Association Between Melatonin Use and Cataract Risk: A Target Trial Emulation Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Data Source
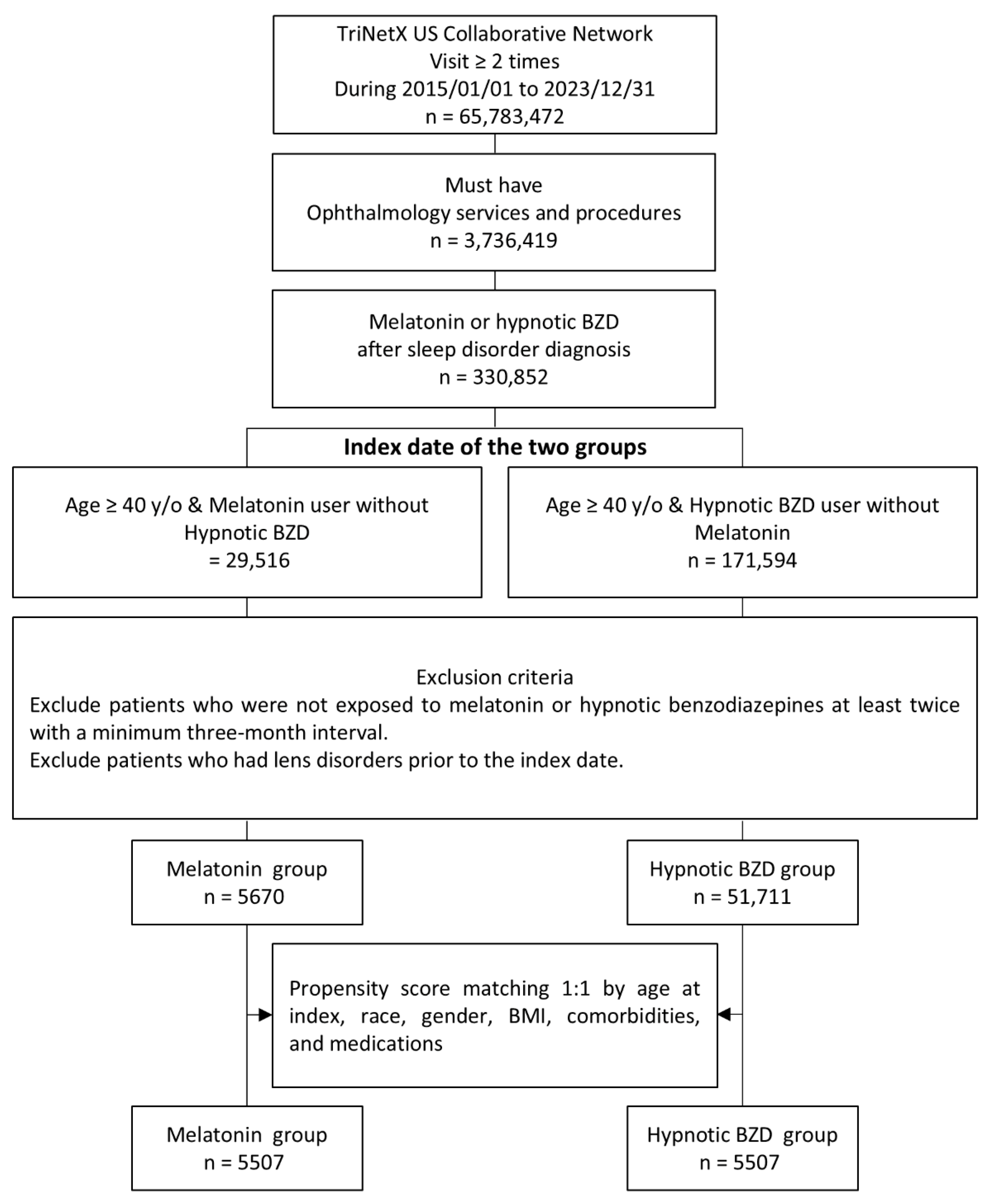
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Subjects
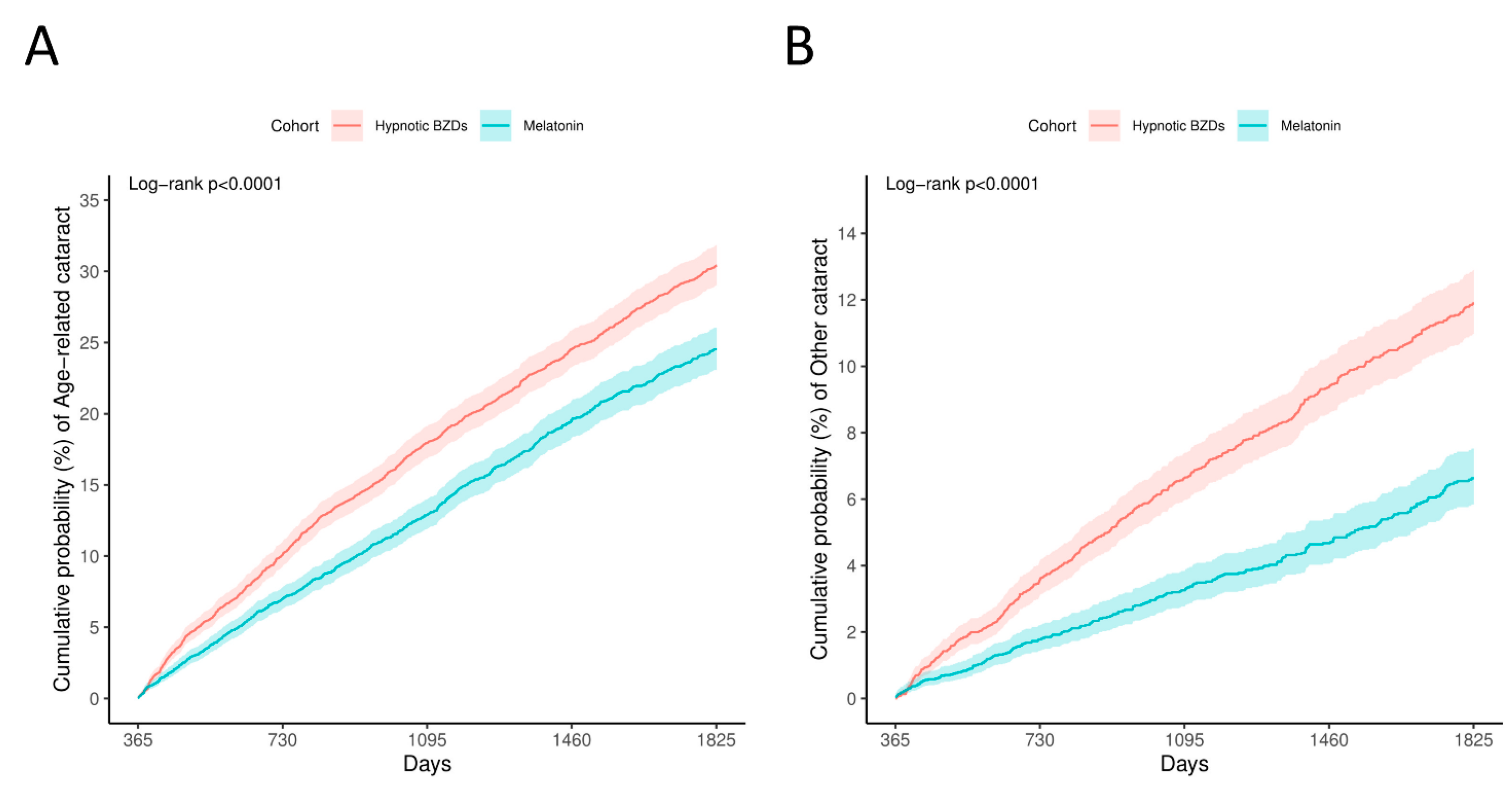
3.2. Primary Outcomes
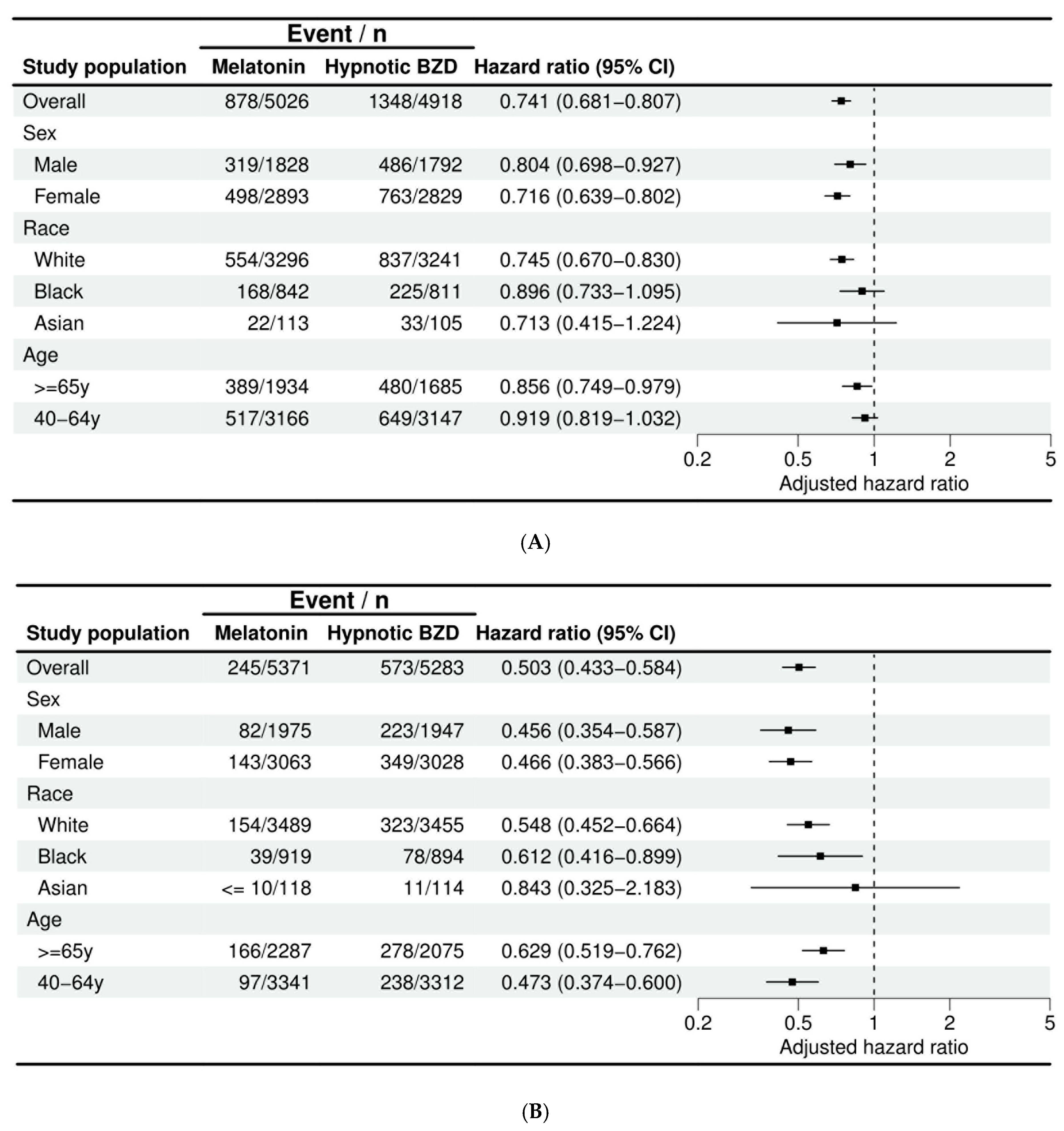
3.3. Subgroup Analysis
3.4. Secondary Outcomes
3.5. Additional Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, A.W.; Bressler, N.M.; Ffolkes, S.; Wittenborn, J.S.; Jorkasky, J. Public Attitudes About Eye and Vision Health. JAMA Ophthalmol. 2016, 134, 1111–1118. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Buchan, J.C.; Nicholson, M.; Varadaraj, V.; Khanna, R.C. Cataracts. Lancet 2023, 401, 377–389. [Google Scholar] [CrossRef]
- Bourne, R.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef] [PubMed]
- Assi, L.; Chamseddine, F.; Ibrahim, P.; Sabbagh, H.; Rosman, L.; Congdon, N.; Evans, J.; Ramke, J.; Kuper, H.; Burton, M.J.; et al. A Global Assessment of Eye Health and Quality of Life: A Systematic Review of Systematic Reviews. JAMA Ophthalmol. 2021, 139, 526–541. [Google Scholar] [CrossRef]
- Azizova, T.V.; Hamada, N.; Grigoryeva, E.S.; Bragin, E.V. Risk of various types of cataracts in a cohort of Mayak workers following chronic occupational exposure to ionizing radiation. Eur. J. Epidemiol. 2018, 33, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Hodge, W.G.; Whitcher, J.P.; Satariano, W. Risk factors for age-related cataracts. Epidemiol. Rev. 1995, 17, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, X.; Wang, Y.H.; Shi, X.; Wei, J.C. Hydroxychloroquine is neutral on incidental cataracts in patients with rheumatoid arthritis. Sci. Rep. 2023, 13, 5576. [Google Scholar] [CrossRef]
- Li, J.; Buonfiglio, F.; Zeng, Y.; Pfeiffer, N.; Gericke, A. Oxidative Stress in Cataract Formation: Is There a Treatment Approach on the Horizon? Antioxidants 2024, 13, 1249. [Google Scholar] [CrossRef]
- Meng, F.; Lu, S.; Li, L.; Qian, T.; Zhang, C.; Liu, X.; Hou, X. Different gender of oxidative balance score on the risk of rheumatoid arthritis in the US population from NHANES. Int. J. Rheum. Dis. 2024, 27, e15237. [Google Scholar] [CrossRef]
- Meng, F.; Lu, S.; Li, Y.; Zhang, C.; Kang, T.; Qian, T.; Tan, C.; Liu, X.; Hou, X. Association between oxidative balance score and risk of gout: The NHANES cross-sectional study, 2007–2018. Int. J. Rheum. Dis. 2024, 27, e15255. [Google Scholar] [CrossRef]
- Casparis, H.; Lindsley, K.; Kuo, I.C.; Sikder, S.; Bressler, N.M. Surgery for cataracts in people with age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 2, CD006757. [Google Scholar] [CrossRef]
- Li, J.; Somers, V.K.; Xu, H.; Lopez-Jimenez, F.; Covassin, N. Trends in Use of Melatonin Supplements Among US Adults, 1999–2018. JAMA 2022, 327, 483–485. [Google Scholar] [CrossRef]
- Rusciano, D.; Russo, C. The Therapeutic Trip of Melatonin Eye Drops: From the Ocular Surface to the Retina. Pharmaceuticals 2024, 17, 441. [Google Scholar] [CrossRef]
- Crooke, A.; Huete-Toral, F.; Colligris, B.; Pintor, J. The role and therapeutic potential of melatonin in age-related ocular diseases. J. Pineal Res. 2017, 63, e12430. [Google Scholar] [CrossRef]
- Lim, J.C.; Suzuki-Kerr, H.; Nguyen, T.X.; Lim, C.J.J.; Poulsen, R.C. Redox Homeostasis in Ocular Tissues: Circadian Regulation of Glutathione in the Lens? Antioxidants 2022, 11, 1516. [Google Scholar] [CrossRef]
- Mi, Y.; Wei, C.; Sun, L.; Liu, H.; Zhang, J.; Luo, J.; Yu, X.; He, J.; Ge, H.; Liu, P. Melatonin inhibits ferroptosis and delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. Biomed. Pharmacother. 2023, 157, 114048. [Google Scholar] [CrossRef]
- Bardak, Y.; Ozertürk, Y.; Ozgüner, F.; Durmuş, M.; Delibaş, N. Effect of melatonin against oxidative stress in ultraviolet-B exposed rat lens. Curr. Eye Res. 2000, 20, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Léveillard, T. Modulating antioxidant systems as a therapeutic approach to retinal degeneration. Redox Biol. 2022, 57, 102510. [Google Scholar] [CrossRef]
- Felder-Schmittbuhl, M.P.; Buhr, E.D.; Dkhissi-Benyahya, O.; Hicks, D.; Peirson, S.N.; Ribelayga, C.P.; Sandu, C.; Spessert, R.; Tosini, G. Ocular Clocks: Adapting Mechanisms for Eye Functions and Health. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4856–4870. [Google Scholar] [CrossRef] [PubMed]
- Ostrin, L.A. Ocular and systemic melatonin and the influence of light exposure. Clin. Exp. Optom. 2019, 102, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Felder-Schmittbuhl, M.P.; Hicks, D.; Ribelayga, C.P.; Tosini, G. Melatonin in the mammalian retina: Synthesis, mechanisms of action and neuroprotection. J. Pineal Res. 2024, 76, e12951. [Google Scholar] [CrossRef]
- Jeong, H.; Shaia, J.K.; Markle, J.C.; Talcott, K.E.; Singh, R.P. Melatonin and Risk of Age-Related Macular Degeneration. JAMA Ophthalmol. 2024, 142, 648–654. [Google Scholar] [CrossRef]
- Monnier, V.M.; Gao, Z.; Gorenflo, M.; Kaelber, D.C.; Xu, R. Melatonin suppresses the long-term risk of cataract surgery in diabetic individuals: An AI based study with clinical corroboration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2465. [Google Scholar]
- Hsu, A.Y.; Kuo, H.T.; Lin, C.J.; Hsia, N.Y.; Kuo, S.C.; Wei, C.C.; Lai, C.T.; Chen, H.S.; Wang, Y.H.; Wei, J.C.; et al. Cataract Development Among Pediatric Patients with Uveitis. JAMA Netw. Open 2024, 7, e2419366. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Hernán, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef]
- Li, H.; Zhou, B.; Wu, J.; Zhang, Y.; Zhang, W.; Doherty, M.; Deng, X.; Wang, N.; Xie, D.; Wang, Y.; et al. Melatonin is a potential novel analgesic agent for osteoarthritis: Evidence from cohort studies in humans and preclinical research in rats. J. Pineal Res. 2024, 76, e12945. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.L.; Richardson, D.B.; Stürmer, T. The active comparator, new user study design in pharmacoepidemiology: Historical foundations and contemporary application. Curr. Epidemiol. Rep. 2015, 2, 221–228. [Google Scholar] [CrossRef]
- Hung, C.H.; Wu, J.Y.; Weng, Y.S.; Hsiao, L.W.; Liu, Y.C.; Chiang, I.T. Impact of sodium-glucose cotransporter-2 inhibitors on ovarian cancer risk in patients with type 2 diabetes mellitus: A multi-institutional TriNetX study. Diabetes Res. Clin. Pract. 2025, 222, 112109. [Google Scholar] [CrossRef]
- Riemann, D.; Espie, C.A.; Altena, E.; Arnardottir, E.S.; Baglioni, C.; Bassetti, C.L.A.; Bastien, C.; Berzina, N.; Bjorvatn, B.; Dikeos, D.; et al. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J. Sleep Res. 2023, 32, e14035. [Google Scholar] [CrossRef] [PubMed]
- Stürmer, T.; Marquis, M.A.; Zhou, H.; Meigs, J.B.; Lim, S.; Blonde, L.; Macdonald, E.; Wang, R.; Lavange, L.M.; Pate, V.; et al. Cancer incidence among those initiating insulin therapy with glargine versus human NPH insulin. Diabetes Care 2013, 36, 3517–3525. [Google Scholar] [CrossRef] [PubMed]
- Arfè, A.; Corrao, G. The lag-time approach improved drug-outcome association estimates in presence of protopathic bias. J. Clin. Epidemiol. 2016, 78, 101–107. [Google Scholar] [CrossRef]
- Khorsand, M.; Akmali, M.; Sharzad, S.; Beheshtitabar, M. Melatonin Reduces Cataract Formation and Aldose Reductase Activity in Lenses of Streptozotocin-induced Diabetic Rat. Iran. J. Med. Sci. 2016, 41, 305–313. [Google Scholar]
- Karslioglu, I.; Ertekin, M.V.; Taysi, S.; Koçer, I.; Sezen, O.; Gepdiremen, A.; Koç, M.; Bakan, N. Radioprotective effects of melatonin on radiation-induced cataract. J. Radiat. Res. 2005, 46, 277–282. [Google Scholar] [CrossRef]
- Yağci, R.; Aydin, B.; Erdurmuş, M.; Karadağ, R.; Gürel, A.; Durmuş, M.; Yiğitoğlu, R. Use of melatonin to prevent selenite-induced cataract formation in rat eyes. Curr. Eye Res. 2006, 31, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Lledó, V.E.; Alkozi, H.A.; Sánchez-Naves, J.; Fernandez-Torres, M.A.; Guzman-Aranguez, A. Melatonin counteracts oxidative damage in lens by regulation of Nrf2 and NLRP3 inflammasome activity. Exp. Eye Res. 2022, 215, 108912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, H.; Cai, Y.; Yoshida, S.; Li, Y.; Zhou, Y. Melatonin: Unveiling the functions and implications in ocular health. Pharmacol. Res. 2024, 205, 107253. [Google Scholar] [CrossRef]
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Melatonin | Hypnotic BZD | SMD | Melatonin | Hypnotic BZD | SMD | |
| N | 5670 | 51711 | 5507 | 5507 | ||
| Age at index (year) | 61.3 ± 12.6 | 57.2 ± 9.5 | 0.3675 | 60.9 ± 12.5 | 60.3 ± 10.1 | 0.0527 |
| Female sex, n (%) | 3417 (60.3%) | 29,412 (56.9%) | 0.0688 | 3327 (60.4%) | 3462 (62.9%) | 0.0504 |
| Race, n (%) | ||||||
| White | 3784 (66.7%) | 35,959 (69.5%) | 0.0601 | 3673 (66.7%) | 3821 (69.4%) | 0.0577 |
| Black or African American | 911 (16.1%) | 8336 (16.1%) | 0.0015 | 890 (16.2%) | 813 (14.8%) | 0.0387 |
| Asian | 140 (2.5%) | 907 (1.8%) | 0.0498 | 135 (2.5%) | 120 (2.2%) | 0.0181 |
| Body mass index (BMI), n (%) | ||||||
| <30 | 2459 (43.4%) | 17,608 (34.1%) | 0.1922 | 2350 (42.7%) | 2410 (43.8%) | 0.0220 |
| 30–40 | 1855 (32.7%) | 20,132 (38.9%) | 0.1299 | 1827 (33.2%) | 1823 (33.1%) | 0.0015 |
| ≧40 | 747 (13.2%) | 9627 (18.6%) | 0.1493 | 745 (13.5%) | 704 (12.8%) | 0.0220 |
| Lifestyle or environmental factor, n (%) | ||||||
| Nicotine dependence | 757 (13.4%) | 6440 (12.5%) | 0.0268 | 746 (13.5%) | 726 (13.2%) | 0.0107 |
| Alcohol related disorders | 390 (6.9%) | 1507 (2.9%) | 0.1845 | 376 (6.8%) | 396 (7.2%) | 0.0142 |
| Comorbidity, n (%) | ||||||
| Diseases of the circulatory system | 3952 (69.7%) | 36,861 (71.3%) | 0.0347 | 3829 (69.5%) | 3695 (67.1%) | 0.0523 |
| Hypertensive diseases | 3354 (59.2%) | 31,487 (60.9%) | 0.0355 | 3252 (59.1%) | 3094 (56.2%) | 0.0581 |
| Diabetes mellitus | 1723 (30.4%) | 15,591 (30.2%) | 0.0052 | 1678 (30.5%) | 1600 (29.1%) | 0.0310 |
| Type 2 diabetes mellitus with ophthalmic complications | 145 (2.6%) | 1210 (2.3%) | 0.0141 | 142 (2.6%) | 118 (2.1%) | 0.0287 |
| Cerebrovascular diseases | 609 (10.7%) | 3294 (6.4%) | 0.1567 | 574 (10.4%) | 591 (10.7%) | 0.0100 |
| Diseases of arteries, arterioles and capillaries | 518 (9.1%) | 4591 (8.9%) | 0.0090 | 500 (9.1%) | 491 (8.9%) | 0.0057 |
| Neoplasms | 1238 (21.8%) | 18,424 (35.6%) | 0.3084 | 1216 (22.1%) | 1158 (21.0%) | 0.0256 |
| Insomnia | 2126 (37.5%) | 11,231 (21.7%) | 0.3509 | 2036 (37.0%) | 2086 (37.9%) | 0.0188 |
| Anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders | 2016 (35.6%) | 13,562 (26.2%) | 0.2029 | 1939 (35.2%) | 1993 (36.2%) | 0.0205 |
| Depressive episode | 1760 (31.0%) | 12,760 (24.7%) | 0.1423 | 1679 (30.5%) | 1662 (30.2%) | 0.0067 |
| Epilepsy and recurrent seizures | 217 (3.8%) | 1057 (2.0%) | 0.1058 | 208 (3.8%) | 185 (3.4%) | 0.0225 |
| Parkinson’s disease | 247 (4.4%) | 224 (0.4%) | 0.2587 | 159 (2.9%) | 164 (3.0%) | 0.0054 |
| Alzheimer’s disease | 108 (1.9%) | 48 (0.1%) | 0.1830 | 46 (0.8%) | 40 (0.7%) | 0.0124 |
| Medications, n (%) | ||||||
| Benzodiazepine related drugs | 691 (12.2%) | 6722 (13.0%) | 0.0245 | 685 (12.4%) | 683 (12.4%) | 0.0011 |
| Other hypnotics and sedatives | 113 (2.0%) | 3960 (7.7%) | 0.2667 | 112 (2.0%) | 106 (1.9%) | 0.0078 |
| Event Number | |||
|---|---|---|---|
| Outcome | Melatonin Cohort (n = 5507) | Hypnotic BZD Cohort (n = 5507) | Hazard Ratio (95% CI) |
| Age-related cataract | 876 | 1348 | 0.741, (0.681, 0.807) |
| Other cataract | 245 | 573 | 0.503, (0.433, 0.584) |
| Traumatic cataract | ≤10 | ≤10 | 0.840, (0.140, 5.045) |
| Complicated cataract | ≤10 | ≤10 | 0.890, (0.148, 5.339) |
| Drug induced cataract | 0 | ≤10 | NA |
| Secondary cataract | 62 | 178 | 0.428, (0.321, 0.572) |
| Other specified cataract | 27 | 41 | 0.804, (0.495, 1.309) |
| Unspecified cataract | 175 | 418 | 0.498, (0.417, 0.594) |
| Negative outcome | |||
| Urinary tract infection | 284 | 391 | 0.962, (0.825, 1.121) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, C.-H.; Huang, J.-Y.; Hung, Y.-C.; Hsu, M.-Y.; Wei, J.C.-C. Association Between Melatonin Use and Cataract Risk: A Target Trial Emulation Retrospective Cohort Study. Antioxidants 2025, 14, 1016. https://doi.org/10.3390/antiox14081016
Hung C-H, Huang J-Y, Hung Y-C, Hsu M-Y, Wei JC-C. Association Between Melatonin Use and Cataract Risk: A Target Trial Emulation Retrospective Cohort Study. Antioxidants. 2025; 14(8):1016. https://doi.org/10.3390/antiox14081016
Chicago/Turabian StyleHung, Cheng-Hsien, Jing-Yang Huang, Yu-Chien Hung, Min-Yen Hsu, and James Cheng-Chung Wei. 2025. "Association Between Melatonin Use and Cataract Risk: A Target Trial Emulation Retrospective Cohort Study" Antioxidants 14, no. 8: 1016. https://doi.org/10.3390/antiox14081016
APA StyleHung, C.-H., Huang, J.-Y., Hung, Y.-C., Hsu, M.-Y., & Wei, J. C.-C. (2025). Association Between Melatonin Use and Cataract Risk: A Target Trial Emulation Retrospective Cohort Study. Antioxidants, 14(8), 1016. https://doi.org/10.3390/antiox14081016









