Anti-Toxoplasma and Antioxidant Activity of a Terpene and Methyl-Ester-Rich Subfraction from Pleopeltis crassinervata
Abstract
1. Introduction
2. Materials and Methods
2.1. Natural Product Obtention
2.2. Animals
2.3. Parasites
2.4. Cell Culture
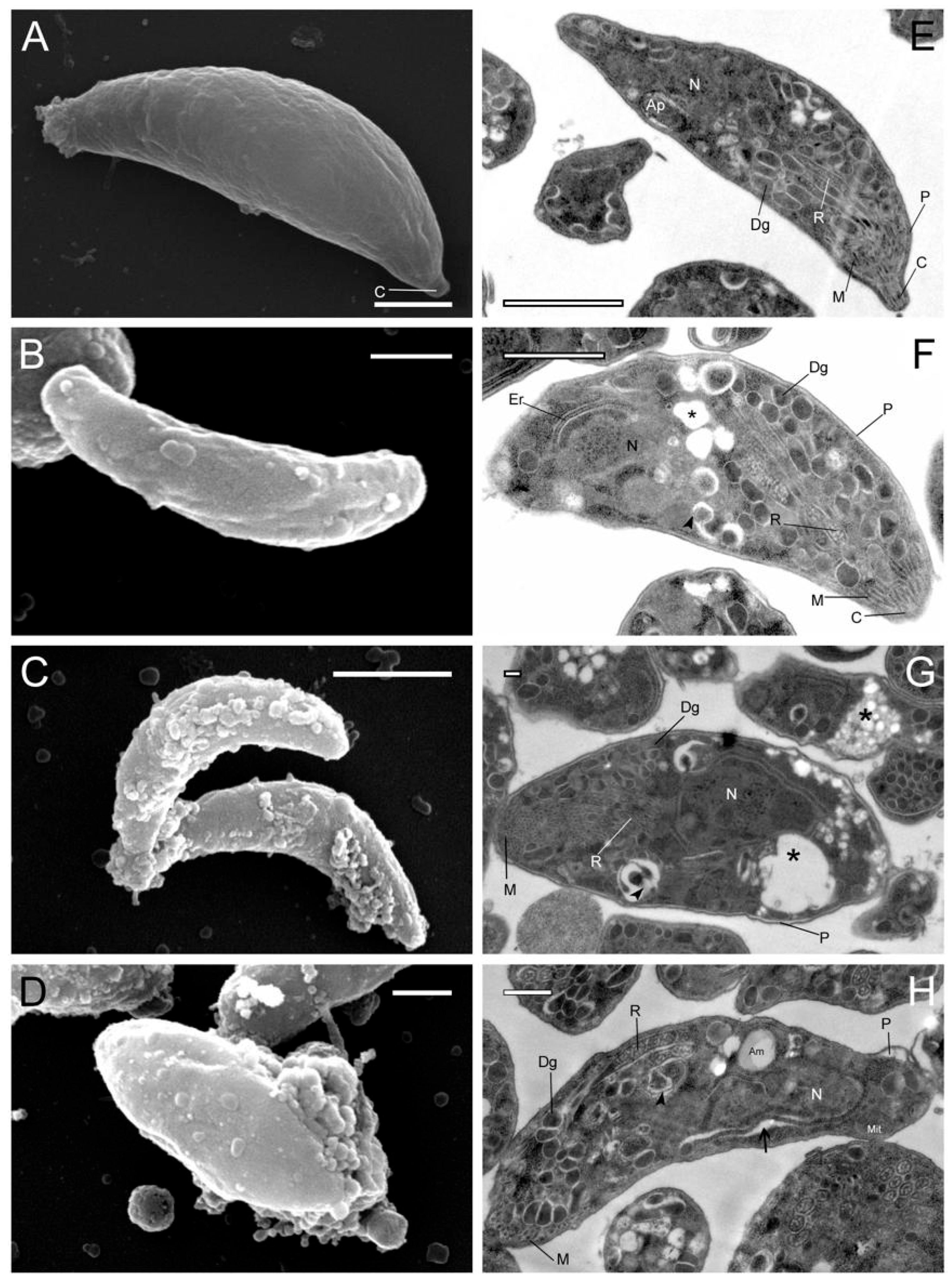
2.5. Electron Microscopy
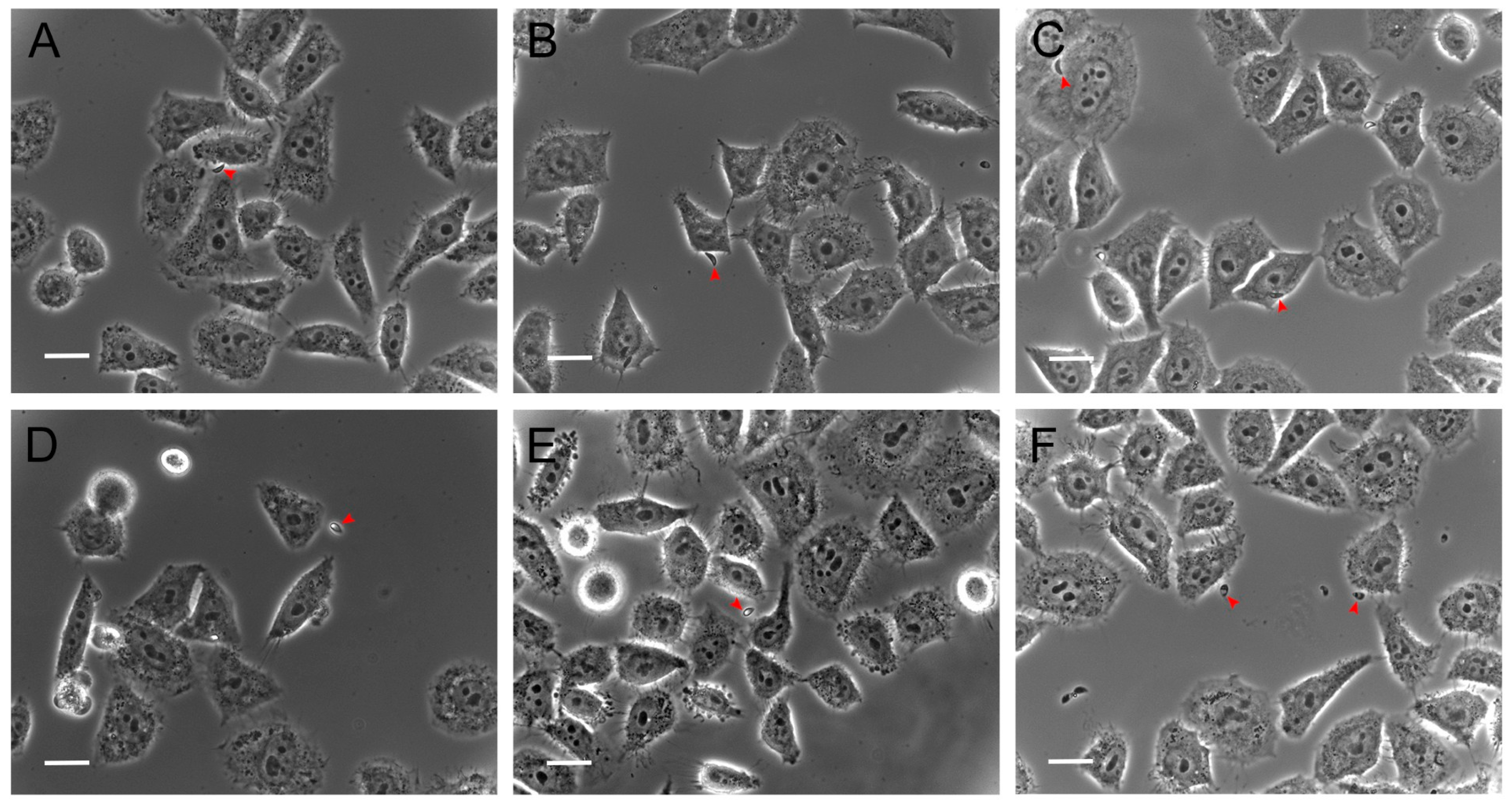
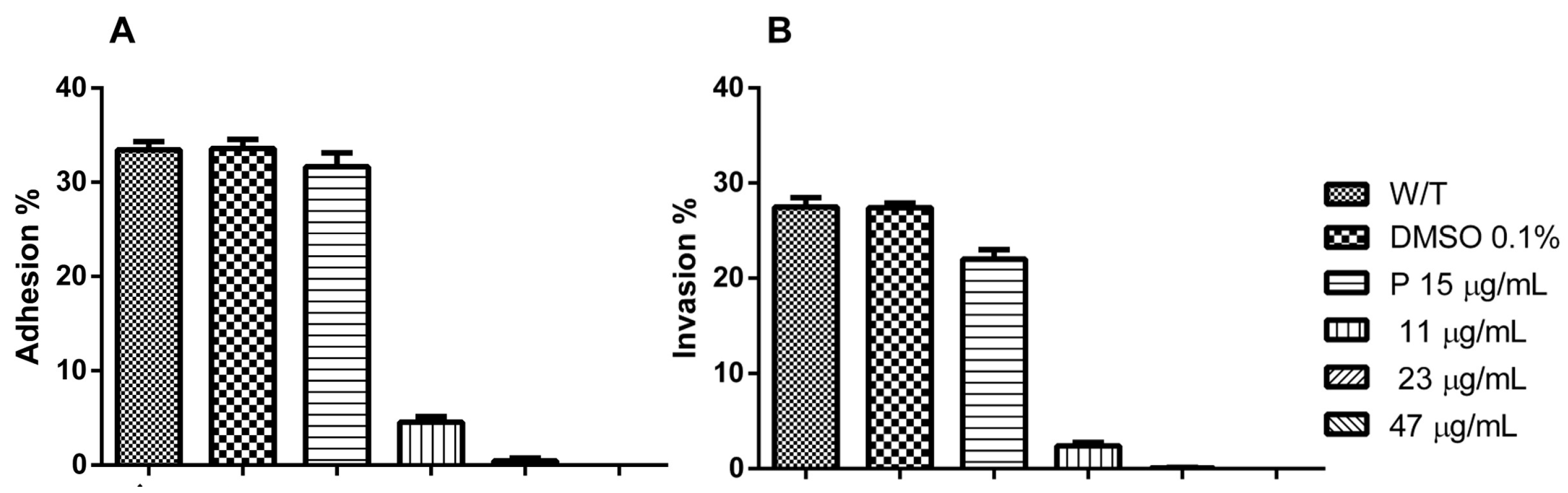
2.6. Hsf1 Effect on Tachyzoite Adhesion
2.7. Hsf1 Effect on Tachyzoite Invasion
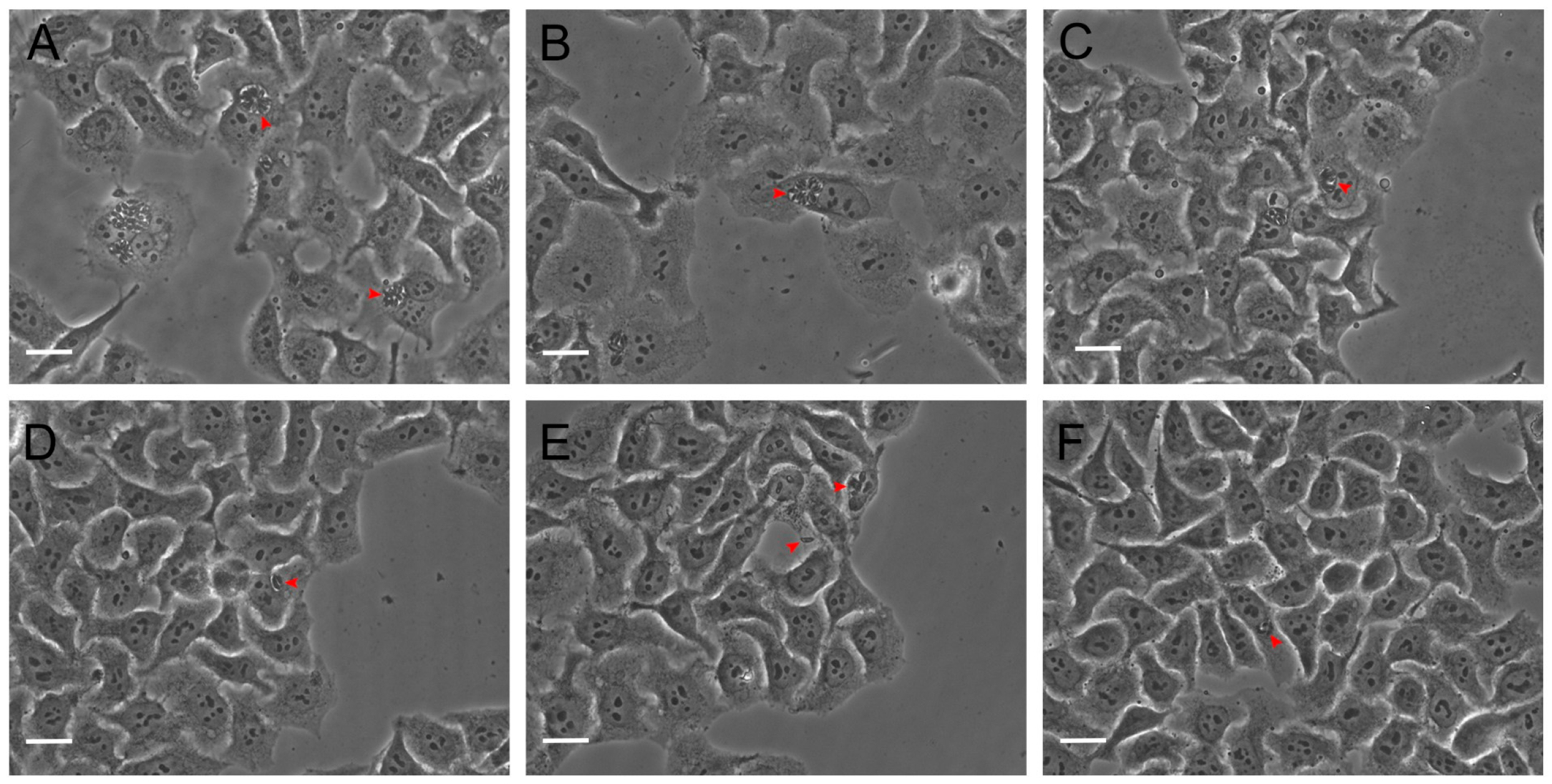
2.8. Hsf1 Effect on Tachyzoite Proliferation
2.9. Phytol and Hexadecanoic Acid Methyl Ester Effect on Tachyzoite Viability
2.10. Hsf1, Phytol, and Hexadecanoic Acid Methyl Ester Antioxidant Capacity
3. Results
3.1. Effect of Hsf1 on the Ultrastructure of T. gondii
3.2. Hsf1 Significantly Alters the Adhesion and Invasion of Infected Cell Cultures
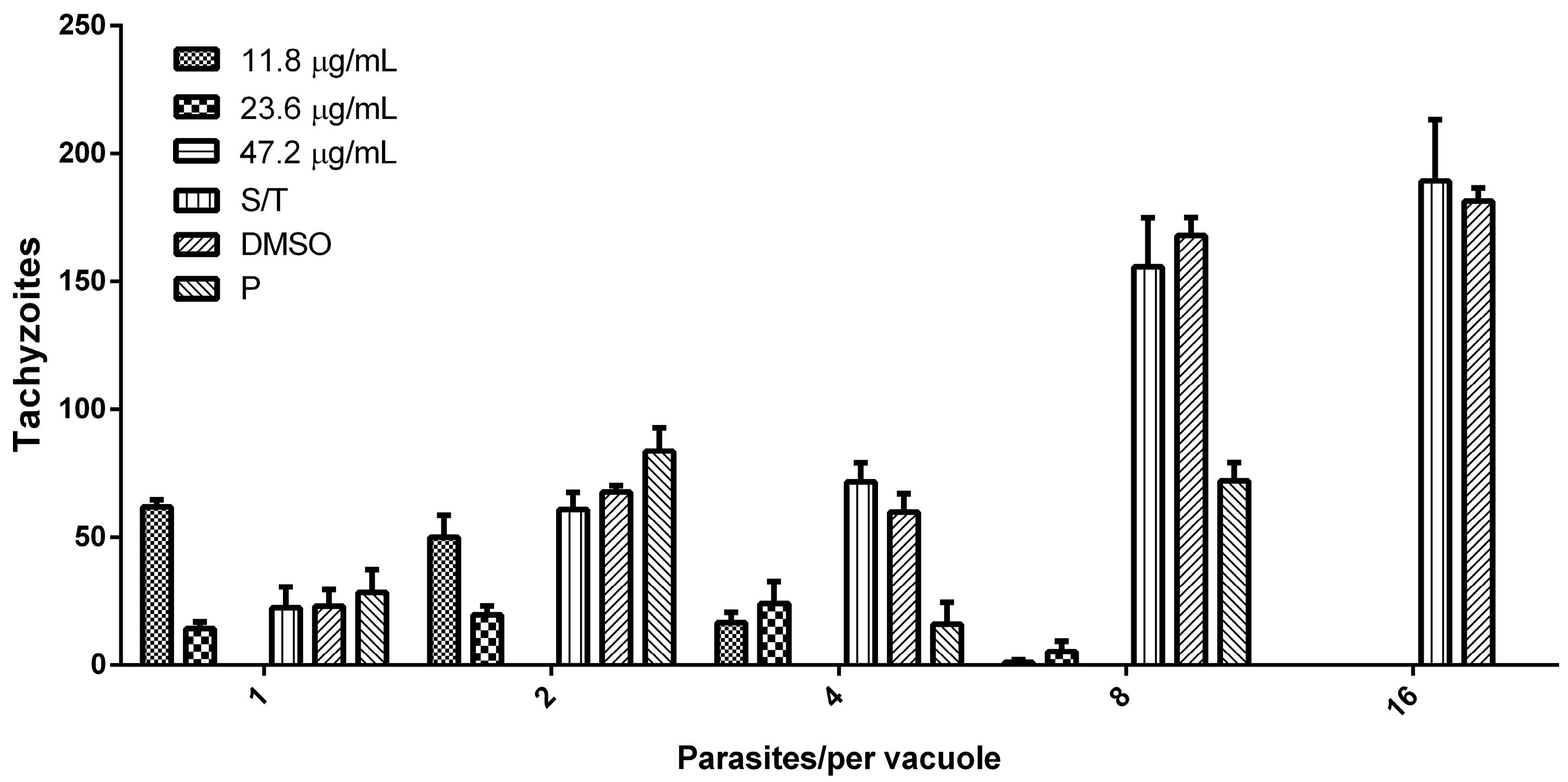
3.3. Hsf1 Affects the Proliferation Rate of Tachyzoites
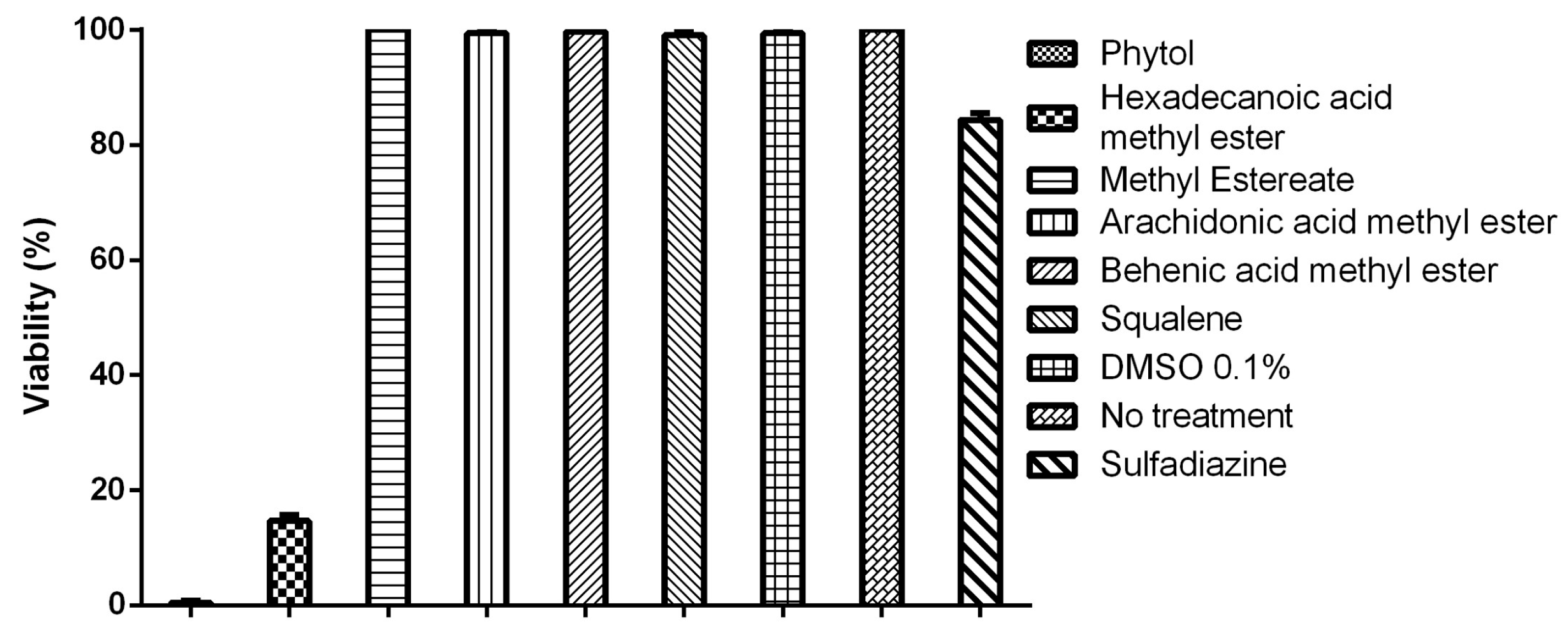
3.4. Tachyzoite Viability Is Affected by Terpenes and Fatty Acids
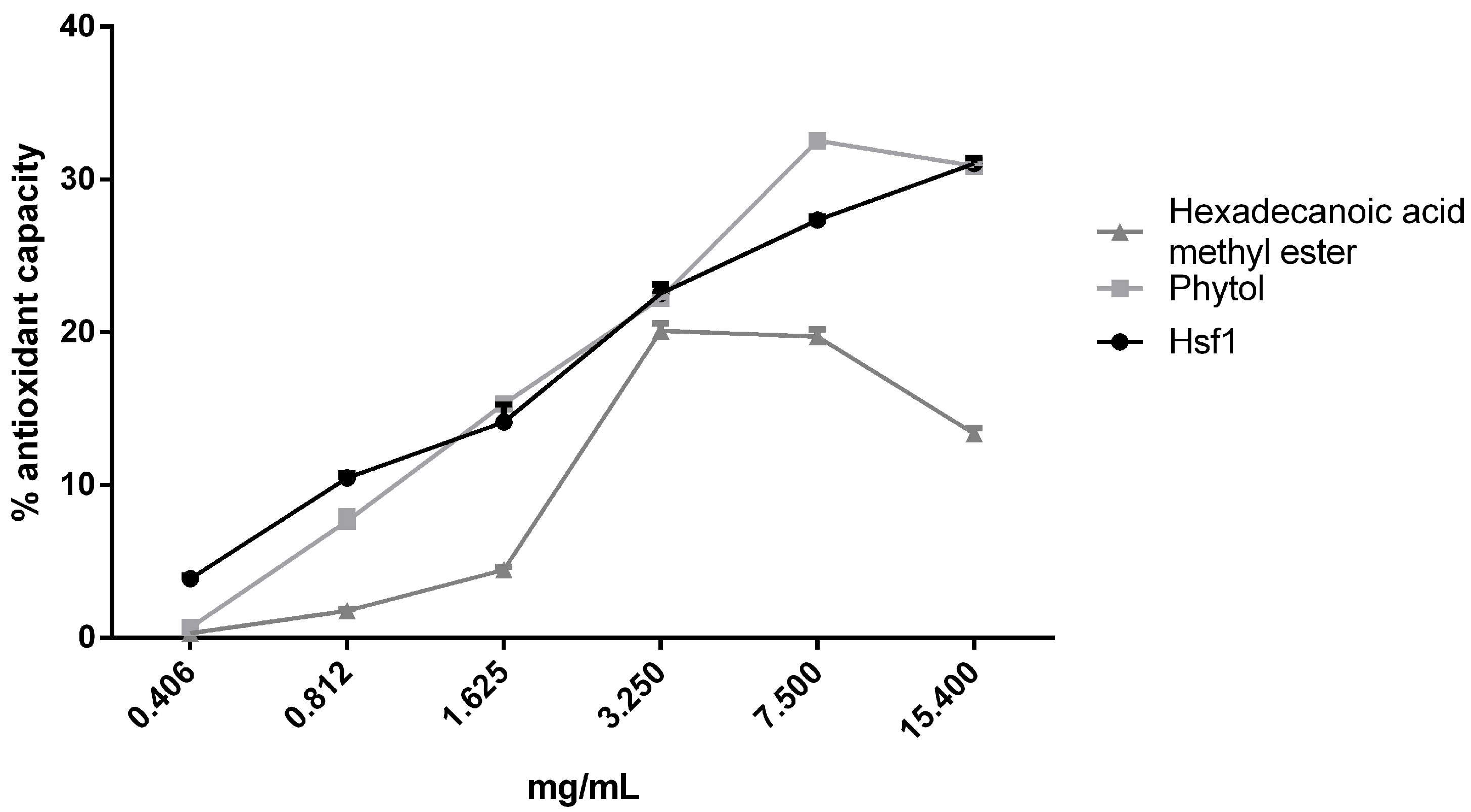
3.5. Antioxidant Effect of Hsf1 and Anti-Toxoplasma Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bigna, J.J.; Tochie, J.N.; Tounouga, D.N.; Bekolo, A.O.; Ymele, N.S.; Youda, E.L.; Sime, P.S.; Nansseu, J. Global, regional, and country seroprevalence of Toxoplasma gondii in pregnant women: A systematic review, modelling and meta-analysis. Sci. Rep. 2020, 10, 12102. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, I.; García-Izquierdo, S.M. Toxoplasmosis en el hombre. Bioquimia 2003, 28, 19–27. [Google Scholar]
- Mamaghani, A.J.; Fathollahi, A.; Arab-Mazar, Z.; Kohansal, K.; Fathollahi, M.; Spotin, A.; Bashiri, H.; Bozorgomid, A. Toxoplasma gondii vaccine candidates: A concise review. Ir. J. Med. Sci. 2023, 192, 231–261. [Google Scholar] [CrossRef]
- Hlaváčová, J.; Flegr, J.; Řežábek, K.; Calda, P.; Kaňková, Š. Male-to-Female Presumed Transmission of Toxoplasmosis Between Sexual Partners. Am. J. Epidemiol. 2021, 190, 386–392. [Google Scholar] [CrossRef]
- Dubey, J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasit. Vectors 2021, 14, 263. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Amouei, A.; Sharif, M.; Sarvi, S.; Galal, L.; Javidnia, J.; Pagheh, A.S.; Gholami, S.; Mizani, A.; Daryani, A. Human toxoplasmosis: A systematic review for genetic diversity of Toxoplasma gondii in clinical samples. Epidemiol. Infect. 2019, 147, e36. [Google Scholar] [CrossRef]
- Smith, N.C.; Goulart, C.; Hayward, J.A.; Kupz, A.; Miller, C.M.; van Dooren, G.G. Control of human toxoplasmosis. Int. J. Parasitol. 2021, 51, 95–121. [Google Scholar] [CrossRef]
- Deganich, M.; Boudreaux, C.; Benmerzouga, I. Toxoplasmosis infection during pregnancy. Trop. Med. Infect. Dis. 2022, 8, 3. [Google Scholar] [CrossRef]
- Espinoza-Rojas, J.; López-Mora, E.; Dabanch-Peña, J.; Cruz-Choappa, R. Recomendaciones para el diagnóstico y tratamiento de la infección por Toxoplasma gondii. Rev. Chil. Infectología 2022, 39, 132–137. [Google Scholar] [CrossRef]
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [PubMed]
- Boy, H.I.A.; Rutilla, A.J.; Santos, K.; Matthew, A.; Yu, A.; Mahboob, T.; Tangpoong, J.; Nissapatorn, V. Recommended Medicinal Plants as Source of Natural Products: A Review. Digit. Chin. Med. 2018, 1, 131–142. [Google Scholar] [CrossRef]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Anacleto-Santos, J.; López-Camacho, P.Y.; Mondragón-Flores, R.; Vega-Ávila, E.; Basurto-Islas, G.; Mondragón-Castelán, M.; Carrasco-Ramírez, E.; Rivera-Fernández, N. Anti-toxoplasma, antioxidant and cytotoxic activities of Pleopeltis crassinervata (Fée) T. Moore hexane fraction. Saudi J. Biol. Sci. 2020, 27, 812–819. [Google Scholar] [CrossRef]
- Anacleto-Santos, J.; Calzada, F.; López-Camacho, P.Y.; López-Pérez, T.J.; Carrasco-Ramírez, E.; Casarrubias-Tabarez, B.; Fortoul, T.; Rojas-Lemus, M.; López-Valdés, N.; Rivera-Fernández, N. Evaluation of the Anti-Toxoplasma gondii Efficacy, Cytotoxicity, and GC/MS Profile of Pleopeltis crassinervata Active Subfractions. Antibiotics 2023, 12, 889. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra, S.M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Paraboni, M.L.R.; Manfredini, V.; Schreiner, G.E.; Gonçalves, I.L.; Silveira, C.; Commodaro, A.G.; Belfort, R., Jr. Comparative study of oxidative stress and antioxidative markers in patients infected with Toxoplasma gondii. Parasitol. Int. 2022, 91, 102645. [Google Scholar] [CrossRef]
- Szewczyk-Golec, K.; Pawłowska, M.; Wesołowski, R.; Wróblewski, M.; Mila-Kierzenkowska, C. Oxidative Stress as a Possible Target in the Treatment of Toxoplasmosis: Perspectives and Ambiguities. Int. J. Mol. Sci. 2021, 11, 5705. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Kuraishi, A.H.; Al-Windy, S.; Al-Gareeb, A.I. Toxoplasmosis and Risk of Endothelial Dysfunction: Role of Oxidative Stress and Pro-Inflammatory Mediators. Arch. Clin. Infect. Dis. 2019, 14, e95563. [Google Scholar] [CrossRef]
- Diario Oficial de La Federación. Norma Oficial Mexicana NOM-062-ZOO-1999. Especificaciones Técnicas para la Producción, Cuidado y Uso de Los Animales de Laboratori. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 20 February 2021).
- Rivera-Fernández, N.; Mondragón-Castelán, M.; González-Pozos, S.; Ramírez-Flores, C.J.; Mondragón-González, R.; Gómez de León, C.T.; Castro-Elizalde, K.N.; Marrero-Ponce, Y.; Arán, V.J.; Martins-Alho, M.A.; et al. A new type of quinoxalinone derivatives affects viability, invasion, and intracellular growth of Toxoplasma gondii tachyzoites in vitro. Parasitol. Res. 2016, 115, 2081–2096. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Mangoba, M.A.A.; Alvindia, D.d.G. Acaricidal activities of Cymbopogon citratus (DC) Stapf against Blomia tropicalis van Bronswijk, Cock & Oshima (Astigmata: Echimyopodidae). Int. J. Acarol. 2022, 48, 686–690. [Google Scholar] [CrossRef]
- Sanchez, S.G.; Besteiro, S. The pathogenicity and virulence of Toxoplasma gondii. Virulence 2021, 12, 3095–3114. [Google Scholar] [CrossRef] [PubMed]
- Da Gama, L.M.; Ribeiro-Gomes, F.L.; Guimarães, U.J.; Arnholdt, A.C. Reduction in adhesiveness to extracellular matrix components, modulation of adhesion molecules and in vivo migration of murine macrophages infected with Toxoplasma gondii. Microbes Infect. 2004, 6, 1287–1296. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Davoodbasha, M.; Edachery, B.; Nooruddin, T.; Lee, S.Y.; Kim, J.W. An evidence of C16 fatty acid methyl esters extracted from microalga for effective antimicrobial and antioxidant property. Microb. Pathog. 2018, 115, 233–238. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, e1800136. [Google Scholar] [CrossRef]
- Attias, M.; Teixeira, D.C.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The life-cycle of Toxoplasma gondii reviewed using animations. Parasit. Vectors 2020, 13, 588. [Google Scholar] [CrossRef]
- Lagal, V.; Binder, E.M.; Huynh, M.H.; Kafsack, B.F.; Harris, P.K.; Diez, R.; Chen, D.; Cole, R.N.; Carruthers, V.B.; Kim, K. Toxoplasma gondii protease TgSUB1 is required for cell surface processing of micronemal adhesive complexes and efficient adhesion of tachyzoites. Cell. Microbiol. 2010, 12, 1792–1808. [Google Scholar] [CrossRef]
- Tomavo, S.; Schwarz, R.T.; Dubremetz, J.F. Evidence for glycosyl-phosphatidylinositol anchoring of Toxoplasma gondii major surface antigens. Mol. Cell. Biol. 1989, 9, 4576–4580. [Google Scholar] [CrossRef]
- Martínez, A.F.F.; Teixeira, S.C.; de Souza, G.; Rosini, A.M.; Júnior, J.P.L.; Melo, G.N.; Blandón, K.O.E.; Gomes, A.O.; Ambrósio, S.R.; Veneziani, R.C.S.; et al. Leaf hydroalcoholic extract and oleoresin from Copaifera multijuga control Toxoplasma gondii infection in human trophoblast cells and placental explants from third-trimester pregnancy. Front. Cell. Infect. Microbiol. 2023, 13, 1113896. [Google Scholar] [CrossRef]
- Sumayya, S.S.; Lubaina, A.S.; Murugan, K. Bactericidal Potentiality of Purified Terpenoid Extracts from the Selected Sea Weeds and its Mode of Action. J. Trop. Life Sci. 2020, 10, 197–205. [Google Scholar] [CrossRef]
- da Silva, J.M.; Antinarelli, L.M.; Ribeiro, A.; Coimbra, E.S.; Scio, E. The effect of the phytol-rich fraction from Lacistema pubescens against Leishmania amazonensis is mediated by mitochondrial dysfunction. Exp. Parasitol. 2015, 159, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Islam, M.T.; Santos, P.S.; Ferreira, P.B.; Oliveira, G.L.; Alencar, M.V.; Paz, M.F.; Ferreira, E.L.; Feitosa, C.M.; Citó, A.M.; et al. Evaluation of antioxidant activity of phytol using non-and pre-clinical models. Curr. Pharm. Biotechnol. 2016, 17, 1278–1284. [Google Scholar] [CrossRef]
- Okpala, E.; Onocha, P.; Ali, M. Antioxidant activity of phytol dominated stem bark and leaf essential oils of Celtis zenkeri. Engl. Trends Phytochem. Res. 2022, 2, 137. [Google Scholar] [CrossRef]
- Jeong, S.H. Anti-oxidant activities of phytol on keratinocytes. Asian J. Beauty Cosmetol. 2017, 15, 457–465. [Google Scholar] [CrossRef]
- Gutbrod, K.; Romer, J.; Dörmann, P. Phytol metabolism in plants. Prog. Lipid Res. 2019, 74, 1–17. [Google Scholar] [CrossRef]
- Santos, C.C.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.; de Oliveira, G.A.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Dincel, G.C.; Atmaca, H.T. Role of oxidative stress in the pathophysiology of Toxoplasma gondii infection. Int. J. Immunopathol. Pharmacol. 2016, 29, 226–240. [Google Scholar] [CrossRef]
- Atolani, O.; Oguntoye, H.; Areh, E.T.; Adeyemi, O.S.; Kambizi, L. Chemical composition, anti-toxoplasma, cytotoxicity, antioxidant, and anti-inflammatory potentials of Cola gigantea seed oil. Pharm. Biol. 2019, 57, 154–160. [Google Scholar] [CrossRef]


| Compounds Within Hsf1 with Biological Activities | |||
|---|---|---|---|
| Compound | Structure | Activity | Reference |
| Phytol | 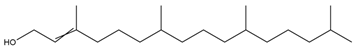 | Cytotoxic, antioxidant, antinociceptive, anti-inflammatory, and antimicrobial | [16] |
| Hexadecanoic acid methyl ester | 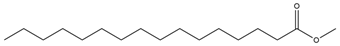 | Antimicrobial and antioxidant | [26] |
| Squalene | 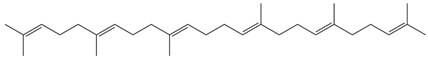 | Antioxidant, anti-inflammatory, and anti-atherosclerotic | [27] |
| 6a,14a-methanopicene; perhydro-1,2,4a,6b,9,9,12a-heptamethyl-10-hydroxy | 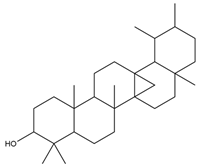 | Acaricidal | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anacleto-Santos, J.; Mondragón-Flores, R.; López-Camacho, P.Y.; Rivera-Vivanco, M.I.; López-Pérez, T.d.J.; Casarrubias-Tabares, B.; Mondragón-Castelán, M.; González-Pozos, S.; Calzada, F.; Vega-Ávila, E.; et al. Anti-Toxoplasma and Antioxidant Activity of a Terpene and Methyl-Ester-Rich Subfraction from Pleopeltis crassinervata. Antioxidants 2025, 14, 342. https://doi.org/10.3390/antiox14030342
Anacleto-Santos J, Mondragón-Flores R, López-Camacho PY, Rivera-Vivanco MI, López-Pérez TdJ, Casarrubias-Tabares B, Mondragón-Castelán M, González-Pozos S, Calzada F, Vega-Ávila E, et al. Anti-Toxoplasma and Antioxidant Activity of a Terpene and Methyl-Ester-Rich Subfraction from Pleopeltis crassinervata. Antioxidants. 2025; 14(3):342. https://doi.org/10.3390/antiox14030342
Chicago/Turabian StyleAnacleto-Santos, Jhony, Ricardo Mondragón-Flores, Perla Yolanda López-Camacho, María Isabel Rivera-Vivanco, Teresa de Jesús López-Pérez, Brenda Casarrubias-Tabares, Mónica Mondragón-Castelán, Sirenia González-Pozos, Fernando Calzada, Elisa Vega-Ávila, and et al. 2025. "Anti-Toxoplasma and Antioxidant Activity of a Terpene and Methyl-Ester-Rich Subfraction from Pleopeltis crassinervata" Antioxidants 14, no. 3: 342. https://doi.org/10.3390/antiox14030342
APA StyleAnacleto-Santos, J., Mondragón-Flores, R., López-Camacho, P. Y., Rivera-Vivanco, M. I., López-Pérez, T. d. J., Casarrubias-Tabares, B., Mondragón-Castelán, M., González-Pozos, S., Calzada, F., Vega-Ávila, E., & Rivera-Fernández, N. (2025). Anti-Toxoplasma and Antioxidant Activity of a Terpene and Methyl-Ester-Rich Subfraction from Pleopeltis crassinervata. Antioxidants, 14(3), 342. https://doi.org/10.3390/antiox14030342








