Sowing Methods and Strigolactones Alleviate Damage to the Photosynthetic System of Rice Seedlings Under Salt Stress by Enhancing Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Measurement Items and Methods
2.3.1. Measurement of Morphological Indices
2.3.2. Measurement of Leaf Gas Exchange Parameters
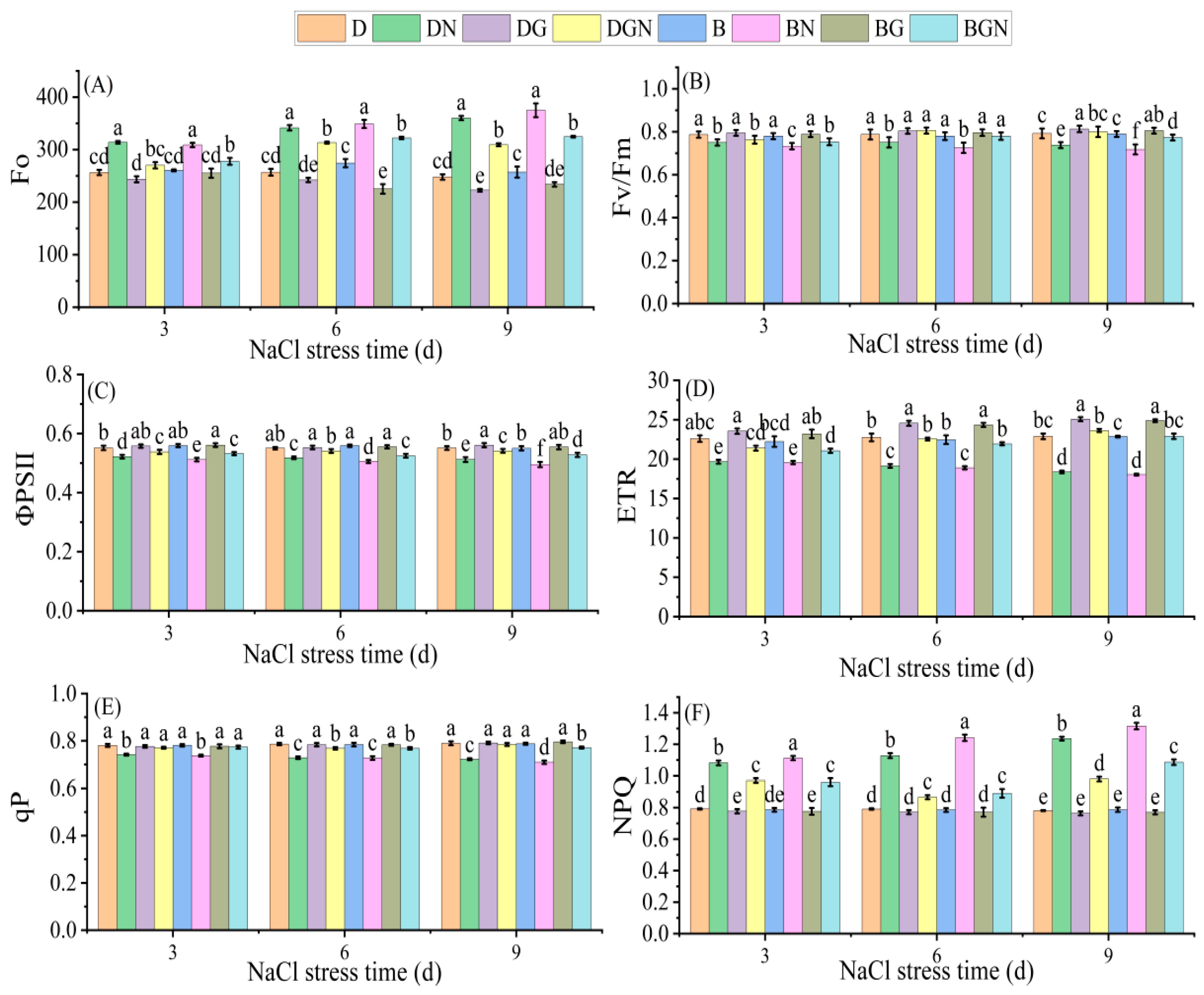
2.3.3. Measurement of Chlorophyll Fluorescence Parameters
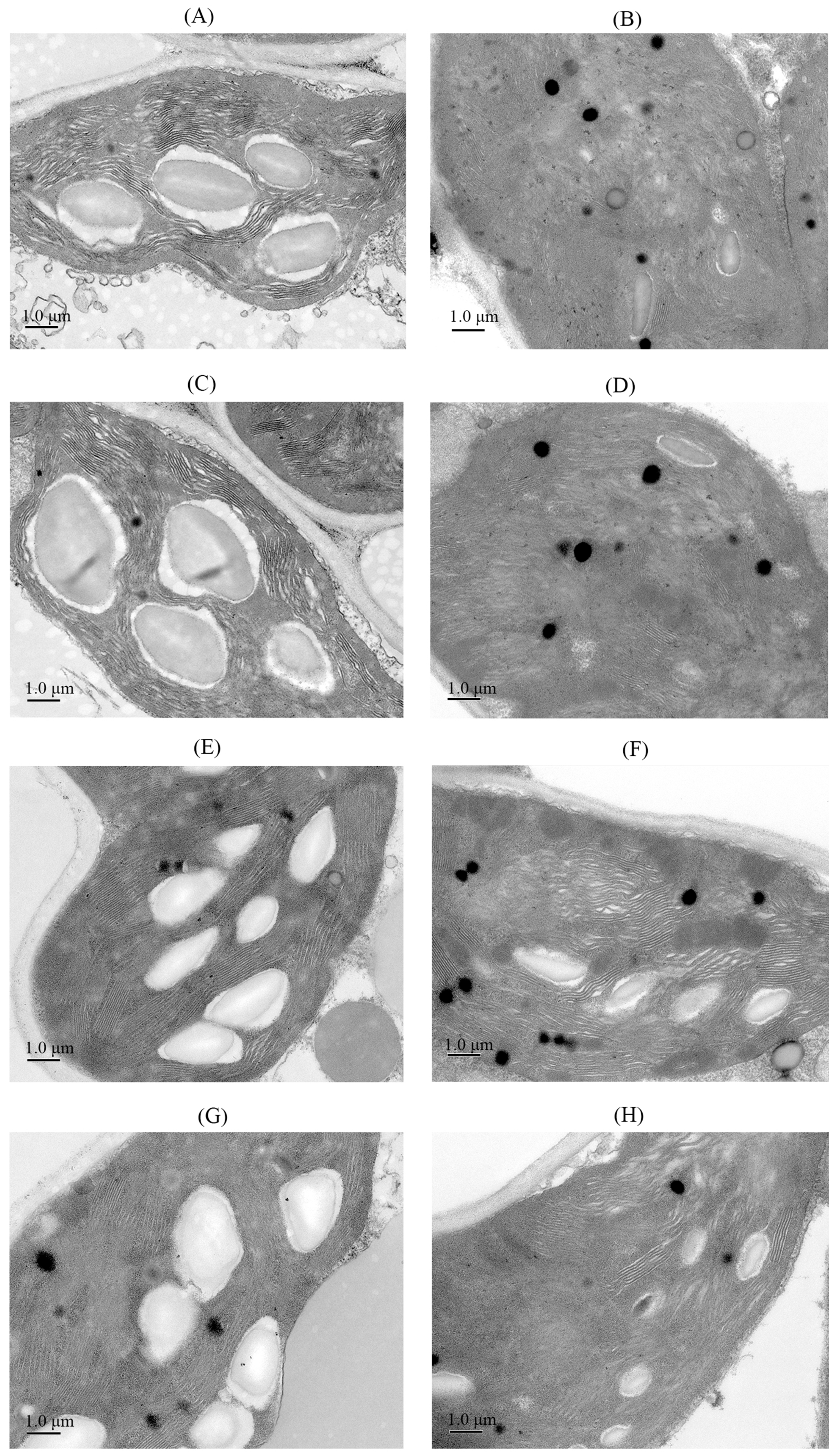
2.3.4. Observation of Chloroplast Ultrastructure
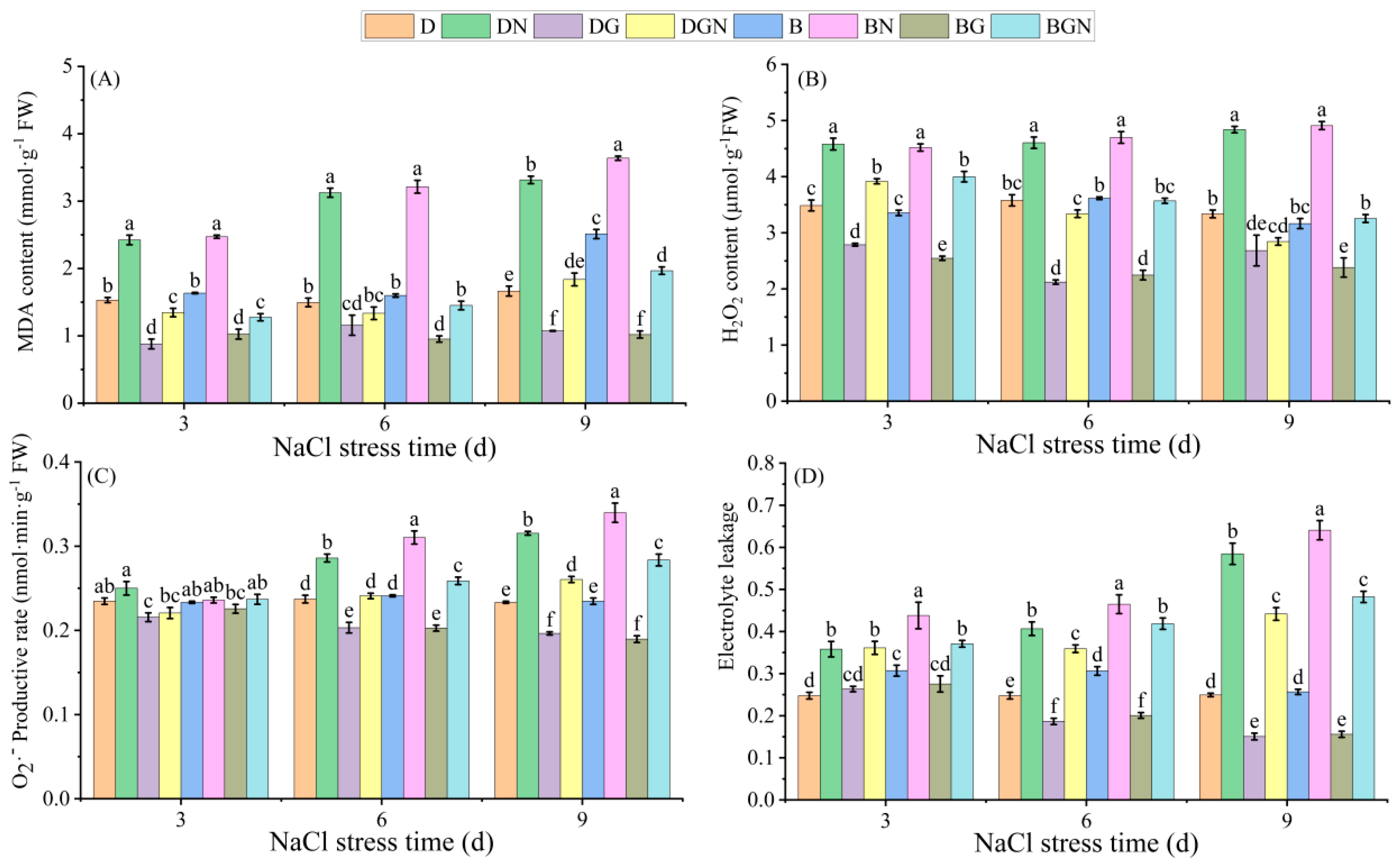
2.3.5. Determination of Leaf Malondialdehyde (MDA), Hydrogen Peroxide (H2O2), and Superoxide Anion (O2·−) Generation Rate
2.3.6. Determination of Leaf Relative Electrolyte Conductivity (REC)
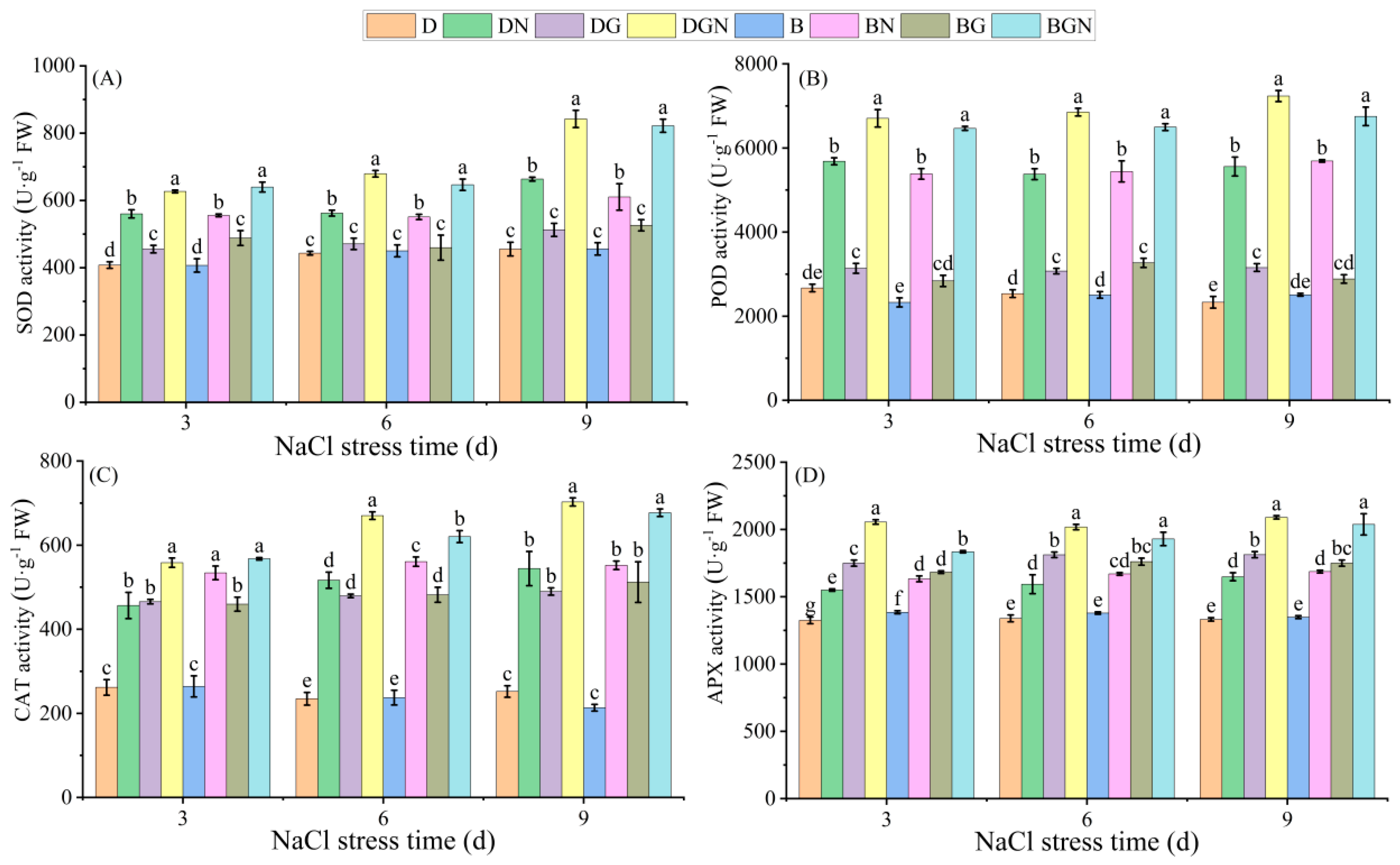
2.3.7. Determination of Leaf Antioxidant Enzyme Activities
2.3.8. Determination of Key Enzyme Activities in the ASA-GSH Cycle and Non-Enzymatic Antioxidants
2.3.9. Determination of Leaf Total Phenolic, Flavonoid Contents, and PAL and PPO Activities
2.3.10. Determination of Endogenous Hormone Content in Leaves
2.3.11. Measurement of Ion Contents in Rice Seedling Leaves
2.4. Data Analysis
3. Results
3.1. Effects of Sowing Methods and GR24 on Seedling Morphology Under Salt Stress
3.2. Effects of Sowing Methods and GR24 on Gas Exchange Parameters of Seedlings Under Salt Stress
3.3. Effects of Sowing Methods and GR24 on Chlorophyll Fluorescence Parameters of Seedlings Under Salt Stress
3.4. Effects of Sowing Methods and GR24 on Chloroplast Ultrastructure of Seedlings Under Salt Stress
3.5. Effects of Sowing Methods and GR24 on Membrane Damage in Seedlings Under Salt Stress
3.6. Effects of Sowing Methods and GR24 on Antioxidant Enzyme Activities in Seedling Leaves Under Salt Stress
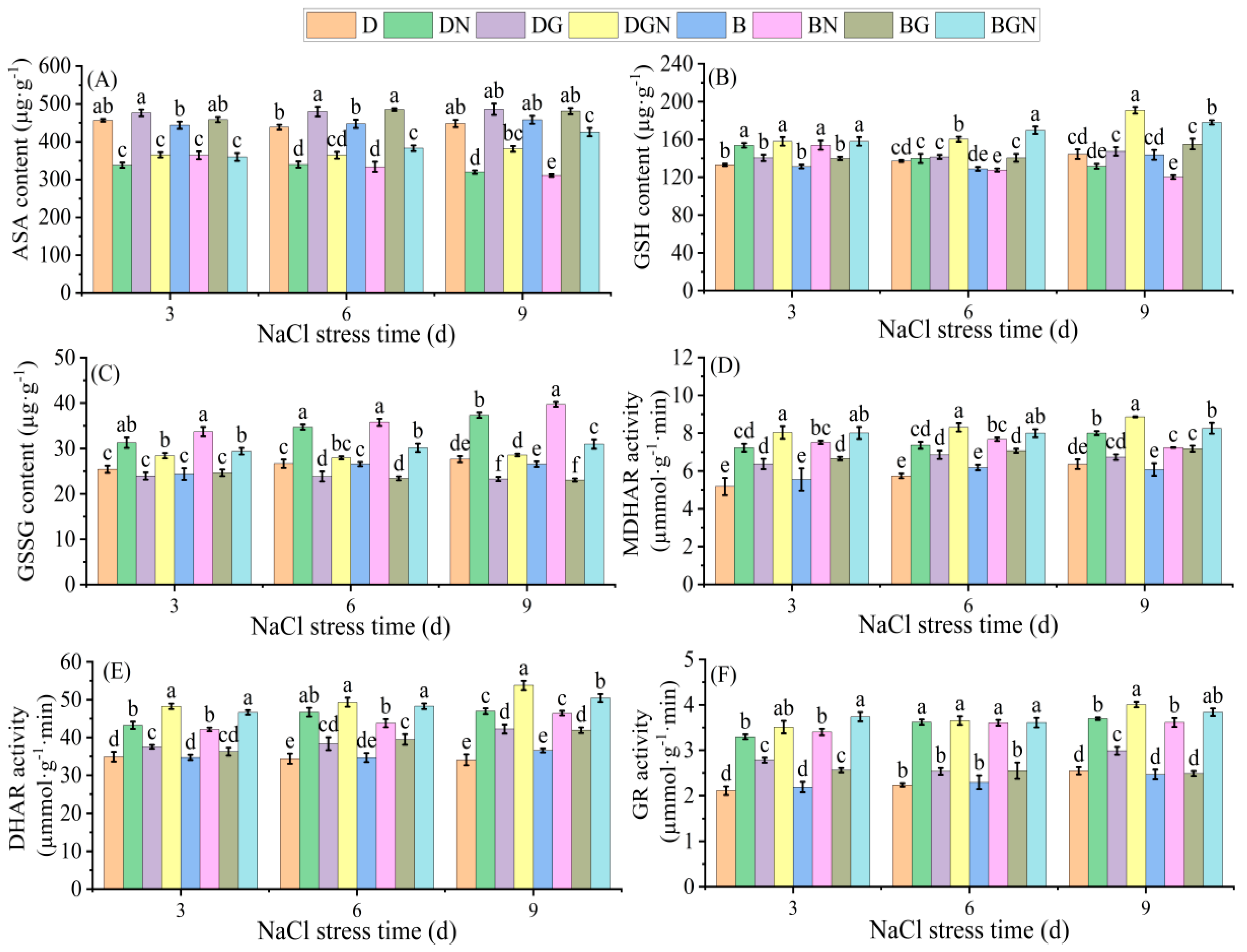
3.7. Effects of Sowing Methods and Exogenous GR24 on Key AsA-GSH Cycle Enzyme Activities and Non-Enzymatic Antioxidants in Seedling Leaves Under Salt Stress
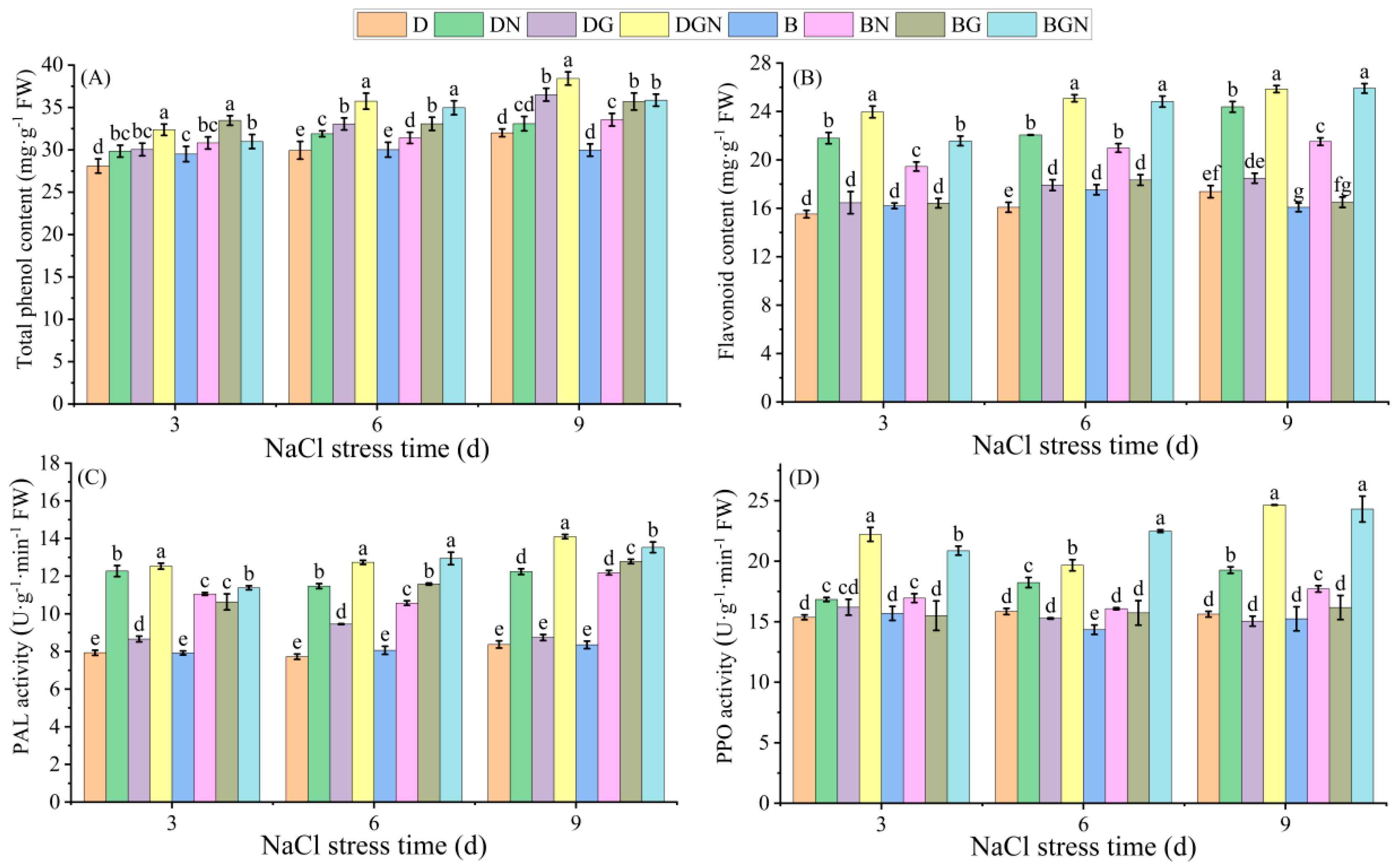
3.8. Effects of Sowing Methods and GR24 on Total Phenolics, Flavonoids, and Related Enzyme Activities in Seedling Leaves Under Salt Stress
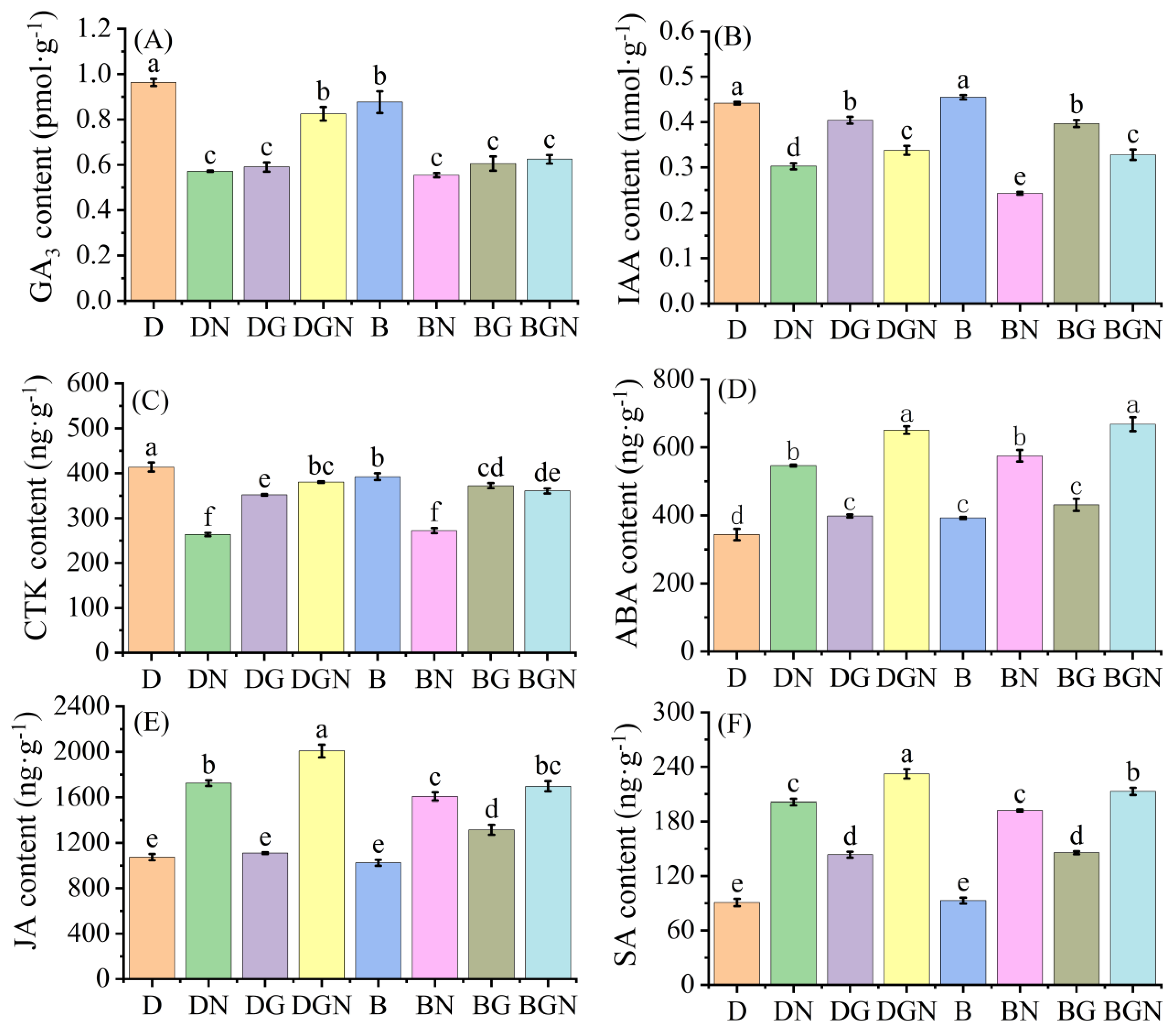
3.9. Effects of Sowing Methods and GR24 on Endogenous Hormone Content in Seedlings Under Salt Stress
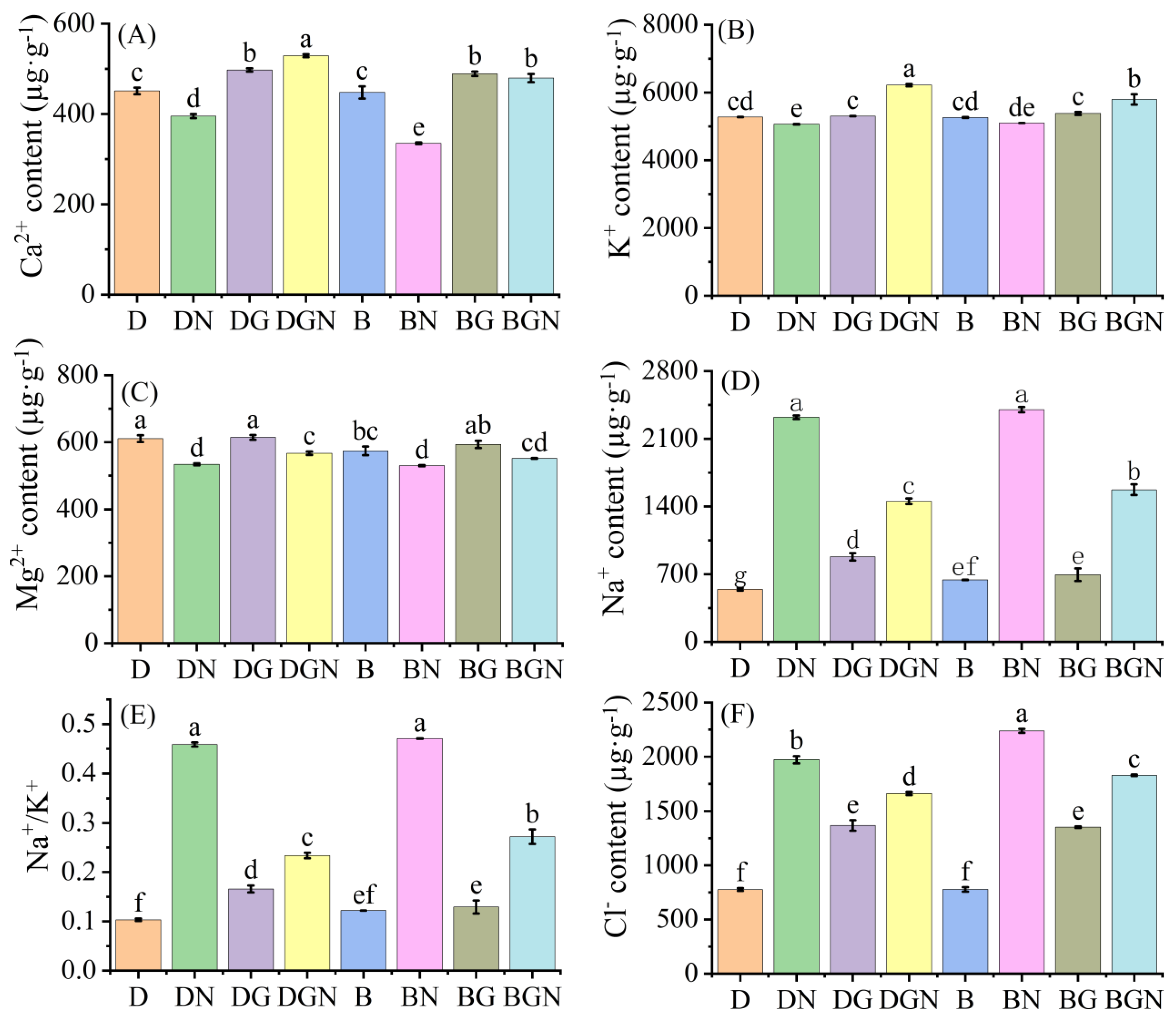
3.10. Effects of Sowing Methods and GR24 on Ion Content in Seedlings Under Salt Stress
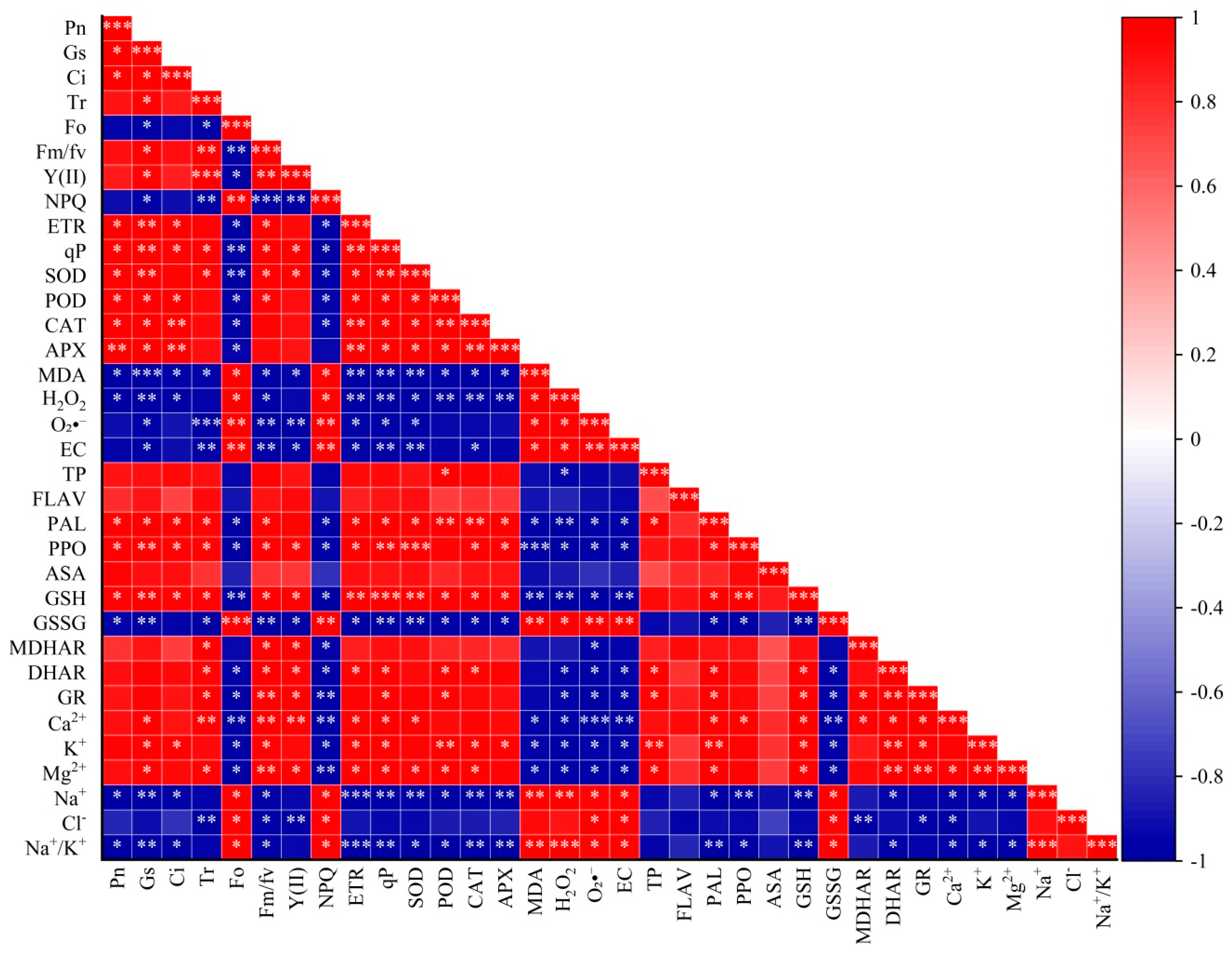
3.11. Correlation Between Various Indicators
4. Discussion
4.1. Alleviating Effects of Sowing Method and GR24 on Seedling Growth Under Salt Stress
4.2. Alleviating Effects of Sowing Method and GR24 on the Photosynthetic System of Seedlings Under Salt Stress
4.3. Sowing Methods and GR24 Reduce Oxidative Damage in Seedlings by Enhancing Antioxidant Enzyme Activity and Non-Enzymatic Antioxidant Contents
4.4. Sowing Methods and GR24 Alleviate Salt Stress by Regulating Hormone Content in Seedlings
4.5. Sowing Methods and GR24 Alleviate Salt Stress by Balancing Ion Content in Seedlings
4.6. Correlation Analysis Between Various Indicators of Rice Seedlings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| SLs | strigolactones |
| POD | peroxidase |
| SOD | superoxide dismutase |
| GSH | glutathione |
| AsA | ascorbic acid |
| ETR | electron transport rate |
| NPQ | non-photochemical quenching coefficient |
| PPO | polyphenol oxidase |
| PAL | phenylalanine ammonia-lyase |
| ELISA | enzyme-linked immunosorbent assay |
| ANOVA | analysis of variance |
| Sm | sowing method |
| Rf | regulatory factor |
| Pn | net photosynthetic rate |
| Gs | stomatal conductance |
| Ci | intercellular CO2 concentration |
| Tr | transpiration rate |
| Fo | initial fluorescence |
| Fv/Fm | maximum photochemical efficiency of PSII |
| ΦPSII | actual quantum yield of PSII |
| qP | photochemical quenching coefficient |
| H2O2 | hydrogen peroxide |
| O·− | superoxide anion generation rate |
| APX | ascorbate peroxidase |
| CAT | catalase |
| GSSG | oxidized glutathione |
| GR | glutathione reductase |
| MDHAR | monodehydroascorbate reductase |
| DHAR | dehydroascorbate reductase |
References
- Ganie, S.A.; Molla, K.A.; Henry, R.J.; Bhat, K.V.; Mondal, T.K. Advances in understanding salt tolerance in rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef]
- Zou, Y.B.; Huang, M. Opportunities and challenges in crop production development during the transition period. Acta Agron. Sin. 2018, 44, 791–795. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, D.; Xiang, J.; Chen, H.; Zhang, Y.; Xu, Y.; Zhang, Y. Characteristics of seedling raising and mechanized transplanting of hybrid rice with a low seeding rate by precise seeding method. Chin. J. Rice Sci. 2020, 34, 332. [Google Scholar] [CrossRef]
- Zhu, J.; Cui, Z.; Wu, C.; Deng, C.; Chen, J.; Zhang, H. Research advances and prospect of saline and alkali land greening in China. World For. Res. 2018, 31, 70–75. [Google Scholar]
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of Land and Water Resources: Human Causes, Extent, Management and Case Studies; CABI Publishing: Oxfordshire, UK, 1995. [Google Scholar]
- Xu, P.Z.; Dai, W.J.; Huang, X.; Lin, B.S.; Zeng, Z.B.; Zhang, Q.; Xie, K.Z. Characteristics and improvement strategies of coastal saline soils in Guangdong Province. Guangdong Agric. Sci. 2023, 50, 87–94. [Google Scholar] [CrossRef]
- Hao, M.Y.; Ding, G.D.; Zheng, H.B.; Zhang, L.; Jia, L.N.; Zhang, J.Y.; Cui, L.Q. Study on the effect of rainwater harvesting measures on water and salt movement in coastal saline-alkali forestland. J. Soil Water Conserv. 2008, 22, 52–56. [Google Scholar] [CrossRef]
- Puniran-Hartley, N.; Hartley, J.; Shabala, L.; Shabala, S. Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: In planta evidence for cross-tolerance. Plant Physiol. Biochem. 2014, 83, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xue, P.; Zhang, Y.; Zhan, X.; Wu, W.; Yu, P.; Chen, D.; Fu, J.; Hong, Y.; Shen, X.; et al. OsCPK12 phosphorylates OsCATA and OsCATC to regulate H(2)O(2) homeostasis and improve oxidative stress tolerance in rice. Plant Commun. 2024, 5, 100780. [Google Scholar] [CrossRef]
- da Rocha Mendes, K.; Bonifácio, A.; Martins, M.O.; Sousa, R.H.V.; Monteiro, M.V.; Silveira, J.A. Heat combined with salinity stimulate antioxidant defense but induce severe impairment in photosynthesis of rice plants. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, M.; Lu, J.; Li, X.; Liu, D.; Li, X.e.; Wang, L. GR24 alleviates the adverse effects of drought stress on physiology and photosystem II function in alfalfa (Medicago sativa L.). Grassl. Sci. 2023, 69, 113–119. [Google Scholar] [CrossRef]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.; Zhang, C. Strigolactones Improve Plant Growth, Photosynthesis, and Alleviate Oxidative Stress under Salinity in Rapeseed (Brassica napus L.) by Regulating Gene Expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Sun, Y.; Zheng, S.; Wang, J.; Zhang, T. Hydrogen peroxide is involved in strigolactone induced low temperature stress tolerance in rape seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.W.; Zhang, C.; Wang, N.H.; Mao, W.; Wu, F. Strigolactone GR24 improves cadmium tolerance by regulating cadmium uptake, nitric oxide signaling and antioxidant metabolism in barley (Hordeum vulgare L.). Environ. Pollut. 2021, 273, 116486. [Google Scholar] [CrossRef]
- Chaffey, N. Principles and Techniques of Electron Microscopy: Biological Applications, 4th ed.; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, C.B. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: In gel enzyme activity assays. Plant Sci. 2000, 159, 75–85. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, K.; Sathi, K.S.; Alam, M.M.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Supplemental selenium and boron mitigate salt-induced oxidative damages in Glycine max L. Plants 2021, 10, 2224. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Su, C.; Hu, D.; Li, F.; Lu, Q.; Zhang, T.; Xu, Q. Molecular distribution and toxicity assessment of praseodymium by Spirodela polyrrhiza. J. Hazard. Mater. 2016, 312, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Tyburski, J.; Tretyn, A. Glutathione and glutathione disulfide affect adventitious root formation and growth in tomato seedling cuttings. Acta Physiol. Plant. 2010, 32, 411–417. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol. Biochem. 2020, 147, 10–20. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Shan, C.; Liu, R. Exogenous hydrogen peroxide up-regulates the contents of ascorbate and glutathione in the leaves of Vigna radiata (Linn.) Wilczek. exposed to salt stress. Braz. J. Bot. 2017, 40, 583–589. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef]
- Luh, B.; Phithakpol, B. Characteristics of polyphenoloxidase related to browning in cling peaches. J. Food Sci. 1972, 37, 264–268. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.a.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, D.; Singh, K. Effect of selenium application on arsenic uptake in rice (Oryza sativa L.). Environ. Monit. Assess. 2017, 189, 430. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hadia, E.; Slama, A.; Romdhane, L.; Cheikh M’Hamed, H.; Fahej, M.A.S.; Radhouane, L. Seed priming of bread wheat varieties with growth regulators and nutrients improves salt stress tolerance particularly for the local genotype. J. Plant Growth Regul. 2023, 42, 304–318. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Dong, G.; Zhu, G.; Zhou, G. Progress of research on the physiology and molecular regulation of sorghum growth under salt stress by gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef]
- Lu, X.; Liu, X.; Xu, J.; Liu, Y.; Chi, Y.; Yu, W.; Li, C. Strigolactone-mediated trehalose enhances salt resistance in tomato seedlings. Horticulturae 2023, 9, 770. [Google Scholar] [CrossRef]
- Zuo, G.; Zhang, R.; Feng, N.; Zheng, D. Photosynthetic Responses to Salt Stress in Two Rice (Oryza sativa L.) Varieties. Agronomy 2024, 14, 2134. [Google Scholar] [CrossRef]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in Hordeum vulgare. Photosynth. Res. 2012, 111, 269–283. [Google Scholar] [CrossRef]
- Ling, F.; Su, Q.; Jiang, H.; Cui, J.; He, X.; Wu, Z.; Zhang, Z.; Liu, J.; Zhao, Y. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci. Rep. 2020, 10, 6183. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Ma, C.; Su, M.; Wang, J.; Zheng, S.; Zhang, T. Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci. Hortic. 2022, 297, 110962. [Google Scholar] [CrossRef]
- Riaz, M.; Arif, M.S.; Ashraf, M.A.; Mahmood, R.; Yasmeen, T.; Shakoor, M.B.; Shahzad, S.M.; Ali, M.; Saleem, I.; Arif, M. A comprehensive review on rice responses and tolerance to salt stress. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–158. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of Chlorophyll a Fluorescence in Natural Compound-Induced Stress Detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K.; Wang, Y.; Zhang, Z.; Lu, F.; Yu, H.; Zou, J. Changes in photosynthetic and chlorophyll fluorescence characteristics of sorghum under drought and waterlogging stress. Photosynthetica 2019, 57, 1156–1164. [Google Scholar] [CrossRef]
- Du, X.L.; Feng, N.J.; Zheng, D.F.; Lin, Y.; Zhou, H.; Li, J.H.; Yang, X.H.; Huo, J.X.; Mei, W.Q. Effects of exogenous Uniconazole (S3307) on oxidative damage and carbon metabolism of rice under salt stress. BMC Plant Biol. 2025, 25, 541. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Ye, S.; Huang, Z.; Zhao, G.; Zhai, R.; Ye, J.; Wu, M.; Yu, F.; Zhu, G.; Zhang, X. Differential physiological responses to salt stress between salt-sensitive and salt-tolerant japonica rice cultivars at the post-germination and seedling stages. Plants 2021, 10, 2433. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Sui, N. Sensitivity and responses of chloroplasts to salt stress in plants. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Bian, C.; Liu, W.; Sun, Z.; Xi, X.; Guo, D.; Liu, X.; Tian, Y.; Wang, C.; Zheng, X. Strigolactone alleviates the salinity-alkalinity stress of Malus hupehensis seedlings. Front. Plant Sci. 2022, 13, 901782. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Faizan, M.; Tonny, S.H.; Rajput, V.D.; Minkina, T.; Alatar, A.A.; Pathirana, R. Strigolactone-mediated mitigation of negative effects of salinity stress in Solanum lycopersicum through reducing the oxidative damage. Sustainability 2023, 15, 5805. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, C.; Li, Y.; Meng, F.; Du, Y.; Zhang, S.; Jiang, W.; Feng, N.; Zhao, L.; Zheng, D. 5-ALA, DTA-6, and Nitrogen Mitigate NaCl Stress by Promoting Photosynthesis and Carbon Metabolism in Rice Seedlings. Metabolites 2024, 14, 142. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kunert, K. The ascorbate-glutathione cycle coming of age. J. Exp. Bot. 2024, 75, 2682–2699. [Google Scholar] [CrossRef]
- Lu, J.; Shen, Y.; Yang, Y.; Li, X.; Li, X.e.; Liu, D.; Wang, L. Synthetic strigolactone (rac-GR24) alleviates the photosynthetic inhibition and oxidative damage in alfalfa (Medicago sativa L.) under salt stress. Grassl. Sci. 2024, 70, 23–34. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Ahsan, M.; Zulfiqar, H.; Farooq, M.A.; Ali, S.; Tufail, A.; Kanwal, S.; Shaheen, M.R.; Sajid, M.; Gul, H.; Jamal, A. Strigolactone (GR24) Application positively regulates photosynthetic attributes, stress-related metabolites and antioxidant enzymatic activities of ornamental sunflower (Helianthus annuus cv. Vincent’s Choice) under salinity stress. Agriculture 2022, 13, 50. [Google Scholar] [CrossRef]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the study of the function and mechanism of the action of flavonoids in plants under environmental stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Wang, Y.; Ding, Y.; Qian, W.; Qiu, C.; Xie, H.; Sun, L.; Jiang, Z.; Ma, Q.; Wang, L.; et al. Exogenous abscisic acid induces the lipid and flavonoid metabolism of tea plants under drought stress. Sci. Rep. 2020, 10, 12275. [Google Scholar] [CrossRef]
- Struk, S.; Braem, L.; Matthys, C.; Walton, A.; Vangheluwe, N.; Van Praet, S.; Jiang, L.; Baster, P.; De Cuyper, C.; Boyer, F.D.; et al. Transcriptional Analysis in the Arabidopsis Roots Reveals New Regulators that Link rac-GR24 Treatment with Changes in Flavonol Accumulation, Root Hair Elongation and Lateral Root Density. Plant Cell Physiol. 2022, 63, 104–119. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Tang, N.; Ma, S.; Zong, W.; Yang, N.; Lv, Y.; Yan, C.; Guo, Z.; Li, J.; Li, X.; Xiang, Y. MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate ABA signaling and drought resistance in rice. Plant Cell 2016, 28, 2161–2177. [Google Scholar] [CrossRef]
- Sakya, A.; Purnomo, J.; Bima, D. Application of GA3 and PGPRs on growth and antioxidant content of Parijoto (Medinilla verrucosa) in peat soil. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bandung, Indonesia, 18–19 April 2022; p. 012009. [Google Scholar]
- Xiang, N.; Hu, J.G.; Yan, S.; Guo, X. Plant Hormones and Volatiles Response to Temperature Stress in Sweet Corn (Zea mays L.) Seedlings. J. Agric. Food Chem. 2021, 69, 6779–6790. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, N.; Zheng, D.; Khan, A.; Du, Y.; Wang, Y.; Deng, R.; Wu, J.; Xiong, J.; Sun, Z. Strigolactone Alleviates NaCl Stress by Regulating Antioxidant Capacity and Hormone Levels in Rice (Oryza sativa L.) Seedlings. Agriculture 2024, 14, 1662. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Yang, Y.; Shen, X.; Zhu, M.; Shen, J.; Zhang, W.; Hu, H.; Wang, Y.F. Multiple cyclic nucleotide-gated channels function as ABA-activated Ca2+ channels required for ABA-induced stomatal closure in Arabidopsis. Plant Cell 2023, 35, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.D.; Froehlich, J.E.; Strand, D.D.; Buck, S.M.; Kramer, D.M.; Larkin, R.M. GUN4-porphyrin complexes bind the ChlH/GUN5 subunit of Mg-Chelatase and promote chlorophyll biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1449–1467. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.; Shahbaz, M.; Wahid, M.A. Potential role of foliage applied strigolactone (GR24) on photosynthetic pigments, gas exchange attributes, mineral nutrients and yield components of Zea mays (L.) under saline regimes. Gesunde Pflanz. 2023, 75, 577–591. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ma, C.; Zhang, L.; Su, M.; Wang, J.; Zheng, S.; Zhang, T.G. GR24-mediated enhancement of salt tolerance and roles of H(2)O(2) and Ca(2+) in regulating this enhancement in cucumber. J. Plant Physiol. 2022, 270, 153640. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Talubaghi, M.J.; Daliri, M.S.; Mazloum, P.; Rameeh, V.; Mousavi, A. Effect of salt stress on growth, physiological and biochemical parameters and activities of antioxidative enzymes of rice cultivars. Cereal Res. Commun. 2023, 51, 403–411. [Google Scholar] [CrossRef]
- Aloufi, S.; Alharbi, B.M.; Sattar, A.; Abbas, Z.K.; Alzuaibr, F.M.; Al-Harbi, N.A.; Alqurashi, M.; Eldin Darwish, D.B. Melatonin and Strigolactone Combined Application Mitigate Salinity Stress Through Modulation of Antioxidants Defense Mechanism, Osmotic Adjustment and Ions Homeostasis in Maize Seedlings. J. Soil Sci. Plant Nutr. 2025, 1–17. [Google Scholar] [CrossRef]
- Farooq, M.; Rafique, S.; Zahra, N.; Rehman, A.; Siddique, K.H. Root system architecture and salt stress responses in cereal crops. J. Agron. Crop Sci. 2024, 210, e12776. [Google Scholar] [CrossRef]

| Salinity Sampling Time (d) | Treatments | Pattern Indicator | ||||
|---|---|---|---|---|---|---|
| Plant Height (cm) | Length of Root (cm) | Stem Diameter (mm) | Leaf Area (cm2) | Fresh Weight (×10−2 g) | ||
| D | 18.57 ± 0.36 ab | 9.16 ± 0.28 a | 1.90 ± 0.02 cd | 462.23 ± 9.44 cd | 19.64 ± 0.40 bc | |
| DN | 15.11 ± 0.46 c | 8.19 ± 0.17 cd | 1.75 ± 0.03 e | 409.35 ± 8.37 de | 17.61 ± 0.12 d | |
| DG | 19.71 ± 0.57 a | 9.08 ± 0.22 ab | 2.10 ± 0.02 a | 567.17 ± 27.51 a | 21.10 ± 0.30 a | |
| 3 | DGN | 17.57 ± 0.25 b | 8.76 ± 0.04 ab | 1.93 ± 0.04 cd | 503.42 ± 20.21 bc | 18.91 ± 0.30 c |
| B | 18.22 ± 0.39 b | 8.82 ± 0.06 ab | 1.86 ± 0.03 d | 447.12 ± 7.41 cde | 19.53 ± 0.10 c | |
| BN | 14.86 ± 0.38 c | 7.77 ± 0.14 d | 1.73 ± 0.02 d | 395.01 ± 11.87 e | 16.21 ± 0.35 e | |
| BG | 19.52 ± 0.16 a | 9.19 ± 0.25 a | 2.06 ± 0.04 ab | 548.19 ± 28.95 ab | 20.48 ± 0.11 ab | |
| BGN | 16.02 ± 0.54 c | 8.53 ± 0.10 bc | 1.98 ± 0.04 bc | 405.07 ± 16.97 de | 17.39 ± 0.47 d | |
| D | 21.42 ± 0.36 b | 10.54 ± 0.28 a | 2.23 ± 0.02 b | 615.46 ± 9.44 b | 24.76 ± 0.37 b | |
| DN | 16.63 ± 0.46 d | 8.71 ± 0.17 e | 1.94 ± 0.03 d | 512.58 ± 8.37 c | 21.82 ± 0.12 d | |
| DG | 23.80 ± 0.50 a | 10.68 ± 0.22 a | 2.39 ± 0.02 a | 740.83 ± 31.61 a | 27.32 ± 0.32 a | |
| 6 | DGN | 19.34 ± 0.51 c | 9.77 ± 0.01 bc | 2.14 ± 0.04 bc | 595.52 ± 18.58 b | 23.12 ± 0.29 c |
| B | 21.07 ± 0.39 b | 10.18 ± 0.06 ab | 2.16 ± 0.04 bc | 595.24 ± 7.41 b | 24.72 ± 0.10 b | |
| BN | 16.28 ± 0.38 d | 8.98 ± 0.14 de | 1.91 ± 0.02 d | 497.32 ± 11.87 c | 20.42 ± 0.35 e | |
| BG | 22.41 ± 0.21 b | 10.71 ± 0.24 a | 2.35 ± 0.05 a | 705.73 ± 20.95 a | 26.70 ± 0.09 a | |
| BGN | 18.09 ± 0.59 c | 9.42 ± 0.09 cd | 2.10 ± 0.04 c | 519.36 ± 15.72 c | 21.59 ± 0.46 d | |
| D | 24.42 ± 0.36 bc | 12.00 ± 0.28 a | 2.58 ± 0.02 b | 880.77 ± 9.44 c | 31.44 ± 0.28 b | |
| DN | 18.05 ± 0.46 f | 9.32 ± 0.17 e | 2.15 ± 0.03 e | 662.63 ± 11.87 e | 26.95 ± 0.12 d | |
| DG | 26.80 ± 0.70 a | 12.48 ± 0.22 a | 2.83 ± 0.03 a | 1051.12 ± 37.27 a | 34.10 ± 0.31 a | |
| 9 | DGN | 22.92 ± 0.26 d | 11.02 ± 0.02 bc | 2.33 ± 0.02 d | 777.49 ± 18.81 d | 29.21 ± 0.29 c |
| B | 24.02 ± 0.39 cd | 11.43 ± 0.06 b | 2.47 ± 0.04 c | 860.55 ± 7.41 c | 31.39 ± 0.10 b | |
| BN | 17.50 ± 0.38 f | 10.25 ± 0.14 d | 2.12 ± 0.02 e | 648.89 ± 20.83 e | 25.55 ± 0.35 e | |
| BG | 25.65 ± 0.17 ab | 12.36 ± 0.24 a | 2.75 ± 0.04 a | 971.37 ± 28.22 b | 33.48 ± 0.11 a | |
| BGN | 20.23 ± 0.56 e | 10.58 ± 0.09 cd | 2.32 ± 0.06 d | 755.67 ± 16.28 d | 27.68 ± 0.47 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.; Zhao, L.; Chen, W.; Zhang, Q.; Ya, J.; Zhong, W.; Shang, Q.; Tu, J.; Xiang, H.; Zhang, J.; et al. Sowing Methods and Strigolactones Alleviate Damage to the Photosynthetic System of Rice Seedlings Under Salt Stress by Enhancing Antioxidant Capacity. Antioxidants 2025, 14, 1020. https://doi.org/10.3390/antiox14081020
Duan S, Zhao L, Chen W, Zhang Q, Ya J, Zhong W, Shang Q, Tu J, Xiang H, Zhang J, et al. Sowing Methods and Strigolactones Alleviate Damage to the Photosynthetic System of Rice Seedlings Under Salt Stress by Enhancing Antioxidant Capacity. Antioxidants. 2025; 14(8):1020. https://doi.org/10.3390/antiox14081020
Chicago/Turabian StyleDuan, Shaobiao, Liming Zhao, Weinan Chen, Qicheng Zhang, Jiangyuan Ya, Wenji Zhong, Qianqian Shang, Jinji Tu, Hongtao Xiang, Jianqin Zhang, and et al. 2025. "Sowing Methods and Strigolactones Alleviate Damage to the Photosynthetic System of Rice Seedlings Under Salt Stress by Enhancing Antioxidant Capacity" Antioxidants 14, no. 8: 1020. https://doi.org/10.3390/antiox14081020
APA StyleDuan, S., Zhao, L., Chen, W., Zhang, Q., Ya, J., Zhong, W., Shang, Q., Tu, J., Xiang, H., Zhang, J., & Zhang, J. (2025). Sowing Methods and Strigolactones Alleviate Damage to the Photosynthetic System of Rice Seedlings Under Salt Stress by Enhancing Antioxidant Capacity. Antioxidants, 14(8), 1020. https://doi.org/10.3390/antiox14081020





