Enhancing Antioxidants Performance of Ceria Nanoparticles in Biological Environment via Surface Engineering with o-Quinone Functionalities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Oleyl Amine-Capped CeO2−x NPs (CeO2−x@OAm)
2.3. Phase Transfer of Organic-Capped CeO2−x Nanoparticles in Aqueous Medium by Ligand-Exchange Reaction with Citrate (CeO2−x@Cit)
2.4. Functionalization of CeO2−x@Cit NPs with Dopamine (CeO2−x@Dopa)
2.5. Spectrophotometric Characterization of CeO2−x Nanoparticles Treated with H2O2
2.6. Spectrophotometric Analysis of 1,1-diphenyl-2-picrylhydrazil (DPPH·) Radical Scavenging by CeO2−x Nanoparticles
2.7. Evaluation of Cell Viability
2.8. Intracellular Reactive Oxygen Species Detection
2.9. Characterization Techinques
3. Results and Discussion
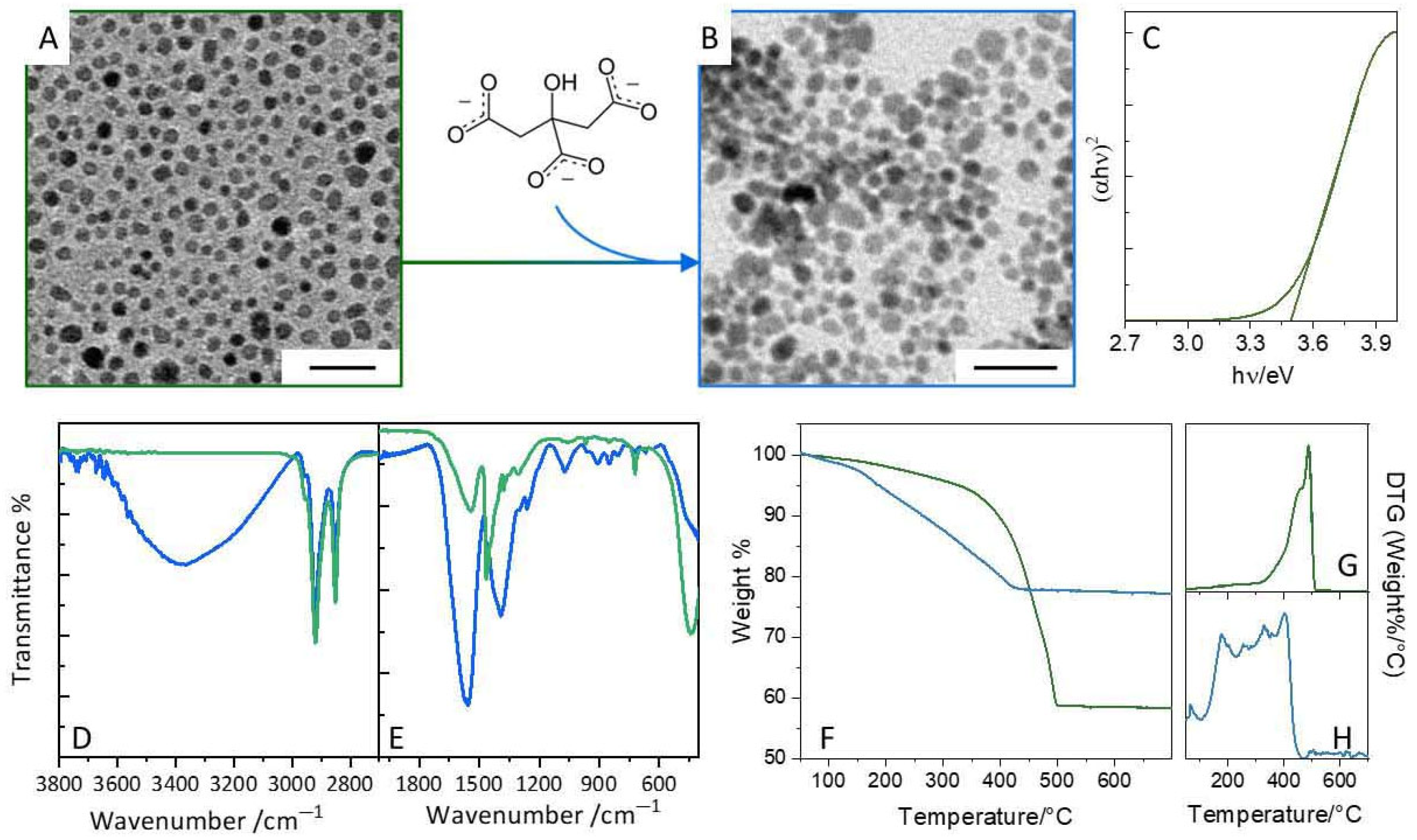
3.1. Synthesis and Aqueous Phase Transfer of CeO2−x@OAm Nanoparticles via Citrate Ligand Exchange
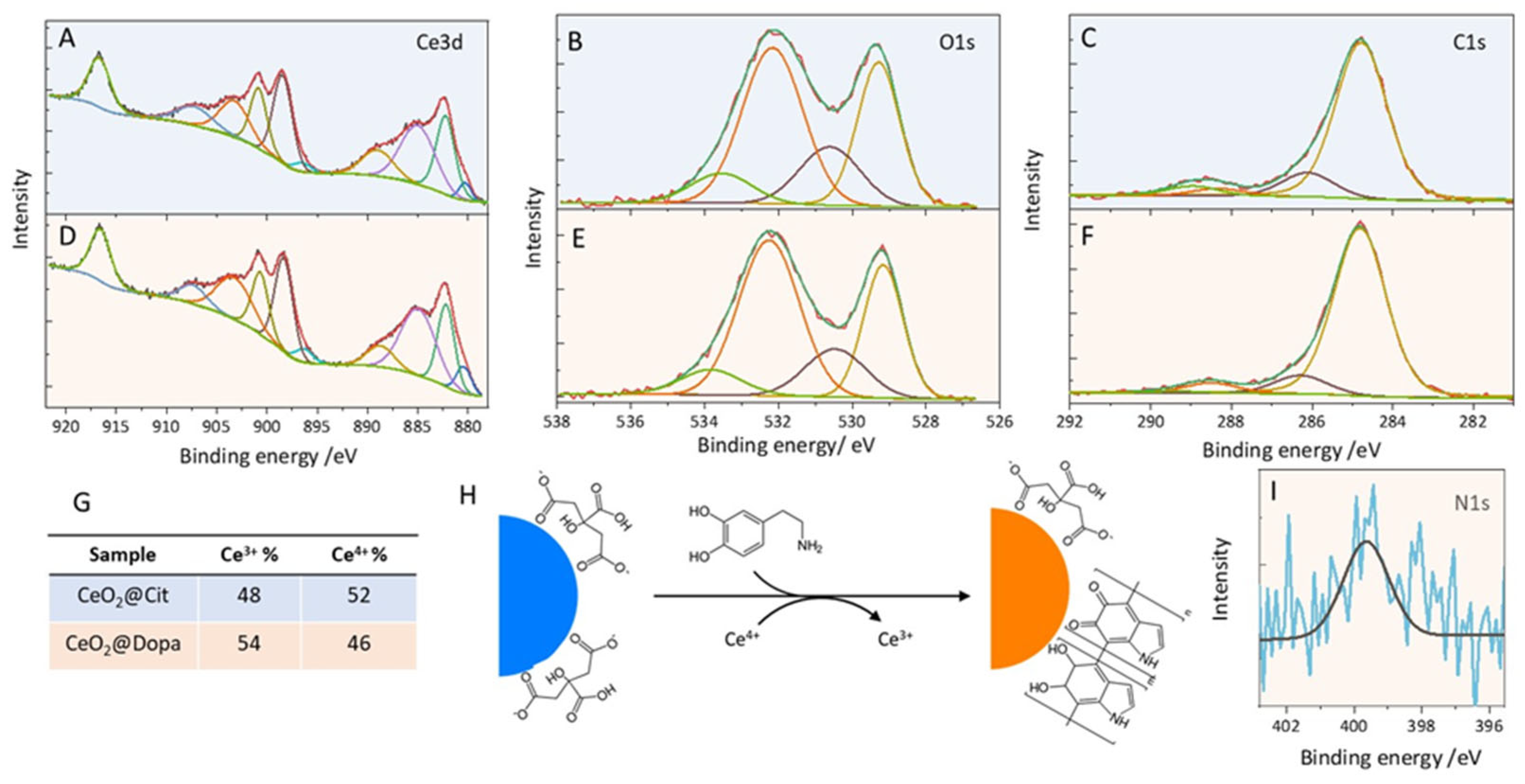
3.2. Ligand-Exchange with Dopamine and Characterization of CeO2−x@Dopa
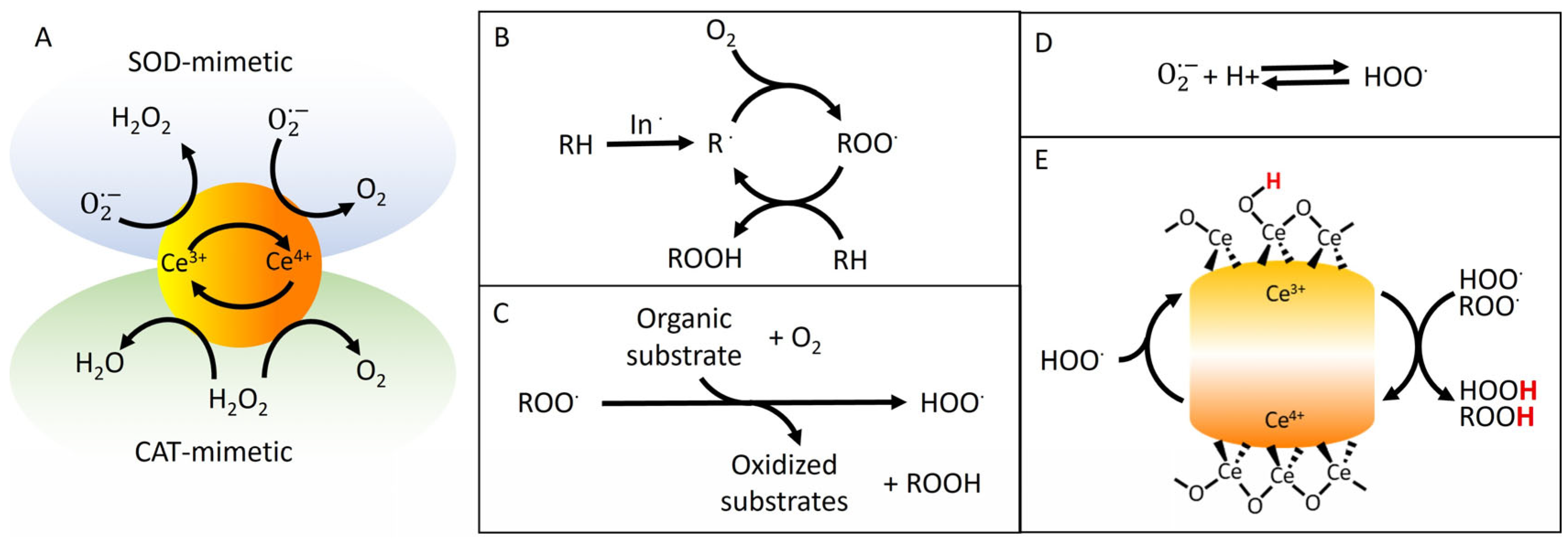
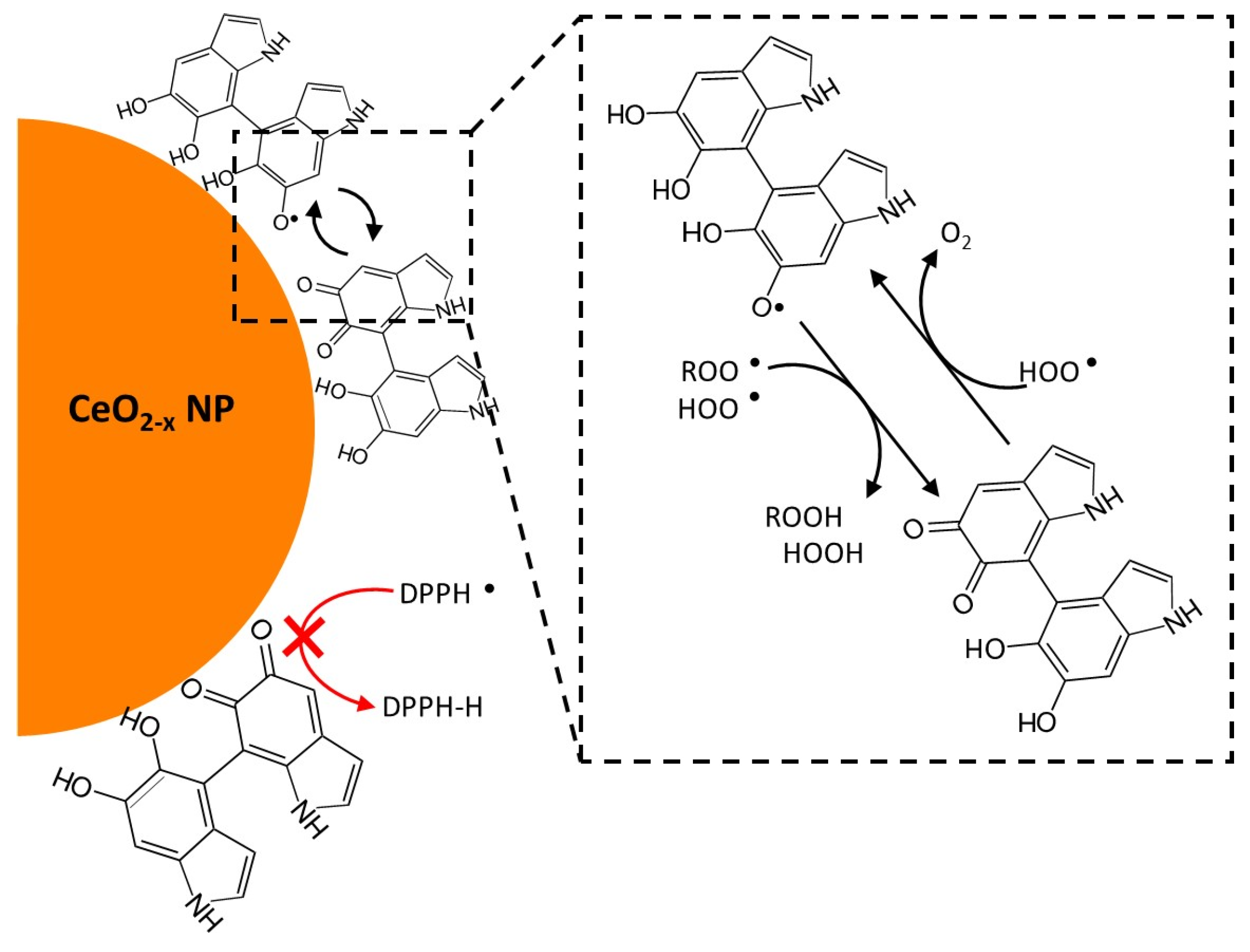
3.3. Catalytic and Radical Scavenging Activity of CeO2−x@Cit and CeO2−x@Dopa
3.3.1. Catalytic Activity of CeO2−x Nanoparticles Towards H2O2
3.3.2. Assessment of DPPH· Scavenging Activity
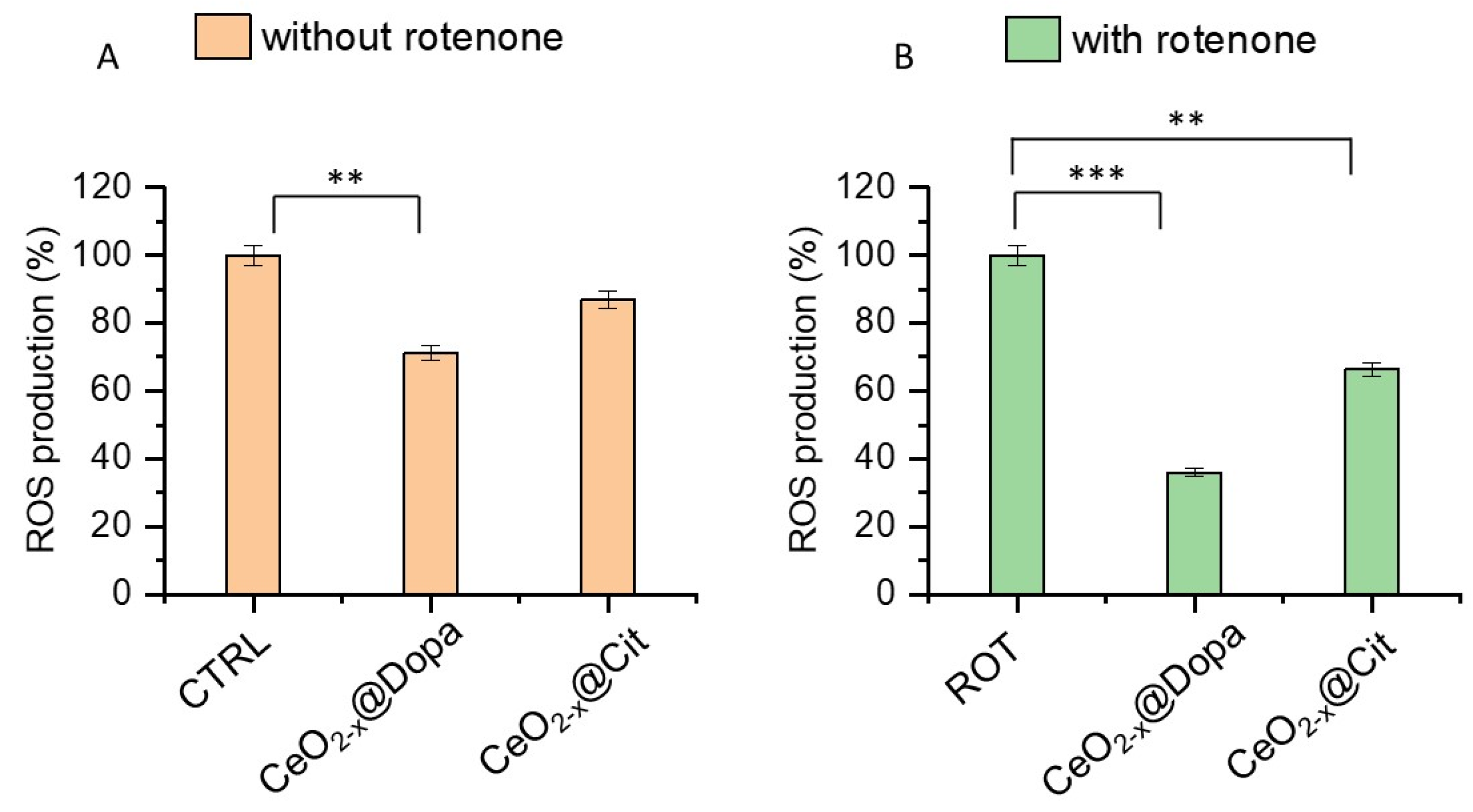
3.3.3. Antioxidant Effect of CeO2−x@Cit and CeO2−x@Dopa on DITNC1 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damatov, D.; Laga, S.M.; Mader, E.A.; Peng, J.; Agarwal, R.G.; Mayer, J.M. Redox Reactivity of Colloidal Nanoceria and Use of Optical Spectra as an In Situ Monitor of Ce Oxidation States. Inorg. Chem. 2018, 57, 14401–14408. [Google Scholar] [CrossRef]
- Eriksson, P.; Tal, A.A.; Skallberg, A.; Brommesson, C.; Hu, Z.; Boyd, R.D.; Olovsson, W.; Fairley, N.; Abrikosov, I.A.; Zhang, X.; et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci. Rep. 2018, 8, 6999. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Yang, F.; Wu, L.; Xia, D.; Liu, Y. CeO2 nanoparticles to promote wound healing: A systematic review. Adv. Healthc. Mater. 2024, 13, 2302858. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Amorati, R.; Guo, Y.; Budhlall, B.M.; Barry, C.F.; Cao, D.; Challa, S.S.R.K. Tandem Hydroperoxyl–Alkylperoxyl Radical Quenching by an Engineered Nanoporous Cerium Oxide Nanoparticle Macrostructure (NCeONP): Toward Efficient Solid-State Autoxidation Inhibitors. ACS Omega 2023, 8, 40174–40183. [Google Scholar] [CrossRef]
- Grulke, E.; Reed, K.; Beck, M.; Huang, X.; Cormack, A.; Seal, S. Nanoceria: Factors affecting its pro- and anti-oxidant properties. Environ. Sci. Nano 2014, 1, 429–444. [Google Scholar] [CrossRef]
- Datta, A.; Mishra, S.; Manna, K.; Saha, K.D.; Mukherjee, S.; Roy, S. Pro-oxidant therapeutic activities of cerium oxide nanoparticles in colorectal carcinoma cells. ACS Omega 2020, 5, 9714–9723. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Gao, X.; Zhao, Y. Simultaneous enzyme mimicking and chemical reduction mechanisms for nanoceria as a bio-antioxidant: A catalytic model bridging computations and experiments for nanozymes. Nanoscale 2019, 11, 13289–13299. [Google Scholar] [CrossRef]
- Othman, A.; Gowda, A.; Andreescu, D.; Hassan, M.H.; Babu, S.; Seo, J.; Andreescu, S. Two decades of ceria nanoparticles research: Structure, properties and emerging applications. Mater. Horiz. 2024, 11, 3213–3266. [Google Scholar] [CrossRef]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.-F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce 3+ surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Amorati, R. Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review. Antioxidants 2021, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Zongxin, J.; and Amorati, R. Hydroperoxyl radical (HOO•) as a reducing agent: Unexpected synergy with antioxidants. A review. Free Radic. Res. 2023, 57, 115–129. [Google Scholar] [CrossRef]
- Damatov, D.; Mayer, J.M. (Hydro)peroxide ligands on colloidal cerium oxide nanoparticles. Chem. Commun. 2016, 52, 10281–10284. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Garcia-Rojas, D.; Ling, V.; Masterson, C.M.; Bi, Y.; Xiao, Z.; Guo, X.; Villanova, J.; Dunn, J.; et al. Increasing the antioxidant capacity of ceria nanoparticles with catechol-grafted poly(ethylene glycol). J. Mater. Chem. B 2022, 10, 10042–10053. [Google Scholar] [CrossRef]
- Chen, L.; Fleming, P.; Morris, V.; Holmes, J.D.; Morris, M.A. Size-Related Lattice Parameter Changes and Surface Defects in Ceria Nanocrystals. J. Phys. Chem. C 2010, 114, 12909–12919. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Lord, M.S.; Berret, J.F.; Singh, S.; Vinu, A.; Karakoti, A.S. Redox Active Cerium Oxide Nanoparticles: Current Status and Burning Issues. Small 2021, 17, 2102342. [Google Scholar] [CrossRef]
- Goujon, G.; Baldim, V.; Roques, C.; Bia, N.; Seguin, J.; Palmier, B.; Graillot, A.; Loubat, C.; Mignet, N.; Margaill, I. Antioxidant activity and toxicity study of cerium oxide nanoparticles stabilized with innovative functional copolymers. Adv. Healthc. Mater. 2021, 10, 2100059. [Google Scholar] [CrossRef]
- Baldim, V.; Yadav, N.; Bia, N.; Graillot, A.; Loubat, C.; Singh, S.; Karakoti, A.S.; Berret, J.-F. Polymer-Coated Cerium Oxide Nanoparticles as Oxidoreductase-like Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 42056–42066. [Google Scholar] [CrossRef]
- Lee, S.S.; Song, W.; Cho, M.; Puppala, H.L.; Nguyen, P.; Zhu, H.; Segatori, L.; Colvin, V.L. Antioxidant Properties of Cerium Oxide Nanocrystals as a Function of Nanocrystal Diameter and Surface Coating. ACS Nano 2013, 7, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, G.; Hayat, A.; Liu, X.; Andreescu, S. Reactivity of nanoceria particles exposed to biologically relevant catechol-containing molecules. RSC Adv. 2016, 6, 60007–60014. [Google Scholar] [CrossRef]
- Hayat, A.; Andreescu, D.; Bulbul, G.; Andreescu, S. Redox reactivity of cerium oxide nanoparticles against dopamine. J. Colloid Interface Sci. 2014, 418, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Mollica, F.; Lucernati, R.; Amorati, R. Expanding the spectrum of polydopamine antioxidant activity by nitroxide conjugation. J. Mater. Chem. B 2021, 9, 9980–9988. [Google Scholar] [CrossRef]
- Lee, S.S.; Zhu, H.; Contreras, E.Q.; Prakash, A.; Puppala, H.L.; Colvin, V.L. High Temperature Decomposition of Cerium Precursors To Form Ceria Nanocrystal Libraries for Biological Applications. Chem. Mater. 2012, 24, 424–432. [Google Scholar] [CrossRef]
- Berestok, T.; Guardia, P.; Blanco, J.; Nafria, R.; Torruella, P.; López-Conesa, L.; Estradé, S.; Ibáñez, M.; de Roo, J.; Luo, Z.; et al. Tuning Branching in Ceria Nanocrystals. Chem. Mater. 2017, 29, 4418–4424. [Google Scholar] [CrossRef]
- Yu, T.; Park, Y.I.; Kang, M.C.; Joo, J.; Park, J.K.; Won, H.Y.; Kim, J.J.; Hyeon, T. Large-scale synthesis of water dispersible ceria nanocrystals by a simple sol–gel process and their use as a chemical mechanical planarization slurry. Small 2008, 6, 855–858. [Google Scholar] [CrossRef]
- Monnier, C.A.; Lattuada, M.; Burnand, D.; Crippa, F.; Martinez-Garcia, J.C.; Hirt, A.M.; Rothen-Rutishauser, B.; Bonmarin, M.; Petri-Fink, A. A lock-in-based method to examine the thermal signatures of magnetic nanoparticles in the liquid, solid and aggregated states. Nanoscale 2016, 8, 13321–13332. [Google Scholar] [CrossRef]
- Latronico, T.; Rizzi, F.; Panniello, A.; Laquintana, V.; Arduino, I.; Denora, N.; Fanizza, E.; Milella, S.; Mastroianni, C.M.; Striccoli, M. Luminescent PLGA nanoparticles for delivery of darunavir to the brain and inhibition of matrix metalloproteinase-9, a relevant therapeutic target of HIV-associated neurological disorders. ACS Chem. Neurosci. 2021, 12, 4286–4301. [Google Scholar] [CrossRef]
- Petraglia, T.; Latronico, T.; Pepe, A.; Crescenzi, A.; Liuzzi, G.M.; Rossano, R. Increased Antioxidant Performance of Lignin by Biodegradation Obtained from an Extract of the Mushroom Pleurotus eryngii. Molecules 2024, 29, 5575. [Google Scholar] [CrossRef]
- Giancaspro, M.; Grisorio, R.; Alò, G.; Margiotta, N.; Panniello, A.; Suranna, G.P.; Depalo, N.; Striccoli, M.; Curri, M.L.; Fanizza, E. Molecular insights into the growth and time evolution of surface states of CsPbBr3 nanoparticles synthesized using a scalable room temperature approach. Mater. Chem. Front. 2023, 7, 2637–2650. [Google Scholar] [CrossRef]
- Kim, J.; Hong, G.; Mazaleuskaya, L.; Hsu, J.C.; Rosario-Berrios, D.N.; Grosser, T.; Cho-Park, P.F.; Cormode, D.P. Ultrasmall Antioxidant Cerium Oxide Nanoparticles for Regulation of Acute Inflammation. ACS Appl. Mater. Interfaces 2021, 13, 60852–60864. [Google Scholar] [CrossRef] [PubMed]
- Parak, W.; Gerion, D.; Pellegrino, T.; Zanchet, D.; Micheel, C.; Williams, S.; Boudreau, R.; Gros, M.; Larabell, C.; Alivisatos, A. Biological Applications of Colloidal Nanocrystals. Nanotechnology 2003, 14, R15. [Google Scholar] [CrossRef]
- Hancock, M.L.; Yokel, R.A.; Beck, M.J.; Calahan, J.L.; Jarrells, T.W.; Munson, E.J.; Olaniyan, G.A.; Grulke, E.A. The characterization of purified citrate-coated cerium oxide nanoparticles prepared via hydrothermal synthesis. Appl. Surf. Sci. 2021, 535, 147681. [Google Scholar] [CrossRef]
- Thistlethwaite, P.; Hook, M. Diffuse Reflectance Fourier Transform Infrared Study of the Adsorption of Oleate/Oleic Acid onto Titania. Langmuir 2000, 16, 4993–4998. [Google Scholar] [CrossRef]
- Fanizza, E.; Depalo, N.; Clary, L.; Agostiano, A.; Striccoli, M.; Curri, M.L. A combined size sorting strategy for monodisperse plasmonic nanostructures. Nanoscale 2013, 5, 3272–3282. [Google Scholar] [CrossRef]
- Altomare, M.; Fanizza, E.; Corricelli, M.; Comparelli, R.; Striccoli, M.; Curri, M.L. Patterned assembly of luminescent nanocrystals: Role of the molecular chemistry at the interface. J. Nanoparticle Res. 2014, 16, 1–14. [Google Scholar] [CrossRef]
- Phoka, S.; Laokul, P.; Swatsitang, E.; Promarak, V.; Seraphin, S.; Maensiri, S. Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater. Chem. Phys. 2009, 115, 423–428. [Google Scholar] [CrossRef]
- Park, J.-W.; Shumaker-Parry, J.S. Structural study of citrate layers on gold nanoparticles: Role of intermolecular interactions in stabilizing nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef]
- Nara, M.; Torii, H.; Tasumi, M. Correlation between the Vibrational Frequencies of the Carboxylate Group and the Types of Its Coordination to a Metal Ion: An ab Initio Molecular Orbital Study. J. Phys. Chem. 1996, 100, 19812–19817. [Google Scholar] [CrossRef]
- Andreescu, D.; Matijević, E.; Goia, D.V. Formation of uniform colloidal ceria in polyol. Colloids Surf. Physicochem. Eng. Asp. 2006, 291, 93–100. [Google Scholar] [CrossRef]
- Culica, M.E.; Chibac-Scutaru, A.L.; Melinte, V.; Coseri, S. Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications. Materials 2020, 13, 2955. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, L.; Liu, W.; Song, Z. Surface modification of ceria nanoparticles and their chemical mechanical polishing behavior on glass substrate. Appl. Surf. Sci. 2010, 256, 3856–3861. [Google Scholar] [CrossRef]
- Baranchikov, A.E.; Sozarukova, M.M.; Mikheev, I.V.; Egorova, A.A.; Proskurnina, E.V.; Poimenova, I.A.; Krasnova, S.A.; Filippova, A.D.; Ivanov, V.K. Biocompatible ligands modulate nanozyme activity of CeO2 nanoparticles. New J. Chem. 2023, 47, 20388–20404. [Google Scholar] [CrossRef]
- Ioannou, M.E.; Pouroutzidou, G.K.; Chatzimentor, I.; Tsamesidis, I.; Florini, N.; Tsiaoussis, I.; Lymperaki, E.; Komninou, P.; Kontonasaki, E. Synthesis and Characterization of Cerium Oxide Nanoparticles: Effect of Cerium Precursor to Gelatin Ratio. Appl. Sci. 2023, 13, 2676. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Menelaou, M.; Fiuza-Maneiro, N.; Zheng, G.; Wei, S.; Pérez-Juste, J.; Polavarapu, L.; Sofer, Z. Oleic acid/oleylamine ligand pair: A versatile combination in the synthesis of colloidal nanoparticles. Nanoscale Horiz. 2022, 7, 941–1015. [Google Scholar] [CrossRef]
- Pinna, A.; Cali, E.; Kerherve, G.; Galleri, G.; Maggini, M.; Innocenzi, P.; Malfatti, L. Fulleropyrrolidine-functionalized ceria nanoparticles as a tethered dual nanosystem with improved antioxidant properties. Nanoscale Adv. 2020, 2, 2387–2396. [Google Scholar] [CrossRef]
- Seal, S.; Jeyaranjan, A.; Neal, C.J.; Kumar, U.; Sakthivel, T.S.; Sayle, D.C. Engineered defects in cerium oxides: Tuning chemical reactivity for biomedical, environmental, & energy applications. Nanoscale 2020, 12, 6879–6899. [Google Scholar] [CrossRef]
- Allahgholi, A.; Flege, J.I.; Thieß, S.; Drube, W.; Falta, J. Oxidation-State Analysis of Ceria by X-ray Photoelectron Spectroscopy. ChemPhysChem 2015, 16, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.M.; Maier, R.A.; Johnston-Peck, A.C.; Gorham, J.M.; Hackley, V.A.; Nelson, B.C. Approaches for the quantitative analysis of oxidation state in cerium oxide nanomaterials. Nanotechnology 2019, 30, 085703. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, P.; Koberstein, J.; Khalid, S.; Chan, S.-W. Cerium oxidation state in ceria nanoparticles studied with X-ray photoelectron spectroscopy and absorption near edge spectroscopy. Surf. Sci. 2004, 563, 74–82. [Google Scholar] [CrossRef]
- Morgan, D.J. Photoelectron spectroscopy of ceria: Reduction, quantification and the myth of the vacancy peak in XPS analysis. Surf. Interface Anal. 2023, 55, 845–850. [Google Scholar] [CrossRef]
- Ma, W.; Mashimo, T.; Tamura, S.; Tokuda, M.; Yoda, S.; Tsushida, M.; Koinuma, M.; Kubota, A.; Isobe, H.; Yoshiasa, A. Cerium oxide (CeO2−x) nanoparticles with high Ce3+ proportion synthesized by pulsed plasma in liquid. Ceram. Int. 2020, 46, 26502–26510. [Google Scholar] [CrossRef]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef]
- Yadav, S.V.; Rathod, V.K. Oxidase-like activity of magnetically separable nano ceria for catechol detection. SN Appl. Sci. 2019, 1, 1071. [Google Scholar] [CrossRef]
- Kim, H.; Yook, S.H.; Kim, H.Y.; Choi, Y.; Lim, Y.; Hwang, Y.; Kim, J.; Lee, K.Y.; Jang, S.S.; Park, J.; et al. Tailor-Made Charged Catechol-Based Polymeric Ligands to Build Robust Fuel Cells Containing Antioxidative Nanoparticles. Adv. Electron. Mater. 2022, 8, 2200171. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO. to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Andreescu, D.; Bulbul, G.; Özel, R.E.; Hayat, A.; Sardesai, N.; Andreescu, S. Applications and implications of nanoceria reactivity: Measurement tools and environmental impact. Environ. Sci. Nano 2014, 1, 445–458. [Google Scholar] [CrossRef]
- Lazić, V.; Živković, L.S.; Sredojević, D.; Fernandes, M.M.; Lanceros-Mendez, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Tuning Properties of Cerium Dioxide Nanoparticles by Surface Modification with Catecholate-type of Ligands. Langmuir 2020, 36, 9738–9746. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Ju, X.; Mezghrani, B.; Berret, J.-F. Cerium Oxide Catalyzed Disproportionation of Hydrogen Peroxide: A Closer Look at the Reaction Intermediate. Chem. A Eur. J. 2024, 30, e202304012. [Google Scholar] [CrossRef]
- Goia, S.; Richings, G.W.; Turner, M.A.P.; Woolley, J.M.; Tully, J.J.; Cobb, S.J.; Burriss, A.; Robinson, B.R.; Macpherson, J.V.; Stavros, V.G. Ultrafast Spectroelectrochemistry of the Catechol/o-Quinone Redox Couple in Aqueous Buffer Solution. ChemPhotoChem 2024, 8, e202300325. [Google Scholar] [CrossRef]
- Boutaybi, M.E.; Titi, A.; Alzahrani, A.Y.; Bahari, Z.; Tillard, M.; Hammouti, B.; Touzani, R. Aerial Oxidation of Phenol/Catechol in the Presence of Catalytic Amounts of [(Cl) 2Mn (RCOOET)], RCOOET= Ethyl-5-Methyl-1-(((6-Methyl-3-Nitropyridin-2-yl) Amino) Methyl)-1 H-Pyrazole-3-Carboxylate. Catalysts 2022, 12, 1642. [Google Scholar] [CrossRef]
- Seminko, V.; Maksimchuk, P.; Grygorova, G.; Okrushko, E.; Avrunin, O.; Semenets, V.; Malyukin, Y. Mechanism and Dynamics of Fast Redox Cycling in Cerium Oxide Nanoparticles at High Oxidant Concentration. J. Phys. Chem. C 2021, 125, 4743–4749. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Popov, A.L.; Shcherbakov, A.B.; Zholobak, N.; Baranchikov, A.Y.; Ivanov, V.K. Cerium dioxide nanoparticles as third-generation enzymes (nanozymes). Nanosyst. Phys. Chem. Math. 2017, 8, 760–781. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Hoss, U.; Heller, A. Mixed-Valence Metal Oxide Nanoparticles as Electrochemical Half-Cells: Substituting the Ag/AgCl of Reference Electrodes by CeO2–x Nanoparticles. J. Am. Chem. Soc. 2012, 134, 20783–20787. [Google Scholar] [CrossRef]
- Liu, H.; Qu, X.; Tan, H.; Song, J.; Lei, M.; Kim, E.; Payne, G.F.; Liu, C. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta Biomater. 2019, 88, 181–196. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef]
- Baxter, P.S.; Hardingham, G.E. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef]
- Damle, M.A.; Jakhade, A.P.; Chikate, R.C. Modulating Pro- and Antioxidant Activities of Nanoengineered Cerium Dioxide Nanoparticles against Escherichia coli. ACS Omega 2019, 4, 3761–3771. [Google Scholar] [CrossRef]
- Moglianetti, M.; Pedone, D.; Udayan, G.; Retta, S.F.; Debellis, D.; Marotta, R.; Turco, A.; Rella, S.; Malitesta, C.; Bonacucina, G. Intracellular antioxidant activity of biocompatible citrate-capped palladium nanozymes. Nanomaterials 2020, 10, 99. [Google Scholar] [CrossRef]
- Cao, G.; Chen, Y.; Jiang, C.; Xu, J.; Xue, W.; Zhang, H.; Tian, Y. Synthesis of Citrate-Capped Sn Nanoparticles with Excellent Oxidation Resistance for High-Performance Electrically Conductive Adhesives. ACS Appl. Electron. Mater. 2023, 5, 1164–1173. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasala, P.; Latronico, T.; Mattia, U.; Matteucci, R.M.; Milella, A.; Grattieri, M.; Liuzzi, G.M.; Petrosillo, G.; Panniello, A.; Depalo, N.; et al. Enhancing Antioxidants Performance of Ceria Nanoparticles in Biological Environment via Surface Engineering with o-Quinone Functionalities. Antioxidants 2025, 14, 916. https://doi.org/10.3390/antiox14080916
Lasala P, Latronico T, Mattia U, Matteucci RM, Milella A, Grattieri M, Liuzzi GM, Petrosillo G, Panniello A, Depalo N, et al. Enhancing Antioxidants Performance of Ceria Nanoparticles in Biological Environment via Surface Engineering with o-Quinone Functionalities. Antioxidants. 2025; 14(8):916. https://doi.org/10.3390/antiox14080916
Chicago/Turabian StyleLasala, Pierluigi, Tiziana Latronico, Umberto Mattia, Rosa Maria Matteucci, Antonella Milella, Matteo Grattieri, Grazia Maria Liuzzi, Giuseppe Petrosillo, Annamaria Panniello, Nicoletta Depalo, and et al. 2025. "Enhancing Antioxidants Performance of Ceria Nanoparticles in Biological Environment via Surface Engineering with o-Quinone Functionalities" Antioxidants 14, no. 8: 916. https://doi.org/10.3390/antiox14080916
APA StyleLasala, P., Latronico, T., Mattia, U., Matteucci, R. M., Milella, A., Grattieri, M., Liuzzi, G. M., Petrosillo, G., Panniello, A., Depalo, N., Curri, M. L., & Fanizza, E. (2025). Enhancing Antioxidants Performance of Ceria Nanoparticles in Biological Environment via Surface Engineering with o-Quinone Functionalities. Antioxidants, 14(8), 916. https://doi.org/10.3390/antiox14080916










