The Protective Effect of Thiamine and Thiamine Pyrophosphate Against Linezolid-Induced Oxidative Liver Damage and Lactic Acidosis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Chemicals
2.3. Experimental Groups
2.4. Experimental Procedure
2.5. Biochemical Analysis
2.5.1. Determination of MDA, GSH Levels, SOD and CAT Activities in Liver Tissue
2.5.2. Determination of Lactate Levels
2.5.3. Determination of LDH Activities
2.5.4. Determination of ALT and AST Activities
2.5.5. Determination of TPP Levels
2.6. Histopathological Method
2.7. Statistical Analysis
3. Results
3.1. Biochemical Results
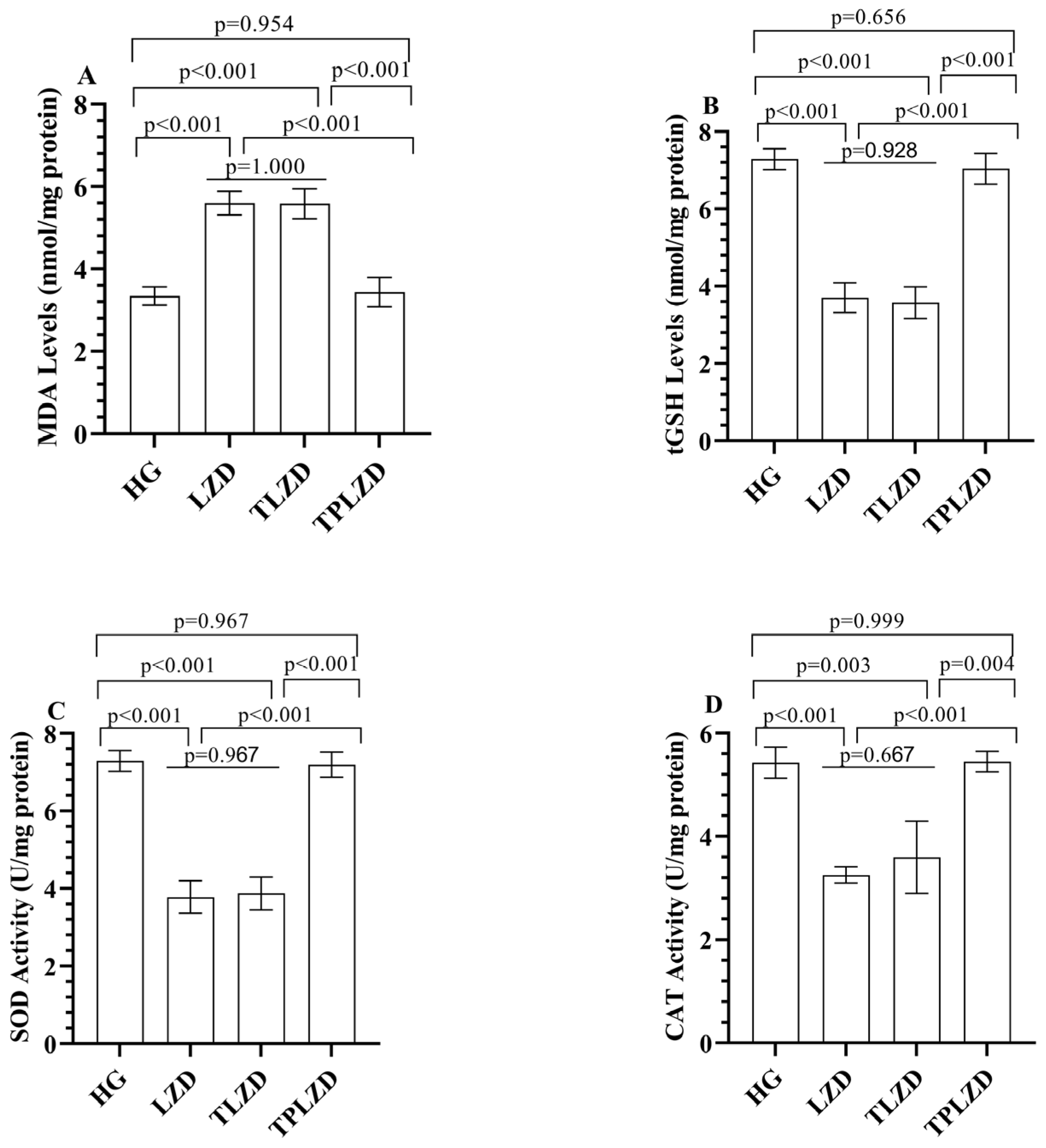
3.1.1. MDA and tGSH Level Analysis Results
3.1.2. SOD and CAT Analysis Results
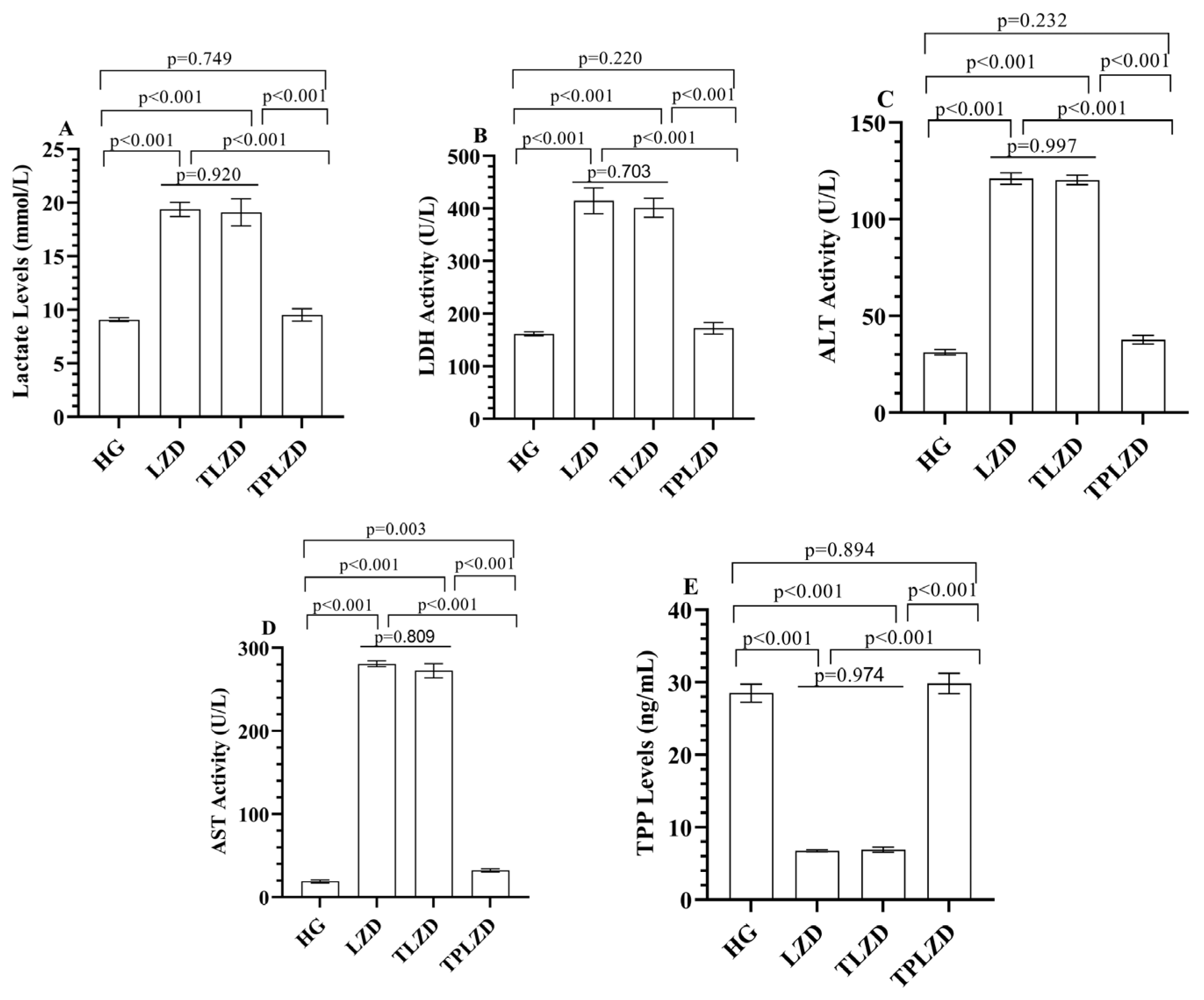
3.1.3. Lactate Level Analysis Results
3.1.4. LDH Activity Analysis Results
3.1.5. ALT and AST Activities Analysis Results
3.1.6. TPP Level Analysis Results
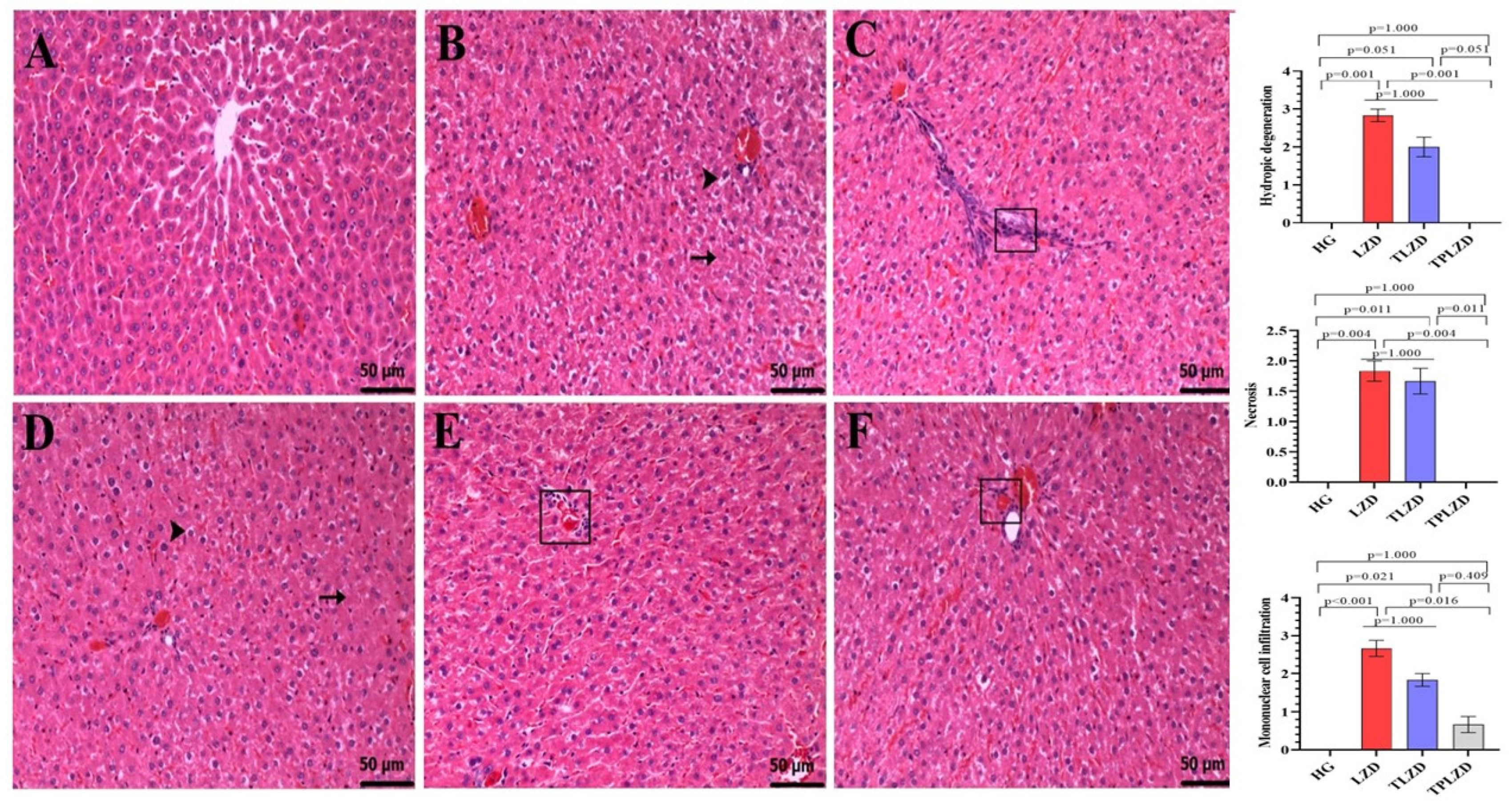
3.2. Histopathological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TPP | Thiamine Pyrophosphate |
| MDA | Malondialdehyde |
| tGSH | Total Glutathione |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| LDH | Lactate Dehydrogenase |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| FDA | Food and Drug Administration |
References
- Paladino, J.A. Linezolid: An oxazolidinone antimicrobial agent. Am. J. Health Syst. Pharm. 2002, 59, 2413–2425. [Google Scholar] [CrossRef]
- Elbarbry, F.; Moshirian, N. Linezolid-associated serotonin toxicity: A systematic review. Eur. J. Clin. Pharmacol. 2023, 79, 875–883. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Feng, R.; Ren, J.; Xu, X.; Sun, S. The in vitro antimicrobial activity of linezolid against unconventional pathogens. PeerJ 2025, 13, e18825. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Devel. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef]
- Veerman, K.; Goosen, J.; Spijkers, K.; Jager, N.; Heesterbeek, P.; Telgt, D. Prolonged use of linezolid in bone and joint infections: A retrospective analysis of adverse effects. J. Antimicrob. Chemother. 2023, 78, 2660–2666. [Google Scholar] [CrossRef]
- Rao, G.G.; Konicki, R.; Cattaneo, D.; Alffenaar, J.W.; Marriott, D.J.; Neely, M.; IATDMCT Antimicrobial Scientific Committee. Therapeutic drug monitoring can improve linezolid dosing regimens in current clinical practice: A review of linezolid pharmacokinetics and pharmacodynamics. Ther. Drug Monit. 2020, 42, 83–92. [Google Scholar] [CrossRef]
- Shaikh, A.; McHugh, J. Linezolid use and drug-induced liver injury. Proc. (Bayl. Univ. Med. Cent.) 2020, 34, 316–317. [Google Scholar] [CrossRef]
- Kendir-Demirkol, Y.; Jenny, L.A.; Demirkol, A.; Özen, M.; Ayata, A.; Canatan, D. The Protective Effects of Pyridoxine on Linezolid-Induced Hematological Toxicity, Hepatotoxicity, and Oxidative Stress in Rats. Turk. Arch. Pediatr. 2023, 58, 298–301. [Google Scholar] [CrossRef]
- Sharma, K.; Keri, V.C.; Kumar, T.P.; Sethi, P.; Dass, J.; Mishra, R.K. Mitochondrial toxicity induced by linezolid causing lactic acidosis. Trop. Dr. 2023, 53, 315–316. [Google Scholar] [CrossRef]
- De Bus, L.; Depuydt, P.; Libbrecht, L.; Vandekerckhove, L.; Nollet, J.; Benoit, D.; Vogelaers, D.; Van Vlierberghe, H. Severe drug-induced liver injury associated with prolonged use of linezolid. J. Med. Toxicol. 2010, 6, 322–326. [Google Scholar] [CrossRef]
- Laus, F.; Faillace, V.; Tesei, B.; Paggi, E.; Serri, E.; Marini, C.; Vullo, C.; Spaterna, A. Effect of Thiamine Pyrophosphate (Bicarbossilasi®) Administration on the Exercising Horse Metabolism. Isr. J. Vet. Med. 2017, 72, 15–21. [Google Scholar]
- Gochhayat, G.; Kumar, M.; Bhatt, S.; Saini, V.; Malik, A. Thiamine: A Key Role in Human Health. Int. J. Sci. Technol. Res. 2019, 8, 42–51. [Google Scholar]
- Huang, W.; Qin, J.; Liu, D.; Wang, Y.; Shen, X.; Yang, N.; Zhou, H.; Cai, X.T.; Wang, Z.L.; Yu, D.; et al. Reduced thiamine binding is a novel mechanism for TPK deficiency disorder. Mol. Genet. Genom. 2019, 294, 409–416. [Google Scholar] [CrossRef]
- Attaluri, P.; Castillo, A.; Edriss, H.; Nugent, K. Thiamine Deficiency: An Important Consideration in Critically Ill Patients. Am. J. Med. Sci. 2018, 356, 382–390. [Google Scholar] [CrossRef]
- Smirnov, A.; Semionov, M.; Ishay, S.Y.; Zlotnik, A.; Fraifeld, V.E.; Frank, D. Severe Intra- and Post-Operative Lactic Acidosis in a Patient Who Underwent Robotic Thoracoscopic Surgery. Biomedicines 2025, 13, 568. [Google Scholar] [CrossRef]
- Connor, H.; Woods, H.; Ledingham, J. Comparison of the kinetics and utilisation of D (−)-and L (+)-sodium lactate in normal man. Ann. Nutr. Metab. 1983, 27, 481–487. [Google Scholar] [CrossRef]
- Perriello, G.; Jorde, R.; Nurjhan, N.; Stumvoll, M.; Dailey, G.; Jenssen, T.; Bier, D.M.; Gerich, J.E. Estimation of glucose-alanine-lactate-glutamine cycles in postabsorptive humans: Role of skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 1995, 269, E443–E450. [Google Scholar] [CrossRef]
- Turan, M.I.; Siltelioglu Turan, I.; Mammadov, R.; Altınkaynak, K.; Kisaoglu, A. The effect of thiamine and thiamine pyrophosphate on oxidative liver damage induced in rats with cisplatin. Biomed. Res. Int. 2013, 2013, 783809. [Google Scholar] [CrossRef]
- Wang, T.; Guo, D.; Dong, X.; Mu, L. Effect of linezolid on hematological and oxidative parameters in rats. J. Antibiot. 2014, 67, 433–437. [Google Scholar] [CrossRef]
- Ciftel, S.; Ciftel, S.; Altuner, D.; Huseynova, G.; Yucel, N.; Mendil, A.S.; Sarigul, C.; Suleyman, H.; Bulut, S. Effects of adenosine triphosphate, thiamine pyrophosphate, melatonin, and liv-52 on subacute pyrazinamide proliferation hepatotoxicity in rats. BMC Pharmacol. Toxicol. 2025, 26, 67. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Hamouda, A.G.; Abd-Allah, E.R.; Mahmoud, A.A. Linezolid administration to lactating Wistar rats affects the health of their offspring. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 1–11. [Google Scholar] [CrossRef]
- Vivekanandan, L.; Sheik, H.; Singaravel, S.; Thangavel, S. Ameliorative effect of silymarin against linezolid-induced hepatotoxicity in methicillin-resistant Staphylococcus aureus (MRSA) infected Wistar rats. Biomed. Pharmacother. 2018, 108, 1303–1312. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Goc, Z.; Szaroma, W.; Kapusta, E.; Dziubek, K. Protective effects of melatonin on the activity of SOD, CAT, GSH-Px and GSH content in organs of mice after administration of SNP. Chin. J. Physiol. 2017, 60, 1–10. [Google Scholar] [CrossRef]
- Azzam, S.M.; Anwar, H.M.; Abd El-Slam, A.H.; Diab, M.S.; Ibrahim, H.M.; Yousef, A.M.; Sabry, F.; Darwish, I.; Kaliyamoorthy, K.; Salem, G.E.M.; et al. The Protective Role of Vitamin C against Linezolid Induced Hepato-Renal Toxicity in a Rat Model. Front. Pharmacol. 2025, 16, 1551062. [Google Scholar] [CrossRef]
- Kalas, M.A.; Chavez, L.; Leon, M.; Taweesedt, P.T.; Surani, S. Abnormal liver enzymes: A review for clinicians. World J. Hepatol. 2021, 13, 1688–1698. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase; StatPearls Publishing: Petersburg, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557536/ (accessed on 15 May 2025).
- Alshehri, F.S.; Alorfi, N.M. Protective role of resveratrol against VCM-induced hepatotoxicity in male wistar rats. Front. Pharmacol. 2023, 14, 1130670. [Google Scholar] [CrossRef]
- Thakur, S.; Kumar, V.; Das, R.; Sharma, V.; Mehta, D.K. Biomarkers of Hepatic Toxicity: An Overview. Curr. Ther. Res. Clin. Exp. 2024, 100, 100737. [Google Scholar] [CrossRef]
- Ferreira, Â.; Sobrosa, P.; Costa, M.; Miranda, I.; Guerra, D. Linezolid Toxicity: A Clinical Case Report. Cureus 2024, 16, e55672. [Google Scholar] [CrossRef]
- Pajtler, K. Hepatotoxicity associated with eculizumab in a patient with at ypical hemolytic uremic syndrome. Nefrologia 2018, 38, 438–457. [Google Scholar]
- Dutta, S.; Chakravarty, P.; Borah, A. Haloperidol induced hepatotoxicity: A case report. Ann. Clin. Case Rep. 2020, 5, 1827. [Google Scholar]
- Santos-Vazquez, G.; Aldana-Vazquez, J.Y.; Seniscal-Arredondo, D.A.; Salgado-de la Mora, M. Linezolid-Induced Pancreatitis, Hypoglycemia, and Lactic Acidosis: Case Report and Literature Review. Ann. Intern. Med. Clin. Cases 2025, 4, e240924. [Google Scholar] [CrossRef]
- Tobias, P.E.; Varughese, C.A.; Hanson, A.P.; Gurnani, P.K. A Case of Linezolid Induced Toxicity. J. Pharm. Pract. 2020, 33, 222–225. [Google Scholar] [CrossRef]
- Akter, F.; Bozell, H.; Neumann, T.; Chung, C. Linezolid-Induced Lactic Acidosis Presenting As Acute Cholecystitis: A Case Report and Systematic Review. Cureus 2024, 16, e70794. [Google Scholar] [CrossRef]
- Al Amri, U.; Al Shukri, Z.; Al Habsi, N.; Al Noumani, J.; Elbingawi, H.; Bilkhair, A. Linezolid induced lactic acidosis: A case report and literature review of a rare side effect. Oman Med. J. 2024. advance online publication. [Google Scholar] [CrossRef]
- Wiener, M.; Guo, Y.; Patel, G.; Fries, B.C. Lactic acidosis after treatment with linezolid. Infection 2007, 35, 278–281. [Google Scholar] [CrossRef]
- Turan, M.; Cetin, N.; Siltelioglu Turan, I.; Ozgeris, F.; Suleyman, H. Effects of thiamine and thiamine pyrophosphate on oxidative stress by methotrexate in the rat brain. Lat. Am. J. Pharm. 2013, 32, 203–207. [Google Scholar]
- Cinici, E.; Ahiskali, I.; Cetin, N.; Suleyman, B.; Kukula, O.; Altuner, D.; Coban, A.; Balta, H.; Kuzucu, M.; Suleyman, H. Effect of thiamine pyrophosphate on retinopathy induced by hyperglycemia in rats: A biochemical and pathological evaluation. Indian J. Ophthalmol. 2016, 64, 434–439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isik, B.; Ates, I.; Yucel, N.; Suleyman, B.; Mendil, A.S.; Sezgin, E.T.; Suleyman, H. The Protective Effect of Thiamine and Thiamine Pyrophosphate Against Linezolid-Induced Oxidative Liver Damage and Lactic Acidosis in Rats. Antioxidants 2025, 14, 920. https://doi.org/10.3390/antiox14080920
Isik B, Ates I, Yucel N, Suleyman B, Mendil AS, Sezgin ET, Suleyman H. The Protective Effect of Thiamine and Thiamine Pyrophosphate Against Linezolid-Induced Oxidative Liver Damage and Lactic Acidosis in Rats. Antioxidants. 2025; 14(8):920. https://doi.org/10.3390/antiox14080920
Chicago/Turabian StyleIsik, Bahar, Irem Ates, Nurinisa Yucel, Bahadir Suleyman, Ali Sefa Mendil, Esra Tuba Sezgin, and Halis Suleyman. 2025. "The Protective Effect of Thiamine and Thiamine Pyrophosphate Against Linezolid-Induced Oxidative Liver Damage and Lactic Acidosis in Rats" Antioxidants 14, no. 8: 920. https://doi.org/10.3390/antiox14080920
APA StyleIsik, B., Ates, I., Yucel, N., Suleyman, B., Mendil, A. S., Sezgin, E. T., & Suleyman, H. (2025). The Protective Effect of Thiamine and Thiamine Pyrophosphate Against Linezolid-Induced Oxidative Liver Damage and Lactic Acidosis in Rats. Antioxidants, 14(8), 920. https://doi.org/10.3390/antiox14080920





