Abstract
Alzheimer’s disease (AD), the leading cause of dementia, remains poorly understood despite decades of intensive research, which continues to hinder the development of effective treatments. As a complex multifactorial disorder, AD lacks a cure to halt the progressive neurodegeneration, and the precise mechanisms underlying its onset and progression remain elusive, limiting therapeutic options. Due to the challenges of studying neuronal cells in vivo, technologies such as clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) and human-induced pluripotent stem cells (hiPSCs) are key for identifying therapeutic targets, although they face technical and ethical hurdles in their early stages. CRISPR/Cas9 and hiPSCs are promising for disease modeling and therapy, but off-target effects and the complexity of gene editing in the brain limit their use. CRISPR technology enables specific genetic modifications in key AD-related genes, such as APP, PSEN1, PSEN2, and APOE, providing valuable insights into disease mechanisms. iPSC-derived neurons, astrocytes, microglia, and 3D organoids can recapitulate key aspects of human AD pathology, but they do not fully replicate the complexity of the human brain, limiting clinical applicability. These technologies advance studies of amyloid processing, tau aggregation, neuroinflammation, and oxidative stress, yet translating them into clinical therapies remains challenging. Despite the promise of CRISPR/Cas9 and iPSCs for precision medicine, gaps in knowledge about their long-term safety and efficacy must be addressed before clinical implementation.
1. Introduction
Alzheimer’s disease (AD) is a chronic, degenerative condition that significantly affects brain function and represents the leading cause of dementia worldwide, particularly among women aged over 65 years [1]. Typically, progressive neuronal damage causes cognitive decline, affecting memory, language, orientation, and behavior, primarily due to irreversible brain atrophy [2]. As the prevalence of AD continues to rise, there is an urgent need to develop effective treatments or preventive strategies. Currently, no curative treatment exists, and the available options offer only limited symptomatic relief [1].
The main pathological hallmarks of AD include the accumulation of beta-amyloid (Aβ) peptides and the abnormal phosphorylation of Tau protein. These alterations lead to extracellular plaques and intracellular neurofibrillary tangles in the brain, respectively. These phenomena are typically associated with neurodegeneration [3,4,5].
Beyond the accumulation of Aβ and Tau, additional processes such as neurodegeneration and synaptic degeneration are linked to disease progression [6,7,8]. Chronic glial activation can exacerbate neuronal loss, while plaques and tangles can disrupt communication, impairing neuronal plasticity. However, the molecular basis of these processes is still not fully understood [9]. Among these molecular processes, oxidative stress has emerged as a critical contributor to AD pathogenesis, as it exacerbates neuronal injury through the accumulation of reactive oxygen species and disruption of mitochondrial function [10].
The complex nature of AD, with diverse genetic, molecular, and clinical manifestations, complicates the development of a unified model [11]. In most cases, late-onset AD is genetically and etiologically heterogeneous, with innumerable genes and environmental factors involved in disease progression. In contrast, familial AD (FAD) is rare (<1%) and results from autosomal dominant mutations in genes such as amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) [12].
A key obstacle in AD research concerning therapeutic strategies arises from the difficulties associated with in vivo models, which often fail to replicate human neuropathology [13,14] accurately. Genotype–environment interactions and differences in experimental approaches further prevent animal models from simulating human disease, limiting the translational impact of preclinical findings [11,15]. Although some therapies have shown promise, their clinical efficacy remains modest, and side effects pose concerns. Thus, innovative strategies are needed to better understand the molecular mechanisms underlying AD and to identify new, more effective interventions [16].
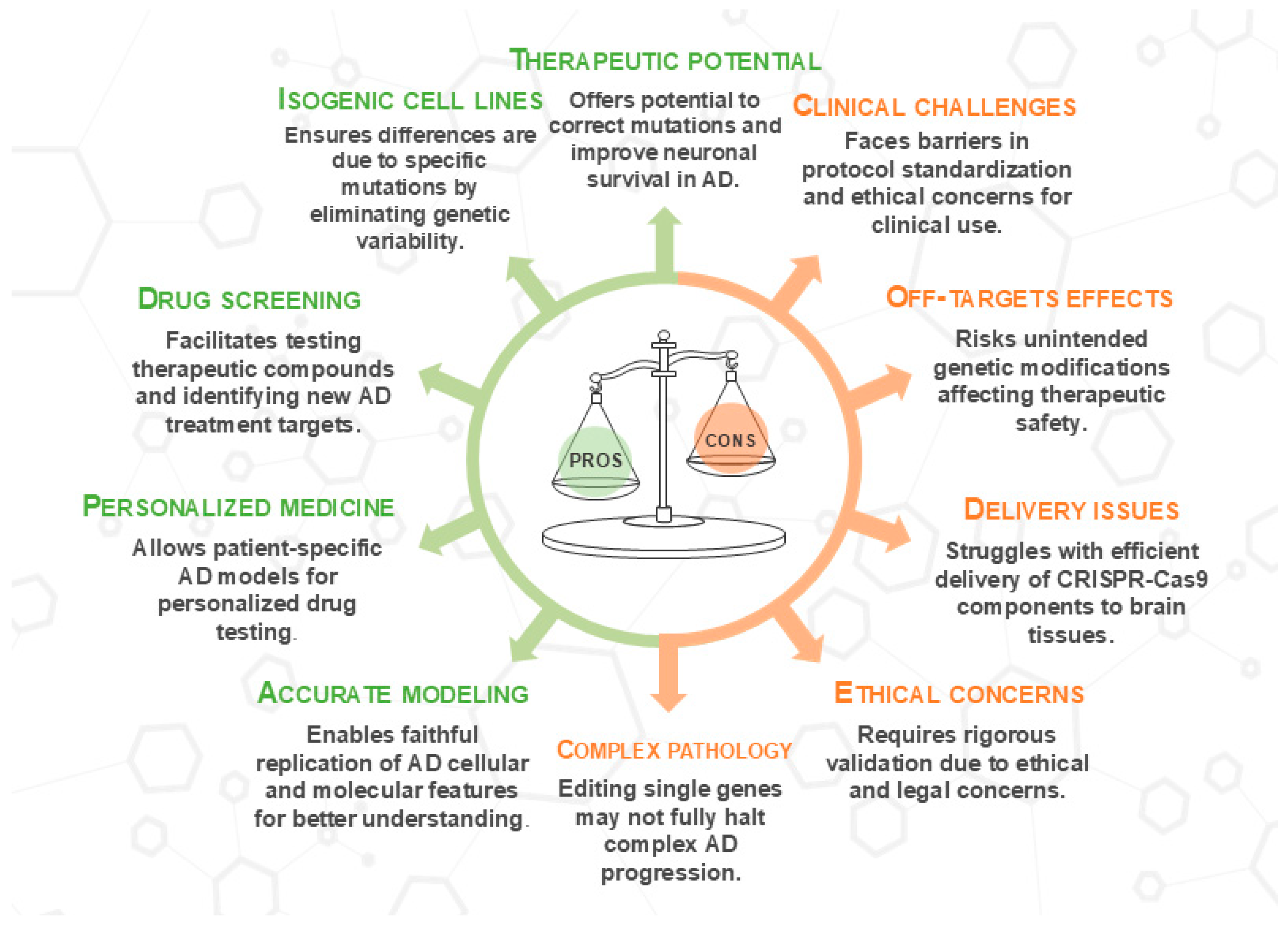
In this context, combining genetic and cellular therapies has emerged as a promising direction, slowing disease progression and possibly restoring specific cellular functions [17]. As explored in Section 2, Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9 (CRISPR/Cas9) has revolutionized gene editing approaches in AD by enabling targeted manipulation of pathogenic loci [18]. Preclinical studies in animal models of AD reported that gene therapy could lower Aβ deposition and enhance cognitive function, alongside a reduction in inflammation and neurodegeneration [19]. At the same time, stem cell-based approaches have gained attention for their potential in neurodegeneration. Several stem cell types, including neural stem cells (NSCs), mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), and human-induced pluripotent stem cells (hiPSCs), have been studied for their ability to differentiate into functional neuronal and glial cells [17]. Moreover, three-dimensional (3D) brain organoids derived from hiPSCs have shown the capacity to replicate key AD processes. They are used to test the efficacy of drugs despite limitations in mimicking complex brain interactions, cell maturation, and aging [20]. CRISPR, combined with hiPSCs, enables precise introduction or correction of genetic variants, such as single nucleotide polymorphisms (SNPs), without altering the entire genome. This approach provides a powerful platform to investigate the functional consequences of specific genetic alterations, enhancing the accuracy of disease modeling, drug screening, and gene function analysis [20]. Despite its potential, the limitations of the model must be considered. Challenges include off-target effects, delivery inefficiency, and mosaicism for CRISPR, as well as developmental immaturity, genomic instability, and cellular heterogeneity in neurons and iPSC-derived organoids. These technical and biological constraints reduce the fidelity of the model and limit translational applicability.
This review aims to provide a comprehensive and critical view of the opportunities offered by integrated therapies to overcome the limitations of current therapies, improving the management and treatment of AD.
2. CRISPR-Based Gene Editing in AD: Target Genes, Therapeutic Potential, and Challenges
CRISPR/Cas9 is a powerful gene-editing tool with growing potential to study and treat neurodegenerative diseases such as AD, which currently lack effective therapies [21,22,23,24]. CRISPR/Cas9 has been applied to identify and edit genes involved in oxidative stress, a key contributor to AD pathogenesis, offering potential therapeutic avenues to mitigate related neuronal damage [25,26]. Since most mutations in the APP, PSEN1, and PSEN2 genes are associated with increased Aβ production, the use of CRISPR/Cas9 to correct these mutations in brain cells could help reduce Aβ accumulation [27].
This, in turn, may mitigate downstream pathological processes such as oxidative stress and other hallmarks of aging and neurodegenerative diseases.
2.1. Mechanism of CRISPR/Cas9
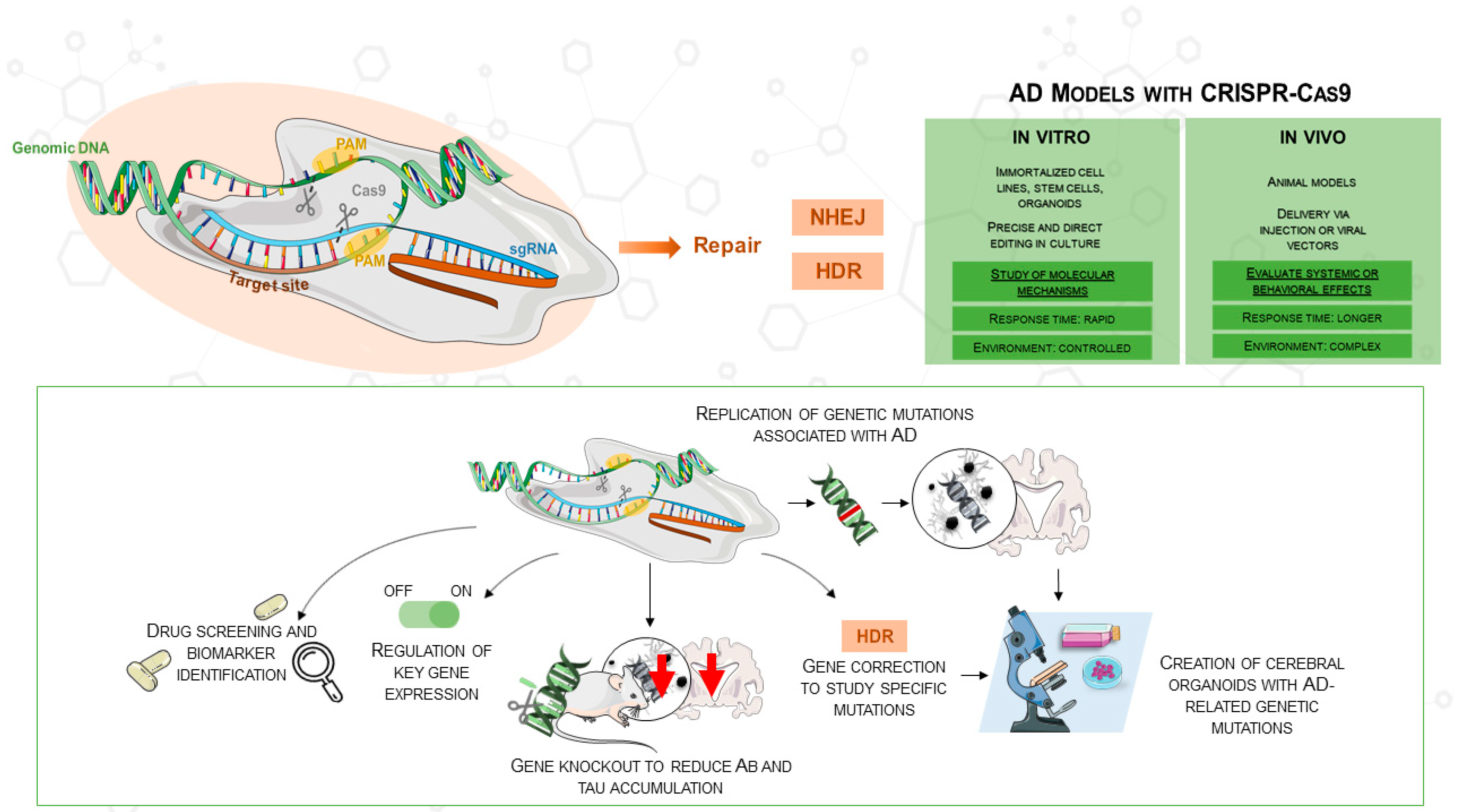
Various neurodegenerative diseases have been investigated with well-established methods such as TALENs and zinc-finger nucleases. However, CRISPR/Cas9 has become a more easily available and flexible method, especially fit for AD research [28]. Originally discovered in the bacterial adaptive immune system, CRISPR/Cas9 targets DNA sequences with the Cas9 nuclease in concert with a guide RNA (gRNA). A double-stranded break (DSB) is produced when the nuclease binds to its target, activating intrinsic repair mechanisms that might produce either homology-directed repair (HDR) or non-homologous end joining (NHEJ). This technique can be used to introduce or fix mutations connected to diseases. CRISPR/Cas9 consists of two main components: a single-guide RNA (sgRNA) that guides Cas9 to the matching target DNA sequence and a Cas9 protein that cleaves the target DNA. Usually 5′-NGG-3′, a brief protospacer adjacent motif (PAM), is necessary immediately downstream of the target for Cas9 recognition. Reprogramming CRISPR/Cas9 usually requires the design of a new sgRNA rather than changing a protein scaffold; thus, this RNA-based targeting mechanism offers many more advantages than gene-editing techniques dependent on engineered protein–DNA interactions [28]. Moreover, since CRISPR/Cas9 allows the combination of multiple sgRNAs in a single experiment, multiplex gene editing is enhanced, enabling the simultaneous targeting of several genes involved in Alzheimer’s disease (Figure 1). This approach not only accelerates research but also facilitates the creation of more accurate disease models, as shown in the comparison of in vitro and in vivo applications in the figure.
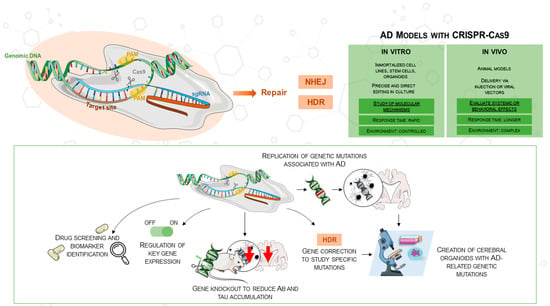
Figure 1.
The figure illustrates the use of CRISPR/Cas9 in AD research, highlighting its application in both in vitro and in vivo models for gene knockouts, mutations, and gene correction. CRISPR/Cas9 enables the replication of AD mutations, the regulation of gene expression, and the creation of cerebral organoids from hiPSCs. The comparison table outlines in vitro (e.g., cell lines, organoids) and in vivo (e.g., animal models) models in AD research, depicting CRISPR/Cas9’s role in advancing therapeutic development. This image was created using the image bank of Servier Medical Art (Available online: http://smart.servier.com/; accessed on 30 May 2025) licensed under a Creative Commons Attribution 3.0 Unported License (available online: https://creativecommons.org/licenses/by/3.0/, accessed on 30 May 2025). AD: Alzheimer’s disease; CRISPR/Cas9: Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9; DSB: double-strand breaks; sgRNA: single guide RNA; NHEJ: non-homologous end joining; HDR: homology-directed repair; Aβ: amyloid beta.
2.2. Gene-Editing Potential of CRISPR/Cas9 in AD Models
CRISPR/Cas9 technology has emerged as a versatile and promising tool for investigating and manipulating key genetic mechanisms involved in AD pathogenesis [29,30]. Precise genetic manipulation holds promise for improving patient outcomes [31]. CRISPR/Cas9 enables the creation of genetically modified models (in vivo and in vitro) to target key AD mechanisms, such as Aβ plaque deposition, Tau hyperphosphorylation, and neuroinflammation. For further details, refer to Table 1.
Kwart et al. used CRISPR/Cas9 to alter Aβ production in mice with mutant APP, reducing toxic Aβ peptides without impairing brain function. In a separate set of experiments, they also targeted other genes implicated in AD pathology. Specifically, they used CRISPR/Cas9 to downregulate BACE1, a key enzyme involved in Aβ generation, and to modulate genes involved in neuroinflammation. Additionally, CRISPR-based editing increased the expression of neurotrophic factors such as brain-derived neurotrophic factors (BDNF), contributing to enhanced neuronal protection and synaptic repair. Overall, these genetic modifications not only improved synaptic plasticity but also led to significant cognitive improvements in behavioral tests [32]. In addition, by reducing Aβ accumulation, these genetic corrections may also alleviate oxidative stress, which is known to contribute to neurodegeneration in AD [33]. The connection between Aβ and oxidative stress has been well established: Aβ peptides can bind redox-active metal ions such as copper and iron, which catalyze the formation of reactive oxygen species (ROS), contributing to cellular damage [34]. Several molecular mechanisms have been described in which oxidative stress and the two hallmark proteins, Aβ and tau, could be associated, creating a vicious cycle that accelerates disease progression, impairing neural and cognitive function [35]. It is likely that Aβ-dependent oxidative damage may be caused by the inhibition of mitochondrial oxidative phosphorylation by Aβ, accelerating neurodegeneration and highlighting the role of ROS in Aβ toxicity [36].
Aβ production begins with the cleavage of APP by the enzyme β-secretase, which releases C-terminal membrane amino acid fragments. The membrane-bound C-terminal fragment is further degraded by γ-secretase to produce the Aβ1-40 and Aβ1-42 isoforms [37]. The Aβ1-42 isoform contributes to amyloid plaque formation due to its propensity for self-aggregation. Aβ1-42 production is linked to mutations in APP, PSEN1, and PSEN2 genes, with APOE4 increasing Aβ accumulation and AD risk [38,39]. CRISPR/Cas9 has been used to manipulate PSEN1 and PSEN2, key components of γ-secretase involved in Aβ production, by creating knockout cell lines in mouse neuroblastoma cells (N2A). These models have been used to study genetic variants commonly found in hereditary AD, providing insight into Aβ production and oxidative stress [40]. However, the effects of PSEN1 mutations on γ-secretase activity are complex. While some mutations increase the Aβ42/Aβ40 ratio and contribute to disease, others may reduce Aβ production or cause an imbalance in unexpected ways. Sun et al. (2017) demonstrated that certain PSEN1 mutations alter γ-secretase activity, with varying effects on Aβ production, highlighting the need to consider mutation-specific variability when exploring γ-secretase modulation in AD therapies [41]. In another study, the PSEN1M146L allele, known to promote Aβ42 generation and cause early-onset AD, was selectively ablated in human fibroblasts using CRISPR/Cas9. The technology successfully mutated more than 50% of the alleles harboring the mutation across samples from multiple families, resulting in a decreased Aβ42/40 ratio in the culture medium. Thus, the use of CRISPR/Cas9 by selectively targeting the PSEN1M146L allele could have the ability to partially restore the Aβ42/40 ratio imbalance and counteract the AD-associated phenotype [42]. A similar result was obtained in another study in which CRISPR/Cas9 technology was used to correct the PSEN2N141I mutation associated with autosomal dominant early-onset FAD [43]. Another target for the CRISPR/Cas9 system is mutations in the APP gene. CRISPR/Cas9 was applied to selectively eliminate the APPswe (Swedish) allele both ex vivo and in vivo, reducing Aβ levels [44]. However, while this approach shows promise, the long-term effects and off-target risks of such genetic modifications require further evaluation. In contrast, another study utilized CRISPR/Cas9 technology to introduce a new mutation (E674K) in the APP gene. The A673T mutation was successfully introduced in 53% of HEK293T cells, resulting in reduced Aβ levels [45]. However, the long-term safety and potential off-target effects of such mutations remain major concerns that require further validation. While these findings suggest that CRISPR/Cas9 could be a valuable therapeutic tool for AD patients with APPswe and other point mutations increasing Aβ levels, the risks of off-target effects and the need for rigorous validation before clinical application are significant concerns.
In another study, CRISPR/Cas9 was used to induce SORL1 deficiency in a preclinical AD model in pigs. Knockout of the SORL1 gene slowed endosomal recycling, indirectly increasing the levels of Aβ and tau in cerebrospinal fluid. These results highlight the importance of CRISPR/Cas9 in studying early disease stages and testing preventive treatments [28]. The use of this technology can be extended to identify new mutations. In fact, in one study, a mutation (p.T209S) was induced in the ZDHHC21 gene, associated with a Chinese family affected by FAD. The mutation in this gene increased the palmitoylation of key proteins, such as APP and FYN, contributing to the accumulation of Aβ and phosphorylation of tau. In addition, mutant mice showed significant cognitive decline, as well as reduced synaptic plasticity and neuronal loss, effects related to the hyperpalmitoylation of FYN and the activation of the NMDAR2B receptor, causing neuronal excitotoxicity. Using CRISPR/Cas9 to alter post-translational modifications like palmitoylation, researchers identified a previously unexplored mechanism in AD pathogenesis. Moreover, the use of palmitoylation inhibitors like 2-BP improved synaptic plasticity and reduced neurodegeneration, suggesting a potential therapeutic strategy for AD patients [46].
The link between Aβ and oxidative stress is well established: Aβ binds redox-active metals, such as copper and iron, facilitating the production of ROS that contribute to neuronal damage. CRISPR/Cas9 interventions targeting APP, PSEN1/2, and BACE1 have been shown to reduce ROS generation and inflammation, thereby improving synaptic plasticity and neuronal viability [47]. Beyond amyloid pathology, CRISPR/Cas9 has also been employed to modulate neuroinflammatory responses, a central feature of AD progression. Activated microglia, in response to Aβ and tau, can produce pro-inflammatory cytokines that exacerbate neuronal injury. Gene editing strategies targeting TREM2, a key modulator of microglial activation, have yielded mixed outcomes [48,49].
In a study using TauPS2APP (which develop both amyloid and tau pathology) and P301L homozygous (which develop only tau pathology without Aβ) mouse models, researchers examined early and late disease stages, monitoring tau accumulation, brain atrophy, and microglial response over the lifespan of the mice (from 9 to 17 months). Deletion of the TREM2 gene using gene editing worsened tau accumulation and accelerated brain atrophy, especially in the presence of Aβ. These results indicate the potential of CRISPR/Cas9 to intervene in TREM2, regulate the microglial response, reduce inflammation, and slow AD progression [50]. In compliance with these data, in another study, TREM2 knockouts showed abnormal microglial activation and altered inflammatory responses, particularly in a pro-inflammatory context stimulated with peripheral lipopolysaccharide (LPS) [51]. In a recent study, researchers introduced the Y38C variant into the Trem2 gene, showing how its mutation leads to impaired synaptic function and altered microglial responses, even in early stages and in the absence of pathological triggers such as Aβ [52]. These findings underline the importance of gene editing in modulating inflammatory processes and microglial responses in AD models.
Another study has further explored the effect of gene editing on neuroinflammation and synaptic plasticity. CRISPR/Cas9 was used to delete the CysLT1R gene in an APP/PS1 mouse model of AD, which showed Aβ accumulation and cognitive decline. Knocking out this gene significantly reduced the accumulation of neurotoxic and unstable Aβ species in the brain, leading to a decrease in plaque formation and associated pathological changes. This reduction reduces the levels of pro-inflammatory cytokines such as TNF-α and IL-6, which modulate the microglial response, thus reducing the immune response. Indeed, gene modification reduced NF-κB activation, a key transcription factor in inflammation, decreasing amyloidosis and enhancing synaptic protection. Thus, CysLT1R knockout mice showed improved synaptic plasticity and cognitive performance and increased long-term potentiation [53].
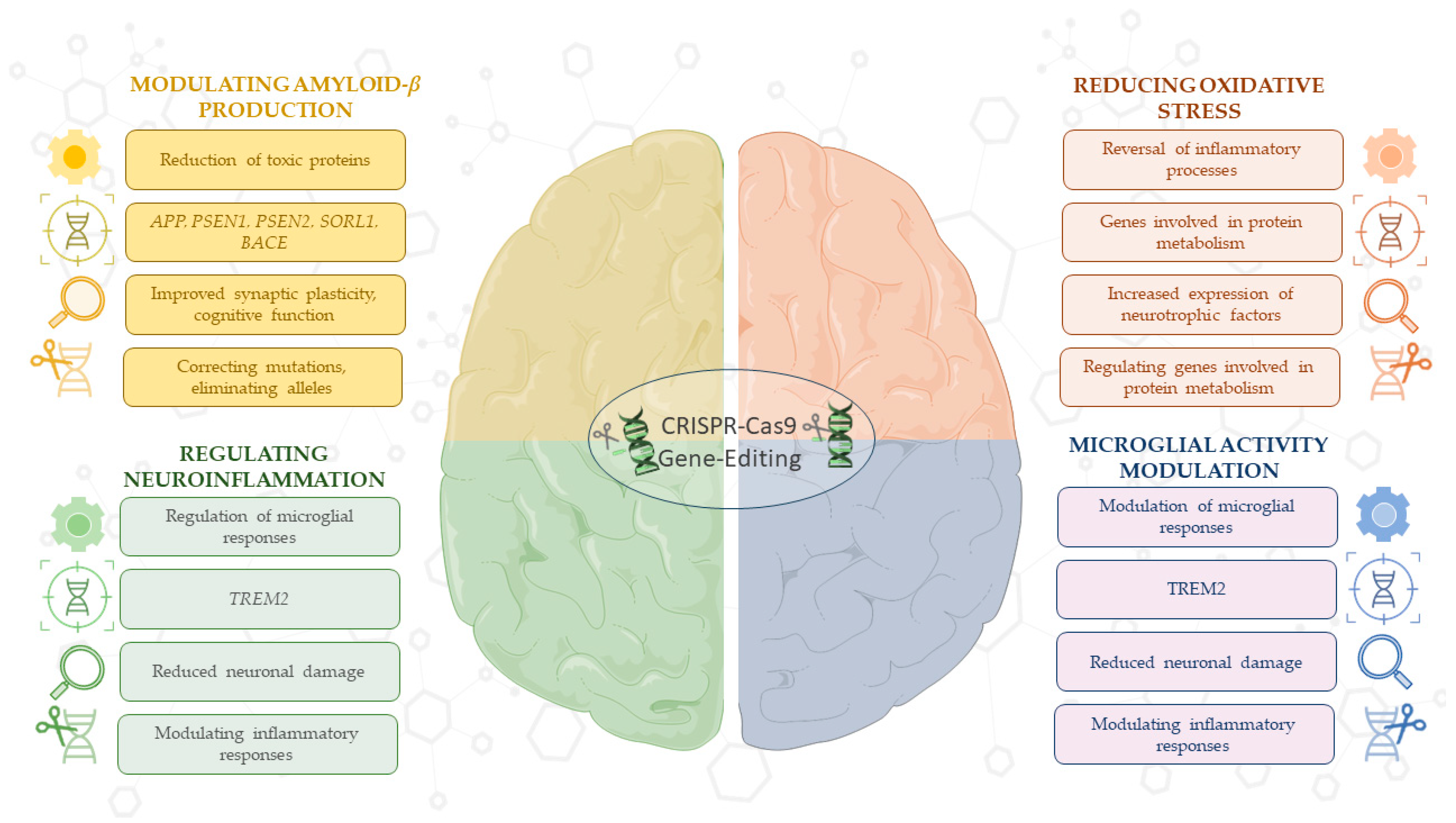
Overall, the results of these preclinical studies emphasize the importance of using CRISPR/Cas9 technology to modulate molecular and cellular processes, such as Aβ production and reduction of oxidative stress, underlying the pathogenesis of AD. This technology, alone or in combination with other treatments, could identify promising therapeutic strategies aimed at halting or even reversing AD progression (Figure 2).
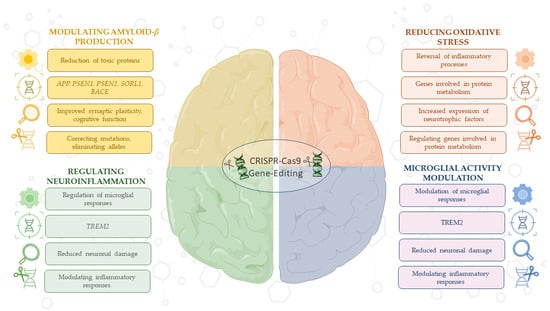
Figure 2.
This schematic illustrates the therapeutic versatility of CRISPR/Cas9 in targeting key processes in AD. CRISPR/Cas9 enables the regulation of Aβ production through gene editing in critical AD-related genes, such as APP, PSEN1, PSEN2, SORL1, and BACE, while also enhancing synaptic function. Technology modulates neuroinflammation and microglia activity, particularly through genes like TREM2, to reduce neuronal damage. Additionally, CRISPR/Cas9 reduces oxidative stress by reversing inflammatory pathways, regulating genes involved in protein metabolism, and promoting neurotrophic factor release. However, challenges remain in the efficiency of CRISPR/Cas9 delivery, including limitations in targeting specific tissues and concerns about off-target effects. These applications highlight CRISPR’s potential as a therapeutic strategy for AD, despite current delivery barriers. For detailed procedures, refer to Table 1. This image was created using the image bank of Servier Medical Art (Available online: http://smart.servier.com/; accessed on 30 May 2025) licensed under a Creative Commons Attribution 3.0 Unported License (available online: https://creativecommons.org/licenses/by/3.0/, accessed on 30 May 2025). APP: Amyloid precursor protein; PSEN1: presenilin 1; PSEN2: presenilin 2; SORL1: sortilin-related receptor 1; BACE: beta-site APP-cleaving enzyme; TREM2: triggering receptor expressed on myeloid cells 2; CRISPR/Cas9: clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9.

Table 1.
The table summarizes the main preclinical evidence for CRISPR/Cas9 gene editing in AD research. It includes studies focusing on the modulation of Aβ production and oxidative stress, as well as studies targeting neuroinflammation and microglial activity. In detail, the table outlines the objective of the studies, the model and experimental design used, together with the main results and their therapeutic implications. These studies highlight how precise gene editing can advance our understanding of AD’s pathogenesis and offer promising prospects for targeted interventions.
Table 1.
The table summarizes the main preclinical evidence for CRISPR/Cas9 gene editing in AD research. It includes studies focusing on the modulation of Aβ production and oxidative stress, as well as studies targeting neuroinflammation and microglial activity. In detail, the table outlines the objective of the studies, the model and experimental design used, together with the main results and their therapeutic implications. These studies highlight how precise gene editing can advance our understanding of AD’s pathogenesis and offer promising prospects for targeted interventions.
| Study | Technology | Aim | Model | Experimental Design | Result | Implications | Ref. |
|---|---|---|---|---|---|---|---|
| CRISPR/Cas9 in mouse models to edit APP and PSEN | CRISPR/Cas9 | Correct genetic mutations in APP and PSEN1/PSEN2 genes to reduce Aβ production | Transgenic mouse models | Mouse models with correction of APP/PSEN mutations, measurement of Aβ reduction and cognitive improvement | Reduction in beta-amyloid plaques and improvement in cognitive function | Therapeutic potential of CRISPR/Cas9 to reduce the production of toxic proteins | [32] |
| Knockout of PSEN1/PSEN2 in murine N2A cells | CRISPR/Cas9 | Knockout PSEN1/PSEN2 to study γ-secretase’s role in APP metabolism and Aβ production | Murine N2A cell lines | Creation of PSEN1/PSEN2 knockout in N2A cells and study of genetic variants and their effects on the Aβ42/Aβ40 ratio | Elimination of Aβ production and identification of variants increasing the Aβ42/Aβ40 ratio | Platform for testing genetic mutations in the presenilin genes | [40] |
| CRISPR/Cas9-mediated PSEN1 knockout in N2a cells | CRISPR/Cas9 | Assess the impact of 138 PSEN1 mutations on Aβ40 and Aβ42 production | In vitro reconstitution in N2A-PSEN1/2KO-8/71 cells | Expression of wild-type and mutant PSEN1; measurement of Aβ levels | 90% of mutations reduced Aβ40 and Aβ42 production, while 10% decreased the Aβ42/Aβ40 ratio | Provides insights into how PSEN1 mutations affect γ-secretase activity, informing CRISPR-based strategies to correct or compensate for these mutations | [41] |
| CRISPR/Cas9 corrects the PSEN1M146L mutation in a FAD model | CRISPR/Cas9 | To evaluate whether CRISPR/Cas9 can selectively disrupt the PSEN1M146L allele in human fibroblasts and normalize the Aβ 42/40 ratio, a hallmark of AD | Human fibroblasts with the PSEN1M146L mutation were transfected with CRISPR/Cas9 plasmids | In vitro use of CRISPR/Cas9 to correct the PSEN1M146L mutation, followed by analysis of Aβ production and neuronal function | Correcting the mutation restored the Aβ42/40 ratio, reducing amyloid plaque formation and improving electrophysiological properties of neurons | This study emphasizes the potential of CRISPR/Cas9 in FAD treatment by restoring normal amyloid precursor protein cleavage | [42] |
| CRISPR/Cas9-correctable mutation-related molecular and physiological phenotypes in iPSC-derived Alzheimer’s PSEN2N141I neurons | CRISPR/Cas9 | To model AD in vitro using PSEN2N141I mutant iPSC-derived BFCNs and assess whether CRISPR/Cas9 correction reverses molecular and functional phenotypes | Human iPSCs from PSEN2N141I mutation carriers and controls | PSCs were differentiated into BFCNs; Aβ42/40 levels and electrophysiological activity were measured; CRISPR/Cas9 was used to correct PSEN2N141I; functional, biochemical, and molecular assays compared mutant, control, and corrected lines | PSEN2N141I neurons showed an increased Aβ42/40 ratio and reduced spike frequency/amplitude; CRISPR/Cas9 correction normalized both molecular and physiological phenotypes | Demonstrates that PSEN2N141I contributes to AD-related dysfunctions in human neurons and that these defects are reversible through gene editing.; supports the amyloid hypothesis and use of iPSC-BFCNs for AD modeling | [43] |
| Human fibroblasts from APPswe mutation carriers and APPWT controls | CRISPR/Cas9 | Disrupt the APPswe to reduce Aβ production in AD | Human fibroblasts from APPswe mutation carriers and APPWT controls | CRISPR/Cas9 was used to target the APPswe or APPWT alleles in human fibroblasts with selective gRNAs | There was a 60% reduction in secreted Aβ40 in fibroblasts. In vivo disruption of the APPswe allele in a mouse model led to decreased Aβ production. | It proves CRISPR/Cas9 can selectively disrupt APP mutations in AD models, offering a potential gene therapy for FAD | [44] |
| Base editing strategy for insertion of the A673T mutation in the APP gene to prevent AD development in vitro | CRISPR/Cas9 | To insert the A673T mutation in the APP gene to reduce Aβ accumulation and prevent AD development | HEK293T and SH-SY5Y cell lines | Used Cas9 nickase-based base editors for insertion of A673T mutation in the 7 gene; quantification of Aβ levels and deep sequencing to evaluate editing efficiency | The A673T mutation was successfully inserted in 53% of HEK293T cells. This resulted in reduced Aβ peptide accumulation, particularly Aβ40 and Aβ42. | It suggests that base editing could be used to prevent the development of AD by modifying the APP gene and reducing toxic Aβ accumulation | [45] |
| Preclinical AD model in pigs (SORL1 knockout) | CRISPR/Cas9 | Create a preclinical AD model in pigs with SORL1 deficiency to study the effect on Aβ and tau production | Pigs with SORL1 knockout | Creation of SORL1 knockout pigs to evaluate preclinical AD biomarkers, including Aβ and tau in cerebrospinal fluid | Increase in Aβ and tau in cerebrospinal fluid, without formation of amyloid plaques | Useful model for exploring the preclinical phase of AD and testing new treatments | [28] |
| ZDHHC21 mutation and the role of palmitoylation | CRISPR/Cas9 | Introduce the ZDHHC21 p.T209S mutation to study the effect of palmitoylation on APP and AD pathology | Mouse models with ZDHHC21 mutation | Introduction of ZDHHC21 mutation in mouse models to study the effect of palmitoylation on Aβ and tau | Increase in palmitoylation, Aβ production, and tau phosphorylation, leading to cognitive decline | Alternative mechanism in AD pathogenesis with therapeutic potential linked to palmitoylation | [46] |
| TREM2 deletion and microglial regulation | CRISPR/Cas9 | Study the role of TREM2 in regulating microglial response and tau pathology in the presence of amyloid | TauPS2 APP mice (Aβ and tau pathologies), P301Lhomo mice (tauopathy) | TREM2 deletion in TauPS2APP mice to study the impact on microglial function and tau pathology in the presence of Aβ | TREM2 plays a protective role in limiting tau pathology, suggesting it as a target for gene editing therapies | Worsening tau accumulation and brain atrophy in the presence of Aβ, impaired microglial function | [50] |
| Behavioral and transcriptomic analysis of Trem2-null mice | CRISPR/Cas9 | Investigate the impact of TREM2 deficiency on neuroinflammation and microglial response in AD models | Trem2 knockout mice (VelociGene and CRISPR/Cas9 versions) | Gene editing was used to create TREM2-deficient mice; transcriptomic analysis and behavioral tests were performed | No significant behavioral or cognitive differences were observed in TREM2 knockout mice; microglial activation was delayed following an LPS challenge. | The study shows that TREM2 is crucial for modulating microglial response in neuroinflammation and suggests caution when interpreting the effects of specific gene knockout strategies in AD research | [51] |
| TREM2 Y38C mutation and loss of TREM2 impairs neuronal synapses in adult mice | CRISPR/Cas9 | Investigate the impact of TREM2 Y38C mutation and TREM2 loss on neuronal synapse function and microglial activity in mice | Trem2 Y38C homozygous (Trem2Y38C/Y38C) and Trem2−/− mice | CRISPR/Cas9 was used to generate the Trem2 Y38C mutation in the Trem2 gene of mice; mice were analyzed for synaptic protein levels, microglial morphology, and gene expression changes | TREM2 Y38C mutation impaired synaptic plasticity and myelination in hippocampal regions | The study underscores the role of TREM2 in the development of presenile dementia and highlights the importance of CRISPR/Cas9 for modeling genetic mutations linked to AD and related diseases | [52] |
| CysLT1R deletion and its effects on neuroinflammation and synaptic plasticity | CRISPR/Cas9 | Evaluate the impact of CysLT1R deletion on amyloidosis, synaptic plasticity, cognition, and neuroinflammation | CysLT1R gene knockout in APP/PS1 mice to study its effects on Aβ accumulation, synaptic plasticity, and cognitive function | Reduction in toxic Aβ levels, lowered pro-inflammatory cytokines (TNF-α, IL-6), improved synaptic plasticity and cognitive performance | Another pathway through which gene editing can alleviate AD symptoms by reducing inflammation and improving synaptic protection | Reduction in toxic Aβ levels, lowered pro-inflammatory cytokines (TNF-α, IL-6), improved synaptic plasticity and cognitive performance | [53] |
Aβ: Amyloid beta; AD: Alzheimer’s disease; APP: amyloid precursor protein; APPswe: amyloid precursor protein Swedish mutation; Cas9: CRISPR-associated protein 9; CRISPR: clustered regularly interspaced short palindromic repeats; CysLT1R: cysteinyl leukotriene receptor 1; FAD: familial AD; gRNA: guide RNA; IL-6: interleukin 6; LPS: lipopolysaccharide; N2A: neuro2A; PSEN1: presenilin 1; PSEN2: presenilin 2; SH-SY5Y: human neuroblastoma cell line; SORL1: sortilin-related receptor 1; TNF-α: tumor necrosis factor alpha; TREM2: triggering receptor expressed on myeloid cells 2; WT: wild-type; ZDHHC21: zinc finger DHHC-type containing 21.
2.3. Translating Gene Therapy into Clinical Practice for AD
While there are currently no active clinical trials using CRISPR/Cas9 for AD, gene editing technology has already been successfully tested in other neurological settings. NTLA-2001 and nexiguran ziclumeran, both CRISPR/Cas9-based therapies, showed a significant and long-lasting reduction of transthyretin protein in the serum of patients with hereditary transthyretin amyloidosis and cardiomyopathy (NCT04601051). Phase 1 results demonstrated a good safety profile, with mild and transient adverse events highlighting the feasibility of in vivo gene editing in humans [54,55].
In the field of AD and related dementias, several gene therapy trials not based on direct genome editing are currently ongoing or completed. CERE-110 (adeno-associated virus 2, AAV2- nerve growth factor, NGF, NCT00087789, NCT00876863) is an adeno-associated vector carrying the NGF gene. It demonstrated good tolerability in Phase 1 and 2 trials, with persistent gene expression over time, but limited vector uptake and imprecise targeting compromised its clinical efficacy. However, a long-lasting neuronal trophic response, including axonal sprouting and cellular hypertrophy, was observed [56,57].
A Phase 1 AAV-human telomerase reverse transcriptase trial (NCT04133454) explores the use of human telomerase reverse transcriptase as a strategy to extend cell life and counteract the degenerative mechanisms of AD, with potential effects on cognition and quality of life. In parallel, AAV2-BDNF (NCT05040217) aims to evaluate whether BDNF, administered continuously via viral vector, can slow neuronal loss in patients with AD or mild cognitive impairment.
In the field of frontotemporal dementia with progranulin mutations (FTD-GRN), two gene therapy approaches are currently being tested: AVB-101 (NCT06064890) and PR006 (NCT04408625). Both aim to restore progranulin levels. In mouse models, PR006 improved lysosomal and inflammatory pathological markers. Preliminary data from a Phase 1/2 trial indicate that a single intracisternal administration was generally safe and well tolerated, with increased progranulin levels in the cerebrospinal fluid in all patients. Adverse events associated with PR006 include cerebrospinal fluid pleocytosis and a transient increase in neurofilament light chain, likely associated with mild toxicity in the dorsal root ganglia [58].
Table 2 summarizes the major clinical trials of gene therapy approaches in AD and related dementias, highlighting the use of CRISPR/Cas9 and AAV vectors for therapeutic targets such as NGF, BDNF, human telomerase reverse transcriptase, and progranulin. The table provides an overview of the clinical phases, outcomes, and limitations of these trials, illustrating the therapeutic potential and challenges associated with these approaches.

Table 2.
Gene therapy approaches for AD and related dementias. Summary of major clinical trials of gene therapy in AD, amyloid transthyretin amyloidosis, and FTD-GRN, including CRISPR/Cas9 and AAV vectors for NGF, BDNF, human telomerase reverse transcriptase, and progranulin. Preliminary results show good safety and therapeutic potential, despite limitations in targeting and tolerability.
3. Stem Cell Therapies in AD: Cell Types, Mechanisms, and Applications in Research
3.1. Types of Stem Cells Used in AD Therapy and Research
Stem cells represent a promising tool for AD research and therapy, owing to their regenerative potential and utility in disease modeling. Combined with genome editing tools such as CRISPR/Cas9, stem cells enable the creation of precise models to investigate AD mechanisms and personalize therapeutic strategies [59]. Their self-renewal and multipotency make stem cells fundamental in preclinical research and potential candidates for autologous transplantation strategies [60]. Stem cell therapies are under investigation for several neurodegenerative disorders [61,62,63], including AD [64], Parkinson’s disease (PD) [65], amyotrophic lateral sclerosis [66], and multiple sclerosis [67]. Based on developmental origin, stem cells are classified as embryonic, fetal, perinatal (e.g., umbilical cord-derived stem cells), and adult [68]. Based the differentiation potential, stem cells are classified as totipotent (e.g., zygote-derived stem cells) [68,69], pluripotent (e.g., ESCs, iPSCs) [70], multipotent (e.g., MSCs, NSCs), oligopotent, and unipotent, with restricted differentiation capabilities [71,72]. ESCs, derived from blastocysts, can differentiate into all cell types of the three germ layers [73,74]. In AD models, the implantation of ESC-derived cholinergic and GABAergic neurons has been shown to improve memory and promote synaptic integration. However, tumorigenic risk and ethical concerns limit clinical application [75,76]. MSCs, isolated from bone marrow, adipose tissue, or umbilical cord, are clinically attractive due to low immunogenicity and paracrine secretion of neurotrophic and anti-inflammatory factors [77]. In AD models, implantation of MSCs promotes neurogenesis, reduces Aβ burden, and modulates microglial activity [78,79,80]. NSCs differentiate into neurons, astrocytes, and oligodendrocytes [81,82]. They may support the repair of damage to blood vessels in the brain [83] and regulate immune responses [84] by replacing lost neurons, restoring neurotransmitter levels, and providing neurotrophic factors that support synaptic function and cell survival [85]. Despite being used in AD research, NSCs are not as effective as hiPSCs. NSCs are less suited for researching the intricacies of cellular interactions in AD since they are already committed to the neural lineage and have a limited ability to differentiate, mostly toward neurons and astrocytes [86,87]. NSC transplantation in AD models enhances synaptic repair and neurogenesis and reduces glial activation and pro-inflammatory markers [88,89]. The iPSCs reprogrammed from somatic cells allow patient-specific modeling of AD [90]. iPSC-derived neurons replicate pathological hallmarks, such as Aβ accumulation and tau phosphorylation, and provide platforms for drug screening and gene correction via CRISPR/Cas9.
Despite progress, major translation barriers persist. ESCs and iPSCs carry risks of uncontrolled growth and teratoma formation, influenced by reprogramming methods, transplantation site, and host background. To address this, rigorous differentiation and purification protocols are essential [91]. The immunogenicity of stem cells represents another major challenge. Even autologous iPSCs can elicit immune responses due to the expression of abnormal antigens. Developing universal stem cell lines with reduced immunogenicity may help overcome this challenge [92]. Producing clinical-grade stem cells with consistent quality and differentiation efficiency remains a major hurdle. A potential solution is the development of universal cell lines with reduced immunogenicity or the use of immunosuppressive strategies to prevent rejection in allogeneic transplants [93]. One of the main obstacles to the clinical implementation of stem cell therapies is the need for standardized protocols to produce large-scale, reproducible cell populations. Variability in differentiation methods and the quality of generated cells can impact therapeutic efficacy and treatment safety [94].
In summary, stem cells hold transformative potential in preclinical research by enabling the development of personalized and regenerative therapies. However, rigorous preclinical validation and technological advancements are essential to fully harness their capabilities and overcome the existing barriers to their clinical application (Table 3).

Table 3.
Characteristics and therapeutic potential of stem cells in neurological disorders.
3.2. Clinical Trials on Stem Cell Therapies for AD
Stem cell therapies for AD are rapidly advancing, with several clinical trials demonstrating promising safety profiles and some early signs of efficacy. Various approaches use autologous or allogeneic MSCs from sources such as adipose tissue, umbilical cord blood, bone marrow, or perinatal tissues. Despite promising preclinical results and acceptable tolerability, stem cell therapies for AD have yet to demonstrate meaningful clinical efficacy. Advancing these approaches will require larger, well-designed studies with extended follow-up and careful consideration of translational, regulatory, and ethical challenges [95].
Autologous adipose-derived MSCs are among the most widely studied, as seen in the AstroStem trial (NCT03117738). Although well-tolerated, this approach showed no significant cognitive benefit compared to placebo, with serious adverse events, including pulmonary embolism and esophageal carcinoma. A follow-up study (NCT04482413) is ongoing. In contrast, a parallel trial (NCT05827757) demonstrated that these MSCs can reduce inflammatory cytokines in patients with age-related low-grade inflammation, suggesting an immunomodulatory benefit.
Studies with hUCB-MSCs, such as NEUROSTEM®-AD (NCT02054208), showed good tolerability and mild side effects that were resolved quickly. These results, along with related trials (e.g., NCT01297218) [96], support the safety of hUCB-MSCs, with new trials exploring secretomes and extracellular vesicles derived from these cells.
Allogeneic MSC therapies have also shown promise, including Lomecel-B (NCT04040348) and laromestrocel (NCT05233774), both of which demonstrated neurocognitive and neuroimaging improvements. In addition, significant reduction in brain atrophy was noted with laromestrocel. Both therapies are safe, supporting larger-scale trials [97].
Other research includes autologous BMSCs (NCT03724136, NCT02795052) aimed at improving cognitive function as well as amniotic or cord tissue-based therapies (NCT03899298), which are still in early stages. HiPSCs, such as those used in the NCT00874783 study, are being explored for disease modeling and preclinical discovery, offering promising future platforms, although they are not yet suitable for clinical use.
Table 4 summarizes the results and limitations of clinical trials investigating stem cell-based therapies for AD, including autologous adipose-derived MSCs, human umbilical cord blood-derived MSCs, and allogeneic MSCs, highlighting key outcomes such as safety, efficacy, and trial phases.

Table 4.
Clinical trials of stem cell-based therapies for AD: outcomes, limitations, and study phases.
3.3. Emerging Strategies to Enhance Stem Cell Therapies
Despite the challenges of applying stem cells as a treatment against AD, several types of new innovative approaches are being developed to improve their efficacy, specificity, and safety to a significant degree. These emerging strategies hold strong potential to enhance stem cell-based interventions for neurodegenerative diseases, paving the way for more effective and clinically applicable treatments.
The use of genome editing technologies, particularly CRISPR/Cas9, is a viable approach for creating highly precise genetic changes in iPSCs. As discussed in the next chapter, one advantage is the ability to generate isogenic cell lines; this is achieved by introducing or correcting disease-associated mutations without altering the overall genetic background of the individual from whom the cells are derived [98].
CRISPR/Cas9 allows for the creation of more accurate disease models and increases the safety of transplants [99]. Moreover, CRISPR/Cas9 technology can be utilized to knock out oncogenes that are causing tumor growth in iPSCs, thus avoiding teratomas upon implantation, which is a significant concern in cell therapies [100].
The application of stem cell-derived exosomes as a “cell-free” therapy is another form of treatment that does not involve transplanting the stem cells themselves. Exosomes are tiny vesicles secreted by cells that carry molecules that have neuroprotective (neuroprotective functions) and immunomodulatory (modulate the immune system) functions. They can promote the survival and regeneration of nerve cells. The advantage of this strategy is that it does not involve the risks typically associated with whole-cell transplantation, such as immune system rejection by the recipient or uncontrolled cell division. In laboratory experiments using animal models of AD, exosomes obtained from MSCs have been shown to reduce the accumulation of Aβ protein in the brain and improve cognitive function [101].
The use of these scaffold biomaterials is being explored for improving some critical parameters of cell transplantation: transplanted cell survival, their engraftment (i.e., integration into the host tissue), and their proper differentiation into the desired cell types. The scaffolds are three-dimensional materials that attempt to mimic the highly complex structure and composition of the extracellular matrix in the brain. They work by providing structural support and a favorable microenvironment that promotes the incorporation of transplanted stem cells [102]. This increased support enables the therapeutic incorporation and function of transplanted cells in damaged brain disease.
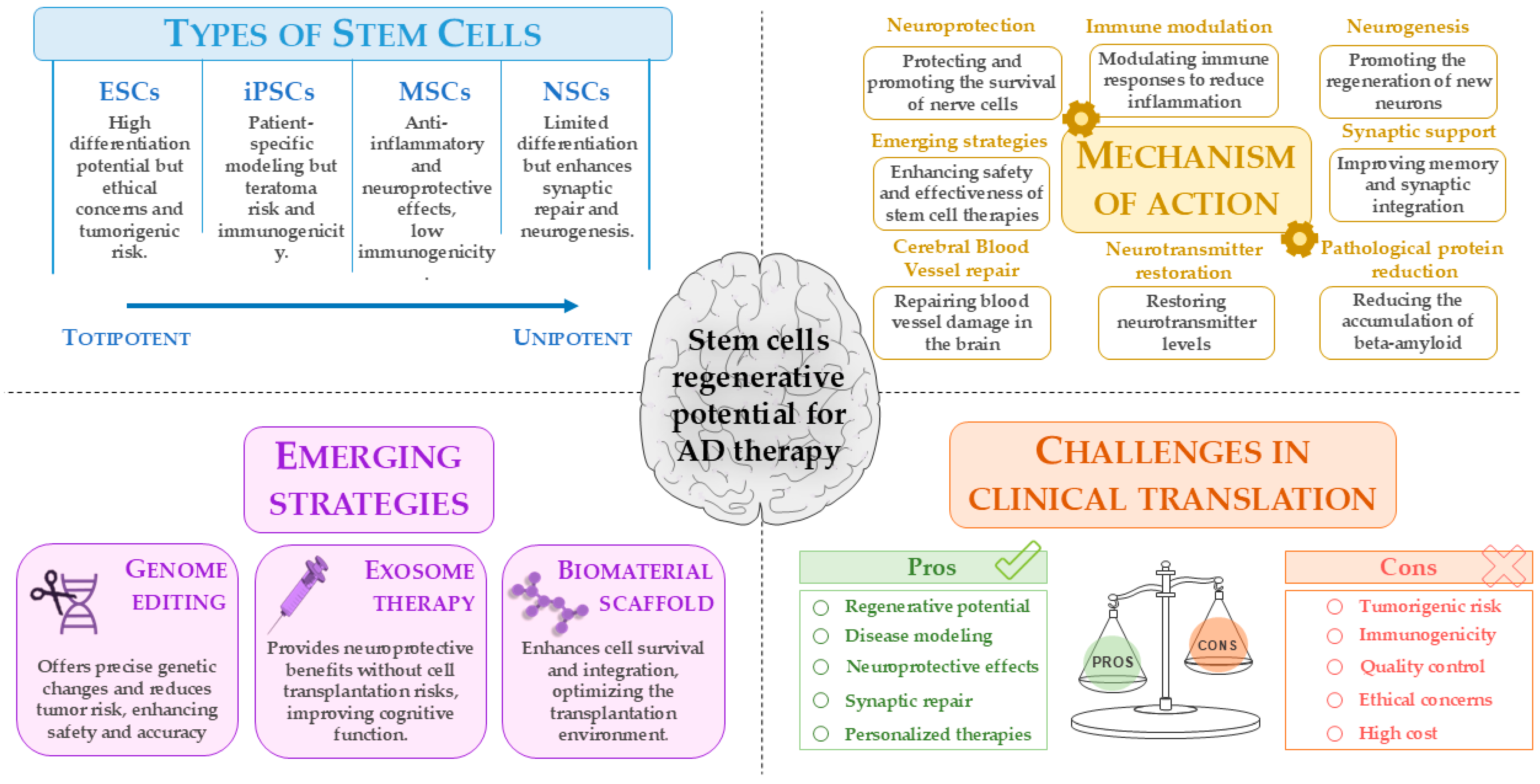
These innovative strategies aim to overcome the current limitations of stem cell therapies for AD using an approach that combines genetic precision, the use of safer cellular components, and optimization of the environment in which the cells are transplanted, potentially leading to more effective treatments for this complex disease (Figure 3).
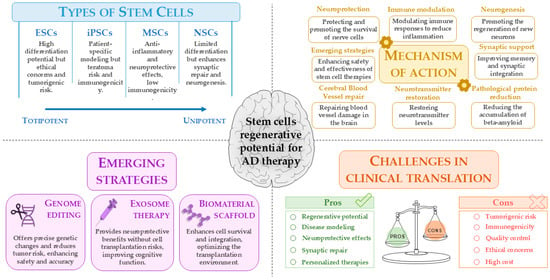
Figure 3.
Stem cell-based approaches for AD therapy: opportunities and challenges. This figure summarizes the types of stem cells and their regenerative potential for AD treatment, highlighting mechanisms of action, emerging strategies such as genome editing and exosome therapy, and key clinical translation challenges. While stem cell therapies show promise in neuroprotection, neurogenesis, and synaptic repair, concerns remain regarding tumorigenicity, immunogenicity, and quality control. This image was created using the image bank of Servier Medical Art (Available online: http://smart.servier.com/; accessed on 30 May 2025) licensed under a Creative Commons Attribution 3.0 Unported License (available online: https://creativecommons.org/licenses/by/3.0/, accessed on 30 May 2025). AD: Alzheimer’s disease; ESCs: embryonic stem cells; iPSCs: induced pluripotent stem cells; MSCs: mesenchymal stem cells; NSCs: neural stem cells.
4. Emerging Experimental Models for AD: The Potential of CRISPR/Cas9 and hiPSCs
According to recent studies, hiPSC-derived brain cells represent a valid and reliable in vitro platform for studying cerebral development and functions. These cells can self-organize into functional neuronal networks, displaying electrophysiological properties and pharmacological responses comparable to those observed in mouse models, including an active inhibitory system. hiPSCs represent an emerging approach to AD, mainly used to model the disease and test drugs in vitro. Although not yet clinically applied, studies explore their potential in the preclinical setting, with the future goal of using them in personalized transplantation therapies. However, it must be emphasized that in vitro and/or in vivo models cannot fully replicate the structural and functional complexity of the human brain [103,104].
Currently, available data do not allow us to determine whether iPSCs or hESCs are intrinsically superior for the study of AD. However, iPSCs offer the key advantage of being derived directly from patients with specific genetic mutations, allowing the generation of cellular models that reproduce the genetic background of the disease [105]. Neurons and other brain cells generated from patient-derived cells provide a human-relevant context for studying AD, especially its familial or genetic forms [106]. Unaffected control iPSCs can serve as a basis for introducing mutations via genetic knock-in methods [107,108]. Moreover, iPSCs overcome the ethical issues linked to hESCs, as they are generated from adult somatic cells without involving embryos, facilitating the development of new therapeutic strategies. Recent evidence suggests that using CRISPR/Cas9 to introduce AD-associated mutations in stem cell-derived models enables researchers to study genetic variations more effectively, shedding light on the molecular mechanisms of AD [109]. Findings revealed that the combination of iPSCs and CRISPR/Cas9 is a promising approach for assessing pathological features typical of AD, including protein aggregation, oxidative stress, and neuronal death [110]. However, as we will see in the next chapter, several methodological limitations must be carefully considered. iPSCs, although offering a relevant human model, present challenges related to inter- and intra-lineage variability, epigenetic memory of the tissue of origin, and potential genomic alterations acquired in culture, including tumorigenesis. Two-dimensional models often do not reproduce complex phenotypes such as neurodegeneration or tau pathology, while more mature and physiologically relevant 3D organoids suffer from heterogeneity, lack of vascularization, and limited representation of cells such as microglia. Generation of specific neuronal subtypes or multicellular co-cultures also remains technically challenging and poorly standardized. With respect to genome editing, CRISPR/Cas9 carries inherent risks such as off-target effects, mosaicism, and DNA damage response. Efficient delivery of editing components into post-mitotic neurons remains a challenge, as does the generation of precise knock-in mutations. Furthermore, lengthy protocols, use of antibiotics and plasmids, and analytical biases in single-cell data further complicate the interpretation of results [see Section 5].
This chapter outlines recent advances in AD research combining hiPSCs with CRISPR/Cas9 gene editing.
4.1. Building an AD Model Using CRISPR/Cas9 and hiPSCs
CRISPR/Cas9 technology is increasingly used to reduce genetic variability within the same cell types through precise genome editing. In the case of AD research, the use of stem cells facilitates genomic modifications that help uncover specific pathological alterations linked to mutations, allowing for a clearer understanding of how these genetic changes contribute to disease progression.
In brief, iPSCs from patients or healthy individuals are grown under specific conditions to keep them able to change into different types of cells [111,112]. Then, hiPSCs are dissociated with reagents like Accutase, and ROCK inhibitors are added to the medium to optimize conditions [113,114,115,116]. Quality control on iPSC lines is crucial and should be conducted by verifying pluripotency through markers, such as Oct4 and Sox2, and performing karyotype analysis to identify chromosomal abnormalities [107,117].
A sgRNA targeting specific genomic regions is introduced into human iPSCs using nucleofection, transfection, or lipofection methods in conjunction with CRISPR/Cas9 technology [113,118,119,120]. Notably, online tools facilitate the selection of high-specificity sequences, and utilizing Cas9 “nickases” has been shown to reduce off-target effects by cutting one DNA strand, allowing for more precise modifications [119]. Then, sgRNA sequences are carefully inserted into specific plasmid vectors and checked using Sanger sequencing to confirm proper insertion [118].
After CRISPR/Cas9 delivery, clonal expansion of hiPSCs using fluorescence-activated cell sorting or pharmacological selection facilitates the isolation of edited cells, which is important for obtaining isogenic lines [116,121,122]. Thus, edited cells are genetically confirmed using PCR and Sanger sequencing to verify that insertion or deletion has occurred and to determine their status (homozygous, heterozygous, or unmodified) [109]. A controlled and physiologically appropriate environment is then created, in which the properly modified hiPSCs are differentiated into brain derivatives, such as neurons or brain organoids, which can better mimic neurological systems [123,124]. In general, the evaluation of biological and molecular processes of cells under analysis exploits multi-omic techniques that allow a better understanding of the mechanisms related to the disease [125]. Functional validation allows, indeed, a detailed characterization of the genetically modified cells, allowing the comparison between healthy and pathological states and ensuring a more repeatable and ethical model of neurodegenerative disorders [113].
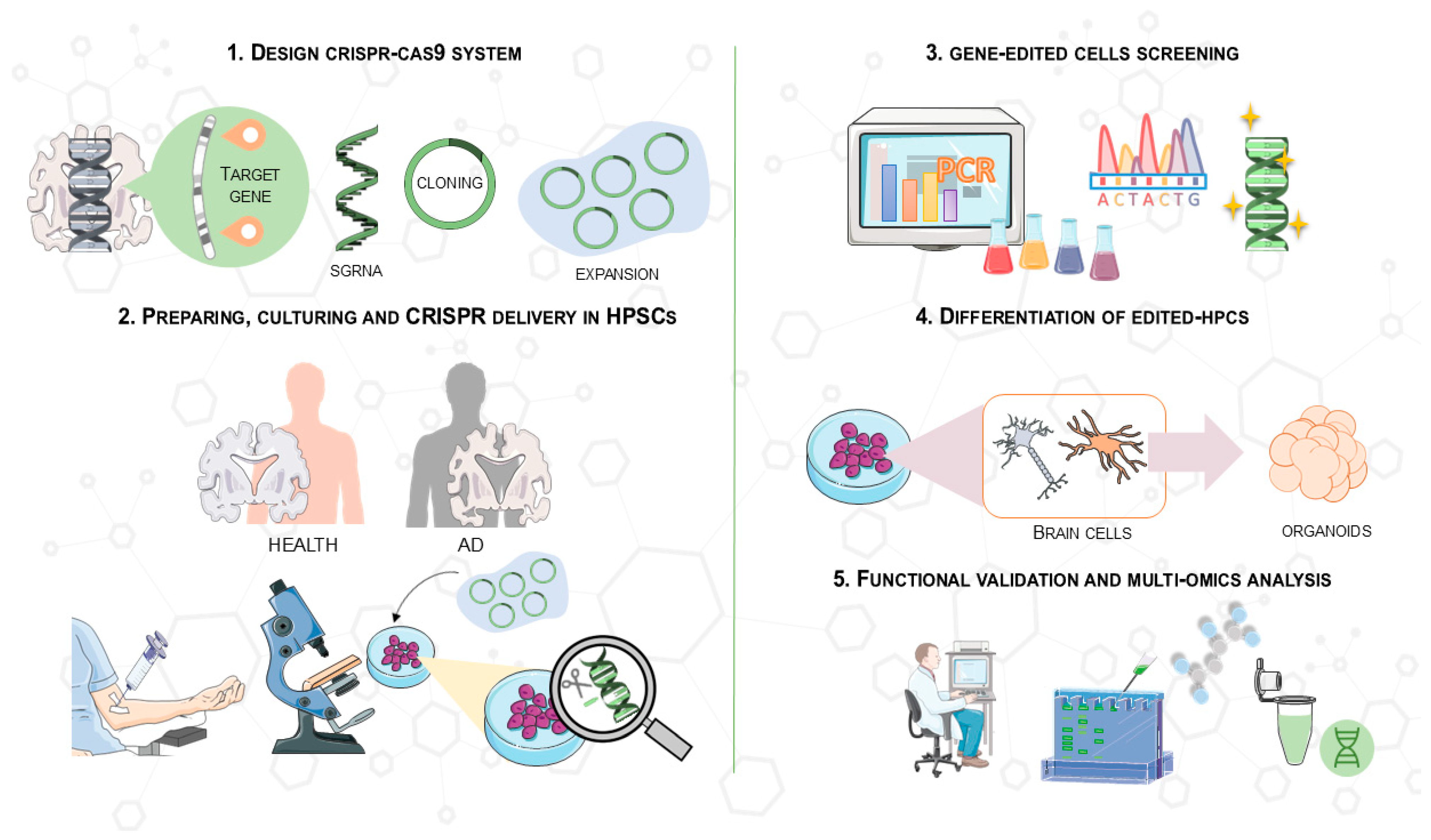
A schematic of this process is shown in Figure 4, which illustrates the main steps of CRISPR/Cas9 gene editing in hiPSCs. These steps include the synthesis of specific sgRNAs targeting AD-related genes, the editing of hiPSCs using CRISPR/Cas9 delivery, confirmation of genetic modifications, differentiation into brain cells, and the generation of 3D organoids for functional analysis. This protocol allows for precise modeling of AD in vitro, facilitating drug screening and the study of pathological mechanisms.
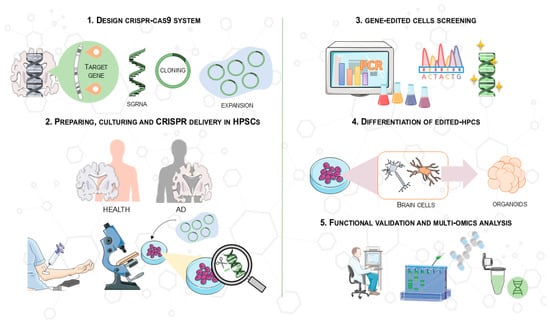
Figure 4.
Overview of the CRISPR/Cas9 protocol used in hiPSCs for drug screening and clinical application in AD models. This process involves (1) synthesizing an optimal sgRNA targeting an AD-related gene (e.g., APOE) and cloning it into a CRISPR/Cas9 vector; (2) deriving and culturing hPSCs from patients or controls, followed by CRISPR/Cas9 delivery (e.g., transfection, electroporation); (3) confirming genetic modifications in expanded clones using PCR and Sanger sequencing; (4) differentiating gene-modified hPSCs into brain cells and generating 3D organoids; and (5) investigating molecular pathways and pathological mechanisms to study the effects of genomic modifications in hPSCs. This image was created using the image bank of Servier Medical Art (Available online: http://smart.servier.com/; accessed on 30 May 2025) licensed under a Creative Commons Attribution 3.0 Unported License (available online: https://creativecommons.org/licenses/by/3.0/, accessed on 30 May 2025). CRISPR/Cas9: Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9; AD: Alzheimer’s disease; ESCs: embryonic stem cells; hiPSCs: human induced pluripotent stem cells.
4.2. The Rise of hiPSCs for Disease Modeling of AD
Gene editing can include different techniques to investigate the pathological mechanisms of AD in iPSC-derived cells [126,127]. The development of human neurons with disease-associated phenotypes helps researchers to uncover critical pathways in AD pathology and model genetic risk factors and protective variants beyond amyloid and tau [25]. As mentioned above, one strategy is the knock-in or the correction of specific mutations in AD-associated genes. A main advantage is the ability to reproduce in vitro models of FAD and associated risk variants, improving the study of pathological characteristics that are difficult to analyze [128]. Recent evidence indicates that cerebral organoids derived from iPSCs provide a more complex and physiologically relevant model for AD. This advancement addresses the limitations of 2D cultures, which fail to replicate intricate cell–cell interactions and do not adequately represent the accumulation of extracellular aggregates [117]. Compared to transgenic mouse models of AD, organoids offer several unique advantages. While mouse models have been invaluable for elucidating disease mechanisms, they often do not fully replicate the human-specific features of AD pathology, such as the exact sequence and structure of Aβ peptides, species-specific tau isoforms, and differences in immune responses. Organoids, being derived from human cells, overcome these species-related limitations and allow the modeling of AD in a human genetic and epigenetic context. Three-dimensional brain organoids have become a powerful tool in modeling AD, offering insights into the molecular mechanisms underlying AD. These organoids replicate the cellular environment of the brain and provide a promising platform for studying disease progression and therapeutic interventions. To enhance their relevance to AD research, it is crucial that 3D brain organoids account for spatiotemporal factors, such as the timing of disease onset (early or late- onset) and the aging process. Early- and late-onset forms of AD present distinct pathological and molecular characteristics, which can be modeled by manipulating the timing of cellular and molecular changes in organoids. Furthermore, since AD is strongly associated with aging, it is essential to incorporate age-related cellular changes into the organoid models [129]. This approach would allow researchers to better understand how the progression of the disease is influenced by age and the timing of onset, ultimately advancing therapeutic strategies tailored to these variations. When combined with CRISPR/Cas9, organoids allow researchers to introduce or correct AD-related mutations and study their effects in a 3D human cell context [130]. Combining this with CRISPR/Cas9 technology and iPSCs to create isogenic cell lines during brain organogenesis offers the ability to generate highly specific models to explore the molecular and cellular mechanisms behind AD [131], with an added advantage of generating a spatiotemporal transcriptomic atlas to map how genetic and epigenetic factors influence brain development and disease progression [132].
Such an atlas would provide insights into how genetic and epigenetic factors contribute to AD progression, including the effects of early- and late-onset AD and aging. By combining iPSCs, CRISPR/Cas9, and spatiotemporal transcriptomics, these models replicate key pathological features of AD, offering a more accurate representation of human disease mechanisms compared to traditional animal models [133,134].
This approach advances the development of more personalized therapeutic strategies for AD. In addition to considering spatiotemporal factors, it is crucial to recognize the role of key signaling pathways, such as Wnt, Shh, BMP/TGF-β/SMAD, and Notch, in shaping the spatial domain identity during brain organogenesis. These signaling pathways are integral to the regulation of cell fate specification, tissue patterning, and the establishment of brain region-specific identities. For example, Wnt signaling plays a critical role in regulating neuronal differentiation and axial patterning, while Shh signaling is pivotal in the patterning of the forebrain and the differentiation of specific neuronal populations. Similarly, BMP/TGF-β/SMAD signaling governs the differentiation of neural progenitors and neuronal specification, whereas Notch signaling regulates neurogenesis and the maintenance of neural stem cells. These pathways interact in complex ways to establish the spatial and temporal organization of the brain. By incorporating these signaling networks into 3D brain organoids derived from iPSCs, researchers can better model how developmental cues influence cellular differentiation and tissue architecture [135].
Manipulating these pathways in organoids can help mimic the spatiotemporal dynamics of the brain in both health and disease, offering a more accurate platform for studying neurodegenerative diseases like Alzheimer’s. Furthermore, by combining these models with CRISPR/Cas9 technology, it is possible to create isogenic cell lines that can be used to explore the specific effects of genetic mutations on the expression and function of these key signaling pathways in a human context.
The potential of these models lies in the ability to generate several human cell populations relevant to AD from a controllable source, the iPSCs, overcoming existing limitations. Thus, the roles of individual brain cell types and their interactions can be studied, including in co-culture models that better mimic neurodegenerative disease [136]. In particular, exploring the role of glial cells can help elucidate pathological features, such as ROS production [136]. For instance, studies using iPSC-derived microglia have shown the crucial regulatory roles of the CX3C chemokine receptor 1 in inflammation and phagocytosis, highlighting its potential for studying neuron–glia interactions during disease [137]. An efficient and reproducible method has recently enabled the generation of iPSC-derived microglia with characteristics closely resembling primary microglia. Haq I. et al. generated a CRISPR ON/OFF system for accurate temporal gene control, without inducing DNA double-strand breaks that can cause genomic rearrangement [120].
Moreover, the ability to recreate specific disease phenotypes in a human context makes these models more predictive of drug efficacy than traditional preclinical models. Key clinical characteristics of AD are replicated in cerebral organoids produced from sporadic individuals, who generally have higher amyloid and tau levels [117]. Notably, organoids with specific mutations, such as PSEN2N141I, have shown responses to drugs that increase neuronal activity, indicating their potential to assess the efficacy of new or existing drugs [112].
Isogenic Lines in CRISPR/Cas9 and hiPSC-Based Models: Enhancing Precision in AD Research
One of the main challenges in human disease modeling is the genetic variability among individuals, which can complicate the interpretation of experimental results. In the context of neurodegenerative diseases, the creation of iPSCs from patients or healthy individuals has been proposed as a potential strategy to study the effect of a specific genetic modification [138]. Gene editing techniques further enhance this approach by enabling the generation of isogenic lines, genetically identical cells that differ only in the variant of interest, allowing direct comparisons regardless of whether they originate from a patient or a healthy donor [139]. Comparing an edited line to its isogenic control boosts confidence that observed differences are due to specific genetic manipulation, not other genetic variations. For instance, Ng, B. et al. generated isogenic tau-depleted iPSC lines from two healthy individuals using CRISPR/Cas9, which were further differentiated into cortical neurons. Tau-depleted neurons showed decreased activity and neurite outgrowth in comparison to controls. In contrast, Aβ-induced toxicity was reduced, including neurodegeneration, hyperactivity, and deficiencies in mitochondrial transport. These results support chronic tau-lowering strategies as a potential treatment for AD [140], validate the use of animal models such as Mapt−/− mice, and underline tau’s role in Aβ-driven pathology. Similar findings were reported by another group, further supporting tau-lowering strategies in AD [141].
The use of CRISPR/Cas9 to generate genetically engineered hiPSC lines with identical genetic backgrounds represents a significant advance in biomedical research, providing highly controlled and reproducible disease models [112]. The main result is a reduction in genetic background due to variability between different individuals [142]. Another study used iPSC-derived cerebral organoids from two FAD patients carrying the PSEN1E280A mutation to investigate the protective role of the APOE3 Christchurch variant. By introducing or removing APOE3Ch through gene editing, the researchers created isogenic-like comparisons that allowed them to isolate the effects of APOE3Ch on disease pathology in a 3D human brain model [143]. APOE is a major genetic risk factor for late-onset AD, although its pathological role across different brain cell types remains unclear. Notably, CRISPR/Cas9-mediated APOE deletion appears to prevent cellular senescence, highlighting APOE as a promising therapeutic target in aging-related diseases like AD [144]. The introduction of hiPSCs combined with CRISPR/Cas9 technology has enabled the generation of isogenic lines carrying either the APOE3 or APOE4 allele, thereby allowing for a precise attribution of observed phenotypes to the specific variant under study [125]. Differentiation of these lines into neurons, astrocytes, and microglia revealed that APOE4 promotes early neuronal maturation, increases Aβ42 secretion, alters astrocyte lipid metabolism, and impairs glial amyloid clearance. In brain organoids, APOE4 leads to the accumulation of Aβ and phosphorylated tau, although with slower kinetics compared to FAD models [118]. Importantly, genomic conversion of APOE4 to APOE3 attenuated most of these phenotypes, confirming the central role of APOE4 in sAD pathology [145].
Another critical example involves different modifications of the APP gene, which is pivotal in AD pathology. Specifically, iPSC cells harboring the V717I (London) and KM670/671NL (Swedish) mutations into the APP gene, commonly used in animal models such as 5xFAD, showed increased Aβ production similar to AD patients [146]. Researchers applied CRISPR/Cas9 to edit the CRTC1 gene in hiPSC with the APPSwe mutation, enhancing neuronal functionality. The results were also reproduced in vivo, as injection of the modified CRTC1 into 5xFAD mice restored synaptic plasticity, crucial for memory [147]. Notably, gene knockout models of APP highlighted defects in neuronal development and synaptic function, implicating cholesterol transport and distribution in neurons [148]. Furthermore, Ye, T. et al. demonstrated that correcting APP gene dosage using paired Cas9 nickases in hiPSC-derived neurons reduced Aβ production, Tau hyperphosphorylation, and neuronal loss [119].
Researchers used hiPSC and CRISPR/Cas9 technology to study whether the loss of the SORL1 gene, which is involved in APP cellular trafficking, causes problems in early endosomes in neurons and other affected cell types [149]. The pathophysiology of AD is significantly influenced by endosomal trafficking disturbance. Another study revealed that rare mutations in the SORL1 gene could disrupt the maturation and trafficking of a protein called SorLA in neurons derived from hiPSCs. Due to its retention in the endoplasmic reticulum, these alterations decrease the quantity of SorLA in the endosomal system and plasma membrane, which eventually results in greater release of Aβ [150].
Furthermore, generating a homozygous ABCA7-knockout iPSC line using CRISPR/Cas9 gene editing is an effective approach to explore the role ABCA7 loss plays in AD [115]. This iPSC line displays pluripotency markers, maintains a stable karyotype, and can develop into all three germ layers [151]. Moreover, iPSC-derived cortical organoids with ABCA7 knockout demonstrate that loss of this AD risk gene leads to mitochondrial oxidative stress and apoptosis. ABCA7 deficiency increased ROS production and activated caspase 3, a key mediator of oxidative stress-induced apoptosis. Upregulation of pro-apoptotic genes such as APAF1, BAK1, and XIAP was also observed, linking ABCA7 loss to impaired mitochondrial lipid metabolism, neuronal apoptosis, and disrupted synapse formation [152].
Overall, there is a growing interest in analyzing the impact of CRISPR/Cas9 and hiPSC technologies. Interestingly, using different gene targets appears useful in generating more accurate models (as summarized in Table 5), allowing the exploration of complex pathogenic mechanisms and potential therapeutic targets. Nevertheless, in the case of AD, these models have significant limitations, as we will see in the next chapter. Creating an in vitro system closely representing the human brain is difficult. In addition to the in vitro models, high spatiotemporal resolution imaging techniques, such as functional magnetic resonance imaging (fMRI), can provide valuable insights into the spatial and temporal dynamics of brain activity in AD. fMRI allows for the real-time monitoring of brain regions involved in cognitive functions and their alterations throughout the disease. By combining fMRI with cellular and molecular models, researchers can gain a more comprehensive understanding of functional changes associated with AD pathology, particularly the progressive nature of amyloid accumulation, tau aggregation, and their effects on brain network connectivity. This approach can aid in better characterizing disease stages, improving diagnostic accuracy, and evaluating the efficacy of therapeutic interventions [153]. Continued progress in this field could play a crucial role in the development of personalized treatments and in improving therapeutic intervention strategies for AD. All these issues highlight the urgent need for more rigorous protocols, validated systems at multiple levels (cell, organoid, animal), and increased standardization to improve the reliability of in vitro models. A more detailed overview of these limitations is provided in Chapter 5 and summarized in Table 5.

Table 5.
Summary of selected studies using CRISPR/Cas9-edited iPSC models to investigate AD. The table includes the aim of each study, the genome editing strategy employed, the specific genetic targets, validation methods, key findings, and reported limitations. References are provided for further details.
5. Challenges and Future Directions in CRISPR and hiPSCs-Based Approaches for AD
Stem cell-derived models, especially those based on hiPSCs and hESCs, have significantly advanced AD research by enabling the study of human neurons in vitro. These models facilitate the investigation of genetic mutations and disease mechanisms in a controlled environment, overcoming some limitations of animal models. Despite their great potential, hiPSC-derived models, often combined with CRISPR/Cas9-based genetic engineering to study AD-associated mutations, still face significant limitations that define areas for future development [103,118,152]. However, there is not yet a shared reference line, as in the case of C57Bl/6J mice, mainly because a thorough and comparative analysis of the different lines has not yet been conducted [154].
Moreover, the high cost of specialized reagents, consumables, sophisticated equipment, laborious protocols, extensive quality controls, and model complexity significantly increase the costs of research using isogenic hiPSC-based and CRISPR/Cas9 lines. Furthermore, in addition to regulatory complexity, ethical considerations regarding the application of hiPSCs and CRISPR/Cas9 technology in AD research are currently under discussion. The potential for germline leakage, unintentional edits in reproductive cells, raises long-term safety questions that cannot yet be fully addressed with current technologies [155]. Furthermore, informed consent becomes more nuanced in the context of irreversible genome editing or experimental stem cell transplants. Thus, while CRISPR- and iPSC-based models offer transformative potential, their clinical adoption requires not only technical refinement but also robust frameworks for safety monitoring, ethical oversight, and fair distribution.
While in the previous chapter we explored the potential in more detail, here we will evaluate the major limitations currently present in the CRISPR/Cas9 and hiPSC-based disease modeling.
5.1. Bioethical Implications of Stem Cell and Genome Editing Technologies in AD
Despite encouraging preclinical results, the real-world clinical translatability of CRISPR/Cas9 and stem cell-based approaches for AD remains constrained by several regulatory, technical, and ethical barriers. A critical distinction must be made between laboratory efficacy, often demonstrated under highly controlled in vitro conditions, and the rigorous requirements for clinical approval. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require comprehensive data on safety, reproducibility, and long-term outcomes before authorizing trials in humans [156]. Detailed characterization of the product, including vectors and modified cells, is mandatory, and high standards of quality, purity and traceability must be guaranteed. The FDA’s guidance on somatic genome editing emphasizes the need for validated delivery methods, detailed off-target analysis, and long-term follow-up, particularly in sensitive tissues like the brain. Similarly, the EMA classifies CRISPR-based and stem cell therapies as Advanced Therapy Medicinal Products, requiring extensive quality controls, traceability, and compliance with Good Manufacturing Practices [157,158].
First, the origin of human stem cells remains a foundational ethical issue. While the use of iPSCs, reprogrammed from somatic cells, circumvents some of the more controversial ethical debates associated with ESCs, the derivation, storage, and use of human biological material still require strict ethical oversight and adherence to national and institutional regulations. The use of patient-derived hiPSCs for disease modeling further emphasizes the need for responsible handling of human tissue and transparency in research protocols [105].
Second, the application of CRISPR/Cas9 for genome editing in human cells raises broader ethical questions regarding the manipulation of the human genome. In the reviewed literature, genome editing is primarily employed to generate disease models by introducing or correcting specific mutations in hiPSC lines. While these modifications are confined to in vitro research and potential somatic (non-heritable) therapies, they nonetheless prompt ongoing debate over the moral boundaries of altering human genetic material. Concerns grow sharper when considering future therapeutic applications, where safety, long-term consequences and broader societal impacts must be considered [103,114,142,143].
A key concern is technical safety, particularly the risk of off-target effects, which are unintended genetic alterations outside the intended site. This poses ethical dilemmas, especially in therapy, as such modifications could lead to harmful outcomes. Using isogenic controls helps minimize variability and identify off-target effects, but bioethical issues surrounding CRISPR/Cas9 remain unresolved. Comprehensive global regulations, developed with input from various stakeholders, are essential for safe and responsible use [159].
Lastly, the issues of informed consent and data privacy are central when working with iPSCs derived from patients with identifiable conditions. The guidelines for reporting research require specifying the measures taken to ensure reproducibility, randomization and blinding of experiments, which are fundamental aspects of research ethics [144]. For example, studies involving rare genetic variants such as the APOE3 Christchurch mutation inherently involve sensitive genetic information. While the primary literature may not always detail the consent procedures, ethical research practice demands comprehensive informed consent processes and careful attention to participant privacy, especially when genomic data are involved [143]. Another critical issue is equitable access: these therapies involve high development and administration costs and may not be readily available to patients outside of highly specialized clinical centers [160].
5.2. Current Limitations of Gene Editing and Stem Cell Technologies in AD Modeling and Therapy Development
5.2.1. Limitations of hiPSC-Derived In Vitro Models for AD
Protocols for differentiating hiPSCs into AD-relevant cell types, such as cortical neurons or microglia, can be time-consuming. Many existing protocols for differentiating iPSCs into microglia typically take more than 30 days, some take up to 74 or 75 days. Although shorter protocols exist, some utilize the integration of plasmids and antibiotics that can activate microglia [120]. Additionally, the need to generate specific neuronal subtypes, such as induced glutamatergic neurons, or to obtain specific cell populations (e.g., deep or higher cortical neurons), adds complexity and potentially time to the differentiation process [110].
One major limitation is the reduced biological complexity of in vitro models compared to the human brain. Key cellular components such as mature glial cells, particularly microglia, crucial players in neuroinflammation, are often absent or poorly represented. Even with the incorporation of iPSC-derived microglia, the full replication of in vivo behavior has yet to be demonstrated. Similarly, the lack of vascularization is a critical gap, as vascular dysfunctions are implicated in AD pathology [114,142]. Many models also focus on specific neuronal subtypes, such as glutamatergic neurons, failing to capture the full cellular diversity of the brain [110]. A further challenge is the difficulty of modeling the late-onset and progressive nature of AD, as most in vitro systems cannot reproduce the decades-long disease course or the aging-related phenotypes that characterize the clinical manifestation of the disorder [107]. The relatively short lifespan of in vitro cultures, combined with the developmental immaturity of iPSC-derived cells, limits their utility in modeling long-term disease progression and age-dependent pathological features [138].
Compared to animal models, 2D cell cultures, such as hiPSC-derived neurons, possess a human genetic background, but at the same time have intrinsic limitations and fail to show extracellular deposition of amyloid aggregates [112]. In addition to biological limitations, there is high variability in differentiation outcomes and the composition and maturation of brain organoids [130]. The variability can affect the reproducibility of studies and the efficiency of differentiation, thus standardization of protocols is needed to produce reproducible cell populations at scale [143]. The generation of isogenic iPSC lines involves several steps, including designing and introducing CRISPR components, selecting edited cells, subcloning single cells, and expanding clones. It is essential to inspect the clones for the desired edits and verify that they maintain a normal karyotype and express pluripotency markers [117]. The results obtained from in vitro models based on CRISPR/Cas9-edited iPSCs often require external validation in more complex systems, such as co-cultures including different cell populations, such as mature glia, or in vivo animal models, to confirm their physiological relevance and therapeutic potential [148]. Therefore, producing iPSC-based models, especially complex brain organoids, at a scale sufficient for high-throughput drug screening or future clinical applications presents scalability challenges. Another important limitation of hiPSC-based models is that they do not fully capture the aging process, which plays a central role in the development and progression of AD, even in cases caused by genetic mutations like those in the APP gene. Because iPSCs are reprogrammed into a youthful, embryonic-like state, they lose many of the typical features of aged cells, such as DNA damage, mitochondrial dysfunction, and impaired protein regulation. These age-related changes are crucial to understanding how the disease unfolds over time [161,162]. For this reason, long-lived systems like animal models are still essential for studying the mechanisms that emerge with aging and for testing treatments that need to work in the context of an aging brain.
5.2.2. Limitations and Challenges of CRISPR/Cas9 Technologies in AD Research
While CRISPR/Cas9 is a powerful tool for precise genetic modification, the challenges of ensuring that there are no unwanted changes and achieving efficient and safe delivery to brain tissue remain crucial obstacles to its full clinical application in AD.
Currently, there are no active, publicly registered human clinical trials using CRISPR/Cas9 technology to treat AD. The most advanced research is still in the preclinical or animal model development phase, with promising results but not yet in human clinical trials.
Clinical trials must be designed to monitor pharmacodynamics, pharmacokinetics, and duration of effects, with long-term follow-up to detect any late adverse effects. Furthermore, accurate informed consent is essential, given the innovative nature and ethical risks (such as possible hereditary alterations), and policies that promote equitable access to therapies, which are often expensive and technologically complex. These conditions make the clinical application of CRISPR/Cas9 in neurodegenerative diseases such as AD particularly challenging [163].
A significant concern for the therapeutic use of CRISPR/Cas9 is the unwanted genetic modifications that may occur at sites other than the intended target, with unexpected consequences [107]. Cas9 nucleases can induce a substantial number of off-target mutations in human cells, underscoring the need for rigorous validation and safety evaluation [112]. Although the use of isogenic controls helps to minimize potential off-target effects, the possibility of their existence cannot be completely excluded [143]. Efforts to improve specificity using high-fidelity Cas9 variants, base editing, and prime editing are ongoing [109,150]. For instance, using the coupled Cas9 nickase significantly reduces off-target effects, although it may have a lesser impact on gene editing efficiency and targetability [119]. Furthermore, generating knockout cells or introducing specific mutations often results in relatively low success rates, making these approaches impractical for many laboratories. Alteration of essential genes, critical for maintaining pluripotency and promoting differentiation of specific cell lineages, can significantly impact the outcomes of functional studies. Inducible Cas9 systems, such as those regulated by doxycycline, represent a potential solution to this challenge, allowing temporal control of Cas9 expression and genome editing [107,109].
The manipulation of large-scale gene expression, which is essential for understanding the molecular role of AD-related genes, is challenged by the low insertion efficiency of CRISPR/Cas9 vectors and high cell mortality. As a result, the use of CRISPR/Cas-mediated gene editing in cell pool experiments is limited [25]. Moreover, although not specifically related to the CRISPR/Cas9 mechanism itself, the ethical and legal implications of gene editing pose significant challenges, particularly for clinical applications and translation of the technology. This requires thorough validation before clinical trials can proceed.
5.3. Future Direction
Despite the transformative potential of CRISPR/Cas9 and hPSCs for AD research and therapy, challenges related to safety (e.g., off-target) and standardization, coupled with the complex ethical and legal considerations of human gene editing and stem cell use, represent significant difficulties that must be addressed before these technologies can be widely translated into effective clinical interventions for AD [105,143]. Moreover, the clinical application of hiPSC-derived technologies, often integrated with gene editing such as CRISPR/Cas9, and the associated ethical considerations, present several significant challenges. As mentioned in Chapter 3, the survival and functional integration of transplanted cells in the often hostile environment of the brain affected by neurodegenerative diseases is a fundamental challenge for therapies based on cell transplantation.
Future research efforts are expected to focus on overcoming the current limitations of CRISPR/Cas9 and stem cell-based models for AD. One of the key priorities is the development of more complex and physiologically relevant systems, including brain organoids and co-culture models that incorporate mature microglia, other glial cell types, and vascular structures [112,117,118,136]. Although significant progress has been made with iPSC-based models in bypassing the differences between different organisms, limitations remain, particularly regarding the potential loss of age-dependent cellular phenotypes. Therefore, integrating in vitro results with data from in vivo studies and human brain tissue will further strengthen the translational relevance of these models [107,148]. A valid choice could be the use of the KOLF2.1J hiPSC subline, which has obtained good results. Several experiments have shown that KOLF2.1J cells can maintain genetic stability; even after multiple CRISPR/Cas9 modifications, these cells are free of variants that could compromise neuronal function [154].
Expanding research to include a broader range of neuronal subtypes and investigating cellular interactions using multi-omic approaches may also uncover novel mechanisms and therapeutic targets [125]. For instance, edited iPSC-derived models can be used to investigate the impact of genetic variations on specific cellular processes, such as oxidative stress, mitochondrial function, the endolysosomal system, proteostasis, synaptic plasticity, and signaling pathways (e.g., Wnt/β-catenin) [114,143,152]. These advancements aim to better recapitulate the cellular diversity and interactions present in the human brain. However, improving the modeling of aging and disease progression remains a critical goal, with strategies being explored to induce age-related phenotypes or extend culture duration to capture long-term neurodegenerative processes. The development of more disease-relevant functional assays capable of capturing specific pathological features of AD and evaluating long-term treatment effects represents an important step forward [143]. Three-dimensional cultures are becoming increasingly recognized as more relevant to disease models compared to 2D cultures, as they provide an environment that more closely resembles the human brain, allowing for better reproduction of complex cell-cell interactions and disease phenotypes [114]. Future research efforts should continue to employ these models to evaluate the efficacy of novel drug candidates or to explore the repositioning of existing approved therapies [112]. Enhancing the robustness and reproducibility of differentiation protocols, alongside the standardization of functional assays, will be essential to reduce variability between cell lines and experimental batches, thus improving the reliability of findings across studies [103]. Furthermore, the combination of organoid-based models with computational analysis and mathematical modeling offers a strategic approach for advancing precision medicine in the treatment of AD [117]. Overall, the ability to evaluate neuronal activity and its responses to drugs makes organoids valuable tools for drug screening efforts [112].
Moreover, future work should continue to refine functional validation strategies following genetic editing, ensuring that the biological impact of introduced mutations is thoroughly characterized. Although isogenic lines reduce the potential off-target effects of CRISPR technology, future studies are needed to further investigate their utility for functional validation and therapeutic screening. Furthermore, manipulation of gene dosage, such as creating heterozygous knockout cells that better mimic partial reduction of gene expression, is not currently rapid and efficient in iPSCs, indicating an area for future methodological development [143].
As illustrated in Figure 5, the balance between the advantages and limitations of CRISPR/Cas9 and iPSC-based models in AD research remains delicate. Despite these continued constraints, the integration of CRISPR/Cas9 with hiPSC-based systems remains of great promise. The ever-growing body of in vitro and in vivo evidence underlines the viewpoint that, ultimately, the therapeutic benefits may transcend the current technological and ethical constraints. The figure reflects the optimistic perspective that the potential benefits may outweigh the current limitations, especially in the long term.
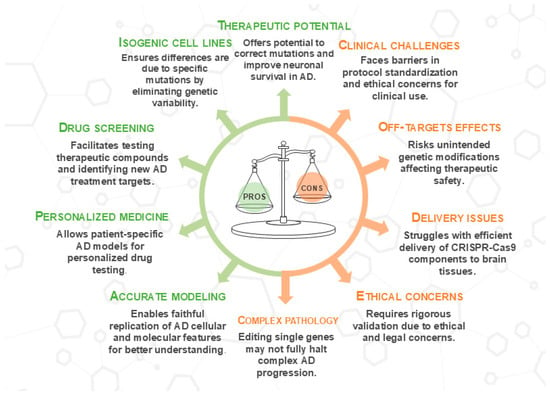
Figure 5.
Advantages and disadvantages of combining CRISPR/Cas9 and stem cells for AD. The figure schematizes the key points of using CRISPR/Cas9 technology and stem cells in AD research. Advantages (PROS) are shown on the left, indicated by green arrows, while disadvantages (CONS) are on the right, marked by orange arrows. This image was created using the image bank of Servier Medical Art (Available online: http://smart.servier.com/; accessed on 30 May 2025) licensed under a Creative Commons Attribution 3.0 Unported License (available online: https://creativecommons.org/licenses/by/3.0/, accessed on 30 May 2025). CRISPR/Cas9: clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9; AD: Alzheimer’s disease.
6. Conclusions
The combination of CRISPR gene editing technology, stem cell-based models, and neuroengineering technologies is revolutionizing AD research and therapeutic development. CRISPR/Cas9 enables precise modeling of pathogenic mutations in human-relevant systems, such as patient-derived neurons and cerebral organoids. These platforms are essential for studying key AD mechanisms, including Aβ and tau aggregation, mitochondrial dysfunction, synaptic alterations, and neuroinflammation. The use of isogenic cell lines minimizes background variability, allowing direct attribution of phenotypic differences to specific genetic edits. The combined approach allows for creating specific genetic models by introducing or correcting AD-associated mutations in genes such as APP, PSEN1, PSEN2, APOE, SORL1, ABCA7, and others, providing insights into the mechanisms underlying disease progression. However, the mechanisms driving tau pathogenesis may involve additional factors, including epigenetic modifications that influence tau expression and phosphorylation. These modifications may represent a potential mechanistic gap, with epigenetic regulation playing a role in how tau is modified independently of the genetic mutations driving Aβ accumulation. Further studies into epigenetic regulation may provide insights into this discrepancy.
While numerous CRISPR/Cas9-based studies and hiPSC-derived models have significantly advanced our understanding of AD pathology, critical evaluation of their limitations is essential for interpreting their translational value. Although genetic targets such as APP, PSEN1, MAPT, and APOE have shown promise in modulating core disease pathways, most findings remain confined to in vitro systems or preclinical models, with limited validation across multiple platforms. Similarly, while iPSC-derived neurons, astrocytes, and microglia enable patient-specific modeling and functional exploration, they present variability, incomplete maturation, and challenges in replicating aging-related phenotypes. While 3D brain organoids are more faithful models of human AD pathology compared to 2D cultures, they still suffer from batch-to-batch heterogeneity and incomplete cellular complexity, which must be considered when extrapolating findings for therapeutic development. Despite promising preclinical results, the clinical translation of these findings remains complex, and their true potential will depend on the continued refinement of modeling systems and integration of cross-validation strategies to bridge the gap between experimental insight and clinical application. No active clinical trials currently apply CRISPR/Cas9 directly to AD, largely due to the disease’s multifactorial nature, challenges in CNS-targeted delivery, and unresolved safety concerns, particularly regarding off-target effects and long-term outcomes. Rigorous standards for vector quality, genomic integrity, and follow-up underscore the need to bridge the gap between laboratory feasibility and therapeutic approval are required. Similarly, stem cell therapies have demonstrated safety in early-phase trials, but with limited cognitive benefit to date. More refined cell sources (e.g., hiPSC-derived neural progenitors) and delivery strategies may offer enhanced efficacy but will need careful validation in controlled settings. Ultimately, while gene and stem cell-based therapies hold significant potential for AD, a cautious and critically informed approach is essential. Future research should prioritize translational rigor, standardized outcome metrics, and long-term safety monitoring to responsibly bridge the gap from innovation to clinical application.
Author Contributions
Conceptualization, S.S. and S.O.; methodology, S.S., I.R., G.L.C. and I.A.; investigation, S.S., I.R., G.L.C. and I.A.; data curation, S.S., I.R., G.L.C. and I.A.; writing—original draft preparation, S.S. and I.R.; writing—review and editing, S.S. and S.O.; visualization, S.S. and I.R.; supervision, S.S. and S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Current Research Funds 2025 (RRC-2025-23686388), Ministry of Health, Italy.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors would like to thank the Ministry of Health, Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bomasang-Layno, E.; Bronsther, R. Diagnosis and Treatment of Alzheimer’s Disease: An Update. Del. J. Public Health 2021, 7, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Soria Lopez, J.A.; Gonzalez, H.M.; Leger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Wildsmith, K.R.; Holley, M.; Savage, J.C.; Skerrett, R.; Landreth, G.E. Evidence for impaired amyloid β clearance in Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 33. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef]
- Li, K.; Wei, Q.; Liu, F.-F.; Hu, F.; Xie, A.-J.; Zhu, L.-Q.; Liu, D. Synaptic Dysfunction in Alzheimer’s Disease: Aβ, Tau, and Epigenetic Alterations. Mol. Neurobiol. 2018, 55, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Walsh, D.M. Alzheimer’s disease: Synaptic dysfunction and Aβ. Mol. Neurodegener. 2009, 4, 48. [Google Scholar] [CrossRef]
- Amelimojarad, M.; Amelimojarad, M.; Cui, X. The emerging role of brain neuroinflammatory responses in Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1391517. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Akhtar, A.; Gupta, S.M.; Dwivedi, S.; Kumar, D.; Shaikh, M.F.; Negi, A. Preclinical Models for Alzheimer’s Disease: Past, Present, and Future Approaches. ACS Omega 2022, 7, 47504–47517. [Google Scholar] [CrossRef]
- Kafkafi, N.; Agassi, J.; Chesler, E.J.; Crabbe, J.C.; Crusio, W.E.; Eilam, D.; Gerlai, R.; Golani, I.; Gomez-Marin, A.; Heller, R.; et al. Reproducibility and replicability of rodent phenotyping in preclinical studies. Neurosci. Biobehav. Rev. 2018, 87, 218–232. [Google Scholar] [CrossRef]
- Hajjo, R.; Sabbah, D.A.; Abusara, O.H.; Al Bawab, A.Q. A Review of the Recent Advances in Alzheimer’s Disease Research and the Utilization of Network Biology Approaches for Prioritizing Diagnostics and Therapeutics. Diagnostics 2022, 12, 2975. [Google Scholar] [CrossRef]
- Ou, C.M.; Xue, W.W.; Liu, D.; Ma, L.; Xie, H.T.; Ning, K. Stem cell therapy in Alzheimer’s disease: Current status and perspectives. Front. Neurosci. 2024, 18, 1440334. [Google Scholar] [CrossRef]
- Brookhouser, N.; Raman, S.; Potts, C.; Brafman, D.A. May I Cut in? Gene Editing Approaches in Human Induced Pluripotent Stem Cells. Cells 2017, 6, 5. [Google Scholar] [CrossRef]
- Yoo, T.J. Anti-Inflammatory Gene Therapy Improves Spatial Memory Performance in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2022, 85, 1001–1008. [Google Scholar] [CrossRef]
- Papaspyropoulos, A.; Tsolaki, M.; Foroglou, N.; Pantazaki, A.A. Modeling and Targeting Alzheimer’s Disease with Organoids. Front. Pharmacol. 2020, 11, 396. [Google Scholar] [CrossRef]
- Cozzi, A.; Orellana, D.I.; Santambrogio, P.; Rubio, A.; Cancellieri, C.; Giannelli, S.; Ripamonti, M.; Taverna, S.; Di Lullo, G.; Rovida, E.; et al. Stem Cell Modeling of Neuroferritinopathy Reveals Iron as a Determinant of Senescence and Ferroptosis during Neuronal Aging. Stem Cell Rep. 2019, 13, 832–846. [Google Scholar] [CrossRef]
- Yuva-Aydemir, Y.; Almeida, S.; Krishnan, G.; Gendron, T.F.; Gao, F.B. Transcription elongation factor AFF2/FMR2 regulates expression of expanded GGGGCC repeat-containing C9ORF72 allele in ALS/FTD. Nat. Commun. 2019, 10, 5466. [Google Scholar] [CrossRef]
- Wang, H.; Rangaswamy, S.; Kodavati, M.; Mitra, J.; Guo, W.; Guerrero, E.N.; Van Den Bosch, L.; Hegde, M.L. RT(2) PCR array screening reveals distinct perturbations in DNA damage response signaling in FUS-associated motor neuron disease. Mol. Brain 2019, 12, 103. [Google Scholar] [CrossRef]
- Soni, N.; Kar, I.; Narendrasinh, J.D.; Shah, S.K.; Konathala, L.; Mohamed, N.; Kachhadia, M.P.; Chaudhary, M.H.; Dave, V.A.; Kumar, L.; et al. Role and application of CRISPR-Cas9 in the management of Alzheimer’s disease. Ann. Med. Surg. 2024, 86, 1517–1521. [Google Scholar] [CrossRef]
- Pavlou, S.; Foskolou, S.; Patikas, N.; Field, S.F.; Papachristou, E.K.; Santos, C.; Edwards, A.R.; Kishore, K.; Ansari, R.; Rajan, S.S.; et al. CRISPR-Cas9 genetic screen leads to the discovery of L-Moses, a KAT2B inhibitor that attenuates Tunicamycin-mediated neuronal cell death. Sci. Rep. 2023, 13, 3934. [Google Scholar] [CrossRef]
- Raikwar, S.P.; Thangavel, R.; Dubova, I.; Selvakumar, G.P.; Ahmed, M.E.; Kempuraj, D.; Zaheer, S.A.; Iyer, S.S.; Zaheer, A. Targeted Gene Editing of Glia Maturation Factor in Microglia: A Novel Alzheimer’s Disease Therapeutic Target. Mol. Neurobiol. 2019, 56, 378–393. [Google Scholar] [CrossRef]
- Barman, N.C.; Khan, N.M.; Islam, M.; Nain, Z.; Roy, R.K.; Haque, A.; Barman, S.K. CRISPR-Cas9: A Promising Genome Editing Therapeutic Tool for Alzheimer’s Disease—A Narrative Review. Neurol. Ther. 2020, 9, 419–434. [Google Scholar] [CrossRef]
- Andersen, O.M.; Bøgh, N.; Landau, A.M.; Pløen, G.G.; Jensen, A.M.G.; Monti, G.; Ulhøi, B.P.; Nyengaard, J.R.; Jacobsen, K.R.; Jørgensen, M.M.; et al. A genetically modified minipig model for Alzheimer’s disease with SORL1 haploinsufficiency. Cell Rep. Med. 2022, 3, 100740. [Google Scholar] [CrossRef]
- Sun, J.; Roy, S. Gene-based therapies for neurodegenerative diseases. Nat. Neurosci. 2021, 24, 297–311. [Google Scholar] [CrossRef]
- Yang, W.; Tu, Z.; Sun, Q.; Li, X.J. CRISPR/Cas9: Implications for Modeling and Therapy of Neurodegenerative Diseases. Front. Mol. Neurosci. 2016, 9, 30. [Google Scholar] [CrossRef]
- Thapar, N.; Eid, M.A.F.; Raj, N.; Kantas, T.; Billing, H.S.; Sadhu, D. Application of CRISPR/Cas9 in the management of Alzheimer’s disease and Parkinson’s disease: A review. Ann. Med. Surg. 2024, 86, 329–335. [Google Scholar] [CrossRef]
- Kwart, D.; Gregg, A.; Scheckel, C.; Murphy, E.A.; Paquet, D.; Duffield, M.; Fak, J.; Olsen, O.; Darnell, R.B.; Tessier-Lavigne, M. A Large Panel of Isogenic APP and PSEN1 Mutant Human iPSC Neurons Reveals Shared Endosomal Abnormalities Mediated by APP β-CTFs, Not Aβ. Neuron 2019, 104, 256–270.e5. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-beta Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 62, 1345–1367. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2019, 72, 981–1017. [Google Scholar] [CrossRef]
- Karapetyan, G.; Fereshetyan, K.; Harutyunyan, H.; Yenkoyan, K. The synergy of beta amyloid 1-42 and oxidative stress in the development of Alzheimer’s disease-like neurodegeneration of hippocampal cells. Sci. Rep. 2022, 12, 17883. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- Shea, Y.F.; Chu, L.W.; Chan, A.O.; Ha, J.; Li, Y.; Song, Y.Q. A systematic review of familial Alzheimer’s disease: Differences in presentation of clinical features among three mutated genes and potential ethnic differences. J. Formos. Med. Assoc. Taiwan Yi Zhi 2016, 115, 67–75. [Google Scholar] [CrossRef]
- Pimenova, A.A.; Goate, A.M. Novel presenilin 1 and 2 double knock-out cell line for in vitro validation of PSEN1 and PSEN2 mutations. Neurobiol. Dis. 2020, 138, 104785. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, R.; Yang, G.; Shi, Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Abeta42 and Abeta40 peptides by gamma-secretase. Proc. Natl. Acad. Sci. USA 2017, 114, E476–E485. [Google Scholar] [CrossRef]
- Konstantinidis, E.; Molisak, A.; Perrin, F.; Streubel-Gallasch, L.; Fayad, S.; Kim, D.Y.; Petri, K.; Aryee, M.J.; Aguilar, X.; Gyorgy, B.; et al. CRISPR-Cas9 treatment partially restores amyloid-beta 42/40 in human fibroblasts with the Alzheimer’s disease PSEN 1 M146L mutation. Mol. Therapy Nucleic Acids 2022, 28, 450–461. [Google Scholar] [CrossRef]
- Ortiz-Virumbrales, M.; Moreno, C.L.; Kruglikov, I.; Marazuela, P.; Sproul, A.; Jacob, S.; Zimmer, M.; Paull, D.; Zhang, B.; Schadt, E.E.; et al. CRISPR/Cas9-Correctable mutation-related molecular and physiological phenotypes in iPSC-derived Alzheimer’s PSEN2 (N141I) neurons. Acta Neuropathol. Commun. 2017, 5, 77. [Google Scholar] [CrossRef]
- Gyorgy, B.; Loov, C.; Zaborowski, M.P.; Takeda, S.; Kleinstiver, B.P.; Commins, C.; Kastanenka, K.; Mu, D.; Volak, A.; Giedraitis, V.; et al. CRISPR/Cas9 Mediated Disruption of the Swedish APP Allele as a Therapeutic Approach for Early-Onset Alzheimer’s Disease. Mol. Therapy Nucleic Acids 2018, 11, 429–440. [Google Scholar] [CrossRef]
- Guyon, A.; Rousseau, J.; Begin, F.G.; Bertin, T.; Lamothe, G.; Tremblay, J.P. Base editing strategy for insertion of the A673T mutation in the APP gene to prevent the development of AD in vitro. Mol. Therapy Nucleic Acids 2021, 24, 253–263. [Google Scholar] [CrossRef]
- Li, W.; Pang, Y.; Wang, Y.; Mei, F.; Guo, M.; Wei, Y.; Li, X.; Qin, W.; Wang, W.; Jia, L.; et al. Aberrant palmitoylation caused by a ZDHHC21 mutation contributes to pathophysiology of Alzheimer’s disease. BMC Med. 2023, 21, 223. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Khan, M.S.; Qureshi, N.; Khan, R.; Son, Y.O.; Maqbool, T. CRISPR/Cas9-Based therapeutics as a promising strategy for management of Alzheimer’s disease: Progress and prospects. Front. Cell. Neurosci. 2025, 19, 1578138. [Google Scholar] [CrossRef]
- Singh, A.K.; Mishra, G.; Maurya, A.; Awasthi, R.; Kumari, K.; Thakur, A.; Rai, A.; Rai, G.K.; Sharma, B.; Kulkarni, G.T.; et al. Role of TREM2 in Alzheimer’s Disease and its Consequences on beta- Amyloid, Tau and Neurofibrillary Tangles. Curr. Alzheimer Res. 2019, 16, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Meilandt, W.J.; Xie, L.; Gandham, V.D.; Ngu, H.; Barck, K.H.; Rezzonico, M.G.; Imperio, J.; Lalehzadeh, G.; Huntley, M.A.; et al. Trem2 restrains the enhancement of tau accumulation and neurodegeneration by beta-amyloid pathology. Neuron 2021, 109, 1283–1301.e6. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kurti, A.; Baker, K.E.; Liu, C.C.; Colonna, M.; Ulrich, J.D.; Holtzman, D.M.; Bu, G.; Fryer, J.D. Behavioral and transcriptomic analysis of Trem2-null mice: Not all knockout mice are created equal. Hum. Mol. Genet. 2018, 27, 211–223. [Google Scholar] [CrossRef]
- Jadhav, V.S.; Lin, P.B.C.; Pennington, T.; Di Prisco, G.V.; Jannu, A.J.; Xu, G.; Moutinho, M.; Zhang, J.; Atwood, B.K.; Puntambekar, S.S.; et al. Trem2 Y38C mutation and loss of Trem2 impairs neuronal synapses in adult mice. Mol. Neurodegener. 2020, 15, 62. [Google Scholar] [CrossRef]
- Chen, F.; Fang, S.; Du, Y.; Ghosh, A.; Reed, M.N.; Long, Y.; Suppiramaniam, V.; Tang, S.; Hong, H. CRISPR/Cas9-mediated CysLT1R deletion reverses synaptic failure, amyloidosis and cognitive impairment in APP/PS1 mice. Aging 2021, 13, 6634–6661. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef]
- Fontana, M.; Solomon, S.D.; Kachadourian, J.; Walsh, L.; Rocha, R.; Lebwohl, D.; Smith, D.; Taubel, J.; Gane, E.J.; Pilebro, B.; et al. CRISPR-Cas9 Gene Editing with Nexiguran Ziclumeran for ATTR Cardiomyopathy. N. Engl. J. Med. 2024, 391, 2231–2241. [Google Scholar] [CrossRef]
- Rafii, M.S.; Baumann, T.L.; Bakay, R.A.; Ostrove, J.M.; Siffert, J.; Fleisher, A.S.; Herzog, C.D.; Barba, D.; Pay, M.; Salmon, D.P.; et al. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014, 10, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.J.; Baltanas, F.C.; Kovacs, I.; Nagahara, A.H.; Barba, D.; Tuszynski, M.H. Postmortem Analysis in a Clinical Trial of AAV2-NGF Gene Therapy for Alzheimer’s Disease Identifies a Need for Improved Vector Delivery. Hum. Gene Ther. 2020, 31, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Uspenskaya, O.; Heckman, L.D.; Wong, L.C.; Hatch, D.A.; Tewari, A.; Vandenberghe, R.; Irwin, D.J.; Saracino, D.; Le Ber, I.; et al. Progranulin AAV gene therapy for frontotemporal dementia: Translational studies and phase 1/2 trial interim results. Nat. Med. 2024, 30, 1406–1415. [Google Scholar] [CrossRef]
- Park, H.; Oh, J.; Shim, G.; Cho, B.; Chang, Y.; Kim, S.; Baek, S.; Kim, H.; Shin, J.; Choi, H.; et al. In vivo neuronal gene editing via CRISPR-Cas9 amphiphilic nanocomplexes alleviates deficits in mouse models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 524–528. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J. The potential of alternate sources of cells for neural grafting in Parkinson’s and Huntington’s disease. Neurodegener. Dis. Manag. 2014, 4, 297–307. [Google Scholar] [CrossRef]
- Menasché, P. Cell therapy trials for heart regeneration—Lessons learned and future directions. Nat. Rev. Cardiol. 2018, 15, 659–671. [Google Scholar] [CrossRef]
- Stoltz, J.-F.; de Isla, N.; Li, Y.; Bensoussan, D.; Zhang, L.; Huselstein, C.; Chen, Y.; Decot, V.; Magdalou, J.; Li, N. Stem cells and regenerative medicine: Myth or reality of the 21th century. Stem Cells Int. 2015, 2015, 734731. [Google Scholar] [CrossRef] [PubMed]
- Rajabzadeh, N.; Fathi, E.; Farahzadi, R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.-A.; Lee, S.-P.; Yang, J.-Y.; Park, Y.-S. Current Perspectives regarding Stem Cell-Based Therapy for Alzheimer’s Disease. Stem Cells Int. 2018, 2018, 6392986. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M. Towards stem cell based therapies for Parkinson’s disease. Development 2018, 145, dev156117. [Google Scholar] [CrossRef]
- Robinson, R. At the bench-amyotrophic lateral sclerosis: In the lab, regulatory T cells slow ALS. Neurol. Today 2018, 18, 1–19. [Google Scholar] [CrossRef]
- Shirani, A.; Stüve, O. Natalizumab: Perspectives from the bench to bedside. Cold Spring Harb. Perspect. Med. 2018, 8, a029066. [Google Scholar] [CrossRef]
- Kalra, K.; Tomar, P.C. Stem cell: Basics, classification and applications. Am. J. Phytomed. Clin. Ther. 2014, 2, 919–930. [Google Scholar]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Beck, S.; Lee, B.-K.; Kim, J. Multi-layered global gene regulation in mouse embryonic stem cells. Cell. Mol. Life Sci. 2015, 72, 199–216. [Google Scholar] [CrossRef][Green Version]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Kote Amol, P.; Pawar Sanjay, D.; Dhonde Satish, M. An overview of stem cell. Pharmacologyonline 2011, 3, 1155–1170. [Google Scholar]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weick, J.P.; Liu, H.; Krencik, R.; Zhang, X.; Ma, L.; Zhou, G.-m.; Ayala, M.; Zhang, S.-C. Medial ganglionic eminence–like cells derived from human embryonic stem cells correct learning and memory deficits. Nat. Biotechnol. 2013, 31, 440–447. [Google Scholar] [CrossRef]
- Rippon, H.; Bishop, A. Embryonic stem cells. Cell Prolif. 2004, 37, 23–34. [Google Scholar] [CrossRef]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics 2017, 7, 106. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.; Chang, E.H.; Kim, J.H.; Hwang, J.W.; Kim, J.-Y.; Kyung, J.W.; Kim, S.H.; Oh, J.S.; Shim, S.M. GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer’s disease model. Stem Cells Dev. 2015, 24, 2378–2390. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Kim, J.; Lee, D.; Jeon, H.; Kwon, S.; Kim, S.; Yoo, Y.; Lee, E.; Choi, S. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ. 2012, 19, 680–691. [Google Scholar] [CrossRef]
- Lim, H.; Lee, D.; Choi, W.K.; Choi, S.J.; Oh, W.; Kim, D.H. Galectin-3 secreted by human umbilical cord blood-derived mesenchymal stem cells reduces aberrant tau phosphorylation in an Alzheimer disease model. Stem Cells Int. 2020, 2020, 8878412. [Google Scholar] [CrossRef]
- Moghadam, F.H.; Alaie, H.; Karbalaie, K.; Tanhaei, S.; Esfahani, M.H.N.; Baharvand, H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation 2009, 78, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Song, J. Treating brain disorders by targeting adult neural stem cells. Trends Mol. Med. 2018, 24, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.C.; Hamblin, M.H.; Lee, J.-P. Neural stem cell therapy for neurovascular injury in Alzheimer’s disease. Exp. Neurol. 2020, 324, 113112. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Zhao, F.; Aschner, M.; Chen, J.; Luo, W. The interaction between microglia and neural stem/precursor cells. Brain Res. Bull. 2014, 109, 32–38. [Google Scholar] [CrossRef]
- Marsh, S.E.; Blurton-Jones, M. Neural stem cell therapy for neurodegenerative disorders: The role of neurotrophic support. Neurochem. Int. 2017, 106, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Fransson, L.A.; Mani, K. Interplay between glypican-1, amyloid-beta and tau phosphorylation in human neural stem cells. Neuroscience 2024, 553, 121–127. [Google Scholar] [CrossRef]
- Ruetz, T.J.; Pogson, A.N.; Kashiwagi, C.M.; Gagnon, S.D.; Morton, B.; Sun, E.D.; Na, J.; Yeo, R.W.; Leeman, D.S.; Morgens, D.W.; et al. CRISPR-Cas9 screens reveal regulators of ageing in neural stem cells. Nature 2024, 634, 1150–1159. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, N.; Hu, N.; Jiang, R.; Lu, H.; Xuan, A.; Long, D.; Chen, Y. Neural stem cell transplantation improves learning and memory by protecting cholinergic neurons and restoring synaptic impairment in an amyloid precursor protein/presenilin 1 transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2020, 21, 1172–1180. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, H.h.; Wang, Y.; Gu, G.j.; Zhang, W.; Xia, R. Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2016, 136, 815–825. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 2009, 27, 743–745. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef]
- Haworth, R.; Sharpe, M. Accept or reject: The role of immune tolerance in the development of stem cell therapies and possible future approaches. Toxicol. Pathol. 2021, 49, 1308–1316. [Google Scholar] [CrossRef]
- Kropp, C.; Massai, D.; Zweigerdt, R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process Biochem. 2017, 59, 244–254. [Google Scholar] [CrossRef]
- Rash, B.G.; Ramdas, K.N.; Agafonova, N.; Naioti, E.; McClain-Moss, L.; Zainul, Z.; Varnado, B.; Peterson, K.; Brown, M.; Leal, T.; et al. Allogeneic mesenchymal stem cell therapy with laromestrocel in mild Alzheimer’s disease: A randomized controlled phase 2a trial. Nat. Med. 2025, 31, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Seo, S.W.; Chang, J.W.; Lee, J.I.; Kim, C.H.; Chin, J.; Choi, S.J.; Kwon, H.; Yun, H.J.; Lee, J.M.; et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimer’s Dement. 2015, 1, 95–102. [Google Scholar] [CrossRef]
- Brody, M.; Agronin, M.; Herskowitz, B.J.; Bookheimer, S.Y.; Small, G.W.; Hitchinson, B.; Ramdas, K.; Wishard, T.; McInerney, K.F.; Vellas, B.; et al. Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 261–273. [Google Scholar] [CrossRef]
- Zhang, M.L.; Li, H.B.; Jin, Y. Application and perspective of CRISPR/Cas9 genome editing technology in human diseases modeling and gene therapy. Front. Genet. 2024, 15, 1364742. [Google Scholar] [CrossRef]
- Ben Jehuda, R.; Shemer, Y.; Binah, O. Genome editing in induced pluripotent stem cells using CRISPR/Cas9. Stem Cell Rev. Rep. 2018, 14, 323–336. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.; Zhan, S.; Huang, Y. Genome editing in insects: Current status and challenges. Natl. Sci. Rev. 2019, 6, 399–401. [Google Scholar] [CrossRef]
- Wang, A.Y.L. Human induced pluripotent stem cell-derived exosomes as a new therapeutic strategy for various diseases. Int. J. Mol. Sci. 2021, 22, 1769. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.U.; Mehta, A.; Vũ, T.T.; Yeo, G.C. Cellular modifications and biomaterial design to improve mesenchymal stem cell transplantation. Biomater. Sci. 2023, 11, 4752–4773. [Google Scholar] [CrossRef]
- Hyvarinen, T.; Hyysalo, A.; Kapucu, F.E.; Aarnos, L.; Vinogradov, A.; Eglen, S.J.; Yla-Outinen, L.; Narkilahti, S. Functional characterization of human pluripotent stem cell-derived cortical networks differentiated on laminin-521 substrate: Comparison to rat cortical cultures. Sci. Rep. 2019, 9, 17125. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Kobayashi, H.; Tatsumi, L.; Tomita, T. Mouse Models of Alzheimer’s Disease. Front. Mol. Neurosci. 2022, 15, 912995. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Plews, J.; Wu, J.C. Comparison of human induced pluripotent and embryonic stem cells: Fraternal or identical twins? Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 635–638. [Google Scholar] [CrossRef]
- Tay, S.H.; Winanto; Khong, Z.J.; Koh, Y.H.; Ng, S.Y. Generation of Cortical, Dopaminergic, Motor, and Sensory Neurons from Human Pluripotent Stem Cells. Methods Mol. Biol. 2022, 2549, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.M.; Tan, Y.; Tong, J.; Ke, M.; Zhang, K.; Yan, L.; Cen, X.; Lu, J.H.; Chen, G.; Su, H.; et al. Presenilin-1 F105C mutation leads to tau accumulation in human neurons via the Akt/mTORC1 signaling pathway. Cell Biosci. 2022, 12, 131. [Google Scholar] [CrossRef]
- Ford, E.; Pearlman, J.; Ruan, T.; Manion, J.; Waller, M.; Neely, G.G.; Caron, L. Human Pluripotent Stem Cells-Based Therapies for Neurodegenerative Diseases: Current Status and Challenges. Cells 2020, 9, 2517. [Google Scholar] [CrossRef]
- Thamodaran, V.; Rani, S.; Velayudhan, S.R. Gene Editing in Human Induced Pluripotent Stem Cells Using Doxycycline-Inducible CRISPR-Cas9 System. Methods Mol. Biol. 2022, 2454, 755–773. [Google Scholar] [CrossRef]
- Borquez, D.A.; Castro, F.; Nunez, M.T.; Urrutia, P.J. New Players in Neuronal Iron Homeostasis: Insights from CRISPRi Studies. Antioxidants 2022, 11, 1807. [Google Scholar] [CrossRef]
- Arnst, N.; Belio-Mairal, P.; Garcia-Gonzalez, L.; Arnaud, L.; Greetham, L.; Nivet, E.; Rivera, S.; Dityatev, A. Deficiency in MT5-MMP Supports Branching of Human iPSCs-Derived Neurons and Reduces Expression of GLAST/S100 in iPSCs-Derived Astrocytes. Cells 2021, 10, 1705. [Google Scholar] [CrossRef]
- Yin, J.; VanDongen, A.M. Enhanced Neuronal Activity and Asynchronous Calcium Transients Revealed in a 3D Organoid Model of Alzheimer’s Disease. ACS Biomater. Sci. Eng. 2021, 7, 254–264. [Google Scholar] [CrossRef]
- Feuer, K.L.; Peng, X.; Yovo, C.K.; Avramopoulos, D. DPYSL2/CRMP2 isoform B knockout in human iPSC-derived glutamatergic neurons confirms its role in mTOR signaling and neurodevelopmental disorders. Mol. Psychiatry 2023, 28, 4353–4362. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.J.; Ivanyuk, D.; Panagiotakopoulou, V.; Di Napoli, G.; Kalb, S.; Brunetti, D.; Al-Shaana, R.; Kaeser, S.A.; Fraschka, S.A.; Jucker, M.; et al. Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer’s disease-like pathology in human cerebral organoids. Mol. Psychiatry 2021, 26, 5733–5750. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chai, Y.; Yi, R.; Chen, Y.; Ip, J.P.K.; Ye, T.; Chen, Y. Generation of a homozygous ABCA7-knockout human iPSC line using the CRISPR/Cas9 system. Stem Cell Res. 2023, 66, 103000. [Google Scholar] [CrossRef] [PubMed]
- Canals, I.; Comella-Bolla, A.; Cepeda-Prado, E.; Avaliani, N.; Crowe, J.A.; Oburoglu, L.; Bruzelius, A.; King, N.; Pajares, M.A.; Perez-Sala, D.; et al. Astrocyte dysfunction and neuronal network hyperactivity in a CRISPR engineered pluripotent stem cell model of frontotemporal dementia. Brain Commun. 2023, 5, fcad158. [Google Scholar] [CrossRef]
- Park, J.C.; Jang, S.Y.; Lee, D.; Lee, J.; Kang, U.; Chang, H.; Kim, H.J.; Han, S.H.; Seo, J.; Choi, M.; et al. A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids. Nat. Commun. 2021, 12, 280. [Google Scholar] [CrossRef]
- Lin, Y.T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141–1154.e7. [Google Scholar] [CrossRef]
- Ye, T.; Duan, Y.; Tsang, H.W.S.; Xu, H.; Chen, Y.; Cao, H.; Chen, Y.; Fu, A.K.Y.; Ip, N.Y. Efficient manipulation of gene dosage in human iPSCs using CRISPR/Cas9 nickases. Commun. Biol. 2021, 4, 195. [Google Scholar] [CrossRef]
- Haq, I.; Ngo, J.C.; Roy, N.; Pan, R.L.; Nawsheen, N.; Chiu, R.; Zhang, Y.; Fujita, M.; Soni, R.K.; Wu, X.; et al. An integrated toolkit for human microglia functional genomics. Stem Cell Res. Ther. 2024, 15, 104. [Google Scholar] [CrossRef]
- Canals, I.; Ahlenius, H. CRISPR/Cas9 Genome Engineering in Human Pluripotent Stem Cells for Modeling of Neurological Disorders. Methods Mol. Biol. 2021, 2352, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhou, Z.; Liu, C.; Kang, X.; Zhong, X.; Zhang, Q.; Xu, Y. Generation of a DAPK1 knockout first (conditional ready) human embryonic stem cell line (ZSSYe001-A) by CRISPR-Cas9 technology. Stem Cell Res. 2020, 43, 101693. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.H.W.; Sampurno, L.; Little, C.B.; Lamande, S.R.; Bateman, J.F. Generation of a miR-26b stem-loop knockout human iPSC line, MCRIi019-A-1, using CRISPR/Cas9 editing. Stem Cell Res. 2020, 50, 102118. [Google Scholar] [CrossRef]
- Martens, Y.A.; Xu, S.; Tait, R.; Li, G.; Zhao, X.C.; Lu, W.; Liu, C.C.; Kanekiyo, T.; Bu, G.; Zhao, J. Generation and validation of APOE knockout human iPSC-derived cerebral organoids. STAR Protoc. 2021, 2, 100571. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Barahona-Torres, N.; Jang, S.Y.; Mok, K.Y.; Kim, H.J.; Han, S.H.; Cho, K.H.; Zhou, X.; Fu, A.K.Y.; Ip, N.Y.; et al. Multi-Omics-Based Autophagy-Related Untypical Subtypes in Patients with Cerebral Amyloid Pathology. Adv. Sci. 2022, 9, e2201212. [Google Scholar] [CrossRef]
- Stolzenburg, L.R.; Esmaeeli, S.; Kulkarni, A.S.; Murphy, E.; Kwon, T.; Preiss, C.; Bahnassawy, L.; Stender, J.D.; Manos, J.D.; Reinhardt, P.; et al. Functional characterization of a single nucleotide polymorphism associated with Alzheimer’s disease in a hiPSC-based neuron model. PLoS ONE 2023, 18, e0291029. [Google Scholar] [CrossRef]
- Laverde-Paz, M.J.; Nuytemans, K.; Wang, L.; Vance, J.M.; Pericak-Vance, M.A.; Dykxhoorn, D.M.; Cukier, H.N. Derivation of stem cell line UMi028-A-2 containing a CRISPR/Cas9 induced Alzheimer’s disease risk variant p.S1038C in the TTC3 gene. Stem Cell Res. 2021, 52, 102258. [Google Scholar] [CrossRef]
- Cenini, G.; Hebisch, M.; Iefremova, V.; Flitsch, L.J.; Breitkreuz, Y.; Tanzi, R.E.; Kim, D.Y.; Peitz, M.; Brustle, O. Dissecting Alzheimer’s disease pathogenesis in human 2D and 3D models. Mol. Cell. Neurosci. 2021, 110, 103568. [Google Scholar] [CrossRef]
- Hossain, M.K.; Kim, H.R.; Chae, H.J. Aging phenotype in AD brain organoids: Track to success and challenges. Ageing Res. Rev. 2024, 96, 102256. [Google Scholar] [CrossRef]
- Hernandez, D.; Rooney, L.A.; Daniszewski, M.; Gulluyan, L.; Liang, H.H.; Cook, A.L.; Hewitt, A.W.; Pebay, A. Culture Variabilities of Human iPSC-Derived Cerebral Organoids Are a Major Issue for the Modelling of Phenotypes Observed in Alzheimer’s Disease. Stem Cell Rev. Rep. 2022, 18, 718–731. [Google Scholar] [CrossRef]
- Konishi, C.T.; Mulaiese, N.; Butola, T.; Zhang, Q.; Kagan, D.; Yang, Q.; Pressler, M.; Dirvin, B.G.; Devinsky, O.; Basu, J.; et al. Modeling and correction of protein conformational disease in iPSC-derived neurons through personalized base editing. Mol. Therapy Nucleic Acids 2025, 36, 102441. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Liu, H.; Xie, F.; Fischer, S.; Adkins, R.S.; Aldridge, A.I.; Ament, S.A.; Bartlett, A.; Behrens, M.M.; Van den Berge, K.; et al. A transcriptomic and epigenomic cell atlas of the mouse primary motor cortex. Nature 2021, 598, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Brauninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 2015, 112, 15672–15677. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sousa, A.M.M.; Gao, T.; Skarica, M.; Li, M.; Santpere, G.; Esteller-Cucala, P.; Juan, D.; Ferrandez-Peral, L.; Gulden, F.O.; et al. Spatiotemporal transcriptomic divergence across human and macaque brain development. Science 2018, 362, eaat8077. [Google Scholar] [CrossRef]
- Tang, X.Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human organoids in basic research and clinical applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Zhang, S.; Sudwarts, A.; Zhang, H.; Kozlova, A.; Moulton, M.J.; Goodman, L.D.; Pang, Z.P.; Sanders, A.R.; et al. Alzheimer’s disease protective allele of Clusterin modulates neuronal excitability through lipid-droplet-mediated neuron-glia communication. Mol. Neurodegener. 2025, 20, 51. [Google Scholar] [CrossRef]
- Murai, N.; Mitalipova, M.; Jaenisch, R. Functional analysis of CX3CR1 in human induced pluripotent stem (iPS) cell-derived microglia-like cells. Eur. J. Neurosci. 2020, 52, 3667–3678. [Google Scholar] [CrossRef]
- Liu, Z.; Chao, J.; Wang, C.; Sun, G.; Roeth, D.; Liu, W.; Chen, X.; Li, L.; Tian, E.; Feng, L.; et al. Astrocytic response mediated by the CLU risk allele inhibits OPC proliferation and myelination in a human iPSC model. Cell Rep. 2023, 42, 112841. [Google Scholar] [CrossRef]
- Chen, K.; Martens, Y.A.; Meneses, A.; Ryu, D.H.; Lu, W.; Raulin, A.C.; Li, F.; Zhao, J.; Chen, Y.; Jin, Y.; et al. LRP1 is a neuronal receptor for alpha-synuclein uptake and spread. Mol. Neurodegener. 2022, 17, 57. [Google Scholar] [CrossRef]
- Ng, B.; Vowles, J.; Bertherat, F.; Abey, A.; Kilfeather, P.; Beccano-Kelly, D.; Stefana, M.I.; O’Brien, D.P.; Bengoa-Vergniory, N.; Carling, P.J.; et al. Tau depletion in human neurons mitigates Abeta-driven toxicity. Mol. Psychiatry 2024, 29, 2009–2020. [Google Scholar] [CrossRef]
- Buchholz, S.; Kabbani, M.A.A.; Bell-Simons, M.; Kluge, L.; Cagmak, C.; Klimek, J.; Haag, N.; Iohan, L.C.; Coulon, A.; Costa, M.R.; et al. The tau isoform 1N4R confers vulnerability of MAPT knockout human iPSC-derived neurons to amyloid beta and phosphorylated tau-induced neuronal dysfunction. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2025, 21, e14403. [Google Scholar] [CrossRef] [PubMed]
- Corsi, G.I.; Gadekar, V.P.; Haukedal, H.; Doncheva, N.T.; Anthon, C.; Ambardar, S.; Palakodeti, D.; Hyttel, P.; Freude, K.; Seemann, S.E.; et al. The transcriptomic landscape of neurons carrying PSEN1 mutations reveals changes in extracellular matrix components and non-coding gene expression. Neurobiol. Dis. 2023, 178, 105980. [Google Scholar] [CrossRef]
- Perez-Corredor, P.; Vanderleest, T.E.; Vacano, G.N.; Sanchez, J.S.; Villalba-Moreno, N.D.; Marino, C.; Krasemann, S.; Mendivil-Perez, M.A.; Aguillon, D.; Jimenez-Del-Rio, M.; et al. APOE3 Christchurch modulates beta-catenin/Wnt signaling in iPS cell-derived cerebral organoids from Alzheimer’s cases. Front. Mol. Neurosci. 2024, 17, 1373568. [Google Scholar] [CrossRef]
- Zhao, H.; Ji, Q.; Wu, Z.; Wang, S.; Ren, J.; Yan, K.; Wang, Z.; Hu, J.; Chu, Q.; Hu, H.; et al. Destabilizing heterochromatin by APOE mediates senescence. Nat. Aging 2022, 2, 303–316. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.R.; Holst, B.; Ramakrishna, S.; Muddashetty, R.; Schmid, B.; Freude, K. Generation of two iPSC lines with either a heterozygous V717I or a heterozygous KM670/671NL mutation in the APP gene. Stem Cell Res. 2019, 34, 101368. [Google Scholar] [CrossRef]
- Zhang, X.; Vlkolinsky, R.; Wu, C.; Dolatabadi, N.; Scott, H.; Prikhodko, O.; Zhang, A.; Blanco, M.; Lang, N.; Pina-Crespo, J.; et al. S-Nitrosylation of CRTC1 in Alzheimer’s disease impairs CREB-dependent gene expression induced by neuronal activity. Proc. Natl. Acad. Sci. USA 2025, 122, e2418179122. [Google Scholar] [CrossRef]
- Mesa, H.; Zhang, E.Y.; Wang, Y.; Zhang, Q. Human neurons lacking amyloid precursor protein exhibit cholesterol-associated developmental and presynaptic deficits. J. Cell. Physiol. 2024, 239, e30999. [Google Scholar] [CrossRef] [PubMed]
- Knupp, A.; Mishra, S.; Martinez, R.; Braggin, J.E.; Szabo, M.; Kinoshita, C.; Hailey, D.W.; Small, S.A.; Jayadev, S.; Young, J.E. Depletion of the AD Risk Gene SORL1 Selectively Impairs Neuronal Endosomal Traffic Independent of Amyloidogenic APP Processing. Cell Rep. 2020, 31, 107719. [Google Scholar] [CrossRef]
- Rovelet-Lecrux, A.; Feuillette, S.; Miguel, L.; Schramm, C.; Pernet, S.; Quenez, O.; Segalas-Milazzo, I.; Guilhaudis, L.; Rousseau, S.; Riou, G.; et al. Impaired SorLA maturation and trafficking as a new mechanism for SORL1 missense variants in Alzheimer disease. Acta Neuropathol. Commun. 2021, 9, 196. [Google Scholar] [CrossRef]
- Li, J.; Yin, L.; Wang, C.; Xu, Y. Generation of a homozygous ABCA7 knockout cell line (AHMUCNi002-A) in human iPSCs using CRISPR/Cas9. Stem Cell Res. 2025, 85, 103700. [Google Scholar] [CrossRef]
- Kawatani, K.; Holm, M.L.; Starling, S.C.; Martens, Y.A.; Zhao, J.; Lu, W.; Ren, Y.; Li, Z.; Jiang, P.; Jiang, Y.; et al. ABCA7 deficiency causes neuronal dysregulation by altering mitochondrial lipid metabolism. Mol. Psychiatry 2024, 29, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Loosen, A.M.; Kato, A.; Gu, X. Revisiting the role of computational neuroimaging in the era of integrative neuroscience. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2024, 50, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, C.B.; Yang, A.; Lara, E.; McDonough, J.A.; Blauwendraat, C.; Peng, L.; Oguro, H.; Kanaujiya, J.; Zou, J.; Sebesta, D.; et al. A reference human induced pluripotent stem cell line for large-scale collaborative studies. Cell Stem Cell 2022, 29, 1685–1702.e22. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.X.; Zhai, H.; Shi, Y.; Liu, G.; Lowry, J.; Liu, B.; Ryan, E.B.; Yan, J.; Yang, Y.; Zhang, N.; et al. Efficacy and long-term safety of CRISPR/Cas9 genome editing in the SOD1-linked mouse models of ALS. Commun. Biol. 2021, 4, 396. [Google Scholar] [CrossRef]
- Grant, E.V. FDA Regulation of Clinical Applications of CRISPR-CAS Gene-Editing Technology. Food Drug Law J. 2016, 71, 608–633. [Google Scholar]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Tavridou, A.; Rogers, D.; Farinelli, G.; Gravanis, I.; Jekerle, V. Genome-editing medicinal products: The EMA perspective. Nat. reviews. Drug Discov. 2024, 23, 242–243. [Google Scholar] [CrossRef]
- Ayanoglu, F.B.; Elcin, A.E.; Elcin, Y.M. Bioethical issues in genome editing by CRISPR-Cas9 technology. Turk. J. Biol. Turk Biyol. Derg. 2020, 44, 110–120. [Google Scholar] [CrossRef]
- Lee, T.L.; Sawai, T. Navigating equity in global access to genome therapy expanding access to potentially transformative therapies and benefiting those in need requires global policy changes. Front. Genet. 2024, 15, 1381172. [Google Scholar] [CrossRef]
- Pitrez, P.R.; Monteiro, L.M.; Borgogno, O.; Nissan, X.; Mertens, J.; Ferreira, L. Cellular reprogramming as a tool to model human aging in a dish. Nat. Commun. 2024, 15, 1816. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.; Henry, K.W.; Fitzgerald, M.Q.; Muralidharan, K.; Caldwell, A.B.; Ramachandran, S.; Goldstein, L.S.B.; Mobley, W.C.; Galasko, D.R.; Subramaniam, S. Limitations of the human iPSC-derived neuron model for early-onset Alzheimer’s disease. Mol. Brain 2023, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Graf, A.; Brady, C.; De Santi, S.; Florian, H.; Landen, J.; Pontecorvo, M.; Randolph, C.; Sink, K.; Carrillo, M.; et al. Operationalizing selection criteria for clinical trials in Alzheimer’s disease: Biomarker and clinical considerations: Proceedings from the Alzheimer’s Association Research Roundtable (AARR) Fall 2021 meeting. Alzheimer’s Dement. 2025, 11, e70038. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).