Oxidative Stress and Nutritional Antioxidants in Renal Diseases: A Narrative Review
Abstract
1. Introduction
2. Acute Kidney Injury
2.1. Pathogenesis
2.2. Nutrients
3. Chronic Kidney Disease
3.1. Pathogenesis
- -
- inflammation, apoptosis, and fibrosis [62];
- -
- harming the glomerular filtration barrier [63];
- -
- hypertension (HTN), with a reduction in vasodilators such as NO (through inactivation) and an increase in vasoconstrictors, such as some byproducts of arachidonic acid oxidation [64]. In animals with CKD, an increase in NADH (nicotinamide adenine dinucleotide hydrogen)/NADPH (nicotinamide adenine dinucleotide phosphate) oxidase (NOX) activity and a decrease in SOD action lead to OxSt, which perpetuates HTN and endothelial dysfunction [62].
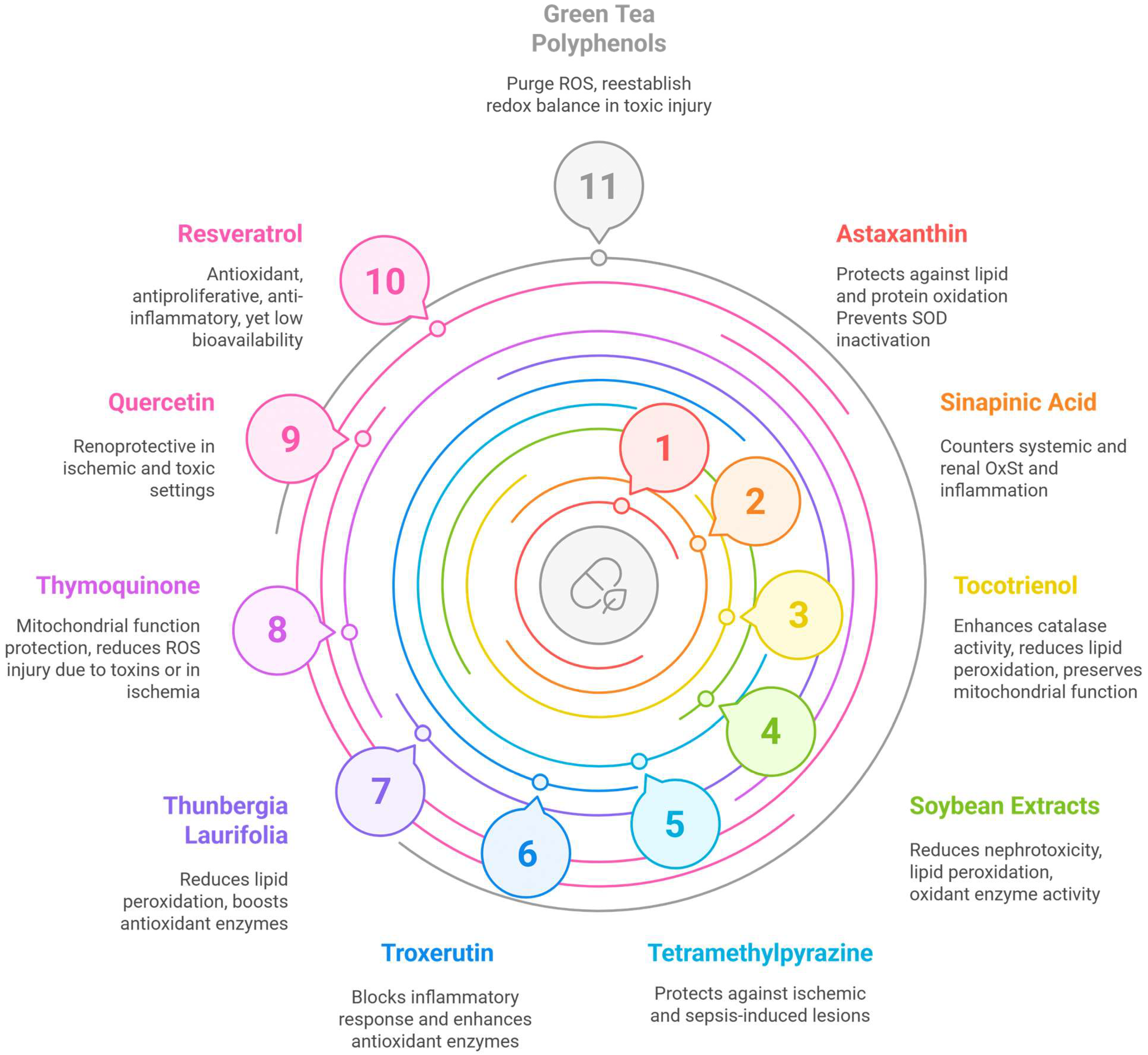
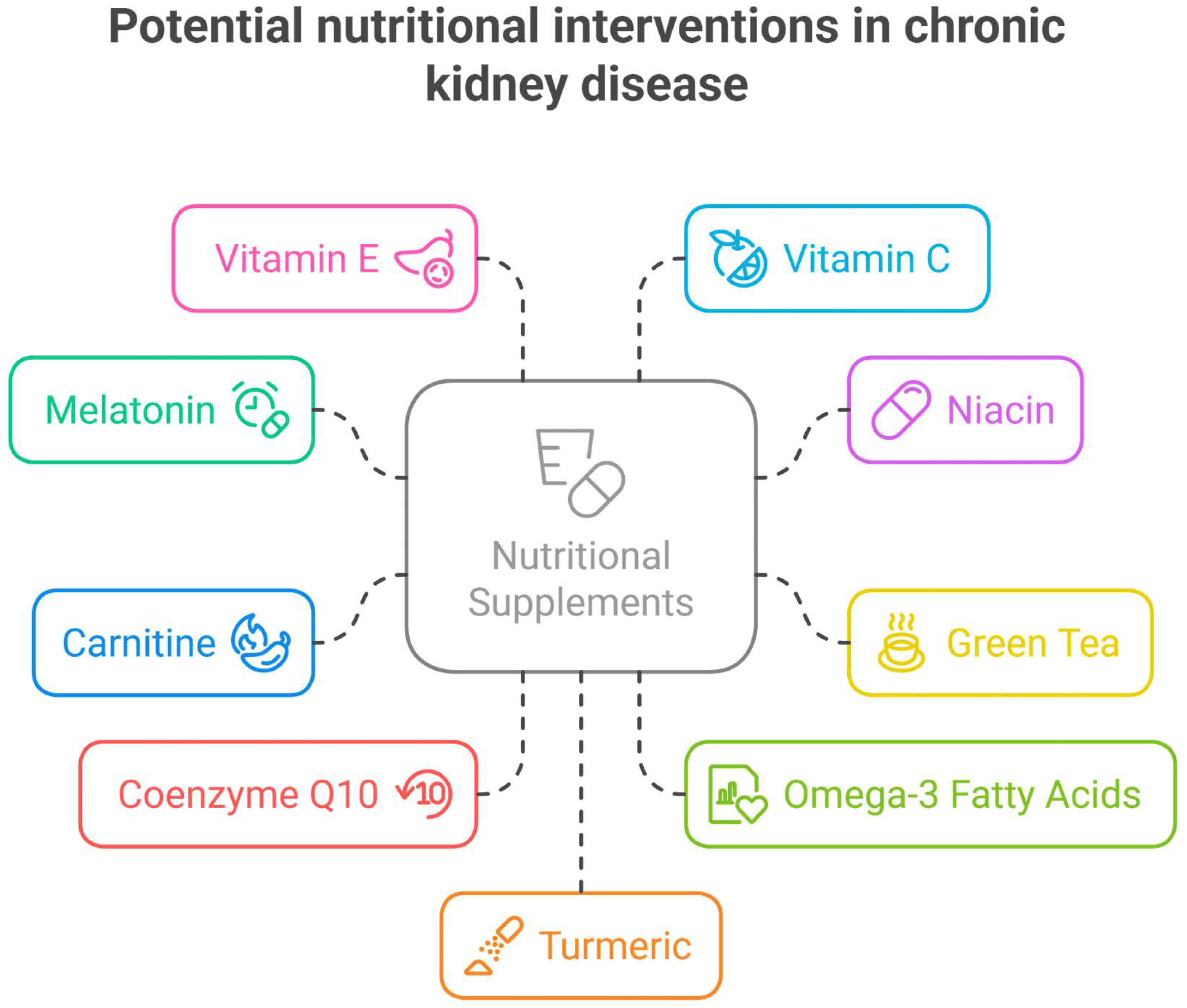
3.2. Nutrients
4. Renal Senescence
4.1. Pathogenesis
4.2. Nutrients
5. The Kidney and Obesity
5.1. Pathogenesis
5.2. Nutrients
6. Kidney and Cardiovascular Disease
6.1. Pathogenesis
6.2. Nutrients
7. Diabetic Nephropathy
7.1. Pathogenesis
7.2. Nutrients
8. Glomerulopathies
8.1. Pathogenesis
8.2. Nutrients
9. Nephrolithiasis and Obstructive Nephropathies
9.1. Pathogenesis
9.2. Nutrients
10. Discussion
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | alpha smooth muscle actin |
| ACE | angiotensin-converting enzyme |
| AGEs | advanced glycation end products |
| AKI | acute kidney injury |
| AMPK | adenosine monophosphate-activated protein kinase |
| AOPPs | advanced oxidation protein products |
| ARE | antioxidant response element |
| AT2 | angiotensin II |
| ATP | adenosine triphosphate |
| Bax | bcl-2-like protein 4 |
| Bcl-2 | B-cell lymphoma/leukemia 2 protein |
| Bid | BH3 interacting-domain death agonist |
| CKD | chronic kidney disease |
| CIN | contrast-induced nephropathy |
| CoQ10 | coenzyme Q10 |
| COX-2 | cyclooxygenase-2 |
| DN | diabetic nephropathy |
| ECG | epicatechin gallate |
| EGCG | epigallocatechin-3-gallate |
| eGFR | estimated glomerular filtration rate |
| ERK | extracellular signal-regulated kinase |
| ESRD | end-stage renal disease |
| FcRn | neonatal fragment crystallizable (Fc) receptor |
| FSGS | focal segmental glomerulosclerosis |
| GBM | glomerular basement membrane |
| Gd-IgA1 | aberrantly glycosylated IgA1 |
| GFR | glomerular filtration rate |
| GP | glomerulopathy |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | glutathione |
| HMOX1 | heme oxygenase-1 |
| HTN | hypertension |
| hs-CRP | high-sensitivity C-reactive protein |
| IgAN | IgA nephropathy |
| IL-6 | interleukin 6 |
| IRF1 | interferon regulatory factor 1 |
| JNK | jun-N-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LDL | low-density lipoproteins |
| LOX | lipoxygenase |
| MAPK | mitogen-activated protein kinases |
| MCD | minimal change disease |
| MDA | malondialdehyde |
| MN | membranous nephropathy/glomerulopathy |
| MPO | myeloperoxidase |
| NADH | nicotinamide adenine dinucleotide hydrogen |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor kB |
| NO | nitric oxide |
| NOX | NADH/NADPH oxidase |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| OxSt | oxidative stress |
| PAN | puromycin aminonucleoside nephrosis |
| PARP | poly(ADP-ribose)polymerase |
| PKC | protein kinase C |
| RAAS | renin-angiotensin-aldosterone system |
| RAGE | receptor for advanced glycation end products |
| Smad3 | mothers against decapentaplegic homolog 3 |
| SOD | superoxide dismutase |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| tBid | truncated BH3 interacting domain death agonist |
| TGF-β | transforming growth factor-beta |
| TNF-α | tumor necrosis factor-alpha |
| TRPV1 | transient receptor potential vanilloid channel 1 |
| UrOb | ureteral obstruction |
| XO | xanthine oxidase |
References
- Nath, K.A.; Norby, S.M. Reactive Oxygen Species and Acute Renal Failure. Am. J. Med. 2000, 109, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Paller, M.S.; Hoidal, J.R.; Ferris, T.F. Oxygen Free Radicals in Ischemic Acute Renal Failure in the Rat. J. Clin. Investig. 1984, 74, 1156–1164. [Google Scholar] [CrossRef]
- Li, C.; Jackson, R.M. Reactive Species Mechanisms of Cellular Hypoxia-Reoxygenation Injury. AJP Cell Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef]
- Wang, Y.; John, R.; Chen, J.; Richardson, J.A.; Shelton, J.M.; Bennett, M.; Zhou, X.J.; Nagami, G.T.; Zhang, Y.; Wu, Q.Q.; et al. IRF-1 Promotes Inflammation Early after Ischemic Acute Kidney Injury. J. Am. Soc. Nephrol. JASN 2009, 20, 1544–1555. [Google Scholar] [CrossRef]
- Liss, P.; Nygren, A.; Hansell, P. Hypoperfusion in the Renal Outer Medulla after Injection of Contrast Media in Rats. Acta Radiol. 1999, 40, 521–527. [Google Scholar] [CrossRef]
- Heyman, S.N.; Reichman, J.; Brezis, M. Pathophysiology of Radiocontrast Nephropathy: A Role for Medullary Hypoxia. Investig. Radiol. 1999, 34, 685–691. [Google Scholar] [CrossRef]
- Persson, P.B.; Hansell, P.; Liss, P. Pathophysiology of Contrast Medium–Induced Nephropathy. Kidney Int. 2005, 68, 14–22. [Google Scholar] [CrossRef]
- Liss, P.; Carlsson, P.-O.; Nygren, A.; Palm, F.; Hansell, P. Et-A Receptor Antagonist BQ123 Prevents Radiocontrast Media-Induced Renal Medullary Hypoxia. Acta Radiol. 2003, 44, 111–117. [Google Scholar] [CrossRef]
- Hosohata, K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef]
- Arany, I.; Safirstein, R.L. Cisplatin Nephrotoxicity. Semin. Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef]
- Liu, H.; Baliga, R. Cytochrome P450 2E1 Null Mice Provide Novel Protection against Cisplatin-Induced Nephrotoxicity and Apoptosis. Kidney Int. 2003, 63, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Kruidering, M.; Van de Water, B.; de Heer, E.; Mulder, G.J.; Nagelkerke, J.F. Cisplatin-Induced Nephrotoxicity in Porcine Proximal Tubular Cells: Mitochondrial Dysfunction by Inhibition of Complexes I to IV of the Respiratory Chain. J. Pharmacol. Exp. Ther. 1997, 280, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.A.; Nick, H.S.; Agarwal, A. Manganese Superoxide Dismutase Attenuates Cisplatin-Induced Renal Injury: Importance of Superoxide. J. Am. Soc. Nephrol. JASN 2001, 12, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Petejova, N.; Martinek, A. Acute Kidney Injury Due to Rhabdomyolysis and Renal Replacement Therapy: A Critical Review. Crit. Care 2014, 18, 224. [Google Scholar] [CrossRef]
- Shah, S.V.; Walker, P.D. Evidence Suggesting a Role for Hydroxyl Radical in Glycerol-Induced Acute Renal Failure. Am. J. Physiol. 1988, 255, F438–F443. [Google Scholar] [CrossRef]
- Holt, S.; Moore, K. Pathogenesis of Renal Failure in Rhabdomyolysis: The Role of Myoglobin. Exp. Nephrol. 2000, 8, 72–76. [Google Scholar] [CrossRef]
- Moore, K.P.; Holt, S.G.; Patel, R.P.; Svistunenko, D.A.; Zackert, W.; Goodier, D.; Reeder, B.J.; Clozel, M.; Anand, R.; Cooper, C.E.; et al. A Causative Role for Redox Cycling of Myoglobin and Its Inhibition by Alkalinization in the Pathogenesis and Treatment of Rhabdomyolysis-Induced Renal Failure. J. Biol. Chem. 1998, 273, 31731–31737. [Google Scholar] [CrossRef]
- Reeder, B.J.; Wilson, M.T. The Effects of pH on the Mechanism of Hydrogen Peroxide and Lipid Hydroperoxide Consumption by Myoglobin: A Role for the Protonated Ferryl Species. Free Radic. Biol. Med. 2001, 30, 1311–1318. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Pevzner, I.B.; Isaev, N.K.; Zorov, D.B. Myoglobin Causes Oxidative Stress, Increase of NO Production and Dysfunction of Kidney’s Mitochondria. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2009, 1792, 796–803. [Google Scholar] [CrossRef]
- Augusti, P.R.; Conterato, G.M.M.; Somacal, S.; Sobieski, R.; Spohr, P.R.; Torres, J.V.; Charão, M.F.; Moro, A.M.; Rocha, M.P.; Garcia, S.C.; et al. Effect of Astaxanthin on Kidney Function Impairment and Oxidative Stress Induced by Mercuric Chloride in Rats. Food Chem. Toxicol. 2008, 46, 212–219. [Google Scholar] [CrossRef]
- Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin Improves Glucose Metabolism and Reduces Blood Pressure in Patients with Type 2 Diabetes Mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef]
- Shokri-Mashhadi, N.; Tahmasebi, M.; Mohammadi-Asl, J.; Zakerkish, M.; Mohammadshahi, M. The Antioxidant and Anti-Inflammatory Effects of Astaxanthin Supplementation on the Expression of miR-146a and miR-126 in Patients with Type 2 Diabetes Mellitus: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Clin. Pract. 2021, 75, e14022. [Google Scholar] [CrossRef]
- Fassett, R.G.; Healy, H.; Driver, R.; Robertson, I.K.; Geraghty, D.P.; Sharman, J.E.; Coombes, J.S. Astaxanthin vs Placebo on Arterial Stiffness, Oxidative Stress and Inflammation in Renal Transplant Patients (Xanthin): A Randomised Controlled Trial. BMC Nephrol. 2008, 9, 17. [Google Scholar] [CrossRef]
- Coombes, J.S.; Sharman, J.E.; Fassett, R.G. Astaxanthin Has No Effect on Arterial Stiffness, Oxidative Stress, or Inflammation in Renal Transplant Recipients: A Randomized Controlled Trial (the XANTHIN Trial). Am. J. Clin. Nutr. 2016, 103, 283–289. [Google Scholar] [CrossRef]
- Mehmood, A.; Soliman, M.M.; Almalki, D.A.; Alotaibi, K.S.; Youssef, G.B.A.; Althobaiti, S. Ameliorative Impacts of Sinapic Acid against Mercuric Chloride-Induced Renal Toxicity: Role of Antioxidants and Inflammatory Cytokines. Toxicol. Res. 2024, 13, tfae066. [Google Scholar] [CrossRef]
- Khan, M.R.; Siddiqui, S.; Parveen, K.; Javed, S.; Diwakar, S.; Siddiqui, W.A. Nephroprotective Action of Tocotrienol-Rich Fraction (TRF) from Palm Oil against Potassium Dichromate (K2Cr2O7)-Induced Acute Renal Injury in Rats. Chem. -Biol. Interact. 2010, 186, 228–238. [Google Scholar] [CrossRef]
- Nowak, G.; Megyesi, J. γ-Tocotrienol Protects against Mitochondrial Dysfunction, Energy Deficits, Morphological Damage, and Decreases in Renal Functions after Renal Ischemia. Int. J. Mol. Sci. 2021, 22, 12674. [Google Scholar] [CrossRef]
- Ekor, M.; Emerole, G.O.; Farombi, E.O. Phenolic Extract of Soybean (Glycine max) Attenuates Cisplatin-Induced Nephrotoxicity in Rats. Food Chem. Toxicol. 2010, 48, 1005–1012. [Google Scholar] [CrossRef]
- Feng, L.; Ke, N.; Cheng, F.; Guo, Y.; Li, S.; Li, Q.; Li, Y. The Protective Mechanism of Ligustrazine Against Renal Ischemia/Reperfusion Injury. J. Surg. Res. 2011, 166, 298–305. [Google Scholar] [CrossRef]
- Fan, S.-H.; Zhang, Z.-F.; Zheng, Y.-L.; Lu, J.; Wu, D.-M.; Shan, Q.; Hu, B.; Wang, Y.-Y. Troxerutin Protects the Mouse Kidney from D-Galactose-Caused Injury through Anti-Inflammation and Anti-Oxidation. Int. Immunopharmacol. 2009, 9, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Palipoch, S.; Jiraungkoorskul, W.; Tansatit, T.; Preyavichyapugdee, N.; Jaikua, W.; Kosai, P. Effect of Thunbergia laurifolia (Linn) Leaf Extract Dietary Supplement Against Lead Toxicity in Nile Tilapia (Oreochromis niloticus). World J. Fish Mar. Sci. 2011, 3, 1–9. [Google Scholar]
- Badary, O.A.; Taha, R.A.; Gamal El-Din, A.M.; Abdel-Wahab, M.H. Thymoquinone Is a Potent Superoxide Anion Scavenger. Drug Chem. Toxicol. 2003, 26, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Severina, I.I.; Severin, F.F.; Korshunova, G.A.; Sumbatyan, N.V.; Ilyasova, T.M.; Simonyan, R.A.; Rogov, A.G.; Trendeleva, T.A.; Zvyagilskaya, R.A.; Dugina, V.B.; et al. In Search of Novel Highly Active Mitochondria-Targeted Antioxidants: Thymoquinone and Its Cationic Derivatives. FEBS Lett. 2013, 587, 2018–2024. [Google Scholar] [CrossRef]
- Sayed-Ahmed, M.M.; Nagi, M.N. Thymoquinone Supplementation Prevents the Development of Gentamicin-Induced Acute Renal Toxicity in Rats. Clin. Exp. Pharmacol. Physiol. 2007, 34, 399–405. [Google Scholar] [CrossRef]
- Hammad, F.T.; Lubbad, L. The Effect of Thymoquinone on the Renal Functions Following Ischemia-Reperfusion Injury in the Rat. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 152–159. [Google Scholar]
- Morand, C.; Crespy, V.; Manach, C.; Besson, C.; Demigné, C.; Rémésy, C. Plasma Metabolites of Quercetin and Their Antioxidant Properties. Am. J. Physiol. 1998, 275, R212–R219. [Google Scholar] [CrossRef]
- Plumb, G.W.; Price, K.R.; Williamson, G. Antioxidant Properties of Flavonol Glycosides from Green Beans. Redox Rep. 1999, 4, 123–127. [Google Scholar] [CrossRef]
- Fiorani, M.; De Sanctis, R.; Menghinello, P.; Cucchiarini, L.; Cellini, B.; Dachà, M. Quercetin Prevents Glutathione Depletion Induced by Dehydroascorbic Acid in Rabbit Red Blood Cells. Free Radic. Res. 2001, 34, 639–648. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The Correlation between Active Oxygens Scavenging and Antioxidative Effects of Flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Shoskes, D.A. Effect of Bioflavonoids Quercetin and Curcumin on Ischemic Renal Injury: A New Class of Renoprotective Agents. Transplantation 1998, 66, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Erboga, M.; Aktas, C.; Erboga, Z.F.; Donmez, Y.B.; Gurel, A. Quercetin Ameliorates Methotrexate-Induced Renal Damage, Apoptosis and Oxidative Stress in Rats. Ren. Fail. 2015, 37, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, P.D.; Lopez-Hernandez, F.J.; Perez-Barriocanal, F.; Morales, A.I.; Lopez-Novoa, J.M. Quercetin Reduces Cisplatin Nephrotoxicity in Rats without Compromising Its Anti-Tumour Activity. Nephrol. Dial. Transplant. 2011, 26, 3484–3495. [Google Scholar] [CrossRef] [PubMed]
- Sabaghian, T.; Gheydari, M.E.; Divani, F. Evaluation of Curcumin (Turmeric Extract) Effect on Prevention of CIN in Patient Under Elective Coronary Angiography, a Randomized Double Blind Placebocontrolled Clinical Trial. Iran. J. Kidney Dis. 2020, 14, 198–205. [Google Scholar]
- Hami, M.; Bigdeli, A.; Khameneh Bagheri, R.; Rajabi, O.; Salehi, M.; Zahedi Avval, F. The Effect of Curcumin in Prevention of Contrast Nephropathy Following Coronary Angiography or Angioplasty in CKD Patients. Iran. J. Kidney Dis. 2019, 13, 304–309. [Google Scholar]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol Scavenges Reactive Oxygen Species and Effects Radical-Induced Cellular Responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Sgambato, A.; Ardito, R.; Faraglia, B.; Boninsegna, A.; Wolf, F.I.; Cittadini, A. Resveratrol, a Natural Phenolic Compound, Inhibits Cell Proliferation and Prevents Oxidative DNA Damage. Mutat. Res. 2001, 496, 171–180. [Google Scholar] [CrossRef]
- Mokni, M.; Elkahoui, S.; Limam, F.; Amri, M.; Aouani, E. Effect of Resveratrol on Antioxidant Enzyme Activities in the Brain of Healthy Rat. Neurochem. Res. 2007, 32, 981–987. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-Antioxidant Signaling Attenuates NF-κB-Inflammatory Response and Elicits Apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and Non-Canonical Mechanisms of Nrf2 Activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Smoliga, J.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability Is the Problem, What Is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Heredia, A.; Song, H.; Zhang, Z.; Yu, B.; Davis, C.; Redfield, R. Resveratrol Glucuronides as the Metabolites of Resveratrol in Humans: Characterization, Synthesis, and Anti-HIV Activity. J. Pharm. Sci. 2004, 93, 2448–2457. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and Bioavailability of Trans-Resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef]
- Abdollahi, S.; Vajdi, M.; Meshkini, F.; Vasmehjani, A.A.; Sangsefidi, Z.S.; Clark, C.C.T.; Soltani, S. Resveratrol May Mildly Improve Renal Function in the General Adult Population: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Nutr. Res. 2023, 113, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-Gallate Attenuates Oxidative Stress and Inflammation in Obstructive Nephropathy via NF-κB and Nrf2/HO-1 Signalling Pathway Regulation. Basic Clin. Pharmacol. Toxicol. 2015, 117, 164–172. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on Protective Mechanisms of Four Components of Green Tea Polyphenols against Lipid Peroxidation in Synaptosomes. Biochim. Et Biophys. Acta 1996, 1304, 210–222. [Google Scholar] [CrossRef]
- Salem, E.A.; Salem, N.A.; Kamel, M.; Maarouf, A.M.; Bissada, N.K.; Hellstrom, W.J.G.; ElAdl, M. Amelioration of Gentamicin Nephrotoxicity by Green Tea Extract in Uninephrectomized Rats as a Model of Progressive Renal Failure. Ren. Fail. 2010, 32, 1210–1215. [Google Scholar] [CrossRef]
- Shah, S.V. Oxidants and Iron in Progressive Kidney Disease. J. Ren. Nutr. 2006, 16, 185–189. [Google Scholar] [CrossRef]
- Granata, S.; Dalla Gassa, A.; Tomei, P.; Lupo, A.; Zaza, G. Mitochondria: A New Therapeutic Target in Chronic Kidney Disease. Nutr. Metab. 2015, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Roles of Oxidative Stress and Antioxidant Therapy in Chronic Kidney Disease and Hypertension. Curr. Opin. Nephrol. Hypertens. 2004, 13, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Whaley-Connell, A.T.; Chowdhury, N.A.; Hayden, M.R.; Stump, C.S.; Habibi, J.; Wiedmeyer, C.E.; Gallagher, P.E.; Tallant, E.A.; Cooper, S.A.; Link, C.D.; et al. Oxidative Stress and Glomerular Filtration Barrier Injury: Role of the Renin-Angiotensin System in the Ren2 Transgenic Rat. Am. J. Physiol. Ren. Physiol. 2006, 291, F1308–F1314. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Oveisi, F.; Ding, Y. Role of Increased Oxygen Free Radical Activity in the Pathogenesis of Uremic Hypertension. Kidney Int. 1998, 53, 1748–1754. [Google Scholar] [CrossRef]
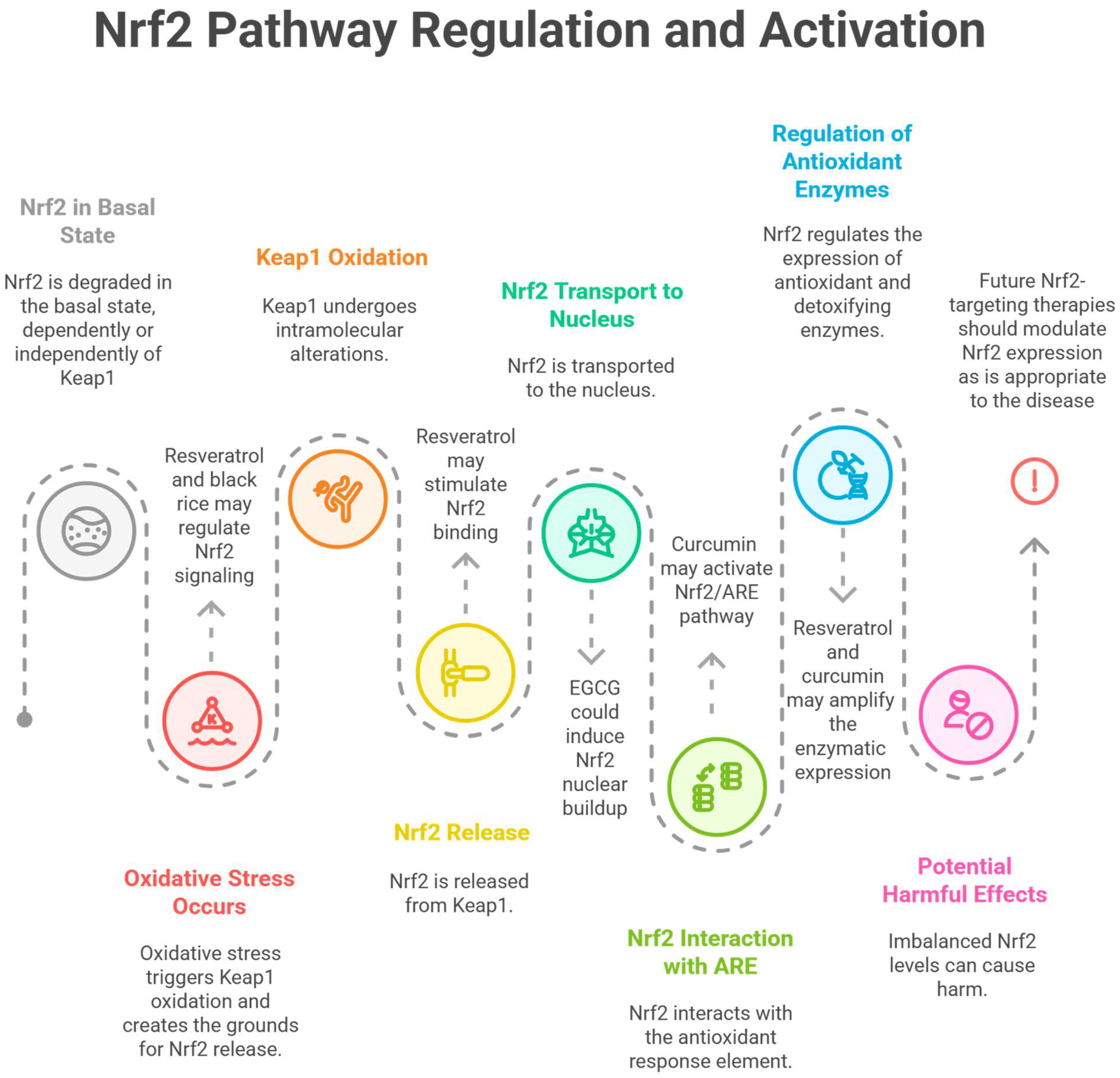
- Kim, H.J.; Vaziri, N.D. Contribution of Impaired Nrf2-Keap1 Pathway to Oxidative Stress and Inflammation in Chronic Renal Failure. Am. J. Physiol. Ren. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef]
- Copple, I.M. The Keap1-Nrf2 Cell Defense Pathway—A Promising Therapeutic Target? Adv. Pharmacol. 2012, 63, 43–79. [Google Scholar] [CrossRef]
- Rush, B.M.; Bondi, C.D.; Stocker, S.D.; Barry, K.M.; Small, S.A.; Ong, J.; Jobbagy, S.; Stolz, D.B.; Bastacky, S.I.; Chartoumpekis, D.V.; et al. Genetic or Pharmacologic Nrf2 Activation Increases Proteinuria in Chronic Kidney Disease in Mice. Kidney Int. 2021, 99, 102–116. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza-Chaverri, J.; Scholze, A. Nrf2 Activation in Chronic Kidney Disease: Promises and Pitfalls. Antioxidants 2022, 11, 1112. [Google Scholar] [CrossRef]
- Stępniewska, J.; Gołembiewska, E.; Dołęgowska, B.; Domański, M.; Ciechanowski, K. Oxidative Stress and Antioxidative Enzyme Activities in Chronic Kidney Disease and Different Types of Renal Replacement Therapy. Curr. Protein Pept. Sci. 2015, 16, 243–248. [Google Scholar] [CrossRef]
- Xu, G.; Luo, K.; Liu, H.; Huang, T.; Fang, X.; Tu, W. The Progress of Inflammation and Oxidative Stress in Patients with Chronic Kidney Disease. Ren. Fail. 2015, 37, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The Elephant in Uremia: Oxidant Stress as a Unifying Concept of Cardiovascular Disease in Uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Giray, B.; Kan, E.; Bali, M.; Hincal, F.; Basaran, N. The Effect of Vitamin E Supplementation on Antioxidant Enzyme Activities and Lipid Peroxidation Levels in Hemodialysis Patients. Clin. Chim. Acta Int. J. Clin. Chem. 2003, 338, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Taccone-Gallucci, M.; Finazzi-Agrò, A. 5-Lipoxygenase-Mediated Mitochondrial Damage and Apoptosis of Mononuclear Cells in ESRD Patients. Kidney Int. 2003, 63, S33–S36. [Google Scholar] [CrossRef]
- Boaz, M.; Smetana, S.; Weinstein, T.; Matas, Z.; Gafter, U.; Iaina, A.; Knecht, A.; Weissgarten, Y.; Brunner, D.; Fainaru, M.; et al. Secondary Prevention with Antioxidants of Cardiovascular Disease in Endstage Renal Disease (SPACE): Randomised Placebo-Controlled Trial. Lancet 2000, 356, 1213–1218. [Google Scholar] [CrossRef]
- Boudouris, G.; Verginadis, I.I.; Simos, Y.V.; Zouridakis, A.; Ragos, V.; Karkabounas, S.C.; Evangelou, A.M. Oxidative Stress in Patients Treated with Continuous Ambulatory Peritoneal Dialysis (CAPD) and the Significant Role of Vitamin C and E Supplementation. Int. Urol. Nephrol. 2013, 45, 1137–1144. [Google Scholar] [CrossRef]
- Ishigaki, S.; Ohashi, N.; Isobe, S.; Tsuji, N.; Iwakura, T.; Ono, M.; Sakao, Y.; Tsuji, T.; Kato, A.; Miyajima, H.; et al. Impaired Endogenous Nighttime Melatonin Secretion Relates to Intrarenal Renin–Angiotensin System Activation and Renal Damage in Patients with Chronic Kidney Disease. Clin. Exp. Nephrol. 2016, 20, 878–884. [Google Scholar] [CrossRef]
- Takahashi, R.; Bui, T.-A.; Elali, I.; Tran, D.; Sumida, K.; Thomas, F.; Dukkipati, R.; Shah, A.; Rhee, C.M.; Kovesdy, C.P.; et al. The Association of Niacin Use with Kidney Outcomes and Mortality. Am. J. Nephrol. 2025. [Google Scholar] [CrossRef]
- Yokozawa, T.; Oura, H.; Shibata, T.; Ishida, K.; Kaneko, M.; Hasegawa, M. Effects of Green Tea Tannin in Dialysis Patients. J. Tradit. Med. 1996, 13, 124–131. [Google Scholar]
- Guo, S.; Yamagishi, K.; Kihara, T.; Muraki, I.; Tamakoshi, A.; Iso, H. Green Tea, Other Teas and Coffee Consumption and Risk of Death from Chronic Kidney Disease as the Underlying Cause among Japanese Men and Women: The JACC Study. Environ. Health Prev. Med. 2025, 30, 13. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.L.; Ou-Yang, X.L.; Zhang, X.J.; Li, Y.; Sun, S.N.; Wang, L.J.; Yang, Z.Q.; Ni, S.H.; Lu, L. Association of Tea Consumption with All-Cause/Cardiovascular Disease Mortality in the Chronic Kidney Disease Population: An Assessment of Participation in the National Cohort. Ren. Fail. 2025, 47, 2449578. [Google Scholar] [CrossRef] [PubMed]
- Pertosa, G.; Grandaliano, G.; Simone, S.; Soccio, M.; Schena, F.P. Inflammation and Carnitine in Hemodialysis Patients. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2005, 15, 8–12. [Google Scholar] [CrossRef]
- Hamedi-kalajahi, F.; Imani, H.; Mojtahedi, S.; Shabbidar, S. Effect of L-Carnitine Supplementation on Inflammatory Markers and Serum Glucose in Hemodialysis Children: A Randomized, Placebo-Controlled Clinical Trial. J. Ren. Nutr. 2022, 32, 144–151. [Google Scholar] [CrossRef]
- Yee, J. L-Carnitine for Anemia in Hemodialysis Patients: A Last Resort. Clin. J. Am. Soc. Nephrol. 2012, 7, 1746–1748. [Google Scholar] [CrossRef]
- Kliger, A.S.; Foley, R.N.; Goldfarb, D.S.; Goldstein, S.L.; Johansen, K.; Singh, A.; Szczech, L. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am. J. Kidney Dis. 2013, 62, 849–859. [Google Scholar] [CrossRef]
- KDIGO 2025 Anemia in CKD Guideline Available for Public Review—KDIGO. Available online: https://kdigo.org/kdigo-2025-anemia-in-ckd-guideline-available-for-public-review/ (accessed on 23 April 2025).
- Sakata, T.; Furuya, R.; Shimazu, T.; Odamaki, M.; Ohkawa, S.; Kumagai, H. Coenzyme Q10 Administration Suppresses Both Oxidative and Antioxidative Markers in Hemodialysis Patients. Blood Purif. 2008, 26, 371–378. [Google Scholar] [CrossRef]
- Bakhshayeshkaram, M.; Lankarani, K.B.; Mirhosseini, N.; Tabrizi, R.; Akbari, M.; Dabbaghmanesh, M.H.; Asemi, Z. The Effects of Coenzyme Q10 Supplementation on Metabolic Profiles of Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2018, 24, 3710–3723. [Google Scholar] [CrossRef]
- Fallah, M.; Askari, G.; Soleimani, A.; Feizi, A.; Asemi, Z. Clinical Trial of the Effects of Coenzyme Q10 Supplementation on Biomarkers of Inflammation and Oxidative Stress in Diabetic Hemodialysis Patients. Int. J. Prev. Med. 2019, 10, 12. [Google Scholar] [CrossRef]
- Mori, T.A.; Burke, V.; Puddey, I.; Irish, A.; Cowpland, C.A.; Beilin, L.; Dogra, G.; Watts, G.F. The Effects of ω3 Fatty Acids and Coenzyme Q10 on Blood Pressure and Heart Rate in Chronic Kidney Disease: A Randomized Controlled Trial. J. Hypertens. 2009, 27, 1863–1872. [Google Scholar] [CrossRef]
- Ong, K.L.; Marklund, M.; Huang, L.; Rye, K.-A.; Hui, N.; Pan, X.-F.; Rebholz, C.M.; Kim, H.; Steffen, L.M.; van Westing, A.C.; et al. Association of Omega 3 Polyunsaturated Fatty Acids with Incident Chronic Kidney Disease: Pooled Analysis of 19 Cohorts. BMJ 2023, 380, e072909. [Google Scholar] [CrossRef]
- Bouzidi, N.; Mekki, K.; Boukaddoum, A.; Dida, N.; Kaddous, A.; Bouchenak, M. Effects of Omega-3 Polyunsaturated Fatty-Acid Supplementation on Redox Status in Chronic Renal Failure Patients with Dyslipidemia. J. Ren. Nutr. 2010, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Taccone-Gallucci, M.; Manca-di-Villahermosa, S.; Battistini, L.; Stuffler, R.G.; Tedesco, M.; Maccarrone, M. N-3 PUFAs Reduce Oxidative Stress in ESRD Patients on Maintenance HD by Inhibiting 5-Lipoxygenase Activity. Kidney Int. 2006, 69, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Saglimbene, V.M.; Wong, G.; van Zwieten, A.; Palmer, S.C.; Ruospo, M.; Natale, P.; Campbell, K.; Teixeira-Pinto, A.; Craig, J.C.; Strippoli, G.F.M. Effects of Omega-3 Polyunsaturated Fatty Acid Intake in Patients with Chronic Kidney Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2020, 39, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Thabet, M.A.; Chan, J.C.M. Vitamin E in Renal Therapeutic Regiments. Pediatr. Nephrol. 2006, 21, 1790–1801. [Google Scholar] [CrossRef]
- Koay, Y.Y.; Tan, G.C.J.; Phang, S.C.W.; Ho, J.-I.; Chuar, P.F.; Ho, L.S.; Ahmad, B.; Abdul Kadir, K. A Phase IIb Randomized Controlled Trial Investigating the Effects of Tocotrienol-Rich Vitamin E on Diabetic Kidney Disease. Nutrients 2021, 13, 258. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Pu, Y.; Dai, H.; Peng, F. Association between Dietary Vitamin E Intake and Chronic Kidney Disease Events in US Adults: A Cross-Sectional Study from NHANES 2009–2016. Clin. Kidney J. 2023, 16, 2559–2566. [Google Scholar] [CrossRef]
- Bergin, P.; Leggett, A.; Cardwell, C.R.; Woodside, J.V.; Thakkinstian, A.; Maxwell, A.P.; McKay, G.J. The Effects of Vitamin E Supplementation on Malondialdehyde as a Biomarker of Oxidative Stress in Haemodialysis Patients: A Systematic Review and Meta-Analysis. BMC Nephrol. 2021, 22, 126. [Google Scholar] [CrossRef]
- Kirmizis, D.; Papagianni, A.; Belechri, A.-M.; Memmos, D. Effects of Vitamin E-Coated Membrane Dialyser on Markers of Oxidative Stress and Inflammation in Patients on Chronic Haemodialysis. Nephrol. Dial. Transplant. 2011, 26, 2296–2301. [Google Scholar] [CrossRef]
- Takouli, L.; Hadjiyannakos, D.; Metaxaki, P.; Sideris, V.; Filiopoulos, V.; Anogiati, A.; Vlassopoulos, D. Vitamin E-Coated Cellulose Acetate Dialysis Membrane: Long-Term Effect on Inflammation and Oxidative Stress. Ren. Fail. 2010, 32, 287–293. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hsu, S.-P.; Wu, M.-S.; Hsu, S.-M.; Chien, C.-T. Effects of Vitamin C Infusion and Vitamin E-Coated Membrane on Hemodialysis-Induced Oxidative Stress. Kidney Int. 2006, 69, 706–714. [Google Scholar] [CrossRef]
- Kitamura, Y.; Kamimura, K.; Yoshioka, N.; Hosotani, Y.; Tsuchida, K.; Koremoto, M.; Minakuchi, J. The Effect of Vitamin E-Bonded Polysulfone Membrane Dialyzer on a New Oxidative Lipid Marker. J. Artif. Organs 2013, 16, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Rosati, A.; Paoletti, S.; Ferrandello, P.; Migliori, M.; Beati, S.; Bernabini, G.; Daini, R.; Casani, A.; Angelini, D.; et al. A Vitamin E-Coated Polysulfone Membrane Reduces Serum Levels of Inflammatory Markers and Resistance to Erythropoietin-Stimulating Agents in Hemodialysis Patients: Results of a Randomized Cross-Over Multicenter Trial. Blood Purif. 2011, 32, 7–14. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, G.; Baggetta, R.; Tripepi, G.; Galli, F.; Bolignano, D. Effects of Vitamin E-Coated versus Conventional Membranes in Chronic Hemodialysis Patients: A Systematic Review and Meta-Analysis. Blood Purif. 2017, 43, 101–122. [Google Scholar] [CrossRef]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in Randomized Trials of Antioxidant Supplements for Primary and Secondary Prevention: Systematic Review and Meta-Analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Berry, D.; Wathen, J.K.; Newell, M. Bayesian Model Averaging in Meta-Analysis: Vitamin E Supplementation and Mortality. Clin. Trials 2009, 6, 28–41. [Google Scholar] [CrossRef]
- Abner, E.L.; Schmitt, F.A.; Mendiondo, M.S.; Marcum, J.L.; Kryscio, R.J. Vitamin E and All-Cause Mortality: A Meta-Analysis. Curr. Aging Sci. 2011, 4, 158–170. [Google Scholar] [CrossRef]
- Sarandol, E.; Erdinc, S.; Senol, E.; Ersoy, A.; Surmen-Gur, E. Effects of Vitamin C Supplementation on Oxidative Stress and Serum Paraoxonase/Arylesterase Activities in Patients on Long-Term Hemodialysis. Nefrología 2023, 43, 351–359. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Pakfetrat, M.; Akmali, M.; Malekmakan, L.; Dabaghimanesh, M.; Khorsand, M. Role of Turmeric in Oxidative Modulation in End-Stage Renal Disease Patients. Hemodial. Int. 2015, 19, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.M.F.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of Curcumin Supplementation on Expression of Inflammatory Transcription Factors in Hemodialysis Patients: A Pilot Randomized, Double-Blind, Controlled Study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, J.J.; Bowden, R.G.; Deike, E.; Griggs, J.; Wilson, R.; Shelmadine, B.; Cooke, M.; Beaujean, A. The Use of an Anti-Inflammatory Supplement in Patients with Chronic Kidney Disease. J. Complement. Integr. Med. 2013, 10, 143–152. [Google Scholar] [CrossRef]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverri, J. The Effect of Dietary Supplementation with Curcumin on Redox Status and Nrf2 Activation in Patients with Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef]
- Shoskes, D.; Lapierre, C.; Cruz-Corerra, M.; Muruve, N.; Rosario, R.; Fromkin, B.; Braun, M.; Copley, J. Beneficial Effects of the Bioflavonoids Curcumin and Quercetin on Early Function in Cadaveric Renal Transplantation: A Randomized Placebo Controlled Trial. Transplantation 2005, 80, 1556. [Google Scholar] [CrossRef]
- Casanova, A.G.; López-Hernández, F.J.; Vicente-Vicente, L.; Morales, A.I. Are Antioxidants Useful in Preventing the Progression of Chronic Kidney Disease? Antioxidants 2021, 10, 1669. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; García-Mayorga, E.A.; Díaz-Avila, D.L.; Garza-Veloz, I.; Martinez-Fierro, M.L.; González-Mateo, G.T. Potential Therapeutic Effects of Natural Plant Compounds in Kidney Disease. Molecules 2021, 26, 6096. [Google Scholar] [CrossRef]
- Papa, S.; Skulachev, V.P. Reactive Oxygen Species, Mitochondria, Apoptosis and Aging. Mol. Cell. Biochem. 1997, 174, 305–319. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Ruiz-Torres, P.; Lucio, J.; González-Rubio, M.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D. Oxidant/Antioxidant Balance in Isolated Glomeruli and Cultured Mesangial Cells. Free Radic. Biol. Med. 1997, 22, 49–56. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Kanji, V.; Racusen, L.C.; Schmidt, A.M.; Yan, S.D.; Marrow, J.; Roberts, L.J.; Salahudeen, A.K. Vitamin E Ameliorates Enhanced Renal Lipid Peroxidation and Accumulation of F2-Isoprostanes in Aging Kidneys. Am. J. Physiol. 1998, 274, R767–R774. [Google Scholar] [CrossRef] [PubMed]
- Mitobe, M.; Yoshida, T.; Sugiura, H.; Shirota, S.; Tsuchiya, K.; Nihei, H. Oxidative Stress Decreases Klotho Expression in a Mouse Kidney Cell Line. Nephron. Exp. Nephrol. 2005, 101, e67–e74. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.I.; Ruiz-Torres, P.; del Moral, R.G.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D. Age-Related Progressive Renal Fibrosis in Rats and Its Prevention with ACE Inhibitors and Taurine. Am. J. Physiol. Ren. Physiol. 2000, 278, F122–F129. [Google Scholar] [CrossRef] [PubMed]
- Camici, M.; Carpi, A.; Cini, G.; Galetta, F.; Abraham, N. Podocyte Dysfunction in Aging–Related Glomerulosclerosis. Front. Biosci. 2011, 3, 995–1006. [Google Scholar] [CrossRef]
- Adler, S.; Huang, H.; Wolin, M.S.; Kaminski, P.M. Oxidant Stress Leads to Impaired Regulation of Renal Cortical Oxygen Consumption by Nitric Oxide in the Aging Kidney. J. Am. Soc. Nephrol. JASN 2004, 15, 52–60. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.-A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 Activator, Ameliorates Aging-Related Progressive Renal Injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Jo, M.J.; Kim, J.E.; Bae, S.Y.; Cho, E.; Ahn, S.Y.; Kwon, Y.J.; Ko, G.-J. Impaired NRF2 Inhibits Recovery from Ischemic Reperfusion Injury in the Aging Kidney. Antioxidants 2023, 12, 1440. [Google Scholar] [CrossRef]
- Yamori, Y.; Liu, L.; Mori, M.; Sagara, M.; Murakami, S.; Nara, Y.; Mizushima, S. Taurine as the Nutritional Factor for the Longevity of the Japanese Revealed by a World-Wide Epidemiological Survey. Adv. Exp. Med. Biol. 2009, 643, 13–25. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, K.J.; Yu, B.P.; Cho, C.G.; Chung, H.Y. Influence of Aging and Calorie Restriction on MAPKs Activity in Rat Kidney. Exp. Gerontol. 2002, 37, 1041–1053. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, K.J.; Kim, J.W.; Kim, H.J.; Yu, B.P.; Chung, H.Y. Suppression of Apoptosis by Calorie Restriction in Aged Kidney. Exp. Gerontol. 2004, 39, 1361–1368. [Google Scholar] [CrossRef]
- Jongbloed, F.; de Bruin, R.W.F.; Steeg, H.V.; Beekhof, P.; Wackers, P.; Hesselink, D.A.; Hoeijmakers, J.H.J.; Dollé, M.E.T.; IJzermans, J.N.M. Protein and Calorie Restriction May Improve Outcomes in Living Kidney Donors and Kidney Transplant Recipients. Aging 2020, 12, 12441–12467. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, S.A.; Brown, K.E.; Kregel, K.C. Renal Iron Accumulation and Oxidative Injury with Aging: Effects of Treatment with an Iron Chelator. J. Gerontol. Ser. A 2020, 75, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ulloa, A.; Nogueira, L.; Rodriguez, A.; Barboza, J.; Hogan, M.C.; Ceballos, G.; Villarreal, F.; Ramirez-Sanchez, I. Recovery of Indicators of Mitochondrial Biogenesis, Oxidative Stress, and Aging with (−)-Epicatechin in Senile Mice. J. Gerontol. Ser. A 2015, 70, 1370–1378. [Google Scholar] [CrossRef]
- Munguia, L.; Rubio-Gayosso, I.; Ramirez-Sanchez, I.; Ortiz, A.; Hidalgo, I.; Gonzalez, C.; Meaney, E.; Villarreal, F.; Najera, N.; Ceballos, G. High Flavonoid Cocoa Supplement Ameliorates Plasma Oxidative Stress and Inflammation Levels While Improving Mobility and Quality of Life in Older Subjects: A Double-Blind Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1620–1627. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa Flavanol Consumption Improves Cognitive Function, Blood Pressure Control, and Metabolic Profile in Elderly Subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A Randomized Controlled Trial1234. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in Cognitive Function, Blood Pressure, and Insulin Resistance through Cocoa Flavanol Consumption in Elderly Subjects with Mild Cognitive Impairment: The Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef]
- Rao, A.; Pandya, V.; Whaley-Connell, A. Obesity and Insulin Resistance in Resistant Hypertension: Implications for the Kidney. Adv. Chronic Kidney Dis. 2015, 22, 211–217. [Google Scholar] [CrossRef]
- Cui, W.; Wang, Y.; Chen, Q.; Sun, W.; Cai, L.; Tan, Y.; Kim, K.-S.; Kim, K.H.; Kim, Y.H. Magnolia Extract (BL153) Ameliorates Kidney Damage in a High Fat Diet-Induced Obesity Mouse Model. Oxidative Med. Cell. Longev. 2013, 2013, 367040. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Mak, C.H.; Chen, H.; Zaky, A.A.; Wong, M.G.; Pollock, C.A.; Saad, S. SIRT1 Attenuates Kidney Disorders in Male Offspring Due to Maternal High-Fat Diet. Nutrients 2019, 11, 146. [Google Scholar] [CrossRef]
- Inoue, R.; Jensen, L.J.; Shi, J.; Morita, H.; Nishida, M.; Honda, A.; Ito, Y. Transient Receptor Potential Channels in Cardiovascular Function and Disease. Circ. Res. 2006, 99, 119–131. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by Dietary Capsaicin Improves Endothelium-Dependent Vasorelaxation and Prevents Hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Llarena, M.; Andrade, F.; Hasnaoui, M.; Portillo, M.P.; Pérez-Matute, P.; Arbones-Mainar, J.M.; Hijona, E.; Villanueva-Millán, M.J.; Aguirre, L.; Carpéné, C.; et al. Potential Renoprotective Effects of Piceatannol in Ameliorating the Early-Stage Nephropathy Associated with Obesity in Obese Zucker Rats. J. Physiol. Biochem. 2016, 72, 555–566. [Google Scholar] [CrossRef]
- Laorodphun, P.; Arjinajarn, P.; Thongnak, L.; Promsan, S.; Swe, M.T.; Thitisut, P.; Mahatheeranont, S.; Jaturasitha, S.; Lungkaphin, A. Anthocyanin-rich Fraction from Black Rice, Oryza sativa L. Var. Indica “Luem Pua,” Bran Extract Attenuates Kidney Injury Induced by High-fat Diet Involving Oxidative Stress and Apoptosis in Obese Rats. Phytother. Res. 2021, 35, 5189–5202. [Google Scholar] [CrossRef]
- Garrison, R.; Chambliss, W.G. Effect of a Proprietary Magnolia and Phellodendron Extract on Weight Management: A Pilot, Double-Blind, Placebo-Controlled Clinical Trial. Altern. Ther. Health Med. 2006, 12, 50–54. [Google Scholar]
- Zsiborás, C.; Mátics, R.; Hegyi, P.; Balaskó, M.; Pétervári, E.; Szabó, I.; Sarlós, P.; Mikó, A.; Tenk, J.; Rostás, I.; et al. Capsaicin and Capsiate Could Be Appropriate Agents for Treatment of Obesity: A Meta-Analysis of Human Studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1419–1427. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Bhat, B.; Ansari, M.; Pandey, A.; Bani, S.; Mundkur, L. The Anti-Obesity Potential of Cyperus Rotundus Extract Containing Piceatannol, Scirpusin A and Scirpusin B from Rhizomes: Preclinical and Clinical Evaluations. Diabetes Metab. Syndr. Obes. 2022, 15, 369–382. [Google Scholar] [CrossRef]
- Jung, A.J.; Sharma, A.; Lee, S.-H.; Lee, S.-J.; Kim, J.-H.; Lee, H.-J. Efficacy of Black Rice Extract on Obesity in Obese Postmenopausal Women: A 12-Week Randomized, Double-Blind, Placebo-Controlled Preliminary Clinical Trial. Menopause 2021, 28, 1391–1399. [Google Scholar] [CrossRef]
- Manucha, W.; Vallés, P.G. Apoptosis Modulated by Oxidative Stress and Inflammation during Obstructive Nephropathy. Inflamm. Allergy Drug Targets 2012, 11, 303–312. [Google Scholar] [CrossRef]
- Nishi, E.E.; Oliveira-Sales, E.B.; Bergamaschi, C.T.; Oliveira, T.G.C.; Boim, M.A.; Campos, R.R. Chronic Antioxidant Treatment Improves Arterial Renovascular Hypertension and Oxidative Stress Markers in the Kidney in Wistar Rats. Am. J. Hypertens. 2010, 23, 473–480. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.; Kim, Y.H.; Chung, W.-S.; Suh, J.K.; Kim, S.J. Antioxidant Effect of Captopril and Enalapril on Reactive Oxygen Species-Induced Endothelial Dysfunction in the Rabbit Abdominal Aorta. Korean J. Thorac. Cardiovasc. Surg. 2013, 46, 14–21. [Google Scholar] [CrossRef] [PubMed]
- de Cavanagh, E.M.; Inserra, F.; Ferder, L.; Romano, L.; Ercole, L.; Fraga, C.G. Superoxide Dismutase and Glutathione Peroxidase Activities Are Increased by Enalapril and Captopril in Mouse Liver. FEBS Lett. 1995, 361, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Zafari, A.M.; Fukui, T.; Ishizaka, N.; Griendling, K.K. P22phox Is a Critical Component of the Superoxide-Generating NADH/NADPH Oxidase System and Regulates Angiotensin II-Induced Hypertrophy in Vascular Smooth Muscle Cells. J. Biol. Chem. 1996, 271, 23317–23321. [Google Scholar] [CrossRef] [PubMed]
- McCurley, A.; Jaffe, I.Z. Mineralocorticoid Receptors in Vascular Function and Disease. Mol. Cell. Endocrinol. 2012, 350, 256–265. [Google Scholar] [CrossRef]
- Rubattu, S.; Mennuni, S.; Testa, M.; Mennuni, M.; Pierelli, G.; Pagliaro, B.; Gabriele, E.; Coluccia, R.; Autore, C.; Volpe, M. Pathogenesis of Chronic Cardiorenal Syndrome: Is There a Role for Oxidative Stress? Int. J. Mol. Sci. 2013, 14, 23011–23032. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, S.; Wang, W.; Wang, Y.; Zhang, P.; Zhu, C.; Ding, G.; Liu, B.; Yang, T.; Zhang, A. Activation of Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α Ameliorates Mitochondrial Dysfunction and Protects Podocytes from Aldosterone-Induced Injury. Kidney Int. 2012, 82, 771–789. [Google Scholar] [CrossRef]
- Fodor, K.; Tit, D.M.; Pasca, B.; Bustea, C.; Uivarosan, D.; Endres, L.; Iovan, C.; Abdel-Daim, M.M.; Bungau, S. Long-Term Resveratrol Supplementation as a Secondary Prophylaxis for Stroke. Oxidative Med. Cell. Longev. 2018, 2018, 4147320. [Google Scholar] [CrossRef]
- Gimblet, C.J.; Kruse, N.T.; Geasland, K.; Michelson, J.; Sun, M.; Mandukhail, S.R.; Wendt, L.H.; Eyck, P.T.; Pierce, G.L.; Jalal, D.I. Effect of Resveratrol on Endothelial Function in Patients with CKD and Diabetes. Clin. J. Am. Soc. Nephrol. 2024, 19, 161–168. [Google Scholar] [CrossRef]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.-C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef]
- Zhao, D.; Liang, Y.; Dai, S.; Hou, S.; Liu, Z.; Liu, M.; Dong, X.; Zhan, Y.; Tian, Z.; Yang, Y. Dose-Response Effect of Coenzyme Q10 Supplementation on Blood Pressure among Patients with Cardiometabolic Disorders: A Grading of Recommendations Assessment, Development, and Evaluation (GRADE)-Assessed Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 2180–2194. [Google Scholar] [CrossRef]
- Gao, L.; Mao, Q.; Cao, J.; Wang, Y.; Zhou, X.; Fan, L. Effects of Coenzyme Q10 on Vascular Endothelial Function in Humans: A Meta-Analysis of Randomized Controlled Trials. Atherosclerosis 2012, 221, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Langsjoen, P.; Langsjoen, P.; Willis, R.; Folkers, K. Treatment of Essential Hypertension with Coenzyme Q10. Mol. Asp. Med. 1994, 15, s265–s272. [Google Scholar] [CrossRef]
- Daei, S.; Ildarabadi, A.; Goodarzi, S.; Mohamadi-Sartang, M. Effect of Coenzyme Q10 Supplementation on Vascular Endothelial Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. High Blood Press. Cardiovasc. Prev. 2024, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Joshi, S.; Semwal, D.K.; Verma, K.; Dwivedi, J.; Sharma, S. Role of Curcumin in Ameliorating Hypertension and Associated Conditions: A Mechanistic Insight. Mol. Cell. Biochem. 2022, 477, 2359–2385. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Rezaei Kelishadi, M.; Bagheri, R.; Moosavian, S.P.; Wong, A.; Davoodi, S.H.; Khalili, P.; Dutheil, F.; Suzuki, K.; Asbaghi, O. The Effects of Nano-Curcumin Supplementation on Risk Factors for Cardiovascular Disease: A GRADE-Assessed Systematic Review and Meta-Analysis of Clinical Trials. Antioxidants 2021, 10, 1015. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between Oxidative Stress and Inflammatory Cytokines in Diabetic Nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, S.; Deng, B.; Qian, L.; Chen, J.; Zou, J.; Zheng, J.; Yang, L.; Zhang, C.; Chen, X.; et al. High Glucose Induces Renal Mesangial Cell Proliferation and Fibronectin Expression through JNK/NF-κB/NADPH Oxidase/ROS Pathway, Which Is Inhibited by Resveratrol. Int. J. Biochem. Cell Biol. 2012, 44, 629–638. [Google Scholar] [CrossRef]
- Arora, M.K.; Singh, U.K. Oxidative Stress: Meeting Multiple Targets in Pathogenesis of Diabetic Nephropathy. Curr. Drug Targets 2014, 15, 531–538. [Google Scholar] [CrossRef]
- Ahmed, S.; Mundhe, N.; Borgohain, M.; Chowdhury, L.; Kwatra, M.; Bolshette, N.; Ahmed, A.; Lahkar, M. Diosmin Modulates the NF-kB Signal Transduction Pathways and Downregulation of Various Oxidative Stress Markers in Alloxan-Induced Diabetic Nephropathy. Inflammation 2016, 39, 1783–1797. [Google Scholar] [CrossRef]
- Pal, P.B.; Sinha, K.; Sil, P.C. Mangiferin Attenuates Diabetic Nephropathy by Inhibiting Oxidative Stress Mediated Signaling Cascade, TNFα Related and Mitochondrial Dependent Apoptotic Pathways in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2014, 9, e107220. [Google Scholar] [CrossRef]
- Suzuki, D.; Miyata, T.; Saotome, N.; Horie, K.; Inagi, R.; Yasuda, Y.; Uchida, K.; Izuhara, Y.; Yagame, M.; Sakai, H.; et al. Immunohistochemical Evidence for an Increased Oxidative Stress and Carbonyl Modification of Proteins in Diabetic Glomerular Lesions. J. Am. Soc. Nephrol. JASN 1999, 10, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.-I.; Matsui, T. Advanced Glycation End Products, Oxidative Stress and Diabetic Nephropathy. Oxidative Med. Cell. Longev. 2010, 3, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, E.S.; Choi, R.; Nawaboot, J.; Lee, M.Y.; Lee, E.Y.; Kim, H.S.; Chung, C.H. Protective Effects of Curcumin on Renal Oxidative Stress and Lipid Metabolism in a Rat Model of Type 2 Diabetic Nephropathy. Yonsei Med. J. 2016, 57, 664. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; Ogborne, R.M.; Charalambos, C.A.; O’Connell, M.A. Role of Protein Kinase C δ in Curcumin-Induced Antioxidant Response Element-Mediated Gene Expression in Human Monocytes. Biochem. Biophys. Res. Commun. 2006, 341, 1007–1016. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological Basis for the Role of Curcumin in Chronic Diseases: An Age-Old Spice with Modern Targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Subudhi, U.; Chainy, G.B.N. Curcumin and Vitamin E Modulate Hepatic Antioxidant Gene Expression in PTU-Induced Hypothyroid Rats. Mol. Biol. Rep. 2012, 39, 9849–9861. [Google Scholar] [CrossRef]
- Jeong, G.-S.; Oh, G.-S.; Pae, H.-O.; Jeong, S.-O.; Kim, Y.-C.; Shin, M.-K.; Seo, B.Y.; Han, S.Y.; Lee, H.S.; Jeong, J.-G.; et al. Comparative Effects of Curcuminoids on Endothelial Heme Oxygenase-1 Expression: Ortho-Methoxy Groups Are Essential to Enhance Heme Oxygenase Activity and Protection. Exp. Mol. Med. 2006, 38, 393–400. [Google Scholar] [CrossRef]
- Ye, S.; Hou, Z.; Zhong, L.; Zhang, Q. Effect of Curcumin on the Induction of Glutathione S-Transferases and NADP(H):Quinone Oxidoreductase and Its Possible Mechanism of Action. Yao Xue Xue Bao = Acta Pharm. Sin. 2007, 42, 376–380. [Google Scholar]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral Supplementation of Turmeric Attenuates Proteinuria, Transforming Growth Factor-β and Interleukin-8 Levels in Patients with Overt Type 2 Diabetic Nephropathy: A Randomized, Double-Blind and Placebo-Controlled Study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef]
- Deng, J.; Zheng, C.; Hua, Z.; Ci, H.; Wang, G.; Chen, L. Diosmin Mitigates High Glucose-Induced Endoplasmic Reticulum Stress through PI3K/AKT Pathway in HK-2 Cells. BMC Complement. Med. Ther. 2022, 22, 116. [Google Scholar] [CrossRef]
- Wang, X.; Gao, L.; Lin, H.; Song, J.; Wang, J.; Yin, Y.; Zhao, J.; Xu, X.; Li, Z.; Li, L. Mangiferin Prevents Diabetic Nephropathy Progression and Protects Podocyte Function via Autophagy in Diabetic Rat Glomeruli. Eur. J. Pharmacol. 2018, 824, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Kishore, L.; Kaur, N.; Singh, R. Renoprotective Effect of Bacopa monnieri via Inhibition of Advanced Glycation End Products and Oxidative Stress in STZ-Nicotinamide-Induced Diabetic Nephropathy. Ren. Fail. 2016, 38, 1528–1544. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kim, H.M.; Kang, J.S.; Lee, E.Y.; Yadav, D.; Kwon, M.-H.; Kim, Y.M.; Kim, H.S.; Chung, C.H. Oleanolic Acid and N-Acetylcysteine Ameliorate Diabetic Nephropathy through Reduction of Oxidative Stress and Endoplasmic Reticulum Stress in a Type 2 Diabetic Rat Model. Nephrol. Dial. Transplant. 2016, 31, 391–400. [Google Scholar] [CrossRef]
- He, T.; Guan, X.; Wang, S.; Xiao, T.; Yang, K.; Xu, X.; Wang, J.; Zhao, J. Resveratrol Prevents High Glucose-Induced Epithelial–Mesenchymal Transition in Renal Tubular Epithelial Cells by Inhibiting NADPH Oxidase/ROS/ERK Pathway. Mol. Cell. Endocrinol. 2015, 402, 13–20. [Google Scholar] [CrossRef]
- Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Mxinwa, V.; Mokgalaboni, K.; Orlando, P.; Silvestri, S.; Louw, J.; Tiano, L.; Dludla, P.V. A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy. Molecules 2020, 25, 5645. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y. Effects of Resveratrol Therapy on Glucose Metabolism, Insulin Resistance, Inflammation, and Renal Function in the Elderly Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Clinical Trial Protocol. Medicine 2022, 101, e30049. [Google Scholar] [CrossRef]
- Yamabe, N.; Yokozawa, T.; Oya, T.; Kim, M. Therapeutic Potential of (-)-Epigallocatechin 3-O-Gallate on Renal Damage in Diabetic Nephropathy Model Rats. J. Pharmacol. Exp. Ther. 2006, 319, 228–236. [Google Scholar] [CrossRef]
- Ladeira, L.C.M.; dos Santos, E.C.; Santos, T.A.; da Silva, J.; de Almeida Lima, G.D.; Machado-Neves, M.; da Silva, R.C.; Freitas, M.B.; Maldonado, I.R.d.S.C. Green Tea Infusion Prevents Diabetic Nephropathy Aggravation in Recent-Onset Type 1 Diabetes Regardless of Glycemic Control. J. Ethnopharmacol. 2021, 274, 114032. [Google Scholar] [CrossRef]
- Borges, C.M.; Papadimitriou, A.; Duarte, D.A.; Lopes de Faria, J.M.; Lopes de Faria, J.B. The Use of Green Tea Polyphenols for Treating Residual Albuminuria in Diabetic Nephropathy: A Double-Blind Randomised Clinical Trial. Sci. Rep. 2016, 6, 28282. [Google Scholar] [CrossRef]
- Sourris, K.C.; Harcourt, B.E.; Tang, P.H.; Morley, A.L.; Huynh, K.; Penfold, S.A.; Coughlan, M.T.; Cooper, M.E.; Nguyen, T.-V.; Ritchie, R.H.; et al. Ubiquinone (Coenzyme Q10) Prevents Renal Mitochondrial Dysfunction in an Experimental Model of Type 2 Diabetes. Free Radic. Biol. Med. 2012, 52, 716–723. [Google Scholar] [CrossRef]
- Persson, M.F.; Franzén, S.; Catrina, S.-B.; Dallner, G.; Hansell, P.; Brismar, K.; Palm, F. Coenzyme Q10 Prevents GDP-Sensitive Mitochondrial Uncoupling, Glomerular Hyperfiltration and Proteinuria in Kidneys from Db/Db Mice as a Model of Type 2 Diabetes. Diabetologia 2012, 55, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, H.; Wang, X.; Gao, Q.; Li, Z.; Huang, H. CoQ10 Ameliorates Mitochondrial Dysfunction in Diabetic Nephropathy through Mitophagy. J. Endocrinol. 2019, 240, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, Z.; Liu, Q.; Quan, H.; Cheng, X. Effects of Coenzyme Q10 Intervention on Diabetic Kidney Disease. Medicine 2019, 98, e15850. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Fathallah, L.; Liu, E.; Nourooz-Zadeh, J. Early Oxidative Stress in the Diabetic Kidney: Effect of DL-Alpha-Lipoic Acid. Free Radic. Biol. Med. 2003, 34, 186–195. [Google Scholar] [CrossRef]
- Kamt, S.F.; Liu, J.; Yan, L.-J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients 2023, 15, 1732. [Google Scholar] [CrossRef]
- Melhem, M.F.; Craven, P.A.; Liachenko, J.; DeRubertis, F.R. Alpha-Lipoic Acid Attenuates Hyperglycemia and Prevents Glomerular Mesangial Matrix Expansion in Diabetes. J. Am. Soc. Nephrol. JASN 2002, 13, 108–116. [Google Scholar] [CrossRef]
- Sun, F.; Jiang, D.; Cai, J. Effects of Valsartan Combined with α-Lipoic Acid on Renal Function in Patients with Diabetic Nephropathy: A Systematic Review and Meta-Analysis. BMC Endocr. Disord. 2021, 21, 178. [Google Scholar] [CrossRef]
- Chen, T.-S.; Liou, S.-Y.; Wu, H.-C.; Tsai, F.-J.; Tsai, C.-H.; Huang, C.-Y.; Chang, Y.-L. Efficacy of Epigallocatechin-3-Gallate and Amla (Emblica officinalis) Extract for the Treatment of Diabetic-Uremic Patients. J. Med. Food 2011, 14, 718–723. [Google Scholar] [CrossRef]
- Túri, S.; Németh, I.; Torkos, A.; Sághy, L.; Varga, I.; Matkovics, B.; Nagy, J. Oxidative Stress and Antioxidant Defense Mechanism in Glomerular Diseases. Free Radic. Biol. Med. 1997, 22, 161–168. [Google Scholar] [CrossRef]
- Wang, J.S.; Ger, L.P.; Tseng, H.H. Expression of Glomerular Antioxidant Enzymes in Human Glomerulonephritis. Nephron 1997, 76, 32–38. [Google Scholar] [CrossRef]
- Lee, H.S. Pathogenic Role of TGF-β in the Progression of Podocyte Diseases. Histol. Histopathol. 2011, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Mechanisms and Consequences of TGF-ß Overexpression by Podocytes in Progressive Podocyte Disease. Cell Tissue Res. 2012, 347, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Lu, K.-C.; Lin, Y.-F.; Chen, J.-S.; Huang, C.-F.; Chen, C.-C.; Lin, S.-H.; Chu, P.; Sytwu, H.-K. Pathogenic Role of Effector Cells and Immunoglobulins in Cationic Bovine Serum Albumin-Induced Membranous Nephropathy. J. Clin. Immunol. 2012, 32, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Neale, T.J.; Ojha, P.P.; Exner, M.; Poczewski, H.; Rüger, B.; Witztum, J.L.; Davis, P.; Kerjaschki, D. Proteinuria in Passive Heymann Nephritis Is Associated with Lipid Peroxidation and Formation of Adducts on Type IV Collagen. J. Clin. Investig. 1994, 94, 1577–1584. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lu, K.-C.; Chen, J.-S.; Hsieh, H.-Y.; Lin, S.-H.; Chu, P.; Wang, J.-Y.; Sytwu, H.-K.; Lin, Y.-F. HO-1 Induction Ameliorates Experimental Murine Membranous Nephropathy: Anti-Oxidative, Anti-Apoptotic and Immunomodulatory Effects. Nephrol. Dial. Transplant. 2008, 23, 3082–3090. [Google Scholar] [CrossRef]
- Buelli, S.; Perico, L.; Galbusera, M.; Abbate, M.; Morigi, M.; Novelli, R.; Gagliardini, E.; Tentori, C.; Rottoli, D.; Sabadini, E.; et al. Mitochondrial-Dependent Autoimmunity in Membranous Nephropathy of IgG4-Related Disease. EBioMedicine 2015, 2, 456–466. [Google Scholar] [CrossRef]
- Prunotto, M.; Carnevali, M.L.; Candiano, G.; Murtas, C.; Bruschi, M.; Corradini, E.; Trivelli, A.; Magnasco, A.; Petretto, A.; Santucci, L.; et al. Autoimmunity in Membranous Nephropathy Targets Aldose Reductase and SOD2. J. Am. Soc. Nephrol. JASN 2010, 21, 507–519. [Google Scholar] [CrossRef]
- Sugimoto, K.; Miyazawa, T.; Miyazaki, K.; Yanagida, H.; Enya, T.; Nishi, H.; Wada, N.; Okada, M.; Takemura, T. Minimal Change Nephrotic Syndrome and Prohibitin-2 Gene Polymorphism. Clin. Exp. Nephrol. 2016, 21, 665–670. [Google Scholar] [CrossRef]
- Mocan, H.; Aksoy, A.; Uydu, H.A.; Mocan, M.C. Oxidative Damage of Erythrocyte Membrane in Nephrotic Syndrome. Pediatr. Nephrol. 1999, 13, 326–332. [Google Scholar] [CrossRef]
- Zima, T.; Tesar, V.; Crkovská, J.; Stejskalová, A.; Pláteník, J.; Temínová, J.; Nemecek, K.; Janebová, M.; Stípek, S. ICRF-187 (Dexrazoxan) Protects from Adriamycin-Induced Nephrotic Syndrome in Rats. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 1998, 13, 1975–1979. [Google Scholar]
- Oteki, T.; Nagase, S.; Yokoyama, H.; Ohya, H.; Akatsuka, T.; Tada, M.; Ueda, A.; Hirayama, A.; Koyama, A. Evaluation of Adriamycin Nephropathy by an in Vivo Electron Paramagnetic Resonance. Biochem. Biophys. Res. Commun. 2005, 332, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Hirayama, A.; Nagase, S.; Inoue, M.; Oteki, T.; Aoyama, M.; Yokoyama, H. In Vivo Detection of Intrinsic Reactive Oxygen Species Using Acyl-Protected Hydroxylamine in Puromycin Nephrosis. Free Radic. Res. 2007, 41, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bigler, S.A.; Henegar, J.R.; Baliga, R. Cytochrome P450 2B1 Mediates Oxidant Injury in Puromycin-Induced Nephrotic Syndrome. Kidney Int. 2002, 62, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Arany, I.; Waxman, D.J.; Baliga, R. Cytochrome-P450 2B1 Gene Silencing Attenuates Puromycin Aminonucleoside-Induced Cytotoxicity in Glomerular Epithelial Cells. Kidney Int. 2010, 78, 182–190. [Google Scholar] [CrossRef]
- Kinugasa, S.; Tojo, A.; Sakai, T.; Tsumura, H.; Takahashi, M.; Hirata, Y.; Fujita, T. Selective Albuminuria via Podocyte Albumin Transport in Puromycin Nephrotic Rats Is Attenuated by an Inhibitor of NADPH Oxidase. Kidney Int. 2011, 80, 1328–1338. [Google Scholar] [CrossRef]
- Casalena, G.; Krick, S.; Daehn, I.; Yu, L.; Ju, W.; Shi, S.; Tsai, S.-Y.; D’Agati, V.; Lindenmeyer, M.; Cohen, C.D.; et al. Mpv17 in Mitochondria Protects Podocytes against Mitochondrial Dysfunction and Apoptosis in Vivo and in Vitro. AJP Ren. Physiol. 2014, 306, F1372–F1380. [Google Scholar] [CrossRef]
- Kuo, H.-T.; Kuo, M.-C.; Chiu, Y.-W.; Chang, J.-M.; Guh, J.-Y.; Chen, H.-C. Increased Glomerular and Extracellular Malondialdehyde Levels in Patients and Rats with Focal Segmental Glomerulosclerosis. Eur. J. Clin. Investig. 2005, 35, 245–250. [Google Scholar] [CrossRef]
- Coppo, R.; Camilla, R.; Amore, A.; Peruzzi, L. Oxidative Stress in IgA Nephropathy. Nephron Clin. Pract. 2010, 116, c196–c199. [Google Scholar] [CrossRef]
- Pei, Y.; Xu, Y.; Ruan, J.; Rong, L.; Jiang, M.; Mo, Y.; Jiang, X. Plasma Oxidative Stress Level of IgA Nephropathy in Children and the Effect of Early Intervention with Angiotensin-Converting Enzyme Inhibitors. J. Renin-Angiotensin-Aldosterone Syst. 2016, 17, 147032031664724. [Google Scholar] [CrossRef]
- Camilla, R.; Suzuki, H.; Dapra, V.; Loiacono, E.; Peruzzi, L.; Amore, A.; Ghiggeri, G.M.; Mazzucco, G.; Scolari, F.; Gharavi, A.G.; et al. Oxidative Stress and Galactose-Deficient IgA1 as Markers of Progression in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1903–1911. [Google Scholar] [CrossRef]
- Vas, T.; Wagner, Z.; Jenei, V.; Varga, Z.; Kovács, T.; Wittmann, I.; Schinzel, R.; Balla, G.; Balla, J.; Heidland, A.; et al. Oxidative Stress and Non-Enzymatic Glycation in IgA Nephropathy. Clin. Nephrol. 2005, 64, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Kashem, A.; Endoh, M.; Yamauchi, F.; Yano, N.; Nomoto, Y.; Sakai, H.; Pronai, L.; Tanaka, M.; Nakazawa, H. Superoxide Dismutase Activity in Human Glomerulonephritis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1996, 28, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.Z.; Gheita, T.A.; Kenawy, S.A.; Fahim, A.T.; El-Sorougy, I.M.; Abdou, M.S. Oxidative Stress in Systemic Lupus Erythematosus and Rheumatoid Arthritis Patients: Relationship to Disease Manifestations and Activity. Int. J. Rheum. Dis. 2011, 14, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Novembrino, C.; Quaglini, S.; De Giuseppe, R.; Gallelli, B.; Uva, V.; Montanari, V.; Messa, P.; Bamonti, F. Oxidative Stress and Homocysteine Metabolism in Patients with Lupus Nephritis. Lupus 2010, 19, 65–72. [Google Scholar] [CrossRef]
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. Nrf2 Suppresses Lupus Nephritis through Inhibition of Oxidative Injury and the NF-κB-Mediated Inflammatory Response. Kidney Int. 2014, 85, 333–343. [Google Scholar] [CrossRef]
- Lalwani, P.; de Souza, G.K.B.B.; de Lima, D.S.N.; Passos, L.F.S.; Boechat, A.L.; Lima, E.S. Serum Thiols as a Biomarker of Disease Activity in Lupus Nephritis. PLoS ONE 2015, 10, e0119947. [Google Scholar] [CrossRef]
- Wu, C.-C.; Huang, Y.-S.; Chen, J.-S.; Huang, C.-F.; Su, S.-L.; Lu, K.-C.; Lin, Y.-F.; Chu, P.; Lin, S.-H.; Sytwu, H.-K. Resveratrol Ameliorates Renal Damage, Increases Expression of Heme Oxygenase-1, and Has Anti-Complement, Anti-Oxidative, and Anti-Apoptotic Effects in a Murine Model of Membranous Nephropathy. PLoS ONE 2015, 10, e0125726. [Google Scholar] [CrossRef]
- Chan, W.; Krieg, R.J.; Norkus, E.P.; Chan, J.C.M. α-Tocopherol Reduces Proteinuria, Oxidative Stress, and Expression of Transforming Growth Factor Β1 in IgA Nephropathy in the Rat. Mol. Genet. Metab. 1998, 63, 224–229. [Google Scholar] [CrossRef]
- Kuemmerle, N.B.; Krieg, R.J.; Chan, W.; Trachtman, H.; Norkus, E.P.; Chan, J.C. Influence of Alpha-Tocopherol over the Time Course of Experimental IgA Nephropathy. Pediatr. Nephrol. 1999, 13, 108–112. [Google Scholar] [CrossRef]
- Lan, Q.-G.; Liang, Y.; Liu, L.; Xie, H.-L.; Wang, R.; Zhao, J.-H.; Liang, B. Causal Relationships between Vitamin E and Multiple Kidney Diseases: Evidence from Trans-Ethnic Mendelian Randomization Study. Eur. J. Nutr. 2024, 63, 2779–2788. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, K.; Zhang, X.; Cai, X.; Chen, Y.; Deng, Y. Nephrokeli, a Chinese Herbal Formula, May Improve IgA Nephropathy through Regulation of the Sphingosine-1-Phosphate Pathway. PLoS ONE 2015, 10, e0116873. [Google Scholar] [CrossRef]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G.-R. Oral Supplementation of Turmeric Decreases Proteinuria, Hematuria, and Systolic Blood Pressure in Patients Suffering from Relapsing or Refractory Lupus Nephritis: A Randomized and Placebo-Controlled Study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Is Oxidative Stress, a Link between Nephrolithiasis and Obesity, Hypertension, Diabetes, Chronic Kidney Disease, Metabolic Syndrome? Urol. Res. 2012, 40, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Dendooven, A.; Ishola, D.A.; Nguyen, T.Q.; Van der Giezen, D.M.; Kok, R.J.; Goldschmeding, R.; Joles, J.A.; Joles, J.A. Oxidative Stress in Obstructive Nephropathy. Int. J. Exp. Pathol. 2011, 92, 202–210. [Google Scholar] [CrossRef]
- Jones, E.A.; Shahed, A.; Shoskes, D.A. Modulation of Apoptotic and Inflammatory Genes by Bioflavonoids and Angiotensin II Inhibition in Ureteral Obstruction. Urology 2000, 56, 346–351. [Google Scholar] [CrossRef]
- Lin, K.C.; Krieg, R.J.; Saborio, P.; Chan, J.C.M. Increased Heat Shock Protein-70 in Unilateral Ureteral Obstruction in Rats. Mol. Genet. Metab. 1998, 65, 303–310. [Google Scholar] [CrossRef]
- Kinter, M.; Wolstenholme, J.T.; Thornhill, B.A.; Newton, E.A.; Mccormick, M.L.; Chevalier, R.L. Unilateral Ureteral Obstruction Impairs Renal Antioxidant Enzyme Activation during Sodium Depletion. Kidney Int. 1999, 55, 1327–1334. [Google Scholar] [CrossRef]
- Manucha, W.; Carrizo, L.; Ruete, C.; Molina, H.; Vallés, P. Angiotensin II Type I Antagonist on Oxidative Stress and Heat Shock Protein 70 (HSP 70) Expression in Obstructive Nephropathy. Cell. Mol. Biol. 2005, 51, 547–555. [Google Scholar]
- Sugiyama, H.; Kobayashi, M.; Wang, D.-H.; Sunami, R.; Maeshima, Y.; Yamasaki, Y.; Masuoka, N.; Kira, S.; Makino, H. Telmisartan Inhibits Both Oxidative Stress and Renal Fibrosis after Unilateral Ureteral Obstruction in Acatalasemic Mice. Nephrol. Dial. Transplant. 2005, 20, 2670–2680. [Google Scholar] [CrossRef]
- Rabbani, N.; Sebekova, K.; Sebekova, K.; Heidland, A.; Thornalley, P.J. Accumulation of Free Adduct Glycation, Oxidation, and Nitration Products Follows Acute Loss of Renal Function. Kidney Int. 2007, 72, 1113–1121. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Obama, A.; Hiroi, J.; Miura, H.; Watanabe, M.; Kumai, T.; Ohtani-Kaneko, R.; Hirata, K.; Kimura, K. Liver-Type Fatty Acid-Binding Protein Attenuates Renal Injury Induced by Unilateral Ureteral Obstruction. Am. J. Pathol. 2006, 169, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Kawada, N.; Moriyama, T.; Ando, A.; Fukunaga, M.; Miyata, T.; Kurokawa, K.; Imai, E.; Hori, M. Increased Oxidative Stress in Mouse Kidneys with Unilateral Ureteral Obstruction. Kidney Int. 1999, 56, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Pat, B.; Yang, T.; Kong, C.; Watters, D.; Johnson, D.W.; Gobe, G. Activation of ERK in Renal Fibrosis after Unilateral Ureteral Obstruction: Modulation by Antioxidants. Kidney Int. 2005, 67, 931–943. [Google Scholar] [CrossRef]
- Omori, H.; Kawada, N.; Inoue, K.; Ueda, Y.; Yamamoto, R.; Matsui, I.; Kaimori, J.; Takabatake, Y.; Moriyama, T.; Isaka, Y.; et al. Use of Xanthine Oxidase Inhibitor Febuxostat Inhibits Renal Interstitial Inflammation and Fibrosis in Unilateral Ureteral Obstructive Nephropathy. Clin. Exp. Nephrol. 2012, 16, 549–556. [Google Scholar] [CrossRef]
- Chen, H.; Sun, F.; Zhong, X.; Shao, Y.; Yoshimura, A.; Liu, Y. Eplerenone-Mediated Aldosterone Blockade Prevents Renal Fibrosis by Reducing Renal Inflammation, Interstitial Cell Proliferation and Oxidative Stress. Kidney Blood Press. Res. 2013, 37, 557–566. [Google Scholar] [CrossRef]
- Yaxley, J.; Yaxley, W. Obstructive Uropathy—Acute and Chronic Medical Management. World J. Nephrol. 2023, 12, 1–9. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Liu, X.; Feng, Y.; Gui, Y.; Yang, J.; He, W.; Dai, C. Quercetin Inhibits Fibroblast Activation and Kidney Fibrosis Involving the Suppression of Mammalian Target of Rapamycin and β-Catenin Signaling. Sci. Rep. 2016, 6, 23968. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Ricardo, S.D.; Bertram, J.F.; Nikolic-Paterson, D.J. Resveratrol Inhibits Renal Fibrosis in the Obstructed Kidney: Potential Role in Deacetylation of Smad3. Am. J. Pathol. 2010, 177, 1065–1071. [Google Scholar] [CrossRef]
- Liang, J.; Tian, S.; Han, J.; Xiong, P. Resveratrol as a Therapeutic Agent for Renal Fibrosis Induced by Unilateral Ureteral Obstruction. Ren. Fail. 2014, 36, 285–291. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Zhang, M.-Z.; You, L.; Davis, L.S.; Fan, H.; Yang, H.-C.; Fogo, A.B.; Zent, R.; Harris, R.C.; et al. Sirt1 Activation Protects the Mouse Renal Medulla from Oxidative Injury. J. Clin. Investig. 2010, 120, 1056–1068. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.F.; Zhao, C.G.; Sui, F.X.; Teng, X.; Wu, Y. Bin Therapeutic Potential of EGCG on Acute Renal Damage in a Rat Model of Obstructive Nephropathy. Mol. Med. Rep. 2013, 7, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-Gallate Attenuates Unilateral Ureteral Obstruction-Induced Renal Interstitial Fibrosis in Mice. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2015, 63, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Saborio, P.; Krieg, R.J.; Kuemmerle, N.B.; Norkus, E.P.; Schwartz, C.C.; Chan, J.C. Alpha-Tocopherol Modulates Lipoprotein Cytotoxicity in Obstructive Nephropathy. Pediatr. Nephrol. 2000, 14, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Akin, M.; Demirbilek, S.; AY, S.; Gurunluoglu, K.; Turkmen, E.; Tas, E.; Aksoy, R.T.; Baykarabulut, A.; Edali, M.N. Attenuation of Ureteral Obstruction-Induced Renal Injury by Polyenylphosphatidylcholine. Int. J. Urol. 2007, 14, 350–356. [Google Scholar] [CrossRef]
- Ozbek, E.; Ilbey, Y.O.; Ozbek, M.; Simsek, A.; Cekmen, M.; Somay, A. Melatonin Attenuates Unilateral Ureteral Obstruction–Induced Renal Injury by Reducing Oxidative Stress, iNOS, MAPK, and NF-kB Expression. J. Endourol. 2009, 23, 1165–1173. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Sun, N.; Liu, X.; Song, E.; Zhang, Z.; Wen, J.; Zheng, T. Melatonin Therapy Protects against Renal Injury before and after Release of Bilateral Ureteral Obstruction in Rats. Life Sci. 2019, 229, 104–115. [Google Scholar] [CrossRef]
- Ardakani Movaghati, M.R.; Yousefi, M.; Saghebi, S.A.; Sadeghi Vazin, M.; Iraji, A.; Mosavat, S.H. Efficacy of Black Seed (Nigella sativa L.) on Kidney Stone Dissolution: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Phytother. Res. 2019, 33, 1404–1412. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragoș, D.; Enache, I.I.; Manea, M.M. Oxidative Stress and Nutritional Antioxidants in Renal Diseases: A Narrative Review. Antioxidants 2025, 14, 757. https://doi.org/10.3390/antiox14070757
Dragoș D, Enache II, Manea MM. Oxidative Stress and Nutritional Antioxidants in Renal Diseases: A Narrative Review. Antioxidants. 2025; 14(7):757. https://doi.org/10.3390/antiox14070757
Chicago/Turabian StyleDragoș, Dorin, Iulia I. Enache, and Maria M. Manea. 2025. "Oxidative Stress and Nutritional Antioxidants in Renal Diseases: A Narrative Review" Antioxidants 14, no. 7: 757. https://doi.org/10.3390/antiox14070757
APA StyleDragoș, D., Enache, I. I., & Manea, M. M. (2025). Oxidative Stress and Nutritional Antioxidants in Renal Diseases: A Narrative Review. Antioxidants, 14(7), 757. https://doi.org/10.3390/antiox14070757





