Dietary Carnosic Acid Supplementation Improves the Growth Performance, the Antioxidant Status, and Diversity of Intestinal Microbiota in Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimental Design
2.2. Diets and Chicken Husbandry
2.3. Measurement of Growth Performance
2.4. Sample Collection
2.5. Calculation of Relative Weights of Immune Organs
2.6. Determination of Meat Quality
2.7. Determination of Antioxidant Variables
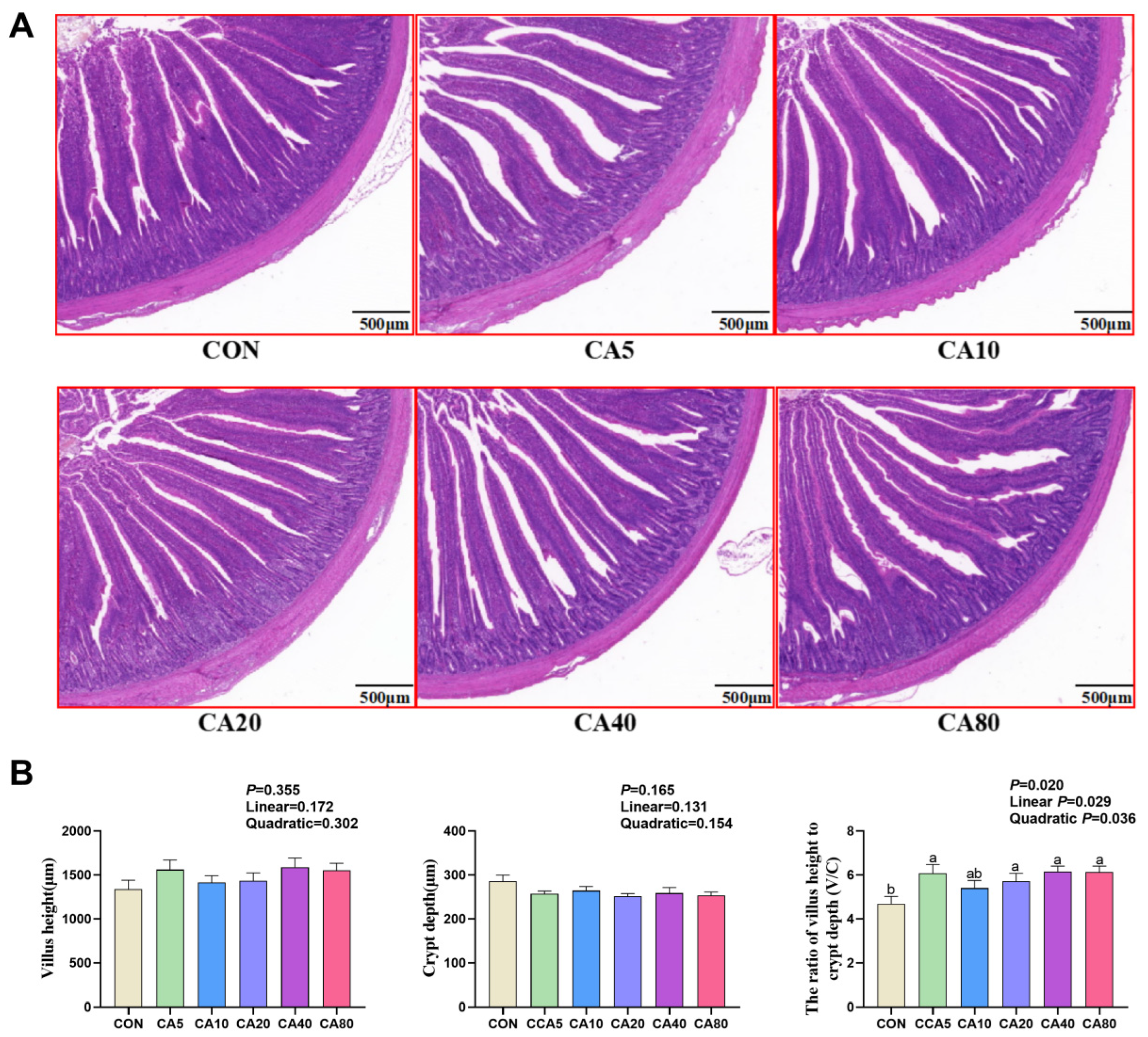
2.8. Intestinal Morphological Observation
2.9. Real-Time PCR Analysis
2.10. 16S rRNA Gene Sequencing
2.11. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Relative Weights of Immune Organs
3.3. Meat Quality
3.4. Jejunal Morphology
3.5. Antioxidant Capacity
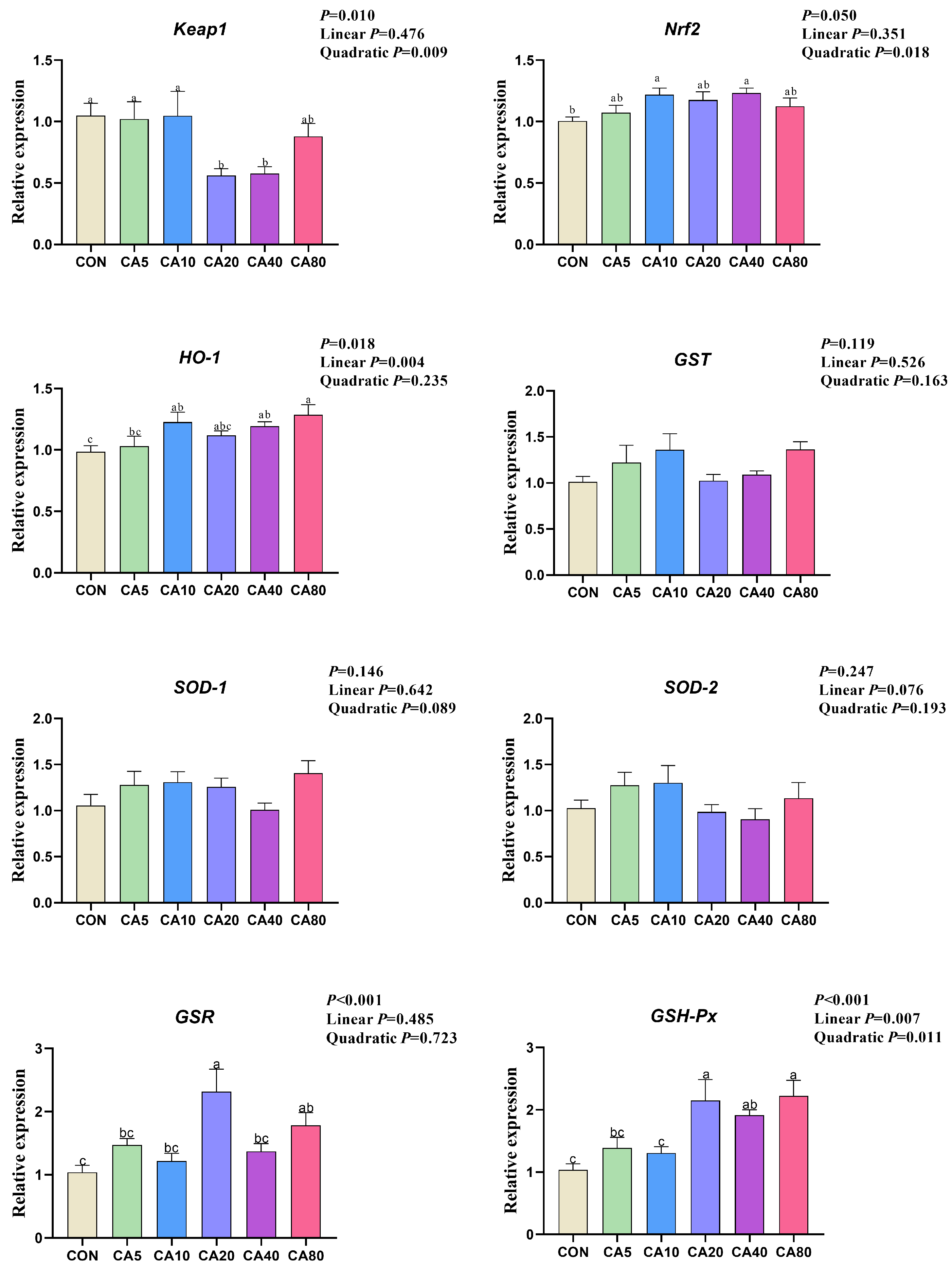
3.6. The Relative Expression of Antioxidative Genes
3.7. Regression Analysis of Optimal Supplemental Level of CA Based on Quadratic Regression
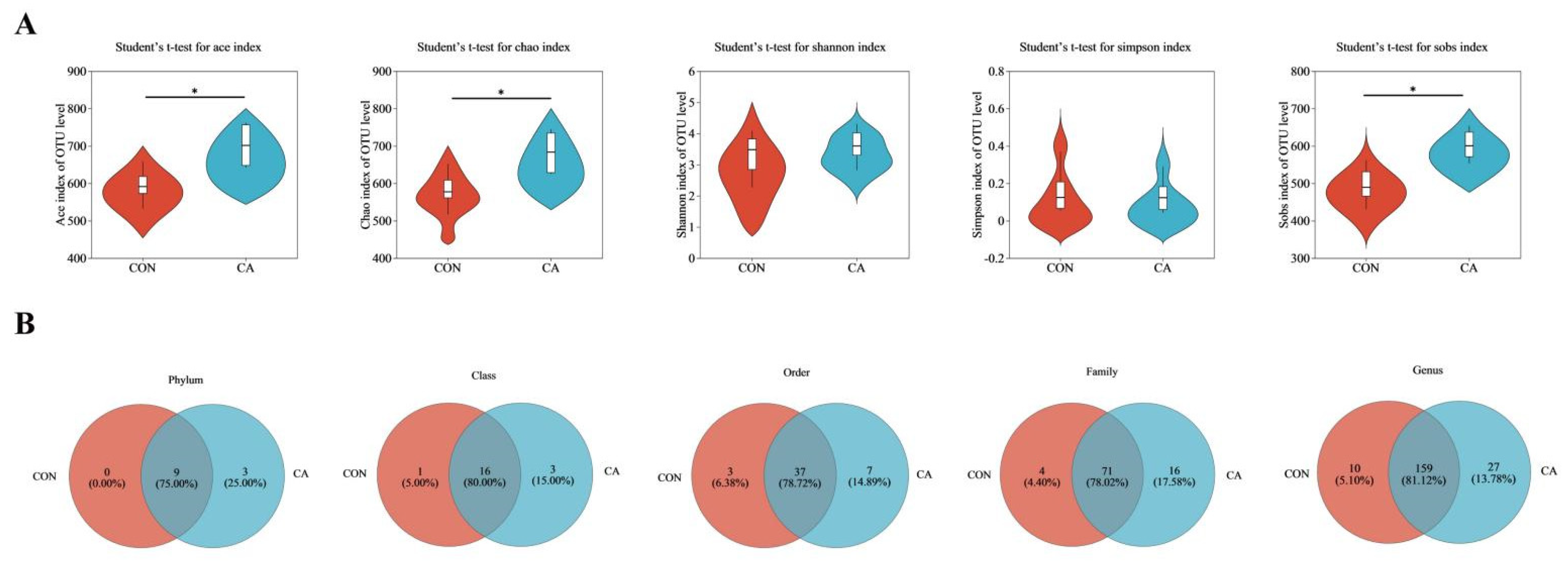
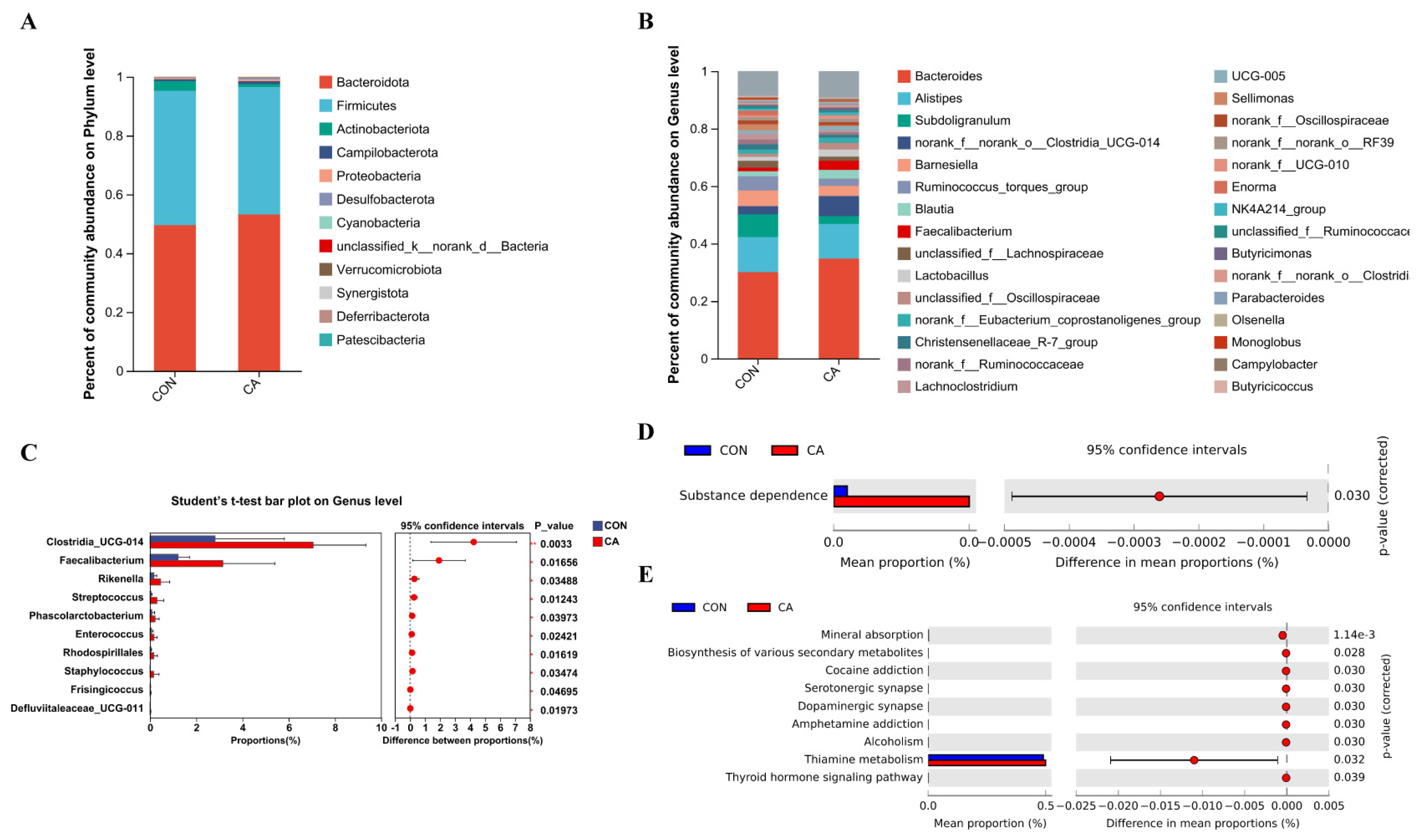
3.8. Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da Silva, B.D.; Conte-Junior, C.A. Perspectives on cultured meat in countries with economies dependent on animal production: A review of potential challenges and opportunities. Trends Food Sci. Technol. 2024, 149, 104551. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Zhao, J.; Wang, Y.; Hao, X.; Liu, K.; Liu, H. Protective effects of chlorogenic acid on the meat quality of oxidatively stressed broilers revealed by integrated metabolomics and antioxidant analysis. Food Funct. 2022, 13, 2238–2252. [Google Scholar] [CrossRef]
- Wei, R.; Lin, Q.; Adu, M.; Huang, H.; Yan, Z.; Shao, F.; Zhong, G.; Zhang, Z.; Sang, Z.; Cao, L.; et al. The sources, properties, extraction, biosynthesis, pharmacology, and application of lycopene. Food Funct. 2023, 14, 9974–9998. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Wu, S.; Zhou, W.; Chen, X.; Zhang, Q.; Wang, L. Dietary supplementation with Neolamarckia cadamba leaf extract improves broiler meat quality by enhancing antioxidant capacity and regulating metabolites. Anim. Nutr. 2024, 17, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Cai, G.; Ma, L.; Yan, L.; Wang, T.; Shi, Z.; Wang, C.; Chen, Z. Effects of different levels of Lycium barbarum flavonoids on growth performance, immunity, intestinal barrier and antioxidant capacity of meat ducks. Antioxidants 2025, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Silva-Guillen, Y.V.; Arellano, C.; Wiegert, J.; Boyd, R.D.; Martinez, G.E.; Van Heugten, E. Supplementation of vitamin E or a botanical extract as antioxidants to improve growth performance and health of growing pigs housed under thermoneutral or heat-stressed conditions. J. Anim. Sci. Biotechnol. 2024, 15, 27. [Google Scholar] [CrossRef]
- Lv, Y.; Zeng, N.; Feng, Y.; Zhang, S.; Zhou, X.; Gao, C. Eugenol accelerates intestinal stem cell regeneration to protect the intestinal barrier integrity through inhibiting JAK2/STAT3 signaling pathway in Salmonella enteritidis-challenged broiler chicks. J. Anim. Sci. Biotechnol. 2025, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A Quantitative Structure-Property Relationship (QSPR) study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Kumar, R.; Sherkhane, B.; Gundu, C.; Arruri, V.K.; Kumar, A. Bardoxolone methyl ameliorates hyperglycemia induced mitochondrial dysfunction by activating the Keap1-Nrf2-ARE pathway in experimental diabetic neuropathy. Mol. Neurobiol. 2020, 57, 3616–3631. [Google Scholar] [CrossRef]
- Lee, D.K.; Jang, H.D. Carnosic acid attenuates an early increase in ROS levels during adipocyte differentiation by suppressing translation of Nox4 and inducing translation of antioxidant enzymes. Int. J. Mol. Sci. 2021, 22, 6096. [Google Scholar] [CrossRef]
- Wan, S.; Li, X.; Liu, S.; Tang, S. The function of carnosic acid in lipopolysaccharides-induced hepatic and intestinal inflammation in poultry. Poult. Sci. 2024, 103, 103415. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, J.; Xiang, H.; Hu, R.; Yang, X.; Lv, J.; Zhang, W.; Liu, M.; Deng, X.; Yuan, X.; et al. Effects of main active components of rosemary on growth performance, meat quality and lipid metabolism in finishing pigs. Anim. Nutr. 2023, 15, 341–349. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Chen, Y.; Zhou, Y.; Meng, T.; Tan, B.; He, W.; Fu, X.; Xiao, D. Dietary supplementation with mulberry leaf flavonoids and carnosic acid complex enhances the growth performance and antioxidant capacity via regulating the p38 MAPK/Nrf2 pathway. Front. Nutr. 2024, 11, 1428577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Li, G.; Zang, Y.; Fan, Q.; Ye, J.; Wang, Y.; Jiang, S. Protective effects of carnosic acid on growth performance, intestinal barrier, and cecal microbiota in yellow-feathered broilers under lipopolysaccharide challenge. Poultry Sci. 2025, 104, 104688. [Google Scholar] [CrossRef]
- Zhang, L.; Ge, J.; Gao, F.; Yang, M.; Li, H.; Xia, F.; Bai, H.; Piao, X.; Sun, Z.; Shi, L. Rosemary leaf powder improves egg quality, antioxidant status, gut barrier function, and cecal microbiota and metabolites of late-phase laying hens. Anim. Nutr. 2024, 17, 325–334. [Google Scholar] [CrossRef]
- Bahadoran, S.; Teymouri, Y.; Hassanpour, H.; Mohebbi, A.; Akbari, M.R. Effect of sage (Salvia officinalis L.) extract in antioxidant status and intestinal morphology of pulmonary hypertensive chickens. Vet. Med. Sci. 2023, 9, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, C.; Luo, D.; Yu, W.; Li, S.; Xiao, Y.; Yuan, B.; Liang, S.; Yao, X.; Kim, N.H.; et al. Carnosic acid improves porcine early embryonic development by inhibiting the accumulation of reactive oxygen species. J. Reprod. Dev. 2020, 66, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture of the People’s Republic of China (PRC). Nutrient Requirements of Yellow Chicken; China Agricultural Press: Beijing, China, 2020. [Google Scholar]
- Wang, Y.; Wang, Y.; Wang, B.; Mei, X.; Jiang, S.; Li, W. Protocatechuic acid improved growth performance, meat quality, and intestinal health of Chinese yellow-feathered broilers. Poult. Sci. 2019, 98, 3138–3149. [Google Scholar] [CrossRef]
- Zerehdaran, S.; Vereijken, A.L.; Van Arendonk, J.A.; Van der Waaijt, E.H. Estimation of genetic parameters for fat deposition and carcass traits in broilers. Poult. Sci. 2004, 83, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gou, Z.; Lin, X.; Fan, Q.; Ye, J.; Jiang, S. Optimal Level of Supplemental manganese for yellow-feathered broilers during the growth phase. Animals 2021, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
- Abu Hafsa, S.H.; Ibrahim, S.A. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J. Anim. Physiol. Anim. Nutr. 2018, 102, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Lu, M.; Guo, Y.; Liang, S.; Mou, R.; He, Y.; Tang, L. Resveratrol relieves chronic heat stress-induced liver oxidative damage in broilers by activating the Nrf2-Keap1 signaling pathway. Ecotoxicol. Environ. Saf. 2023, 249, 114411. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, W.; Guo, Y.; Wang, D.; Zhang, Y.; Zhi, Y.; Li, D.; Li, W.; Li, Z.; Jiang, R.; et al. Dynamic alternations of three-dimensional chromatin architecture contribute to phenotypic characteristics of breast muscle in chicken. Commun. Biol. 2024, 7, 910. [Google Scholar] [CrossRef]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for monitoring intestinal health in poultry:present status and future perspectives. Vet. Res. 2018, 49, 43. [Google Scholar] [CrossRef]
- Grego-Bessa, J.; Bloomekatz, J.; Castel, P.; Omelchenko, T.; Baselga, J.; Anderson, K.V. The tumor suppressor PTEN and the PDK1 kinase regulate formation of the columnar neural epithelium. eLife 2016, 5, e12034. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Du, E.; Jin, F.; Zheng, C.; Fan, Q.; Zhao, N.; Guo, W.; Zhang, W.; Huang, S.; et al. Effects of magnolol on egg production, egg quality, antioxidant capacity, and intestinal health of laying hens in the late phase of the laying cycle. Poult. Sci. 2021, 100, 835–843. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Zhao, N.; Du, E.; Jin, F.; Fan, Q.; Guo, W.; Huang, S.; Wei, J. Effects of magnolol and honokiol blend on performance, egg quality, hepatic lipid metabolism, and intestinal morphology of hens at late laying cycle. Animal 2022, 16, 100532. [Google Scholar] [CrossRef]
- Aryal, B.; Kwakye, J.; Ariyo, O.W.; Ghareeb, A.F.A.; Milfort, M.C.; Fuller, A.L.; Khatiwada, S.; Rekaya, R.; Aggrey, S.E. Major Oxidative and Antioxidant Mechanisms During Heat Stress-Induced Oxidative Stress in Chickens. Antioxidants 2025, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Oke, O.E.; Akosile, O.A.; Oni, A.I.; Opowoye, I.O.; Ishola, C.A.; Adebiyi, J.O.; Odeyemi, A.J.; Adjei-Mensah, B.; Uyanga, V.A.; Abioja, M.O. Oxidative stress in poultry production. Poult. Sci. 2024, 103, 104003. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Cao, Q.; Zhang, C.; Dong, Z.; Feng, D.; Ye, H.; Zuo, J. Dietary catalase supplementation alleviates deoxynivalenol-induced oxidative stress and gut microbiota dysbiosis in broiler chickens. Toxins 2022, 14, 830. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, K.; Li, C. Oxidative stress in poultry and the therapeutic role of herbal medicine in intestinal health. Antioxidants 2024, 13, 1375. [Google Scholar] [CrossRef] [PubMed]
- Kouvedaki, I.; Pappas, A.C.; Surai, P.F.; Zoidis, E. Nutrigenomics of natural antioxidants in broilers. Antioxidants 2024, 13, 270. [Google Scholar] [CrossRef]
- He, R.; Liu, B.; Xiong, R.; Geng, B.; Meng, H.; Lin, W.; Hao, B.; Zhang, L.; Wang, W.; Jiang, W.; et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Tschuck, J.; Theilacker, L.; Rothenaigner, I.; Weiss, S.A.I.; Akdogan, B.; Van Thanh, L.; Mueller, C.; Graf, R.; Brandner, S.; Puetz, C.; et al. Farnesoid X receptor activation by bile acids suppresses lipid peroxidation and ferroptosis. Nat. Commun. 2023, 14, 6908. [Google Scholar] [CrossRef]
- Luo, J.; Wu, X.; Chen, D.; Yu, B.; He, J. Dietary ferulic acid supplementation enhances antioxidant capacity and alleviates hepatocyte pyroptosis in diquat challenged piglets. J. Anim. Sci. Biotechnol. 2024, 15, 134. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, Y.; Luo, Y.; Zhang, J.; Zhu, X.; Xiao, J. Ganoderic acid D prevents oxidative stress-induced senescence by targeting 14-3-3ε to activate CaM/CaMKII/NRF2 signaling pathway in mesenchymal stem cells. Aging Cell 2022, 21, e13686. [Google Scholar] [CrossRef]
- Zhan, S.; Wu, L.; Lv, Y.; Huang, W.; Ge, C.; Hu, Z.; Shen, X.; Lin, G.; Yu, D.; Liu, B. Lactobacillus reuteri alleviates diquat induced hepatic impairment and mitochondrial dysfunction via activation of the Nrf2 antioxidant system and suppression of NF-κB inflammatory response. Poult. Sci. 2025, 104, 104997. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) methanolic extracts. J. Anim. Sci. Biotechnol. 2012, 60, 9603–9608. [Google Scholar] [CrossRef]
- Karagianni, K.; Pettas, S.; Kanata, E.; Lioulia, E.; Thune, K.; Schmitz, M.; Tsamesidis, I.; Lymperaki, E.; Xanthopoulos, K.; Sklaviadis, T.; et al. Carnosic acid and carnosol display antioxidant and anti-prion properties in in vitro and cell-free models of prion diseases. Antioxidants 2022, 11, 726. [Google Scholar] [CrossRef]
- Geertsema, S.; Bourgonje, A.R.; Fagundes, R.R.; Gacesa, R.; Weersma, R.K.; Van Goor, H.; Mann, G.E.; Dijkstra, G.; Faber, K.N. The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol. Med. 2023, 29, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. Nrf2: A main responsive element in cells to mycotoxin-induced toxicity. Arch. Toxicol. 2021, 95, 1521–1533. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Zheng, Y.; Lang, D.; Wang, J.; Yan, S.; Chen, Y. EGCG-NPs inhibition HO-1-mediated reprogram iron metabolism against ferroptosis after subarachnoid hemorrhage. Redox Biol. 2024, 70, 103075. [Google Scholar] [CrossRef]
- Biondini, M.; Lehuédé, C.; Tabariès, S.; Annis, M.G.; Pacis, A.; Ma, E.H.; Tam, C.; Hsu, B.E.; Audet-Delage, Y.; Abu-Thuraia, A.; et al. Metastatic breast cancer cells are metabolically reprogrammed to maintain redox homeostasis during metastasis. Redox Biol. 2024, 75, 103276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiu, R.; Li, Z.; Wang, Q.; Lei, X.; Chen, J.; Liu, H.; Liu, J. Tyrosine-modified tilapia skin antioxidant peptides and their hydroxyl radical quenching activities. J. Mater. Chem. B. 2025, 13, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Da Costa Ferreira, G.; Peres, A.; Dal Bosco, S.M. Carnosic acid suppresses the H2O2-induced mitochondria-related bioenergetics disturbances and redox impairment in Sh-Sy5 y cells: Role for nrf2. Mol. Neurobiol. 2018, 55, 968–979. [Google Scholar] [CrossRef]
- De Souza, I.C.C.; Gobbo, R.C.B.; De Almeida, F.J.S.; Luckachaki, M.D.; De Oliveira, M.R. Carnosic acid depends on glutathione to promote mitochondrial protection in methylglyoxal-exposed SH-SY5 Y cells. Metab. Brain Dis. 2021, 36, 471–481. [Google Scholar] [CrossRef]
- AlKahtane, A.A.; Ghanem, E.; Bungau, S.G.; Alarifi, S.; Ali, D.; AlBasher, G.; Alkahtani, S.; Aleya, L.; Abdel-Daim, M.M. Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues. Environ. Sci. Pollut. Res. 2020, 27, 11663–11670. [Google Scholar] [CrossRef]
- Miller, D.M.; Singh, I.N.; Wang, J.; Hall, E.D. Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp. Neurol. 2015, 264, 103–110. [Google Scholar] [CrossRef]
- Glendinning, L.; Wu, Z.; Vervelde, L.; Watson, M.; Balic, A. Infectious bronchitis virus vaccination, but not the presence of XCR1, is correlated with large differences in chicken caecal microbiota. Microb. Genom. 2024, 10, 001289. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Zhang, L.; Waqas, M.; Mehmood, K.; Iqbal, M.; Can, M.; Li, Z.; Lian, Y.; Sizhu, S.; et al. Probiotic potential of Lactobacillus on the intestinal microflora against Escherichia coli induced mice model through high-throughput sequencing. Microb. Pathog. 2019, 137, 103760. [Google Scholar] [CrossRef]
- Zhang, J.; Han, H.; Zhang, L.; Wang, T. Dietary bisdemethoxycurcumin supplementation attenuates lipopolysaccharide-induced damages on intestinal redox potential and redox status of broilers. Poult. Sci. 2021, 100, 101061. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.Q.; Moon, S.K.; Wang, Y.Q.; Xie, L.M.; Cho, H.S.; Kim, S.K. Supplemental effects of different production methods of pine needle additives on growth performance, intestinal environment, meat quality and serum of broiler chickens. Anim. Biosci. 2024, 37, 1263–1276. [Google Scholar] [CrossRef]
- Wu, L.; Niu, Y.; Ren, B.; Wang, S.; Song, Y.; Wang, X.; Zhao, K.; Yue, Z.; Li, Y.; Gao, J. Naringenin promotes gastrointestinal motility in mice by impacting the SCF/c-Kit pathway and gut microbiota. Foods 2024, 13, 2520. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Won, T.H.; Li, T.T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.B.; et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 2022, 611, 578–584. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Liu, J.; Zhong, F.; Zhang, C.; Chen, Y.; Sun, T.; Ji, C.; Ma, D. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat. Commun. 2022, 13, 2522. [Google Scholar] [CrossRef]
- Lei, L.; Yang, Y.; Yang, Y.; Wu, S.; Ma, X.; Mao, M.; Hu, T. Mechanisms by which small rnas affect bacterial activity. J. Dent. Res. 2019, 98, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Zimatkina, T.I.; Chernikevich, I.P.; Zimatkin, S.M.; Deitrich, R.A. Thiamine status in liver and brain of rats genetically selected for different sensitivity to hypnotic effect of alcohol. Alcohol Clin. Exp. Res. 2000, 24, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Behura, S.K.; Kelleher, A.M.; Spencer, T.E. Evidence for functional interactions between the placenta and brain in pregnant mice. FASEB J. 2019, 33, 4261–4272. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, Y.; Wang, X.; Woodson, K.; Kemper, C.; Disney, E.; Wang, J. Alcohol intake enhances glutamatergic transmission from D2 receptor-expressing afferents onto D1 receptor-expressing medium spiny neurons in the dorsomedial striatum. Neuropsychopharmacology 2019, 44, 1123–1131. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The impact of ellagitannins and their metabolites through gut microbiome on the gut health and brain wellness within the gut-brain axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wei, H.; Yuan, S.; Kong, Y.; Yang, H.; Zhang, Y.; Cui, X.; Chen, W.; Liu, J.; Zhang, Y. Probiotic pediococcus pentosaceus ameliorates MPTP-induced oxidative stress via regulating the gut microbiota-gut-brain axis. Front. Cell. Infect. Microbiol. 2022, 12, 1022879. [Google Scholar] [CrossRef] [PubMed]
| Items | 1 to 21 Days of Age | 22 to 53 Days of Age |
|---|---|---|
| Ingredient, % | ||
| Corn | 56.50 | 63.40 |
| Soybean meal | 35.90 | 30.40 |
| Soybean oil | 2.60 | 1.50 |
| L-Lys HCl (78.8%) | 0.20 | 0.20 |
| DL-Met (99.0%) | 0.30 | 0.30 |
| Talcum powder | 1.00 | 1.00 |
| Dicalcium phosphate | 2.20 | 1.90 |
| NaCl | 0.30 | 0.30 |
| Premix 1 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Nutrient levels 2 | ||
| Nitrogen-corrected metabolizable energy, MJ/kg | 11.92 | 12.34 |
| Crude protein, % | 20.94 | 17.81 |
| Calcium, % | 1.01 | 0.92 |
| Total phosphorus, % | 0.72 | 0.67 |
| Non-phytate phosphorus, % | 0.47 | 0.41 |
| Lysine, % | 1.29 | 1.15 |
| Methionine, % | 0.52 | 0.48 |
| Methionine + Cysteine, % | 0.93 | 0.85 |
| Gene | Primer Sequence (5′ to 3′) | GenBank ID |
|---|---|---|
| β-actin | F: GAGAAATTGTGCGTGACATCA R: CCTGAACCTCTCATTGCCA | NM_001101.5 |
| GST | F: GATGAACGTCGTCCAACCAG R: TCATGTCCGTGGTCCTTCAA | NM_001001777.2 |
| SOD1 | F: GGTGCTCACTTTAATCCTG R: CTACTTCTGCCACTCCTCC | NM_205064.2 |
| SOD2 | F: TGGTGTTCAAGGATCAGGCT R: CCCAGCAATGGAATGAGACC | NM_204211.2 |
| GSR | F: ATACCCGGCGTCAGGTTTAA R: CCTGTCGCAATGAGGATGTG | XM_040671422.2 |
| Keap1 | F: CAACTTCGCCGAGCAGA R: CGTGGAACACCTCCGACT | XM_040671565.2 |
| HO-1 | F: CTTCGCACAAGGAGTGTTAAC R: CATCCTGCTTGTCCTCTCAC | NM_205344.2 |
| Nrf2 | F: ATCACCTCTTCTGCACCGAA R: GCTTTCTCCCGCTCTTTCTG | XM_046921122.1 |
| GSH-Px | F: AAGTGCCAGGTGAACGGGAAGG R: AGGGCTGTAGCGGCGGAAAG | NM_001277854.3 |
| Items | CON | CA5 | CA10 | CA20 | CA40 | CA80 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 to 21 days of age | ||||||||||
| Initial BW, g | 40.90 | 40.83 | 40.83 | 40.87 | 40.92 | 40.88 | 0.03 | 0.266 | 0.340 | 0.552 |
| Final BW, g | 468.67 | 462.33 | 461.50 | 464.50 | 470.00 | 465.00 | 4.24 | 0.816 | 0.789 | 0.901 |
| ADG, g | 21.39 | 21.07 | 21.03 | 21.18 | 21.45 | 21.21 | 0.21 | 0.814 | 0.789 | 0.905 |
| ADFI, g | 36.17 | 35.54 | 35.93 | 35.67 | 35.53 | 35.26 | 0.34 | 0.661 | 0.141 | 0.329 |
| F/G | 1.68 | 1.70 | 1.69 | 1.69 | 1.66 | 1.66 | 0.01 | 0.122 | 0.017 | 0.057 |
| Survival rate, % | 100.00 | 100.00 | 98.81 | 100.00 | 100.00 | 97.62 | 0.71 | 0.186 | 0.043 | 0.064 |
| 22 to 53 days of age | ||||||||||
| Final BW, g | 2013.06 b | 2056.04 ab | 2077.70 a | 2085.06 a | 2074.50 a | 2040.36 ab | 15.21 | 0.019 | 0.980 | 0.012 |
| ADG, g | 48.18 b | 49.77 a | 50.37 a | 50.52 a | 49.98 a | 49.15 ab | 0.52 | 0.044 | 0.914 | 0.049 |
| ADFI, g | 119.19 | 120.05 | 121.92 | 122.23 | 121.16 | 118.69 | 1.47 | 0.618 | 0.501 | 0.256 |
| F/G | 2.46 | 2.42 | 2.42 | 2.41 | 2.48 | 2.39 | 0.02 | 0.122 | 0.427 | 0.292 |
| Survival rate, % | 100.00 a | 98.61 a | 98.61 a | 100.00 a | 98.61 a | 90.15 b | 1.68 | <0.001 | <0.001 | <0.001 |
| 1 to 53 days of age | ||||||||||
| ADG, g | 35.86 b | 36.64 ab | 37.03 a | 37.17 a | 36.97 a | 36.35 ab | 0.37 | 0.019 | 0.979 | 0.011 |
| ADFI, g | 90.09 | 90.90 | 92.44 | 91.99 | 91.58 | 90.12 | 1.38 | 0.640 | 0.088 | 0.007 |
| F/G | 2.51 | 2.48 | 2.50 | 2.48 | 2.53 | 2.45 | 0.03 | 0.171 | 0.216 | 0.107 |
| Survival rate, % | 100.00 a | 98.61 a | 95.83 a | 100.00 a | 98.61 a | 88.89 b | 1.88 | <0.001 | <0.001 | <0.001 |
| Items | CON | CA5 | CA10 | CA20 | CA40 | CA80 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|---|---|
| 21 days of age | ||||||||||
| Liver/BW | 3.27 | 3.25 | 2.94 | 3.15 | 3.28 | 3.35 | 0.12 | 0.728 | 0.112 | 0.245 |
| Spleen/BW | 0.17 | 0.17 | 0.14 | 0.16 | 0.16 | 0.17 | 0.01 | 0.670 | 0.703 | 0.390 |
| Thymus/BW | 0.48 | 0.38 | 0.42 | 0.41 | 0.44 | 0.43 | 0.03 | 0.330 | 0.936 | 0.537 |
| Bursa of Fabricius/BW | 0.27 | 0.25 | 0.23 | 0.24 | 0.25 | 0.25 | 0.02 | 0.307 | 0.287 | 0.568 |
| 53 days of age | ||||||||||
| Liver/BW | 2.29 ab | 2.24 bc | 2.37 a | 2.26 b | 2.28 ab | 2.14 c | 0.02 | 0.010 | 0.002 | 0.003 |
| Spleen/BW | 0.22 | 0.23 | 0.21 | 0.22 | 0.22 | 0.20 | 0.01 | 0.380 | 0.059 | 0.160 |
| Items | CON | CA5 | CA10 | CA20 | CA40 | CA80 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|---|---|
| Pectoral muscle rate, % | 12.67 | 12.42 | 12.23 | 12.58 | 12.63 | 12.85 | 0.14 | 0.195 | 0.059 | 0.103 |
| Shear force24h, N | 22.55 | 24.32 | 24.73 | 23.70 | 24.22 | 26.42 | 0.88 | 0.894 | 0.176 | 0.207 |
| Drip loss24h, % | 1.83 | 1.63 | 1.69 | 1.70 | 1.55 | 1.57 | 0.04 | 0.396 | 0.207 | 0.176 |
| a*45min | 13.94 | 13.54 | 13.87 | 14.00 | 13.30 | 13.48 | 0.10 | 0.268 | 0.298 | 0.556 |
| b*45min | 9.75 | 9.95 | 9.97 | 9.82 | 9.69 | 9.50 | 0.11 | 0.851 | 0.245 | 0.508 |
| L*45min | 49.95 | 50.29 | 50.31 | 49.81 | 50.57 | 49.76 | 0.18 | 0.771 | 0.775 | 0.822 |
| pH45min | 6.13 | 6.08 | 6.05 | 5.95 | 6.19 | 6.16 | 0.03 | 0.123 | 0.264 | 0.527 |
| a*24h | 13.92 | 13.58 | 13.19 | 13.18 | 13.17 | 13.37 | 0.23 | 0.928 | 0.550 | 0.621 |
| b*24h | 10.10 | 10.11 | 10.26 | 10.46 | 10.02 | 10.21 | 0.13 | 0.952 | 0.928 | 0.995 |
| L*24h | 55.03 | 53.31 | 54.46 | 54.72 | 54.28 | 54.22 | 0.29 | 0.658 | 0.784 | 0.683 |
| pH24h | 5.65 | 5.68 | 5.65 | 5.66 | 5.67 | 5.64 | 0.01 | 0.855 | 0.176 | 0.207 |
| Intramuscular fat, % | 8.51 a | 8.19 a | 8.66 a | 8.13 ab | 3.78 b | 8.62 a | 0.82 | 0.001 | 0.340 | 0.001 |
| Item | CON | CA5 | CA10 | CA20 | CA40 | CA80 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | ||||||||||
| MDA, nmol/mL | 2.49 | 2.34 | 2.39 | 2.50 | 2.70 | 2.59 | 0.11 | 0.253 | 0.499 | 0.752 |
| GSH-Px, U/mL | 1491.86 bc | 1548.84 b | 1439.53 c | 1405.81 c | 1469.77 bc | 1768.6 a | 19.02 | <0.001 | <0.001 | <0.001 |
| T-SOD, U/mL | 164.19 a | 163.66 a | 164.73 a | 164.53 a | 164.06 a | 162.05 b | 0.19 | <0.001 | 0.014 | <0.001 |
| T-AOC, U/mL | 1.34 c | 1.37 c | 2.14 ab | 1.22 c | 1.62 bc | 2.41 a | 0.27 | 0.004 | 0.004 | 0.011 |
| Liver | ||||||||||
| MDA, nmol/mg prot | 0.29 a | 0.22 ab | 0.24 a | 0.21 ab | 0.21 ab | 0.13 b | 0.01 | 0.038 | 0.002 | 0.130 |
| GSH-Px, U/mg prot | 75.47 | 82.89 | 84.19 | 77.66 | 76.72 | 74.49 | 1.49 | 0.313 | 0.172 | 0.390 |
| T-SOD, U/mg prot | 23.24 | 24.89 | 24.88 | 24.38 | 24.08 | 23.56 | 0.42 | 0.480 | 0.424 | 0.717 |
| T-AOC, U/mg prot | 0.13 ab | 0.13 ab | 0.14 a | 0.13 ab | 0.12 bc | 0.11 c | 0.02 | 0.002 | 0.001 | 0.004 |
| CAT, U/mg prot | 74.72 b | 82.92 a | 82.22 a | 76.29 ab | 76.43 ab | 75.11 b | 1.01 | 0.048 | 0.070 | 0.155 |
| Duodenum | ||||||||||
| MDA, nmol/mg prot | 0.89 | 0.70 | 0.71 | 0.73 | 0.96 | 0.99 | 0.09 | 0.069 | 0.023 | 0.075 |
| GSH-Px, U/mg prot | 12.90 c | 19.74 bc | 21.54 ab | 31.35 a | 23.61 ab | 30.51 a | 1.52 | <0.001 | 0.002 | 0.002 |
| T-SOD, U/mg prot | 29.20 c | 38.94 a | 37.42 ab | 33.52 bc | 39.07 a | 40.34 a | 0.78 | <0.001 | 0.001 | 0.003 |
| T-AOC, U/mg prot | 2.61 c | 3.83 a | 3.29 ab | 2.84 bc | 3.38 ab | 3.36 ab | 0.08 | <0.001 | 0.414 | 0.678 |
| CAT, U/mg prot | 1.15 | 1.18 | 1.32 | 1.44 | 0.76 | 1.11 | 0.19 | 0.188 | 0.231 | 0.430 |
| Jejunum | ||||||||||
| T-SOD, U/mg prot | 22.99 ab | 21.93 ab | 21.97 ab | 22.92 ab | 21.54 b | 23.59 a | 0.20 | 0.047 | 0.185 | 0.084 |
| GSH-Px, U/mg prot | 119.54 | 111.93 | 137.00 | 119.48 | 108.26 | 131.58 | 3.37 | 0.093 | 0.731 | 0.394 |
| T-AOC, U/mg prot | 0.24 | 0.22 | 0.23 | 0.24 | 0.21 | 0.25 | 0.01 | 0.164 | 0.281 | 0.318 |
| Ileum | ||||||||||
| MDA, nmol/mg prot | 0.11 | 0.13 | 0.14 | 0.17 | 0.12 | 0.12 | 0.02 | 0.289 | 0.602 | 0.424 |
| T-SOD, U/mg prot | 8.52 | 7.86 | 7.83 | 8.41 | 8.24 | 7.77 | 0.28 | 0.246 | 0.307 | 0.466 |
| GSH-Px, U/mg prot | 14.22 b | 28.51 a | 23.49 a | 24.15 a | 25.24 a | 9.80 b | 1.18 | <0.001 | 0.002 | <0.001 |
| T-AOC, U/mg prot | 0.15 abc | 0.16 a | 0.15 ab | 0.15 abc | 0.13 bc | 0.13 c | 1.17 | 0.009 | <0.001 | 0.003 |
| CAT | 2.62 | 2.74 | 2.79 | 3.30 | 2.36 | 2.77 | 1.00 | 0.176 | 0.878 | 0.727 |
| Item | CON | CA5 | CA10 | CA20 | CA40 | CA80 | SEM | p-Value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | ||||||||||
| MDA, nmol/mL | 9.40 a | 9.31 a | 8.17 a | 4.10 b | 3.71 b | 4.26 b | 1.76 | 0.027 | 0.007 | 0.003 |
| GSH-Px, U/mL | 1376.60 | 1379.49 | 1355.69 | 1360.74 | 1386.70 | 1329.01 | 40.42 | 0.940 | 0.319 | 0.486 |
| T-SOD, U/mL | 151.32 | 152.92 | 149.89 | 147.19 | 129.51 | 130.52 | 7.70 | 0.085 | 0.002 | 0.006 |
| T-AOC, U/mL | 6.18 | 6.23 | 6.54 | 6.51 | 5.84 | 6.34 | 0.36 | 0.798 | 0.766 | 0.900 |
| Liver | ||||||||||
| MDA, nmol/mg prot | 1.36 a | 0.82 b | 0.80 b | 0.75 bc | 0.55 c | 0.70 bc | 0.11 | <0.001 | 0.001 | <0.001 |
| GSH-Px, U/mg prot | 1436.44 | 1551.88 | 1626.89 | 1389.36 | 1712.59 | 1392.63 | 124.57 | 0.349 | 0.675 | 0.391 |
| T-SOD, U/mg prot | 577.78 b | 576.59 b | 584.04 b | 579.68 ab | 607.88 a | 609.67 a | 8.06 | 0.001 | <0.001 | <0.001 |
| T-AOC, U/mg prot | 2.46 | 2.46 | 2.68 | 2.20 | 2.56 | 2.88 | 0.17 | 0.092 | 0.064 | 0.110 |
| CAT, U/mg prot | 4.72 ab | 4.80 a | 4.80 a | 4.72 ab | 4.56 b | 4.58 b | 0.06 | 0.007 | 0.001 | 0.052 |
| Duodenum | ||||||||||
| MDA, nmol/mg prot | 0.66 | 0.49 | 0.72 | 0.52 | 0.56 | 0.64 | 0.08 | 0.326 | 0.995 | 0.760 |
| GSH-Px, U/mg prot | 2302.82 | 3340.94 | 2676.97 | 2824.52 | 4463.37 | 4510.80 | 758.35 | 0.182 | 0.019 | 0.051 |
| T-SOD, U/mg prot | 825.25 | 825.74 | 806.00 | 770.15 | 755.95 | 762.21 | 27.94 | 0.273 | 0.042 | 0.051 |
| T-AOC, U/mg prot | 21.43 | 18.43 | 18.50 | 19.06 | 19.67 | 19.91 | 0.39 | 0.260 | 0.066 | 0.086 |
| CAT, U/mg prot | 1.15 | 1.18 | 1.32 | 1.44 | 0.76 | 1.11 | 0.19 | 0.183 | 0.271 | 0.469 |
| Jejunum | ||||||||||
| MDA, nmol/mg prot | 1.36 a | 0.57 b | 0.61 ab | 0.70 ab | 0.64 a | 0.31 b | 0.29 | 0.048 | 0.039 | 0.066 |
| GSH-Px, U/mg prot | 35.69 | 36.62 | 43.24 | 43.05 | 33.87 | 36.71 | 4.56 | 0.638 | 0.653 | 0.896 |
| T-SOD, U/mg prot | 20.46 | 20.61 | 20.32 | 20.55 | 19.92 | 20.84 | 0.52 | 0.894 | 0.893 | 0.680 |
| T-AOC, U/mg prot | 0.25 | 0.25 | 0.28 | 0.29 | 0.27 | 0.27 | 0.02 | 0.488 | 0.180 | 0.279 |
| CAT, U/mg prot | 3.76 | 3.72 | 3.56 | 4.05 | 3.05 | 3.80 | 0.47 | 0.785 | 0727 | 0.800 |
| Ileum | ||||||||||
| MDA, nmol/mg prot | 0.96 | 1.09 | 1.07 | 0.98 | 0.91 | 1.04 | 0.07 | 0.564 | 0.619 | 0.651 |
| GSH-Px, U/mg prot | 21.50 c | 45.26 a | 47.88 a | 41.02 ab | 27.09 c | 32.35 bc | 4.62 | <0.001 | 0.298 | 0.471 |
| T-SOD, U/mg prot | 15.45 | 15.56 | 15.32 | 15.31 | 16.12 | 15.14 | 0.30 | 0.464 | 0.896 | 0.243 |
| T-AOC, U/mg prot | 0.24 | 0.25 | 0.25 | 0.25 | 0.27 | 0.24 | 0.01 | 0.581 | 0.825 | 0.070 |
| CAT, U/mg prot | 2.39 c | 2.57 bc | 3.11 bc | 3.58 ab | 2.80 bc | 3.84 a | 0.30 | 0.009 | 0.025 | 0.062 |
| Item | p-Value | R2 | Regression Equation | CA Supplemental Level (mg/kg) 1 |
|---|---|---|---|---|
| Final BW (53 d), g | 0.012 | 0.243 | y = −0.0356117 CA2 + 2.88631 CA + 2034.329 | 40.53 |
| ADG (22 to 53 d), g | 0.049 | 0.171 | y = −0.0010260 CA2 + 0.08236 CA + 49.211 | 40.14 |
| ADG (1 to 53 d), g | 0.011 | 0.244 | y = −0.006492 CA2 + 0.05261 CA + 36.244 | 40.52 |
| 21 d | ||||
| Hepatic T-AOC, U/mg prot | 0.004 | 0.160 | y = −0.0000020 CA2 + 0.00009 CA + 0.130 | 24.17 |
| Duodenal T-SOD, U/mg prot | 0.003 | 0.188 | y = −0.0012707 CA2 + 0.18963 CA + 33.018 | 74.61 |
| Duodenal GSH-Px, U/mg prot | 0.002 | 0.208 | y = −0.0042395 CA2 + 0.50918 CA + 15.977 | 60.05 |
| Ileal GSH-Px, U/mg prot | <0.001 | 0.407 | y = −0.0067097 CA2 + 0.41349 CA + 19.535 | 30.81 |
| 53 d | ||||
| Relative weight of liver, % | 0.003 | 0.165 | y = −0.0000429 CA2 + 0.00164 CA + 2.281 | 19.11 |
| Intramuscular fat, % | 0.001 | 0.432 | y = −0.0022318 CA2 + 0.19212 CA + 9.439 | 43.04 |
| Jejunal V/C | 0.036 | 0.143 | y = −0.0003450 CA2 + 0.03950 CA + 5.169 | 57.28 |
| Plasma MDA, nmol/mL | 0.003 | 0.234 | y = 0.0023638 CA2 − 0.26109 CA +10.047 | 55.23 |
| Hepatic MDA, nmol/mg prot | <0.001 | 0.483 | y = 0.0002654 CA2 − 0.02685 CA + 1.161 | 50.58 |
| Hepatic T-SOD, U/mg prot | <0.001 | 0.329 | y = −0.0075841 CA2 + 1.08413 CA + 566.677 | 71.47 |
| Hepatic Keap1, relative expression | 0.009 | 0.293 | y = 0.0003332 CA2 − 0.0300 CA + 1.194 | 45.02 |
| Hepatic Nrf2, relative expression | 0.018 | 0.215 | y = −0.0001084 CA2 + 0.00962 CA + 1.044 | 44.35 |
| Hepatic HO-1, relative expression | 0.012 | 0.235 | y = −0.00004167 CA2 + 0.00640 CA + 1.034 | 76.85 |
| Hepatic GSH-Px, relative expression | 0.011 | 0.345 | y = −0.0003055 CA2 + 0.037626 CA + 1.133 | 61.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, Q.; Dong, J.; Li, G.; Niu, K.; Pan, J.; Xia, L.; Wang, Y.; Jiang, S. Dietary Carnosic Acid Supplementation Improves the Growth Performance, the Antioxidant Status, and Diversity of Intestinal Microbiota in Broilers. Antioxidants 2025, 14, 1026. https://doi.org/10.3390/antiox14081026
Zhang S, Wang Q, Dong J, Li G, Niu K, Pan J, Xia L, Wang Y, Jiang S. Dietary Carnosic Acid Supplementation Improves the Growth Performance, the Antioxidant Status, and Diversity of Intestinal Microbiota in Broilers. Antioxidants. 2025; 14(8):1026. https://doi.org/10.3390/antiox14081026
Chicago/Turabian StyleZhang, Sheng, Qin Wang, Jingjing Dong, Guanhuo Li, Kaiyuan Niu, Junhao Pan, Linghan Xia, Yibing Wang, and Shouqun Jiang. 2025. "Dietary Carnosic Acid Supplementation Improves the Growth Performance, the Antioxidant Status, and Diversity of Intestinal Microbiota in Broilers" Antioxidants 14, no. 8: 1026. https://doi.org/10.3390/antiox14081026
APA StyleZhang, S., Wang, Q., Dong, J., Li, G., Niu, K., Pan, J., Xia, L., Wang, Y., & Jiang, S. (2025). Dietary Carnosic Acid Supplementation Improves the Growth Performance, the Antioxidant Status, and Diversity of Intestinal Microbiota in Broilers. Antioxidants, 14(8), 1026. https://doi.org/10.3390/antiox14081026





