Effect of Supplementation of Antioxidant Lipids Synthetized by Enzymatic Acidolysis with EPA/DHA Concentrate and Maqui (Aristotelia chilensis (Mol.) Stuntz) Seed Oil for Mitigating High-Fat Diet-Induced Obesity and Metabolic Disorders in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Extraction of Belly Oil from Rainbow Trout
2.3. LCPUFAn-3 Concentrate Preparation
2.4. Synthesis of Antioxidant Lipids
2.5. Experimental Design Using Response Surface Methodology (RSM) for Enzymatic Acidolysis Under Supercritical CO2 Condition
2.6. Optimization of Enzymatic Acidolysis Process Variables Using Supercritical CO2 to Obtain ALω-3
2.7. Validation of the Optimal Antioxidant Lipid Formulation
2.8. Identification and Quantification of Fatty Acids Using Gas Liquid Chromatography (GLC)
2.9. Identification and Quantification of Tocopherols and Tocotrienols
2.10. Thin-Layer Chromatography (TLC)
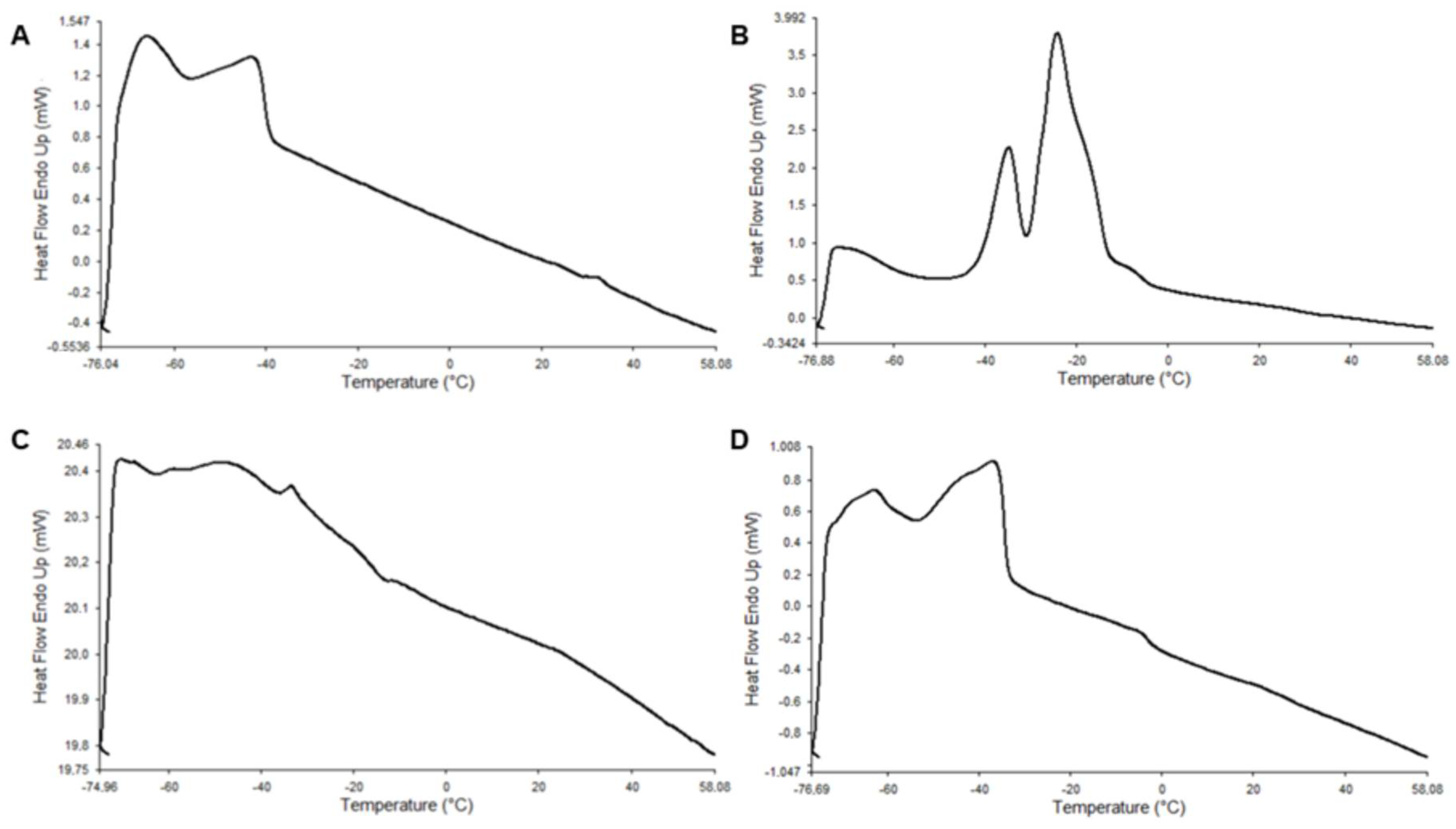
2.11. Differential Scanning Calorimetry (DSC)
2.12. Feeding Trial
2.13. Measurements of Biochemical Serum Parameters
2.14. Histological Assessment
2.15. Quantitative PCR
2.16. Total Antioxidant Capacity Assay Based on ABTS Oxidation
2.17. Statistics
3. Results
3.1. Enzymatic Acidolysis Under Supercritical CO2 of RTBO Concentrate and MO Using the Experimental Design by RSM
3.2. Optimization of Enzymatic Acidolysis Process Variables for RTBO Concentrate and MO Using Supercritical CO2 to Obtain an ALω-3
3.3. Multiple Response Optimization of Enzymatic Acidolysis and Desirability
3.4. Experimental Validation of the Optimal ALω-3 Formulation
3.5. Analysis of Characterization and Fatty Acid Composition of ALω-3
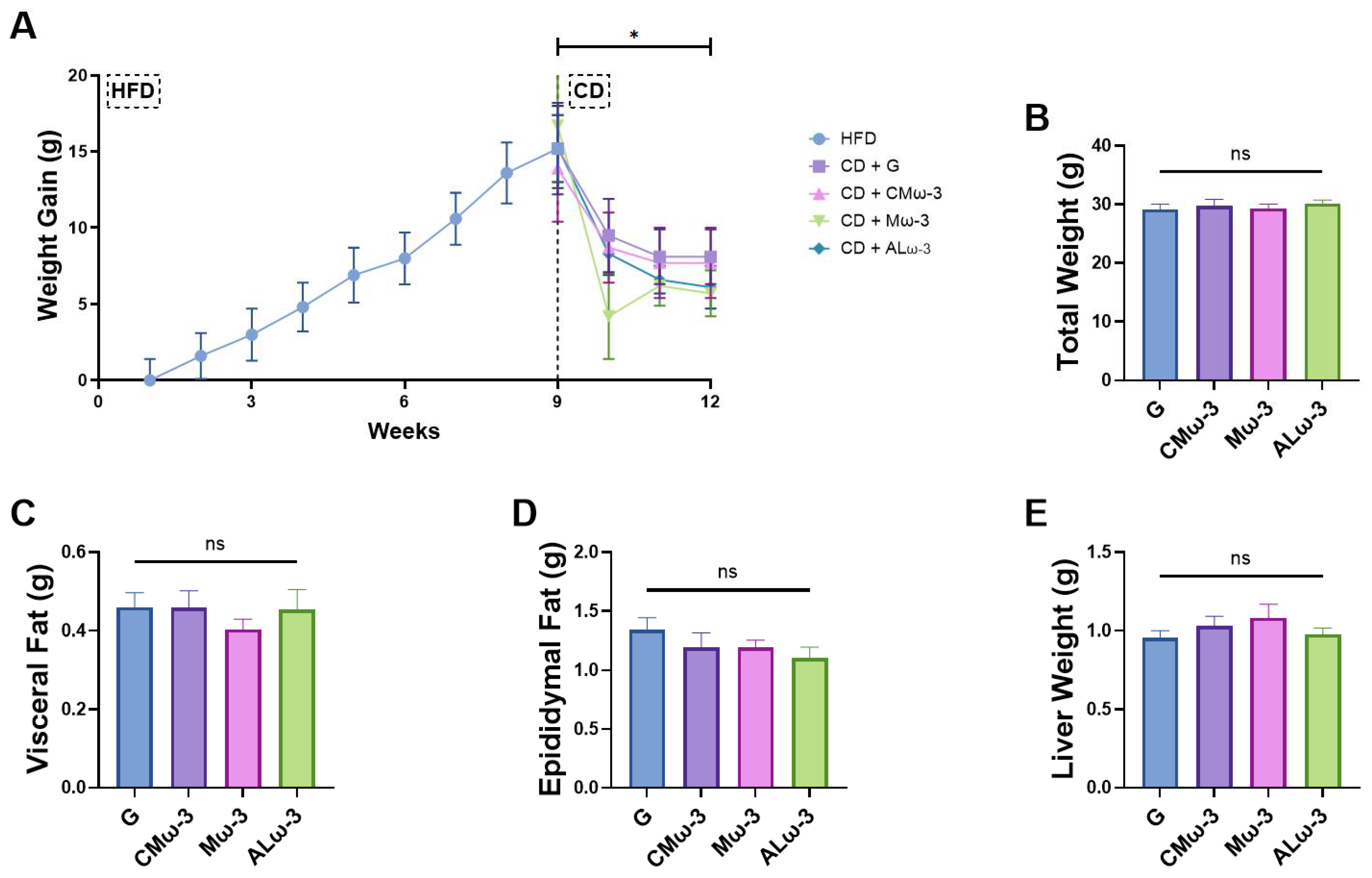
3.6. ALω-3 Effects on Tissue Weight of HFD Murine Model
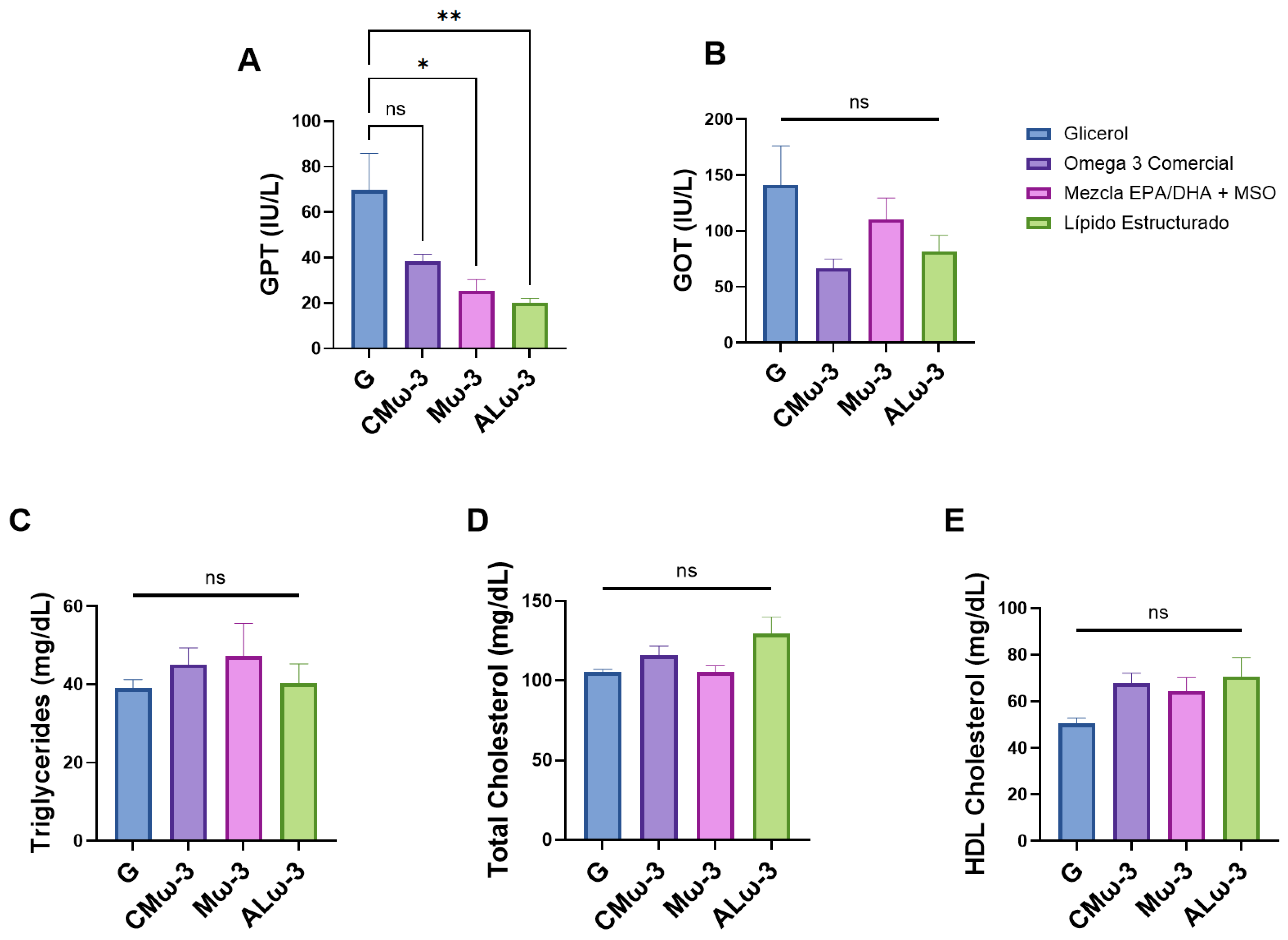
3.7. ALω-3 Effects on Biochemical Parameters of HFD Murine Model
3.8. Effect of ALω-3 on MASLD-Associated Liver Damage
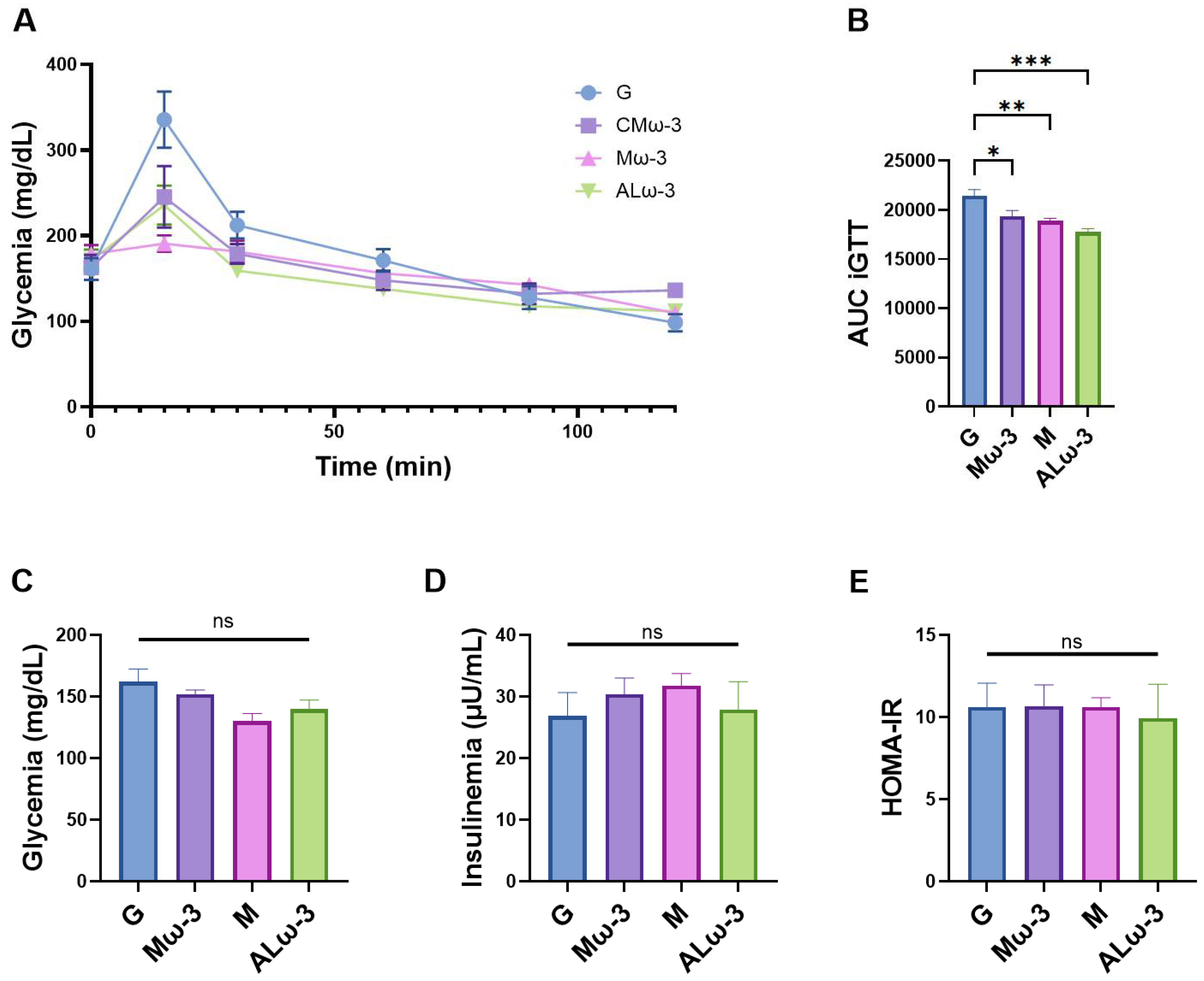
3.9. Evaluation of Glucose Homeostasis After ALω-3 Supplementation
3.10. Effect of Supplementation with ALω-3 on the Transcription of Pro-Inflammatory Genes
3.11. Evaluation of Total Antioxidant Capacity (TAC) in Serum After ALω-3 Supplementation
4. Discussion
4.1. Optimization of Enzymatic Acidolysis Conditions Using Supercritical CO₂
4.2. Characterization and Thermal Analysis of ALω-3
4.3. Impact of ALω-3 Supplementation on Body Weight and Fat Distribution in a High-Fat Diet-Induced Obesity Murine Model
4.4. Effects of ALω-3 on Biochemical Parameters Related to MASLD and Lipid Profile in HFD-Fed Mice
4.5. Effect of ALω-3 Supplementation on Glucose Homeostasis and Insulin Resistance in HFD-Fed Mice
4.6. Effect of ALω-3 Supplementation on Hepatic and Visceral Fat Pro-Inflammatory Gene Expression in HFD-Fed Mice
4.7. Effect of ALω-3 Supplementation on Serum Total Antioxidant Capacity in HFD-Fed Mice
4.8. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MetS | Metabolic Syndrome |
| T2D | Type 2 diabetes |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAFLD | Non-alcoholic fatty liver disease |
| BC | Bioactive compounds |
| LCPUFAn-3 | Long-chain polyunsaturated fatty acids omega-3 |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| LA | Linoleic acid |
| ALA | α-linolenic acid |
| MAG | Monoacylglycerols |
| DAG | Diacylglycerols |
| TAG | Triacylglycerols |
| SCCO2 | Supercritical carbon dioxide |
| GRAS | Generally Recognized As Safe |
| ALω-3 | Antioxidant lipids |
| RTBO | Rainbow trout belly oil |
| MO | Cold-pressed maqui seed oil |
| CMω-3 | Commercial marine Omega 3 |
| FA | Fatty acids |
| Mω-3 | Mixture of concentrated RTBO and MO in a 70/30 (w/w) ratio |
| PET | Polyethylene terephthalate |
| RSM | Response surface methodology |
| ANOVA | Analysis of variance |
| GLC | Gas liquid chromatography |
| FAME | Fatty acid methyl esters |
| IUPAC | International Union of Pure and Applied Chemistry |
| AOCS | American Oil Chemistry Society |
| HPLC | High-performance liquid chromatography |
| TLC | Thin-layer chromatography |
| FFA | Free fatty acids |
| DSC | Differential scanning calorimetry |
| HFD | High-fat diet |
| CD | Control diet |
| G | Glycerol |
| iGTT | Intraperitoneal Glucose Tolerance Test |
| CICUA | Institutional Animal Care and Use Committee |
| GPT | Glutamate pyruvate transaminase |
| GOT | Glutamate oxaloacetate transaminase |
| TG | Triglycerides |
| T-Chol | Total cholesterol |
| HDL-Chol | High-density lipoprotein cholesterol |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| IL-6 | Interleukin 6 |
| TNFα | Tumor necrosis factor-a |
| TLR4 | Toll like receptor 4 |
| TAC | Total antioxidant capacity |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| H2O2 | Hydrogen peroxide |
| TE | Trolox equivalents |
| ΔAbs | Change in absorbance |
| SEM | Standard error of the mean |
| DPA | Docosapentaenoic acid |
| TSFA | Total saturated fatty acids |
| TMUFA | Total monounsaturated fatty acids |
| TPUFA | Total polyunsaturated fatty acids |
| TFA | Total fatty acids n-3 |
| N.I. | Not identified |
| H&E | Hematoxylin and Eosin |
| AUC | Area under curve |
| sAG | Structured acylglycerols |
| SREBP-1 | Sterol Regulatory Element-Binding Protein-1 |
| PPARγ | Peroxisome Proliferator-Activated Receptor gamma |
| NF-κB | Nuclear factor-kappa B |
| EVOO | Extra virgin olive oil |
| sPAG | Structured phenolic acylglycerols containing gallic acid |
| AHA | American Heart Association |
| IR | Insulin resistance |
| NASH | Nonalcoholic steatohepatitis |
| IL-1β | Interleukin 1β |
| MCD | Methionine- and choline-deficient |
| NAFLD | Non-alcoholic fatty liver disease |
| CDAHFD | Choline-deficient, L-amino acid-defined high-fat diet |
| RM | Rosehip oil |
| TSO | Trichosanthes kirilowii Maxim seed oil |
| FLA | Flavonoids |
| TSOFLA | Trichosanthes kirilowii Maxim seed oil + flavonoids |
| MDA | Serum malondialdehyde |
| SOD | Superoxide dismutase |
| GSH-Px | Glutathione peroxidase |
| TBARS | Thiobarbituric acid reactive substances |
Appendix A
| Process Variables | Response Variables | |||||
|---|---|---|---|---|---|---|
| (Y1) EPA | (Y2) DHA | (Y3) EPA + DHA | ||||
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | |
| Constant | 8.07 | 23.17 | 34.85 | |||
| A | 0.00 | 0.52 | −0.02 | 0.00 | −0.04 | 0.01 |
| B | 0.09 | 0.02 | −0.11 | 0.01 | −0.07 | 0.03 |
| A × B | - | - | 0.00 | 0.00 | −0.00 | 0.04 |
| R2 | 79.04 | 99.93 | 95.18 | |||
| Adjusted R2 | 68.57 | 99.86 | 90.36 | |||
| SE | 0.74 | 0.06 | 0.77 | |||
| MAE | 0.47 | 0.04 | 0.50 | |||
| DW value | 0.75 | 0.58 | 0.58 | |||
References
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome—A New World-Wide Definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. Medicina 2023, 59, 561. [Google Scholar] [CrossRef]
- Masood, B.; Moorthy, M. Causes of Obesity: A Review. Clin. Med. 2023, 23, 284–291. [Google Scholar] [CrossRef]
- Hsu, C.L.; Loomba, R. From NAFLD to MASLD: Implications of the New Nomenclature for Preclinical and Clinical Research. Nat. Metab. 2024, 6, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Fracchiolla, G.; Muraglia, M.; Tardugno, R.; Dibenedetto, R.S.; D’Alessandro, A.G. Metabolic Syndrome: A Narrative Review from the Oxidative Stress to the Management of Related Diseases. Antioxidants 2023, 12, 2091. [Google Scholar] [CrossRef] [PubMed]
- Mallhi, T.H.; Rasheed, M.; Khan, Y.H.; Alzarea, A.I.; Raja, A.A. Bioactive Compounds for the Treatment of Metabolic Disorders. In Endocrine Disrupting Chemicals-induced Metabolic Disorders and Treatment Strategies; Akash, M.S.H., Rehman, K., Hashmi, M.Z., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 489–505. [Google Scholar]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-Metabolism, Absorption, Bioavailability and Health Benefits–A Review. PharmaNutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- CODEX Alimentarius. Codex standard for named vegetable oils. Codex Stan 1999, 210, 1–13. [Google Scholar]
- Sánchez, C.; Rodríguez, A.; Reinoso, F.; Dovale-Rosabal, G.; Romero, N.; Espinosa, A.; Pando, M.E.; Claria, B.; Valenzuela, R.; Char, C.; et al. Optimization of Oil and Tocopherol Extraction from Maqui (Aristotelia chilensis (Mol.) Stuntz) by Supercritical CO2 Procedure. Antioxidants 2024, 13, 845. [Google Scholar] [CrossRef]
- Pando, M.E.; Rodríguez, A.; Galdames, A.; Berríos, M.M.; Rivera, M.; Romero, N.; Valenzuela, M.A.; Ortiz, J.; Aubourg, S.P. Maximization of the Docosahexaenoic and Eicosapentaenoic Acids Content in Concentrates Obtained from a By-Product of Rainbow Trout (Oncorhynchus mykiss) Processing. Eur. Food Res. Technol. 2018, 244, 937–948. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.D.; Jang, Y.J. Delivery Systems Designed to Enhance Stability and Suitability of Lipophilic Bioactive Compounds in Food Processing: A Review. Food Chem. 2024, 437, 137910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E Therapy beyond Cancer: Tocopherol versus Tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Madhujith, T. Current Trends in Applications of Enzymatic Interesterification of Fats and Oils: A Review. LWT 2020, 132, 109880. [Google Scholar] [CrossRef]
- Raederstorff, D.; Wyss, A.; Calder, P.C.; Weber, P.; Eggersdorfer, M. Vitamin E Function and Requirements in Relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122. [Google Scholar] [CrossRef]
- Zhao, Y.; Monahan, F.J.; McNulty, B.A.; Li, K.; Bloomfield, F.J.; Duff, D.J.; Brennan, L.; Nugent, A.P.; Gibney, E.R. Plasma N-3 Polyunsaturated Fatty Status and Its Relationship with Vitamin E Intake and Plasma Level. Eur. J. Nutr. 2017, 56, 1281–1291. [Google Scholar] [CrossRef]
- Cernia, E.; Palocc, C. Lipases in Supercritical Fluids. In Methods in Enzymology; Lipases Part B: Enzyme Characterization and Utilization; Academic Press: Cambridge, MA, USA, 1997; Volume 286, pp. 495–508. [Google Scholar]
- Budisa, N.; Schulze-Makuch, D. Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef]
- Claria, B.; Espinosa, A.; Rodríguez, A.; Dovale-Rosabal, G.; Bucarey, J.L.; Pando, M.E.; Romero, N.; Reinoso, F.; Sánchez, C.; Valenzuela, R.; et al. Cold-Pressed Aristotelia Chilensis (Mol.) Stuntz Seed Oil Prevents Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) in a High-Fat-Diet-Induced Obesity Murine Model. Antioxidants 2024, 13, 1384. [Google Scholar] [CrossRef] [PubMed]
- Reinoso, F.; Rodríguez, A.; Sánchez, C.; Claria, B.; Romero, N.; Espinosa, A.; Pando, M.E.; Valenzuela, R.; Apaza, D.; Dovale-Rosabal, G.; et al. Enzymatic Interesterification of Cold-Pressed Maqui (Aristotelia chilensis (Mol.) Stuntz) Seed Oil and Belly Oil from Rainbow Trout (Oncorhynchus mykiss) Through Supercritical CO2. Mar. Drugs 2024, 22, 547. [Google Scholar] [CrossRef]
- Radin, N.S. Extraction of Tissue Lipids with a Solvent of Low Toxicity. In Methods in Enzymology; Lipids Part D; Academic Press: Cambridge, MA, USA, 1981; Volume 72, pp. 5–7. [Google Scholar]
- Pando, M.E.; Rodríguez, A.; Valenzuela, M.A.; Berríos, M.M.; Rivera, M.; Romero, N.; Barriga, A.; Aubourg, S.P. Acylglycerol Synthesis Including EPA and DHA from Rainbow Trout (Oncorhynchus mykiss) Belly Flap Oil and Caprylic Acid Catalyzed by Thermomyces Lanuginosus Lipase under Supercritical Carbon Dioxide. Eur. Food Res. Technol. 2021, 247, 499–511. [Google Scholar] [CrossRef]
- Zuta, C.P.; Simpson, B.K.; Chan, H.M.; Phillips, L. Concentrating PUFA from Mackerel Processing Waste. J. Amer. Oil Chem. Soc. 2003, 80, 933–936. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Belarbi, E.-H. Purification Process for Cod Liver Oil Polyunsaturated Fatty Acids. J. Amer. Oil Chem. Soc. 2001, 78, 477–484. [Google Scholar] [CrossRef]
- Vázquez, L.; Prados, I.M.; Reglero, G.; Torres, C.F. Identification and Quantification of Ethyl Carbamate Occurring in Urea Complexation Processes Commonly Utilized for Polyunsaturated Fatty Acid Concentration. Food Chem. 2017, 229, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hill, W.J.; Hunter, W.G. A Review of Response Surface Methodology: A Literature Survey. Technometrics 1966, 8, 571–590. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Hong, P.; Ji, H. Concentration of Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) of Tuna Oil by Urea Complexation: Optimization of Process Parameters. J. Food Eng. 2006, 73, 203–209. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Espinosa, A.; Rodríguez, A.; Barriga, A.; Palomino-Calderón, A.; Romero, N.; Troncoso, R.H.; Aubourg, S.P. Effect of Structured Phenolic Lipids with EPA/DHA and Gallic Acid against Metabolic-Associated Fatty Liver Disease (MAFLD) in Mice. Molecules 2022, 27, 7702. [Google Scholar] [CrossRef]
- IUPAC Standard Method 2.301. Preparation of Fatty Acid Methyl Esters. In Standard Methods for Analysis of Oils, Fats and Derivatives, 7th ed.; Blackwell Scientific Publications: Oxford, UK, 1987. [Google Scholar]
- American Oil Chemists’ Society (AOCS). Determination of cis-, trans-, saturated, monounsaturated, and polyunsaturated fatty acids by capillary gas liquid chromatography (GLC). Sampling and analysis of commercial fats and oils. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 7th ed.; Official Method Ce 1j-7; AOCS: Champaign, IL, USA, 2017. [Google Scholar]
- American Oil Chemists’ Society (AOCS). Determination of tocopherols and tocotrienols in vegetable oils and fats by HPLC. In Official Methods and Recommended Practices of the American Oil Chemists Society, 5th ed.; Official method Ce 8-89; AOCS: Champaign, IL, USA, 1997. [Google Scholar]
- Sabally, K.; Karboune, S.; St-Louis, R.; Kermasha, S. Lipase-Catalyzed Synthesis of Phenolic Lipids from Fish Liver Oil and Dihydrocaffeic Acid. Biocatal. Biotransformation 2007, 25, 211–218. [Google Scholar] [CrossRef]
- Fuchs, B.; Süss, R.; Teuber, K.; Eibisch, M.; Schiller, J. Lipid Analysis by Thin-Layer Chromatography—A Review of the Current State. J. Chromatogr 2011, 1218, 2754–2774. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F. Positional Distribution of Fatty Acids in Triacylglycerols of Seal Blubber Oil. J. Food Lipids 1997, 4, 51–64. [Google Scholar] [CrossRef]
- Rodríguez, A.; Castro, E.; Salinas, M.C.; López, R.; Miranda, M. Interesterification of Tallow and Sunflower Oil. J. Am. Oil Chem. Soc. 2001, 78, 431–436. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C. Synthesis of Structured Lipids by Transesterification of Trilinolein Catalyzed by Lipozyme IM60. J. Agric. Food Chem. 2001, 49, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.-H. Mouse Model of the Adipose Organ: The Heterogeneous Anatomical Characteristics. Arch. Pharm. Res. 2021, 44, 857–875. [Google Scholar] [CrossRef]
- Börgeson, E.; Boucher, J.; Hagberg, C.E. Of Mice and Men: Pinpointing Species Differences in Adipose Tissue Biology. Front. Cell Dev. Biol. 2022, 10, 1003118. [Google Scholar] [CrossRef]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An Improvement of the 2ˆ(-Delta Delta CT) Method for Quantitative Real-Time Polymerase Chain Reaction Data Analysis. Biostat Bioinform. Biomath 2013, 3, 71–85. [Google Scholar]
- Wimmer, Z.; Zarevúcka, M. A Review on the Effects of Supercritical Carbon Dioxide on Enzyme Activity. Int. J. Mol. Sci. 2010, 11, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Gießauf, A.; Magor, W.; Steinberger, D.J.; Marr, R. A Study of Hydrolases Stability in Supercritical Carbon Dioxide (SC-CO2). Enzym. Microb. Technol. 1999, 24, 577–583. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Di Pasquale, M.G. The Essentials of Essential Fatty Acids. J. Diet. Suppl. 2009, 6, 143–161. [Google Scholar] [CrossRef]
- Singh, P.K.; Chopra, R.; Dhiman, A.; Chuahan, K.; Garg, M. Development of Omega-3-Rich Structured Lipids Using Perilla Seed Oil and Palm Olein: Optimization and Characterization. Biomass Conv. Bioref. 2024, 14, 23857–23871. [Google Scholar] [CrossRef]
- Bastías-Montes, J.M.; Monterrosa, K.; Muñoz-Fariña, O.; García, O.; Acuña-Nelson, S.M.; Vidal-San Martín, C.; Quevedo-Leon, R.; Kubo, I.; Avila-Acevedo, J.G.; Domiguez-Lopez, M.; et al. Chemoprotective and Antiobesity Effects of Tocols from Seed Oil of Maqui-Berry: Their Antioxidative and Digestive Enzyme Inhibition Potential. Food Chem. Toxicol. 2020, 136, 111036. [Google Scholar] [CrossRef]
- Follegatti-Romero, L.A.; Piantino, C.R.; Grimaldi, R.; Cabral, F.A. Supercritical CO2 Extraction of Omega-3 Rich Oil from Sacha Inchi (Plukenetia volubilis L.) Seeds. J. Supercrit. Fluids 2009, 49, 323–329. [Google Scholar] [CrossRef]
- Melgosa, R.; Sanz, M.T.; Beltrán, S. Supercritical CO2 Processing of Omega-3 Polyunsaturated Fatty Acids—Towards a Biorefinery for Fish Waste Valorization. J. Supercrit. Fluids 2021, 169, 105121. [Google Scholar] [CrossRef]
- Hamam, F.; Shahidi, F. Structured Lipids from High-Laurate Canola Oil and Long-Chain Omega-3 Fatty Acids. J. Am. Oil Chem. Soc. 2005, 82, 731–736. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Rodríguez, A.; Espinosa, A.; Barriga, A.; Aubourg, S.P. Synthesis of EPA- and DHA-Enriched Structured Acylglycerols at the Sn-2 Position Starting from Commercial Salmon Oil by Enzymatic Lipase Catalysis under Supercritical Conditions. Molecules 2021, 26, 3094. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Y.; Wang, Z.; Wang, C.; Wang, Q. Effects of a Novel Medium–Long–Medium-Type Structured Lipid Synthesized Using a Two-Step Enzymatic Method on Lipid Metabolism and Obesity Protection in Experimental Mice. Food Sci. Nutr. 2023, 11, 4516–4529. [Google Scholar] [CrossRef]
- Kok, W.-M.; Chuah, C.-H.; Cheng, S.-F. Enzymatic Synthesis of Structured Lipids with Behenic Acid at the Sn-1, 3 Positions of Triacylglycerols. Food Sci. Biotechnol 2017, 27, 353–366. [Google Scholar] [CrossRef]
- Diao, X.; Sun, W.; Jia, R.; Wang, Y.; Liu, D.; Guan, H. Preparation and Characterization of Diacylglycerol via Ultrasound-Assisted Enzyme-Catalyzed Transesterification of Lard with Glycerol Monolaurate. Ultrason. Sonochem 2023, 95, 106354. [Google Scholar] [CrossRef]
- Ji, T.; Fang, B.; Wu, F.; Liu, Y.; Cheng, L.; Li, Y.; Wang, R.; Zhu, L. Diet Change Improves Obesity and Lipid Deposition in High-Fat Diet-Induced Mice. Nutrients 2023, 15, 4978. [Google Scholar] [CrossRef]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic Fatty Liver Disease: Pathology and Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 145–171. [Google Scholar] [CrossRef]
- Valenzuela, R.; Espinosa, A.; González-Mañán, D.; D’Espessailles, A.; Fernández, V.; Videla, L.A.; Tapia, G. N-3 Long-Chain Polyunsaturated Fatty Acid Supplementation Significantly Reduces Liver Oxidative Stress in High Fat Induced Steatosis. PLoS ONE 2012, 7, e46400. [Google Scholar] [CrossRef]
- Rodriguez-Echevarria, R.; Macias-Barragan, J.; Parra-Vargas, M.; Davila-Rodriguez, J.R.; Amezcua-Galvez, E.; Armendariz-Borunda, J. Diet Switch and Omega-3 Hydroxy-Fatty Acids Display Differential Hepatoprotective Effects in an Obesity/Nonalcoholic Fatty Liver Disease Model in Mice. World J. Gastroenterol. 2018, 24, 461–474. [Google Scholar] [CrossRef]
- Valenzuela, R.; Espinosa, A.; Llanos, P.; Hernandez-Rodas, M.C.; Barrera, C.; Vergara, D.; Romero, N.; Pérez, F.; Ruz, M.; Videla, L.A. Anti-Steatotic Effects of an n-3 LCPUFA and Extra Virgin Olive Oil Mixture in the Liver of Mice Subjected to High-Fat Diet. Food Funct. 2016, 7, 140–150. [Google Scholar] [CrossRef]
- Martínez-Galán, J.P.; Ontibón-Echeverri, C.M.; Campos Costa, M.; Batista-Duharte, A.; Guerso Batista, V.; Mesa, V.; Monti, R.; Veloso de Paula, A.; Martins Baviera, A. Enzymatic Synthesis of Capric Acid-Rich Structured Lipids and Their Effects on Mice with High-Fat Diet-Induced Obesity. Food Res. Int. 2021, 148, 110602. [Google Scholar] [CrossRef]
- la Fuente, F.P.; Quezada, L.; Sepúlveda, C.; Monsalves-Alvarez, M.; Rodríguez, J.M.; Sacristán, C.; Chiong, M.; Llanos, M.; Espinosa, A.; Troncoso, R. Exercise Regulates Lipid Droplet Dynamics in Normal and Fatty Liver. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158519. [Google Scholar] [CrossRef]
- Gaggini, M.; Gorini, F.; Vassalle, C. Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. Int. J. Mol. Sci. 2022, 24, 75. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory from the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Quimby, F.W.; Luong, R.H. Clinical Chemistry of the Laboratory Mouse. Mouse Biomed. Res. 2007, 3, 171–216. [Google Scholar]
- Sherratt, S.C.R.; Mason, R.P.; Libby, P.; Steg, P.G.; Bhatt, D.L. Do Patients Benefit from Omega-3 Fatty Acids? Cardiovasc. Res. 2024, 119, 2884–2901. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The Effects of Omega-3 Fatty Acids in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Prostaglandins leukot. Essent. Fat. Acids 2022, 182, 102456. [Google Scholar] [CrossRef]
- Pérez-Matute, P.; Pérez-Echarri, N.; Martínez, J.A.; Marti, A.; Moreno-Aliaga, M.J. Eicosapentaenoic Acid Actions on Adiposity and Insulin Resistance in Control and High-Fat-Fed Rats: Role of Apoptosis, Adiponectin and Tumour Necrosis Factor-Alpha. Br. J. Nutr. 2007, 97, 389–398. [Google Scholar] [CrossRef]
- Asrih, M.; Jornayvaz, F.R. Inflammation as a Potential Link between Nonalcoholic Fatty Liver Disease and Insulin Resistance. J. Endocrinol. 2013, 218, R25–R36. [Google Scholar] [CrossRef]
- Jia, L.; Vianna, C.R.; Fukuda, M.; Berglund, E.D.; Liu, C.; Tao, C.; Sun, K.; Liu, T.; Harper, M.J.; Lee, C.E.; et al. Hepatocyte Toll-like Receptor 4 Regulates Obesity-Induced Inflammation and Insulin Resistance. Nat. Commun. 2014, 5, 3878. [Google Scholar] [CrossRef]
- Sharifnia, T.; Antoun, J.; Verriere, T.G.C.; Suarez, G.; Wattacheril, J.; Wilson, K.T.; Peek, R.M.; Abumrad, N.N.; Flynn, C.R. Hepatic TLR4 Signaling in Obese NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G270–G278. [Google Scholar] [CrossRef]
- Tordjman, J.; Guerre-Millo, M.; Clément, K. Adipose Tissue Inflammation and Liver Pathology in Human Obesity. Diabetes Metab. 2008, 34, 658–663. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 PUFA and Inflammation: From Membrane to Nucleus and from Bench to Bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef]
- Spooner, M.H.; Jump, D.B. Omega-3 Fatty Acids and Nonalcoholic Fatty Liver Disease in Adults and Children: Where Do We Stand? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 103–110. [Google Scholar] [CrossRef]
- Depner, C.M.; Traber, M.G.; Bobe, G.; Kensicki, E.; Bohren, K.M.; Milne, G.; Jump, D.B. A Metabolomic Analysis of Omega-3 Fatty Acid-Mediated Attenuation of Western Diet-Induced Nonalcoholic Steatohepatitis in LDLR-/- Mice. PLoS ONE 2013, 8, e83756. [Google Scholar] [CrossRef]
- Šmíd, V.; Dvořák, K.; Stehnová, K.; Strnad, H.; Rubert, J.; Stříteský, J.; Staňková, B.; Stránská, M.; Hajšlová, J.; Brůha, R.; et al. The Ameliorating Effects of N-3 Polyunsaturated Fatty Acids on Liver Steatosis Induced by a High-Fat Methionine Choline-Deficient Diet in Mice. Int. J. Mol. Sci. 2023, 24, 17226. [Google Scholar] [CrossRef]
- Todorčević, M.; Hodson, L. The Effect of Marine Derived N-3 Fatty Acids on Adipose Tissue Metabolism and Function. J. Clin. Med. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Noichi, J.; Ishiakawa, T.; Ichi, I.; Fujiwara, Y. Effect of Tocotrienol on the Primary Progression of Nonalcoholic Steatohepatitis in a Mouse Model. J. Clin. Biochem. Nutr. 2022, 70, 140–146. [Google Scholar] [CrossRef]
- Tapia, G.; Silva, D.; Romero, N.; Pettinelli, P.; Dossi, C.G.; de Miguel, M.; González-Mañán, D. Role of Dietary α- and γ-Tocopherol from Rosa Mosqueta Oil in the Prevention of Alterations Induced by High-Fat Diet in a Murine Model. Nutrition 2018, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Hou, Z.; Zhu, L.; Meng, R.; Wang, B. Hypolipidemic and Antioxidant Activities of Trichosanthes Kirilowii Maxim Seed Oil and Flavonoids in Mice Fed with a High-Fat Diet. J. Food Biochem. 2020, 44, e13272. [Google Scholar] [CrossRef]
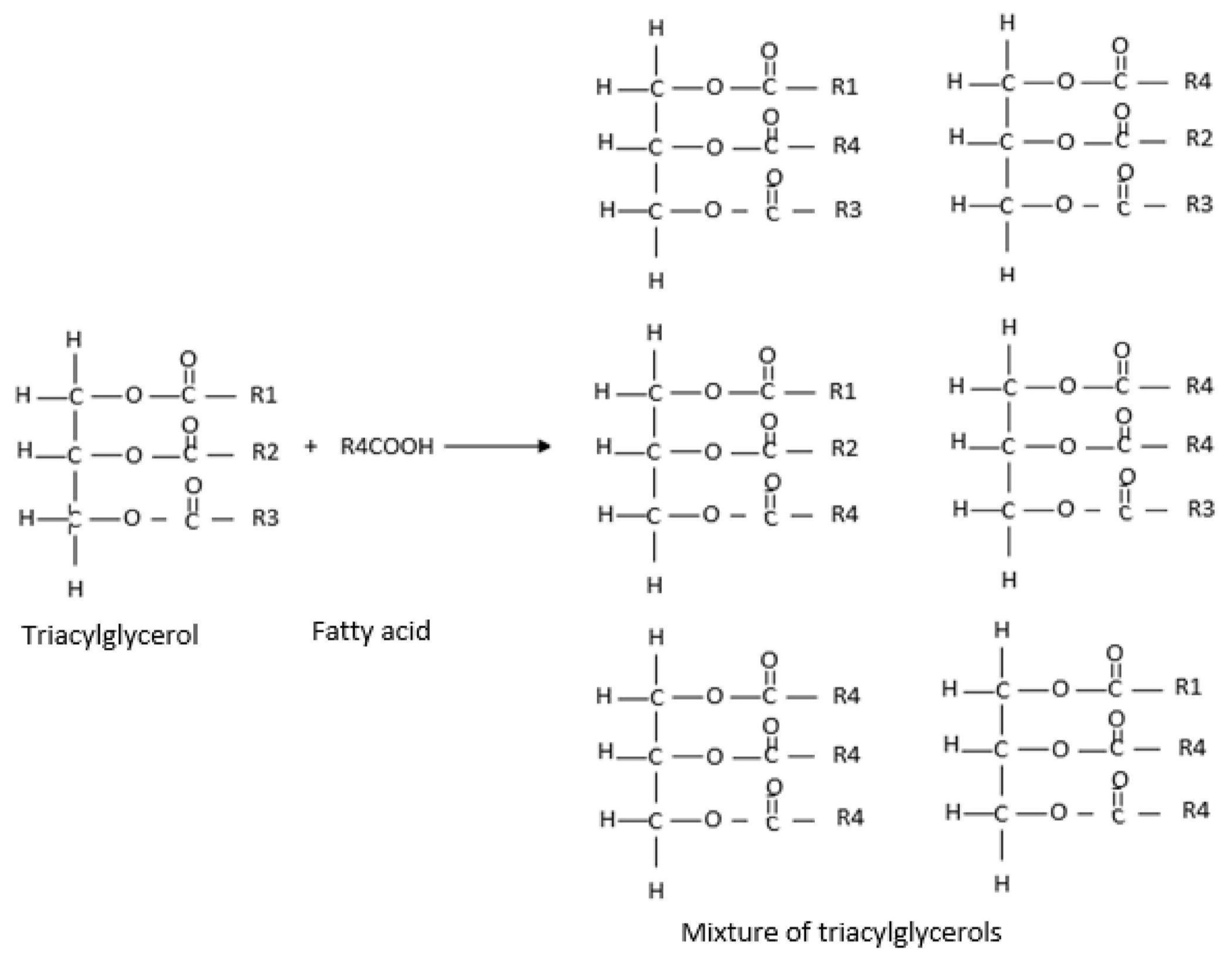
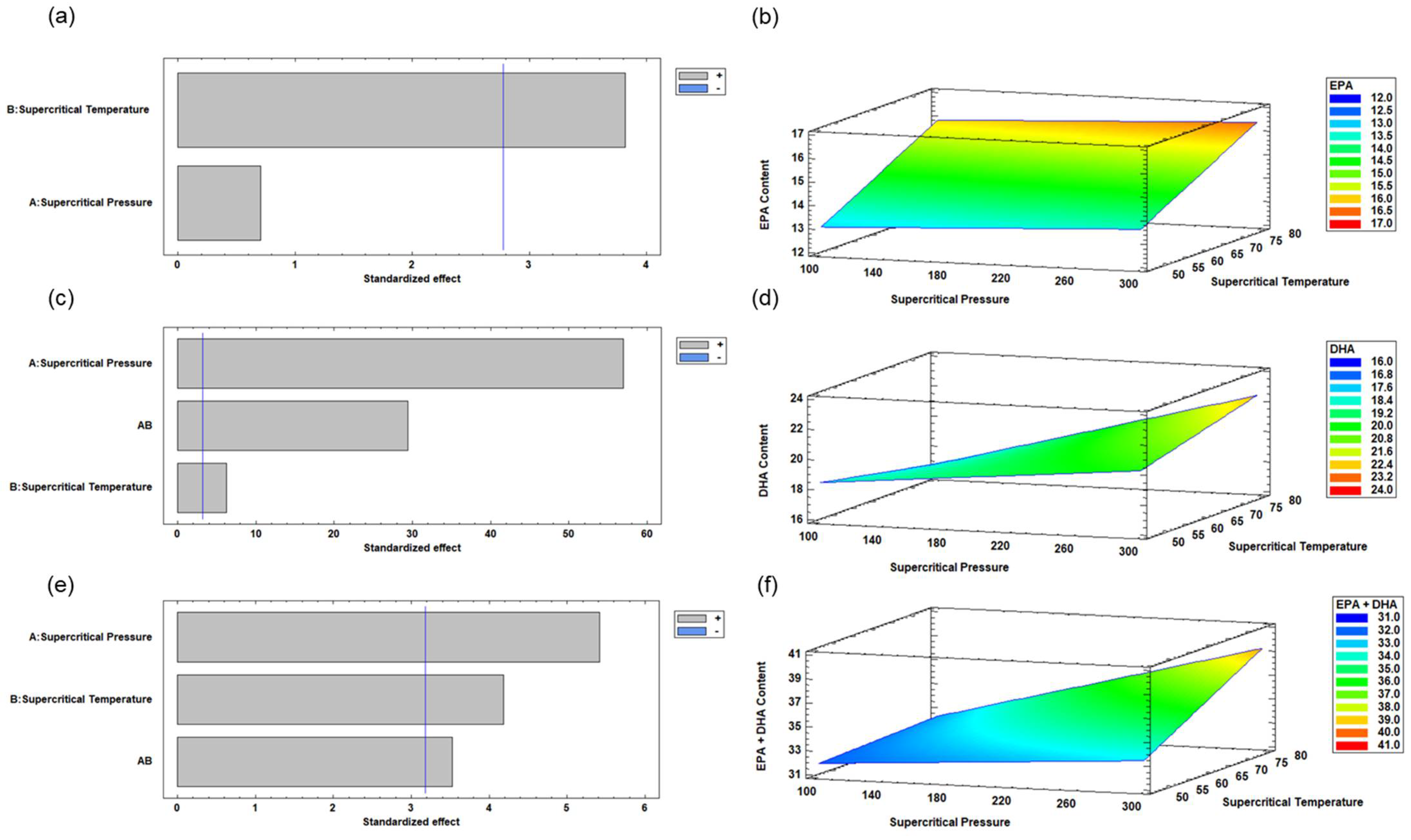
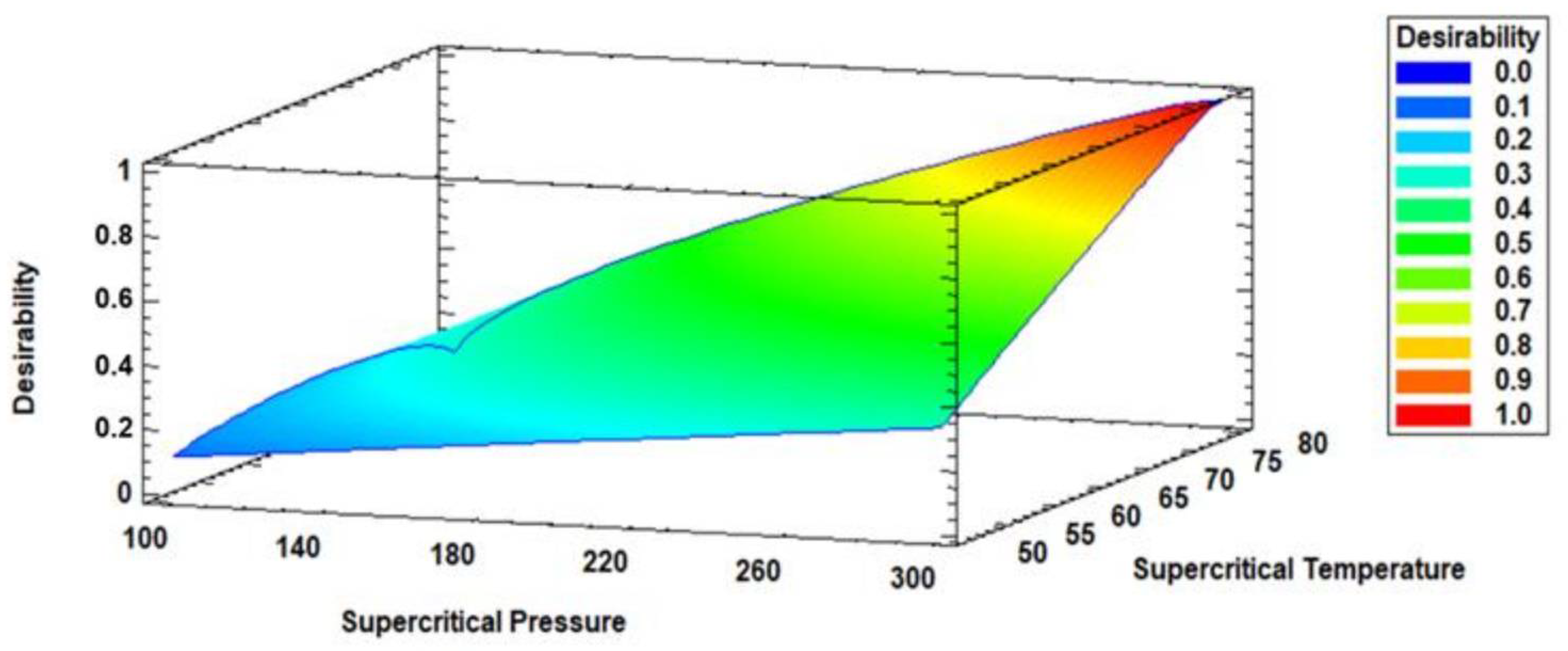
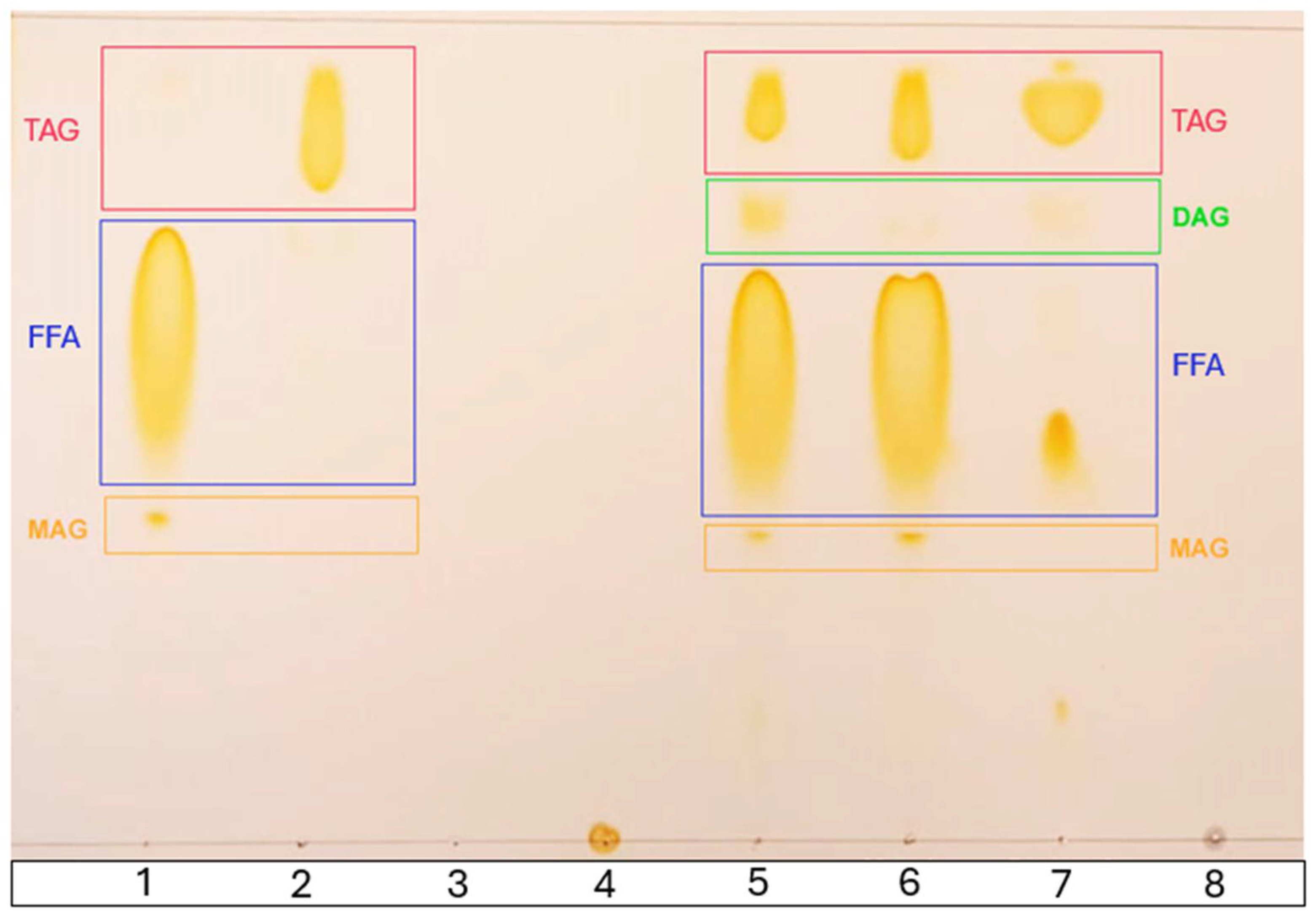


| Experiment (No.) | Supercritical CO2 Pressure (bar) | Supercritical CO2 Temperature (°C) |
|---|---|---|
| 1 | 100 | 50 |
| 2 | 100 | 80 |
| 3 | 300 | 50 |
| 4 | 300 | 80 |
| 5 | 200 | 65 |
| 6 | 200 | 65 |
| 7 | 200 | 65 |
| Gen | Sense | Sequence | %Efficiency |
|---|---|---|---|
| β-actin (NM_007393) | FW | AGGGAAATCGTGCGTGACAT | 100.1 |
| RV | AACCGCTCGTTGCCAATAGT | ||
| IL-6 (DQ788722) | FW | TGATGGATGCTACCAAACTGG | 100.8 |
| RV | TCTCTCTGAAAGACTCTGGCT | ||
| TNFα (NC_000083) | FW | GGTGCCTATGTCTCAGCCTC | 103.4 |
| RV | TGAGGGTCTGGGGCATAGAA | ||
| TLR4 (NM_021297) | FW | CTGGAGAAACTGCTGCCTCA | 101.5 |
| RV | AGCCTGGAAGAAGGTCT |
| Experiment (No.) | EPA | DHA | EPA + DHA |
|---|---|---|---|
| 1 | 13.07 | 18.38 | 31.45 |
| 2 | 12.76 | 20.15 | 32.91 |
| 3 | 15.07 | 16.89 | 31.96 |
| 4 | 16.43 | 22.44 | 38.87 |
| 5 | 15.27 | 19.55 | 34.82 |
| 6 | 15.27 | 19.55 | 34.82 |
| 7 | 15.27 | 19.55 | 34.82 |
| Response Variables | Supercritical CO2 Pressure | Supercritical CO2 Temperature | Stationary Point | Optimized Values |
|---|---|---|---|---|
| Part a: Optimization of the process variables | ||||
| EPA (g/100 g TFA) | 300 | 80 | Maximum | 16.41 |
| DHA (g/100 g TFA) | Maximum | 22.48 | ||
| EPA + DHA (g/100 g TFA) | Maximum | 39.31 | ||
| Part b: Multiple response optimization of the response variables and desirability Optimized process variables to obtain ALω-3 | ||||
| EPA (g/100 g TFA) | 300 | 80 | Maximum | 16.43 |
| DHA (g/100 g TFA) | 22.44 | |||
| EPA + DHA (g/100 g TFA) | 38.87 | |||
| Part c: Experimental validation of the multiple response optimization of the response variables of Part b Optimized process variables by RSM | ||||
| EPA (g/100 g TFA) | 300 | 80 | Maximum | 10.74 |
| DHA (g/100 g TFA) | 16.23 | |||
| EPA + DHA (g/100 g TFA) | 26.97 | |||
| Systematic Name | Abbreviated Name | CMω-3 | Mω-3 | ALω-3 |
|---|---|---|---|---|
| Myristic acid | C14:0 | N.I. | 0.42 ± 0.01 b | 0.60 ± 0.00 a |
| Palmitic acid | C16:0 | 0.53 ± 0.02 c | 2.92 ± 0.08 b | 4.16 ± 0.01 a |
| Cis-Palmitoleic acid | C16:1n-7 | N.I. | 1.02 ± 0.01 b | 1.26 ± 0.01 a |
| Margaric acid | C17:0 | N.I. | 0.17 ± 0.00 | N.I. |
| Margaroleic acid | C17:1 | N.I. | 0.09 ± 0.01 | N.I. |
| Stearic Acid | C18:0 | 3.43 ± 0.08 a | 0.60 ± 0.01 b | 0.54 ± 0.02 b |
| Trans-Vaccenic acid | C18:1n-7t | N.I. | 1.01 ± 0.00 a | 0.86 ± 0.06 b |
| Oleic acid | C18:1n-9 | 5.91 ± 0.08 c | 10.51 ± 0.07 b | 13.36 ± 0.07 a |
| Cis-Vaccenic acid | C18:1n-7 | 2.07 ± 0.00 a | 0.50 ± 0.01 c | 0.75 ± 0.05 b |
| Trans-Linoleic acid | C18:2n-6t | N.I. | 0.07 ± 0.00 | N.I. |
| Cis-Linoleic acid | C18:2n-6 | 1.09 ± 0.02 c | 30.91 ± 0.10 b | 33.86 ± 0.09 a |
| γ-Linolenic acid | C18:3n-6 | N.I. | N.I. | 0.46 ± 0.00 |
| Eicosanoic acid | C20:0 | 0.51 ± 0.00 b | 0.08 ± 0.01 a | N.I. |
| 8- Eicosanoic acid | C20:1n-12 | 1.22 ± 0.03 a | 0.48 ± 0.00 c | 0.60 ± 0.00 b |
| α-Linolenic acid | C18:3n-3 | N.I. | 5.37 ± 0.01 b | 6.34 ± 0.01 a |
| Eicosenoic acid | C20:1n-15 | 0.60 ± 0.02 | N.I. | N.I. |
| Eicosenoic acid | C20:1n-12 | 0.69 ± 0.02 | N.I. | N.I. |
| Eicosenoic acid | C20:1n-9 | 3.65 ± 0.06 c | 4.46 ± 0.01 a | 4.02 ± 0.01 b |
| Eicosadienoic acid | C20:2n-6 | 0.52 ± 0.03 c | 1.47 ± 0.00 a | 1.12 ± 0.03 b |
| Docosanoic acid | C22:0 | N.I. | 0.30 ± 0.00 a | 0.31 ± 0.00 a |
| Cis-11,14,17-Eicosatrienoic acid | C20:3n-3 | N.I. | 0.80 ± 0.00 a | 0.75 ± 0.02 b |
| Erucic acid | C22:1n-1 | 1.87 ± 0.05 | N.I. | N.I. |
| Docosadienoic acid | C22:2n-6 | 1.82 ± 0.03 a | 1.47 ± 0.00 b | 1.32 ± 0.01 c |
| Eicosapentaenoic acid (EPA) | C20:5n-3 | 47.94 ± 0.36 a | 12.58 ± 0.06 b | 10.74 ± 0.13 c |
| Docosatrienoic acid | C22:3n-3 | 1.72 ± 0.03 a | 1.01 ± 0.01 b | 0.80 ± 0.00 c |
| Docosatetraenoic acid | C22:4n-3 | 0.66 ± 0.01 a | 0.34 ± 0.00 b | N.I. |
| Docosapentaenoic acid (DPA) | C22:5n-3 | 3.04 ± 0.04 a | 2.14 ± 0.01 b | 1.91 ± 0.03 c |
| Docosahexaenoic acid (DHA) | C22:6n-3 | 22.65 ± 0.08 a | 20.05 ± 0.15 b | 16.23 ± 0.01 c |
| Total saturated fatty acids (TSFA) | 4.47 c | 4.50 b | 5.61 a | |
| Total monounsaturated fatty acids (TMUFA) | 16.01 c | 18.16 b | 20.85 a | |
| Total polyunsaturated fatty acids (TPUFA) | 79.44 a | 76.22 b | 73.53 c | |
| Total fatty acids n-3 (TFA n-3) | 76.01 a | 42.30 b | 36.77 c | |
| α-Tocopherol | β-Tocopherol | β-Tocotrienol | γ-Tocopherol | γ-Tocotrienol | δ-Tocopherol |
|---|---|---|---|---|---|
| Traces | 22.9 ± 1.4 a | 24.9 ± 0.2 a | 6.8 ± 0.7 b | 22.9 ± 1.7 a | 22.4 ± 4.7 a |
| Sample | ΔH (J·g−1) | TOnset (°C) | TEndset (°C) | TPeak1 (°C) | TPeak2 (°C) | TPeak3 (°C) |
|---|---|---|---|---|---|---|
| LCPUFAn-3 RTBO | 131.40 ± 26.52 ab | −73.23 ± 0.79 a | 58.08 ± 0.00 a | −70.30 ± 0.28 bc | −42.51 ± 0.51 c | - |
| MSO | 157.10 ± 8.17 a | −73.41 ± 2.79 a | 58.09 ± 0.01 a | −71.05 ± 2.23 c | −34.37 ± 0.64 a | −24.07 ± 0.18 a |
| Mω-3 | 111.70 ± 17.0 b | −74.56 ± 0.45 a | 58.09 ± 0.01 a | −65.93 ± 0.15 ab | −46.60 ± 0.89 d | −33.41 ± 0.66 b |
| ALω-3 | 160.90 ± 3.41 a | −75.78 ± 1.86 a | 58.08 ± 0.00 a | −65.48 ± 2.85 a | −36.44 ± 0.10 b | - |
| Histological Feature | G | CMω-3 | Mω-3 | ALω-3 |
|---|---|---|---|---|
| Steatosis | ||||
| Macrovesicular | 0 | 0 | 0 | 0 |
| Microvesicular | 1 | 1 | 1 | 1 |
| Hypertrophy | 0 | 0 | 0 | 0 |
| Inflammation | ||||
| Number of inflammatory foci/field | 0 | 0 | 0 | 0 |
| Total score | 1/12 | 1/12 | 1/12 | 1/12 |
| Pro-Inflammatory Marker | G | CMω-3 | Mω-3 | ALω-3 |
|---|---|---|---|---|
| Fold G | ||||
| Liver | ||||
| IL-6 | 1.0 ± 0.2 a | 1.4 ± 0.3 a | 1.1 ± 0.1 a | 0.9 ± 0.2 a |
| TNF-α | 1.0 ± 0.2 a | 0.8 ± 0.1 a | 0.8 ± 0.2 a | 0.8 ± 0.2 a |
| TLR4 | 1.0 ± 0.1 a | 0.9 ± 0.1 a | 0.8 ± 0.1 a | 0.7 ± 0.1 a |
| Visceral Fat | ||||
| IL-6 | 1.0 ± 0.3 a | 0.8 ± 0.2 a | 0.4 ± 0.1 b | 1.0 ± 0.4 a |
| TNF-α | 1.0 ± 0.1 a | 0.9 ± 0.2 a | 1.1 ± 0.3 a | 0.9 ± 0.2 a |
| TLR4 | 1.0 ± 0.2 a | 0.7 ± 0.0 a | 0.6 ± 0.1 a | 0.9 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claria, B.; Espinosa, A.; Rodríguez, A.; Pando, M.E.; Dovale-Rosabal, G.; Romero, N.; Mayorga, K.; Tapia, E.; Saez, J.; Tsuchida, M.; et al. Effect of Supplementation of Antioxidant Lipids Synthetized by Enzymatic Acidolysis with EPA/DHA Concentrate and Maqui (Aristotelia chilensis (Mol.) Stuntz) Seed Oil for Mitigating High-Fat Diet-Induced Obesity and Metabolic Disorders in Mice. Antioxidants 2025, 14, 790. https://doi.org/10.3390/antiox14070790
Claria B, Espinosa A, Rodríguez A, Pando ME, Dovale-Rosabal G, Romero N, Mayorga K, Tapia E, Saez J, Tsuchida M, et al. Effect of Supplementation of Antioxidant Lipids Synthetized by Enzymatic Acidolysis with EPA/DHA Concentrate and Maqui (Aristotelia chilensis (Mol.) Stuntz) Seed Oil for Mitigating High-Fat Diet-Induced Obesity and Metabolic Disorders in Mice. Antioxidants. 2025; 14(7):790. https://doi.org/10.3390/antiox14070790
Chicago/Turabian StyleClaria, Benjamín, Alejandra Espinosa, Alicia Rodríguez, María Elsa Pando, Gretel Dovale-Rosabal, Nalda Romero, Katherynne Mayorga, Evelyn Tapia, Jenifer Saez, Melissa Tsuchida, and et al. 2025. "Effect of Supplementation of Antioxidant Lipids Synthetized by Enzymatic Acidolysis with EPA/DHA Concentrate and Maqui (Aristotelia chilensis (Mol.) Stuntz) Seed Oil for Mitigating High-Fat Diet-Induced Obesity and Metabolic Disorders in Mice" Antioxidants 14, no. 7: 790. https://doi.org/10.3390/antiox14070790
APA StyleClaria, B., Espinosa, A., Rodríguez, A., Pando, M. E., Dovale-Rosabal, G., Romero, N., Mayorga, K., Tapia, E., Saez, J., Tsuchida, M., Vásquez, K., Valenzuela, R., Pérez, Á., Díaz, P., & Aubourg, S. P. (2025). Effect of Supplementation of Antioxidant Lipids Synthetized by Enzymatic Acidolysis with EPA/DHA Concentrate and Maqui (Aristotelia chilensis (Mol.) Stuntz) Seed Oil for Mitigating High-Fat Diet-Induced Obesity and Metabolic Disorders in Mice. Antioxidants, 14(7), 790. https://doi.org/10.3390/antiox14070790








