Identification, Quantification, and Antioxidant Evaluation of Phenolic Compounds from Colored Opuntia ficus-indica (L.) Roots Using UHPLC-DAD-ESI-MS/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sampling of Roots
2.3. Solid–Liquid Extraction of Phenolics Compounds
2.4. Determination of Total Phenolic and Flavonoid Compounds
2.5. UHPLC-DAD-ESI/MSn Investigation
2.6. Antioxidant Activities
2.7. Relative Antioxidant Capacity Index (RACI)
2.8. Preparation of Extract-Enriched Soap Formulations
2.9. Color Analysis
2.10. Statistics
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Content
3.2. Identification of Phenolic Compounds
3.2.1. Organic Acids
3.2.2. Phenylpyruvic Acids
3.2.3. Glycosylated Flavonols
3.2.4. Flavanols and Flavones
3.2.5. Phenolic Acid Derivatives
3.2.6. Flavonoid Derivatives
3.2.7. Other Identified Compounds
3.3. Antioxidant Evaluation
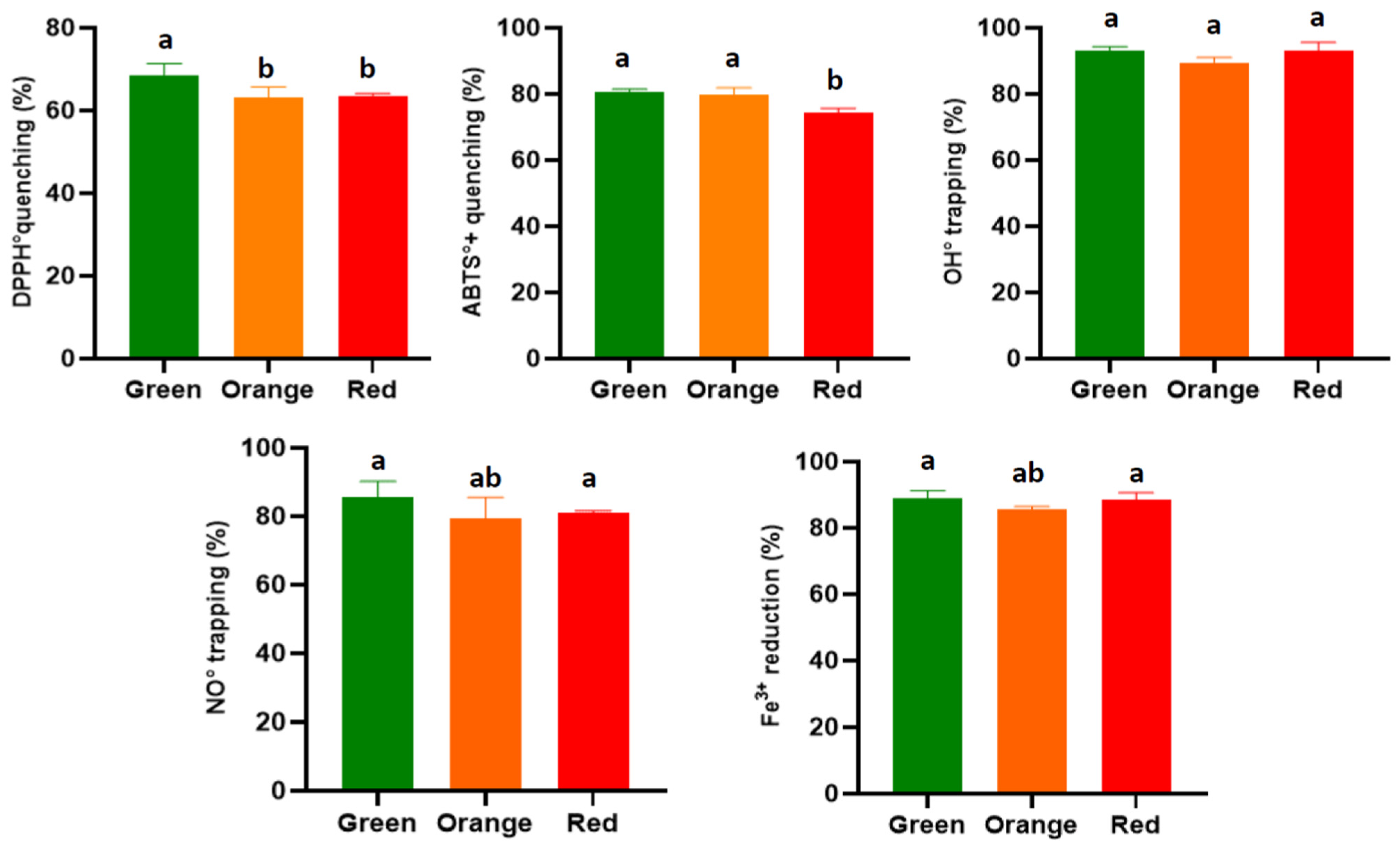
3.3.1. DPPH Radical
3.3.2. ABTS Radical
3.3.3. Hydroxyl Radical OH
3.3.4. Nitric Oxide
3.3.5. Reduction of Ferric Iron
3.4. Relative Antioxidant Capacity Index—RACI
3.5. Development of Extract-Enriched Soap Formulations
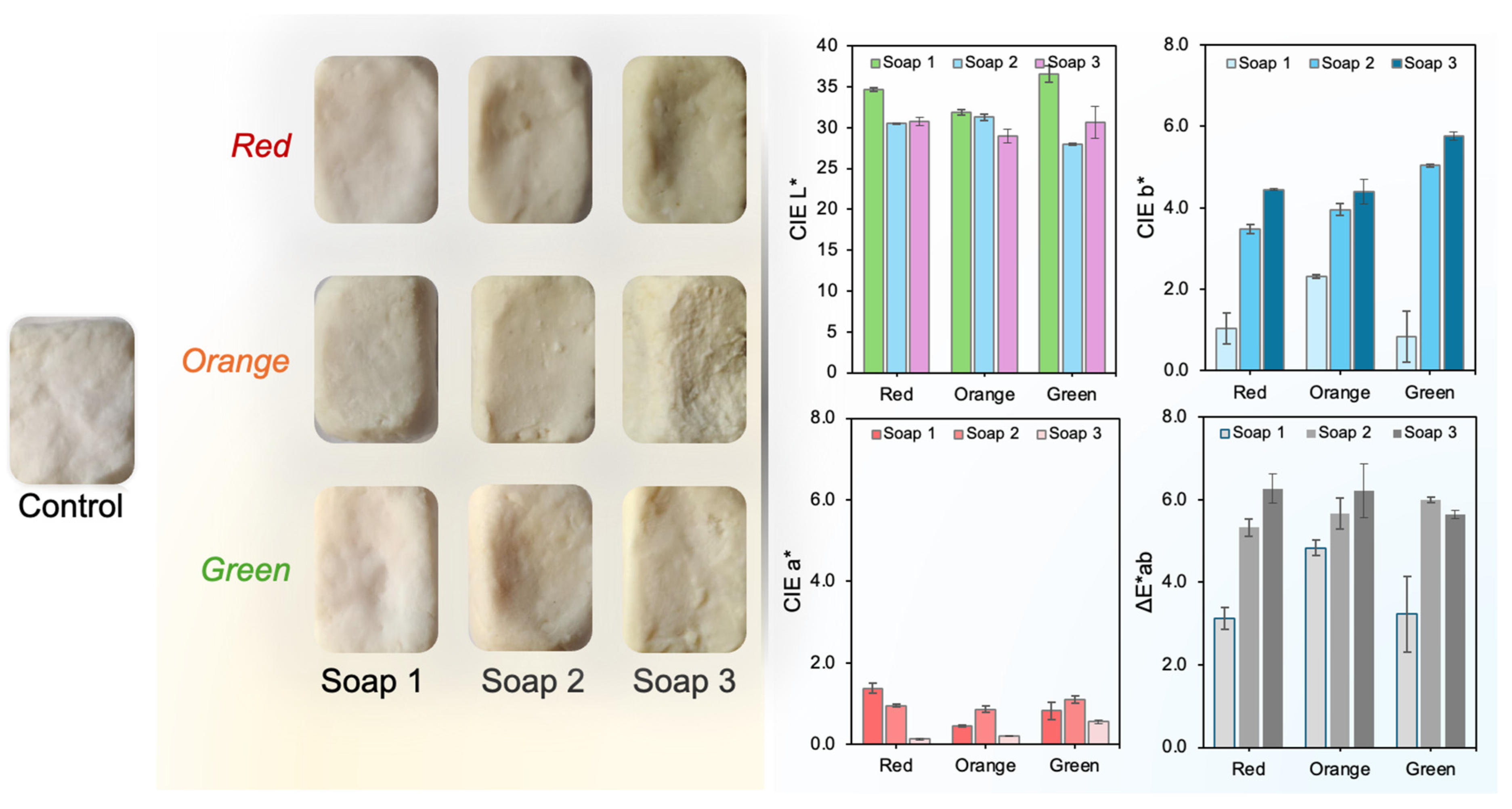
3.5.1. Colorimetric Evaluation (CIELab)
3.5.2. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; De Vries, H. Critical Success and Risk Factors for Circular Business Models Valorising Agricultural Waste and By-Products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food Waste Biomass: A Resource for High-Value Chemicals. Green Chem. 2013, 15, 307. [Google Scholar] [CrossRef]
- Coêlho, D.d.L.; Dubeux, J.C.B., Jr.; Santos, M.V.F.d.; Mello, A.C.L.d.; Cunha, M.V.d.; Santos, D.C.d.; Freitas, E.V.d.; Santos, E.R.d.S. Soil and Root System Attributes of Forage Cactus under Different Management Practices in the Brazilian Semiarid. Agronomy 2023, 13, 743. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaiedh, N.; Bouoni, Z.; Hfaiedh, M.; Sakly, M.; Zourgui, L.; Rhouma, K. Ben Antioxidant and Antiulcerogenic Activities of Opuntia ficus indica f. Inermis Root Extract in Rats. Phytomedicine 2010, 17, 1120–1126. [Google Scholar] [CrossRef]
- Benramdane, E.; Chougui, N.; Ramos, P.A.B.; Makhloufi, N.; Tamendjari, A.; Silvestre, A.J.D.; Santos, S.A.O. Lipophilic Compounds and Antibacterial Activity of Opuntia ficus-indica Root Extracts from Algeria. Int. J. Mol. Sci. 2022, 23, 11161. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of Phenolic Components in Polar Extracts of Eucalyptus Globulus Labill. Bark by High-Performance Liquid Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Moreirinha, C.; Silva, S.; Costa, E.M.; Veiga, M.; Coscueta, E.; Santos, S.A.O.; Almeida, A.; Pintado, M.M.; Freire, C.S.R.; et al. The Health-Promoting Potential of Salix Spp. Bark Polar Extracts: Key Insights on Phenolic Composition and in Vitro Bioactivity and Biocompatibility. Antioxidants 2019, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; Del Rio, D.; Hernández, F. Phytochemical Characterization of Different Prickly Pear (Opuntia ficus-indica (L.) Mill.) Cultivars and Botanical Parts: UHPLC-ESI-MSn Metabolomics Profiles and Their Chemometric Analysis. Food Res. Int. 2018, 108, 301–308. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical Properties and Storage Stability of Margarine Containing Opuntia ficus-indica Peel Extract as Antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds Characterization by LC-DAD- ESI/MSn and Bioactive Properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, L.; Wang, L.; Hu, Y.; Zhang, W.; Liu, R. Structural Characterization and Identification of Major Constituents in Jitai Tablets by High-Performance Liquid Chromatography/Diode-Array Detection Coupled with Electrospray Ionization Tandem Mass Spectrometry. Molecules 2012, 17, 10470–10493. [Google Scholar] [CrossRef]
- Zhong, L.; Wu, G.; Fang, Z.; Wahlqvist, M.L.; Hodgson, J.M.; Clarke, M.W.; Junaldi, E.; Johnson, S.K. Characterization of Polyphenols in Australian Sweet Lupin (Lupinus angustifolius) Seed Coat by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2019, 116, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, B.; Shi, Y.; Du, X.; Fan, M.; Sun, Z.; Cui, X.; Huang, C. Bioactivity-Guided Fractionation and Analysis of Compounds with Anti-Influenza Virus Activity from Gardenia jasminoides Ellis. Arch. Pharm. Res. 2012, 35, 9–17. [Google Scholar] [CrossRef]
- Faustino, M.V.A.F.; Faustino, M.V.A.F.; Silva, H.; Cunha, Â.; Silva, A.M.S.; Pinto, D.C.G.A. Puccinellia maritima, Spartina Maritime, and Spartina Patens Halophytic Grasses: Characterization of Polyphenolic and Chlorophyll Profiles and Evaluation of Their Biological Activities. Molecules 2019, 24, 3796. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Illiano, A.; Carpentieri, A.; Spinelli, M.; Melchiorre, C.; Fontanarosa, C.; Serio, M.d.; Amoresano, A. Quantification of Polyphenols and Metals in Chinese Tea Infusions by Mass Spectrometry. Foods 2020, 9, 835. [Google Scholar] [CrossRef]
- Mascherpa, D.; Carazzone, C.; Marrubini, G.; Gazzani, G.; Papetti, A. Identification of Phenolic Constituents in Cichorium endivia Var. Crispum and Var. Latifolium Salads by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ioniziation Tandem Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 12142–12150. [Google Scholar] [CrossRef]
- Maldini, M.; Montoro, P.; Hamed, A.I.; Mahalel, U.A.; Oleszek, W.; Stochmal, A.; Piacente, S. Strong Antioxidant Phenolics from Acacia nilotica: Profiling by ESI-MS and Qualitative–Quantitative Determination by LC–ESI-MS. J. Pharm. Biomed. Anal. 2011, 56, 228–239. [Google Scholar] [CrossRef]
- Mämmelä, P. Phenolics in Selected European Hardwood Species by Liquid Chromatography-Electrospray Ionisation Mass Spectrometry. Analyst 2001, 126, 1535–1538. [Google Scholar] [CrossRef]
- Ling, Y.; Chen, M.; Wang, K.; Sun, Z.; Li, Z.; Wu, B.; Huang, C. Systematic Screening and Characterization of the Major Bioactive Components of Poria Cocos and Their Metabolites in Rats by LC-ESI-MSn. Biomed. Chromatogr. 2012, 26, 1109–1117. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef]
- Marks, S.C.; Mullen, W.; Crozier, A. Flavonoid and Chlorogenic Acid Profiles of English Cider Apples. J. Sci. Food Agric. 2007, 87, 719–728. [Google Scholar] [CrossRef]
- Rodríguez-Medina, I.C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Use of High-Performance Liquid Chromatography with Diode Array Detection Coupled to Electrospray-Qq-Time-of-Flight Mass Spectrometry for the Direct Characterization of the Phenolic Fraction in Organic Commercial Juices. J. Chromatogr. A 2009, 1216, 4736–4744. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, R.C.F.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis “Icterina” and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ye, M.; Xu, M.; Sun, J.; Wang, B.; Guo, D. Characterization of Flavonoids in the Traditional Chinese Herbal Medicine-Huangqin by Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2007, 848, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic Compounds in Cistus incanus Herbal Infusions—Antioxidant Capacity and Thermal Stability during the Brewing Process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and Characterization of Phenolic Compounds in Hydromethanolic Extracts of Sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A Nutraceutical Extract from Inula viscosa Leaves: UHPLC-HR-MS/MS Based Polyphenol Profile, and Antioxidant and Cytotoxic Activities. J. Food Drug Anal. 2019, 27, 692–702. [Google Scholar] [CrossRef]
- Moqbel, H.; El Hawary, S.S.E.D.; Sokkar, N.M.; El-Naggar, E.M.B.; El Boghdady, N.; El Halawany, A.M. HPLC-ESI-MS/MS Characterization of Phenolics in Prunus amygdalus, Cultivar “Umm Alfahm” and Its Antioxidant and Hepatoprotective Activity. J. Food Meas. Charact. 2018, 12, 808–819. [Google Scholar] [CrossRef]
- Rini Vijayan, K.P.; Raghu, A.V. Tentative Characterization of Phenolic Compounds in Three Species of the Genus Embelia by Liquid Chromatography Coupled with Mass Spectrometry Analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Touati, R.; Santos, S.A.O.; Rocha, S.M.; Belhamel, K.; Silvestre, A.J.D. Phenolic Composition and Biological Prospecting of Grains and Stems of Retama sphaerocarpa. Ind. Crops Prod. 2017, 95, 244–255. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Kanwal, N.; Thadhani, V.M.; Choudhary, M.I. Rapid Identification of Lichen Compounds Based on the Structure–Fragmentation Relationship Using ESI-MS/MS Analysis. Anal. Methods 2015, 7, 6066–6076. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Felício, L.; Jerónimo, E.; Silvestre, A.J.D.; Neto, C.P.; Duarte, M. Valorization of Olive Mill Residues: Antioxidant and Breast Cancer Antiproliferative Activities of Hydroxytyrosol-Rich Extracts Derived from Olive Oil by-Products. Ind. Crops Prod. 2013, 46, 359–368. [Google Scholar] [CrossRef]
- Cano, A.; Alcaraz, O.; Acosta, M.; Arnao, M.B. On-Line Antioxidant Activity Determination: Comparison of Hydrophilic and Lipophilic Antioxidant Activity Using the ABTS•+ Assay. Redox Report 2002, 7, 103–109. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef]
- Kumaran, A.; Joel Karunakaran, R. In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Senevirathne, M.; Kim, S.-H.; Siriwardhana, N.; Ha, J.-H.; Lee, K.-W.; Jeon, Y.-J. Antioxidant Potential of Ecklonia Cavaon Reactive Oxygen Species Scavenging, Metal Chelating, Reducing Power and Lipid Peroxidation Inhibition. Food Sci. Technol. Int. 2006, 12, 27–38. [Google Scholar] [CrossRef]
- Sun, T.; Tanumihardjo, S.A. An Integrated Approach to Evaluate Food Antioxidant Capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef] [PubMed]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic Compound Profile and Biological Activities of Southern African Opuntia ficus-indica Fruit Pulp and Peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant Properties and Chemical Characterization of Spanish Opuntia ficus-indica Mill. Cladodes and Fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef]
- Gallegos-Infante, J.A.; Rocha-Guzman, N.E.; González-Laredo, R.F.; Reynoso-Camacho, R.; Medina-Torres, L.; Cervantes-Cardozo, V. Effect of Air Flow Rate on the Polyphenols Content and Antioxidant Capacity of Convective Dried Cactus Pear Cladodes (Opuntia ficus indica). Int. J. Food Sci. Nutr. 2009, 60, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, M.S.; Badr, S.E.A.; Elsaid, A.S. Nutritive Value and Chemical Composition of Prickly Pear Seeds (Opuntia ficus indica L.) Growing in Egypt. Int. J. Agric. Policy Res. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Ben Saad, A.; Dalel, B.; Rjeibi, I.; Smida, A.; Ncib, S.; Zouari, N.; Zourgui, L. Phytochemical, Antioxidant and Protective Effect of Cactus Cladodes Extract against Lithium-Induced Liver Injury in Rats. Pharm. Biol. 2017, 55, 516–525. [Google Scholar] [CrossRef]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.-W.; De León-Rodríguez, A.; Fomsgaard, I.S.; Barba de la Rosa, A.P. Proximate Composition, Phenolic Acids, and Flavonoids Characterization of Commercial and Wild Nopal (Opuntia Spp.). J. Food Compos. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and Antibiofilm Activity against Staphylococcus Aureus of Opuntia ficus-indica (L.) Mill. Cladode Polyphenolic Extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and Quantification of Individual Betalain and Phenolic Compounds in Mexican and Spanish Prickly Pear (Opuntia ficus-indica L. Mill) Tissues: A Comparative Study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Ammar, I.; Ben Salem, M.; Harrabi, B.; Mzid, M.; Bardaa, S.; Sahnoun, Z.; Attia, H.; Ennouri, M. Anti-Inflammatory Activity and Phenolic Composition of Prickly Pear (Opuntia ficus-indica) Flowers. Ind. Crops Prod. 2018, 112, 313–319. [Google Scholar] [CrossRef]
- Boutakiout, A.; Elothmani, D.; Hanine, H.; Mahrouz, M.; Le Meurlay, D.; Hmid, I.; Ennahli, S. Effects of Different Harvesting Seasons on Antioxidant Activity and Phenolic Content of Prickly Pear Cladode Juice. J. Saudi Soc. Agric. Sci. 2018, 17, 471–480. [Google Scholar] [CrossRef]
- Figueroa-Pérez, M.G.; Pérez-Ramírez, I.F.; Paredes-López, O.; Mondragón-Jacobo, C.; Reynoso-Camacho, R. Phytochemical Composition and in Vitro Analysis of Nopal (O. ficus-indica) Cladodes at Different Stages of Maturity. Int. J. Food Prop. 2018, 21, 1728–1742. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Simões, M.A.M.; Silva, A.M.S. Genista tridentata L.: A Rich Source of Flavonoids with Anti-Inflammatory Activity. Medicines 2020, 7, 31. [Google Scholar] [CrossRef]
- Simões, M.A.M.; Pinto, D.C.G.A.; Neves, B.M.R.; Silva, A.M.S. Flavonoid Profile of the Genista tridentata L., a Species Used Traditionally to Treat Inflammatory Processes. Molecules 2020, 25, 812. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Extractable and Macromolecular Antioxidants of Opuntia ficus-indica Cladodes: Phytochemical Profiling, Antioxidant and Antibacterial Activities. S. Afr. J. Bot. 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Sobeh, M.; Rezq, S.; Sabry, O.M.; Abdelfattah, M.A.O.; El Raey, M.A.; El-Kashak, W.A.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. Albizia Anthelmintica: HPLC-MS/MS Profiling and in Vivo Anti-Inflammatory, Pain Killing and Antipyretic Activities of Its Leaf Extract. Biomed. Pharmacother. 2019, 115, 108882. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Fernández, X.; Vega-Ahuatzin, A.; Ochoa-Velasco, C.E.; Cid-Pérez, S.; Hernández-Carranza, P.; Ávila-Sosa, R. Physical and Antioxidant Characterization of Edible Films Added with Red Prickly Pear (Opuntia ficus-indica L.) Cv. San Martín Peel and/or Its Aqueous Extracts. Food Bioproc. Tech. 2018, 11, 368–379. [Google Scholar] [CrossRef]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants—Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Jana, B.; Senapati, S.; Ghosh, D.; Bose, D.; Chattopadhyay, N. Spectroscopic Exploration of Mode of Binding of CtDNA with 3-Hydroxyflavone: A Contrast to the Mode of Binding with Flavonoids Having Additional Hydroxyl Groups. J. Phys. Chem. B 2012, 116, 639–645. [Google Scholar] [CrossRef]
- Avila-Nava, A.; Calderón-Oliver, M.; Medina-Campos, O.N.; Zou, T.; Gu, L.; Torres, N.; Tovar, A.R.; Pedraza-Chaverri, J. Extract of Cactus (Opuntia ficus indica) Cladodes Scavenges Reactive Oxygen Species in Vitro and Enhances Plasma Antioxidant Capacity in Humans. J. Funct. Foods 2014, 10, 13–24. [Google Scholar] [CrossRef]
- Jeon, Y.E.; Yin, X.F.; Choi, D.B.; Lim, S.S.; Kang, I.J.; Shim, J.H. Inhibitory Activity of Aromadendrin from Prickly Pear (Opuntia ficus-indica) Root on Aldose Reductase and the Formation of Advanced Glycation End Products. Food Sci. Biotechnol. 2011, 20, 1283–1288. [Google Scholar] [CrossRef]
- Nabil, B.; Ouaabou, R.; Ouhammou, M.; Saadouni, L.; Mahrouz, M. Impact of Particle Size on Functional, Physicochemical Properties and Antioxidant Activity of Cladode Powder (Opuntia ficus-indica). J. Food Sci. Technol. 2020, 57, 943–954. [Google Scholar] [CrossRef]
- Jorge, A.J.; De La Garza, T.H.; Alejandro, Z.C.; Ruth, B.C.; Noé, A.C. The Optimization of Phenolic Compounds Extraction from Cactus Pear (Opuntia ficus-indica) Skin in a Reflux System Using Response Surface Methodology. Asian Pac. J. Trop. Biomed. 2013, 3, 436–442. [Google Scholar] [CrossRef]
- Petruk, G.; Di Lorenzo, F.; Imbimbo, P.; Silipo, A.; Bonina, A.; Rizza, L.; Piccoli, R.; Monti, D.M.; Lanzetta, R. Protective Effect of Opuntia ficus-indica L. Cladodes against UVA-Induced Oxidative Stress in Normal Human Keratinocytes. Bioorg Med. Chem. Lett. 2017, 27, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Kim, H.-R.; Kim, J.; Jang, Y.-S. Antioxidant Property of an Ethanol Extract of the Stem of Opuntia ficus-indica Var. Saboten. J. Agric. Food Chem. 2002, 50, 6490–6496. [Google Scholar] [CrossRef]
- Hfaiedh, M.; Brahmi, D.; Zourgui, M.N.; Zourgui, L. Phytochemical Analysis and Nephroprotective Effect of Cactus (Opuntia ficus-indica) Cladodes on Sodium Dichromate-Induced Kidney Injury in Rats. Appl. Physiol. Nutr. Metab. 2019, 44, 239–247. [Google Scholar] [CrossRef]
- Chaalal, M.; Louaileche, H.; Touati, N.; Bachir Bey, M. Phytochemicals, in Vitro Antioxidant Capacity and Antiradical Potential of Whole and Ground Seeds of Three Prickly Pear Varieties: A Comparative Study. Ind. Crops Prod. 2013, 49, 386–391. [Google Scholar] [CrossRef]
- Izuegbuna, O.; Otunola, G.; Bradley, G. Chemical Composition, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Opuntia stricta Cladodes. PLoS One 2019, 14, e0209682. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; López-Pacheco, F.; Alvarez, M.M.; Serna-Saldívar, S.O. In Vivo Anti-Inflammatory Effects of Isorhamnetin Glycosides Isolated from Opuntia ficus-indica (L.) Mill Cladodes. Ind. Crops Prod. 2015, 76, 803–808. [Google Scholar] [CrossRef]
- Panico, A.M.; Cardile, V.; Garufi, F.; Puglia, C.; Bonina, F.; Ronsisvalle, S. Effect of Hyaluronic Acid and Polysaccharides from Opuntia ficus indica (L.) Cladodes on the Metabolism of Human Chondrocyte Cultures. J. Ethnopharmacol. 2007, 111, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bakari, S.; Daoud, A.; Felhi, S.; Smaoui, S.; Gharsallah, N.; Kadri, A. Proximate Analysis, Mineral Composition, Phytochemical Contents, Antioxidant and Antimicrobial Activities and GC-MS Investigation of Various Solvent Extracts of Cactus Cladode. Food Sci. Technol. 2017, 37, 286–293. [Google Scholar] [CrossRef]
- Mohan, S.; Gupta, D. Phytochemical Analysis and Differential in Vitro Cytotoxicity Assessment of Root Extracts of Inula Racemosa. Biomed. Pharmacother. 2017, 89, 781–795. [Google Scholar] [CrossRef]
- Paputungan, F.; Momuat, L.I.; Suryanto, E. Quality and Antioxidant Activity of Scrub Bath Soap with Addition of Eucheuma Spinosum Algae Powder. J. Ilm. Sains 2023, 23, 55–64. [Google Scholar] [CrossRef]
- Okoh, S.O.; Okoh, O.O.; Okoh, A.I. Inhibitory Effects of Azadirachta Indica Secondary Metabolites Formulated Cosmetics on Some Infectious Pathogens and Oxidative Stress Radicals. BMC Complement. Altern. Med. 2019, 19, 123. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Kumbhakar, M.; Singh, P.K.; Satpati, A.K.; Nath, S.; Pal, H. Ultrafast Electron Transfer Dynamics in Micellar Media Using Surfactant as the Intrinsic Electron Acceptor. J. Phys. Chem. B 2010, 114, 10057–10065. [Google Scholar] [CrossRef]
- Ioannou, I.; Kriznik, A.; Chekir, L.; Ghoul, M. Effect of the Processing Temperature on the Degradation of Food Flavonoids: Kinetic and Calorimetric Studies on Model Solutions. J. Food Eng. Technol. 2019, 8, 91–102. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The Role of Antioxidants in Photoprotection: A Critical Review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Dermal Toxicity and Environmental Contamination: Electron Transfer, Reactive Oxygen Species, Oxidative Stress, Cell Signaling, and Protection by Antioxidants. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2010; pp. 119–138. [Google Scholar]




| Varieties | Total Phenolics | Flavonoids | ||
|---|---|---|---|---|
| mg/g Extract | mg/100 g pr | mg/g Extract | mg/100 g pr | |
| Green | 147.82 ± 10.33 a | 2920.19 ± 42.21 a | 125.65 ± 6.33 a | 2483.17 ± 25.75 b |
| Orange | 100.83 ± 6.11 c | 1392.63 ± 57.95 b | 82.33 ± 7.18 c | 1141.96 ± 33.3 c |
| Red | 120.29 ± 3.51 b | 2944.56 ± 6.31 a | 105.60 ± 3.88 b | 2591.18 ± 5.29 a |
| Peak | RT | Tentative Identification | Green Variety | Orange Variety | Red Variety | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | [M-H] | MS/MS | Peak% | Conc. µg/mg | Peak% | Conc. µg/mg | Peak% | Conc. µg/mg | |||
| 1 | 0.83 | L-malic acid | 133 | 115, 87 | 3.124 | 4.62 a | 2.726 | 2.75 c | 4.062 | 4.89 a | [13] |
| 2 | 1.01 | Citric acid | 191 | 111, 173 | 3.009 | 4.45 a | 2.772 | 2.80 c | 4.123 | 4.96 a | [13] |
| 3 | 3.23 | Piscidic acid | 255 | 165,193, 179, 149 | 1.240 | 1.83 a | 0.943 | 0.95 c | 1.586 | 1.91 a | [14] |
| 4 | 8.41 | Eucomic acid | 239 | 179, 149 | 1.595 | 2.36 a | 1.699 | 1.71 c | 1.647 | 1.98 b | [14] |
| 5 | 10.15 | Myricetin-C-hexoside | 479 | 359 | 0.770 | 1.09 c | ND | ND | 2.148 | 2.57 a | [15] |
| 6 | 11.11 | Carthamidin-5-O-glucosyle-rutinoside | 757 | 595 | 1.504 | 2.22 a | 1.666 | 1.68 c | 1.664 | 2.00 b | [16] |
| 7 | 11.30 | Aromadendrin-6-C-glucopyranosyl-7-O- apiofuranosyl-2-O-glucopyranoside | 743 | 623, 341 | 1.141 | 1.60 b | 0.857 | 0.86 c | 1.363 | 1.64 a | [17] |
| 8 | 11.71 | 6″-O-sinapoyl gardoside | 535 | 329, 205, 367 | 1.202 | 1.78 a | ND | ND | 1.185 | 1.43 c | [18] |
| 9 | 13.93 | Sinapoyl spinacetin | 569 | 551, 345 | 0.989 | 1.46 b | 0.845 | 0.85 c | 1.615 | 1.94 a | [19] |
| 10 | 14.23 | Ferulic acid guaiacylglyceryl derivative | 613 | 569, 417, 193 | 1.245 | 1.84 a | 0.894 | 0.90 c | 1.128 | 1.36 b | [19] |
| 11 | 14.93 | Epicatechin-3-O-gallate | 441 | 289 | 1.208 | 1.79 a | 0.773 | 0.78 c | 1.374 | 1.65 b | [20] |
| 12 | 15.93 | Kaempferol-O-diglucoside | 611 | 449, 287 | 1.197 | 1.77 a | 1.114 | 1.12 c | 1.499 | 1.80 a | [21] |
| 13 | 16.33 | 6-Hydroxykaempferol triglucoside/galactoside | 787 | 625, 301, 463 | 1.435 | 2.12 a | 0.943 | 0.95 c | 1.403 | 1.69 b | [16] |
| 14 | 16.60 | Tetragalloyle-glucose | 787 | 635, 743, 495, 331 | 1.455 | 2.15 a | 0.791 | 0.80 c | 1.411 | 1.70 b | [22,23] |
| 15 | 17.65 | Poricoic acid A | 497 | 423, 453, 479 | 1.065 | 1.57 b | 1.114 | 1.12 c | 1.499 | 1.80 a | [24] |
| 16 | 18.93 | Kaempferol-3-O-hexose-deoxyhexose | 593 | 284, 285 | 0.863 | 1.28 a | 0.905 | 0.91 c | 1.139 | 1.37 a | [25] |
| 17 | 20.32 | Phloretin-2′-xyloside | 567 | 273 | 0.863 | 1.28 b | 0.982 | 0.99 c | 1.493 | 1.80 a | [26,27] |
| 18 | 21.89 | Yunnaneic acid | 597 | 579, 312, 355, 295 | 1.133 | 1.68 b | 1.183 | 1.19 c | 1.517 | 1.83 a | [28] |
| 19 | 22.08 | Procyanidin B1 | 577 | 559, 425, 289 | 1.315 | 1.94 b | 1.253 | 1.26 c | 1.666 | 2.00 a | [29,30] |
| 20 | 24.43 | Apigenin-6-C-glucoside (Isovitexine) | 431 | 413, 341, 311, 269 | 3.086 | 4.56 a | 2.048 | 2.06 c | 3.714 | 4.47 a | [31] |
| 21 | 28.02 | Quercetin-3-O-(6″-acétyle) hexoside | 505 | 463, 301 | 1.178 | 1.74 a | 1.145 | 1.15 c | 1.349 | 1.62 b | [32,33] |
| 22 | 30.97 | Tricin | 329 | 229, 211, 183 | 1.313 | 1.94 a | 1.055 | 1.06 c | 1.673 | 2.01 a | [34] |
| 23 | 31.08 | Isovitexin-3-hydroxy-3- methylglutaroyl | 575 | 507, 431, 513, 473 | 1.164 | 1.72 c | 1.926 | 1.94 b | 1.788 | 2.15 a | [35] |
| 24 | 31.30 | Trihydroxy-octadecadienoic acid | 327 | 309, 291, 239, 195 | 1.335 | 1.97 a | 1.709 | 1.72 c | 1.599 | 1.92 b | [31] |
| 25 | 31.70 | Placodiolic acid | 375 | 347 | 1.296 | 1.92 b | 1.957 | 1.97 a | 1.081 | 1.30 c | [36] |
| 26 | 32.65 | Guaiacyl-(t8-O-4)-guaiacyl-hexoside | 537 | 375, 327, 179, 165 | ND | ND | 1.272 | 1.28 a | ND | ND | [13] |
| Compounds | DPPH• | ABTS•+ | •OH | NO• | RP Fe3+ |
|---|---|---|---|---|---|
| Green | 695.70 ± 59.30 a | 29.38 ± 1.21 a | 125.33 ± 2.66 a | 123.82 ± 4.66 a | 63.77 ± 2.33 a |
| Orange | 830.37 ± 72.33 b | 31.01 ± 1.13 b | 159.22 ± 1.95 b | 189.30 ± 3.33 b | 67.99 ± 1.77 a |
| Red | 852.29 ± 28.23 b | 35.12 ± 0.95 c | 163.45 ± 5.55 b | 182.99 ± 4.56 b | 66.87 ± 5.23 a |
| Trolox | - | 6.70 ± 0.25 | - | - | - |
| Ascorbate | 5.87 ± 0.26 | - | 35.67 ± 0.33 | 32.34 ± 0.66 | 9.55 ± 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benramdane, E.; Mustafa, A.; Chougui, N.; Makhloufi, N.; Tamendjari, A.; Mussagy, C.U. Identification, Quantification, and Antioxidant Evaluation of Phenolic Compounds from Colored Opuntia ficus-indica (L.) Roots Using UHPLC-DAD-ESI-MS/MS. Antioxidants 2025, 14, 1023. https://doi.org/10.3390/antiox14081023
Benramdane E, Mustafa A, Chougui N, Makhloufi N, Tamendjari A, Mussagy CU. Identification, Quantification, and Antioxidant Evaluation of Phenolic Compounds from Colored Opuntia ficus-indica (L.) Roots Using UHPLC-DAD-ESI-MS/MS. Antioxidants. 2025; 14(8):1023. https://doi.org/10.3390/antiox14081023
Chicago/Turabian StyleBenramdane, Elias, Ahmad Mustafa, Nadia Chougui, Nawal Makhloufi, Abderezak Tamendjari, and Cassamo U. Mussagy. 2025. "Identification, Quantification, and Antioxidant Evaluation of Phenolic Compounds from Colored Opuntia ficus-indica (L.) Roots Using UHPLC-DAD-ESI-MS/MS" Antioxidants 14, no. 8: 1023. https://doi.org/10.3390/antiox14081023
APA StyleBenramdane, E., Mustafa, A., Chougui, N., Makhloufi, N., Tamendjari, A., & Mussagy, C. U. (2025). Identification, Quantification, and Antioxidant Evaluation of Phenolic Compounds from Colored Opuntia ficus-indica (L.) Roots Using UHPLC-DAD-ESI-MS/MS. Antioxidants, 14(8), 1023. https://doi.org/10.3390/antiox14081023










