR were similar in young and old mice | Not specified | [106] | |
| TMS | In vitro | One session of 10 series at 5, 10, and 15 Hz | THP-1-derived macrophages | 4 h, 6 h, and 24 h | Time-dependent ↑ NRF2 | 5 min treatment: ↔ inflammatory factors Pre-treatment: ↓ IL-1β and TNF-β | KEAP1 p62 | [44] | |
| TH | In vitro | 8 h at 37 °C or 32 °C | HepG2-ARE stable cells | Directly after TH | ↓ antioxidant genes after treatment with TH | Lower temperatures (27 °C and 22 °C): did not activate NRF2 and HIF1A pathways as efficiently as mild hypothermia | Possibly post-translational mechanism | [107] | |
| DR | Rats | Acute: single diving session Chronic: daily diving sessions for 4 weeks | Brain, kidney, lung | Directly after DR | ↑ NRF2 phosphorylation and nuclear translocation | ↑ GSH/GSSG ↑ SOD, ↑ HO-1, ↑ NQO1, ↓ 4HNE, ↓ nitrotyrosine, ↔ MDA | CGRP→ KEAP1/p62, AMPK, SIRT1, PI3K | [66] | |
| RIPreC | Rats | 1 session of 60 min ischemia followed by 60 min reperfusion | Skin tissue | After flap surgery | ↑ NRF2 | Viable flap area was smaller in groups with serum transfer than those w/o RIC | Not specified | [108] | |
| PBM | In vitro | Bisphenol A (BPA) + photobiomodulation (PHT) wavelength: 660 + 10, output power: 35, energy density (J/cm2): 0.28, for 12 min | ADSCs | Directly after PBM | ↑ NRF2 | Low concentrations of BPA + PHT: induced autophagy | p62 | [67] | |
| PBM | In vitro | 100 mW, wavelength of 808 nm, spot area of 0.5 cm2, and irradiation at 1, 2, and 3 J/cm2s OR PBM + metformin | HPDLSCs | Directly after PBM | 3 J/cm2 PBM + metformin: ↑ NRF2. Was more than 2 J/cm2 PBM + metformin | 3 J/cm2 PBM: ↑ PIK3 3 J/cm2 PBM + metformin: ↓ TNF-α | KEAP1 | [68] | |
| PBM | In vitro | Blue light-500 mW/cm2 | A431 epidermoid carcinoma cells | Directly after PBM | ↑ NRF2 | Average volume of light-treated tumors was significantly lower than that in the untreated controls | Not specified | [109] | |
| PBM | In vitro | 250 mW, 500 mW, 1000 mW for 30 s/8 h for 12 days | Schwann cells | Directly after PBM | ↑ NRF2 | 250 mW and 500 Mw: ↓ apoptosis | PI3K/Akt signaling pathway | [110] | |
| Dietary change—CR | Rats | 24 mo old with lifelong 40% CR | CMVECs | After establishment of CMVECs | ↑ NRF2 | ↓ age-related impairment of angiogenic processes | miR-144 | [103] | |
| Intermittent fasting | Mice | Mice fasted for 24 h | Skeletal gastrocnemius muscle | After fasting | ↑ NRF2 | ↑ Cat, Gclc, Gclm, Gsr, HO-1 and Ucp3, GPX4 | Not specified | [111] | |
| Intermittent fasting | Humans | Fasted during Ramadan. Daily fasting between 23–30 days | Blood sample | Directly before RIF and after Ramadan | ↑ NRF2 | ↑ SOD2, TFAM ↓ SIRT3 | ROS production | [69] | |
| Intermittent fasting | Mice, WT and Tlr4-/- | Food deprived for 24 h every other day, for 30 days | Hippocampus | After regimen | Tlr4-/-: ↓ NRF2 Tlr4-/- + IF: ↓ NRF2 | Tlr4-/- + IF: ↓ memory, depressive-like behavior | Not specified | [71] | |
| Intermittent fasting | Mice with ZEB1 or ZEB2 knockout | Deprived of food but free access to water for 3 days | ZEB1-/- + IF: ↓ NRF2 ZEB12-/- + IF: ↑ NRF2 | Not specified | [70] |
| Model | Intervention Type | Species | Intervention Parameters | Focal Organ/Cell | NRF2 Measurement Timing | NRF2 Modulation | Other Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| HFD-induced non-alcoholic fatty liver disease (NAFLD) | Physical exercise | Zebrafish | Placed in swimming tunnel. Exercised for 5 days per week | Liver | Fresh liver tissue samples | ↑ NRF2 | ↓ oxidative stress and apoptosis | SIRT1/AMPK signaling | [43] |
| Hemiparkinsonism | Physical exercise pre-treatment | Mice | Treadmill, 6 weeks, 5 times/week, time-out on weekends | Striatum | Directly after hemiparkinsonism was induced | PE: ↑ NRF2 | ↓ nigrostriatal neurodegeneration, functional impairment, and supersensitivity of DA receptors | Not specified | [92] |
| Parkinsonism | Physical exercise pre-treatment | Rats | Treadmill, at 70% of maximal oxygen consumption for 60 min/day, 5 days/week for 4 weeks | Striatum, substantia nigra | Directly after parkinsonism was induced | ↑ NRF2 | ↔ glutathione or ratio of GSSG | ROS production | [90] |
| Physical exercise | Rats | After induction of PD. Treadmill, 30 min/day, 5 times a week for 4 weeks | Striatum | After behavioral tests | ↑ NRF2 | ↑ NQO1 and TFAM | Not specified | [88] | |
| A53T α-syn, related to PD | EA | A53T mice | 4 weeks starting at 2 months of age at the 2t36 and SP6 acupoints. Intensity increased stepwise | Brain—midbrain and striatum | ↑ NRF2 | ↑ HO-1 and glutamate-cysteine ligase modifier subunits | Not specified | [91] | |
| Heart failure with preserved ejection fraction (HFpEF) | VNS (non-invasive) | Rats | 20 Hz, 0.2 ms, 2 mA, daily for 30 min over a 4-week period | Subfornical organ, spinal trigeminal nucleus | After VNS | ↑ NRF2 | ↑ NQO1 | Not specified | [112] |
| Huntington’s disease | TMS | Rats | 60 Hz, 0.7 mT, 2 h in the morning and 2 h in the afternoon, 8 consecutive days, starting 4 days before the first injection of 3-NP | Striatum | After ELFEF | ↑ NRF2 | Not specified | [95] | |
| Vascular dementia | TMS | Rats | 10 days, 10 Hz for 2 h per day | Brain tissue | After TMS | ↑ NRF2 | ↑ GPx4 and learning memory ability | Not specified | [47] |
| EA | Rats | Third day after 2VO. Administered at DU20 and ST36 acupoints for two weeks | Hippocampus | After EA | ↑ NRF2 | ↓ microglia activation and cognitive deficits | Not specified | [94] | |
| RIPreC | Mice | Four 10-min cycles | Brain | After RIPreC | ↑ NRF2 | ↑ glutathione reductase | Not specified | [93] | |
| Senescence | TMS | SAMP89 mice | 25 Hz for durations of 14 and 28 days | Hippocampus | ↑ NRF2 | ↓ MDA, ↑ GPX4 | Not specified | [46] | |
| Dietary change—olive oil phenolics | SAMP8 mice | Ate diet with high or low amounts of olive oil phenolics for 4.5 months | Heart | High olive oil phenolics: ↑ NRF2 | High olive oil phenolics: ↑ GST, γ-GCS, NQO1, and PON2 mRNA l | SIRT1 | [101] | ||
| Dietary change—Med diet | Humans | 4 weeks. Med diet, Med diet with CoQ (Med + CoQ), Western diet rich with saturated fats (SFA) | Blood samples—peripheral blood mononuclear cells | 4 h after diet | Med + CoQ diet: ↑ cytoplasmic NRF2, ↓ nuclear NRF2 SFA diet: ↓ cytoplasmic NRF2, ↑ nuclear NRF2 Med diet: intermediate effects | ↓ SOD1 and SOD2, TrxR, NADPH-oxidase (p22phox and p47phox subunits) | KEAP1 | [102] | |
| Physical exercise | Rats | Treadmill exercise. 5 days/week for 6 weeks | Renal proximal tubules | 48 h after PE | ↑ NRF2 | ↓ MDA, CRP ↑ SOD1, IL-10 | Not specified | [89] | |
| CUMS | TMS | Rats | 15 Hz for 15 min for 7 consecutive days | Hippocampus | After TMS | ↑ NRF2 | ↓ depressive and anxiety-like behavior | Not specified | [96] |
| EA pre-treatment | Rats | 1 h before CUMS protocol. Administered at GV23 and GV16 acupoints, every other day for 4 weeks | Hippocampus | After inducing CUMS | ↑NRF2 | ↓depressive behaviors, oxidative stress, and MDA | Not specified | [97] | |
| ECT | Rats | Once daily for 10 days at 100 Hz for 0.5 s at 80 mA | Hippocampus | After ECT | ECT: ↑ NRF2 | ECT: ↓ depressive-like behaviors and hippocampal neuronal ferroptosis | BDNF | [98] | |
| Enhanced single prolonged stress (ESPS)–PTSD | EA pre-treatment | Rats | Given at GV20 acupoint for 30 min daily (frequency: 2/15 Hz, intensity: 1 mA) | Hippocampus | 14 days after ESPS | ↑ NRF2 | ↑ HO-1, BDNF, AMPK, hippocampal neurogenesis ↓ anxiety-like behaviors | KEAP1 | [99] |
| Sleep deprivation (SD) | TMS–cTBS | Mice | 600 pulses of 3 stimuli of 40 s for 7 sessions | Hippocampus | After cTBS | ↑ NRF2 | ↑ spatial learning and memory abilities ↓ oxidative stress, inflammation, and autophagy of hippocampal tissues | Not specified | [113] |
| Sepsis | EA | Rats | Given at ST36 0.5 mA and 15 Hz for 30 min once daily for five days | Hippocampus | After EA | ↑ NRF2 | EA: ↓ MDA | α7nAChR | [87] |
| TH | Rats | 10 h. Maintained at 32–33.9 °C | Lungs | 5 days after model is induced | ↑ NRF2 | TH: ↑ GPX4 via the KEAP1/NRF2/SLC7A11 signaling pathway | PI3K/Akt/GSK3β signaling pathway | [114] | |
| Diabetic encephalopathy | EA | Rats | 30 min alternately at ST36 and EXB3 acupoints once a day for 4 weeks | Hippocampus | After EA | ↑ NRF2 | ↑ HO-1 ↑ learning and memory abilities | Not specified | [84] |
| Complex regional pain syndrome type-I | EA | Rats | Given at ST36 and BL60 acupoints on a daily basis for 7 days | Hind paw | After EA | ↑ NRF2 | ↓ mechanical pain response | Not specified | [85] |
| Ventilator-induced lung injury (VILI) | EA | Mice | Given at BL13 and ST36 acupoints five times a week for 2 weeks | Lung | After EA | ↑ NRF2 | ↑ HO-1. ↓ activation of NLRP3 inflammasome | Not specified | [115] |
| Carcinogenesis | Dietary change—CR | Mice | CR mice were fed 40% less than control | Liver | After carcinogenesis | CR required NRF2 for protection against induced tumors | CR: ↑ HO-1, GCLC, GST A1, and GPx-1 | Not specified | [104] |
| Obesity | Dietary change—diets with virgin olive oil (VOO) | Humans | Each group ingested one breakfast every 2 weeks, until completing the four breakfasts | Blood samples—peripheral blood mononuclear cells | After 12 h fasting and at 2 and 4 h after ingestion of each breakfast | 4 h after VOO, SOX, or SOP: ↓ NRF2 4 h after SFO: ↑ NRF2 | 4 h after VOO: ↓ SOD1, GPx 4 h after SOX: ↓ GPx 4 h after SFO: ↑ NRF2 GSH ↑ higher with VOO, SOX, or SOP in comparison to SFO | Not specified | [116] |
| Intermittent fasting | Humans | Ramadan fasting periods—28 to 30 consecutive days abstaining from food and drink from dawn to sunset | Blood samples | 2–7 days before fasting and after Ramadan | IF: ↑ NRF2 | IF: ↑ SOD2, TFAM, CD163 | Not specified | [117] | |
| Adriamycin (ADR)-induced nephropathy | Intermittent fasting | Rats | Alternate 24-h fasting and feeding periods for 8 weeks | Kidney | After regimen | ↑ NRF2 | ↑ SIRT1, HO-1, ↑ AQP2 | Not specified | [118] |
2.4. Non-Pharmacological NRF2 Activation in Acute Oxidative Stress Paradigms
| Model | Intervention Type | Species | Intervention Parameters | Focal Organ/Cell | NRF2 Measurement Timing | NRF2 Modulation | Other Effects | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Cerebral ischemia—MCAO | Physical exercise | Rats | 30-min treadmill training with either Constraint-induced movement therapy (CIMT) OR unconstrained exercise (UE) | Brain | Directly after PE | CIMT in comparison to UE: ↑ NRF2 | CIMT in comparison to UE: ↓ MDA | KEAP1 | [147] |
| Physical exercise—pre-conditional | Mice | Housed for 6 weeks in a cage with a running wheel | Brain—microglia | Directly after PE | PE: ↑ NRF2 | PE w/o stroke: ↔ CB2R, P2Y12, mafk, and p-NRF2 PE w/stroke: ↑ CB2R, P2Y12, mafk, and p-NRF2 | CB2R | [125] | |
| EA | Rats | 30 min for 7 days; continuous 2/100 Hz, ~2–4 V and 0.5~1.5 mA at the PC6, DU26, SP6, and DU20 acupoints | Brain—cerebral cortex | Directly after EA | ↑ NRF2 | ↑GPX4 and SLC7A11, ↓neuronal damage and neuronal mitochondrial injury, ↓ iron | Not specified | [128] | |
| EA pre-treatment | Mouse | 30 min, 1 mA, 2/15 Hz at the GV20 acupoint | Brain—cerebral cortex | 2 h after MCAO | ↑ NRF2 | ↑ HO-1 and NQO1 | GSK-3β | [42] | |
| TH | Rats | Cold condition (4 °C), isolated cortical temperature of 33 ± 1 °C during ischemia | Brain | 24 h after reperfusion | ↑ NRF2 | ↓ neurological deficit and cerebral cell death | PPARs | [126] | |
| RIPreC | Rats | LRIC (3 cycles) was applied every day up to 14 days before MCAO | Brain | 24 h after reperfusion | ↑ NRF2 | ↑ SOD1 and HO-1 | Not specified | [148] | |
| RIPostC | Mice | 4 cycles lasting 40 min in total and continued every 12 h until execution (treated twice daily for 1, 3, or 7 days) | Brain—cortex | After behavioral testing | ↑ NRF2 | ↑ HO-1, NLRP3, cleaved caspase-1, TAC, SOD, GSH/GSSG levels, neurological function, ↓ MDA | KEAP1 | [119] | |
| RIPostC | Mice | 3 cycles immediately after stroke onset | Brain—cerebral cortex | 24 h after reperfusion | ↑ NRF2 | ↑ in neurological outcome, HO-1, NQO-1, SOD, ↓ MDA | Not specified | [120] | |
| Subarachnoid hemorrhage | Physical exercise | Rats | Pre-conditioning exercise, treadmill, 30 min/day, 5 days/week for 3 weeks | Brain—motor cortex | ↑ NRF2 | ↓ neurological deficits, sensorimotor dysfunction, and consciousness disorder | Not specified | [149] | |
| Intracerebral hemorrhage (ICH) | Intermittent fasting | Mice | Every-other-day feeding | Microglia of ipsilateral basal ganglia | Days 1, 2 | Day 1 and 3 after ICH: ↑ NRF2 Day 7: returned to baseline | ↓ CD16+Iba-1+ microglia activation, IL-1β, TNF-α | SIRT3 | [144] |
| I/R on skin | Dietary change = soybean oil and olive oil | Mice | 6 weeks subjected to either regimen | Blood samples—proteins of wound lysate | 14 days after second IR cycle | ↑ NRF2 | Promoted wound closure at 7, 10, and 14 days | Not specified | [129] |
| MIRI | Delayed RIPreC (DRIPC) | Rats | 4 cycles once per day for 3 days before heart isolation | Heart—left ventricle | 30 min after stabilized perfusion | ↑ NRF2 | ↑ HO-1 | Did not activate PKB/Akt or ERK 1/2 | [150] |
| RIPreC | Rats | Four cycles for a total of 40 min | Heart | 24 h after RIPreC | ↑ NRF2 in young hearts, ↓ in old hearts | HIF-1 α | [151] | ||
| VNS | Rats | Intensity: 0.5 V, frequency: 2.5 Hz, pulse width: 5 ms, duration: 5 min | Heart | After I/R | ↑ NRF2 | ↑ GRPR | KEAP1 | [152] | |
| RIPostC | Mice | 3 cycles at the start of reperfusion period | Heart | After 2 h if cardiac reperfusion | ↑ NRF2 | ↑ Akt, HO-1, SOD1, ↓ MDA | STAT3 | [121] | |
| Hepatic I/R | TH | Rats | Induced by the superfusion of cooled saline at 26 °C onto the ischemic lobes | Liver | Directly after TH | ↓ NRF2 | ↑ NQO1 | KEAP1 | [153] |
| VNS | Rats | 20 Hz for 0.1 millisecond | Liver | Directly after VNS | ↑ NRF2 | ↑ HO-1 | Not specified | [130] | |
| VNS | Rats | Interval of 1 s, a duration of 1 ms, and a frequency of 5 Hz | Lung | After 6 h of reperfusion | ↑ NRF2 | ↓ MPO and MDA | Not specified | [131] | |
| VNS | Rats | 20 Hz, 0.2 ms in duration | Kidneys | After I/R | ↑ NRF2 | ↑ HO-1 | Not specified | [154] | |
| Lung I/R | EA | Rabbits | 15 min once a day for 5 days at BL13 and ST36 acupoints | Lung | After I/R | ↑ NRF2 | ↑ SOD, GPx, and CAT ↓ MDA | p38 MAPK | [127] |
| Renal I/R | RIPostC | Mice | 3 cycles of 5 min during the reperfusion period | Kidney | 24 h after reperfusion | ↑ NRF2 | ↑ HO-1, SOD ↓ MDA | TOPK/Akt | [122] |
| RIPostC | Rats | Began on second post-operative day, lasted 10 min | Retina | After RIPostC | ↑ NRF2 | ↑ HO-1, ↓ GFAP | Not specified | [123] | |
| Hemorrhagic shock/resuscitation (S/R) | RIPreC | Mice, zebrafish | Mice underwent 4 cycles for 10 min. Zebrafish were treated with RIC blood | Liver of mice. Plasma from zebrafish | After hemorrhage (S/R) | RIPreC for mice and zebrafish: ↑ NRF2 | KEAP1 and ERK 1/2 | [145] | |
| Tailfin-cut inflammation | RIPostC | Zebrafish | Fish were treated with RIC plasma injection from wildtype mice (4 cycles of 5 min) and NRF2-knockout mice | ↑ NRF2 | ↑ hmox1a, ↓ neutrophil migration | ROS | [139] | ||
| Endotoxic-shock-induced acute lung injury | EA | Rabbits | Performed throughout the operating steps for 6 h during the experimental day at ST36 and BL13 acupoints | Lung | After EA | ↑ NRF2 | ↑ HO-1, SOD, GPx, and CAT, ↓ MDA | Not specified | [146] |
| Hydrogen-peroxide-induced oxidative stress | RIPreC | In vitro | Human umbilical vein endothelial cells (HUVECs) were treated with rat sera. Rats underwent 3 cycles of 10 min | HUVECs | After sera was injected | ↑ NRF2 | ↓ MDA | Not specified | [155] |
| Inflammation—stimulation with 2,4-dinitrochlorobenzene (DNCB) | PBM | In vitro | 660 nm (red light) or 520 nm (green light), 20 min after DNCB treatment. Exposure time of 250 s | Primary human KCs | After PBM | ↑ NRF2 | ↑ HO-1, NQO1, and GCLC | Not specified | [141] |
| DSS-induced acute colitis | EA | Mice | 30 min at ST36 acupoints | Colon—macrophages | After EA | ↑ NRF2 | EA: ↑ NRF2 HO-1, ↓ NLRP3/IL-1β activation | Not specified | [86] |
| TBI | MNS | Rats | 300 microseconds at 40 Hz for 20 s/min. Continued 8 h per day for 2 weeks | Hippocampus | After MNS | ↑ NRF2 | ↑ GPX4, SLC7A11, VEGF | Not specified | [142] |
| TH | Mice | 4 h at 37 degrees Celsius | Cortex | 24 h after TBI | ↑ NRF2 | ↑ HO-1 and NQO-1 ↓ MDA | Not specified | [143] | |
| Hypothermic circulatory arrest (HCA) | RIPreC | Piglets | 4 cycles of 5-min ischemia followed by 5-min reperfusion | Cortex, hippocampus, thalamus, brainstem, cerebellum | After RIPreC | ↑ NRF2 | ↓ 8-OHdG | HIF-1-α | [133] |
| Cardiac hypoxia | PBM | 8 W/cm2–12 W/cm2 0.15–250 s, varied energy intensity | H9C2 cardiomyocytes | After PBM | ↑ NRF2 | ↓ SOD2, PGC-1α | p62 | [134] | |
| Cardiac arrest | TH | Rats | 4 h after return of spontaneous circulation | Hippocampus | After TH | ↑ NRF2 | ↓ MDA, caspase-3 ↑ SOD | GSK-3β | [135] |
| TH | Minipigs | 24 h after ROSC. TH was maintained for 12 h | Frontal cortex | After TH | ↑ NRF2 | ↓ MDA | Oxidative stress | [136] | |
| TH | Rats | Hypothermia was maintained at 33 ± 0.5 °C for four hours | Lumber spinal cord—motor neurons and glial-like cells | 24 h after CA | ↑ NRF2 | ↑ HO-1 | Not specified | [137] | |
| CA-induced renal I/R | TH | Rats | Administered for 2, 4, 6 h | Kidney | After TH | ↑ NRF2, more ↑ with time | ↑ HO-1, ↓ MDA | Not specified | [138] |
| Cardiopulmonary bypass | EA pre-treatment | Rats | EA at PC6 and LI4 acupoints for 30 min before CPB | Lung | After EA | ↑ NRF2 | ↓ pulmonary neutrophil infiltrations ↓ TNF-α, IL-18, and IL-1β | ROS | [132] |
3. Discussion and Future Perspectives
3.1. Multi-Mechanistic NRF2 Activation: A Strategy to Circumvent Constraints of Current Electrophilic and Non-Electrophilic Activators
3.2. Cell-Specific Oxidative Stress Vulnerability and NRF2 Utilization: Mechanistic Advantages of Non-Electrophilic Activation Pathways
3.2.1. Oligodendrocytes
3.2.2. Neurons
3.2.3. Pericytes and Endothelial Cells
3.2.4. Microglia
3.2.5. Astrocytes
3.3. Therapeutic Applications of Non-Pharmacological NRF2 Activators in Neurodegenerative Pathologies and Psychological Disorders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSC | Adipose-tissue-derived stem cell |
| α7nACHr | Alpha7 nicotinic acetylcholine receptor |
| AD | Alzheimer’s disease |
| AMPK | AMP-activated protein kinase |
| ARE | Antioxidant response element |
| BDNF | Brain-derived neurotrophic factor |
| CR | Caloric restriction |
| CB2R | Cannabinoid receptor 2 |
| CA | Cardiac arrest |
| CMVECs | Cerebromicrovascular endothelial cells |
| CUMS | Chronic unpredictable mild stress |
| cTBS | Continuous theta burst stimulation |
| CRP | C-reactive protein |
| DMF | Dimethyl fumarate |
| DR | Diving reflex |
| EA | Electropuncture |
| ECT | Electroconvulsive therapy |
| ERK | Extracellular signal-regulated kinase |
| GPX4 | Glutathione peroxidase 4 |
| GSK3β | Glycogen synthase kinase 3 beta |
| HO-1 | Heme oxygenase-1 |
| HFD | High-fat diet |
| I/R | Ischemia/reperfusion |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| MDA | Malondialdehyde |
| MNS | Median nerve stimulation |
| MCAO | Middle cerebral artery occlusion |
| MIRI | Myocardial ischemia–reperfusion injury |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PD | Parkinson’s disease |
| PI3K | Phosphoinositide-3-kinase |
| PBM | Photobiomodulation |
| PTSD | Post-traumatic stress disorder |
| Akt | Protein kinase B |
| p38 MAPK | p38 mitogen-activated protein kinase |
| ROS | Reactive oxygen species |
| RIC | Remote ischemic conditioning |
| RIPreC | Remote ischemic pre-conditioning |
| RIPostC | Remote ischemic post-conditioning |
| STAT3 | Signal transducer and activator of transcription 3 |
| SIRT1 | Sirtuin 1 |
| SIRT3 | Sirtuin 3 |
| SOD1 | Superoxide dismutase 1 |
| SOD2 | Superoxide dismutase 2 |
| TH | Therapeutic hypothermia |
| TOPK | T-LAK cell-originated protein kinase |
| TMS | Transcranial magnetic stimulation |
| TBI | Traumatic brain injury |
| VNS | Vagus nerve stimulation |
References
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.-C. The Nrf2-ARE Pathway: An Indicator and Modulator of Oxidative Stress in Neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-Related Factor-2-Dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef]
- Shih, A.Y.; Johnson, D.A.; Wong, G.; Kraft, A.D.; Jiang, L.; Erb, H.; Johnson, J.A.; Murphy, T.H. Coordinate Regulation of Glutathione Biosynthesis and Release by Nrf2-Expressing Glia Potently Protects Neurons from Oxidative Stress. J. Neurosci. 2003, 23, 3394–3406. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Johnson, J.A. Oxidative Damage and the Nrf2-ARE Pathway in Neurodegenerative Diseases. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2014, 1842, 1208–1218. [Google Scholar] [CrossRef]
- Navarro, E.; Esteras, N. Multitarget Effects of Nrf2 Signalling in the Brain: Common and Specific Functions in Different Cell Types. Antioxidants 2024, 13, 1502. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.; Srivastava, S.; Siow, R.C.M.; Cash, D.; Modo, M.; Duchen, M.R.; Fraser, P.A.; Williams, S.C.R.; Mann, G.E. Sulforaphane Preconditioning of the Nrf2/HO-1 Defense Pathway Protects the Cerebral Vasculature against Blood–Brain Barrier Disruption and Neurological Deficits in Stroke. Free Radic. Biol. Med. 2013, 65, 1012–1022. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Oh, Y.; Lee, J.E. Glutathione Suppresses Cerebral Infarct Volume and Cell Death after Ischemic Injury: Involvement of FOXO3 Inactivation and Bcl2 Expression. Oxid. Med. Cell. Longev. 2015, 2015, 426069. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The Emerging Role of Nrf2 in Mitochondrial Function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Liebmann, M.; Korn, L.; Janoschka, C.; Albrecht, S.; Lauks, S.; Herrmann, A.M.; Schulte-Mecklenbeck, A.; Schwab, N.; Schneider-Hohendorf, T.; Eveslage, M.; et al. Dimethyl Fumarate Treatment Restrains the Antioxidative Capacity of T Cells to Control Autoimmunity. Brain 2021, 144, 3126–3141. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Mohsenzadegan, M. Antioxidant Therapy in Multiple Sclerosis. Immunopharmacol. Immunotoxicol. 2009, 31, 13–29. [Google Scholar] [CrossRef]
- Dohare, P.; Hyzinski-García, M.C.; Vipani, A.; Bowens, N.H.; Nalwalk, J.W.; Feustel, P.J.; Keller, R.W.; Jourd’heuil, D.; Mongin, A.A. The Neuroprotective Properties of the Superoxide Dismutase Mimetic Tempol Correlate with Its Ability to Reduce Pathological Glutamate Release in a Rodent Model of Stroke. Free Radic. Biol. Med. 2014, 77, 168–182. [Google Scholar] [CrossRef]
- Huang, H.; Guo, F.; Cao, Y.; Shi, W.; Xia, Q. Neuroprotection by Manganese Superoxide Dismutase (MnSOD) Mimics: Antioxidant Effect and Oxidative Stress Regulation in Acute Experimental Stroke. CNS Neurosci. Ther. 2012, 18, 811–818. [Google Scholar] [CrossRef]
- Owjfard, M.; Bigdeli, M.R.; Safari, A.; Haghani, M.; Namavar, M.R. Effect of Dimethyl Fumarate on the Motor Function and Spatial Arrangement of Primary Motor Cortical Neurons in the Sub-Acute Phase of Stroke in a Rat Model. J. Stroke. Cerebrovasc. Dis. 2021, 30, 105630. [Google Scholar] [CrossRef]
- Hou, X.; Xu, H.; Chen, W.; Zhang, N.; Zhao, Z.; Fang, X.; Zhang, X.; Chen, H.; Xu, Y. Neuroprotective Effect of Dimethyl Fumarate on Cognitive Impairment Induced by Ischemic Stroke. Ann. Transl. Med. 2020, 8, 375. [Google Scholar] [CrossRef]
- Bo, C.; Li, J.; Wang, J.; Zhang, Y.; Wu, T.; Wang, M.; Hou, S.; Liang, Y.; Zhang, X.; Zhao, S.; et al. Dimethyl Fumarate Modulates the Immune Environment and Improves Prognosis in the Acute Phase after Ischemic Stroke. Neuroimmunomodulation 2024, 31, 126–141. [Google Scholar] [CrossRef]
- Krämer, T.; Grob, T.; Menzel, L.; Hirnet, T.; Griemert, E.; Radyushkin, K.; Thal, S.C.; Methner, A.; Schaefer, M.K.E. Dimethyl Fumarate Treatment after Traumatic Brain Injury Prevents Depletion of Antioxidative Brain Glutathione and Confers Neuroprotection. J. Neurochem. 2017, 143, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Campolo, M.; Paterniti, I.; Lanza, M.; Filippone, A.; Cuzzocrea, S.; Esposito, E. Dimethyl Fumarate Attenuates Neuroinflammation and Neurobehavioral Deficits Induced by Experimental Traumatic Brain Injury. J. Neurotrauma. 2018, 35, 1437–1451. [Google Scholar] [CrossRef]
- Powell, K.; Wadolowski, S.; Tambo, W.; Chang, E.H.; Kim, D.; Jacob, A.; Sciubba, D.; AlAbed, Y.; Wang, P.; Li, C. Endogenous CGRP Activates NRF2 Signaling via Non-Electrophilic Mechanisms. bioRxiv 2025. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Chu, X.-Y.; Jiang, L.-H.; Liu, M.-Y.; Mei, Z.-L.; Zhang, H.-Y. Identification of Non-Electrophilic Nrf2 Activators from Approved Drugs. Molecules 2017, 22, 883. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Kwong, M. Impaired Expression of Glutathione Synthetic Enzyme Genes in Mice with Targeted Deletion of the Nrf2 Basic-Leucine Zipper Protein. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2000, 1517, 19–26. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription Factor Nrf2 Coordinately Regulates a Group of Oxidative Stress-Inducible Genes in Macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Lee, J.; Kim, J.; Kirchner, V.A.; Jo, Y.H.; Miura, T.; Kim, N.; Song, G.-W.; Hwang, S.; Lee, S.-G.; et al. Upregulation of Carbonyl Reductase 1 by Nrf2 as a Potential Therapeutic Intervention for Ischemia/Reperfusion Injury during Liver Transplantation. Mol. Cells 2019, 42, 672–685. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Zhao, M.; Wang, B.; Su, Z.; Guo, B.; Qin, L.; Zhang, W.; Zheng, R. The Nrf2-NLRP3-Caspase-1 Axis Mediates the Neuroprotective Effects of Celastrol in Parkinson’s Disease. Redox Biol. 2021, 47, 102134. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, Q.; Fadoul, G.; Alraqmany, N.; Ikonomovic, M.; Zhang, F. Aldo-Keto Reductase 1C15 Characterization and Protection in Ischemic Brain Injury. Antioxidants 2023, 12, 909. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chu, L.; Liu, C.; Zha, Z.; Shu, Y. Protective Effect of GSK-3β/Nrf2 Mediated by Dimethyl Fumarate in Middle Cerebral Artery Embolization Reperfusion Rat Model. Curr. Neurovasc. Res. 2021, 18, 456–464. [Google Scholar] [CrossRef]
- Mao, L.; Yang, T.; Li, X.; Lei, X.; Sun, Y.; Zhao, Y.; Zhang, W.; Gao, Y.; Sun, B.; Zhang, F. Protective Effects of Sulforaphane in Experimental Vascular Cognitive Impairment: Contribution of the Nrf2 Pathway. J. Cereb. Blood Flow Metab. 2019, 39, 352–366. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Yang, Y.; Zhang, L.; Cui, L.; Zhang, C.; Chen, R.; Xie, Y.; He, J.; He, W. Tert-Butylhydroquinone Enhanced Angiogenesis and Astrocyte Activation by Activating Nuclear Factor-E2-Related Factor 2/Heme Oxygenase-1 after Focal Cerebral Ischemia in Mice. Microvasc. Res. 2019, 126, 103891. [Google Scholar] [CrossRef]
- Dordoe, C.; Wang, X.; Lin, P.; Wang, Z.; Hu, J.; Wang, D.; Fang, Y.; Liang, F.; Ye, S.; Chen, J.; et al. Non-Mitogenic Fibroblast Growth Factor 1 Protects against Ischemic Stroke by Regulating Microglia/Macrophage Polarization through Nrf2 and NF-ΚB Pathways. Neuropharmacology 2022, 212, 109064. [Google Scholar] [CrossRef]
- Wu, H.; Che, X.; Zheng, Q.; Wu, A.; Pan, K.; Shao, A.; Wu, Q.; Zhang, J.; Hong, Y. Caspases: A Molecular Switch Node in the Crosstalk between Autophagy and Apoptosis. Int. J. Biol. Sci. 2014, 10, 1072–1083. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Dringen, R.; Hirrlinger, J. Glutathione Pathways in the Brain. Biol. Chem. 2003, 384, 505–516. [Google Scholar] [CrossRef]
- Baba, M.Z.; Gomathy, S.; Wahedi, U. Role of Nrf2 Pathway Activation in Neurological Disorder: A Brief Review. J. Pharmacol. Pharmacother. 2022, 13, 229–238. [Google Scholar] [CrossRef]
- Morris, G.; Walker, A.J.; Walder, K.; Berk, M.; Marx, W.; Carvalho, A.F.; Maes, M.; Puri, B.K. Increasing Nrf2 Activity as a Treatment Approach in Neuropsychiatry. Mol. Neurobiol. 2021, 58, 2158–2182. [Google Scholar] [CrossRef]
- Tutakhail, A.; Nazary, Q.A.; Lebsir, D.; Kerdine-Romer, S.; Coudore, F. Induction of Brain Nrf2-HO-1 Pathway and Antinociception after Different Physical Training Paradigms in Mice. Life Sci. 2018, 209, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Wang, X.; Wang, Y.; Jia, Y.; Zhang, Y.; Wang, K.; Luo, L.; Cai, W.; Li, J.; Li, S.; et al. MiR-125b-5p in Adipose Derived Stem Cells Exosome Alleviates Pulmonary Microvascular Endothelial Cells Ferroptosis via Keap1/Nrf2/GPX4 in Sepsis Lung Injury. Redox Biol. 2023, 62, 102655. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Copple, I.M. Advances and Challenges in Therapeutic Targeting of NRF2. Trends. Pharmacol. Sci. 2023, 44, 137–149. [Google Scholar] [CrossRef]
- Barakat, M.; Han, C.; Chen, L.; David, B.P.; Shi, J.; Xu, A.; Skowron, K.J.; Johnson, T.; Woods, R.A.; Ankireddy, A.; et al. Non-Electrophilic NRF2 Activators Promote Wound Healing in Human Keratinocytes and Diabetic Mice and Demonstrate Selective Downstream Gene Targeting. Sci. Rep. 2024, 14, 25258. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; You, Q.; Jiang, Z. Recent Progress in the Development of Small Molecule Nrf2 Activators: A Patent Review (2017–Present). Expert Opin. Ther. Pat. 2020, 30, 209–225. [Google Scholar] [CrossRef]
- Davis, C.K.; Arruri, V.; Joshi, P.; Vemuganti, R. Non-Pharmacological Interventions for Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2024, 44, 641–659. [Google Scholar] [CrossRef]
- Ni, C.; Huang, B.; Huang, Y.; Wen, Z.; Luo, S. Keap1-Independent GSK-3β/Nrf2 Signaling Mediates Electroacupuncture Inhibition of Oxidative Stress to Induce Cerebral Ischemia-Reperfusion Tolerance. Brain Res. Bull. 2024, 217, 111071. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, Z.; Sun, C.; Yang, D.; Zhou, Z.; Peng, X.; Zheng, L.; Tang, C. Exercise Intervention Mitigates Pathological Liver Changes in NAFLD Zebrafish by Activating SIRT1/AMPK/NRF2 Signaling. Int. J. Med. Stud. 2021, 22, 10940. [Google Scholar] [CrossRef] [PubMed]
- Deramaudt, T.B.; Chehaitly, A.; Charrière, T.; Arnaud, J.; Bonay, M. High-Frequency Repetitive Magnetic Stimulation Activates Bactericidal Activity of Macrophages via Modulation of P62/Keap1/Nrf2 and P38 MAPK Pathways. Antioxidants 2023, 12, 1695. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, E.F.; Del Campo, F.M.; Cortés-Sanabria, L.; Mendoza-Carrera, F.; Avesani, C.M.; Stenvinkel, P.; Lindholm, B.; Cueto-Manzano, A.M. Transcription Factor NRF2 as Potential Therapeutic Target for Preventing Muscle Wasting in Aging Chronic Kidney Disease Patients. J. Nephrol. 2022, 35, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, M.; Zhou, C.; Sun, L.; Cai, W.; Li, X. Ferroptosis and Its Implications in Treating Cognitive Impairment Caused by Aging: A Study on the Mechanism of Repetitive Transcranial Magnetic Stimulation. Exp. Gerontol. 2024, 192, 112443. [Google Scholar] [CrossRef]
- Jin, W.-J.; Zhu, X.-X.; Luo, K.-T.; Wang, S.; Li, J.-A.; Qian, L.-F.; Xu, G.-X. Enhancement of Cognitive Function in Rats with Vascular Dementia Through Modulation of the Nrf2/GPx4 Signaling Pathway by High-Frequency Repetitive Transcranial Magnetic Stimulation. Physiol. Res. 2024, 73, 857–868. [Google Scholar] [CrossRef]
- Campione, E.; Mazzilli, S.; Di Prete, M.; Dattola, A.; Cosio, T.; Lettieri Barbato, D.; Costanza, G.; Lanna, C.; Manfreda, V.; Gaeta Schumak, R.; et al. The Role of Glutathione-S Transferase in Psoriasis and Associated Comorbidities and the Effect of Dimethyl Fumarate in This Pathway. Front. Med. 2022, 9, 760852. [Google Scholar] [CrossRef]
- Glutathione-S-transferase T1 Genotyping and Phenotyping in Psoriasis Patients Receiving Treatment with Oral Fumaric Acid Esters—Gambichler—2014—Journal of the European Academy of Dermatology and Venereology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jdv.12137 (accessed on 2 May 2025).
- Fadoul, G.; Ikonomovic, M.; Zhang, F.; Yang, T. The Cell-specific Roles of Nrf2 in Acute and Chronic Phases of Ischemic Stroke. CNS Neurosci. Ther. 2023, 30, e14462. [Google Scholar] [CrossRef]
- Mathis, B.J.; Kato, H.; Hiramatsu, Y. Induction of Cardiac Pathology: Endogenous versus Exogenous Nrf2 Upregulation. Cells 2022, 11, 3855. [Google Scholar] [CrossRef]
- Lynch, D.R.; Chin, M.P.; Delatycki, M.B.; Subramony, S.H.; Corti, M.; Hoyle, J.C.; Boesch, S.; Nachbauer, W.; Mariotti, C.; Mathews, K.D.; et al. Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study). Ann. Neurol. 2021, 89, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.H.; Jadoul, M.; Block, G.A.; Chin, M.P.; Ferguson, D.A.; Goldsberry, A.; Meyer, C.J.; O’Grady, M.; Pergola, P.E.; Reisman, S.A.; et al. Effects of Bardoxolone Methyl on Hepatic Enzymes in Patients with Type 2 Diabetes Mellitus and Stage 4 CKD. Clin. Transl. Sci. 2021, 14, 299–309. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Amador-Martínez, I.; Hernández-Cruz, E.Y.; Tapia, E.; Pedraza-Chaverri, J. Antioxidants Targeting Mitochondria Function in Kidney Diseases. Mitochondrial Commun. 2024, 2, 21–37. [Google Scholar] [CrossRef]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Nrf2/ARE-Mediated Antioxidant Actions of Pro-Electrophilic Drugs. Free Radic. Biol. Med. 2013, 65, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.M.; Dringen, R. Fumaric Acid Diesters Deprive Cultured Primary Astrocytes Rapidly of Glutathione. Neurochem. Int. 2010, 57, 460–467. [Google Scholar] [CrossRef]
- Dibbert, S.; Clement, B.; Skak-Nielsen, T.; Mrowietz, U.; Rostami-Yazdi, M. Detection of Fumarate–Glutathione Adducts in the Portal Vein Blood of Rats: Evidence for Rapid Dimethylfumarate Metabolism. Arch. Dermatol. Res. 2013, 305, 447–451. [Google Scholar] [CrossRef]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane Reactivates Cellular Antioxidant Defense by Inducing Nrf2/ARE/Prdx6 Activity during Aging and Oxidative Stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro. 2020, 12, 1759091419899782. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic Targeting of the NRF2 and KEAP1 Partnership in Chronic Diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Ulasov, A.V.; Rosenkranz, A.A.; Georgiev, G.P.; Sobolev, A.S. Nrf2/Keap1/ARE Signaling: Towards Specific Regulation. Life Sci. 2022, 291, 120111. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.R.; Kannan, S.; Sadhaasivam, K.; Gounder, S.S.; Davidson, C.J.; Boeheme, C.; Hoidal, J.R.; Wang, L.; Rajasekaran, N.S. Acute Exercise Stress Activates Nrf2/ARE Signaling and Promotes Antioxidant Mechanisms in the Myocardium. Free Radic. Biol. Med. 2012, 52, 366–376. [Google Scholar] [CrossRef]
- Li, T.; He, S.; Liu, S.; Kong, Z.; Wang, J.; Zhang, Y. Effects of Different Exercise Durations on Keap1-Nrf2-ARE Pathway Activation in Mouse Skeletal Muscle. Free Radic. Res. 2015, 49, 1269–1274. [Google Scholar] [CrossRef]
- Done, A.J.; Newell, M.J.; Traustadóttir, T. Effect of Exercise Intensity on Nrf2 Signalling in Young Men. Free Radic. Res. 2017, 51, 646–655. [Google Scholar] [CrossRef]
- Powell, K.; Wadolowski, S.; Tambo, W.; Strohl, J.J.; Kim, D.; Turpin, J.; Al-Abed, Y.; Brines, M.; Huerta, P.T.; Li, C. Intrinsic Diving Reflex Induces Potent Antioxidative Response by Activation of NRF2 Signaling. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ebrahimi-Kia, Y.; Noori-Zadeh, A.; Rajaei, F.; Darabi, S.; Darabi, L.; Ghasemi Hamidabadi, H. The Effect of Bisphenol A and Photobiomodulation Therapy on Autophagy-Related Genes Induction in Adipose Tissue-Derived Stem Cells. J. Lasers Med. Sci. 2022, 13, e15. [Google Scholar] [CrossRef]
- Mohamed Abdelgawad, L.; Abd El-hamed, M.M.; Sabry, D.; Abdelgwad, M. Efficacy of Photobiomodulation and Metformin on Diabetic Cell Line of Human Periodontal Ligament Stem Cells through Keap1/Nrf2/Ho-1 Pathway. Rep. Biochem. Mol. Biol. 2021, 10, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.I.; El-Serafi, A.; Jahrami, H.A.; Sherif, N.M.; Hassan, R.E.; Awadallah, S.; Al-Islam, M.E.; Faris, E. Ramadan Diurnal Intermittent Fasting Modulates SOD2, TFAM, Nrf2, and Sirtuins (SIRT1, SIRT3) Gene Expressions in Subjects with Overweight and Obesity. Diabetes Res. Clin. Pract. 2019, 155, 107801. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, C.; Cortés, M.; Martínez-Campanario, M.C.; Domínguez, V.; Han, L.; Tobías, E.; Esteve-Codina, A.; Enrich, C.; Pintado, B.; Garrabou, G.; et al. The Adaptive Antioxidant Response during Fasting-Induced Muscle Atrophy Is Oppositely Regulated by ZEB1 and ZEB2. Proc. Natl. Acad. Sci. USA 2023, 120, e2301120120. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.R.; Da Paixão, A.G.; Kinoshita, P.F.; Orellana, A.M.; Scavone, C.; Kawamoto, E.M. Toll-like Receptor 4 Signaling Is Critical for the Adaptive Cellular Stress Response Effects Induced by Intermittent Fasting in the Mouse Brain. Neuroscience 2021, 465, 142–153. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Yang, Y.-X.; Zhe, H.; He, Z.-X.; Zhou, S.-F. Bardoxolone Methyl (CDDO-Me) as a Therapeutic Agent: An Update on Its Pharmacokinetic and Pharmacodynamic Properties. Drug Des. Devel. Ther. 2014, 8, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.S.; Zhu, F.; Zhao, Y.; Yao, S.; Lu, X.; Ekuma, O.; Evans, C.; Fisk, J.D.; Marrie, R.A.; Tremlett, H. Adverse Events Associated With Disease-Modifying Drugs for Multiple Sclerosis: A Multiregional Population-Based Study. Neurology 2024, 102, e208006. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Kulick, C.G.; Kortepeter, C.M.; Levin, R.L.; Avigan, M.I. Liver Injury Associated with Dimethyl Fumarate in Multiple Sclerosis Patients. Mult. Scler. 2017, 23, 1947–1949. [Google Scholar] [CrossRef]
- Pasquali, L.; Pecori, C.; Lucchesi, C.; LoGerfo, A.; Iudice, A.; Siciliano, G.; Bonuccelli, U. Plasmatic Oxidative Stress Biomarkers in Multiple Sclerosis: Relation with Clinical and Demographic Characteristics. Clin. Biochem. 2015, 48, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Jüngst, C.; Kim, Y.-J.; Lammert, F. Severe Drug-Induced Liver Injury Related to Therapy with Dimethyl Fumarate. Hepatology 2016, 64, 1367–1369. [Google Scholar] [CrossRef]
- Omaveloxolone. In LiverTox: Clinical and Research Information on. Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Hoefnagel, J.J.; Thio, H.B.; Willemze, R.; Bouwes Bavinck, J.N. Long-Term Safety Aspects of Systemic Therapy with Fumaric Acid Esters in Severe Psoriasis. Br. J. Dermatol. 2003, 149, 363–369. [Google Scholar] [CrossRef]
- Bakić, M.; Klisić, A.; Kocić, G.; Kocić, H.; Karanikolić, V. Oxidative Stress and Metabolic Biomarkers in Patients with Psoriasis. J. Med. Biochem. 2024, 43, 97–105. [Google Scholar] [CrossRef]
- Skoie, I.M.; Dalen, I.; Omdal, R.; Jonsson, G. Malondialdehyde and Advanced Oxidation Protein Products Are Not Increased in Psoriasis: A Controlled Study. Arch. Dermatol. Res. 2019, 311, 299–308. [Google Scholar] [CrossRef]
- Aksoy, M.; Kirmit, A. Thiol/Disulphide Balance in Patients with Psoriasis. Postep. Dermatol. Alergol. 2020, 37, 52–55. [Google Scholar] [CrossRef] [PubMed]
- R Rodríguez, L.; Lapeña, T.; Calap-Quintana, P.; Moltó, M.D.; Gonzalez-Cabo, P.; Navarro Langa, J.A. Antioxidant Therapies and Oxidative Stress in Friedreich’s Ataxia: The Right Path or Just a Diversion? Antioxidants 2020, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Watanabe, H.; Imafuku, T.; Arimura, N.; Fujita, I.; Noguchi, I.; Tanaka, S.; Nakano, T.; Tokumaru, K.; Enoki, Y.; et al. Advanced Oxidation Protein Products Contribute to Chronic Kidney Disease-Induced Muscle Atrophy by Inducing Oxidative Stress via CD36/NADPH Oxidase Pathway. J. Cachexia Sarcopenia Muscle 2021, 12, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, M.; Miao, H.; Gong, X.; Han, F.; Shi, L.; Yan, X.; Xu, Z. Electroacupuncture Improves Learning and Memory Deficits in Diabetic Encephalopathy Rats by Regulating the Nrf2/HO-1 Pathway. Brain Res. 2025, 1847, 149309. [Google Scholar] [CrossRef]
- Li, X.; Yin, C.; Hu, Q.; Wang, J.; Nie, H.; Liu, B.; Tai, Y.; Fang, J.; Du, J.; Shao, X.; et al. Nrf2 Activation Mediates Antiallodynic Effect of Electroacupuncture on a Rat Model of Complex Regional Pain Syndrome Type-I through Reducing Local Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2022, 2022, 8035109. [Google Scholar] [CrossRef]
- Song, S.; An, J.; Li, Y.; Liu, S. Electroacupuncture at ST-36 Ameliorates DSS-Induced Acute Colitis via Regulating Macrophage Polarization Induced by Suppressing NLRP3/IL-1β and Promoting Nrf2/HO-1. Mol. Immunol. 2019, 106, 143–152. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Y.; Wei, Y.; Xie, Y. Remimazolam Attenuates LPS-Derived Cognitive Dysfunction via Subdiaphragmatic Vagus Nerve Target A7nAChR-Mediated Nrf2/HO-1 Signal Pathway. Neurochem. Res. 2024, 49, 1306–1321. [Google Scholar] [CrossRef]
- Monir, D.M.; Mahmoud, M.E.; Ahmed, O.G.; Rehan, I.F.; Abdelrahman, A. Forced Exercise Activates the NrF2 Pathway in the Striatum and Ameliorates Motor and Behavioral Manifestations of Parkinson’s Disease in Rotenone-Treated Rats. Behav. Brain Funct. 2020, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.; George, L.; Lokhandwala, M.F. Exercise Decreases Oxidative Stress and Inflammation and Restores Renal Dopamine D1 Receptor Function in Old Rats. Am. J. Physiol.-Ren. Physiol. 2007, 293, F914–F919. [Google Scholar] [CrossRef]
- Tsou, Y.-H.; Shih, C.-T.; Ching, C.-H.; Huang, J.-Y.; Jen, C.J.; Yu, L.; Kuo, Y.-M.; Wu, F.-S.; Chuang, J.-I. Treadmill Exercise Activates Nrf2 Antioxidant System to Protect the Nigrostriatal Dopaminergic Neurons from MPP+ Toxicity. Exp. Neurol. 2015, 263, 50–62. [Google Scholar] [CrossRef]
- Deng, J.; Lv, E.; Yang, J.; Gong, X.; Zhang, W.; Liang, X.; Wang, J.; Jia, J.; Wang, X. Electroacupuncture Remediates Glial Dysfunction and Ameliorates Neurodegeneration in the Astrocytic α-Synuclein Mutant Mouse Model. J. Neuroinflamm. 2015, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S.; Duzzioni, M.; Remor, A.P.; Tristão, F.S.M.; Matheus, F.C.; Raisman-Vozari, R.; Latini, A.; Prediger, R.D. Moderate-Intensity Physical Exercise Protects Against Experimental 6-Hydroxydopamine-Induced Hemiparkinsonism Through Nrf2-Antioxidant Response Element Pathway. Neurochem. Res. 2016, 41, 64–72. [Google Scholar] [CrossRef]
- He, J.-T.; Li, H.; Yang, L.; Cheng, K.-L. Involvement of Endothelin-1, H2S and Nrf2 in Beneficial Effects of Remote Ischemic Preconditioning in Global Cerebral Ischemia-Induced Vascular Dementia in Mice. Cell. Mol. Neurobiol. 2019, 39, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-R.; Shi, G.-X.; Yang, J.-W.; Yan, C.-Q.; Lin, L.-T.; Du, S.-Q.; Zhu, W.; He, T.; Zeng, X.-H.; Xu, Q.; et al. Acupuncture Ameliorates Cognitive Impairment and Hippocampus Neuronal Loss in Experimental Vascular Dementia through Nrf2-Mediated Antioxidant Response. Free Radic. Biol. Med. 2015, 89, 1077–1084. [Google Scholar] [CrossRef]
- Tasset, I.; Pérez-Herrera, A.; Medina, F.J.; Arias-Carrión, Ó.; Drucker-Colín, R.; Túnez, I. Extremely Low-Frequency Electromagnetic Fields Activate the Antioxidant Pathway Nrf2 in a Huntington’s Disease-like Rat Model. Brain Stimul. 2013, 6, 84–86. [Google Scholar] [CrossRef]
- Tian, L.; Sun, S.-S.; Cui, L.-B.; Wang, S.-Q.; Peng, Z.-W.; Tan, Q.-R.; Hou, W.-G.; Cai, M. Repetitive Transcranial Magnetic Stimulation Elicits Antidepressant- and Anxiolytic-like Effect via Nuclear Factor-E2-Related Factor 2-Mediated Anti-Inflammation Mechanism in Rats. Neuroscience 2020, 429, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-J.; Li, P.; Huang, W.-Y.; Huang, Y.; Chen, W.-J.; Chen, Y.-P.; Shen, J.-L.; Chen, J.-K.; Long, N.-S.; Meng, X.-J. Acupuncture Relieves Stress-Induced Depressive Behavior by Reducing Oxidative Stress and Neuroapoptosis in Rats. Front. Behav. Neurosci. 2022, 15. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Zang, X.; Xing, J.; Mo, X.; Hei, Z.; Gong, C.; Chen, C.; Zhou, S. Etomidate Improves the Antidepressant Effect of Electroconvulsive Therapy by Suppressing Hippocampal Neuronal Ferroptosis via Upregulating BDNF/Nrf2. Mol. Neurobiol. 2023, 60, 6584–6597. [Google Scholar] [CrossRef]
- Zhou, C.; Xue, F.; Xue, S.; Sang, H.; Liu, L.; Wang, Y.; Cai, M.; Zhang, Z.-J.; Tan, Q.; Wang, H.; et al. Electroacupuncture Pretreatment Ameliorates PTSD-Like Behaviors in Rats by Enhancing Hippocampal Neurogenesis via the Keap1/Nrf2 Antioxidant Signaling Pathway. Front. Cell. Neurosci. 2019, 13, 275. [Google Scholar] [CrossRef]
- Morrison, C.D.; Pistell, P.J.; Ingram, D.K.; Johnson, W.D.; Liu, Y.; Fernandez-Kim, S.O.; White, C.L.; Purpera, M.N.; Uranga, R.M.; Bruce-Keller, A.J.; et al. High Fat Diet Increases Hippocampal Oxidative Stress and Cognitive Impairment in Aged Mice: Implications for Decreased Nrf2 Signaling. J. Neurochem. 2010, 114, 1581–1589. [Google Scholar] [CrossRef]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.A.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A Diet Rich in Olive Oil Phenolics Reduces Oxidative Stress in the Heart of SAMP8 Mice by Induction of Nrf2-Dependent Gene Expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Gonzalez-Guardia, L.; Rangel-Zuñiga, O.; Delgado-Casado, N.; Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Caballero, J.; Marin, C.; Gutierrez-Mariscal, F.M.; et al. Postprandial Antioxidant Gene Expression Is Modified by Mediterranean Diet Supplemented with Coenzyme Q10 in Elderly Men and Women. Age (Dordr.) 2013, 35, 159–170. [Google Scholar] [CrossRef]
- Csiszar, A.; Gautam, T.; Sosnowska, D.; Tarantini, S.; Banki, E.; Tucsek, Z.; Toth, P.; Losonczy, G.; Koller, A.; Reglodi, D.; et al. Caloric Restriction Confers Persistent Anti-Oxidative, pro-Angiogenic, and Anti-Inflammatory Effects and Promotes Anti-Aging miRNA Expression Profile in Cerebromicrovascular Endothelial Cells of Aged Rats. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H292–H306. [Google Scholar] [CrossRef]
- Pearson, K.J.; Lewis, K.N.; Price, N.L.; Chang, J.W.; Perez, E.; Cascajo, M.V.; Tamashiro, K.L.; Poosala, S.; Csiszar, A.; Ungvari, Z.; et al. Nrf2 Mediates Cancer Protection but Not Prolongevity Induced by Caloric Restriction. Proc. Natl. Acad. Sci. USA 2008, 105, 2325–2330. [Google Scholar] [CrossRef]
- Ostrom, E.L.; Valencia, A.P.; Marcinek, D.J.; Traustadóttir, T. High Intensity Muscle Stimulation Activates a Systemic Nrf2-Mediated Redox Stress Response. Free Radic. Biol. Med. 2021, 172, 82–89. [Google Scholar] [CrossRef]
- Gounder, S.S.; Kannan, S.; Devadoss, D.; Miller, C.J.; Whitehead, K.J.; Odelberg, S.J.; Firpo, M.A.; Paine, R.; Hoidal, J.R.; Abel, E.D.; et al. Impaired Transcriptional Activity of Nrf2 in Age-Related Myocardial Oxidative Stress Is Reversible by Moderate Exercise Training. PLoS ONE 2012, 7, e45697. [Google Scholar] [CrossRef]
- Eskla, K.-L.; Porosk, R.; Reimets, R.; Visnapuu, T.; Vasar, E.; Hundahl, C.A.; Luuk, H. Hypothermia Augments Stress Response in Mammalian Cells. Free Radic. Biol. Med. 2018, 121, 157–168. [Google Scholar] [CrossRef]
- Orhan, E.; Gündüz, Ö.; Kaya, O.; Öznur, M.; Şahin, E. Transferring the Protective Effect of Remote Ischemic Preconditioning on Skin Flap among Rats by Blood Serum. J. Plast. Surg. Hand Surg. 2019, 53, 198–203. [Google Scholar] [CrossRef]
- Patel, A.D.; Rotenberg, S.; Messer, R.L.W.; Wataha, J.C.; Ogbureke, K.U.E.; Mccloud, V.V.; Lockwood, P.; Hsu, S.; Lewis, J.B. Blue Light Activates Phase 2 Response Proteins and Slows Growth of A431 Epidermoid Carcinoma Xenografts. Anticancer Res. 2014, 34, 6305–6313. [Google Scholar] [PubMed]
- Li, B.; Wang, X. Photobiomodulation Enhances Facial Nerve Regeneration via Activation of PI3K/Akt Signaling Pathway–Mediated Antioxidant Response. Lasers Med. Sci. 2022, 37, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Lettieri-Barbato, D.; Minopoli, G.; Caggiano, R.; Izzo, R.; Santillo, M.; Aquilano, K.; Faraonio, R. Fasting Drives Nrf2-Related Antioxidant Response in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 7780. [Google Scholar] [CrossRef]
- Subramanian, M.; Edwards, L.; Melton, A.; Branen, L.; Herron, A.; Sivasubramanian, M.K.; Monteiro, R.; Stansbury, S.; Balasubramanian, P.; Morris, L.; et al. Non-Invasive Vagus Nerve Stimulation Attenuates Proinflammatory Cytokines and Augments Antioxidant Levels in the Brainstem and Forebrain Regions of Dahl Salt Sensitive Rats. Sci. Rep. 2020, 10, 17576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Dai, Q.; Ma, R. Continuous Theta Burst Stimulation Inhibits Oxidative Stress-Induced Inflammation and Autophagy in Hippocampal Neurons by Activating Glutathione Synthesis Pathway, Improving Cognitive Impairment in Sleep-Deprived Mice. Neuromol. Med. 2024, 26, 40. [Google Scholar] [CrossRef]
- Xu, J.; Tao, L.; Jiang, L.; Lai, J.; Hu, J.; Tang, Z. Moderate Hypothermia Alleviates Sepsis-Associated Acute Lung Injury by Suppressing Ferroptosis Induced by Excessive Inflammation and Oxidative Stress via the Keap1/GSK3β/Nrf2/GPX4 Signaling Pathway. J. Inflamm. Res. 2024, 17, 7687–7704. [Google Scholar] [CrossRef]
- Luo, J.; Yan, R.; Ding, L.; Ning, J.; Chen, M.; Guo, Y.; Liu, J.; Chen, Z.; Zhou, R. Electroacupuncture Attenuates Ventilator-Induced Lung Injury by Modulating the Nrf2/HO-1 Pathway. J. Surg. Res. 2024, 295, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrera, A.; Rangel-Zuñiga, O.A.; Delgado-Lista, J.; Marin, C.; Perez-Martinez, P.; Tasset, I.; Tunez, I.; Quintana-Navarro, G.M.; Lopez-Segura, F.; Luque De Castro, M.D.; et al. The Antioxidants in Oils Heated at Frying Temperature, Whether Natural or Added, Could Protect against Postprandial Oxidative Stress in Obese People. Food Chem. 2013, 138, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.I.; Hassan, R.E.; Sherif, N.M.; Awadallah, S.; Farahat, N.M.; Abdelrahim, D.N.; AlHasan, F.A.; Taneera, J.; Faris, M.E. Changes in Haptoglobin Genotype-Based Gene Expressions upon the Observance of Dawn-to-Dusk Intermittent Fasting: A Prospective Cohort Study on Overweight and Obese Individuals. Front. Nutr. 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, F.H.; Hussein, A.M.; Eid, E.A.; Ammar, O.A.; Khalil, A.A. Effect of Intermittent Fasting on Adriamycin-Induced Nephropathy: Possible Underlying Mechanisms. Tissue Cell 2024, 88, 102360. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Zhu, H.-J.; Zhao, R.-Y.; Zhou, S.-Y.; Wang, M.-Q.; Yang, Y.; Guo, Z.-N. Remote Ischemic Conditioning Attenuates Oxidative Stress and Inflammation via the Nrf2/HO-1 Pathway in MCAO Mice. Redox Biol. 2023, 66, 102852. [Google Scholar] [CrossRef]
- Li, P.; Su, L.; Li, X.; Di, W.; Zhang, X.; Zhang, C.; He, T.; Zhu, X.; Zhang, Y.; Li, Y. Remote Limb Ischemic Postconditioning Protects Mouse Brain against Cerebral Ischemia/Reperfusion Injury via Upregulating Expression of Nrf2, HO-1 and NQO-1 in Mice. Int. J. Neurosci. 2015, 1–8. [Google Scholar] [CrossRef]
- Gao, S.; Zhan, L.; Yang, Z.; Shi, R.; Li, H.; Xia, Z.; Yuan, S.; Wu, Q.; Wang, T.; Yao, S. Remote Limb Ischaemic Postconditioning Protects Against Myocardial Ischaemia/Reperfusion Injury in Mice: Activation of JAK/STAT3-Mediated Nrf2-Antioxidant Signalling. Cell Physiol. Biochem. 2017, 43, 1140–1151. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Li, H.; Xia, Z.; Wu, Q.; Yao, S.; Wang, T.; Yuan, S. Remote Ischemic Postconditioning Protects against Renal Ischemia/Reperfusion Injury by Activation of T-LAK-Cell-Originated Protein Kinase (TOPK)/PTEN/Akt Signaling Pathway Mediated Anti-Oxidation and Anti-Inflammation. Int. Immunopharmacol. 2016, 38, 395–401. [Google Scholar] [CrossRef]
- Zhang, X.; Jizhang, Y.; Xu, X.; Kwiecien, T.D.; Li, N.; Zhang, Y.; Ji, X.; Ren, C.; Ding, Y. Protective Effects of Remote Ischemic Conditioning against Ischemia/Reperfusion-Induced Retinal Injury in Rats. Vis. Neurosci. 2014, 31, 245–252. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Cheng, L.; Han, J.; Mu, J. Hydrogen Sulfide Restores Cardioprotective Effects of Remote Ischemic Preconditioning in Aged Rats via HIF-1α/Nrf2 Signaling Pathway. Korean J. Physiol. Pharmacol. 2021, 25, 239–249. [Google Scholar] [CrossRef]
- He, X.; Yang, X.; Li, G.; Zhao, Y.; Luo, J.; Xu, J.; Zheng, H.; Zhang, L.; Hu, X. Physical Exercise Improves the Neuronal Function in Ischemic Stroke Via Microglial CB2R/P2Y12 Signaling. Mol. Neurobiol. 2025, 62, 2039–2057. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Chen, B.; Wang, X.; Gao, C.; Yu, H. Icariin Enhance Mild Hypothermia-Induced Neuroprotection via Inhibiting the Activation of NF-ΚB in Experimental Ischemic Stroke. Metab. Brain Dis. 2021, 36, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Kan, Y.; Lian, Y.; Dong, S.; Zhao, D.; Shi, J.; Yu, J. Electroacupuncture Attenuates Limb Ischemia-Reperfusion-Induced Lung Injury Via P38 Mitogen-Activated Protein Kinase-Nuclear Factor Erythroid-2-Related Factor-2/Heme Oxygenase Pathway. J. Surg. Res. 2020, 246, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, J.; Han, Y. Electroacupuncture Downregulating Neuronal Ferroptosis in MCAO/R Rats by Activating Nrf2/SLC7A11/GPX4 Axis. Neurochem. Res. 2024, 49, 2105–2119. [Google Scholar] [CrossRef] [PubMed]
- Schanuel, F.S.; Saguie, B.O.; Monte-Alto-Costa, A. Olive Oil Promotes Wound Healing of Mice Pressure Injuries through NOS-2 and Nrf2. Appl. Physiol. Nutr. Metab. 2019, 44, 1199–1208. [Google Scholar] [CrossRef]
- Zhang, Q.; Lai, Y.; Deng, J.; Wang, M.; Wang, Z.; Wang, M.; Zhang, Y.; Yang, X.; Zhou, X.; Jiang, H. Vagus Nerve Stimulation Attenuates Hepatic Ischemia/Reperfusion Injury via the Nrf2/HO-1 Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9549506. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, Y.; Wang, M.; Shao, L.; Deng, C. Activation of Vagovagal Reflex Prevents Hepatic Ischaemia–Reperfusion-Induced Lung Injury via Anti-Inflammatory and Antioxidant Effects. Exp. Physiol. 2021, 106, 2210–2222. [Google Scholar] [CrossRef]
- Dhar, R.; Zhang, L.; Li, Y.; Rana, M.N.; Hu, Z.; Li, Z.; Cui, H.; Tang, H. Electroacupuncture Ameliorates Cardiopulmonary Bypass Induced Apoptosis in Lung via ROS/Nrf2/NLRP3 Inflammasome Pathway. Life Sci. 2019, 238, 116962. [Google Scholar] [CrossRef]
- Arvola, O.; Haapanen, H.; Herajärvi, J.; Anttila, T.; Puistola, U.; Karihtala, P.; Tuominen, H.; Anttila, V.; Juvonen, T. Remote Ischemic Preconditioning Reduces Cerebral Oxidative Stress Following Hypothermic Circulatory Arrest in a Porcine Model. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Bahr, A.C.; Naasani, L.I.S.; De Gregório, E.; Wink, M.R.; Da Rosa Araujo, A.S.; Turck, P.; Dal Lago, P. Photobiomodulation Improves Cell Survival and Death Parameters in Cardiomyocytes Exposed to Hypoxia/Reoxygenation. J. Photochem. B Biol. 2024, 258, 112991. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.-Y.; Zheng, J.; Shan, Y.; Xi, S.; Zhu, Y.; Hu, W.; Lin, Z. Hypothermia Prevents Hippocampal Oxidative Stress and Apoptosis via the GSK-3β/Nrf2/HO-1 Signaling Pathway in a Rat Model of Cardiac Arrest-Induced Brain Damage. Neurol. Res. 2020, 42, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, C.-S.; Hua, R.; Zhao, H.; Tang, Z.-R.; Mei, X.; Zhang, M.-Y.; Cui, J. Mild Hypothermia Attenuates Mitochondrial Oxidative Stress by Protecting Respiratory Enzymes and Upregulating MnSOD in a Pig Model of Cardiac Arrest. PLoS ONE 2012, 7, e35313. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, T.-K.; Kim, D.W.; Shin, M.C.; Cho, J.H.; Lee, J.-C.; Tae, H.-J.; Park, J.H.; Hong, S.; Lee, C.-H.; et al. Therapeutic Hypothermia after Cardiac Arrest Attenuates Hindlimb Paralysis and Damage of Spinal Motor Neurons and Astrocytes through Modulating Nrf2/HO-1 Signaling Pathway in Rats. Cells 2023, 12, 414. [Google Scholar] [CrossRef]
- Jawad, A.; Yoo, Y.-J.; Cho, J.-H.; Yoon, J.C.; Tian, W.; Islam, M.S.; Lee, E.-Y.; Shin, H.-Y.; Kim, S.E.; Kim, K.; et al. Therapeutic Hypothermia Effect on Asphyxial Cardiac Arrest-Induced Renal Ischemia/Reperfusion Injury via Change of Nrf2/HO-1 Levels. Exp. Ther. Med. 2021, 22, 1031. [Google Scholar] [CrossRef]
- Guan, R.; Wen, X.; Leung, C.H.; Ciano-Oliveira, C.D.; Lam, S.; Dai, S.Y.; Karbassi, F.; Mauro, A.; Wang, Y.; Rotstein, O. Plasma Obtained Following Murine Hindlimb Ischemic Conditioning Protects against Oxidative Stress in Zebrafish Models through Activation of Nrf2a and Downregulation of Duox. PLoS ONE 2021, 16, e0260442. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally Relevant Concentrations of Resveratrol and Hydroxytyrosol Mitigate Oxidative Burst of Human Granulocytes and Monocytes and the Production of Pro-Inflammatory Mediators in LPS-Stimulated RAW 264.7 Macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Salman, S.; Guermonprez, C.; Peno-Mazzarino, L.; Lati, E.; Rousseaud, A.; Declercq, L.; Kerdine-Römer, S. Photobiomodulation Controls Keratinocytes Inflammatory Response through Nrf2 and Reduces Langerhans Cells Activation. Antioxidants 2023, 12, 766. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.; Zhao, Y.; Feng, Z.; Liu, Y. Elucidating the Molecular Mechanisms behind the Therapeutic Impact of Median Nerve Stimulation on Cognitive Dysfunction Post-Traumatic Brain Injury. Exp. Gerontol. 2024, 194, 112500. [Google Scholar] [CrossRef]
- Yan, C.; Mao, J.; Yao, C.; Liu, Y.; Yan, H.; Jin, W. Neuroprotective Effects of Mild Hypothermia against Traumatic Brain Injury by the Involvement of the Nrf2/ARE Pathway. Brain Behav. 2022, 12, e2686. [Google Scholar] [CrossRef]
- Dai, S.; Wei, J.; Zhang, H.; Luo, P.; Yang, Y.; Jiang, X.; Fei, Z.; Liang, W.; Jiang, J.; Li, X. Intermittent Fasting Reduces Neuroinflammation in Intracerebral Hemorrhage through the Sirt3/Nrf2/HO-1 Pathway. J. Neuroinflamm. 2022, 19, 122. [Google Scholar] [CrossRef]
- Leung, C.H.; Caldarone, C.A.; Guan, R.; Wen, X.-Y.; Ailenberg, M.; Kapus, A.; Szaszi, K.; Rotstein, O.D. Nuclear Factor (Erythroid-Derived 2)-Like 2 Regulates the Hepatoprotective Effects of Remote Ischemic Conditioning in Hemorrhagic Shock. Antioxid. Redox Signal. 2019, 30, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shi, J.; Gong, L.; Dong, S.; Xu, Y.; Zhang, Y.; Cao, X.; Wu, L. Role of Nrf2/ARE Pathway in Protective Effect of Electroacupuncture against Endotoxic Shock-Induced Acute Lung Injury in Rabbits. PLoS ONE 2014, 9, e104924. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Pan, H.; Huang, L.; Sun, X.; He, C.; Wei, Q. Comparison of the Effects of Constraint-Induced Movement Therapy and Unconstraint Exercise on Oxidative Stress and Limb Function—A Study on Human Patients and Rats with Cerebral Infarction. Brain Sci. 2022, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, S.; Liu, K.; Rajah, G.B.; Zhang, A.; Han, R.; Liu, Y.; Huang, Q.; Li, H.; Ding, Y.; et al. Enhanced Oxidative Stress Response and Neuroprotection of Combined Limb Remote Ischemic Conditioning and Atorvastatin after Transient Ischemic Stroke in Rats. Brain Circ. 2017, 3, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Setoyama, K.; Takada, S.; Nakanishi, K.; Terashi, T.; Norimatsu, K.; Tani, A.; Sakakima, H.; Maruyama, I.; Tancharoen, S.; et al. Preconditioning Exercise in Rats Attenuates Early Brain Injury Resulting from Subarachnoid Hemorrhage by Reducing Oxidative Stress, Inflammation, and Neuronal Apoptosis. Mol. Neurobiol. 2021, 58, 5602–5617. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Yao, Y.; Li, L. Delayed Remote Ischemic Preconditioning Produces an Additive Cardioprotection to Sevoflurane Postconditioning Through an Enhanced Heme Oxygenase 1 Level Partly Via Nuclear Factor Erythroid 2-Related Factor 2 Nuclear Translocation. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 558–566. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xin, Y.; Zhang, J.; Wang, S.; Yang, Z.; Liu, C. Hydrogen Sulfide Alleviates the Anxiety-like and Depressive-like Behaviors of Type 1 Diabetic Mice via Inhibiting Inflammation and Ferroptosis. Life Sci. 2021, 278, 119551. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Y.; Xiong, Z.; Cheng, H.; Du, Y.; Xiong, Y.; Lv, J.; Huang, W.; Qiu, K.; Zhang, S. Bombesin Protects Myocardium against Ischemia/Reperfusion Injury via Activation of the Keap1-Nrf2-HO-1 Signaling Pathway. Peptides 2024, 180, 171279. [Google Scholar] [CrossRef]
- Longo, L.; Sinigaglia-Fratta, L.X.; Weber, G.R.; Janz-Moreira, A.; Kretzmann, N.A.; Grezzana-Filho, T.D.J.M.; Possa-Marroni, N.; Corso, C.O.; Schmidt-Cerski, C.T.; Reverbel-da-Silveira, T.; et al. Hypothermia Is Better than Ischemic Preconditioning for Preventing Early Hepatic Ischemia/Reperfusion in Rats. Ann. Hepatol. 2016, 15, 110–120. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, Y.; Xin, Y.; Hu, X. Vagus Nerve Stimulation Attenuates Acute Kidney Injury Induced by Hepatic Ischemia/Reperfusion Injury in Rats. Sci. Rep. 2022, 12, 21662. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Zhang, X.; Li, J.; Wang, Y.; Fan, Y.; Shi, R. Limb Ischemic Preconditioning Protects Endothelium from Oxidative Stress by Enhancing Nrf2 Translocation and Upregulating Expression of Antioxidases. PLoS ONE 2015, 10, e0128455. [Google Scholar] [CrossRef]
- Yang, H.-L.; Lin, M.-W.; Korivi, M.; Wu, J.-J.; Liao, C.-H.; Chang, C.-T.; Liao, J.-W.; Hseu, Y.-C. Coenzyme Q0 Regulates NFκB/AP-1 Activation and Enhances Nrf2 Stabilization in Attenuation of LPS-Induced Inflammation and Redox Imbalance: Evidence from in Vitro and in Vivo Studies. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2016, 1859, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.-H.; Kim, Y.-D.; Seo, J.H. High Vulnerability of Oligodendrocytes to Oxidative Stress Induced by Ultrafine Urban Particles. Antioxidants 2020, 10, 4. [Google Scholar] [CrossRef]
- Moubarak, M.M.; Pagano Zottola, A.C.; Larrieu, C.M.; Cuvellier, S.; Daubon, T.; Martin, O.C.B. Exploring the Multifaceted Role of NRF2 in Brain Physiology and Cancer: A Comprehensive Review. Neuro-Oncol. Adv. 2024, 6, vdad160. [Google Scholar] [CrossRef]
- Lim, J.L.; Van der Pol, S.M.A.; Baron, W.; McCord, J.M.; De Vries, H.E.; Van Horssen, J. Protandim Protects Oligodendrocytes against an Oxidative Insult. Antioxidants 2016, 5, 30. [Google Scholar] [CrossRef]
- Teske, N.; Liessem, A.; Fischbach, F.; Clarner, T.; Beyer, C.; Wruck, C.; Fragoulis, A.; Tauber, S.C.; Victor, M.; Kipp, M. Chemical Hypoxia-Induced Integrated Stress Response Activation in Oligodendrocytes Is Mediated by the Transcription Factor Nuclear Factor (Erythroid-Derived 2)-like 2 (NRF2). J. Neurochem. 2018, 144, 285–301. [Google Scholar] [CrossRef]
- De Nuccio, C.; Bernardo, A.; Troiano, C.; Brignone, M.S.; Falchi, M.; Greco, A.; Rosini, M.; Basagni, F.; Lanni, C.; Serafini, M.M.; et al. NRF2 and PPAR-γ Pathways in Oligodendrocyte Progenitors: Focus on ROS Protection, Mitochondrial Biogenesis and Promotion of Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 7216. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative Stress and Impaired Oligodendrocyte Precursor Cell Differentiation in Neurological Disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.; Chaudhary, P.; Sahu, M.; Jaiswal, S.; Awasthi, S.; Awasthi, Y.C. Role of 4-Hydroxynonenal in Chemopreventive Activities of Sulforaphane. Free Radic. Biol. Med. 2012, 52, 2177–2185. [Google Scholar] [CrossRef]
- Xie, S.; Zou, W.; Liu, S.; Yang, Q.; Hu, T.; Zhu, W.; Tang, H.; Wang, C. Site 1 Protease Aggravates Acute Kidney Injury by Promoting Tubular Epithelial Cell Ferroptosis through SIRT3-SOD2-mtROS Signaling. FEBS J. 2024, 291, 1575–1592. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, R.; Maccallini, C.; Bellezza, I. Activators of Nrf2 to Counteract Neurodegenerative Diseases. Antioxidants 2023, 12, 778. [Google Scholar] [CrossRef]
- Chen, W.-T.; Dodson, M. The Untapped Potential of Targeting NRF2 in Neurodegenerative Disease. Front. Aging 2023, 4, 1270838. [Google Scholar] [CrossRef] [PubMed]
- Pisani, F.; Castagnola, V.; Simone, L.; Loiacono, F.; Svelto, M.; Benfenati, F. Role of Pericytes in Blood–Brain Barrier Preservation during Ischemia through Tunneling Nanotubes. Cell Death Dis. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Kerkar, S.; Speyer, C.; Tyburski, J.; Steffes, C. Reactive Oxygen Metabolites Induce a Biphasic Contractile Response in Microvascular Lung Pericytes. J. Trauma 2001, 51, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin by Down-Regulating NF-κB and Elevating Nrf2, Reduces Brain Edema and Neurological Dysfunction after Cerebral I/R. Microvasc. Res. 2016, 106, 117–127. [Google Scholar] [CrossRef]
- Kunze, R.; Urrutia, A.; Hoffmann, A.; Liu, H.; Helluy, X.; Pham, M.; Reischl, S.; Korff, T.; Marti, H.H. Dimethyl Fumarate Attenuates Cerebral Edema Formation by Protecting the Blood-Brain Barrier Integrity. Exp. Neurol. 2015, 266, 99–111. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Mo, Y.; Li, R.; Huang, S.; Zhang, A.; Ni, X.; Dai, Q.; Wang, J. DCA Protects against Oxidation Injury Attributed to Cerebral Ischemia-Reperfusion by Regulating Glycolysis through PDK2-PDH-Nrf2 Axis. Oxid. Med. Cell. Longev. 2021, 2021, 5173035. [Google Scholar] [CrossRef]
- Zhao, J.; Moore, A.N.; Redell, J.B.; Dash, P.K. Enhancing Expression of Nrf2-Driven Genes Protects the Blood Brain Barrier after Brain Injury. J. Neurosci. 2007, 27, 10240–10248. [Google Scholar] [CrossRef]
- Sakuma, R.; Kobayashi, M.; Kobashi, R.; Onishi, M.; Maeda, M.; Kataoka, Y.; Imaoka, S. Brain Pericytes Acquire Stemness via the Nrf2-Dependent Antioxidant System. Stem Cells 2022, 40, 641–654. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Wang, W.; Liu, M.; Bao, Y. Differential Effects of Sulforaphane in Regulation of Angiogenesis in a Co-Culture Model of Endothelial Cells and Pericytes. Oncol. Rep. 2017, 37, 2905–2912. [Google Scholar] [CrossRef]
- Li, J.-W.; Xia, L.-J.; Cui, C.-T.; Cheng, J.; Xia, Y.-B. Mechanism of Electroacupuncture in Treating Uterine Endometrial Fibrosis in Intrauterine Adhesions Rats Based on Wnt/β-Catenin Pathway-Mediated Epithelial-Mesenchymal Transition. Zhen Ci Yan Jiu 2024, 49, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Yemisci, M.; Gursoy-Ozdemir, Y.; Vural, A.; Can, A.; Topalkara, K.; Dalkara, T. Pericyte Contraction Induced by Oxidative-Nitrative Stress Impairs Capillary Reflow despite Successful Opening of an Occluded Cerebral Artery. Nat. Med. 2009, 15, 1031–1037. [Google Scholar] [CrossRef]
- Andersson-Sjöland, A.; Karlsson, J.C.; Rydell-Törmänen, K. ROS-Induced Endothelial Stress Contributes to Pulmonary Fibrosis through Pericytes and Wnt Signaling. Lab. Investig. 2016, 96, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Tambo, W.; Powell, K.; Wadolowski, S.; Unadkat, P.; Chang, E.H.; LeDoux, C.; Sciubba, D.; Wang, P.; Huerta, P.; Li, C. Vasoactive Neuropeptide Dysregulation: A Novel Mechanism of Microvascular Dysfunction in Vascular Cognitive Impairment. bioRxiv 2025, 2025.04.25.650644. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 Is a Critical Regulator of the Innate Immune Response and Survival during Experimental Sepsis. J. Clin. Investig. 2016, 116, 984–995. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxidative. Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Ercan, I.; Isci, K.B.; Olcum, M.; Tastan, B.; Gonul, C.P.; Genc, K.; Genc, S. Sulforaphane Inhibits NLRP3 Inflammasome Activation in Microglia through Nrf2-Mediated MiRNA Alteration. Immunol. Lett. 2021, 233, 20–30. [Google Scholar] [CrossRef]
- Tastan, B.; Arioz, B.I.; Tufekci, K.U.; Tarakcioglu, E.; Gonul, C.P.; Genc, K.; Genc, S. Dimethyl Fumarate Alleviates NLRP3 Inflammasome Activation in Microglia and Sickness Behavior in LPS-Challenged Mice. Front. Immunol. 2021, 12, 737065. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Gao, Z.; Luo, K.; Hu, Y.; Niu, Y.; Zhu, X.; Li, S.; Zhang, H. Melatonin Alleviates Chronic Stress-Induced Hippocampal Microglia Pyroptosis and Subsequent Depression-like Behaviors by Inhibiting Cathepsin B/NLRP3 Signaling Pathway in Rats. Transl. Psychiatry 2024, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Wang, Y.; Hu, R. Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid. Redox Signal. 2017, 26, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H. The Influence of Systemic Inflammation on Inflammation in the Brain: Implications for Chronic Neurodegenerative Disease. Brain Behav. Immun. 2004, 18, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Zipp, F.; Aktas, O. The Brain as a Target of Inflammation: Common Pathways Link Inflammatory and Neurodegenerative Diseases. Trends. Neurosci. 2006, 29, 518–527. [Google Scholar] [CrossRef]
- Barros, L.F. How Expensive Is the Astrocyte? J. Cereb. Blood Flow Metab. 2022, 42, 738–745. [Google Scholar] [CrossRef]
- Narayanan, S.V.; Dave, K.R.; Perez-Pinzon, M.A. Ischemic Preconditioning Protects Astrocytes against Oxygen Glucose Deprivation Via the Nuclear Erythroid 2-Related Factor 2 Pathway. Transl. Stroke Res. 2018, 9, 99–109. [Google Scholar] [CrossRef]
- Czyżewski, W.; Mazurek, M.; Sakwa, L.; Szymoniuk, M.; Pham, J.; Pasierb, B.; Litak, J.; Czyżewska, E.; Turek, M.; Piotrowski, B.; et al. Astroglial Cells: Emerging Therapeutic Targets in the Management of Traumatic Brain Injury. Cells 2024, 13, 148. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein Misfolding and Aggregation: Implications in Parkinson’s Disease Pathogenesis. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef]
- Chinta, S.J.; Mallajosyula, J.K.; Rane, A.; Andersen, J.K. Mitochondrial Alpha-Synuclein Accumulation Impairs Complex I Function in Dopaminergic Neurons and Results in Increased Mitophagy In Vivo. Neurosci. Lett. 2010, 486, 235–239. [Google Scholar] [CrossRef]
- Brown, D.R. α-Synuclein as a Ferrireductase. Biochem. Soc. Trans. 2013, 41, 1513–1517. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S.G. Oxidative Stress in Amyotrophic Lateral Sclerosis: Pathophysiology and Opportunities for Pharmacological Intervention. Oxid. Med. Cell. Longev. 2020, 2020, 5021694. [Google Scholar] [CrossRef]
- Hemerková, P.; Vališ, M. Role of Oxidative Stress in the Pathogenesis of Amyotrophic Lateral Sclerosis: Antioxidant Metalloenzymes and Therapeutic Strategies. Biomolecules 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, B.; Zheng, L.; Perry, G.; Smith, M.A.; Zhu, X. The Role of Abnormal Mitochondrial Dynamics in the Pathogenesis of Alzheimer’s Disease. J. Neurochem. 2009, 109, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; et al. Brain Regional Correspondence Between Alzheimer’s Disease Histopathology and Biomarkers of Protein Oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hou, S.; Jiang, J.; Sekutowicz, M.; Kelly, J.; Bacskai, B.J. Rapid Cell Death Is Preceded by Amyloid Plaque-Mediated Oxidative Stress. Proc. Natl. Acad. Sci. USA 2013, 110, 7904–7909. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Hong, X.; Huang, L.; Lei, F.; Li, T.; Luo, Y.; Zeng, M.; Wang, Z. The Role and Pathogenesis of Tau Protein in Alzheimer’s Disease. Biomolecules 2025, 15, 824. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Li, P.; Wei, N.; Zhao, Z.; Liang, H.; Ji, X.; Chen, W.; Xue, M.; Wei, J. The Ambiguous Relationship of Oxidative Stress, Tau Hyperphosphorylation, and Autophagy Dysfunction in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 352723. [Google Scholar] [CrossRef]
- Szabo, L.; Eckert, A.; Grimm, A. Insights into Disease-Associated Tau Impact on Mitochondria. Int. J. Mol. Sci. 2020, 21, 6344. [Google Scholar] [CrossRef]
- Pérez, M.J.; Jara, C.; Quintanilla, R.A. Contribution of Tau Pathology to Mitochondrial Impairment in Neurodegeneration. Front. Neurosci. 2018, 12, 441. [Google Scholar] [CrossRef]
- Unadkat, P.; Rebeiz, T.; Ajmal, E.; De Souza, V.; Xia, A.; Jinu, J.; Powell, K.; Li, C. Neurobiological Mechanisms Underlying Psychological Dysfunction After Brain Injuries. Cells 2025, 14, 74. [Google Scholar] [CrossRef]
- Ren, H.; Han, R.; Liu, X.; Wang, L.; Koehler, R.C.; Wang, J. Nrf2-BDNF-TrkB Pathway Contributes to Cortical Hemorrhage-Induced Depression, but Not Sex Differences. J. Cereb. Blood Flow Metab. 2021, 41, 3288–3301. [Google Scholar] [CrossRef] [PubMed]
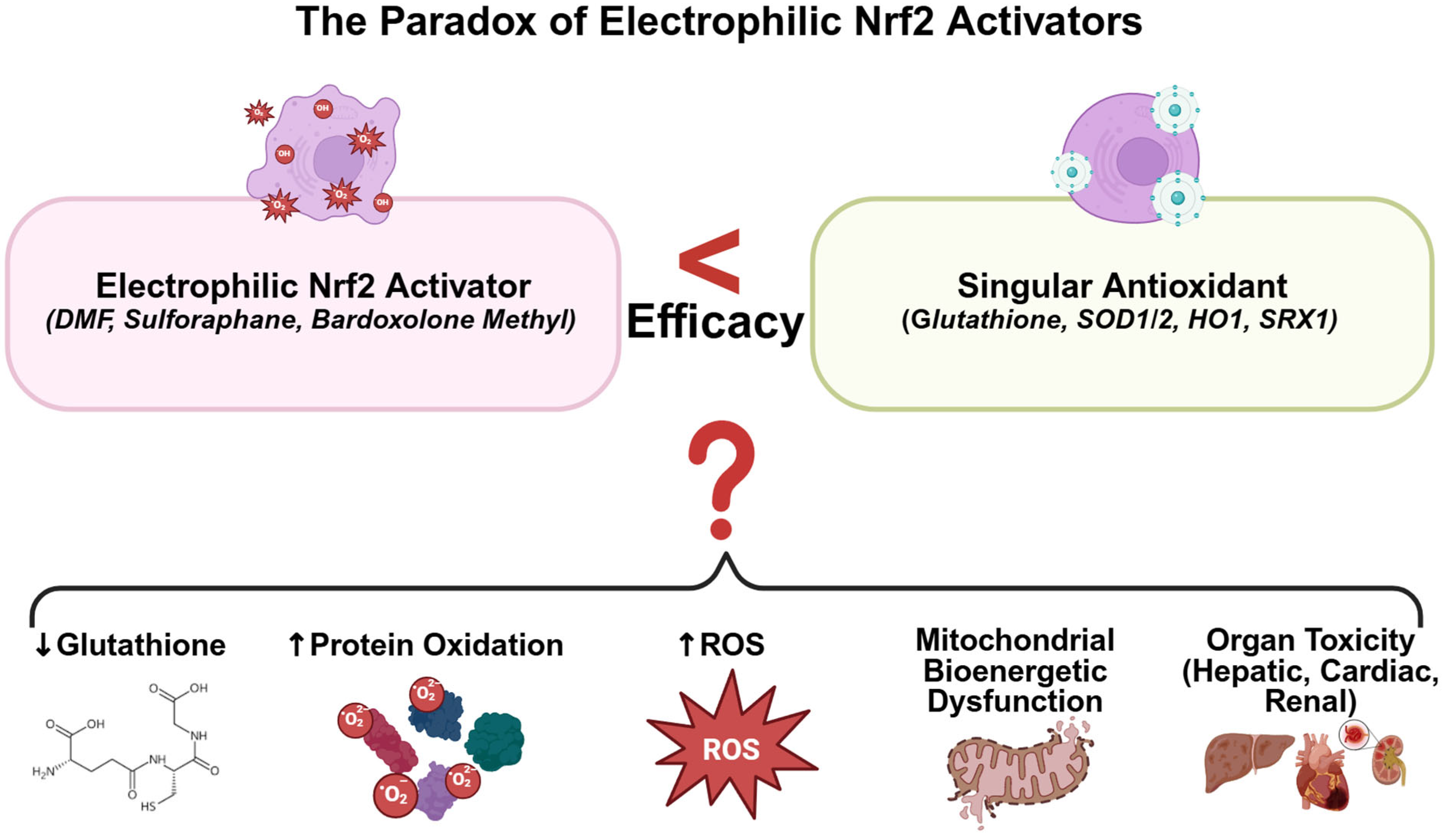
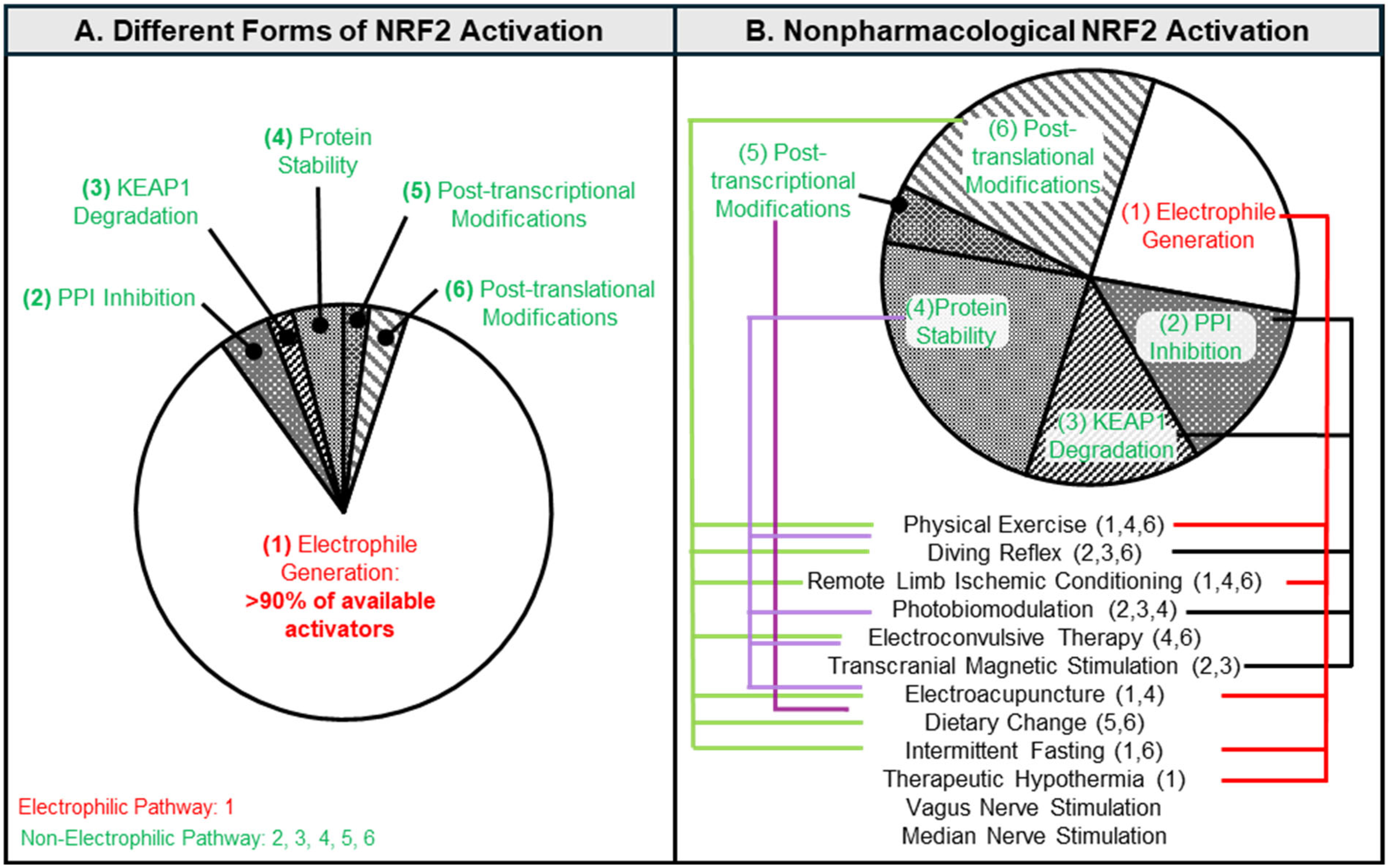
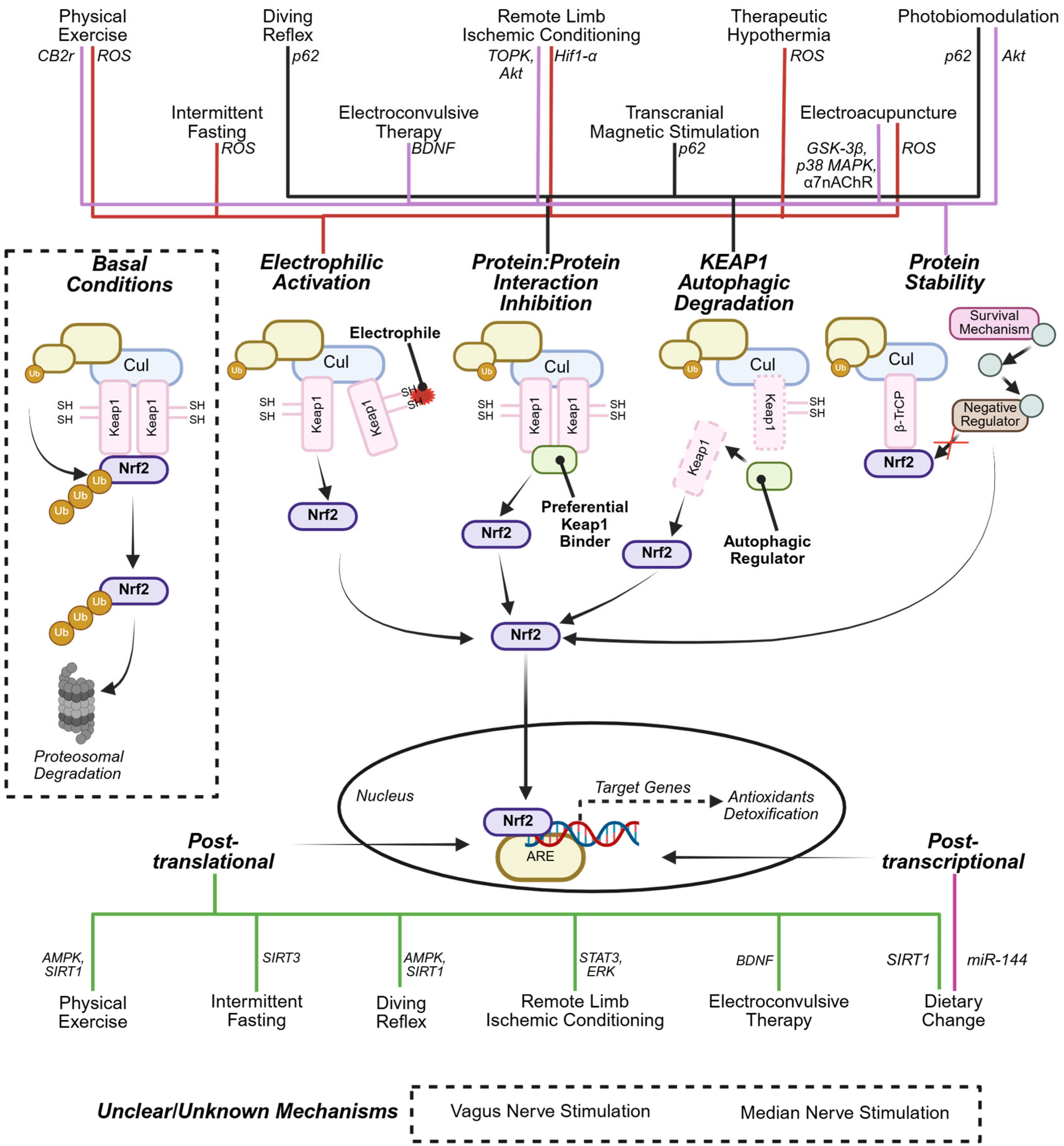
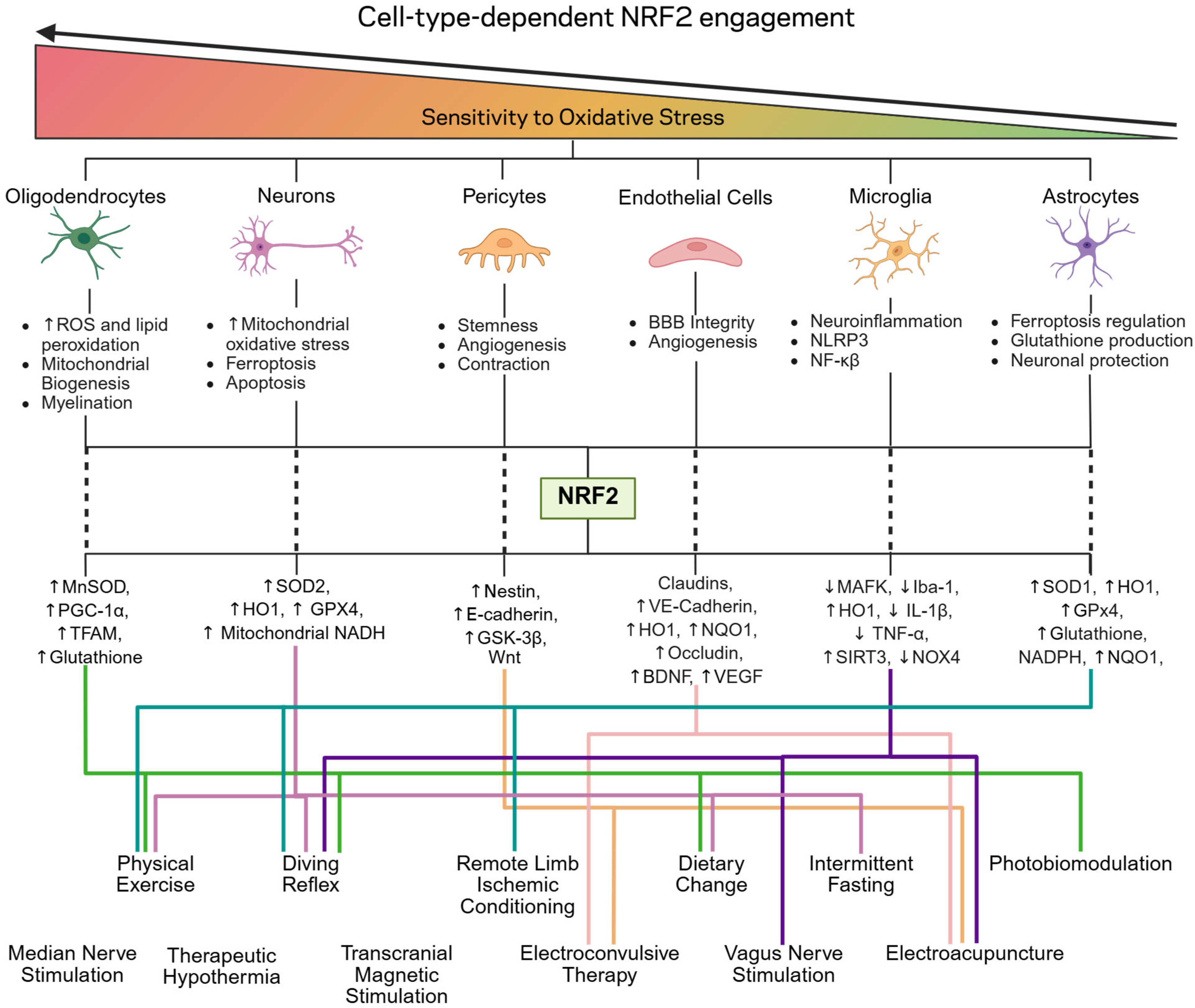
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Powell, K.; Giliberto, L.; LeDoux, C.; d’Abramo, C.; Sciubba, D.; Al Abed, Y. Non-Electrophilic Activation of NRF2 in Neurological Disorders: Therapeutic Promise of Non-Pharmacological Strategies. Antioxidants 2025, 14, 1047. https://doi.org/10.3390/antiox14091047
Li C, Powell K, Giliberto L, LeDoux C, d’Abramo C, Sciubba D, Al Abed Y. Non-Electrophilic Activation of NRF2 in Neurological Disorders: Therapeutic Promise of Non-Pharmacological Strategies. Antioxidants. 2025; 14(9):1047. https://doi.org/10.3390/antiox14091047
Chicago/Turabian StyleLi, Chunyan, Keren Powell, Luca Giliberto, Christopher LeDoux, Cristina d’Abramo, Daniel Sciubba, and Yousef Al Abed. 2025. "Non-Electrophilic Activation of NRF2 in Neurological Disorders: Therapeutic Promise of Non-Pharmacological Strategies" Antioxidants 14, no. 9: 1047. https://doi.org/10.3390/antiox14091047
APA StyleLi, C., Powell, K., Giliberto, L., LeDoux, C., d’Abramo, C., Sciubba, D., & Al Abed, Y. (2025). Non-Electrophilic Activation of NRF2 in Neurological Disorders: Therapeutic Promise of Non-Pharmacological Strategies. Antioxidants, 14(9), 1047. https://doi.org/10.3390/antiox14091047







