Widely Targeted Metabolomics Analysis Reveals Developmental Shifts in Antioxidants and Functional Peptides in Akebia trifoliata
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurement of Fruit Morphological Traits
2.3. Sample Processing and Extraction
2.4. Quantification of the Total Phenolics and Flavonoids Content
2.5. Determination of Antioxidant Activities
2.6. Determination of α-Glucosidase Inhibitory Activity
2.7. UPLC-MS/MS Metabolomics Analysis
2.7.1. UPLC Conditions
2.7.2. ESI-Q TRAP-MS/MS
2.8. Metabolite Identification and Quantification
2.9. Statistical Analysis
3. Results
3.1. Morphological Characterization of Fruits
3.2. Dynamic Changes in Total Phenolics, Total Flavonoids, Antioxidant Activities, and α-Glucosidase Inhibitory Activity at Different Fruit Growth Stages
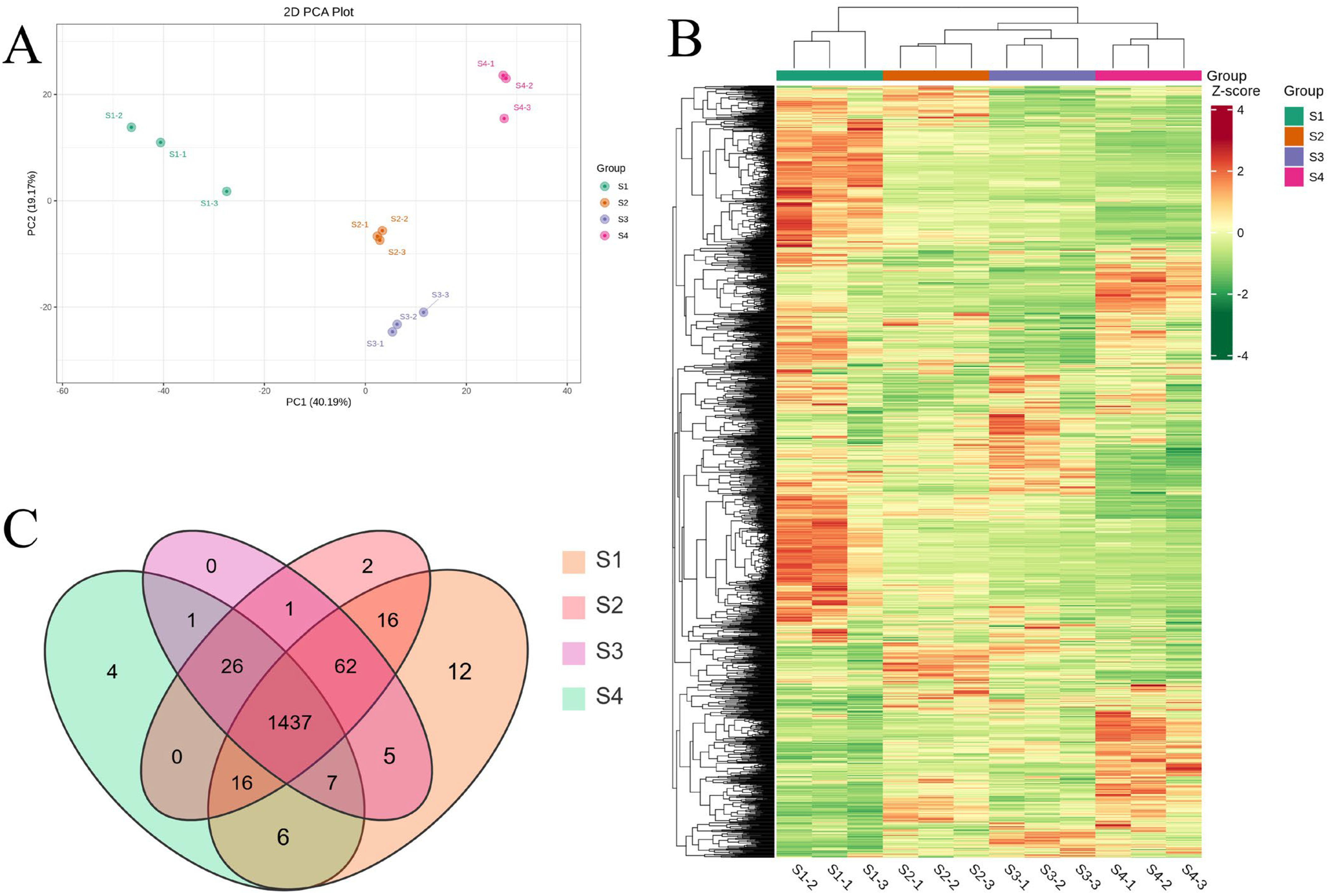
3.3. Metabolite Profiles of A. trifoliata Fruit
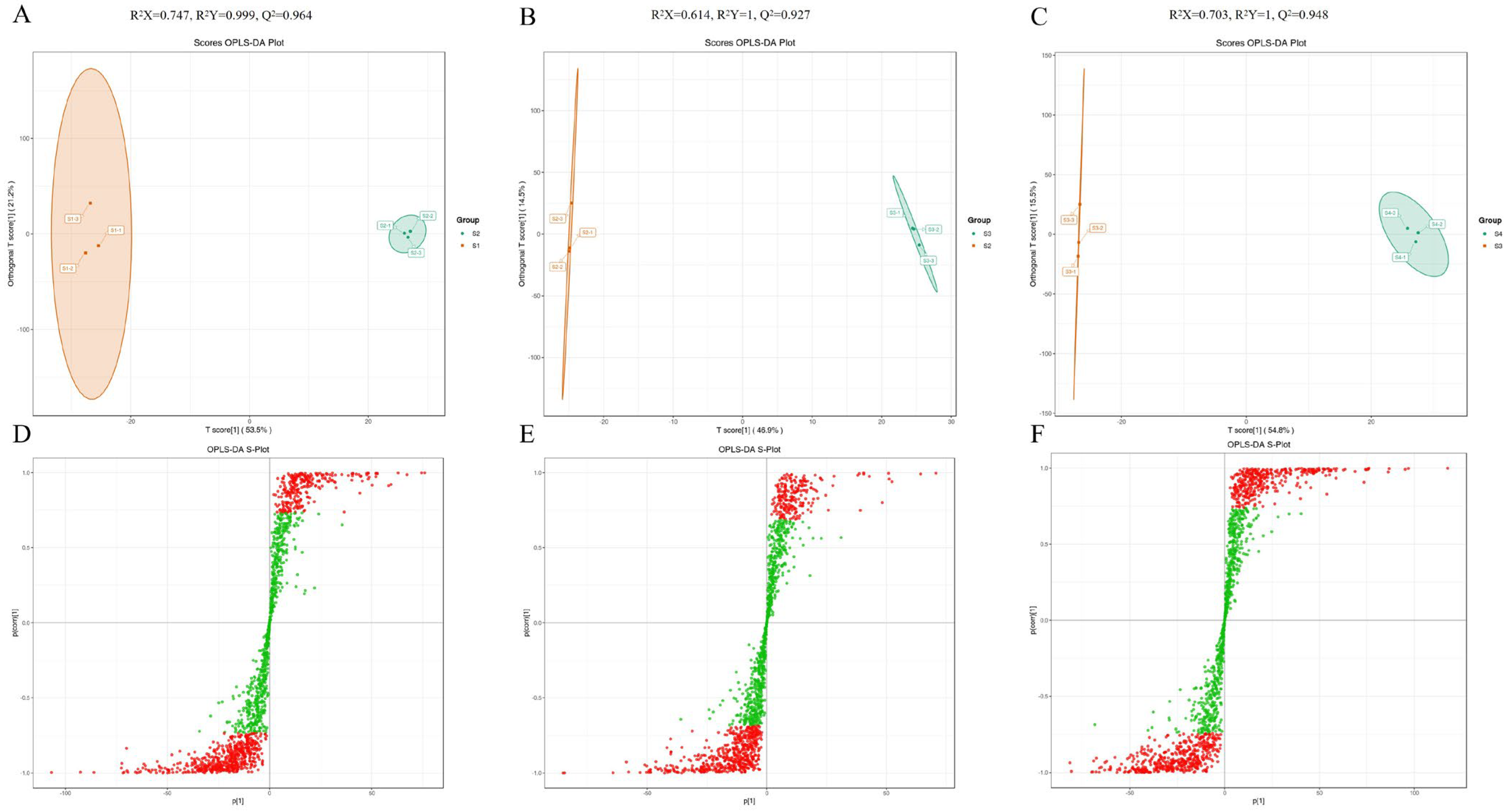
3.4. Multivariate Statistical Analysis of Metabolites
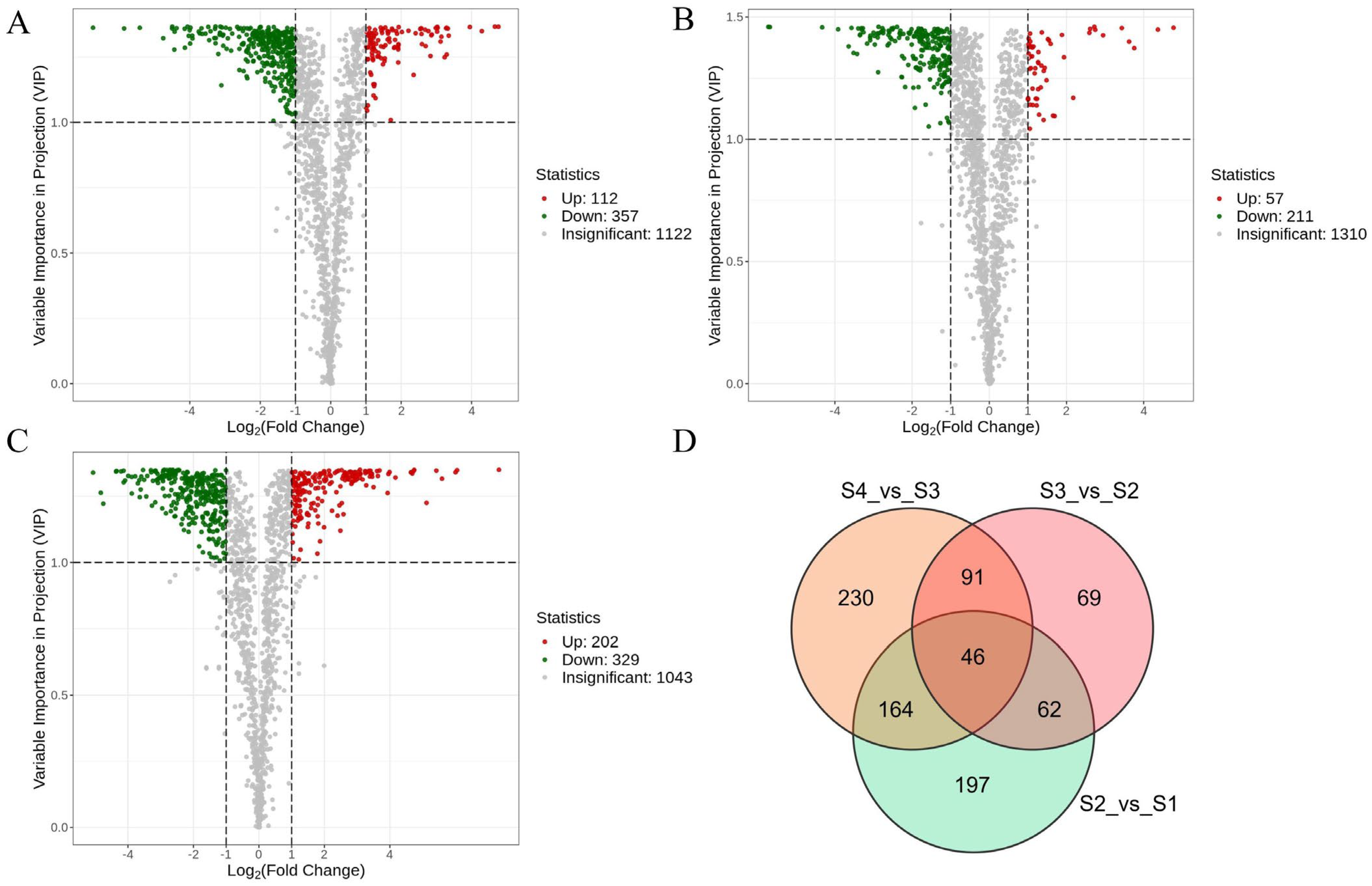
3.5. Differential Metabolite Screening
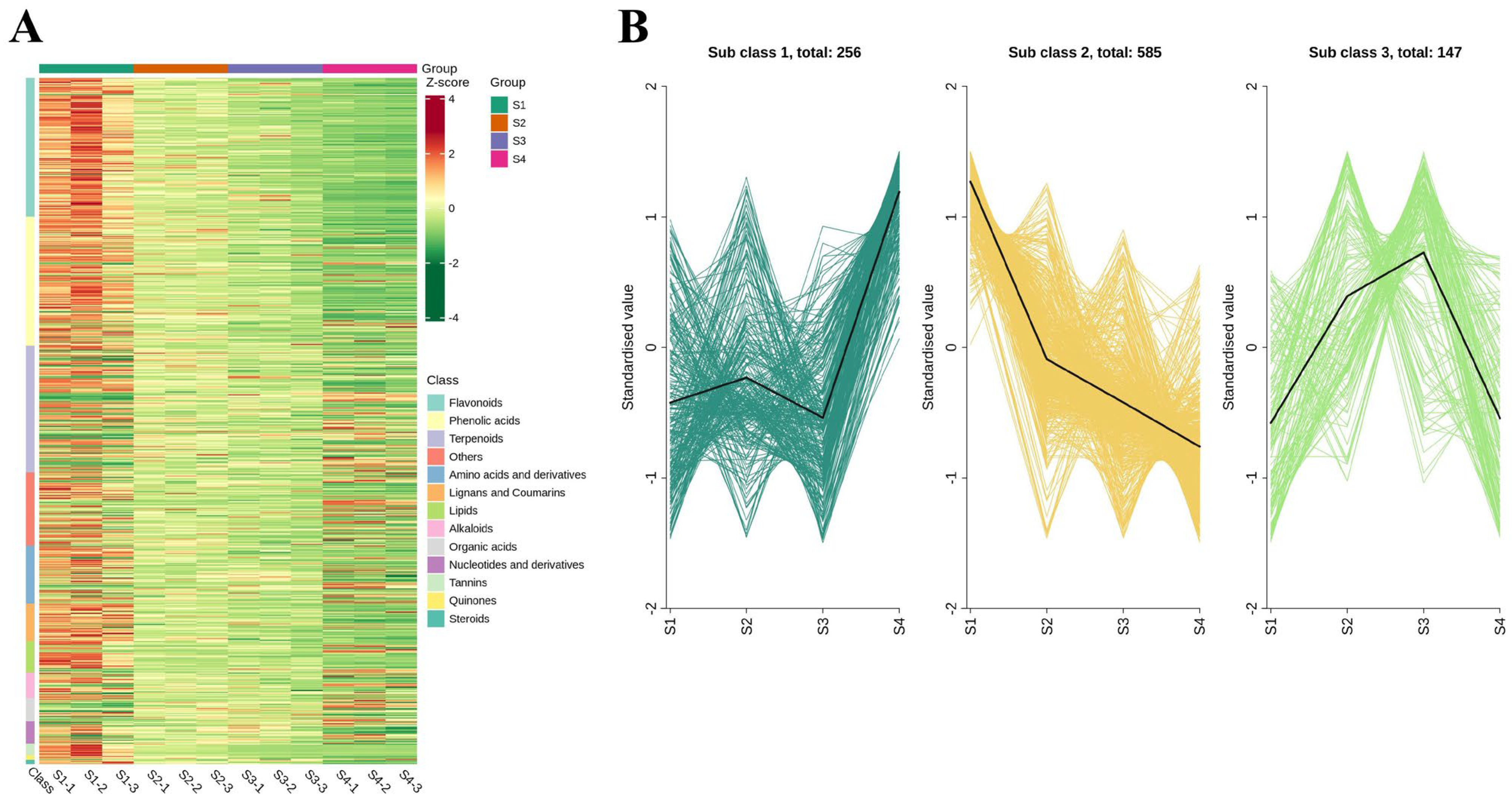
3.6. Dynamics of the Different Metabolites During Fruit Maturation
3.7. Functional Oligopeptide Prediction in A. trifoliata Fruit
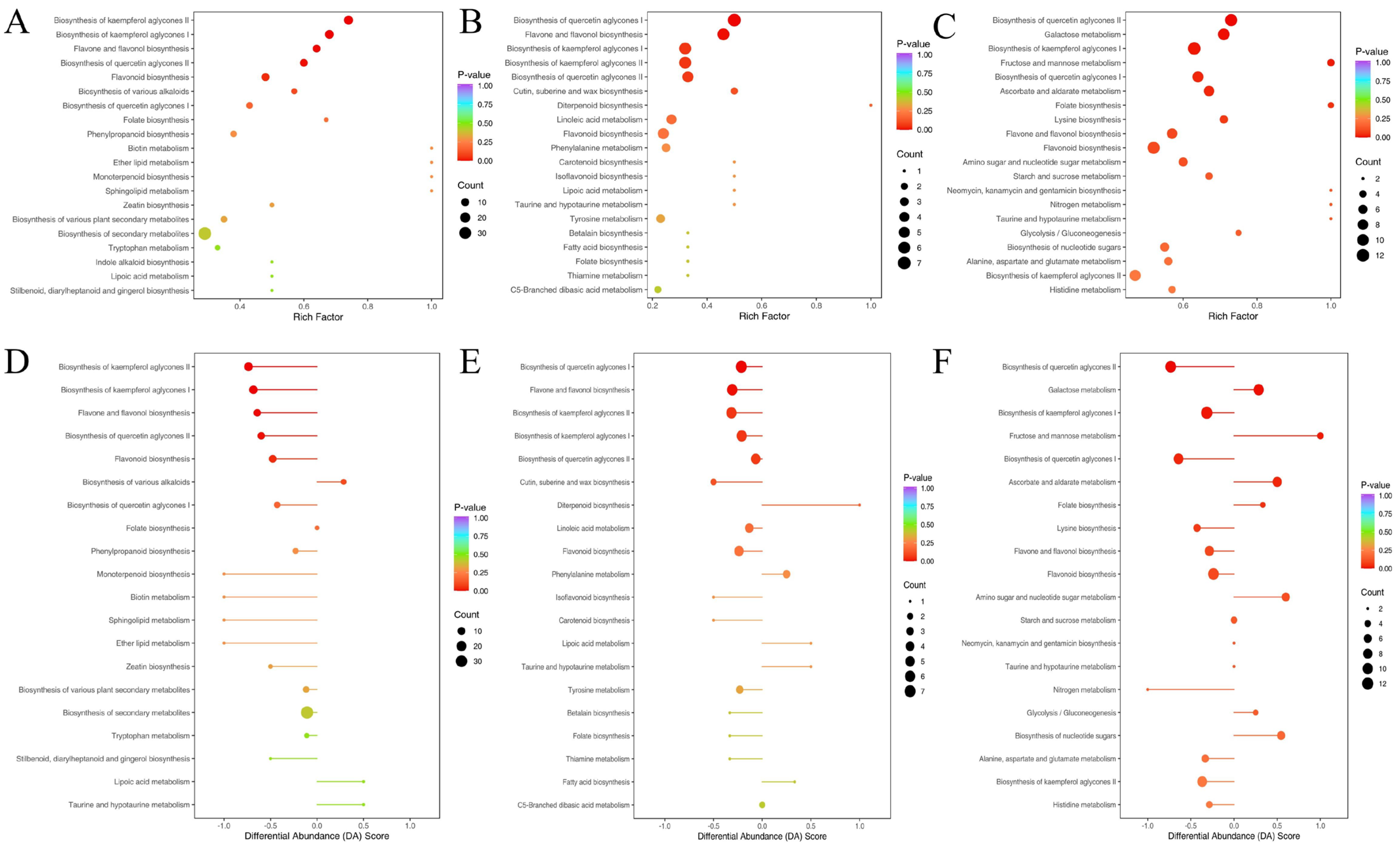
3.8. KEGG Annotation and Enrichment Analysis of Differential Metabolism
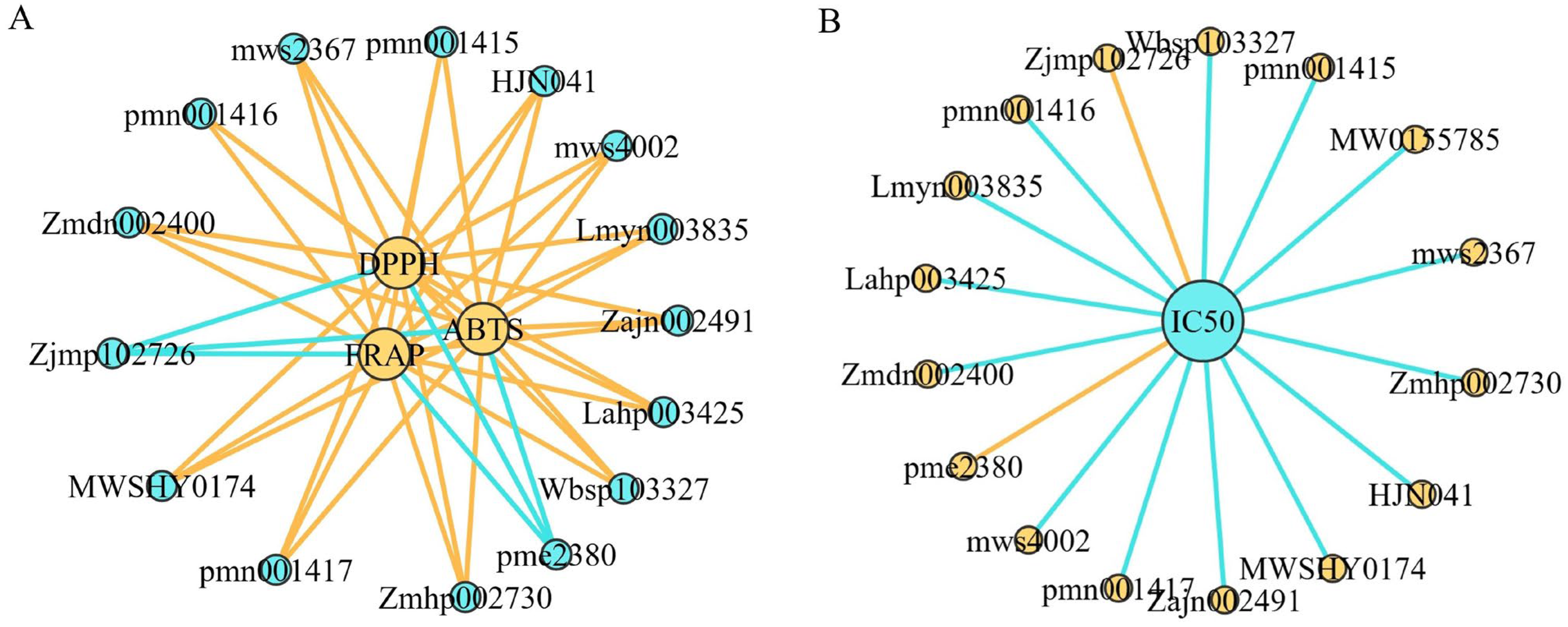
3.9. Correlation Analysis of Secondary Metabolites in A. trifoliata and Antioxidant Activity, α-Glucosidase Inhibitory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, S.Y.; Feng, C.; Gao, P.X.; Li, T.J.; Jia, T.J.; Huang, H. Germplasm resources and genetic improvement of Akebia: A new fruit crop in China. Plant Divers. 2023, 45, 712–721. [Google Scholar] [CrossRef]
- Huang, P.; Zang, F.; Li, C.; Lin, F.; Zang, D.; Li, B.; Zheng, Y. The Akebia Genus as a Novel Forest Crop: A Review of Its Genetic Resources, Nutritional Components, Biosynthesis, and Biological Studies. Front. Plant Sci. 2022, 13, 936571. [Google Scholar] [CrossRef]
- Zou, S.; Gao, P.; Jia, T.; Huang, H. Physicochemical Characteristics and Nutritional Composition during Fruit Ripening of Akebia trifoliata (Lardizabalaceae). Horticulturae 2022, 8, 326. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Gao, T.; Yan, N.N.; Duan, Z.H.; Tang, Z.Z.; Zhou, L.J.; Chen, T.; Feng, S.L.; Ding, C.B.; Yuan, S.; et al. Characterization and Anti-Aging Activity of Polysaccharides from Akebia trifoliata Fruit Separated by an Aqueous Two-Phase System. Plant Foods Hum. Nutr. 2023, 78, 154–159. [Google Scholar] [CrossRef]
- Huang, H.W.; Zou, S.Y.; Cheng, C.S. Domestication and breeding strategy of wild fruit trees on track of plant introduction and domestication history. J. Plant Genet. Resour. 2021, 22, 1463–1473. [Google Scholar]
- Ma, B.; Yang, S.; Tan, T.; Li, J.; Zhang, X.; Ouyang, H.; He, M.; Feng, Y. An integrated study of metabolomics and transcriptomics to reveal the anti-primary dysmenorrhea mechanism of Akebiae Fructus. J. Ethnopharmacol. 2021, 270, 113763. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.H.; Wu, F.; Ju, J.M.; Hua, J.L.; Li, Z.H.; Huo, J.G. Comparatively Study on Content of Hederagenin from Different Parts of Fructus Akebiae. Chin. J. Mod. Appl. Pharm. 2014, 31, 1344–1347. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. PPB 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Pholphana, N.; Rangkadilok, N.; Saehun, J.; Ritruechai, S.; Satayavivad, J. Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian). Chin. Med. 2013, 8, 2. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Yang, Y.; Zhan, Y.; Tu, D. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind. Crops Prod. 2013, 46, 311–316. [Google Scholar] [CrossRef]
- Xu, J.; Yu, Y.; Shi, R.; Xie, G.; Zhu, Y.; Wu, G.; Qin, M. Organ-Specific Metabolic Shifts of Flavonoids in Scutellaria baicalensis at Different Growth and Development Stages. Molecules 2018, 23, 428. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, M.; Luo, L.; Pan, H.; Zhang, Q.; Yu, C. Metabolic profiles, bioactive compounds and antioxidant activity of rosehips from Xinjiang, China. LWT 2023, 174, 114451. [Google Scholar] [CrossRef]
- Zou, S.; Wu, J.; Shahid, M.Q.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Wang, X.; Liao, Q.; Zhang, W.; Liu, J.; Liu, G.; Tang, J. Widely targeted metabolomics analysis reveals the effect of exogenous auxin on postharvest resistance to botrytis cinerea in kiwifruit (Actinidia chinensis L.). Postharvest Biol. Technol. 2023, 195, 112129. [Google Scholar] [CrossRef]
- Gedük, A.; Atsız, S. LC-MS/MS phenolic composition of peach (Prunus persica (L.) batsch) extracts and an evaluation of their antidiabetic, anti-oxidant, and antibacterial activities. S. Afr. J. Bot. 2022, 147, 636–645. [Google Scholar] [CrossRef]
- Yang, H.; Tian, C.; Ji, S.; Ni, F.; Fan, X.; Yang, Y.; Sun, C.; Gong, H.; Zhang, A. Integrative analyses of metabolome and transcriptome reveals metabolomic variations and candidate genes involved in sweet cherry (Prunus avium L.) fruit quality during development and ripening. PLoS ONE 2021, 16, e0260004. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, Z.; Yang, N.; Zhou, K.; Hu, W.; Ou, S.; Liu, P. Widely targeted UHPLC-MS/MS metabolomic analysis on the chemical variation in blueberry-filled pastries during processing. Front. Nutr. 2020, 7, 569172. [Google Scholar] [CrossRef]
- Nazir, M.F.; Jia, T.; Xu, J.; Dai, L.; Zhao, Y.; Zou, S. Metabolomic profiling of Akebia species: Comparative analysis of bioactive compounds in the pulp of A. trifoliata, A. trifoliata ssp. australis, and A. quinata. Food Chem. X 2025, 28, 102531. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, Y.; Yin, H.; Wang, D.; Zhong, Y.; Deng, Y. Metabolic and bioactive comparative analyses reveal the mechanisms of color changes in Akebia trifoliata (Thunb.) Koidz fruit. Sci. Hortic. 2022, 295, 110819. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yu, N.X.; Peng, H.L.; Hu, Z.Y.; Sun, Y.; Zhu, X.M.; Jiang, L.; Xiong, H. The profiling of bioactives in Akebia trifoliata pericarp and metabolites, bioavailability and in vivo anti-inflammatory activities in DSS-induced colitis mice. Food Funct. 2019, 10, 3977–3991. [Google Scholar] [CrossRef]
- Jia, T.; Feng, C.; Zou, S.; Gao, P. The Main Physicochemical Characteristics and Nutrient Composition during Fruit Ripening of Stauntonia Obovatifoliola Subsp. Urophylla (Lardizabalaceae). Horticulturae 2023, 9, 29. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, J.; Zhu, L.; Yu, X.; Zhang, Q.; Li, M.; Yang, X. Metabolomics provide a novel interpretation of the changes in flavonoids during sea buckthorn (Hippophae rhamnoides L.) drying. Food Chem. 2023, 413, 135598. [Google Scholar] [CrossRef]
- Jia, T.; Nazir, M.F.; Zhang, T.; Zhu, Q.; Xu, J.; Dai, L.; Zhao, Y.; Zou, S. Akebia quinata flower is an excellent potential herbal tea: Chemical quality, bioactivity analysis and metabolite profiles of Akebia quinata flower with different drying methods. Appl. Food Res. 2025, 5, 100804. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Dong, X.; Wang, J.; Raghavan, V. Effects of variety, maturity and storage conditions on the allergic potential of kiwifruit and its relationship with antioxidant activity. Food Chem. X 2022, 16, 100467. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Min, H.-J.; Cho, H.-D.; Kim, H.-G.; Park, W.-L.; Kim, D.-H.; Tachibana, H.; Seo, K.-I. Bioactivity Evaluation and Phytochemical Characterization of Euonymus alatus (Thunb.) Siebold Leaf Extract. Biomedicines 2024, 12, 2928. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Jin, H.G.; Liu, K.Y.; Qu, W.H.; Li, T.J.; Liao, L.; Yu, J.M. Isolation and structure identification of chemical constituents from the fruits of Akebiae quinata. Nat. Prod. Res. Dev. 2019, 31, 2077–2081. [Google Scholar] [CrossRef]
- Li, Z.F.; Wang, Q.; Liu, Y.T.; Li, X.; Ouyang, S.; Feng, Y.L.; Yang, S.L. Chemical constituents from Akebia trifoliata. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 56–60. [Google Scholar]
- Wang, J.; Ren, H.; Xu, Q.L.; Zhou, Z.Y.; Wu, P.; Wei, X.Y.; Cao, Y.; Chen, X.X.; Tan, J.W. Antibacterial oleanane-type triterpenoids from pericarps of Akebia trifoliata. Food Chem. 2015, 168, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.F.; Jia, T.; Zhang, Y.; Dai, L.; Xu, J.; Zhao, Y.; Zou, S. Deciphering the Genetic and Biochemical Drivers of Fruit Cracking in Akebia trifoliata. Int. J. Mol. Sci. 2024, 25, 12388. [Google Scholar] [CrossRef]
- Jin, P.; Wu, X.; Xu, F.; Wang, X.L.; Wang, J.; Zheng, Y.H. Enhancing Antioxidant Capacity and Reducing Decay of Chinese Bayberries by Essential Oils. J. Agric. Food Chem. 2012, 60, 3769–3775. [Google Scholar] [CrossRef] [PubMed]
- Kirigia, D.; Winkelmann, T.; Kasili, R.; Mibus, H. Nutritional composition in African nightshade (Solanum scabrum) influenced by harvesting methods, age and storage conditions. Postharvest Biol. Technol. 2019, 153, 142–151. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P.; Sharma, J.; Dixit, A. Flavonoids modulate tight junction barrier functions in hyperglycemic human intestinal Caco-2 cells. Nutrition 2020, 78, 110792. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; Pereira-Netto, A.B.; Beta, T. Comparative analysis of total phenolic content, antioxidant activity, and flavonoids profile of fruits from two varieties of Brazilian cherry (Eugenia uniflora L.) throughout the fruit developmental stages. Food Res. Int. 2011, 44, 2442–2451. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Aradhya, S.M. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005, 93, 319–324. [Google Scholar] [CrossRef]
- Lima, V. Total phenolic and carotenoid contents in acerola genotypes harvested at three ripening stages. Food Chem. 2005, 90, 565–568. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Liu, Z.; Zhao, Z.; Zhao, J.; Wang, Z.; Zhou, G.; Liu, P.; Liu, M. Transcriptome and metabolome profiling unveil the mechanisms of Ziziphus jujuba Mill. peel coloration. Food Chem. 2020, 312, 125903. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Haggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- He, J.G.; Cheng, Y.D.; Guan, J.F.; Ge, W.Y.; Zhao, Z. Changes of chlorogenic acid content and its synthesis-associated genes expression in Xuehua pear fruit during development. J. Integr. Agr. 2017, 16, 471–477. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in Phenolic Compounds and Antioxidant Activity during Development of ‘Qiangcuili’ and ‘Cuihongli’ Fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- Wang, J.R.; Song, X.H.; Li, L.Y.; Gao, S.J.; Shang, F.H.; Zhang, X.M.; Yang, Y. Metabolomic analysis reveals dynamic changes in secondary metabolites of Sophora japonica L. during flower maturation. Front. Plant Sci. 2022, 13, 916410. [Google Scholar] [CrossRef]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Plaza, M.; Pozzo, T.; Liu, J.; Ara, K.Z.G.; Turner, C.; Karlsson, E.N. Substituent Effects on in Vitro Antioxidizing Properties, Stability, and Solubility in Flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Metabolites of Geum aleppicum and Sibbaldianthe bifurca: Diversity and α-Glucosidase Inhibitory Potential. Metabolites 2023, 13, 689. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Wu, J. Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) promote adipocyte differentiation and inhibit inflammation in 3T3-F442A cells. PLoS ONE 2015, 10, e0117492. [Google Scholar] [CrossRef]
- Durrani, R.; Meiyun, Y.; Yang, B.; Durand, E.; Delavault, A.; Bowen, H.; Weiwei, H.; Yiyang, L.; Lili, S.; Fei, G. Identification of novel bioactive proteins and their produced oligopeptides from Torreya grandis nuts using proteomic based prediction. Food Chem. 2023, 405, 134843. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.; Rong, H.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Lu, W.L.; Yang, T.; Song, Q.J.; Fang, Z.Q.; Pan, Z.Q.; Liang, C.; Jia, D.W.; Peng, P.K. Akebia trifoliata (Thunb.) Koidz seed extract inhibits human hepatocellular carcinoma cell migration and invasion in vitro. J. Ethnopharmacol. 2019, 234, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.K.; Dong, L.M.; Xu, Q.L.; Wang, J.; Liu, S.B.; Qian, T.; Yuan, Y.F.; Tan, J.W. Triterpenoids with α-glucosidase inhibitory activity and cytotoxic activity from the leaves of Akebia trifoliata. RSC Adv. 2018, 8, 40483–40489. [Google Scholar] [CrossRef]
- Shin, S.; Son, D.; Kim, M.; Lee, S.; Roh, K.B.; Ryu, D.; Lee, J.; Jung, E.; Park, D. Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products. Nutrients 2015, 7, 9337–9352. [Google Scholar] [CrossRef]
- Song, D.H.; Kim, G.J.; Chung, K.H.; Lee, K.J.; An, J.H. Ormosanine from Akebia quinata suppresses ethanol-induced inflammation and apoptosis and activates antioxidants via the mitogen activated protein kinase signaling pathway. J. Funct. Foods 2018, 48, 357–366. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, D.S.; Kim, H.K. Akebia quinata extract exerts anti-obesity and hypolipidemic effects in high-fat diet-fed mice and 3T3-L1 adipocytes. J. Ethnopharmacol. 2015, 168, 17–24. [Google Scholar] [CrossRef]
- Guan, S.; Xiong, Y.; Song, B.; Song, Y.; Wang, D.; Chu, X.; Chen, N.; Huo, M.; Deng, X.; Lu, J. Protective effects of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice. Immunopharmacol. Immunotoxicol. 2012, 34, 667–672. [Google Scholar] [CrossRef]
- Mao, G.X.; Xing, W.M.; Wen, X.L.; Jia, B.B.; Yang, Z.X.; Wang, Y.Z.; Jin, X.Q.; Wang, G.F.; Yan, J. Salidroside protects against premature senescence induced by ultraviolet B irradiation in human dermal fibroblasts. Int. J. Cosmet. Sci. 2015, 37, 321–328. [Google Scholar] [CrossRef]
- Baranwal, A.; Aggarwal, P.; Rai, A.; Kumar, N. Pharmacological Actions and Underlying Mechanisms of Catechin: A Review. Mini Rev. Med. Chem. 2022, 22, 821–833. [Google Scholar] [CrossRef]
- Kürbitz, C.; Heise, D.; Redmer, T.; Goumas, F.; Arlt, A.; Lemke, J.; Rimbach, G.; Kalthoff, H.; Trauzold, A. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells. Cancer Sci. 2011, 102, 728–734. [Google Scholar] [CrossRef]
- Du, P.; Cao, Y.; Liu, H.; Ji, J.; Sun, W.; Zhang, X.; Xu, J.; Liang, B. Dopamine improves apple replant disease resistance by regulating physiological resilience and rhizosphere microbial community structure. J. Integr. Agric. 2024, 23, 3025–3044. [Google Scholar] [CrossRef]

| Stages | Single Fruit Weight/g | Fruit Length/mm | Fruit Diameter/mm | Dry Matter/% |
|---|---|---|---|---|
| S1 | 75.50 ± 1.15 d | 83.28 ± 1.03 c | 47.63 ± 1.23 c | 19.46 ± 0.73 c |
| S2 | 161.11 ± 16.41 c | 115.81 ± 5.86 b | 47.77 ± 1.09 c | 23.02 ± 1.80 bc |
| S3 | 203.31 ± 11.28 b | 123.87 ± 7.04 b | 52.74 ± 0.71 b | 30.18 ± 1.31 a |
| S4 | 287.07 ± 34.37 a | 148.25 ± 2.85 a | 56.87 ± 1.25 a | 26.19 ± 1.23 ab |
| Stages | Total Phenolics (GAE_mg/g_DW) | Total Flavonoids (RE_mg/g_DW) | ABTS (%) | DPPH (%) | FRAP (μmol_Trolox/g_DW) | IC50 (mg/mL_DW) |
|---|---|---|---|---|---|---|
| S1 | 28.20 ± 0.81 a | 63.43 ± 2.51 a | 97.88 ± 0.86 a | 90.09 ± 0.35 a | 164.66 ± 4.24 a | 0.81 ± 0.11 c |
| S2 | 6.85 ± 0.78 b | 13.84 ± 0.71 b | 33.35 ± 2.68 b | 20.68 ± 0.65 b | 44.90 ± 3.86 b | 2.82 ± 0.57 b |
| S3 | 2.99 ± 0.56 c | 7.86 ± 0.54 c | 20.47 ± 1.31 c | 8.70 ± 0.65 c | 11.58 ± 1.89 c | 10.89 ± 0.72 a |
| S4 | 1.85 ± 0.24 c | 4.61 ± 0.13 c | 15.34 ± 0.52 d | 5.56 ± 0.27 d | 11.99 ± 0.04 c | 11.45 ± 2.16 a |
| Subclass | Oligopeptide Sequences | Activity |
|---|---|---|
| Subclass 1 | ER | ACE inhibitor; neprilysin inhibitor; hypotensive |
| GW | ACE inhibitor; antioxidative; inhibitor of tripeptidyl peptidase II; dipeptidyl peptidase IV inhibitor | |
| KF | ACE inhibitor; renin inhibitor; CaMPDE inhibitor; dipeptidyl peptidase IV inhibitor | |
| KY | ACE inhibitor; dipeptidyl peptidase IV inhibitor | |
| MH | dipeptidyl peptidase IV inhibitor | |
| FH | hypouricemic | |
| FT | renin inhibitor | |
| Subclass 2 | AEL | ACE inhibitor |
| AY | ACE inhibitor; tubulin-tyrosine ligase inhibitor; antioxidative; dipeptidyl peptidase IV inhibitor | |
| DWR | alpha-amylase inhibitor; alpha-glucosidase inhibitor | |
| EP | dipeptidyl peptidase IV inhibitor | |
| EYW | antioxidative | |
| GF | ACE inhibitor; dipeptidyl peptidase III inhibitor; acylaminoacyl peptidase inhibitor; dipeptidyl peptidase IV inhibitor; inhibitor of tripeptidyl peptidase II | |
| GY | ACE inhibitor; dipeptidyl peptidase IV inhibitor; inhibitor of tripeptidyl peptidase II | |
| LP | ACE inhibitor; lactocepin inhibitor; dipeptidyl peptidase IV inhibitor | |
| MYY | ACE inhibitor; antioxidative | |
| PYY | antioxidative | |
| YPY | alpha-glucosidase inhibitor; dipeptidyl peptidase IV inhibitor | |
| VL | stimulating; dipeptidyl peptidase IV inhibitor | |
| VW | ACE inhibitor; antioxidative; alpha-glucosidase inhibitor; dipeptidyl peptidase IV inhibitor; hypouricemic | |
| Subclass 3 | DE | glutamate carboxypeptidase II inhibitor |
| LF | ACE inhibitor | |
| KG | ACE inhibitor; dipeptidyl peptidase IV inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, T.; Nazir, M.F.; Bovio-Zenteno, E.M.; Dai, L.; Xu, J.; Zhao, Y.; Zou, S. Widely Targeted Metabolomics Analysis Reveals Developmental Shifts in Antioxidants and Functional Peptides in Akebia trifoliata. Antioxidants 2025, 14, 1039. https://doi.org/10.3390/antiox14091039
Jia T, Nazir MF, Bovio-Zenteno EM, Dai L, Xu J, Zhao Y, Zou S. Widely Targeted Metabolomics Analysis Reveals Developmental Shifts in Antioxidants and Functional Peptides in Akebia trifoliata. Antioxidants. 2025; 14(9):1039. https://doi.org/10.3390/antiox14091039
Chicago/Turabian StyleJia, Tianjiao, Mian Faisal Nazir, Edgar Manuel Bovio-Zenteno, Longyu Dai, Jie Xu, Yafang Zhao, and Shuaiyu Zou. 2025. "Widely Targeted Metabolomics Analysis Reveals Developmental Shifts in Antioxidants and Functional Peptides in Akebia trifoliata" Antioxidants 14, no. 9: 1039. https://doi.org/10.3390/antiox14091039
APA StyleJia, T., Nazir, M. F., Bovio-Zenteno, E. M., Dai, L., Xu, J., Zhao, Y., & Zou, S. (2025). Widely Targeted Metabolomics Analysis Reveals Developmental Shifts in Antioxidants and Functional Peptides in Akebia trifoliata. Antioxidants, 14(9), 1039. https://doi.org/10.3390/antiox14091039







