Expression of Free Radicals and Reactive Oxygen Species in Endometriosis: Current Knowledge and Its Implications
Abstract
1. Introduction
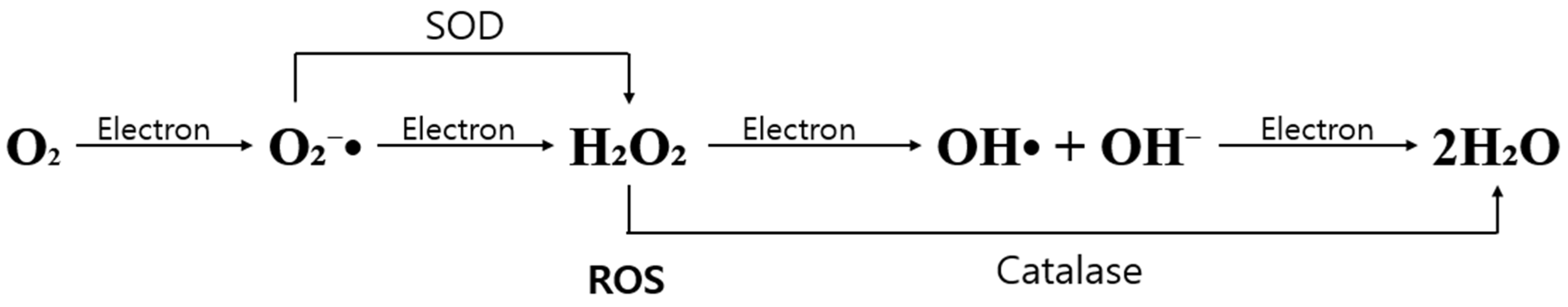
2. Production and Role of Reactive Oxygen Species in Various Diseases
Reactive Oxygen Species
- Oxidizes DNA, leading to the formation of DNA adducts
- Oxidizes lipids, generating lipid peroxides (LPOs), which contribute to cellular toxicity
3. Methods
4. Role of Free Radicals and ROS in Endometriosis
4.1. Studies Indicating That Increased ROS and Free Radicals Contribute to the Pathogenesis of Endometriosis
4.1.1. Studies on ROS and RNS in Endometriosis
Hydrogen Peroxide H2O2
Hydrogen Peroxide (H2O2), Ferric-Orange Xylenol (FOX), Malondialdehyde (MDA), Glutathione (GSH), and Oxidized Glutathione (GSSG)
Lipid Peroxides
Oxidatively Modified Lipid–Protein Complexes
Production of ROS
Free Oxygen Radicals (FORT) and Free Oxidant Radical Defense (FORD)
O2− + SOD + Catalase + GSH
Free Radical or Non-Enzymatically Derived 8-Isoprostane
4.1.2. Studies on Polymorphisms Related to ROS and Free Radicals in Endometriosis
4.1.3. Studies in Which the Use of ROS Scavenging and Detoxifying Enzymes Led to a Reduction in ROS or Free Radicals, Resulting in Symptom Improvement or Therapeutic Effects in Endometriosis Patients (Table 2)
Mitochondrial Superoxide Dismutase 2
| Enzyme | Reaction Catalyzed |
|---|---|
| Catalase | 2H2O2 → 2H2O + O2 |
| Cytochrome c peroxidase | complex + 2cyt-c(Fe2+) → enzyme + 2cyt-c(Fe2+) + 2OH− |
| Glutathione peroxidase | H2O2 + 2GSH → GSSG + 2H2O LOOH + 2GSH → GSSG +H2O + LOH |
| Glutathione S-transferase | RX + GSH → RSG + HX |
| Glutathione reductase | NADPH + GSSG ⇄ NADP+ + 2GSH |
| Superoxide dismutase | O2•− + O2•− + 2H+ → 2H2O2 + O2 |
| Thioredoxin | Trx-(SH)2 + Protein-S2 ⇄ Trx-S2 + Protein-(SH)2 |
Glutathionylated Protein (GSSP), Total GSH, and Carbonic Anhydrase
SOD Activity, Total Antioxidant Status, GPx Activity, and LPO
4.1.4. Studies in Which the Use of Inhibitors Targeting ROS and Free Radicals Led to an Improvement in Endometriosis (Table 3)
Macrophages
| Regulator Type | Agents | Effects |
|---|---|---|
| ROS inhibitors | ERK HIF-2α NRROS Nrf2 PKM2 PGC-1α Ucp2 Vitamin C/E, FHC | Reduce oxidative damage to cardiac cells ROS homeostasis Reduce tissue damage Limit ROS production in tumors Reduce oxidative damage in lung cancer cells Activate antioxidant enzymes Limit inflammation and ROS production in macrophages Suppress ROS accumulation |
| ROS promotors | EST-1 MMP-3 NOX2 P66SHC TNF TLR 1,2,4 UPBEAT1 | Increase ROS generation DNA damage and genomic instability Increase mitochondrial ROS production Increase mitochondrial ROS as an apoptosis signal Enhance macrophage killing and necroptosis Increase ROS generation in macrophages Change cells from proliferation to differentiation |
Vitamins
Genistein
Astaxanthin
miR-455 Targets FABP4
Cerium Oxide Nanoparticles (Nanoceria)
Osteopontin
Sphingosine 1-Phosphate
CAT and NAC
Peripheral Antioxidant Markers
4.2. Studies Suggesting That ROS Are Not Associated with the Pathogenesis of Endometriosis
5. Summary
- (1)
- The primary ROS involved in the pathogenesis of endometriosis is hydrogen peroxide (H2O2), LPOs, and superoxide radicals.
- (2)
- Polymorphism-related studies in endometriosis associated with oxidative stress included genes such as AT-rich interactive domain 1A (ARID1A) and quinone oxidoreductase 1 (NQO1) isoforms.
- (3)
- In studies showing symptom improvement or therapeutic effects in endometriosis patients through the reduction in ROS and/or free radicals, the ROS scavenging and detoxifying enzymes used included SOD, GSH, and GPx.
- (4)
- In studies where inhibitors of ROS and free radicals led to improvement in endometriosis, the agents used included vitamins C and E, astaxanthin, FABP4, cerium oxide nanoparticles (nanoceria), osteopontin, S1P, NAC, CAT, and high antioxidant diets.
- (5)
- In the studies that found no association between ROS and the pathogenesis of endometriosis, the substances examined were lipid peroxidation markers, including MDA, MDA with copper addition, and cholest-3,5-dien-7-one. These markers showed no variations according to the stage of endometriosis and were not associated with reduced fertility in patients with endometriosis.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sampson, J.A. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the pelvic cavity. Am. J. Obs. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Dinulescu, D.M.; Ince, T.A.; Quade, B.J. Role of Kras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat. Med. 2005, 11, 63–70. [Google Scholar] [CrossRef]
- Levander, G.; Normann, P. The pathogenesis of endometriosis: An experimental study. Acta Obs. Gynecol. Scand. 1955, 34, 366–398. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.; Vermeulen, N.; Becker, C. European Society of Human Reproduction and Embryology. ESHRE guideline for the management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Koninckx, P.R.; Piazze, J.; Natili, M.; Colagrande, S.; Cosmi, E.V. Correlation between endometriosis and pelvic pain. J. Am. Assoc. Gynecol. Laparosc. 1999, 6, 429–434. [Google Scholar] [CrossRef]
- Hadfield, R.; Mardon, H.; Barlow, D.; Kennedy, S. Delay in the diagnosis of endometriosis: A survey of women from the USA and the UK. Hum. Reprod. 1996, 11, 878–880. [Google Scholar] [CrossRef]
- May, K.E.; Conduit-Hulbert, S.A.; Villar, J.; Kirtley, S.; Kennedy, S.H.; Becker, C.M. Peripheral biomarkers of endometriosis: A systematic review. Hum. Reprod. Update 2010, 16, 651–674. [Google Scholar] [CrossRef]
- Abrao, M.S.; Podgaec, S.; Pinotti, J.A.; de Oliveira, R.M. Tumor markers in endometriosis. Int. J. Gynaecol. Obs. 1999, 66, 19–22. [Google Scholar] [CrossRef]
- Kim, H.; Cho, S.H. Diagnosis and treatment of endometriosis. J. Korean Med. Assoc. 2019, 62, 513–518. [Google Scholar] [CrossRef]
- Gambone, J.C.; Mittman, B.S.; Munro, M.G.; Scialli, A.R.; Winkel, C.A. Consensus statement for the management of chronic pelvic pain and endometriosis: Proceedings of an expert-panel consensus process. Fertil. Steril. 2002, 78, 961–972. [Google Scholar] [CrossRef]
- Farquhar, C.; Sutton, C. The evidence for the management of endometriosis. Curr. Opin. Obs. Gynecol. 1998, 10, 321–332. [Google Scholar] [CrossRef]
- Taverne, Y.J.; Merkus, D.; Bogers, A.J.; Halliwell, B.; Duncker, D.J.; Lyons, T.W. Reactive Oxygen Species: Radical Factors in the Evolution of Animal Life: A molecular timescale from Earth’s earliest history to the rise of complex life. Bioessays 2018, 40, 1700158. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Defrère, S.; Van Langendonckt, A.; Vaesen, S.; Jouret, M.; González Ramos, R.; Gonzalez, D.; Donnez, J. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum. Reprod. 2006, 21, 2810–2816. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, K.; Luo, F. Reactive oxygen species(ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Lushchack, V. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- D’Autreaux, B.; Toledo, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Yeo, S.G.; Jeong, M.H. Generation and action of reactive oxygen species. Biochem. Mol. Biol. News 2005, 12, 10–15. [Google Scholar]
- Young, I.S. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yon, D.K.; Choi, Y.S.; Lee, J.; Yeo, J.H.; Kim, S.S.; Lee, J.M. Induction of Nitric Oxide and Its Role in Facial Nerve Regeneration According to the Method of Facial Nerve Injury. Antioxidants 2024, 13, 741. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, W.Y. Physical activity and reactive oxygen. Health Sports Med. 2007, 9, 21–31. [Google Scholar]
- Jacob, R.A.; Burri, B.J. Oxidative damage and defense. Am. J. Clin. Nutr. 1996, 63, 985S–990S. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K. A Study on Skin Aging Caused by Free-Radical and on Efficacy of Antioxidant Vitamins. Asian J. Beauty Cosmetol. 2009, 7, 51–62. [Google Scholar]
- Kang, S.W. Role of Reactive Oxygen Species in Cell Death Pathways. Hanyang Med. Rev. 2013, 33, 77–82. [Google Scholar] [CrossRef]
- Andrade, S.S.; Azevedo, A.d.C.; Monasterio, I.C.; Paredes-Gamero, E.J.; Gonçalves, G.A.; Bonetti, T.C.; Albertoni, G.; Schor, E.; Barreto, J.A.; Luiza Oliva, M.; et al. 17β-Estradiol and steady-state concentrations of H2O2: Antiapoptotic effect in endometrial cells from patients with endometriosis. Free Radic. Biol. Med. 2013, 60, 63–72. [Google Scholar] [CrossRef]
- Veal, E.; Day, A. Hydrogen peroxide as a signaling molecule. Antioxid. Redox Signal. 2011, 15, 147–151. [Google Scholar] [CrossRef]
- Urata, Y.; Ihara, Y.; Murata, H.; Goto, S.; Koji, T.; Yodoi, J.; Inoue, S.; Kondo, T. 17 Beta-estradiol protects against oxidative stress-induced cell death through the glutathione/glutaredoxin-dependent redox regulation of Akt in myocardiac H9c2 cells. J. Biol. Chem. 2006, 281, 13092–13102. [Google Scholar] [CrossRef]
- Shanti, A.; Santanam, N.; Morales, A.J.; Parthasarathy, S.; Murphy, A.A. Autoantibodies to markers of oxidative stress are elevated in women with endometriosis. Fertil. Steril. 1999, 71, 1115–1118. [Google Scholar] [CrossRef]
- Yi, L.; Lilan, L.; Haibo, Z. Levels of Lipid Perioxides and Superoxide Dismutase in Peritoneal Fluid if Patients with Endometriosis. J. Tongi Med. Univ. 2001, 21, 166–167. [Google Scholar] [CrossRef]
- Murphy, A.A.; Palinski, W.; Rankin, S.; Morales, A.J.; Parthasarathy, S. Evidence for oxidatively modified lipid-protein complexes in endometrium and endometriosis. Fertil. Steril. 1998, 69, 1092–1094. [Google Scholar] [CrossRef]
- Malvezzi, H.; Cestari, B.A.; Meola, J.; Podgaec, S. Higher Oxidative Stress in Endometriotic Lesions Upregulates Senescence-Associated p16ink4a and β-Galactosidase in Stromal Cells. Int. J. Mol. Sci. 2023, 24, 914. [Google Scholar] [CrossRef]
- Wang, Y.; Goldberg, J.; Sharma, R.K.; Agarwal, A.; Falcone, T. Importance of reactive oxygen species in the peritoneal fluid of women with endometriosis or idiopathic infertility. Fertil. Steril. 1997, 66, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Biasioli, A.; Xholli, A.; Previtera, F.; Balanzo, A.; Capodicasa, V.; Tassi, A.; Londero, A.P.; Cagnacci, A. Systemic Oxidative Stress in Women with Ovarian and Pelvic Endometriosis: Role of Hormonal Therapy. J. Clin. Med. 2022, 11, 7460. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.; Chereau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive Oxygen Species Controls Endometriosis Progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef]
- Ray, K.; Fahrmann, J.; Mitchell, B.; Paul, D.; King, H.; Crain, C.; Cook, C.; Golovko, M.; Brose, S.; Golovko, S.; et al. Oxidation Sensitive Nociception Involved in Endometriosis Associated Pain. Pain 2015, 156, 528–539. [Google Scholar] [CrossRef]
- Hevir, N.; Ribic-Pucelj, M.; Rizner, T.L. Disturbed balance between phase I and II metabolizing enzymes in ovarian endometriosis: A source of excessive hydroxy-estrogens and ROS? Mol. Cell. Endocrinol. 2013, 367, 74–84. [Google Scholar] [CrossRef]
- Chen, H.X.P.; Huang, H.W.; Liu, L.P.; Zhao, L.F. Reactive oxygen species downregulate ARID1A expression via its promoter methylation during the pathogenesis of endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4509–4515. [Google Scholar]
- Chen, C.; Zhou, Y.; Hu, C.; Wang, Y.; Yan, Z.; Li, Z.; Wu, R. Mitochondria and oxidative stress in ovarian endometriosis. Free Radic. Biol. Med. 2019, 136, 22–34. [Google Scholar] [CrossRef]
- Kim, K.S. The role of antioxidants in the treatment of aging. In Proceedings of the Conference of the Korean Society of Clinical Geriatrics; 2004; pp. 379–392. [Google Scholar]
- Addor, F.A.S. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef]
- Andrisani, A.; Dona, G.; Brunati, A.M.; Clari, G.; Armanini, D.; Ragazzi, E.; Ambrosini, G.; Bordin, L. Increased oxidation-related glutathionylation and carbonic anhydrase activity in endometriosis. Reprod. Biomed. Online 2014, 28, 773–779. [Google Scholar] [CrossRef][Green Version]
- Szczepanska, M.; Kozlik, J.; Skrzypczak, J.; Mikolajczyk, M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil. Steril. 2003, 79, 1288–1293. [Google Scholar] [CrossRef]
- Ogawa, K.; Liu, T.; Kawahara, N.; Kobayashi, H. Macrophages Protect Endometriotic Cells Against Oxidative Damage Through a Cross-Talk Mechanism. Reprod. Sci. 2022, 29, 2165–2178. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Wang, M.; Cheng, W. Effects of vitamin C on the outcome of in vitro fertilization-embryo transfer in endometriosis: A randomized controlled study. J. Int. Med. Res. 2018, 46, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Quesada, J.F.; Cambero, O.; Pacheco, A.; Pellicer, A.; Codoceo, R.; Garcia-Velasco, J.A. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012, 98, 126–130. [Google Scholar] [CrossRef]
- Santanam, N.; Kavtaradze, N.; Murphy, A.; Dominguez, C.; Parthasarathy, S. Antioxidant Supplementation Reduces Endometriosis Related Pelvic Pain in Humans. Trans. Res. 2012, 161, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, J.; Genera-Garcia, M.; Jara-Diaz, J.D.; Perichart-Perera, O.; Vadillo-Ortega, F.; Hernandez-Guerrero, C. Effect of vitamins C and E supplementation on peripheral oxidative stress markers and pregnancy rate in women with endometriosis. Int. J. Gynaecol. Obs. 2008, 100, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 2021, 5529741. [Google Scholar] [CrossRef]
- Sutrisno, S.; Miryani, I.; Dwijayasa, P.M.; Suprobo, N.R.; Wiyasa, I.W.A. Genistein administration increases the level of superoxide dismutase and glutathione peroxidase in the endometriosis mice model: An experimental study. Int. J. Reprod. Biomed. 2022, 20, 873–882. [Google Scholar] [CrossRef]
- Rostami, S.; Alyasin, A.; Saedi, M.; Nekoonam, S.; Khodarahmian, M.; Moeini, A.; Amidi, F. Astaxanthin ameliorates inflammation, oxidative stress, and reproductive outcomes in endometriosis patients undergoing assisted reproduction: A randomized, triple-blind placebo-controlled clinical trial. Front. Endocrinol. 2023, 14, 1144323. [Google Scholar] [CrossRef]
- Tang, W.; Chen, O.; Yao, F.; Cui, L. miR-455 targets FABP4 to protect human endometrial stromal cells from cytotoxicity induced by hydrogen peroxide. Mol. Med. Rep. 2019, 20, 4781–4790. [Google Scholar] [CrossRef]
- Chaudhury, K.; Babu, K.N.; Singh, A.K.; Das, S.; Kumar, A.; Seal, S. Mitigation of endometriosis using regenerative cerium oxide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Wu, M.; Lu, J.; Duan, P. Targeting osteopontin alleviates endometriosis and inflammation by inhibiting the RhoA/ROS axis and achieves non-invasive in vitro detection via menstrual blood. Hum. Reprod. 2024, 39, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Seidita, I.; Tusa, I.; Prisinzano, M.; Menconi, A.; Cencetti, F.; Vannuccini, S.; Castiglione, F.; Bruni, P.; Petraglia, F.; Bernacchioni, C.; et al. Sphingosine 1-phosphate elicits a ROS-mediated proinflammatory response in human endometrial stromal cells via ERK5 activation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2023, 37, e23061. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hu, H.; Yang, Y.; Li, S. The inhibition of reactive oxygen species (ROS) by antioxidants inhibits the release of an autophagy marker in ectopic endometrial cells. Taiwan J. Obstet. Gynecol. 2020, 59, 256–261. [Google Scholar] [CrossRef]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Méndez, S.; Jiménez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernández-Guerrero, C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef]
- Arumugam, K.; Dip, Y.C. Endometriosis and infertility: The role of exogenous lipid peroxides in the peritoneal fluid. Fertil. Steril. 1995, 63, 198–199. [Google Scholar] [CrossRef]
- do Amaral, V.F.; Bydlowski, S.P.; Peranovich, T.C.; Navarro, P.A.; Subbiah, M.T.; Ferriani, R.A. Lipid peroxidation in the peritoneal fluid of infertile women with peritoneal endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 119, 72–75. [Google Scholar] [CrossRef]


| Reactive Oxygen Species | Reactive Nitrogen Species | |||
|---|---|---|---|---|
| Free Radicals | O2− HO2− H2O2 HO− 1O2 OCl− O3 | Superoxide radical (or anion) Perhydroxyl radical Hydrogen peroxide Hydroxyl radical Singlet oxygen Hypochlorite Ozone | NO NO2 | Nitric oxide Nitrogen dioxide |
| Non-Free Radicals | H2O2 HOCl 1O2 | Hydrogen peroxide Hypochlorous acid Singlet oxygen | ONOO NO2− NO3− | Peroxynitrite Nitrite Nitrate |
| Author/ Year/ Reference | Study Design | Species and/or Sample | Detection Method | Target Substances | Results/Conclusions |
|---|---|---|---|---|---|
| Andrade et al., 2013 [26] | In vitro | Tissue samples were collected from 7 patients without endometriosis as controls and 11 patients with endometriosis who were reported to suffer from chronic pelvic pain. | Tissue isolation and cell culture, ferric-xylenol orange assay, ultraviolet spectroscopy, lactate dehydrogenase assay, Western blot analysis | H2O2, catalase | When endometrial cells were pretreated with E2 and [H2O2]ss, a decrease in apoptosis was observed compared to control cells (p < 0.01). Endometrial cells from patients with endometriosis subjected to both E2 and [H2O2]ss showed increased ERK phosphorylation. /These findings suggest that H2O2 is a signaling molecule that downregulates apoptosis in endometrial cells, supporting the fact that endometriosis, although a benign disease, shares features with cancer, such as decreased catalase levels. These results link the effects of E2 on [H2O2]ss to resistance to apoptosis and progression of endometriosis. |
| Shanti et al., 1999 [29] | Prospective study | Blood samples were collected from 40 patients aged 18–45 years undergoing laparoscopy or laparotomy. | ELISA | LPO, MDA, oxidized LDL | Mean serum autoantibody titers to the three antigens were as follows: (1) LPO-modified rabbit serum albumin, 0.49 ± 0.12 units in endometriosis patients and 0.2 ± 0.02 units in the controls; (2) oxidized low-density lipoprotein, 0.22 ± 0.005 units in endometriosis patients and 0.18 ± 0.006 units in controls; and (3) malondialdehyde-modified low-density protein, 0.21 ± 0.005 units in endometriosis patients and 0.16 ± 0.003 units in controls. /Autoantibodies to markers of oxidative stress were significantly increased in women with endometriosis. These findings strongly suggest that women with endometriosis have enhanced oxidative stress. |
| Yi et al., 2001 [30] | Comparative study | Peritoneal fluid was collected from 30 infertile women undergoing diagnostic laparoscopy, including 15 patients with endometriosis and 15 control subjects. | ELISA | Lipid peroxide, superoxide dismutase | The level of LPO in peritoneal fluid from patients with endometriosis was significantly higher than that of infertile women with normal pelvises (p < 0.01). The SOD level of peritoneal fluid showed no significant difference between the two groups (p > 0.05). /LPO is abundant and participates in the pathogenesis of endometriosis through cytotoxicity, inflammatory response, and adhesion formation. |
| Murphy et al., 1998 [31] | Controlled clinical study | Five women with endometriosis had laparoscopic resection of endometriosis and underwent biopsy. Five controls underwent endometrial biopsy in the follicular phase of the cycle (endometrial tissue sample). | Immunocytochemistry | Oxidatively modified lipid proteins (HNE-7, MDA2), macrophage (HAM-56), muscle cell actin (HHF-35) | Both endometrium and endometriosis tissues contained stromal cells that immunostained with HAM-56 and showed immunostaining (both intracellular and extracellular) with HNE-7 and MDA2. Some endometriotic implants showed patchy staining with HHF-35. The endometrium was devoid of staining with HHF-35. When stained with nonimmune sera as a control, both tissues were devoid of staining. /These data strongly indicate the presence of oxidative stress in endometriotic tissue. Ectopic endometrium may be a possible source of oxidized lipid proteins in the peritoneal cavity by diffusion or as a result of induction by tissue macrophages. |
| Malvezzi et al., 2023 [32] | Cross-sectional pilot study | Endometrial tissue was collected from 53 patients who were divided into two groups: 33 patients with endometriosis and 20 patients without endometriosis. | FOX assay, protein quantitation, ELISA, IHC, GSH and GSSG absorption assay, stromal cell culture, immunofluorescence, β-galactosidase assay | MDA, H2O2 | Higher oxidative damage was observed in endometriotic lesions than in eutopic endometrium. Concentration assays suggested 0.25 mM to be the ideal H2O2 stimulus, as there was a significant drop (p < 0.05) in cell viability and proliferation when going to higher concentrations. H2O2-treated stromal cells increased the relative expression of p16ink4a. /These results indicate the presence of higher ROS levels in endometriotic lesions and the upregulation of MAPK. In addition, endometriotic lesions in stromal cells stimulated with hydrogen peroxide developed more senescence traits than eutopic and non-endometriosis endometrium. |
| Wang et al., 1997 [33] | Prospective study | Peritoneal fluid was collected from women with endometriosis (n = 15) or idiopathic infertility (n = 11) who underwent laparoscopy for infertility, and from patients undergoing tubal ligation who served as controls (n = 13). | Peroxidase staining test, chemiluminescence | PMN granulocyte, macrophage, ROS | ROS were present in the peritoneal fluid of patients with endometriosis, idiopathic infertility, and tubal ligation. ROS levels did not differ significantly between patients with endometriosis and the control group in unprocessed peritoneal fluid, but did differ significantly between patients with idiopathic infertility and controls in processed peritoneal fluid. /A lack of antioxidant enzymes or low antioxidant capacity may be responsible for the observed increase in ROS levels. |
| Biasioli et al., 2022 [34] | Prospective cohort study | Capillary blood samples were collected from 24 women without endometriosis, 26 women with endometrioma, and 26 women with deep infiltrating endometriosis (DIE), with or without endometrioma. | Colorimetric analysis, spectrophotometer, | Free oxygen radicals (FORT), free oxidant radical defense (FORD) | Women were prescribed contraceptive hormones, and the baseline assessments were repeated at the third month of use, revealing a higher oxidative stress balance (FORT/FORD) in women with endometriosis than in controls (p = 0.05). Regression analysis revealed an independent link between FORT/FORD and endometrioma (p = 0.027) and DIE (p = 0.001) but a negative correlation with HDL-cholesterol (p = 0.043). In women with endometriosis, FORT remained unchanged, but FORD increased (p = 0.004), and the FORT/FORD ratio significantly decreased to values similar to the control levels (p = 0.002). /The results indicate that women with endometriosis, particularly those with DIE, have increased systemic oxidative stress, as assessed by the ratio of free oxygen radicals (FORT) to antioxidants (FORD). The greater oxidative stress seen in these women is improved by the administration of estradiol-based hormonal contraceptives. |
| Ngo et al., 2009 [35] | In vitro, animal study | Endometrium and ovarian endometrioma specimens were obtained from 14 patients with endometriosis undergoing surgical treatment. Twenty-eight 8-week-old female nude mice were studied. | Cell culture, ultraviolet spectroscopy, in vitro cell proliferation and viability assay, immunoblotting | Superoxide anion, hydrogen peroxide, SOD, GSH, CAT | The production of O2− was increased by 39% in stromal endometriotic cells from patients (p < 0.05) and by 35% in stromal endometrial cells (p < 0.05) compared with stromal control cells. SOD activity was three-fold higher in stromal endometriotic cells (p < 0.01) and 2.25-fold higher in stromal endometrial cells from patients (p < 0.05) than in stromal control cells. In epithelial endometrial cells, production of hydrogen peroxide was 7.75-fold higher than in control cells (p < 0.0001) and 3.1-fold higher than in epithelial endometrial cells (p < 0.001). Catalase activity was 2.7-fold lower in stromal endometriotic cells than in control cells (p < 0.01) and 1.8-fold lower than in stromal endometrial cells (p < 0.01). Catalase activity was three-fold lower in epithelial endometriotic cells (p < 0.01) and 1.5-fold lower in epithelial endometrial cells (p < 0.05) compared with control cells. The level of GSH was higher in stromal endometriotic cells than in stromal control cells, and higher in epithelial endometriotic cells compared with epithelial control cells. /This work showed that endometriotic cells from patients with endometriosis have an altered phenotype of ROS production, leading to an increase in the proliferative capabilities of cells. |
| Ray et al., 2015 [36] | Human study, animal study | Peritoneal fluid was collected from women aged 18–60 years undergoing tubal ligation or laparoscopy for endometriosis (50 women per group). Additionally, eight CD-1 mice and Sprague–Dawley rats were used for the study. | Limulus amebocyte lysate assay, LC-MS/MS, spectrophotometry, agarose gel electrophoresis, enzyme immunoassay, body temperature assay, Hargreaves paw withdrawal pain assay | Prostaglandins, lipoproteins | Increased levels of non-enzymatically derived 8-isoprostanes (p = 0.005) were observed in the PF of women with endometriosis compared to controls. Levels of 12,15- or 15-lipoxygenase derived eicosanoids such as 12,15-HETEs (p < 0.05) and 5-HETEs, and cyclooxygenase derived eicosanoids such as PGE2 and PGD2, were higher in endometriosis patients than in control subjects. LC-MS/MS studies confirmed that non-enzymatic oxidation of LDL generates prostaglandin-like molecules. In the animal study, oxidatively modified lipoproteins induced pain-related behavior (significant reductions in withdrawal latencies, indicative of increased pain sensitivity). /This study demonstrated that non-enzymatic oxidatively modified lipoproteins are similar to prostaglandins in their ability to modulate body temperature, induce nociception, and alter the expression of inflammatory and nociceptive genes. |
| Hevir et al., 2013 [37] | In vitro | Sixty specimens were collected: 31 ovarian endometriomas removed via laparoscopic surgery and 29 control eutopic normal endometrium tissue samples. | Quantitative real-time PCR, Western blotting, and immunohistochemical staining | Genes encoding five estrogen hydroxylating, five OH-estrogen conjugating, and three estrogen quinone detoxifying enzymes (CYPB1, COMT, NQO1, GSTP1, SULTs) | Increased expression of CYP1A1, CYP3A7, and COMT, and higher levels of MB-COMT were seen in endometriosis compared to normal endometrium. Expression of CYP1B1, CYP3A5, SULT1A1, and NQO2 was unchanged, with comparable CYP1B1 protein levels. Expression of SULT1E1, SULT2B1, UGT2B7, NQO1, and GSTP1 was decreased. Three NQO1 isoforms were detected; NQO1c appears to be endometriosis-specific. /The disturbed balance between phase I and II enzymes demonstrated by the study may result in excessive OH-estrogens and ROS formation, resulting in the stimulation of the proliferation of ectopic endometrium. |
| Chen et al., 2017 [38] | Experimental study | Thirty endometriosis and 30 normal endometrial tissue samples. | Methylation-specific PCR, RT-PCR, Western blot, cell culture | ARID1A, H2O2, MDA, GPx | The low level of the ARID1A gene was associated with hypermethylation of its promoter. In H2O2-stimulated endometrial cells, ARID1A gene expression was decreased. Finally, ROS regulated ARID1A gene expression by changing the methylation level of the ARID1A gene promoter. Finally, both the mRNA and protein levels of DNMT1 were increased in H2O2-stimulated endometrial cells /In this study, ROS-induced ARID1A gene downregulation was caused by hypermethylation of its promoter. Hypermethylation of the ARID1A gene promoter caused by oxidative stress, leading to low ARID1A gene expression, constitutes a new mechanism of endometriosis. |
| Chen et al., 2019 [39] | Observational study | Thirty-five childbearing-age women undergoing hysteroscopy for other benign gynecological diseases without endometriosis as a control group, and 78 ectopic endometrial tissues obtained from ovarian endometriotic lesions, and their homologous eutopic endometrium (n = 38). | Cell culture, flow cytometry, transmission electron microscopy, Seahorse XF96 extracellular flux analysis, immunohistochemistry, RT-qPCR, Western blotting, cell proliferation assay, cell migration assay | SOD2 | SOD2 expression in ectopic endometrium was higher than that in eutopic endometrium (p < 0.0001) and controlled endometrium (p < 0.0001). SOD2 mRNA expression was significantly elevated in ectopic ESCs compared to eutopic ESCs (p < 0.01) and controlled ESCs (p < 0.01). SOD2 protein expression was also higher in ectopic ESCs than in eutopic and control ESCs. Mitochondrial membrane potential (MMP) was significantly decreased in si-SOD2-treated cells compared to control cells (p < 0.001). /This study identified overexpressed SOD2 coupled with preserved mitochondrial functions but increased mitochondrial superoxide production in endometriosis. The findings indicate that oxidative stress in endometriosis has exhausted the antioxidant mechanisms of the body. Thus, mitochondrial superoxide scavenging can be proposed as a therapeutic option to prevent the development of ovarian endometriosis. |
| Andrisani et al., 2014 [42] | Observational study | Blood samples were collected from 30 women classified as having endometriosis by histological examination and 27 healthy volunteers serving as the control group. | Immunoassay, carbonic anhydrase assay | GSSP, GSH, carbonic anhydrase | In association with an increase in membrane GSSP and a decrease in cytosolic GSH content in endometriosis patients, carbonic anhydrase monomerization and activity significantly increased (p < 0.0001) compared with controls. This oxidation-induced activation of carbonic anhydrase was positively and significantly correlated with the GSH content of RBC (p < 0.001) and with the amount of the 30-kDa monomer of carbonic anhydrase (p < 0.001). /This study investigated the effects of higher oxidation status on cytosolic carbonic anhydrase in RBC from endometriotic patients. The observation of a close positive correlation between the endometriosis-associated increase in systemic oxidative stress and carbonic anhydrase activity is of great interest as it could potentially be used as the basis for a diagnostic tool to monitor patients’ oxidation status. |
| Szczepanska et al., 2003 [43] | Retrospective study | Peritoneal fluid samples were collected from 65 women admitted for diagnostic laparoscopy. | Spectrophotometry, ELISA, colorimetry | SOD, GPx, LPO | SOD activity differed significantly between infertile patients with endometriosis and patients with idiopathic infertility (p < 0.0001) and fertile controls (p < 0.0001). It was lowest in patients with endometriosis and highest in those with idiopathic infertility. Glutathione peroxidase activity was lowest in patients with endometriosis (p < 0.000123) and highest in those with idiopathic infertility (p < 0.00123). Patients with endometriosis had the lowest total antioxidant status, and those with idiopathic infertility had the highest. Lipid peroxidase levels were highest in women with endometriosis (p < 0.039) and lowest in those with idiopathic infertility. /Low antioxidant status and low activity of antioxidant enzymes in the peritoneal fluid of infertile women with endometriosis probably do not influence fertility in these women, but these factors may play a role in the development of the disease. |
| Ogawa et al., 2022 [44] | In vitro | The cyst walls of ovarian endometrioma were collected from 14 women undergoing surgery for endometriosis. | Cell culture, co-culture experiments, immunocytochemistry, Western blotting, ELISA, cell viability assay, cell proliferation analysis | Macrophage, HO-1, TGF-β1 | HO-1 expression was increased in dHTP-1 cells co-cultured with endometriotic cells compared with dHTP-1 monoculture. Co-culturing with dTHP-1 protected endometriotic cells against oxidative injury. Blockade of HO-1 abolished the protective effects of macrophages. /Dynamic cross-talk between endometriotic cells and macrophages may affect the progression of endometriosis through the upregulation of TGF-β1 and HO-1 expression. Macrophage-derived HO-1 protects endometriotic cells from oxidative injury. |
| Lu et al., 2018 [45] | Randomized controlled study | A total of 280 patients with endometriosis who underwent IVF-ET were enrolled in the study and divided into two groups: a vitamin C treatment group (n = 160) and a non-treatment group (n = 120). Additionally, 150 patients without endometriosis who also underwent IVF-ET were enrolled as the control group. Plasma and peritoneal fluid samples were collected for analysis. | Phenanthroline colorimetry, thiobarbituric acid chromatometry, spectrophotometry, xanthine oxidase method | SOD, TAC, MDA, ROS | FF levels of VitC, SOD, and TAC were significantly lower in patients with EMs than in controls (p < 0.05). FF levels of SOD and TAC in these two groups were significantly higher than serum levels (p < 0.05). In contrast, serum MDA level was significantly higher than FF MDA level in patients with EMs and controls (p < 0.05). However, the FF MDA level was higher in EM patients than in controls (p < 0.05). ROS levels in serum and FF were significantly higher in EM patients than in controls (p < 0.05). Treatment with an oral formulation of vitamin C for 2 months improved serum and FF levels of vitamin C in EM patients, but it did not affect oxidative stress markers (SOD, ROS, TAC, MDA). /Treatment with vitamin C oral formulation improved the serum and FF levels of vitamin C but did not affect oxidant stress markers in patients with Ems. |
| Prieto et al., 2012 [46] | Prospective clinical cohort study | Blood samples were collected from 23 infertile women with endometriosis and 68 controls, including women who were infertile due to tubal factor, male factor, or healthy egg donors. | Spectrophotometry, high-performance liquid chromatography(HPLC), ELISA | Antioxidants consisting of vitamins C, E, SOD, and MDA | In women with endometriosis, lower vitamin C concentrations were found in FF (p = 0.03) and a tendency toward reduced SOD activity in plasma (p = 0.059) compared with the control group. Vitamin E plasma levels were significantly higher in women with endometriosis (p = 0.001), whereas FF levels did not differ between endometriosis patients and controls. Plasma MDA levels were higher in controls than in endometriosis patients, but the difference was not significant. Negative correlations were observed between plasma vitamin C level and number of oocytes retrieved, number of mature oocytes, and number of fertilized oocytes (p = 0.036, p = 0.0007, and p = 0.046, respectively). /These findings suggest a lower antioxidant capacity in infertile women with endometriosis. |
| Santanam et al., 2013 [47] | Randomized controlled trial | Fifty-nine women (age 19–41 years) were recruited. Group A (n = 46) was given a combination of vitamins E and C, and group B (n = 13) was given placebo pills. | ELISA | Vitamin C, E | After treatment with antioxidants, 43% of endometriosis patients reported a decrease in chronic pain (p = 0.0055), whereas no patient in the placebo group reported a change in chronic pain. Dysmenorrhea and dyspareunia decreased in 37% and 24% of patients treated with antioxidants, respectively, while in the placebo group, dysmenorrhea-associated pain decreased in 4 patients, and there was no change in chronic pain or dyspareunia. /Natural antioxidants such as vitamins E and C at low doses are a highly efficient alternative therapy for relieving chronic pelvic pain in women with endometriosis. The current study also provided in vivo evidence for the global hypothesis that endometriosis is a disease of oxidative stress. |
| Mier-Cabrera et al., 2008 [48] | Randomized controlled trial | Thirty-four women with endometriosis were randomly assigned to the study (vitamin) group (n = 16) or placebo group (n = 18). | Ferrous-ion-mediated oxidation of xylenol orange assay, thiobarbituric acid reactive substances assay, and spectrophotometry | Malondialdehyde, lipid hydroperoxide | The plasma concentrations of LOOHs and MDA were significantly decreased in patients treated with antioxidants compared to the placebo group at 4 and 6 months (p < 0.05). Significant differences in plasma concentration compared to baseline were observed for MDA at 4 and 6 months (p < 0.05), but only at 6 months for LOOHs (p < 0.05). In the supplementation group, significant differences were observed between baseline concentrations of the two oxidative stress markers in peritoneal fluid and plasma, and the corresponding plasma concentrations at month 4 (p < 0.04). /The findings suggest that vitamin intake influences the peritoneal milieu as well as the peripheral compartment, and that a decrease in peritoneal oxidative stress is to be expected with vitamin intake. |
| Amini et al., 2021 [49] | Randomized Controlled Trial | Serum and FF samples were collected from 60 reproductive-aged women with pelvic pain who were randomized into two groups: Group A, which received a combination of vitamins C and E (n = 30), and Group B, which received placebo pills (n = 30). | ELISA | MDA, vitamin C, vitamin E | Women in group B had significantly lower MDA levels than those in group A before the intervention (p < 0.01) and after vitamin administration. MDA (p = 0.002) and ROS (p < 0.001) were significantly reduced compared to placebo, but no change in TAC level was observed. The intergroup comparison showed a more significant reduction in dysmenorrhea, dyspareunia, and chronic pelvic pain VAS score in group A than group B. /The results suggest that supplementation with vitamins C and E effectively reduces systemic oxidative stress indices in women with endometriosis. |
| Sutrisno et al., 2022 [50] | Animal study | Thirty-two healthy female mice (Mus musculus) were divided into a negative control group, an endometriosis group, and treatment groups administered different doses of genistein. | Quantitative colorimetric determination, colorimetric assay | SOD, GPx | SOD levels in the EMT group were significantly lower than in the control group (p = 0.006). Genistein significantly increased SOD levels at doses of 0.13 mg (p < 0.001) and 0.26 mg (p < 0.001) compared to the EMT group. However, in EMT + G3, EMT + G4, EMT + G5, EMT + G6, the levels of SOD were similar to those of the EMT group. The GPx levels in the EMT group were significantly lower than in the control group (p = 0.023). Genistein increased GPx levels significantly in all groups administered genistein (p = 0.010; p = 0.002; p < 0.001; p < 0.001; p < 0.001, respectively). /Genistein has the potential to increase SOD and GPx levels in the peritoneal fluid of a mouse model of endometriosis to prevent oxidative stress. |
| Rostami et al., 2023 [51] | Randomized controlled trial | Blood serum and FF samples were collected from 50 infertile endometriosis patients presenting for assisted reproductive techniques (ART). | ELISA | MDA, SOD, CAT, TAC, proinflammatory cytokines (IL-1β, IL-6, TNF-α) | Increased serum levels of TAC (p = 0.004) and SOD (p = 0.010) were observed after AST therapy in the treatment group. In addition, serum MDA (p = 0.031) decreased significantly following antioxidant treatment. Furthermore, AST supplementation led to improvements in the number of oocytes retrieved (p = 0.043), the number of mature oocytes (p = 0.041), and the number of high-quality embryos (p = 0.024). /AST could be a promising supplement to combat the oxidative stress and inflammatory reaction associated with endometriosis. AST may also enhance oocyte and embryo quality in endometriosis patients presenting to ART clinics. |
| Tang et al., 2019 [52] | In vitro | Immortalized human endometrial stromal cells (HESCs) | Cell culture and transfection, qPCR, dual-luciferase reporter assay, cell viability assay, apoptosis assay, caspase-3/7 activity assay, Western blotting, flow cytometry | Hydrogen peroxide, CAT, SOD, GPx | The data suggest that miR-455 is likely negatively correlated with H2O2-induced apoptosis in HESCs. Flow cytometric analysis indicated that upregulation of miR-455 significantly reduced H2O2-induced apoptosis in HESCs. miR-455 alleviated the oxidative stress induced by H2O2 in HESCs, and H2O2 significantly decreased the activities of SOD, CAT, and GPx. Silencing of FABP4 generated cytoprotective effects against H2O2 in HESCs. Moreover, overexpression of FABP4 abrogated the miR-455-mediated effects of antioxidative stress in cells. /Given that oxidative stress is implicated in the pathophysiology of endometriosis because it provokes a general inflammatory response in the peritoneal cavity, these findings suggest that miR-455 may be applied as part of a more optimized therapy for endometriosis. |
| Chaudhury et al., 2013 [53] | Animal study | For the first part of the study, 20 CD-1 strain Swiss Albino mice were selected (10 controls and 10 endometriosis-induced mice). For the second part, an additional 40 mice were included (30 endometriosis-induced mice and 10 controls). Peritoneal tissue and blood samples were collected from these mice for analysis. | Nanoceria synthesis, cell proliferation/cytotoxicity assay, histological examination, immunohistochemistry, thiobarbituric acid assay, luminol-mediated chemiluminescence assay, ELISA | LPO, TAC, MDA | ROS and LPO levels were higher in endometriosis-induced mice compared to controls (p < 0.05, 0 < 0.001, respectively), whereas TAC level was higher in controls (p < 0.001). Injection of NAC in endometriosis-induced mice did not reduce ROS levels (p > 0.05). However, ROS levels were significantly reduced in endometriotic mice treated with nanoceria (p < 0.05). A similar trend was observed with regard to LPO and TAC levels; specifically, endometriotic mice treated with nanoceria showed significantly lower LPO and higher TAC levels (p < 0.05). Moreover, nanoceria protected against endometriosis-related adverse effects on oocytes, which is critical for successful pregnancy. /Nanoceria showed promising efficacy against endometriosis-related pathogenesis. |
| Wang et al., 2024 [54] | In vitro, in vivo | Twenty patients were diagnosed with endometriosis, and 10 controls were without endometriosis. Endometriotic stromal cells were isolated from endometrial samples, while menstrual blood endometrial cells (MESCs) were isolated. | Inflammatory factor sequencing, immunohistochemical staining, qRT-PCR, Western blotting, Calcein-AM/PI fluorescence assay, mitochondrial membrane potential assay, glycolytic rate assay, co-immunoprecipitation assay | Osteopontin (OPN) | OPN was significantly upregulated in endometriosis, suggesting that it may play a role in disease progression (p < 0.0001). In vitro assays demonstrated significant upregulation of OPN in EESCs (p < 0.01), and knockdown of OPN effectively inhibited necroptosis and the release of inflammatory factors by inhibiting mitochondrial stress and ROS release. In vivo, targeting of OPN can inhibit the growth of endometriotic lesions. Clinically, OPN was significantly upregulated in the menstrual blood of patients with endometriosis. /This study unveils the critical role of OPN in regulating the RhoA/ROS signaling pathway, thereby controlling necroptosis and the release of inflammatory factors. OPN knockdown effectively impeded the development of endometriosis, providing a promising treatment approach in which inflammation is targeted through OPN regulation. Additionally, measuring OPN levels in menstrual blood may be a non-invasive and specific method for detecting endometriosis. |
| Seidita et al., 2023 [55] | In vitro | Immortalized human endometrial stromal cells (HESC). | Cell culture, Western blotting, cell transfection, qRT-PCR, confocal microscopy, flow cytometry | S1P | Treatment with 100 nM S1P for 10 min potently increased ROS formation in HESCs (p < 0.001). S1P stimulation increased ROS production, and S1P activated ERK5 through S1P1 and S1P3 receptor engagement and downstream activation of SFK and MEK5. Activation of ERK5 by the expression of a constitutively active form of MEK5 significantly increased intracellular ROS, confirming the role of MEK5-dependent phosphorylation of ERK5 in the modulation of ROS levels in HESCs. /The findings indicate that S1P signaling, via ERK5 activation, supports a proinflammatory response in the endometrium and establish a rationale for exploiting innovative therapeutic targets for endometriosis. |
| Lu et al., 2020 [56] | Animal study | Fifty Wistar rats were randomly divided into four groups: an endometriosis group, an endometriosis group treated with normal saline, an endometriosis group treated with N-acetyl-L-cysteine (NAC), and an endometriosis group treated with catalase (CAT). Endometriotic endometrial tissue was collected from these groups for analysis. | Immunofluorescence, Western blotting, ELISA, HE staining | NAC, CAT | LC3 fluorescence levels were significantly lower in the EMs group of rats compared with controls (p < 0.05). Western blot analysis revealed a downregulation of Beclin-1 protein in both the NAC and CAT groups compared with controls (p < 0.05), while ELISA revealed significantly lower ROS levels in the NAC and CAT groups (p < 0.05). /The study demonstrated that hypoxia induced autophagy in EMs cells and resulted in ROS generation, and that these biological changes could be reversed by the antioxidants CAT and NAC. The results may partly explain the mechanism by which the levels of autophagy markers are reduced in response to ROS inhibitors. |
| Cabrera et al., 2009 [57] | Comparative cross-sectional study | Blood samples were collected from women with endometriosis (WEN, n = 83) and without endometriosis (WWE, n = 80), who were interviewed for the study. Among the WEN group, participants were further divided into a control group (n = 35) and a high antioxidant diet group (n = 37). | Xylenol orange assay, thiobarbituric acid assay, electrochemical detection, colorimetric assay | Vitamin A, C, E, zinc, copper, MDA, GPx, SOD | Comparison of antioxidant intake between WWE and WEN showed a lower intake of vitamins A, C, E, zinc, and copper by WEN (p < 0.05). Increases in vitamin concentrations (serum retinol, alpha-tocopherol, leukocyte and plasma ascorbate) and antioxidant enzyme activity (SOD, GPx), as well as decreases in oxidative stress markers (MDA, lipid hydroperoxides) were observed in the HAD group after 2 months of intervention. These phenomena were not observed in the control group. /Application of a HAD in women with endometriosis increased the peripheral enzymatic SOD and GPx activity after 3 months of intervention in comparison to the control diet group. Additionally, application of a HAD in women with endometriosis decreased the peripheral concentrations of MDA and LPOs after 3 months of intervention in comparison to the control diet group. |
| Author/ Year/ Reference | Study Design | Species and/or Sample | Detection Method | Target Substances | Results/Conclusions |
|---|---|---|---|---|---|
| Arumugam et al., 1995 [58] | In vitro | Twelve peritoneal fluid samples from patients with moderate to severe endometriosis, 15 samples from patients with minimal to mild endometriosis, and 13 samples from patients with a normal pelvis | Thiobarbituric acid assay | LPO, MDA | LPO levels were not affected by the presence or severity of endometriosis. The mean values for the MDA were: 1.09 ± 0.14 for moderate to severe endometriosis, 0.95 ± 0.09 for minimal to mild endometriosis, and 1.07 ± 0.15 for normal pelvis. /The TBA assay results show LPO levels in the pelvic cavity are independent of the presence or absence of endometriosis. As such, LPOs in the peritoneal fluid do not appear to play a significant role in the causal relationship between endometriosis and infertility. |
| Amaral et al., 2004 [59] | Prospective study | Peritoneal fluid samples from 21 infertile women with endometriosis and 21 patients undergoing tubal ligation | Thiobarbituric acid assay | MDA, LPO | A significant increase (p < 0.01) in MDA levels was observed in the peritoneal fluid after the addition of copper in both groups (MDA and MDA/Cu2+ = 0.07 and 0.34 nmol/mL, and 0.04 and 0.21 nmol/mL in patients with pelvic endometriosis and controls, respectively). /These data suggest that increased ROS may not be one of the factors responsible for compromised fertility in patients with endometriosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yeo, S.G.; Lee, J.M.; Kim, S.S.; Lee, J.-W.; Chung, N.; Park, D.C. Expression of Free Radicals and Reactive Oxygen Species in Endometriosis: Current Knowledge and Its Implications. Antioxidants 2025, 14, 877. https://doi.org/10.3390/antiox14070877
Lee J, Yeo SG, Lee JM, Kim SS, Lee J-W, Chung N, Park DC. Expression of Free Radicals and Reactive Oxygen Species in Endometriosis: Current Knowledge and Its Implications. Antioxidants. 2025; 14(7):877. https://doi.org/10.3390/antiox14070877
Chicago/Turabian StyleLee, Jeongmin, Seung Geun Yeo, Jae Min Lee, Sung Soo Kim, Jin-Woo Lee, Namhyun Chung, and Dong Choon Park. 2025. "Expression of Free Radicals and Reactive Oxygen Species in Endometriosis: Current Knowledge and Its Implications" Antioxidants 14, no. 7: 877. https://doi.org/10.3390/antiox14070877
APA StyleLee, J., Yeo, S. G., Lee, J. M., Kim, S. S., Lee, J.-W., Chung, N., & Park, D. C. (2025). Expression of Free Radicals and Reactive Oxygen Species in Endometriosis: Current Knowledge and Its Implications. Antioxidants, 14(7), 877. https://doi.org/10.3390/antiox14070877








