The Mechanism of PMC (2,2,5,7,8-Pentamethyl-6-chromanol), a Sterically Hindered Phenol Antioxidant, in Rescuing Oxidized Low-Density-Lipoprotein-Induced Cytotoxicity in Human Retinal Pigment Epithelial Cells
Abstract
1. Introduction
2. Methods
2.1. The Cell Culture
2.2. The Oxidized LDL (Ox-LDL) and PMC Treatments
2.3. The Cytotoxicity Assay
2.4. Bulk RNA Sequencing
2.5. Quantitative PCR
2.6. siRNA Gene Knockdown
2.7. Western Blot
2.8. Immunocytochemistry
2.9. Reactive Oxygen Species (ROS) Detection
2.10. The Statistical Analysis
3. Results
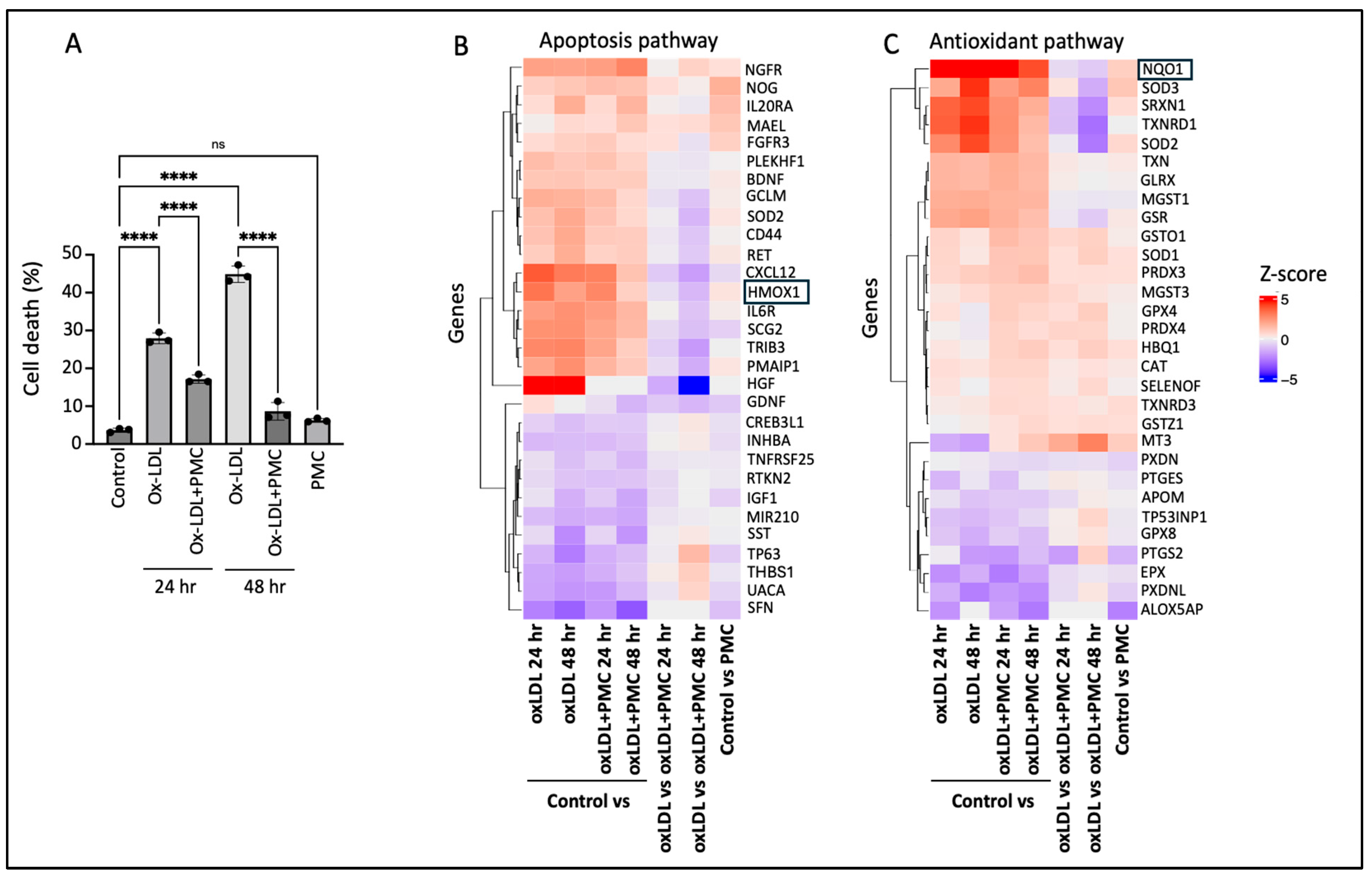
3.1. PMC Protects the RPE Against Ox-LDL-Induced Cytotoxicity
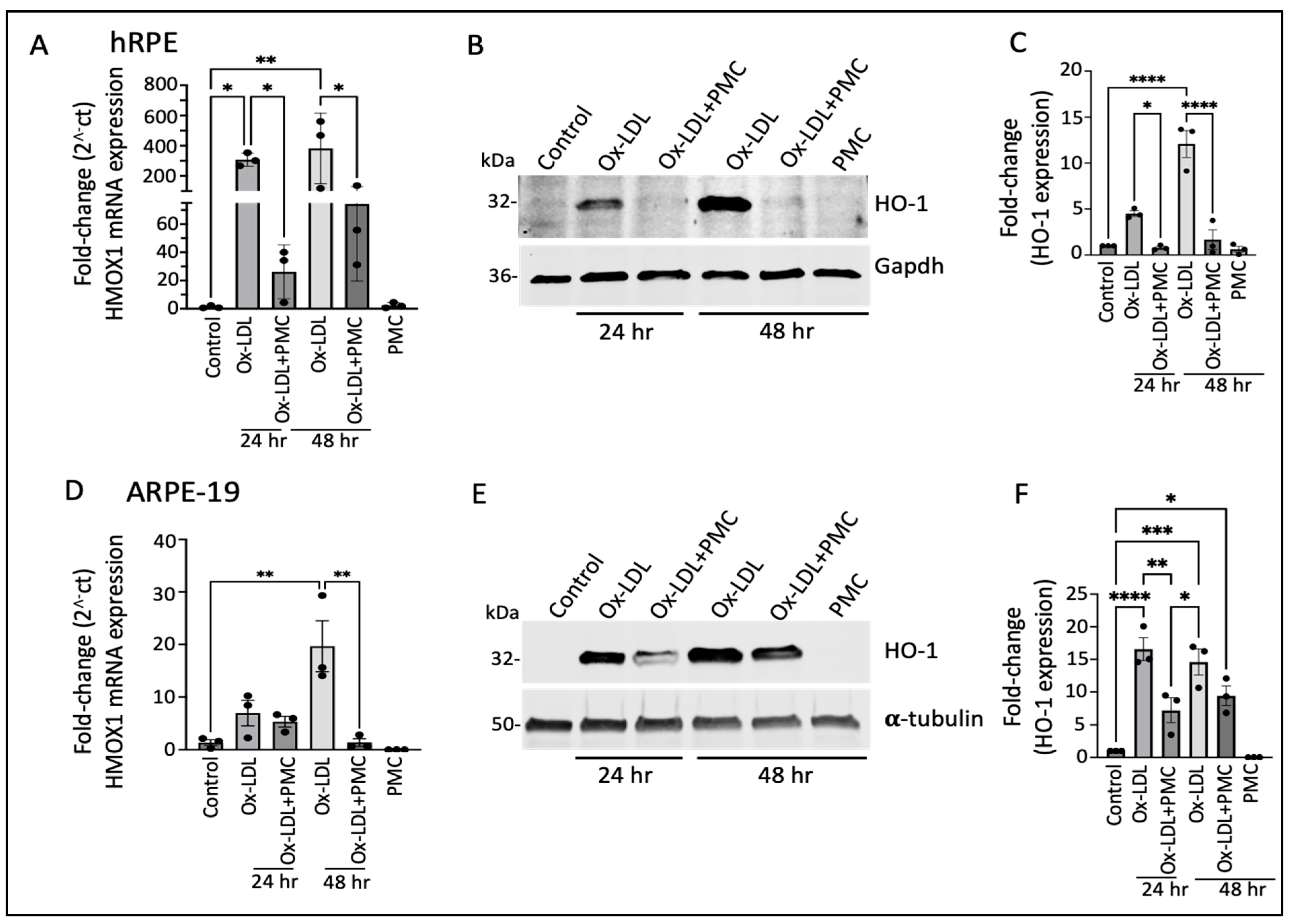
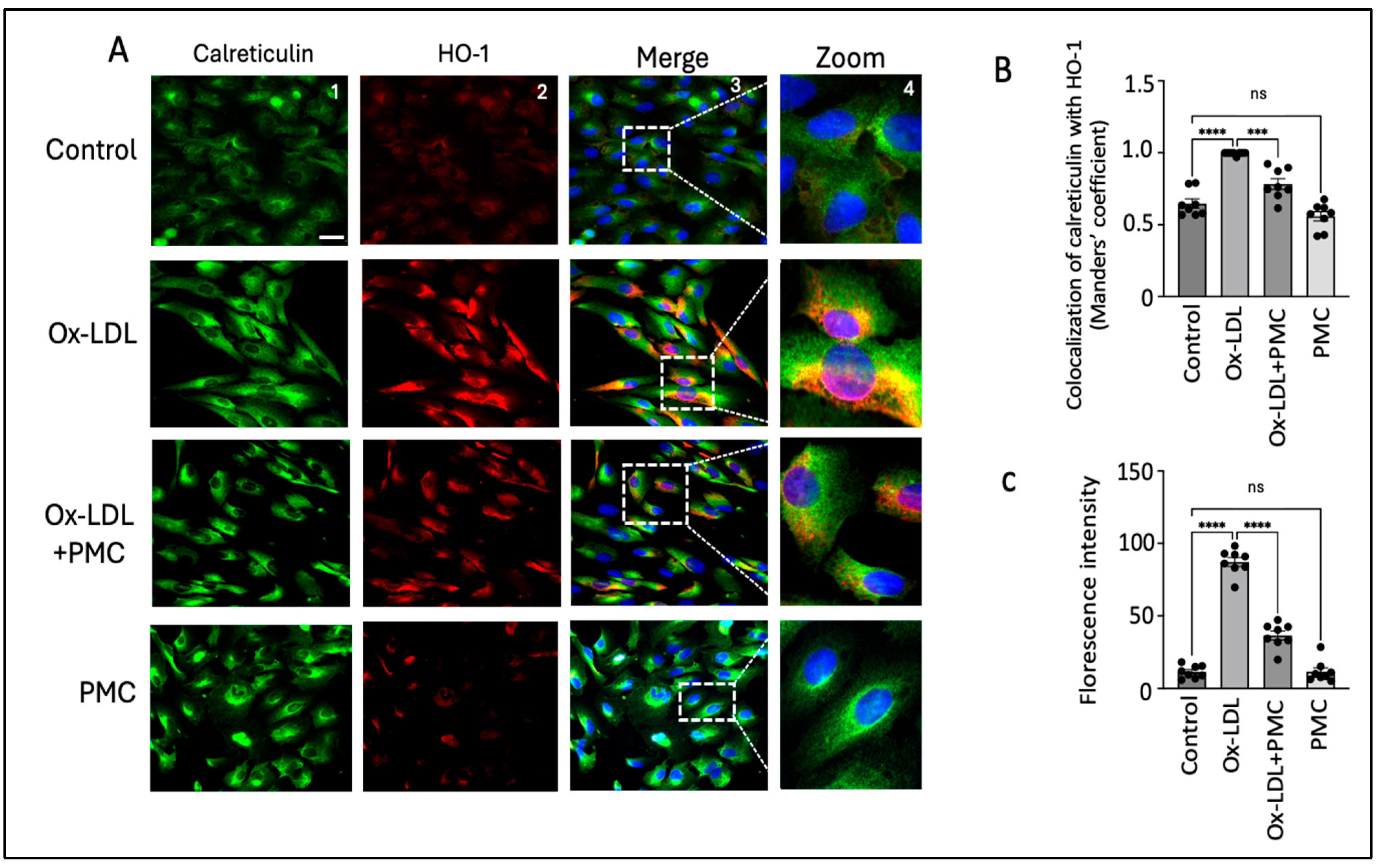
3.2. Ox-LDL Upregulates Heme Oxygenase-1 in RPE Cells
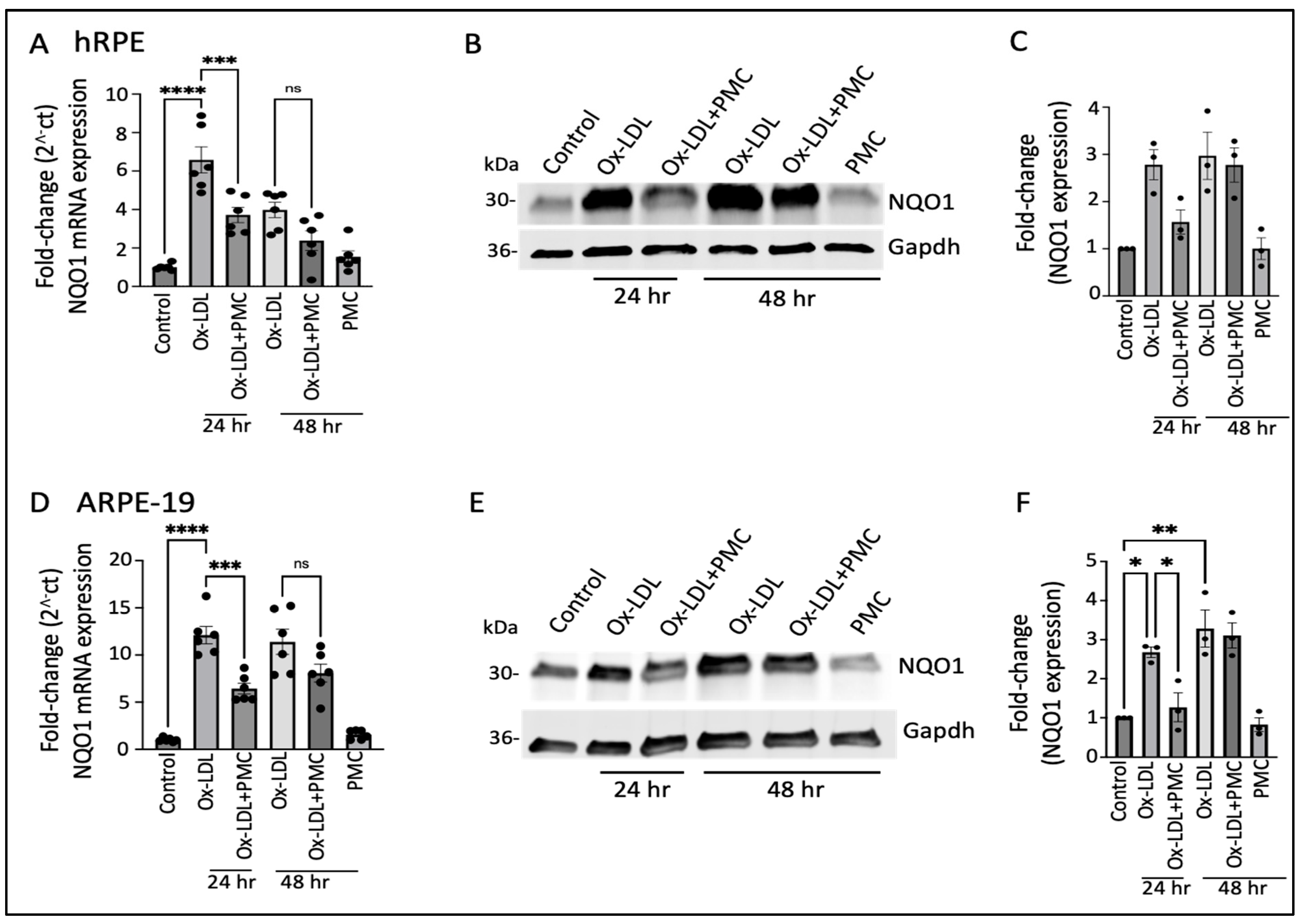
3.3. Ox-LDL Upregulates NQO1 in the RPE, Which Is Lowered by PMC
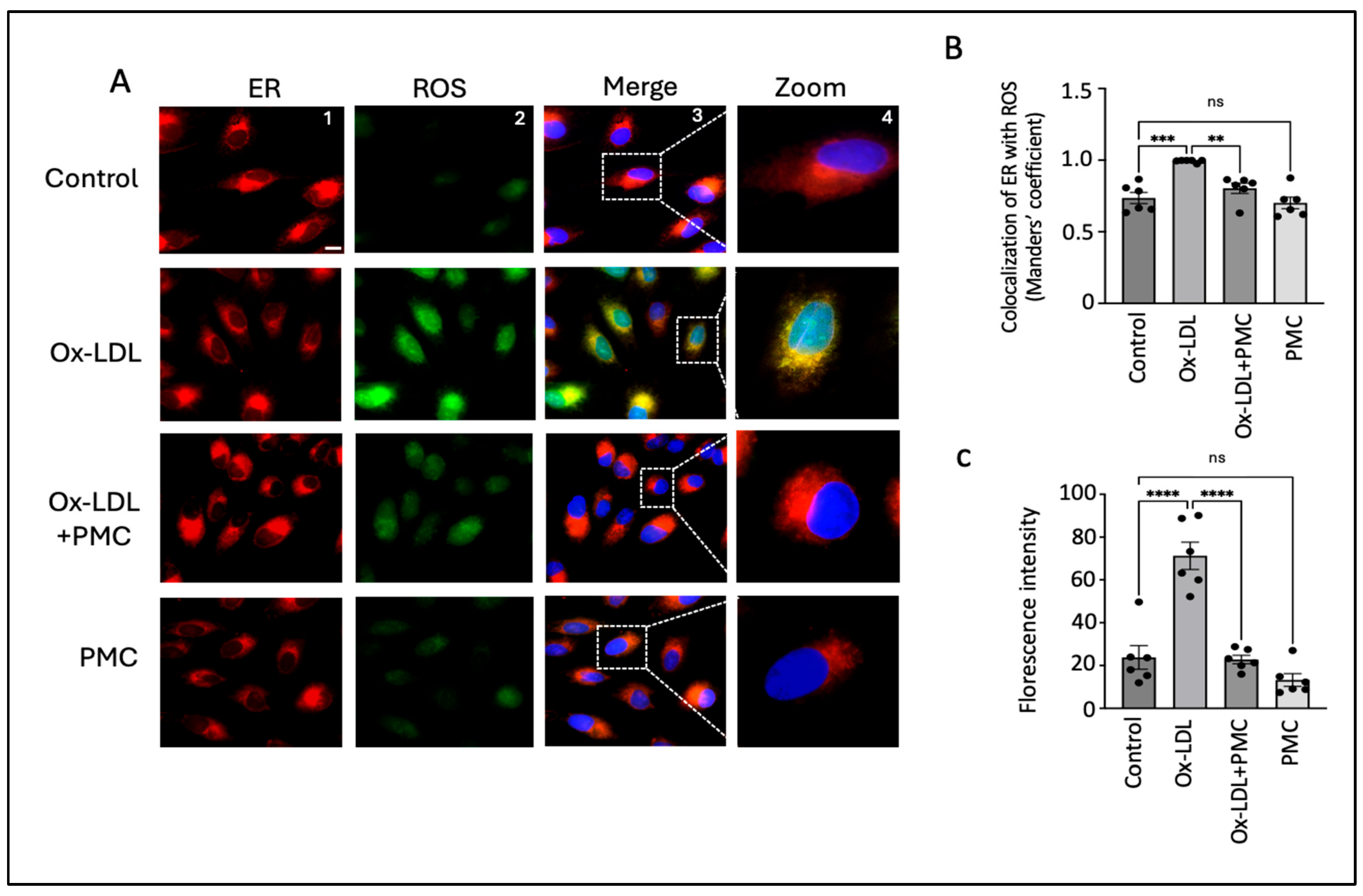
3.4. PMC Reduces Ox-LDL-Induced ROS
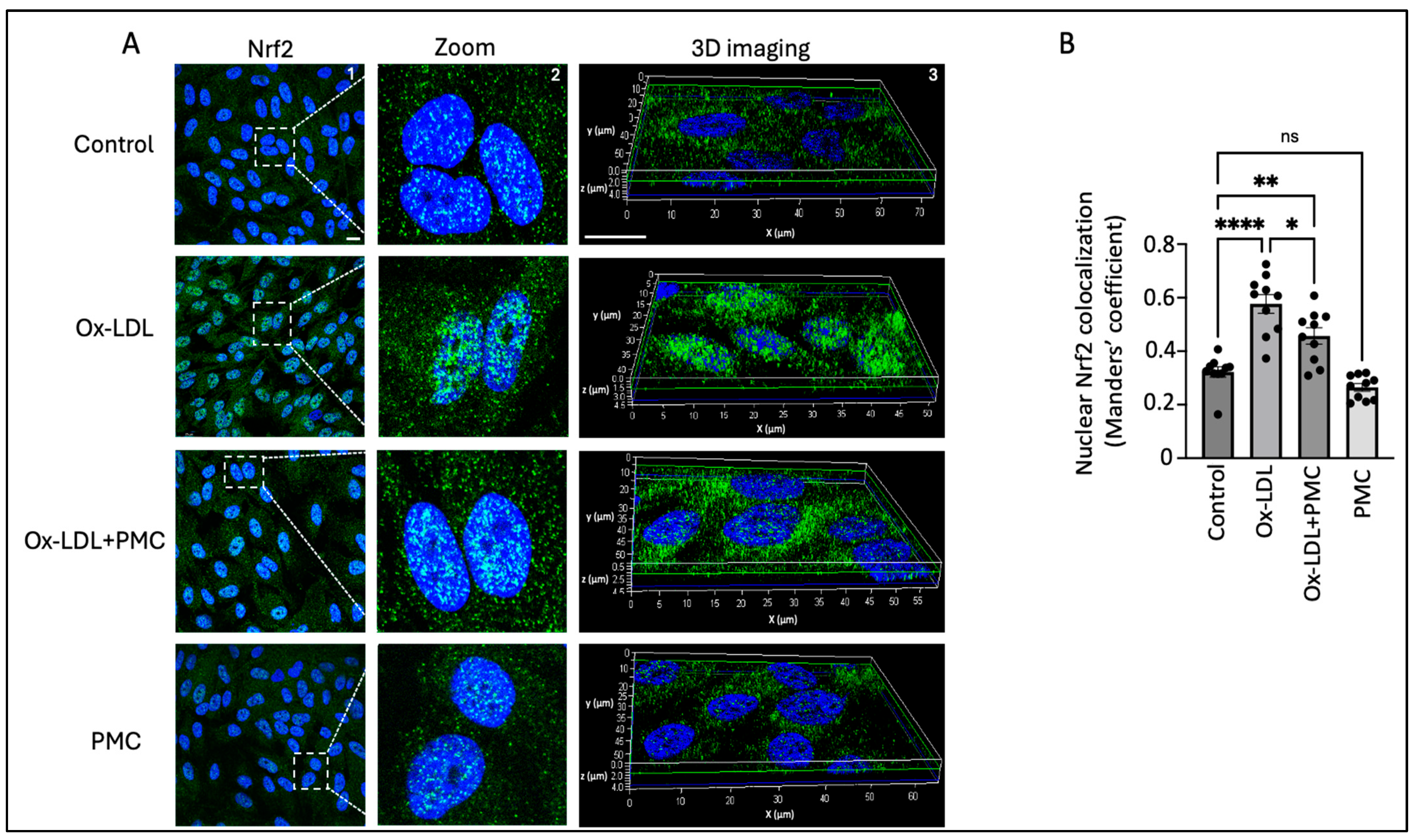
3.5. Ox-LDL Stimulates Nrf2 Nuclear Translocation
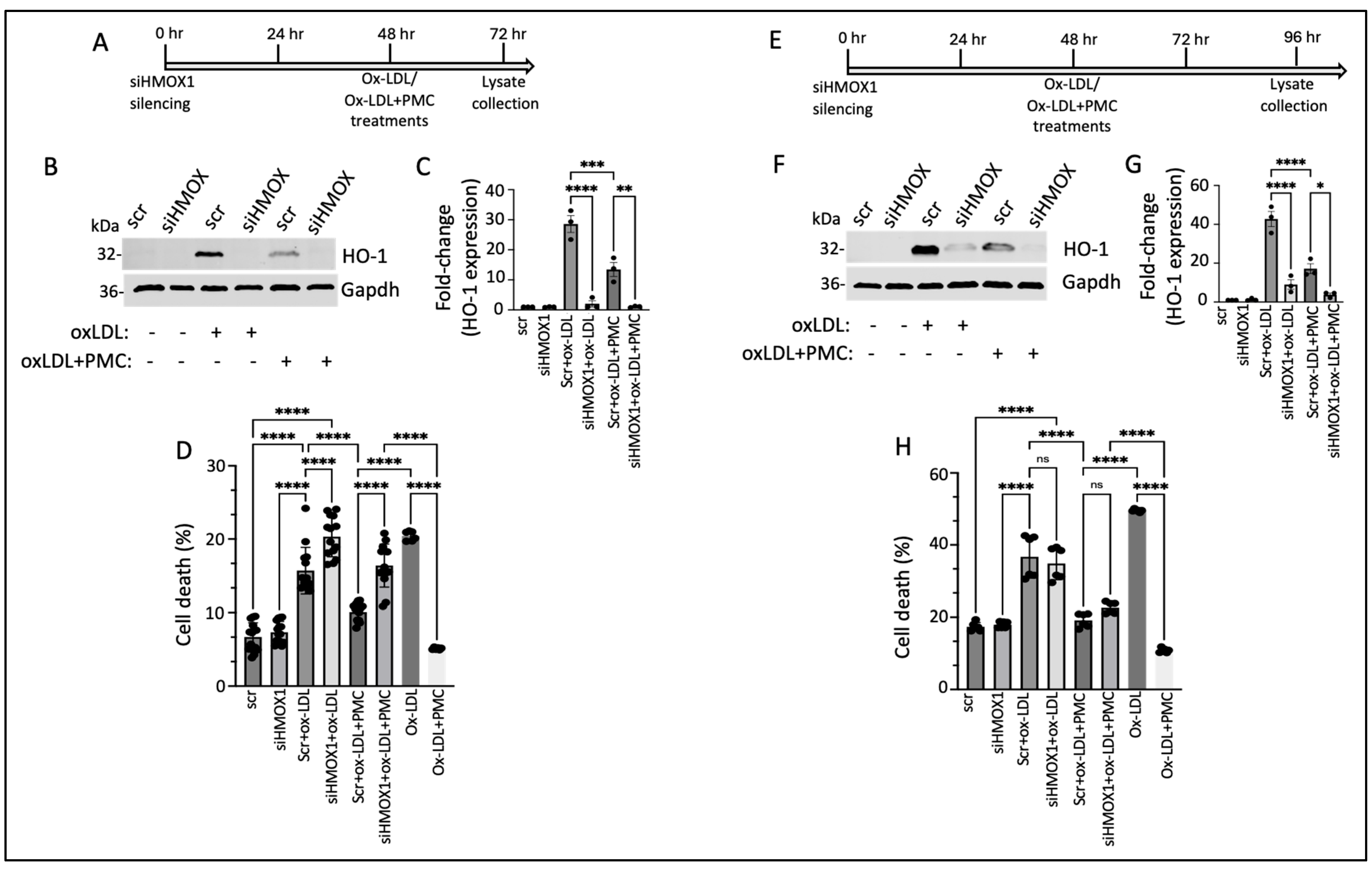
3.6. PMC Protects Against Ox-LDL via the HO-1 Pathway
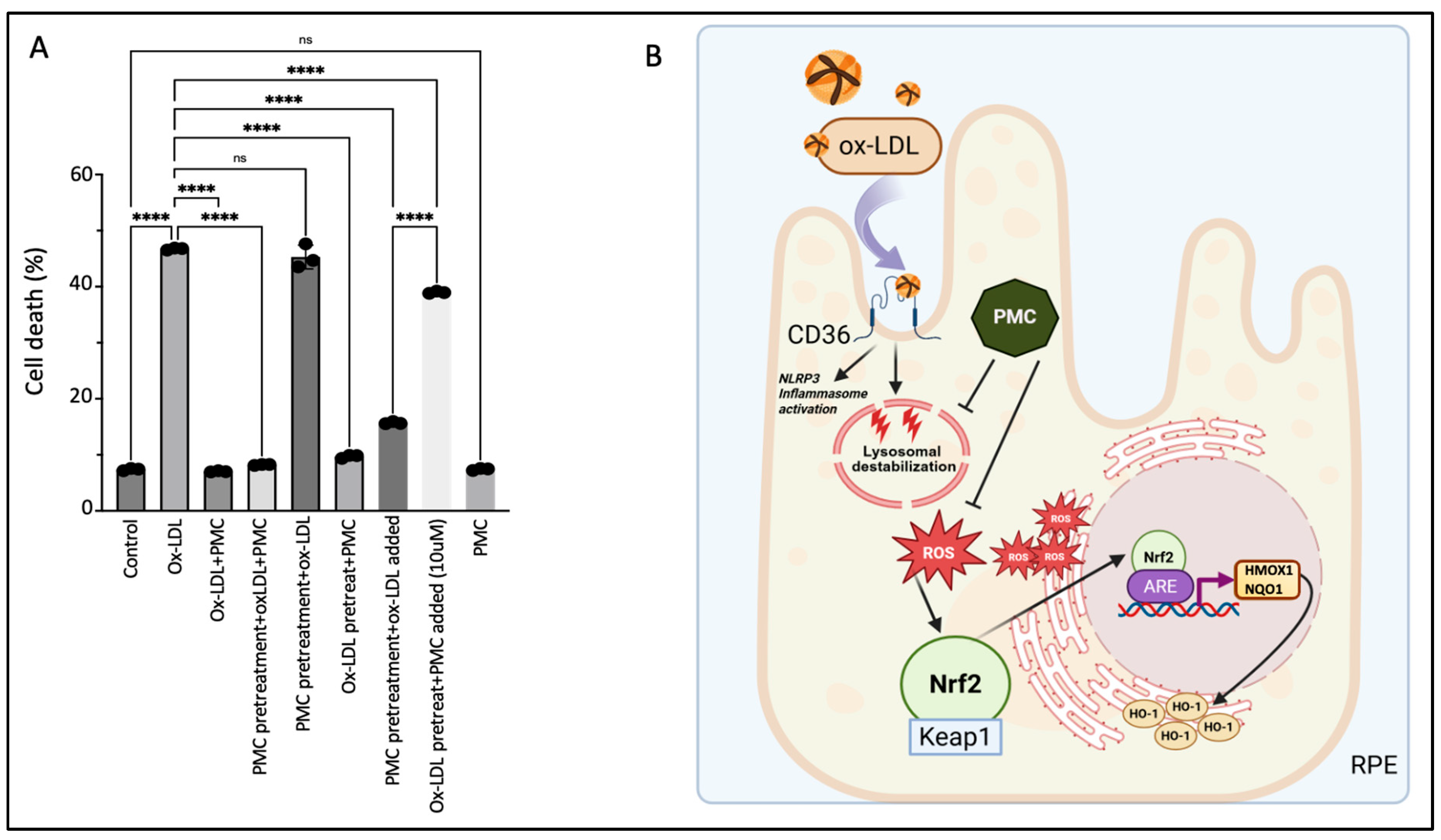
3.7. The Continuous Presence of PMC Provides Optimal Protection to the RPE Against Ox-LDL
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacconi, R.; Corbelli, E.; Querques, L.; Bandello, F.; Querques, G. A review of current and future management of geographic atrophy. Ophthalmol. Ther. 2017, 6, 69–77. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Malek, G. Age-related macular degeneration revisited: From pathology and cellular stress to potential therapies. Front. Cell Dev. Biol. 2021, 8, 612812. [Google Scholar] [CrossRef]
- Ebrahimi, K.B.; Handa, J.T. Lipids, lipoproteins, and age-related macular degeneration. J. Lipids 2011, 2011, 802059. [Google Scholar] [CrossRef]
- Landowski, M.; Bowes Rickman, C. Targeting lipid metabolism for the treatment of age-related macular degeneration: Insights from preclinical mouse models. J. Ocul. Pharmacol. Ther. 2022, 38, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Basyal, D.; Lee, S.; Kim, H.J. Antioxidants and Mechanistic Insights for Managing Dry Age-Related Macular Degeneration. Antioxidants 2024, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Evereklioglu, C.; Er, H.; Doganay, S.; Cekmen, M.; Turkoz, Y.; Otlu, B.; Ozerol, E. Nitric oxide and lipid peroxidation are increased and associated with decreased antioxidant enzyme activities in patients with age-related macular degeneration. Doc. Ophthalmol. 2003, 106, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Abokyi, S.; To, C.-H.; Lam, T.T.; Tse, D.Y. Central role of oxidative stress in age-related macular degeneration: Evidence from a review of the molecular mechanisms and animal models. Oxidative Med. Cell. Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef]
- Gupta, U.; Ghosh, S.; Wallace, C.T.; Shang, P.; Xin, Y.; Nair, A.P.; Yazdankhah, M.; Strizhakova, A.; Ross, M.A.; Liu, H. Increased LCN2 (lipocalin 2) in the RPE decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry AMD. Autophagy 2023, 19, 92–111. [Google Scholar] [CrossRef]
- Suzuki, M.; Kamei, M.; Itabe, H.; Yoneda, K.; Bando, H.; Kume, N.; Tano, Y. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol. Vis. 2007, 13, 772–778. [Google Scholar]
- Kamei, M.; Yoneda, K.; Kume, N.; Suzuki, M.; Itabe, H.; Matsuda, K.-i.; Shimaoka, T.; Minami, M.; Yonehara, S.; Kita, T. Scavenger receptors for oxidized lipoprotein in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1801–1807. [Google Scholar] [CrossRef]
- Xue, C.C.; Teo, K.Y.; Tham, Y.C.; Li, H.; Thakur, S.; Sabanayagam, C.; Fan, Q.; Silver, D.L.; Wang, X.; Cheung, C.M.G. Lipid-lowering drug and complement factor H genotyping–personalized treatment strategy for age-related macular degeneration. iScience 2024, 27, 111344. [Google Scholar] [CrossRef]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.-H. Involvement of Nrf2 in ocular diseases. Oxidative Med. Cell. Longev. 2017, 2017, 1703810. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. The age-related eye disease study (AREDS): Design implications AREDS report no. 1. Control. Clin. Trials 1999, 20, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein+ zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.; Agrón, E.; Keane, P.A.; Domalpally, A.; Chew, E.Y.; Age-Related Eye Disease Study Research Group; Age-Related Eye Disease Study 2 Research Group. Oral antioxidant and lutein/zeaxanthin supplements slow geographic atrophy progression to the fovea in age-related macular degeneration. Ophthalmology 2024, 132, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.M.; Cano, M.; Handa, J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014, 119, 111–114. [Google Scholar] [CrossRef]
- Lambros, M.L.; Plafker, S.M. Oxidative stress and the Nrf2 anti-oxidant transcription factor in age-related macular degeneration. Retin. Degener. Dis. Mech. Exp. Ther. 2016, 854, 67–72. [Google Scholar]
- Catanzaro, M.; Lanni, C.; Basagni, F.; Rosini, M.; Govoni, S.; Amadio, M. Eye-light on age-related macular degeneration: Targeting Nrf2-pathway as a novel therapeutic strategy for retinal pigment epithelium. Front. Pharmacol. 2020, 11, 844. [Google Scholar] [CrossRef]
- Bertram, K.M.; Baglole, C.J.; Phipps, R.P.; Libby, R.T. Molecular regulation of cigarette smoke induced-oxidative stress in human retinal pigment epithelial cells: Implications for age-related macular degeneration. Am. J. Physiol.-Cell Physiol. 2009, 297, C1200–C1210. [Google Scholar] [CrossRef]
- Muangnoi, C.; Sharif, U.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Paraoan, L. Protective effects of curcumin ester prodrug, curcumin diethyl disuccinate against H2O2-induced oxidative stress in human retinal pigment epithelial cells: Potential therapeutic avenues for age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 3367. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Nie, L.; Sun, S.; Liu, J.; Chen, H. Heme oxygenase 1 in erythropoiesis: An important regulator beyond catalyzing heme catabolism. Ann. Hematol. 2023, 102, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, G.; Yoshida, T.; Noguchi, M. Heme oxygenase and heme degradation. Biochem. Biophys. Res. Commun. 2005, 338, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Qu, C.; Zhao, N.; Li, S.; Pu, N.; Tao, Y.; Song, Z. The significance of precisely regulating heme oxygenase-1 expression: Another avenue for treating age-related ocular disease? Ageing Res. Rev. 2024, 97, 102308. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.Y.; Jeong, K.W. Photooxidation of A2E by blue light regulates heme oxygenase 1 expression via NF-κB and lysine methyltransferase 2A in ARPE-19 cells. Life 2022, 12, 1698. [Google Scholar] [CrossRef]
- Yang, P.-M.; Cheng, K.-C.; Yuan, S.-H.; Wung, B.-S. Carbon monoxide-releasing molecules protect against blue light exposure and inflammation in retinal pigment epithelial cells. Int. J. Mol. Med. 2020, 46, 1096–1106. [Google Scholar] [CrossRef]
- Tang, Z.; Ju, Y.; Dai, X.; Ni, N.; Liu, Y.; Zhang, D.; Gao, H.; Sun, H.; Zhang, J.; Gu, P. HO-1-mediated ferroptosis as a target for protection against retinal pigment epithelium degeneration. Redox Biol. 2021, 43, 101971. [Google Scholar] [CrossRef]
- Chen, C.; Yang, K.; He, D.; Yang, B.; Tao, L.; Chen, J.; Wu, Y. Induction of ferroptosis by HO-1 contributes to retinal degeneration in mice with defective clearance of all-trans-retinal. Free Radic. Biol. Med. 2023, 194, 245–254. [Google Scholar] [CrossRef]
- Gnanaguru, G.; Choi, A.R.; Amarnani, D.; D’Amore, P.A. Oxidized lipoprotein uptake through the CD36 receptor activates the NLRP3 inflammasome in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4704–4712. [Google Scholar] [CrossRef]
- Gnanaguru, G.; Mackey, A.; Choi, E.Y.; Arta, A.; Rossato, F.A.; Gero, T.W.; Urquhart, A.J.; Scott, D.A.; D’Amore, P.A.; Ng, Y.S.E. Discovery of sterically-hindered phenol compounds with potent cytoprotective activities against ox-LDL–induced retinal pigment epithelial cell death as a potential pharmacotherapy. Free Radic. Biol. Med. 2022, 178, 360–368. [Google Scholar] [CrossRef]
- Fernandes, M.; McArdle, B.; Schiff, L.; Blenkinsop, T.A. Stem cell–derived retinal pigment epithelial layer model from adult human globes donated for corneal transplants. Curr. Protoc. Stem Cell Biol. 2018, 45, e53. [Google Scholar] [CrossRef]
- Mahaling, B.; Pandala, N.; Wang, H.-C.; Lavik, E.B. Azithromycin protects retinal glia against oxidative stress-induced morphological changes, inflammation, and cell death. ACS Bio Med. Chem. Au 2022, 2, 499–508. [Google Scholar] [CrossRef]
- Gáll, T.; Balla, G.; Balla, J. Heme, heme oxygenase, and endoplasmic reticulum stress—A new insight into the pathophysiology of vascular diseases. Int. J. Mol. Sci. 2019, 20, 3675. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Pae, H.-O.; Back, S.H.; Chung, S.W.; Woo, J.M.; Son, Y.; Chung, H.-T. Heme oxygenase-1 comes back to endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2011, 404, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, oxidized lipids, oxidation-specific epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative stress and antioxidants in age-related macular degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Ikeda, T.; Obayashi, H.; Hasegawa, G.; Nakamura, N.; Yoshikawa, T.; Imamura, Y.; Koizumi, K.; Kinoshita, S. Paraoxonase gene polymorphisms and plasma oxidized low-density lipoprotein level as possible risk factors for exudative age-related macular degeneration. Am. J. Ophthalmol. 2001, 132, 191–195. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Li, C.; Cui, L.; Ma, J.; Hui, Y. The non-canonical effects of heme oxygenase-1, a classical fighter against oxidative stress. Redox Biol. 2021, 47, 102170. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Lu, H.; Matsukura, M.; Zhao, J.; Shinohara, M. Silencing heme oxygenase-1 gene expression in retinal pigment epithelial cells inhibits proliferation, migration and tube formation of cocultured endothelial cells. Biochem. Biophys. Res. Commun. 2013, 434, 492–497. [Google Scholar] [CrossRef]
- Peterson, K.M.; Mishra, S.; Asaki, E.; Powell, J.I.; He, Y.; Berger, A.E.; Rajapakse, D.; Wistow, G. Serum-deprivation response of ARPE-19 cells; expression patterns relevant to age-related macular degeneration. PLoS ONE 2024, 19, e0293383. [Google Scholar] [CrossRef]
- Mishra, S.; Peterson, K.; Yin, L.; Berger, A.; Fan, J.; Wistow, G. Accumulation of cholesterol and increased demand for zinc in serum-deprived RPE cells. Mol. Vis. 2016, 22, 1387. [Google Scholar] [PubMed]
- Kim, H.J.; Zheng, M.; Kim, S.-K.; Cho, J.J.; Shin, C.H.; Joe, Y.; Chung, H.T. CO/HO-1 induces NQO-1 expression via Nrf2 activation. Immune Netw. 2011, 11, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Lian, L.; Zhou, J.; Xiang, S.; Zhai, Y.; Chen, Y.; Ma, X.; Wu, W.; Hou, L. High dose expression of heme oxigenase-1 induces retinal degeneration through ER stress-related DDIT3. Mol. Neurodegener. 2021, 16, 16. [Google Scholar] [CrossRef]
- Kulbay, M.; Wu, K.Y.; Nirwal, G.K.; Bélanger, P.; Tran, S.D. The Role of Reactive Oxygen Species in Age-Related Macular Degeneration: A Comprehensive Review of Antioxidant Therapies. Biomedicines 2024, 12, 1579. [Google Scholar] [CrossRef]
- Wong, K.-H.; Nam, H.-Y.; Lew, S.-Y.; Naidu, M.; David, P.; Kamalden, T.A.; Hadie, S.N.H.; Lim, L.-W. Discovering the potential of natural antioxidants in age-related macular degeneration: A review. Pharmaceuticals 2022, 15, 101. [Google Scholar] [CrossRef]
- Evans, J. Antioxidant vitamin and mineral supplements for age-related macular degeneration. Cochrane Database Syst. Rev. 2002, 13, CD000254. [Google Scholar]
| Gene Name | Forward Sequence | Reverse Sequence | Gene Accession Number |
|---|---|---|---|
| HMOX1 | CAACATCCAGCTCTTTGAGG | GGCAGAATCTTGCACTTTG | NM_002133.3 |
| NQO1 | CCTGCCATTCTGAAAGGCTGGT | GTGGTGATGGAAAGCACTGCCT | NM_000903.3 |
| Gapdh | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTC | NM_001256799.3 |
| Antibody | Host Species * | Species Reactivity * | Company | Catalogue Number | Dilution ** |
|---|---|---|---|---|---|
| HO-1 | m | b, h, m, r, d | Thermo Fisher Scientific, Waltham, MA, USA | MA1-112 | WB: 1:1000 ICC: 1:200 |
| NQO1 | m | h | Abcam, Waltham, MA, USA | ab28947 | WB: 1:500 |
| Calreticulin | Rb | h, m, r | Cell Signaling Technology, Danvers, MA, USA | D3E6 | ICC: 1:200 |
| Nrf2 | m | h | Santa Cruz Biotechnology, Dallas, TX, USA | sc-365949 | WB: 1:500 ICC: 1:100 |
| GAPDH | Rb | h, m, r, mk | Cell Signaling Technology, Danvers, MA, USA | D16H11 | WB: 1:1000 |
| α-tubulin | Rb | h, m, r, mk, b | Cell Signaling Technology, Danvers, MA, USA | 2144 | WB: 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, S.; Moon, J.; Hu, Z.; Kriukov, E.; Pestun, S.; Baranov, P.Y.; Ng, Y.-S.E.; D’Amore, P.A. The Mechanism of PMC (2,2,5,7,8-Pentamethyl-6-chromanol), a Sterically Hindered Phenol Antioxidant, in Rescuing Oxidized Low-Density-Lipoprotein-Induced Cytotoxicity in Human Retinal Pigment Epithelial Cells. Antioxidants 2025, 14, 996. https://doi.org/10.3390/antiox14080996
Chaudhary S, Moon J, Hu Z, Kriukov E, Pestun S, Baranov PY, Ng Y-SE, D’Amore PA. The Mechanism of PMC (2,2,5,7,8-Pentamethyl-6-chromanol), a Sterically Hindered Phenol Antioxidant, in Rescuing Oxidized Low-Density-Lipoprotein-Induced Cytotoxicity in Human Retinal Pigment Epithelial Cells. Antioxidants. 2025; 14(8):996. https://doi.org/10.3390/antiox14080996
Chicago/Turabian StyleChaudhary, Suman, Jean Moon, Zhengping Hu, Emil Kriukov, Sergio Pestun, Petr Y. Baranov, Yin-Shan Eric Ng, and Patricia A. D’Amore. 2025. "The Mechanism of PMC (2,2,5,7,8-Pentamethyl-6-chromanol), a Sterically Hindered Phenol Antioxidant, in Rescuing Oxidized Low-Density-Lipoprotein-Induced Cytotoxicity in Human Retinal Pigment Epithelial Cells" Antioxidants 14, no. 8: 996. https://doi.org/10.3390/antiox14080996
APA StyleChaudhary, S., Moon, J., Hu, Z., Kriukov, E., Pestun, S., Baranov, P. Y., Ng, Y.-S. E., & D’Amore, P. A. (2025). The Mechanism of PMC (2,2,5,7,8-Pentamethyl-6-chromanol), a Sterically Hindered Phenol Antioxidant, in Rescuing Oxidized Low-Density-Lipoprotein-Induced Cytotoxicity in Human Retinal Pigment Epithelial Cells. Antioxidants, 14(8), 996. https://doi.org/10.3390/antiox14080996






