Uric Acid and Preeclampsia: Pathophysiological Interactions and the Emerging Role of Inflammasome Activation
Abstract
1. Introduction
2. Methods
3. Uric Acid: Metabolism and Physiological Implications
4. Uric Acid in Pregnancy
5. Hyperuricemia and Preeclampsia
6. Diagnostic and Predictive Value of Uric Acid in Preeclampsia
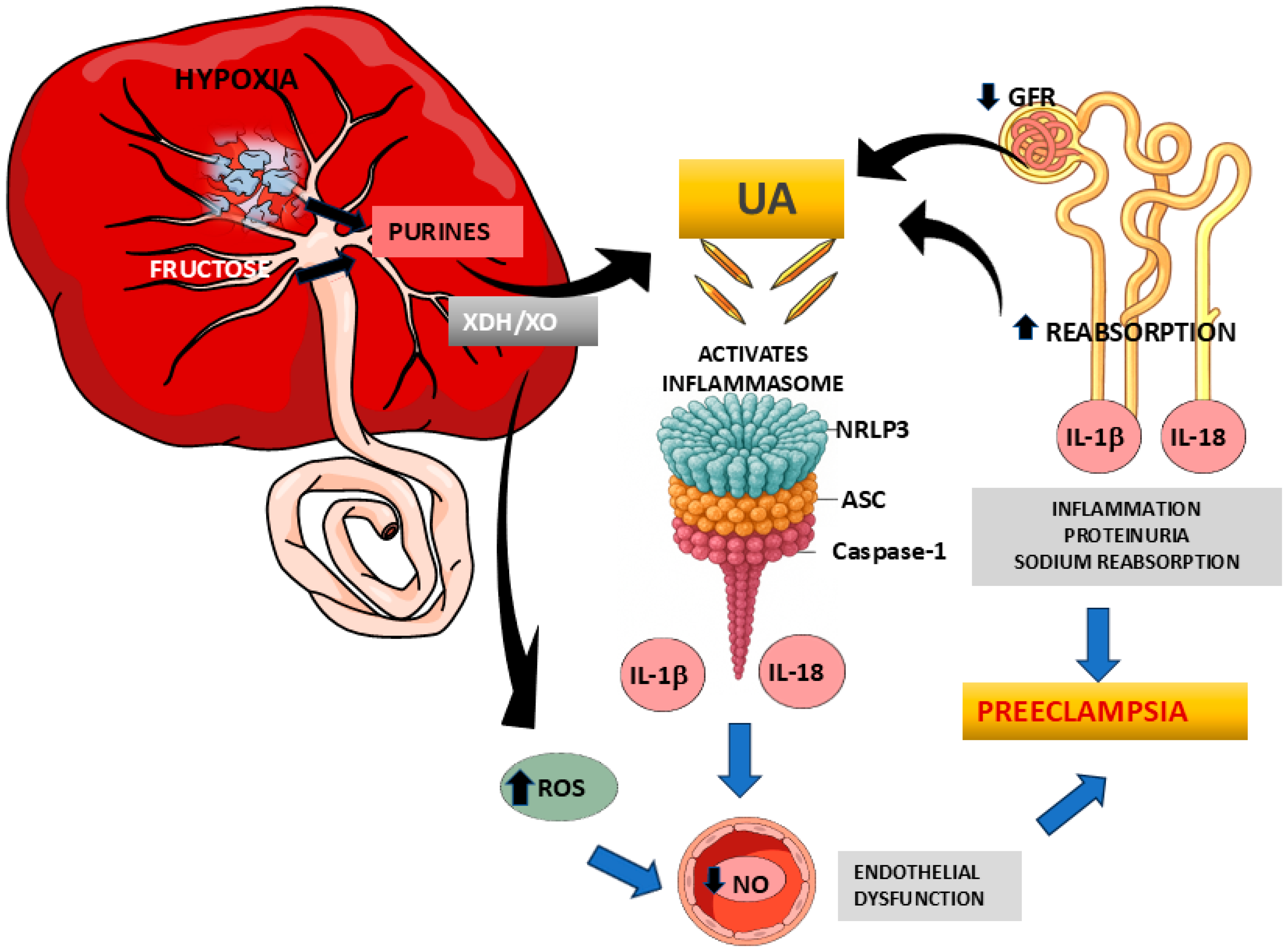
7. The NLRP3 Inflammasome as a Potential Contributor to the Pathophysiology of Preeclampsia
7.1. NLRP3 Inflammasome and Pregnancy
7.2. Evidence of NLRP3 Activation in Preeclampsia
7.3. Renal NLRP3 Inflammasome Activation in Hypertension: Implications for Preeclampsia Pathophysiology
7.4. NLRP3 Inflammasome as a Mediator of Endothelial Dysfunction: Implications for Preeclampsia Pathophysiology
8. Uric Acid and NLRP3 Inflammasome Activation
9. Uric Acid and NLRP3 Inflammasome as Targets of Preeclampsia
| AGENT | MECHANISM OF ACTION | LEVEL OF EVIDENCE | USE IN PREGNANCY | REGULATORY STATUS |
|---|---|---|---|---|
| ALLOPURINOL | Inhibits xanthine oxidase, blocking uric acid and reactive oxygen species production [125,126]. | Preclinical and some clinical studies in women with preeclampsia (PE) or cardiovascular risk [143,144]. | Documented off-label use in pregnant women; no major teratogenic effects reported [144]. | Approved for hyperuricemia and gout; off-label use in PE. FDA Category C: Risk cannot be ruled out. |
| METFORMIN | Activates AMPK; improves insulin sensitivity and exerts anti-inflammatory effects. Indirectly inhibits NLRP3 [100,141]. | Strong clinical evidence in gestational diabetes; beneficial effects in PE animal models [100,141]. | Widely used during pregnancy; considered safe by international guidelines (ACOG, NICE). | Approved by FDA and EMA; widely used in pregnancy. FDA Category B: No evidence of harm in humans. |
| MCC950 | Specific NLRP3 inhibitor; blocks inflammasome oligomerization and IL-1β/IL-18 release [134,135,136]. | Efficacy shown in animal models of salt-sensitive hypertension and PE. No clinical trials in humans [134,135,136]. | Not evaluated in pregnant women; promising preclinical results. | Not approved; in preclinical phase. Clinical development halted due to hepatotoxicity. |
| ANAKINRA | Recombinant IL-1 receptor antagonist; blocks IL-1α and IL-1β signaling, reducing inflammatory cytokine activation and NLRP3 inflammasome downstream effects [139]. | Antihypertensive effect in animal models. Modest reduction in diastolic blood pressure and confirmed anti-inflammatory effects in humans following acute coronary syndrome [138,139]. | No large studies in hypertensive pregnant women. | Approved for inflammatory diseases such as rheumatoid arthritis. FDA Category B: No evidence of risk in humans, but caution is advised. Off-label use in cardiovascular disease. |
10. Conclusion and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Steyn, W.; Zeeman, G.G.; Brown, M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014, 4, 97–104. [Google Scholar] [CrossRef]
- Roberts, J.M.; Rich-Edwards, J.W.; McElrath, T.F.; Garmire, L.; Myatt, L.; Global Pregnancy Collaboration. Subtypes of Preeclampsia: Recognition and Determining Clinical Usefulness. Hypertension 2021, 77, 1430–1441. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence points for multiple pathways. Am. J. Obs. Gynecol. 2022, 226, S907–S927. [Google Scholar] [CrossRef]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef]
- Murray, E.J.; Gumusoglu, S.B.; Santillan, D.A.; Santillan, M.K. Manipulating CD4+ T cell pathways to prevent preeclampsia. Front. Bioeng. Biotechnol. 2021, 9, 811417. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMArca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef]
- Brien, M.E.; Boufaied, I.; Soglio, D.D.; Rey, E.; Leduc, L.; Girard, S. Distinct inflammatory profile in preeclampsia and postpartum preeclampsia reveal unique mechanisms. Biol. Reprod. 2019, 100, 187–194. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, C.; Zhao, Y.; Yang, L.; Wang, R.; Shen, D.; Ren, N.; Zhang, Q. Inflammasomes in human reproductive diseases. Mol. Hum. Reprod. 2023, 29, gaad035. [Google Scholar] [CrossRef]
- Shirasuna, K.; Karasawa, T.; Takahashi, M. Role of the NLRP3 Inflammasome in Preeclampsia. Front. Endocrinol. 2020, 11, 80. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef]
- Chaudhary, K.; Malhotra, K.; Sowers, J.; Aroor, A. Uric Acid—Key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal. Med. 2013, 3, 208–220. [Google Scholar] [CrossRef]
- Major, T.J.; Topless, R.K.; Dalbeth, N.; Merriman, T.R. Evaluation of the diet wide contribution to serum urate levels: Meta-analysis of population-based cohorts. BMJ 2018, 363, k3951. [Google Scholar] [CrossRef]
- Nakagawa, T.; Andrés-Hernando, A.; Kosugi, T.; Sánchez-Lozada, L.G.; Stenvinkel, P.; Kublickiene, K.; Ananth Karumanchi, S.; Kang, D.H.; Kojima, H.; Rodriguez-Iturbe, B.; et al. Fructose might be a clue to the origin of preeclampsia insights from nature and evolution. Hypertens. Res. 2023, 46, 646–653. [Google Scholar] [CrossRef]
- Cantu-Medellin, N.; Kelley, E.E. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol. 2013, 1, 353–358. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14, Erratum in Int. J. Cardiol. 2023, 387, 131126. https://doi.org/10.1016/j.ijcard.2023.131126. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Moe, O.W. Renal transport of uric acid: Evolving concepts and uncertainties. Adv. Chronic Kidney Dis. 2012, 19, 358–371. [Google Scholar] [CrossRef]
- Chung, S.; Kim, G.H. Urate Transporters in the Kidney: What Clinicians Need to Know. Electrolyte Blood Press 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Halperin Kuhns, V.L.; Woodward, O.M. Urate transport in health and disease. Best. Pract. Res. Clin. Rheumatol. 2021, 35, 101717. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.; Zhou, P.; Jiang, T. Uric acid transporters hiding in the intestine. Pharm. Biol. 2016, 54, 3151–3155. [Google Scholar] [CrossRef]
- Cutler, R.G.; Camandola, S.; Feldman, N.H.; Yoon, J.S.; Haran, J.B.; Arguelles, S.; Mattson, M.P. Uric acid enhances longevity and endurance and protects the brain against ischemia. Neurobiol. Aging 2019, 75, 159–168. [Google Scholar] [CrossRef]
- Fabbrini, E.; Serafini, M.; Baric, I.; Hazen, S.L.; Klein, S. Effect of Plasma Uric Acid on Antioxidant Capacity, Oxidative Stress, and Insulin Sensitivity in Obese Subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef]
- Ma, P.; Zhao, M.; Li, Y.; Zhang, G.; Ma, Y.; Shi, Y.; Su, P.; Chen, R.; Tang, Z.G.; Zhang, Y.; et al. The protective effects of uric acid against myocardial ischemia via the Nrf2 pathway. Eur. J. Pharmacol. 2023, 959, 176062. [Google Scholar] [CrossRef]
- Wen, S.; Arakawa, H.; Tamai, I. Uric acid in health and disease: From physiological functions to pathogenic mechanisms. Pharmacol. Ther. 2024, 256, 108615. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. A Possible Therapeutic Application of the Selective Inhibitor of Urate Transporter 1, Dotinurad, for Metabolic Syndrome, Chronic Kidney Disease, and Cardiovascular Disease. Cells 2024, 13, 450. [Google Scholar] [CrossRef]
- Dewulf, J.P.; Sandrine, M.; Nassogne, M.C. Disorders of purine biosynthesis metabolism. Mol. Genet. Metab. 2022, 136, 190–198. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Andrés-Hernándo, A.; García-Arroyo, F.E.; Cicerchi, C.; Li, N.; Kuwabara, M.; Roncal-Jiménez, C.A.; Johnson, R.J.; Lanaspa, M.A. Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J. Biol. Chem. 2019, 294, 4272–4281. [Google Scholar] [CrossRef]
- Grivei, A.; Giuliani, K.; Wang, X.; Ungerer, J.; Francis, L.; Hepburn, K.; John, G.T.; Gois, P.; Kassianos, A.J.; Healy, H. Oxidative stress and inflammasome activation in human rhabdomyolysis-induced acute kidney injury. Free Radic. Biol. Med. 2020, 160, 690–695. [Google Scholar] [CrossRef]
- Kim, G.H.; Jun, J.B. Altered Serum Uric Acid Levels in Kidney Disorders. Life 2022, 12, 1891. [Google Scholar] [CrossRef]
- Khanna, P.; Johnson, R.J.; Marder, B.; LaMoreaux, B.; Kumar, A. Systemic Urate Deposition: An Unrecognized Complication of Gout? J. Clin. Med. 2020, 9, 3204. [Google Scholar] [CrossRef]
- Li, D.; Yuan, S.; Deng, Y.; Wang, X.; Wu, S.; Chen, X.; Li, Y.; Ouyang, J.; Lin, D.; Quan, H.; et al. The dysregulation of immune cells induced by uric acid: Mechanisms of inflammation associated with hyperuricemia and its complications. Front. Immunol. 2023, 14, 1282890. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cristóbal-García, M.; García-Arroyo, F.; Soto, V.; Cruz-Robles, D.; Nakagawa, T.; Yu, M.A.; Kang, D.H.; Johnson, R.J. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp. Nephrol. 2012, 121, e71–e78. [Google Scholar] [CrossRef]
- Mishima, M.; Hamada, T.; Maharani, N.; Ikeda, N.; Onohara, T.; Notsu, T.; Ninomiya, H.; Miyazaki, S.; Mizuta, E.; Sugihara, S.; et al. Effects of Uric Acid on the NO Production of HUVECs and its Restoration by Urate Lowering Agents. Drug Res. 2016, 66, 270–274. [Google Scholar] [CrossRef]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef]
- Huang, Z.; Hong, Q.; Zhang, X.; Xiao, W.; Wang, L.; Cui, S.; Feng, Z.; Lv, Y.; Cai, G.; Chen, X.; et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun. Signal. 2017, 15, 3. [Google Scholar] [CrossRef]
- Many, A.; Hubel, C.A.; Roberts, J.M. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am. J. Obstet. Gynecol. 1996, 174 Pt 1, 288–291. [Google Scholar] [CrossRef]
- Jauniaux, E.; Hempstock, J.; Teng, C.; Battaglia, F.C.; Burton, G.J. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: Maintenance of redox potential in a low oxygen environment. J. Clin. Endocrinol. Metab. 2005, 90, 1171–1175. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Asghar, Z.A.; Thompson, A.; Chi, M.; Cusumano, A.; Scheaffer, S.; Al-Hammadi, N.; Saben, J.L.; Moley, K.H. Maternal fructose drives placental uric acid production leading to adverse fetal outcomes. Sci. Rep. 2016, 6, 25091. [Google Scholar] [CrossRef]
- Lind, T.; Godfrey, K.A.; Otun, H.; Philips, P.R. Changes in serum uric acid concentrations during normal pregnancy. Br. J. Obstet. Gynaecol. 1984, 91, 128–132. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, J.; Liu, X.; Guo, S.; Ming, L. Trimester-Specific Reference Intervals of Serum Urea, Creatinine, and Uric Acid Among Healthy Pregnant Women in Zhengzhou, China. Lab. Med. 2021, 52, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, Q.; Li, Q.; Cao, S.; Zhang, Y.; Liu, Y.; Qin, X. Consecutive reference intervals for biochemical indices related to serum lipid levels and renal function during normal pregnancy. BMC Pregnancy Childbirth 2022, 22, 642. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.B.; Abraham, W.T.; Zamudio, S.; Coffin, C.; Merouani, A.; Young, D.; Johnson, A.; Osorio, F.; Goldberg, C.; Moore, L.G.; et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998, 54, 2056–2063, Erratum in Kidney Int. 1999, 55, 1185. [Google Scholar] [CrossRef] [PubMed]
- Halperin Kuhns, V.L.; Woodward, O.M. Sex Differences in Urate Handling. Int. J. Mol. Sci. 2020, 21, 4269. [Google Scholar] [CrossRef]
- Takiue, Y.; Hosoyamada, M.; Kimura, M.; Saito, H. The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids 2011, 30, 113–119. [Google Scholar] [CrossRef]
- Lam, C.; Lim, K.-H.; Kang, D.-H.; Karumanchi, S.A. Uric acid and preeclampsia. Semin. Nephrol. 2005, 25, 56–60. [Google Scholar] [CrossRef]
- Pertegal, M.; Fenoy, F.J.; Bonacasa, B.; Mendiola, J.; Delgado, J.L.; Hernández, M.; Salom, M.G.; Bosch, V.; Hernández, I. 2-methoxyestradiol plasma levels are associated with clinical severity indices and biomarkers of preeclampsia. Reprod. Sci. 2015, 22, 198–206. [Google Scholar] [CrossRef]
- Powers, R.W.; Bodnar, L.M.; Ness, R.B.; Cooper, K.M.; Gallaher, M.J.; Frank, M.P.; Daftary, A.R.; Roberts, J.M. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am. J. Obstet. Gynecol. 2006, 194, 160. [Google Scholar] [CrossRef]
- Hassen, F.S.; Malik, T.; Dejenie, T.A. Evaluation of serum uric acid and liver function tests among pregnant women with and without preeclampsia at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS ONE 2022, 17, e0272165. [Google Scholar] [CrossRef]
- Adu-Bonsaffoh, K.; Kudaya, D.Q.; Fidelis, B.; Fondjo, L.A.; Ahenkorah, J. Alteration in maternal serum uric acid levels in pre-eclampsia and associated perinatal outcomes: A cross-sectional study in Ghana. Pan Afr. Med. J. 2024, 47, 49. [Google Scholar] [CrossRef]
- Sani, H.M.; Vahed, S.Z.; Ardalan, M. Preeclampsia: A close look at renal dysfunction. Biomed. Pharmacother. 2019, 109, 408–416. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, C.; Li, X.; Sun, L.; Zhu, X.; Zhao, C.; Zhang, Z.; Yang, Z. Serum Uric Acid Levels and Risk of Metabolic Syndrome: A Dose-Response Meta-Analysis of Prospective Studies. J. Clin. Endocrinol. Metab. 2015, 100, 4198–4207. [Google Scholar] [CrossRef]
- Lüscher, B.P.; Surbek, D.V.; Clémençon, B.; Huang, X.; Albrecht, C.; Marini, C.; Hediger, M.A.; Baumann, M.U. Different Pharmacological Properties of GLUT9a and GLUT9b: Potential Implications in Preeclampsia. Cell Physiol. Biochem. 2019, 53, 508–517. [Google Scholar] [CrossRef]
- Lüscher, B.P.; Schoeberlein, A.; Surbek, D.V.; Baumann, M.U. Hyperuricemia during Pregnancy Leads to a Preeclampsia-Like Phenotype in Mice. Cells 2022, 11, 3703. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A.E.; Lacey, H.A.; Jones, C.J.; Huppertz, B.; Baker, P.N.; Crocker, I.P. Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta 2008, 29, 175–186. [Google Scholar] [CrossRef]
- Roland, C.S.; Hu, J.; Ren, C.E.; Chen, H.; Li, J.; Varvoutis, M.S.; Leaphart, L.W.; Byck, D.B.; Zhu, X.; Jiang, S.W. Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia. Cell Mol. Life Sci. 2016, 73, 365–376. [Google Scholar] [CrossRef]
- Carrasco-Wong, I.; Aguilera-Olguín, M.; Escalona-Rivano, R.; Chiarello, D.I.; Barragán-Zúñiga, L.-J.; Sosa-Macías, M.; Galaviz-Hernandez, C.; San Martín, S.; Gutiérrez, J. Syncytiotrophoblast stress in early onset preeclampsia: The issues perpetuating the syndrome. Placenta 2021, 113, 57–66. [Google Scholar] [CrossRef]
- Annesi, L.; Tossetta, G.; Borghi, C.; Piani, F. The Role of Xanthine Oxidase in Pregnancy Complications: A Systematic Review. Antioxidants 2024, 13, 1234. [Google Scholar] [CrossRef]
- Many, A.; Hubel, C.A.; Fisher, S.J.; Roberts, J.M.; Zhou, Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am. J. Pathol. 2000, 156, 321–331. [Google Scholar] [CrossRef]
- San Juan-Reyes, S.; Gómez-Oliván, L.M.; Islas-Flores, H.; Dublán-García, O. Oxidative stress in pregnancy complicated by preeclampsia. Arch. Biochem. Biophys. 2020, 681, 108255. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, J.M.; Noguera, J.A.; Avilés, F.; Hernández-Caselles, T.; de Paco-Matallana, C.; Delgado, J.L.; Cuevas, S.; Llinás, M.T.; Hernández, I. Pravastatin reduces plasma levels of extracellular vesicles in pregnancies at high risk of term preeclampsia. Front. Pharmacol. 2023, 14, 1166123. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef]
- Phan, S.H.; Gannon, D.E.; Ward, P.A.; Karmiol, S. Mechanism of neutrophil-induced xanthine dehydrogenase to xanthine oxidase conversion in endothelial cells: Evidence of a role for elastase. Am. J. Respir. Cell Mol. Biol. 1992, 6, 270–278. [Google Scholar] [CrossRef]
- Cnossen, J.S.; de Ruyter-Hanhijärvi, H.; van der Post, J.A.; Mol, B.W.; Khan, K.S.; ter Riet, G. Accuracy of serum uric acid determination in predicting pre-eclampsia: A systematic review. Acta Obstet. Gynecol. Scand. 2006, 85, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, C.M.; van Pampus, M.G.; Groen, H.; Aarnoudse, J.G.; van den Berg, P.P.; Mol, B.W. Accuracy of serum uric acid as a predictive test for maternal complications in pre-eclampsia: Bivariate meta-analysis and decision analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 8–14. [Google Scholar] [CrossRef]
- Bellos, I.; Pergialiotis, V.; Loutradis, D.; Daskalakis, G. The prognostic role of serum uric acid levels in preeclampsia: A meta-analysis. J. Clin. Hypertens. 2020, 22, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Delić, R.; Stefanović, M. Optimal laboratory panel for predicting preeclampsia. J. Matern. Fetal Neonatal Med. 2010, 23, 96–102. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, X.; Wang, Z.; Hu, Y. Combination of lipids and uric acid in mid-second trimester can be used to predict adverse pregnancy outcomes. J. Matern. Neonatal Med. 2012, 25, 2633–2638. [Google Scholar] [CrossRef]
- Tang, Z.; Ji, Y.; Zhou, S.; Su, T.; Yuan, Z.; Han, N.; Jia, J.; Wang, H. Development and Validation of Multi-Stage Prediction Models for Pre-eclampsia: A Retrospective Cohort Study on Chinese Women. Front. Public Health 2022, 10, 911975. [Google Scholar] [CrossRef]
- Dal, Y.; Karaca, S.G.; Akkuş, F.; Karagün, Ş.; Nessar, A.Z.; Coşkun, A. Evaluation of the diagnostic value of the HALP score, uric acid value, and uric acid-creatinine ratio in preeclampsia. Ceska Gynekol. 2024, 89, 180–187. [Google Scholar] [CrossRef]
- Colmenares-Mejia, C.C.; Quintero-Lesmes, D.C.; Bautista-Niño, P.K.; Guío, E.; Paez, M.C.; Beltrán, M.; Williams, D.; Gray, K.J.; Casas, J.P.; Serrano, N.C. Uric acid and risk of pre-eclampsia: Results from a large case-control study and meta-analysis of prospective studies. Sci. Rep. 2023, 13, 3018. [Google Scholar] [CrossRef]
- Yue, C.; Ying, C.; Li, X. Association of first trimester serum uric acid with preeclampsia: An observational cohort study with propensity score matching. Hypertens. Res. 2023, 46, 377–385. [Google Scholar] [CrossRef]
- de Mendonça, E.L.S.S.; da Silva, J.V.F.; Mello, C.S.; de Oliveira, A.C.M. Serum uric acid levels associated with biochemical parameters linked to preeclampsia severity and to adverse perinatal outcomes. Arch. Gynecol. Obstet. 2022, 305, 1453–1463. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K.; Maini, B. Impact of maternal serum uric acid on perinatal outcome in women with hypertensive disorders of pregnancy: A prospective study. Pregnancy Hypertens. 2017, 10, 220–225. [Google Scholar] [CrossRef]
- Villar, J.; Cavoretto, P.I.; Barros, F.C.; Romero, R.; Papageorghiou, A.T.; Kennedy, S.H. Etiologically Based Functional Taxonomy of the Preterm Birth Syndrome. Clin. Perinatol. 2024, 51, 475–495. [Google Scholar] [CrossRef]
- Yalamati, P.; Bhongir, A.V.; Betha, K.; Verma, R.; Dandge, S. Relationship of serum uric acid, serum creatinine and serum cystatin C with maternal and fetal outcomes in rural Indian pregnant women. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 4, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Ding, M.; Shen, J.; Huang, Y.; Li, J.; Sun, A.; Hong, J.; Yang, Y.; He, S.; Pei, C.; et al. Causal pathways in preeclampsia: A Mendelian randomization study in European populations. Front. Endocrinol. 2024, 15, 1453277. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Warner, N.; Inohara, N.; Núñez, G. NOD1 and NOD2: Signaling, host defense, and inflammatory disease. Immunity 2014, 41, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, Y. Multifaceted roles of ninjurin1 in immunity, cell death, and disease. Front. Immunol. 2025, 16, 1519519. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Motomura, K.; Miller, D.; Garcia-Flores, V.; Galaz, J.; Romero, R. Inflammasomes: Their Role in Normal and Complicated Pregnancies. J. Immunol. 2019, 203, 2757–2769. [Google Scholar] [CrossRef]
- Matias, M.L.; Romão, M.; Weel, I.C.; Ribeiro, V.R.; Nunes, P.R.; Borges, V.T.; Araújo, J.P., Jr.; Peraçoli, J.C.; de Oliveira, L.; Peraçoli, M.T. Endogenous and Uric Acid-Induced Activation of NLRP3 Inflammasome in Pregnant Women with Preeclampsia. PLoS ONE 2015, 10, e0129095. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, B.; Romero, R.; Gomez-Lopez, N.; Xu, Y.; Leng, Y.; Maymon, E.; Pacora, P.; Erez, O.; Yeo, L.; Hassan, S.S.; et al. In vivo evidence of inflammasome activation during spontaneous labor at term. J. Matern. Neonatal Med. 2018, 32, 1978–1991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, R.; Lappas, M. NOD-like receptor pyrin domain-containing-3 (NLRP3) regulates inflammation-induced pro-labor mediators in human myometrial cells. Am. J. Reprod. Immunol. 2018, 79, e12825. [Google Scholar] [CrossRef]
- Zhu, J.; He, M.; Ma, C.; Peng, F.; Su, Y.; Huang, L. Expression and Clinical Significance of NOD-Like Receptor Protein 3 (NLRP3) and Caspase-1 in Fetal Membrane and Placental Tissues of Patients with Premature Rupture of Membrane. Med. Sci. Monit. 2018, 24, 1560–1566. [Google Scholar] [CrossRef]
- Tamura, K.; Ishikawa, G.; Yoshie, M.; Ohneda, W.; Nakai, A.; Takeshita, T.; Tachikawa, E. Glibenclamide inhibits NLRP3 inflammasome-mediated IL-1β secretion in human trophoblasts. J. Pharmacol. Sci. 2017, 135, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M. Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1β secretion. Am. J. Reprod. Immunol. 2013, 71, 189–201. [Google Scholar] [CrossRef]
- Gotsch, F.; Romero, R.; Chaiworapongsa, T.; Erez, O.; Vaisbuch, E.; Espinoza, J.; Kusanovic, J.P.; Mittal, P.; Mazaki-Tovi, S.; Kim, C.J.; et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: A link between the inflammasome and parturition. J. Matern. Neonatal Med. 2008, 21, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Muñoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef]
- Yang, H.; Ai, M.; Guo, Y.; Li, B.; Liu, C.; Qu, D. NLRP3 inflammasome in peripheral blood monocytes as a risk factor for early -onset preeclampsia. BMC Pregnancy Childbirth 2023, 23, 380. [Google Scholar] [CrossRef]
- Weel, I.C.; Romão-Veiga, M.; Matias, M.L.; Fioratti, E.G.; Peraçoli, J.C.; Borges, V.T.; Araujo, J.P., Jr.; Peraçoli, M.T. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol. 2017, 123, 40–47. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Han, T.L.; Zhou, X.; Qi, H.; Baker, P.N.; Zhou, W.; Zhang, H. Endoplasmic reticulum stress may activate NLRP3 inflammasomes via TXNIP in preeclampsia. Cell Tissue Res. 2020, 379, 589–599, Erratum in Cell Tissue Res. 2020, 380, 203. https://doi.org/10.1007/s00441-019-03138-z. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Nakashima, A.; Huber, W.J.; Davis, S.; Banerjee, S.; Huang, Z.; Saito, S.; Sadovsky, Y.; Sharma, S. Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. 2019, 10, 927. [Google Scholar] [CrossRef]
- Garcia-Puente, L.M.; Fraile-Martinez, O.; García-Montero, C.; Bujan, J.; De León-Luis, J.A.; Bravo, C.; Rodríguez-Benitez, P.; Pintado, P.; Ruiz-Labarta, F.J.; Álvarez-Mon, M.; et al. Placentas from Women with Late-Onset Preeclampsia Exhibit Increased Expression of the NLRP3 Inflammasome Machinery. Biomolecules 2023, 13, 1644. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.B.; Gierman, L.M.; Rakner, J.J.; Stødle, G.S.; Mundal, S.B.; Thaning, A.J.; Sporsheim, B.; Elschot, M.; Collett, K.; Bjørge, L.; et al. Cholesterol Crystals and NLRP3 Mediated Inflammation in the Uterine Wall Decidua in Normal and Preeclamptic Pregnancies. Front. Immunol. 2020, 11, 564712. [Google Scholar] [CrossRef]
- Xu, L.; Li, S.; Liu, Z.; Jiang, S.; Wang, J.; Guo, M.; Zhao, X.; Song, W.; Liu, S. The NLRP3 rs10754558 polymorphism is a risk factor for preeclampsia in a Chinese Han population. J. Matern. Fetal Neonatal Med. 2019, 32, 1792–1799. [Google Scholar] [CrossRef]
- Rezaei, M.; Ghasemi, M.; Saravani, M.; Moghadam, R.G.; Shahraki-Ghadimi, H.; Norouzi, M.; Salimi, S. The effects of NLRP3 rs10754558 and rs4612666 polymorphisms on preeclampsia susceptibility, onset, and severity: A case-control study and in silico analysis. Mol. Biol. Res. Commun. 2024, 13, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Han, X.; Zhu, Z.; Yu, S.; Mei, S.; Cheng, X.; Zhang, W.; Zhang, G.; Fang, D. Increased uterine NLRP3 inflammasome and leucocyte infiltration in a rat model of preeclampsia. Am. J. Reprod. Immunol. 2021, 86, e13493. [Google Scholar] [CrossRef]
- Shirasuna, K.; Karasawa, T.; Usui, F.; Kobayashi, M.; Komada, T.; Kimura, H.; Kawashima, A.; Ohkuchi, A.; Taniguchi, S.; Takahashi, M. NLRP3 Deficiency Improves Angiotensin II-Induced Hypertension but Not Fetal Growth Restriction During Pregnancy. Endocrinology 2015, 156, 4281–4292. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Ma, H.; Hao, H.; Wang, F.; Yang, H. Metformin inhibits activation of NLRP3 inflammasome and inflammatory response in preeclamptic rats. Gene 2024, 919, 148509. [Google Scholar] [CrossRef]
- Cui, D.; Liu, S.; Tang, M.; Lu, Y.; Zhao, M.; Mao, R.; Wang, C.; Yuan, Y.; Li, L.; Chen, Y.; et al. Phloretin ameliorates hyperuricemia-induced chronic renal dysfunction through inhibiting NLRP3 inflammasome and uric acid reabsorption. Phytomedicine 2020, 66, 153111. [Google Scholar] [CrossRef]
- Romero, C.A.; Remor, A.; Latini, A.; De Paul, A.L.; Torres, A.I.; Mukdsi, J.H. Uric acid activates NLRP3 inflammasome in an in-vivo model of epithelial to mesenchymal transition in the kidney. Histochem. J. 2017, 48, 209–218. [Google Scholar] [CrossRef]
- Kim, I.Y.; Lee, D.W.; Lee, S.B.; Kwak, I.S. The role of uric acid in kidney fibrosis: Experimental evidences for the causal relationship. Biomed. Res. Int. 2014, 2014, 638732. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, X.L.; Fu, C.; Han, R.; Chen, W.; Lu, Y.; Ye, Z. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int. J. Mol. Med. 2015, 35, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, C.; Pelegrín, P.; Baroja-Mazo, A.; Cuevas, S. Emerging Role of the Inflammasome and Pyroptosis in Hypertension. Int. J. Mol. Sci. 2021, 22, 1064. [Google Scholar] [CrossRef] [PubMed]
- Conley, S.M.; Abais, J.M.; Boini, K.M.; Li, P.L. Inflammasome Activation in Chronic Glomerular Diseases. Curr. Drug Targets 2017, 18, 1019–1029. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, R.; Huang, B.; Hou, J.; Yin, J.; Zhao, T.; Zhou, L.; Wu, R.; Qian, Y.; Wang, F. Tumor necrosis factor-α blockade ameliorates diabetic nephropathy in rats. Clin. Kidney J. 2019, 14, 301–308. [Google Scholar] [CrossRef]
- Tashiro, M.; Sasatomi, Y.; Watanabe, R.; Watanabe, M.; Miyake, K.; Abe, Y.; Yasuno, T.; Ito, K.; Ueki, N.; Hamauchi, A.; et al. IL-1β promotes tubulointerstitial injury in MPO-ANCA-associated glomerulonephritis. Clin. Nephrol. 2016, 86, 190–199. [Google Scholar] [CrossRef]
- Zhang, J.; Rudemiller, N.P.; Patel, M.B.; Karlovich, N.S.; Wu, M.; McDonough, A.A.; Griffiths, R.; Sparks, M.A.; Jeffs, A.D.; Crowley, S.D. Interleukin-1 Receptor Activation Potentiates Salt Reabsorption in Angiotensin II-Induced Hypertension via the NKCC2 Co-transporter in the Nephron. Cell Metab. 2016, 23, 360–368. [Google Scholar] [CrossRef]
- Thomas, J.M.; Ling, Y.H.; Huuskes, B.; Jelinic, M.; Sharma, P.; Saini, N.; Kem-Harper, B.K.; O’Connor, P.M.; Latz, E.; Arumugan, T.V.; et al. IL-18 (Interleukin-18) Produced by Renal Tubular Epithelial Cells Promotes Renal Inflammation and Injury During Deoxycorticosterone/Salt-Induced Hypertension in Mice. Hypertension 2021, 78, 1296–1309. [Google Scholar] [CrossRef]
- Pitzer, A.; Elijovich, F.; Laffer, C.L.; Ertuglu, L.A.; Sahinoz, M.; Saleem, M.; Krishnan, J.; Dola, T.; Aden, L.A.; Sheng, Q.; et al. DC ENaC-Dependent Inflammasome Activation Contributes to Salt-Sensitive Hypertension. Circ. Res. 2022, 131, 328–344. [Google Scholar] [CrossRef]
- Hu, C.C.; Katerelos, M.; Choy, S.W.; Crossthwaite, A.; Walker, S.P.; Pell, G.; Lee, M.; Cook, N.; Mount, P.F.; Paizis, K.; et al. Pre-eclampsia is associated with altered expression of the renal sodium transporters NKCC2, NCC and ENaC in urinary extracellular vesicles. PLoS ONE 2018, 13, e0204514. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, G.; Wu, Q.; Shen, L.; Liu, D.; Wang, Z.; Wang, W.; Ren, Z.; Jia, Y.; Liu, M.; et al. Renal CD81 interacts with sodium potassium 2 chloride cotransporter and sodium chloride cotransporter in rats with lipopolysaccharide-induced preeclampsia. FASEB J. 2023, 37, e22834. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Yang, X.; Liu, K.; Zhang, X.; Zuo, X.; Ye, R.; Wang, Z.; Shi, R.; Meng, Q.; et al. Signaling pathways in vascular function and hypertension: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 168. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Luo, M.; Cheng, Z.; Wang, R.; Liu, Q.; Lv, D.; Yan, J.; Shang, F.; Luo, S.; et al. NLRP3 inflammasome contributes to endothelial dysfunction in angiotensin II-induced hypertension in mice. Microvasc. Res. 2022, 143, 104384. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Pagliaro, P. Endothelial Dysfunction: Redox Imbalance, NLRP3 Inflammasome, and Inflammatory Responses in Cardiovascular Diseases. Antioxidants 2025, 14, 256. [Google Scholar] [CrossRef]
- Lv, Z.; Lv, D.Y.; Meng, J.Y.; Sha, X.Y.; Qian, X.Y.; Chen, Y.S.; Pan, X.Y.; Yu, G.Y.; Liu, H.S. Trophoblastic mitochondrial DNA induces endothelial dysfunction and NLRP3 inflammasome activation: Implications for preeclampsia. Int. Immunopharmacol. 2023, 114, 109523. [Google Scholar] [CrossRef]
- Mulla, M.J.; Myrtolli, K.; Potter, J.; Boeras, C.; Kavathas, P.B.; Sfakianaki, A.K.; Tadesse, S.; Norwitz, E.R.; Guller, S.; Abrahams, V.M. Uric acid induces trophoblast IL-1β production via the inflammasome: Implications for the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 2011, 65, 542–548. [Google Scholar] [CrossRef]
- Matias, M.L.; Gomes, V.J.; Romao-Veiga, M.; Ribeiro, V.R.; Nunes, P.R.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Silibinin Downregulates the NF-κB Pathway and NLRP1/NLRP3 Inflammasomes in Monocytes from Pregnant Women with Preeclampsia. Molecules 2019, 24, 1548. [Google Scholar] [CrossRef]
- Nunes, P.R.; Romao-Veiga, M.; Ribeiro, V.R.; de Oliveira, L.R.C.; de Carvalho Depra, I.; de Oliveira, L.G.; Peracoli, J.C.; Peracoli, M.T.S. Inflammasomes in placental explants of women with preeclampsia cultured with monosodium urate may be modulated by vitamin D. Hypertens. Pregnancy 2022, 41, 139–148. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Farina, A.; Salmeri, N.; Syngelaki, A.; Tan, M.Y.; Nicolaides, K.H. First trimester risk of preeclampsia and rate of spontaneous birth in patients without preeclampsia. Am. J. Obstet. Gynecol. 2024, 231, 452.e1–452.e7. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Kim, J.S. Chronic inflammation of the placenta: Definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.H.; Kim, Y.G.; Kim, S.Y.; Seo, J.W.; Choi, Y.W.; Kim, D.J.; Jeong, K.H.; Lee, T.W.; Ihm, C.G.; et al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am. J. Physiol.-Ren. Physiol. 2015, 308, F993–F1003. [Google Scholar] [CrossRef]
- Foresto-Neto, O.; Ávila, V.F.; Arias, S.C.A.; Zambom, F.F.F.; Rempel, L.C.T.; Faustino, V.D.; Machado, F.G.; Malheiros, D.M.A.C.; Abensur, H.; Camara, N.O.S.; et al. NLRP3 inflammasome inhibition ameliorates tubulointerstitial injury in the remnant kidney model. Lab. Investig. 2018, 98, 773–782. [Google Scholar] [CrossRef]
- MacIsaac, R.L.; Salatzki, J.; Higgins, P.; Walters, M.R.; Padmanabhan, S.; Dominiczak, A.F.; Touyz, R.M.; Dawson, J. Allopurinol and Cardiovascular Outcomes in Adults With Hypertension. Hypertension 2016, 67, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Piani, F.; Agnoletti, D.; Borghi, C. Advances in pharmacotherapies for hyperuricemia. Expert. Opin. Pharmacother. 2023, 24, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Kojima, S.; Uchiyama, K.; Yokota, N.; Tokutake, E.; Wakasa, Y.; Hiramitsu, S.; Waki, M.; Jinnouchi, H.; Kakuda, H.; et al. Febuxostat for Cerebral and Cardiorenovascular Events Prevention Study (FREED) investigators. Effect of febuxostat on clinical outcomes in patients with hyperuricemia and cardiovascular disease. Int. J. Cardiol. 2022, 349, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.D.; Hansell, J.A.; Herrera, E.A.; Allison, B.J.; Niu, Y.; Brain, K.L.; Kaandorp, J.J.; Derks, J.B.; Giussani, D.A. Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy. J. Physiol. 2013, 592, 475–489. [Google Scholar] [CrossRef]
- Derks, J.B.; Oudijk, M.A.; Torrance, H.L.; Rademaker, C.M.; Benders, M.J.; Rosen, K.G.; Cindrova-Davies, T.; Thakor, A.S.; Visser, G.H.; Burton, G.J.; et al. Allopurinol reduces oxidative stress in the ovine fetal cardiovascular system after repeated episodes of ischemia-reperfusion. Pediatr. Res. 2010, 68, 374–380. [Google Scholar] [CrossRef][Green Version]
- Rubinstein, J.; Woo, J.G.; Garcia, A.M.; Alsaied, T.; Li, J.; Lunde, P.K.; Moore, R.A.; Laasmaa, M.; Sammons, A.; Mays, W.A.; et al. Probenecid Improves Cardiac Function in Subjects with a Fontan Circulation and Augments Cardiomyocyte Calcium Homeostasis. Pediatr. Cardiol. 2020, 41, 1675–1688. [Google Scholar] [CrossRef]
- Nakata, T.; Ikeda, S.; Koga, S.; Yonekura, T.; Tsuneto, A.; Doi, Y.; Fukae, S.; Minami, T.; Kawano, H.; Maemura, K. Randomized, Open-Label, Cross-Over Comparison of the Effects of Benzbromarone and Febuxostat on Endothelial Function in Patients with Hyperuricemia. Int. Heart J. 2020, 61, 984–992. [Google Scholar] [CrossRef]
- Akbari, A.; Razmi, M.; Rafiee, M.; Watts, G.F.; Sahebkar, A. The Effect of Statin Therapy on Serum Uric Acid Levels: A Systematic Review and Meta-analysis. Curr. Med. Chem. 2024, 31, 1726–1739. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.I.A.; Azis, M.A.; Riu, D.S.; Wawengkang, E.; Ernawati, E.; Bachnas, M.A.; Sulistyowati, S.; Dachlan, E.G.; Mose, J.C.; Dekker, G. INOVASIA Study: A Multicenter Randomized Clinical Trial of Pravastatin to Prevent Preeclampsia in High-Risk Patients. Am. J. Perinatol. 2024, 41, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Xiao, L.; Sun, G.; Li, M.; Yang, H.; Ming, Z.; Zhao, K.; Shang, X.; Zhang, H.; Liu, C. TMBIM4 Deficiency Facilitates NLRP3 Inflammasome Activation-Induced Pyroptosis of Trophoblasts: A Potential Pathogenesis of Preeclampsia. Biology 2023, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Travis, O.K.; Shields, C.A.; Tardo, G.A.; Giachelli, C.; Nutter, C.W.; Glenn, H.L.; Cooper, O.G.; Davis, T.; Thomas, R.; et al. NLRP3 inhibition improves maternal hypertension, inflammation, and vascular dysfunction in response to placental ischemia. Am. J. Physiol. Integr. Comp. Physiol. 2023, 324, R556–R567. [Google Scholar] [CrossRef]
- Tapia-Abellán, A.; Angosto-Bazarra, D.; Martínez-Banaclocha, H.; de Torre-Minguela, C.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H.; Arostegui, J.I.; Pelegrin, P. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 2019, 15, 560–564, Erratum in Nat. Chem. Biol. 2021, 17, 361. https://doi.org/10.1038/s41589-021-00741-6. [Google Scholar] [CrossRef]
- Ramírez, J.; Cañete, J.D. Anakinra for the treatment of rheumatoid arthritis: A safety evaluation. Expert. Opin. Drug Saf. 2018, 17, 727–732. [Google Scholar] [CrossRef]
- Ling, Y.H.; Krishnan, S.M.; Chan, C.T.; Diep, H.; Ferens, D.; Chin-Dusting, J.; Kemp-Harper, B.K.; Samuel, C.S.; Hewitson, T.D.; Latz, E.; et al. Anakinra reduces blood pressure and renal fibrosis in one kidney/DOCA/salt-induced hypertension. Pharmacol. Res. 2017, 116, 77–86. [Google Scholar] [CrossRef]
- Morton, A.C.; Rothman, A.M.; Greenwood, J.P.; Gunn, J.; Chase, A.; Clarke, B.; Hall, A.S.; Fox, K.; Foley, C.; Banya, W.; et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: The MRC-ILA Heart Study. Eur. Heart J. 2015, 36, 377–384. [Google Scholar] [CrossRef]
- Li, S.; Yu, S.; Mu, Y.; Wang, K.; Liu, Y.; Zhang, M. Metformin ameliorates PM2.5-induced functional impairment of placental trophoblasts by inhibiting ferroptosis. Nan Fang Yi Ke Da Xue Xue Bao 2024, 44, 437. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Hague, W.M.; Gao, W.; Battin, M.R.; Moore, M.P. Metformin versus Insulin for the Treatment of Gestational Diabetes. N. Engl. J. Med. 2008, 358, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Nelson-Piercy, C.; Duley, J.; Florin, T.; Ansari, A. Successful Pregnancies with Thiopurine-Allopurinol Co-Therapy for Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 9, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Crouwel, F.; Simsek, M.; de Boer, M.A.; van Asseldonk, D.P.; Bhalla, A.; Weusthuis, A.L.M.; Gilissen, L.P.L.; Verburg, R.J.; Mares, W.G.N.; Jharap, B.; et al. Multicentre study and systematic review: Allopurinol exposure during pregnancy. Aliment. Pharmacol. Ther. 2024, 60, 503–518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Sánchez, C.; Pérez-Olmos, A.; Reverte, V.; Hernández, I.; Cuevas, S.; Llinás, M.T. Uric Acid and Preeclampsia: Pathophysiological Interactions and the Emerging Role of Inflammasome Activation. Antioxidants 2025, 14, 928. https://doi.org/10.3390/antiox14080928
Arias-Sánchez C, Pérez-Olmos A, Reverte V, Hernández I, Cuevas S, Llinás MT. Uric Acid and Preeclampsia: Pathophysiological Interactions and the Emerging Role of Inflammasome Activation. Antioxidants. 2025; 14(8):928. https://doi.org/10.3390/antiox14080928
Chicago/Turabian StyleArias-Sánchez, Celia, Antonio Pérez-Olmos, Virginia Reverte, Isabel Hernández, Santiago Cuevas, and María Teresa Llinás. 2025. "Uric Acid and Preeclampsia: Pathophysiological Interactions and the Emerging Role of Inflammasome Activation" Antioxidants 14, no. 8: 928. https://doi.org/10.3390/antiox14080928
APA StyleArias-Sánchez, C., Pérez-Olmos, A., Reverte, V., Hernández, I., Cuevas, S., & Llinás, M. T. (2025). Uric Acid and Preeclampsia: Pathophysiological Interactions and the Emerging Role of Inflammasome Activation. Antioxidants, 14(8), 928. https://doi.org/10.3390/antiox14080928








