Enhanced Antioxidant and Anti-Inflammatory Activities of Diospyros lotus Leaf Extract via Enzymatic Conversion of Rutin to Isoquercitrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of D. lotus Leaf Extract
2.3. Hydrolytic Activity
2.4. Environmental Conditions
2.5. Enzymatic Conversion of Rutin to Isoquercitrin in D. lotus Leaf Extract
2.6. DPPH Assay
2.7. Lipoxygenase Assay
2.8. High-Performance Liquid Chromatography Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Substrate Specificity of α-l-Rhamnosidase for Flavonoids
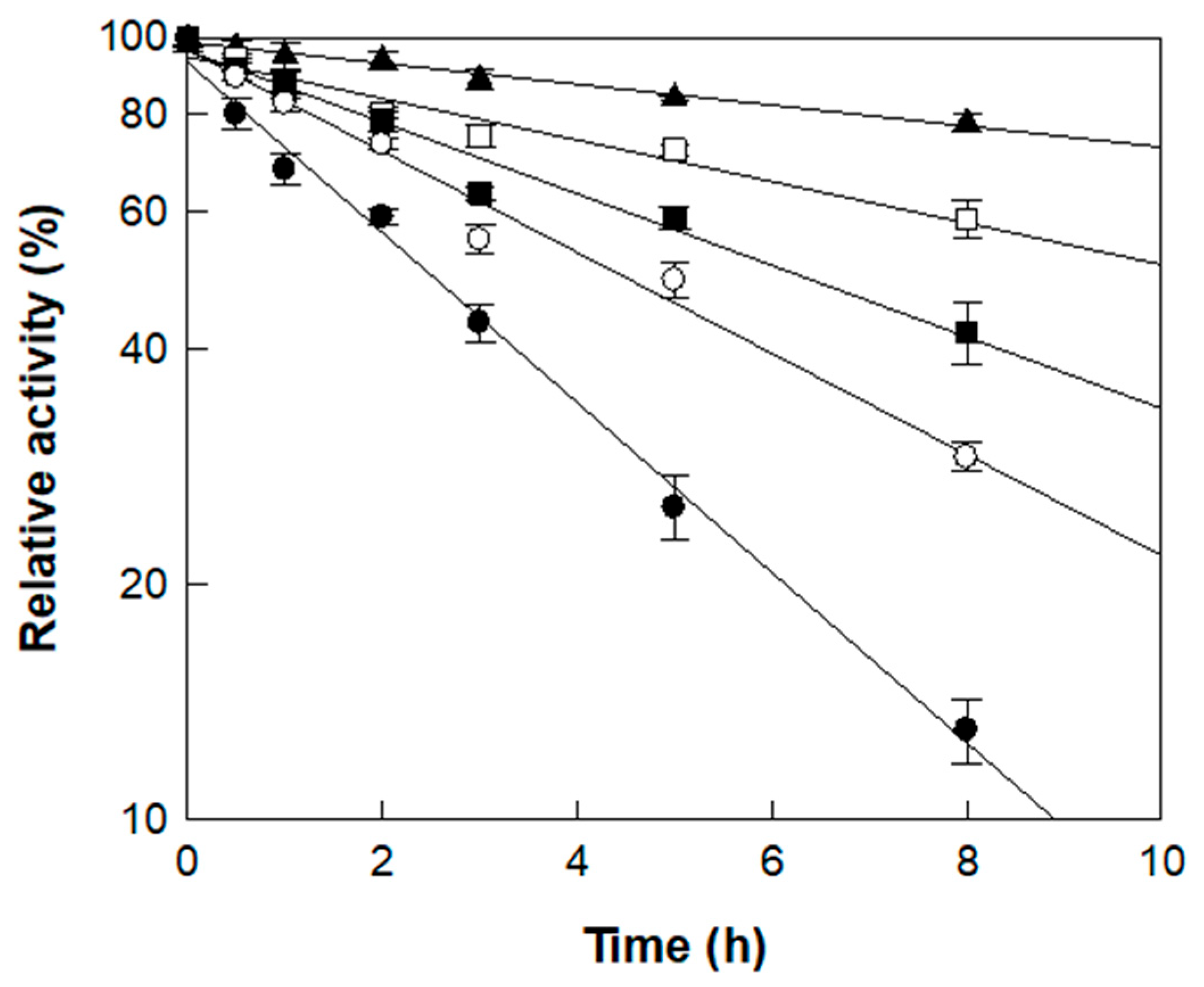
3.2. Effects of pH and Temperature on the Production of Isoquercitrin from Rutin in D. lotus Leaf Extract
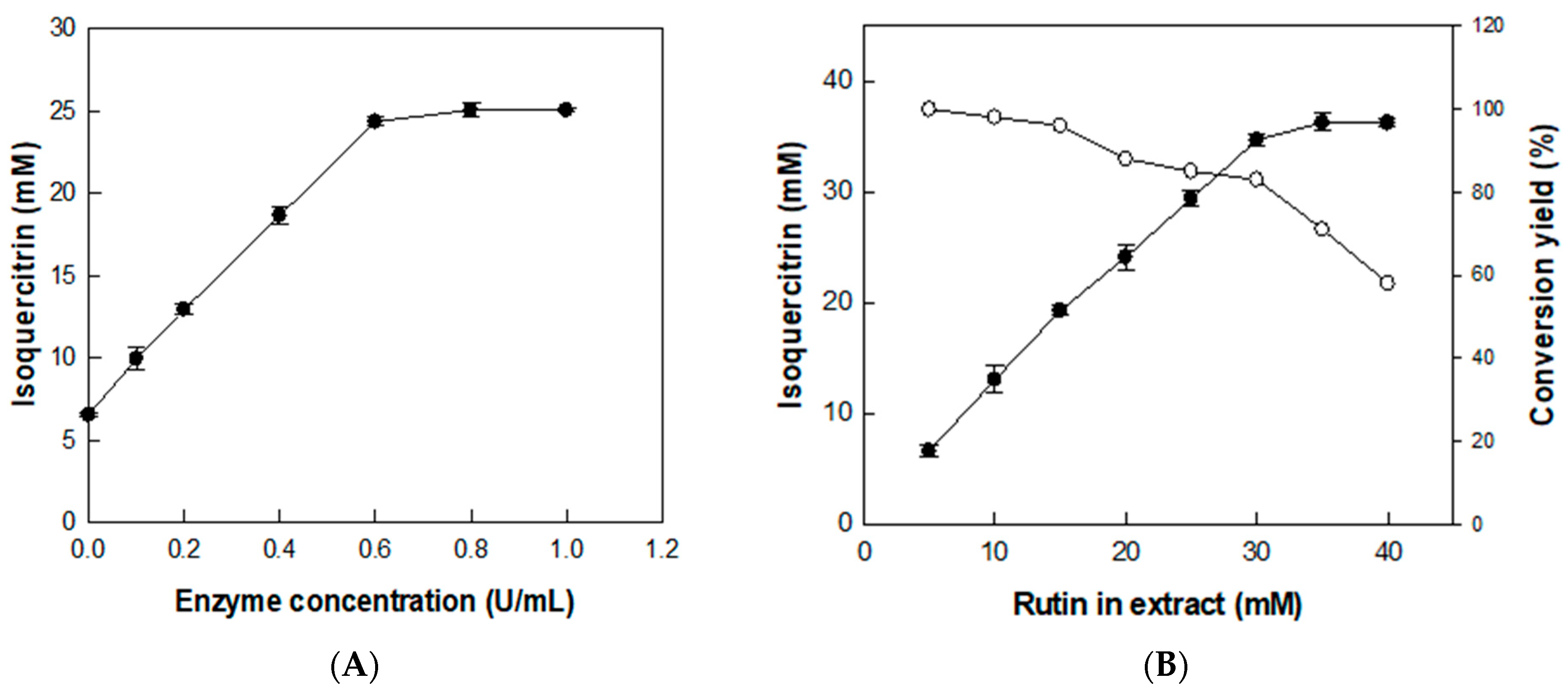
3.3. Effects of Enzyme and Substrate Concentrations on the Production of Isoquercitrin from Rutin in D. lotus Leaf Extract
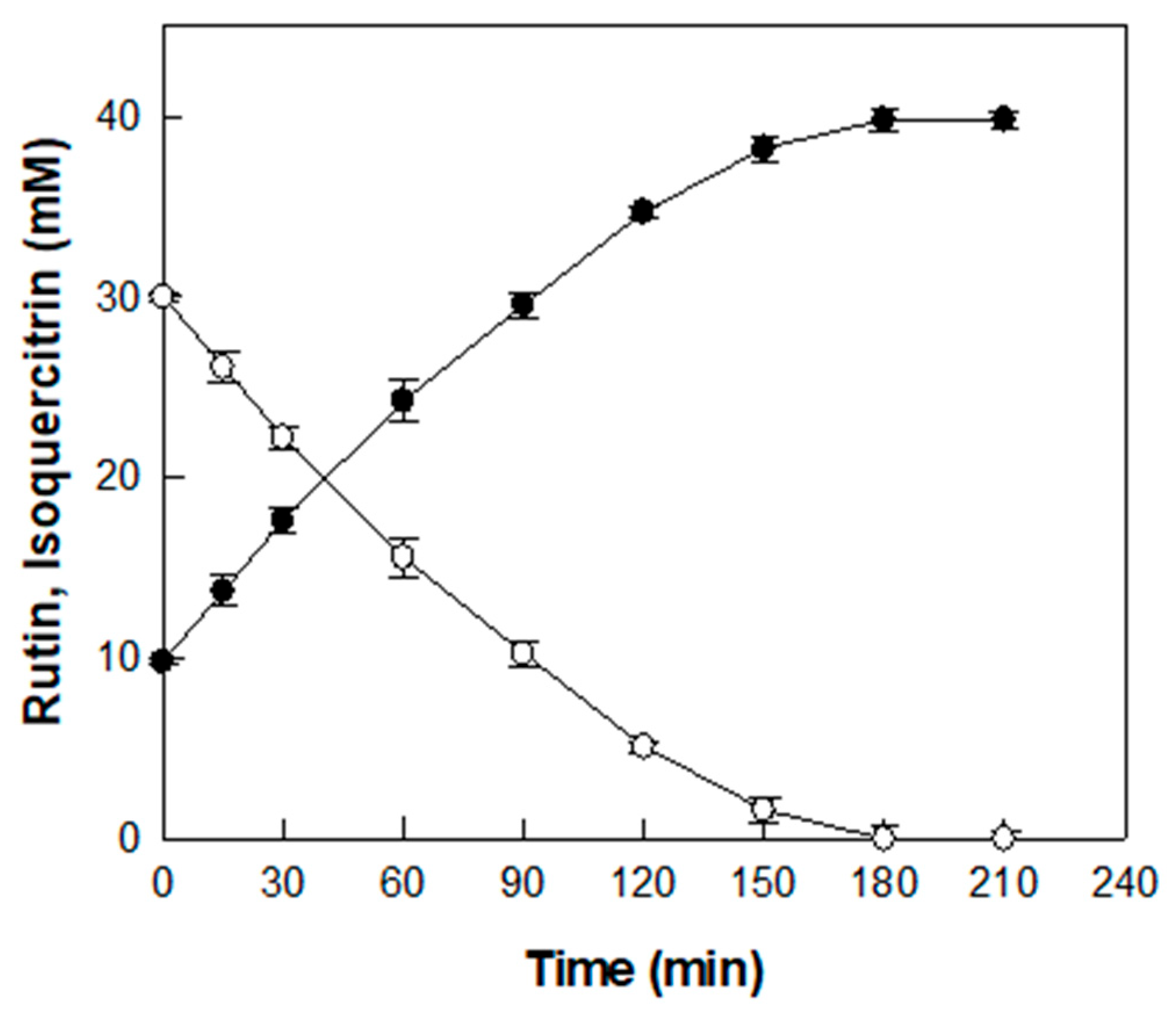
3.4. Time-Course Reaction for the Production of Isoquercitrin from Rutin in D. lotus Leaf Extract
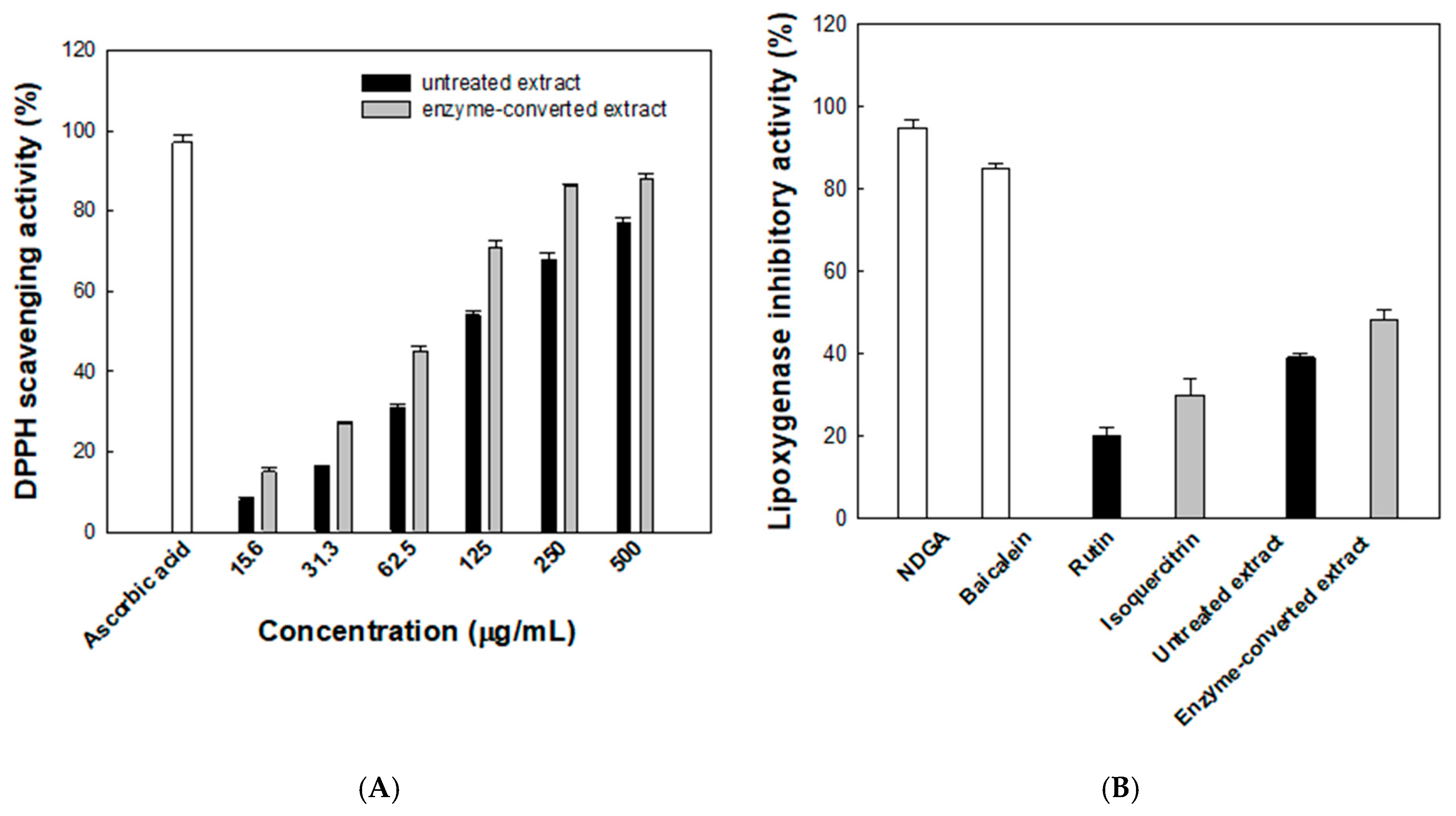
3.5. Antioxidant and Anti-Inflammatory Activities of Enzyme-Converted D. lotus Leaf Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, G.; Rauf, A.; Siddiqui, B.S.; Muhammad, N.; Khan, A.; Shah, S.U. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine 2014, 21, 954–959. [Google Scholar] [CrossRef]
- Rauf, A.; Uddin, G.; Patel, S.; Khan, A.; Halim, S.A.; Bawazeer, S.; Ahmad, K.; Muhammad, N.; Mubarak, M.S. Diospyros, an under-utilized, multi-purpose plant genus: A review. Biomed. Pharmacother. 2017, 91, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, W.; Zhou, J.; Xie, X.; Zhong, X.; Liu, Y.; Shi, L. Extraction, analysis of antioxidant activities and structural characteristics of flavonoids in fruits of Diospyros lotus L. LWT 2024, 201, 116248. LWT 2024, 201, 116248. [Google Scholar] [CrossRef]
- Gao, H.; Cheng, N.; Zhou, J.; Wang, B.; Deng, J.; Cao, W. Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. J. Food Sci. Technol. 2014, 51, 950–956. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of flavonoids on disease prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Chiang, M.C.; Tsai, T.Y.; Wang, C.J. The potential benefits of quercetin for brain health: A review of anti-inflammatory and neuroprotective mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Yin, H.; Ma, J.; Han, J.; Li, M.; Shang, J. Pharmacokinetic comparison of quercetin, isoquercitrin, and quercetin-3-O-β-D-glucuronide in rats by HPLC-MS. PeerJ 2019, 7, e6665. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Coricovac, D.; Pinzaru, I.; Marcovici, I.; Macasoi, I.G.; Semenescu, A.; Lazar, G.; Cinta Pinzaru, S.; Radulov, I.; Alexa, E.; et al. Rutin bioconjugates as potential nutraceutical prodrugs: An in vitro and in ovo toxicological screening. Front. Pharmacol. 2022, 13, 1000608. [Google Scholar] [CrossRef]
- Zhang, H.; Hassan, Y.I.; Liu, R.; Mats, L.; Yang, C.; Liu, C.; Tsao, R. Molecular mechanisms underlying the absorption of aglycone and glycosidic flavonoids in a CacO-2 BBe1 cell model. ACS Omega 2020, 5, 10782–10793. [Google Scholar] [CrossRef]
- Owczarek-Januszkiewicz, A.; Magiera, A.; Olszewska, M.A. Enzymatically modified isoquercitrin: Production, metabolism, bioavailability, toxicity, pharmacology, and related molecular mechanisms. Int. J. Mol. Sci. 2022, 23, 14784. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Montoya, C.A.; Mace, L.; Rettedal, E.A.; Fraser, K.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. Biotransformation of Rutin in in vitro porcine ileal and colonic fermentation models. J. Agric. Food Chem. 2023, 71, 12487–12496. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M.; Chrétien, M. Isoquercetin as an anti-COVID-19 medication: A potential to realize. Front. Pharmacol. 2022, 13, 830205. [Google Scholar] [CrossRef] [PubMed]
- Paulke, A.; Eckert, G.P.; Schubert-Zsilavecz, M.; Wurglics, M. Isoquercitrin provides better bioavailability than quercetin: Comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Pharmazie 2012, 67, 991–996. [Google Scholar]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Protective effects of quercetin glycosides, rutin, and isoquercetrin against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in rat pheochromocytoma (PC-12) cells. Int. J. Immunopathol. Pharmacol. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6″-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef]
- Wang, H.; Xia, W.; Long, G.; Pei, Z.; Li, Y.; Wu, M.; Wang, Q.; Zhang, Y.; Jia, Z.; Chen, H. Isoquercitrin ameliorates cisplatin-induced nephrotoxicity via the inhibition of apoptosis, inflammation, and oxidative stress. Front. Pharmacol. 2020, 11, 599416. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Yao, Y.; Ru, G.; Lan, C.; Li, L.; Huang, T. Protective effect of isoquercitrin on UVB-induced injury in HaCaT cells and mice skin through anti-inflammatory, antioxidant, and regulation of MAPK and JAK2-STAT3 pathways. Photochem. Photobiol. 2024, 100, 1507–1518. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.-H.; Liu, G.-R.; Liu, C.; Dong, Y.-M. Isoquercitrin suppresses the expression of histamine and prO-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. Chin. J. Nat. Med. 2016, 14, 407–412. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically modified isoquercitrin improves endothelial function in volunteers at risk of cardiovascular disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Chen, L.; Shen, T.; Zhang, C.P.; Xu, B.L.; Qiu, Y.Y.; Xie, X.Y.; Wang, Q.; Lei, T. Quercetin and isoquercitrin inhibiting hepatic gluconeogenesis through LKB1-AMPKα pathway. Acta Endocrinol. 2020, 16, 9–14. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Hostetler, G.; Riedl, K.; Cardenas, H.; Diosa-Toro, M.; Arango, D.; Schwartz, S.; Doseff, A.I. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol. Nutr. Food Res. 2012, 56, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Kornpointner, C.; Scheibelreiter, J.; Halbwirth, H. Snailase: A promising tool for the enzymatic hydrolysis of flavonoid glycosides from plant extracts. Front. Plant Sci. 2022, 13, 889184. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Kapešová, J.; Valentová, K. ‘Sweet Flavonoids’: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Shin, K.C.; Nam, H.K.; Oh, D.K. Hydrolysis of flavanone glycosides by β-glucosidase from Pyrococcus furiosus and its application to the production of flavanone aglycones from citrus extracts. J. Agric. Food Chem. 2013, 61, 11532–11540. [Google Scholar] [CrossRef]
- Ge, L.; Chen, A.; Pei, J.; Zhao, L.; Fang, X.; Ding, G.; Wang, Z.; Xiao, W.; Tang, F. Enhancing the thermostability of α-L-rhamnosidase from Aspergillus terreus and the enzymatic conversion of rutin to isoquercitrin by adding sorbitol. BMC Biotechnol. 2017, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.J.; Ji, Y.J.; Hu, J.L.; Hu, C.N.; Yang, F.; Yang, G.E. Biotransformation of Rutin using crude enzyme from Rhodopseudomonas palustris. Curr. Microbiol. 2017, 74, 431–436. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, P.; Chen, P.; Wu, D. Highly efficient enzymatic conversion of Rutin to isoquercitrin and l-rhamnose using deep eutectic solvents. ACS Sustain. Chem. Eng. 2020, 8, 14905–14913. [Google Scholar] [CrossRef]
- Li, L.J.; Liu, X.Q.; Du, X.P.; Wu, L.; Jiang, Z.D.; Ni, H.; Li, Q.B.; Chen, F. Preparation of isoquercitrin by biotransformation of rutin using α-L-rhamnosidase from Aspergillus niger JMU-TS528 and HSCCC purification. Prep. Biochem. Biotechnol. 2020, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Guo, Y.; Zhang, M.; Xie, H.; Xia, G.; Xu, L.; Yang, H.; Shen, Y. Preparation of isoquercitrin and rhamnose from readily accessible rutin by a highly specific recombinant α-L-rhamnosidase (r-Rha1). Nat. Prod. Res. 2025, 39, 2566–2571. [Google Scholar] [CrossRef]
- Shin, K.C.; Seo, M.J.; Oh, D.K.; Choi, M.N.; Kim, D.W.; Kim, Y.S.; Park, C.S. Cloning and characterization of α-L-rhamnosidase from Chloroflexus aurantiacus and its application in the production of isoquercitrin from rutin. Biotechnol. Lett. 2019, 41, 419–426. [Google Scholar] [CrossRef]
- Kim, D.Y.; Yeom, S.J.; Park, C.S.; Kim, Y.S. Effect of high hydrostatic pressure treatment on isoquercetin production from rutin by commercial α-L-rhamnosidase. Biotechnol. Lett. 2016, 38, 1775–1780. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, D.H. Purification and characterization of alpha-L-rhamnosidase from Bacteroides JY-6, a human intestinal bacterium. Biol. Pharm. Bull. 1996, 19, 1546–1549. [Google Scholar] [CrossRef]
- Miake, F.; Satho, T.; Takesue, H.; Yanagida, F.; Kashige, N.; Watanabe, K. Purification and characterization of intracellular alpha-L-rhamnosidase from Pseudomonas paucimobilis FP2001. Arch Microbiol. 2000, 173, 65–70. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Kim, D. Purification and characterization of quercitrin-hydrolyzing alpha-L-rhamnosidase from Fusobacterium K-60, a human intestinal bacterium. J. Microbiol. Biotechnol. 2005, 15, 519–524. [Google Scholar]
- Franco, E.P.D.; Contesini, F.J.; Lima da Silva, B.; Alves de Piloto Fernandes, A.M.; Wielewski Leme, C.; Gonçalves Cirino, J.P.; Bueno Campos, P.R.; de Oliveira Carvalho, P. Enzyme-assisted modification of flavonoids from Matricaria chamomilla: Antioxidant activity and inhibitory effect on digestive enzymes. J. Enzym. Inhib. Med. Chem. 2020, 35, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Kim, Y.E.; Yang, H.J.; Ko, S.; Hong, G.P. Influence of autoclave treatment and enzymatic hydrolysis on the antioxidant activity of Opuntia ficus-indica fruit extract. Food Sci. Biotechnol. 2017, 26, 581–590. [Google Scholar] [CrossRef] [PubMed]
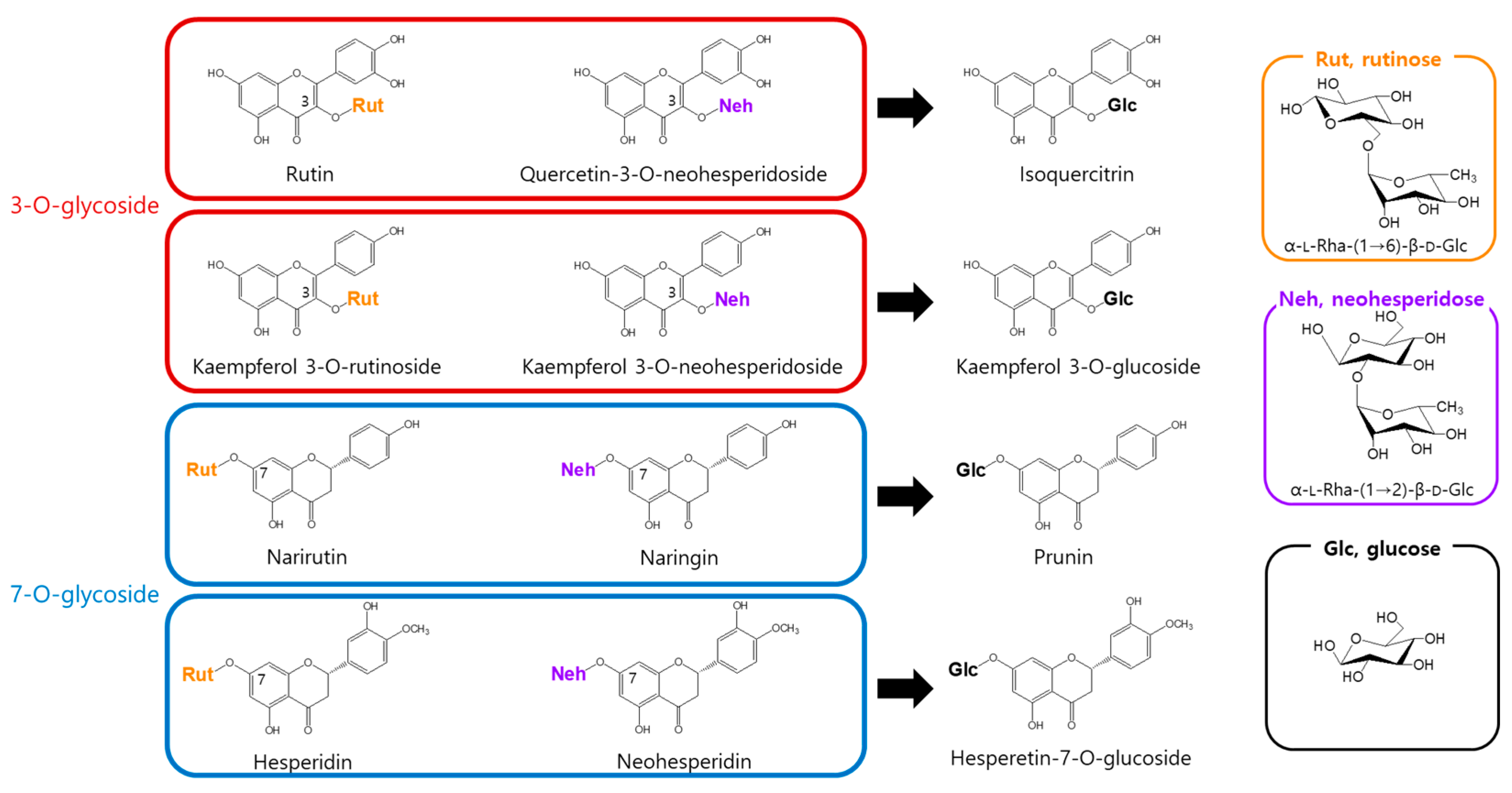

| Substrate | Specific Activity (nmol/min/mg) |
|---|---|
| Rutin | 94.04 ± 1.23 |
| Quercetin-3-O-neohesperidoside | 14.15 ± 2.38 |
| Kaempferol-3-O-rutinoside | 91.05 ± 0.89 |
| Kaempferol-3-O-neohesperidoside | 16.35 ± 1.64 |
| Narirutin | 71.35 ± 2.19 |
| Naringin | 23.51 ± 1.90 |
| Hesperidin | 66.10 ± 2.41 |
| Neohesperidin | 21.87 ± 0.96 |
| Temperature (°C) | kd (h−1) | t1/2 (h) |
|---|---|---|
| 45 | 3.08 × 10−2 | 22.5 |
| 50 | 6.11 × 10−2 | 11.3 |
| 55 | 1.05 × 10−1 | 6.6 |
| 60 | 1.49 × 10−1 | 4.7 |
| 65 | 2.05 × 10−1 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Na, C.S.; Shin, K.-C. Enhanced Antioxidant and Anti-Inflammatory Activities of Diospyros lotus Leaf Extract via Enzymatic Conversion of Rutin to Isoquercitrin. Antioxidants 2025, 14, 950. https://doi.org/10.3390/antiox14080950
Kim Y-S, Na CS, Shin K-C. Enhanced Antioxidant and Anti-Inflammatory Activities of Diospyros lotus Leaf Extract via Enzymatic Conversion of Rutin to Isoquercitrin. Antioxidants. 2025; 14(8):950. https://doi.org/10.3390/antiox14080950
Chicago/Turabian StyleKim, Yeong-Su, Chae Sun Na, and Kyung-Chul Shin. 2025. "Enhanced Antioxidant and Anti-Inflammatory Activities of Diospyros lotus Leaf Extract via Enzymatic Conversion of Rutin to Isoquercitrin" Antioxidants 14, no. 8: 950. https://doi.org/10.3390/antiox14080950
APA StyleKim, Y.-S., Na, C. S., & Shin, K.-C. (2025). Enhanced Antioxidant and Anti-Inflammatory Activities of Diospyros lotus Leaf Extract via Enzymatic Conversion of Rutin to Isoquercitrin. Antioxidants, 14(8), 950. https://doi.org/10.3390/antiox14080950






