Pathways to the Brain: Impact of Fine Particulate Matter Components on the Central Nervous System
Abstract
1. Introduction
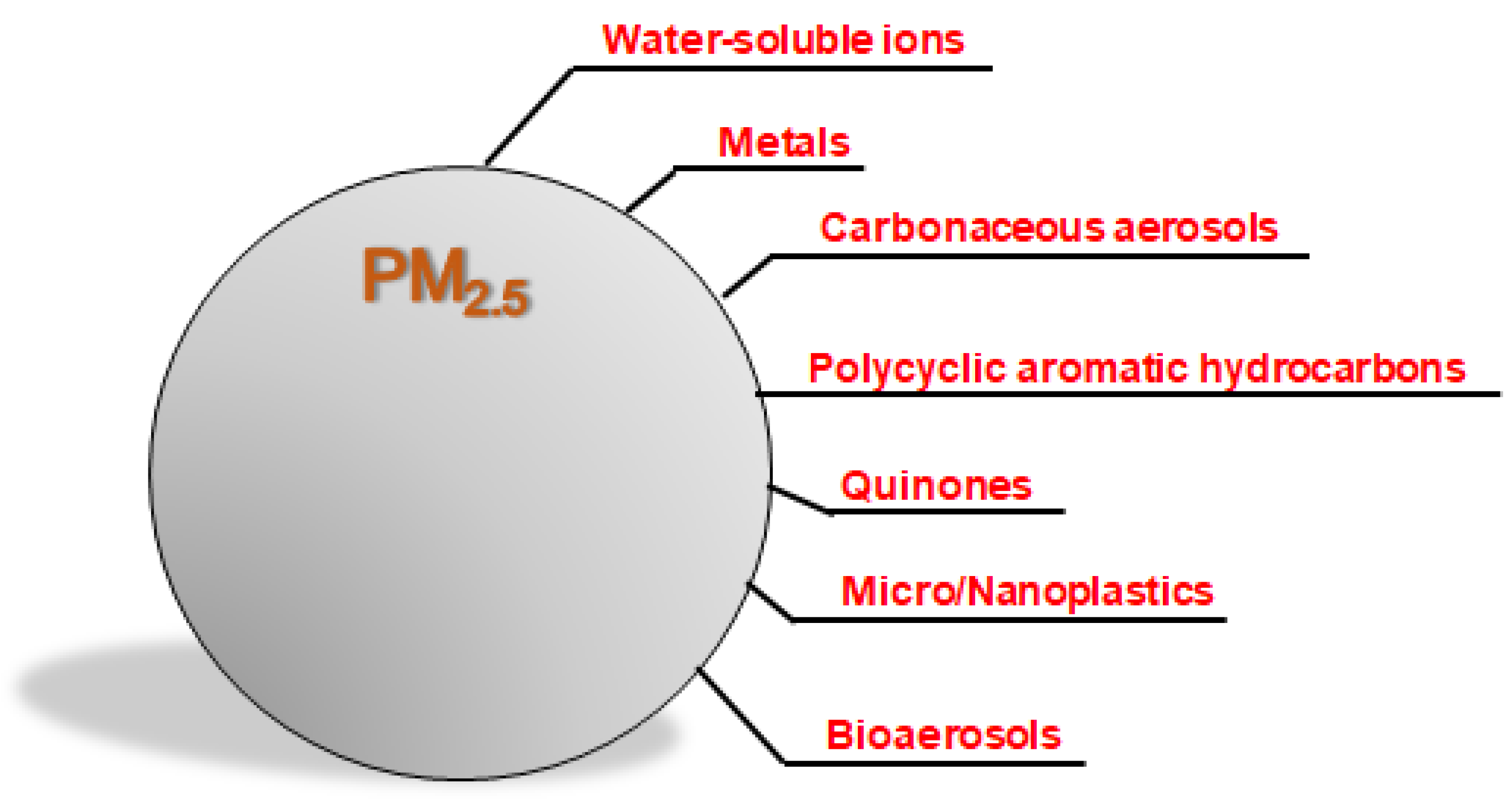
2. Components of PM2.5 and Their Biological Effects
2.1. Water-Soluble Ions
2.2. Metals
2.3. Carbonaceous Aerosols
2.4. PAHs
2.5. Quinones
2.6. Plastics
2.7. Bioaerosols
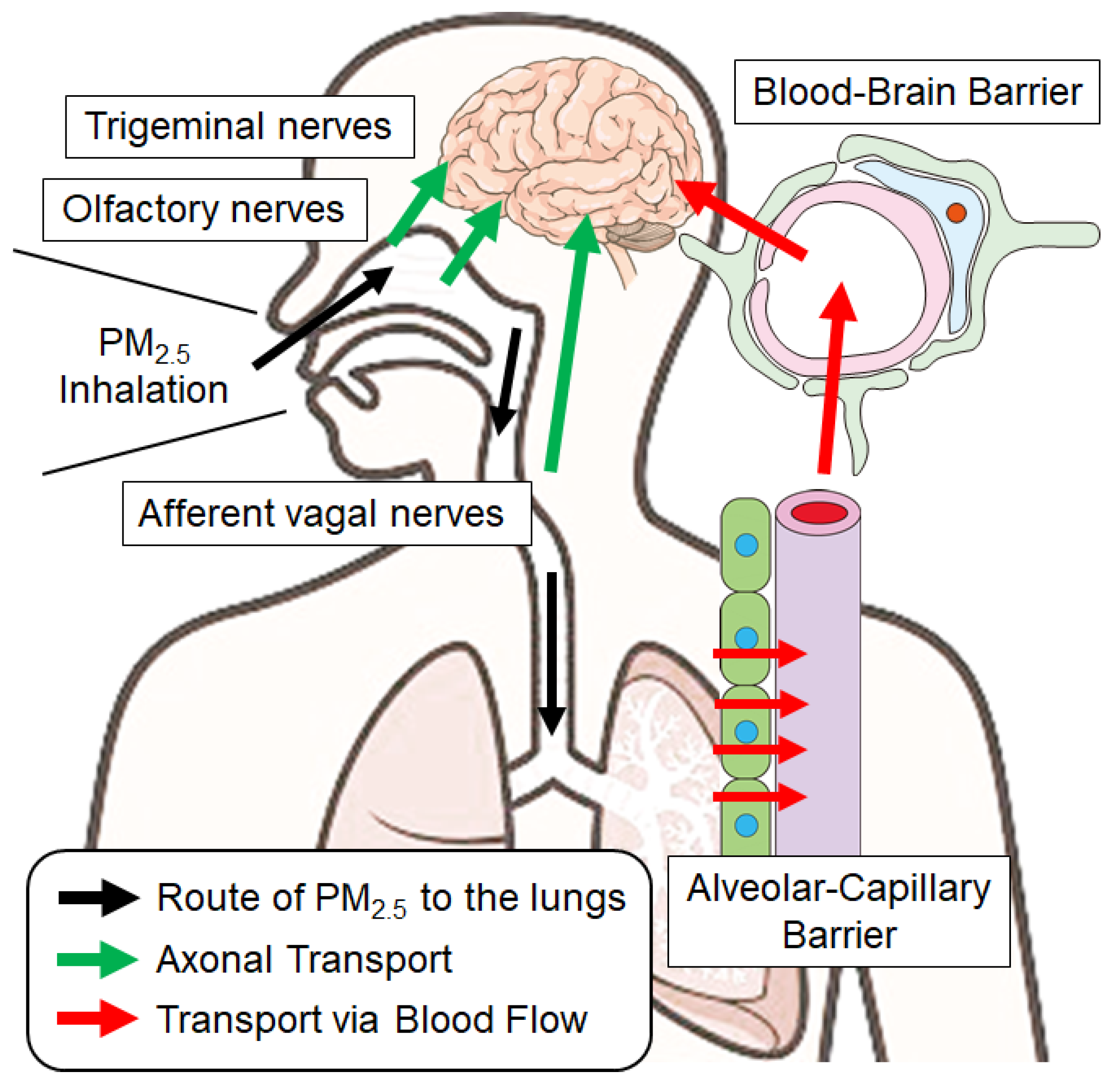
3. Routes of PM2.5 Components into the Brain
3.1. Solid Particles: Pathways via the Olfactory Epithelium
3.2. Solid Particles: Passing Through the Alveolar Barrier into the Blood
3.3. Solid Particles: Passing Through the Blood–Brain Barrier into the Brain
3.4. Transport via Biological Fluids
3.5. Alternative Pathways of PM2.5 Neurotoxicity
4. Action of PM2.5 Components in the Brain
4.1. Action Mechanisms of Particles Delivered to the Brain
4.2. Indirect Effects of PM2.5-Induced Peripheral Oxidative Stress and Inflammation
4.3. Particle Exposure and Neurological Disorders
| Endpoints | Pollutant | Year of Study | Location | Sample Sizes | Reference |
|---|---|---|---|---|---|
| (No. of Cases or Participants) | |||||
| Migraine, Headache | PM2.5, PM10, SO2, NO2, O3, CO | 1992–2002 | Canada | 56,241 a | [181] |
| 48,022 a | |||||
| Migraine | PM2.5, PM10, SO2, NO2, O3, CO | 2006–2011 | Taiwan | 1,000,000 b | [182] |
| Ischemic stroke | PM2.5 | 2003–2008 | Canada | 9,202 a | [183] |
| Ischemic stroke | PM2.5, SO2, NO2, O3, CO | 2006–2010 | Taiwan | 40,009 a | [184] |
| Ischemic stroke | PM2.5, O3 | 2000–2012 | USA | 2948 a | [185] |
| Ischemic stroke | PM2.5 | 2007–2015 | USA | 31,414 b | [186] |
| Ischemic stroke | PM2.5, SO2, NO2, O3, CO | 2014–2016 | China | 2,032,667 a | [187] |
| Ischemic stroke | total hydrocarbons, | 2000–2013 | Taiwan | 283,666 a | [188] |
| nonmethane hydrocarbons | |||||
| PM2.5, PM10, SO2, NO2, O3, CO, | |||||
| CO2, NOX, NO, CH4 | |||||
| Ischemic stroke | PM2.5 | 2006–2013 | Taiwan | 10,035 a | [189] |
| Ischemic stroke | PM2.5 | 2015–2017 | China | 155,616 a | [190] |
| Hemorrhagic stroke | PM2.5 | 2010–2015 | Portugal | 308 a | [191] |
| Hemorrhagic stroke | PM2.5 | 2002–2013 | Republic of Korea | 62,676 b | [192] |
| Hemorrhagic stroke | PM2.5, SO2, NO2 | 2012–2014 | China | 6412 a | [193] |
| Hemorrhagic stroke | PM2.5, PM10, SO2, NO2, O3, CO | 2017–2018 | Republic of Korea | 92 a | [194] |
| Ischemic, Hemorrhagic stroke | PM2.5 SO42−, NO3−, OC, EC | 2004–2008 | Taiwan | 12,982 a | [195] |
| 3362 a | |||||
| Ischemic, Hemorrhagic stroke | PM2.5, PM10, PM2.5abs | 2000–2012 | Germany | 4433 b | [196] |
| Ischemic, Hemorrhagic stroke | PM1, PM2.5, PM10, OC, EC, | 2007–2011 | China | 9066 a | [197] |
| SO2, NO2, NH4+ SO42−, NO3−, | |||||
| Na+, Cl− | |||||
| Ischemic, Hemorrhagic stroke | PM2.5, PM10, NO, NO2, NOX, O3 | 2005–2012 | United Kingdom | 1800 a | [198] |
| Ischemic, Hemorrhagic stroke | PM2.5 | 2013–2015 | China | 1356 a | [199] |
| Ischemic, Hemorrhagic stroke | PM2.5, PM10, SO2, NO2 | 2013–2015 | China | 84,535 a | [200] |
| Ischemic, Hemorrhagic stroke | PM2.5, PM10, SO2, NO2, O3, CO | 2016–2017 | China | 1063 a | [201] |
| Ischemic, Hemorrhagic stroke | PM2.5, PM2.5abs, NO2, NOX | 1996–2012 | Australia | 1778 a | [202] |
| Ischemic, Hemorrhagic stroke | PM2.5 | 2014–2018 | Israel | 74,052 a | [203] |
| Ischemic, Hemorrhagic stroke | PM2.5, EC, NO2 | 2005–2017 | Denmark | 94,256 a | [204] |
| PD | PM2.5 | 1999–2010 | USA | 6,982,678 b | [205] |
| PD | PM2.5, PM10 | 1990–2008 | USA | 508 a | [206] |
| PD | PM2.5, O3 | 1993–2010 | USA | 301 a | [207] |
| PD | PM2.5, PM10, NO2 | 1995–2006 | USA | 1556 a | [208] |
| PD | PM2.5, NO2, O3 | 2001–2013 | Canada | 38,475 a | [209] |
| PD | PM2.5, PM10, SO2, NO2, O3, CO | 2002–2015 | Republic of Korea | 338 a | [210] |
| PD | PM2.5, BC, organic matter, | 2000–2014 | USA | 197,545 a | [176] |
| NO3−, SO42−, sea salt, soil particle | |||||
| PD | PM2.5 | 2006–2013 | Taiwan | 137 a | [211] |
| PD | PM2.5, NO2 | 2009–2018 | USA | 163 a | [212] |
| PD | PM2.5, NO2 | 1998–2015 | USA | 346 a | [213] |
| Dementia | PM2.5, PM10, NO2, O3 | 2001–2009 | Spain | 1175 a | [214] |
| Dementia | PM2.5, NOX | 2001–2013 | Sweden | 364 a | [215] |
| Dementia | PM2.5 | 2001–2013 | Sweden | 2253 b | [216] |
| Dementia | PM2.5, NO2 | 2008–2012 | USA | 398 a | [217] |
| Dementia | PM2.5, PMcoarse, PM2.5abs, NO2 | 2006–2018 | United Kingdom | 1394 a | [218] |
| Dementia | PM2.5, PM10, PM2.5abs, NO2, NOX | 2010–2018 | Netherlands | 545 a | [219] |
| Dementia | PM2.5, black carbon, organic matter, | 2000–2018 | USA | 309,842 a | [177] |
| NO3−, SO42−, NH4+, soil dust | |||||
| Dementia | PM2.5 | 1998–2016 | USA | 4105 a | [220] |
| Dementia | PM2.5 | 2006–2019 | USA | 80,993 a | [221] |
| Dementia | PM2.5, NO2, O3 | 2007–2013 | USA | 1011 a | [222] |
| Dementia, AD | PM2.5 | 1993–2010 | Sweden | 302 a | [223] |
| Dementia, AD | PM2.5, NO2 | 2000–2012 | Canada | 251,641 a | [224] |
| Dementia, AD | PM2.5 | 1994–2018 | USA | 1136 a | [225] |
| Dementia, AD | PM2.5, black carbon, organic matter, | 2000–2017 | USA | ~5.8 million a | [226] |
| NO3−, SO42−, NH4+, soil dust | ~2.8 million a | ||||
| Dementia, AD | PM2.5, NO2, O3 | 2000–2018 | USA | 2,025,130 a | [227] |
| 804,668 a | |||||
| AD | PM2.5, PM10, SO2, NO2, O3, CO | 2001–2010 | Taiwan | 1399 a | [228] |
| AD | PM2.5 | 2008–2013 | Taiwan | 3803 a | [229] |
| AD | PM2.5 | 1996–2010 | USA | 998 b | [230] |
| AD | PM2.5 | 1994–2018 | USA | 832 a | [231] |
| AD | PM2.5 | 2018–2020 | China | 1545 b | [232] |
| AD | PM2.5, PM10, SO2, NO2, O3, CO | 2010–2014 | USA | 57,990 a | [233] |
| Cognitive decline | PM2.5, PM10, SO2, NO2, O3, CO | 2007–2018 | Republic of Korea | 398,889 b | [234] |
| Mild cognitive impairment | PM2.5, PM10, SO2, O3 | 2015–2018 | China | 782 a | [235] |
| Dementia, AD, vascular dementia | PM2.5, black carbon, organic matter, | 2006–2021 | United Kingdom | 5768 a | [236] |
| NO3−, SO42−, NH4+ | 1860 a | ||||
| 1071 a | |||||
| AD, non-AD dementia, PD | PM2.5 | 2007–2014 | USA | 1503 a | [237] |
| 4955 a | |||||
| 570 a | |||||
| Brain tumors | PM2.5, PM10, NO2, NOx | 1993–2013 | Denmark | 121 a | [238] |
| Brain tumors | UFP (<0.1 µm), PM2.5, PM10 | 1991–2016 | Canada | 1400 a | [239] |
| Epilepsy | PM2.5, SO2, NO2, O3 | 2013–2014 | China | 20,368 a | [240] |
| Epilepsy | PM2.5, PM10, SO2, NO2, O3, CO, NO, | 2009–2013 | Taiwan | 108,175 a | [178] |
| CH4, non-methane hydrocarbons | |||||
| Multiple sclerosis | PM2.5, PM10, SOX, NOX, NH3, CO | 1990–2015 | Italy | 927 a | [241] |
| Multiple sclerosis | PM2.5 | 1998–2018 | Italy | 683 a | [242] |
| Multiple sclerosis | PM2.5 | 2017 | 195 countries | 1,761,078 a | [243] |
| Multiple sclerosis | PM2.5, PM10 | 2013–2022 | Thailand | 126 a | [244] |
| Schizophrenia | PM2.5 | 2010–2015 | USA, China | 46 a | [245] |
| Depression | PM2.5, SO2, NO2, NOX, O3, CO, | 2010–2115 | Poland | 318,779 a | [246] |
| benzo(a)pyrene, Pb | |||||
| Sleep disorder | PM1, PM2.5, PM10, SO2, NO2, | 2012–2013 | China | 2,304 a | [247] |
| O3, CO | |||||
| Sleep disorder | PM2.5 | 2012–2015 | USA | 51,562 b | [248] |
| Sleep disorder | PM2.5, PM10, SO2, NO2, O3 | 2018–2019 | China | 87,734 a | [249] |
| Hearing loss | PM2.5, PM10, SO2, NO2, O3 | 2015 | Republic of Korea | 817 a | [250] |
| Hearing loss | PM2.5, SO2, NO2 O3, CO | 2011–2019 | Taiwan | 850 a | [251] |
| Olfactory decline | PM2.5, PM10 | 2016–2017 | Mexico | 120 b | [252] |
| Olfactory decline | PM2.5 | 2001–2016 | Sweden | 1774 b | [253] |
| Anosmia | PM2.5 | 2013–2016 | USA | 538 a | [254] |
| Amyotrophic Lateral Sclerosis | PM2.5 | 1989–2013 | Denmark | 3983 a | [255] |
| Developmental disorders | PM2.5, SO2, NO2, O3, CO, | 2016–2020 | Republic of Korea | 843,134 b | [256] |
| Pb, Cd, Cr, Cu, Mn, Fe, Ni, As | |||||
| Nervous system anomalies | PM2.5 | 2004–2018 | Taiwan | 12,383 a | [257] |
| Cerebral palsy | PM2.5, NO2, O3 | 2002–2017 | Canada | 3170 a | [258] |
| Tic disorders | PM2.5 | 2004–2017 | Taiwan | 5902 a | [259] |
| Delirium | PM2.5, PM10, SO2, NO2, CO | 2014–2015 | China | 559 a | [260] |
| Brain volume, Covert brain infarcts | PM2.5 | 1998–2001 | USA | 943 b | [261] |
| Gray/white matter volumes | PM2.5 | 1996–2006 | USA | 1403 b | [262] |
| Cerebral vascular resistance | PM2.5 | 2005–2008 | USA | 482 b | [263] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dockery, D.W.; Speizer, F.E.; Stram, D.O.; Ware, J.H.; Spengler, J.D.; Ferris, B.G., Jr. Effects of inhalable particles on respiratory health of children. Am. Rev. Respir. Dis. 1989, 139, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lim, H.J.; Kim, Y.Y. Publication trends in research on particulate matter and health impact over a 10-year period: 2009–2018. Environ. Anal. Health Toxicol. 2021, 36, e2021005. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A., 3rd; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Burnett, R.T.; Goldberg, M.; Hoover, K.; Siemiatycki, J.; Abrahamowicz, M.; Villeneuve, P.J.; White, W. Reanalysis of the Harvard Six Cities Study, part II: Sensitivity analysis. Inhal. Toxicol. 2005, 17, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Burnett, R.T.; Goldberg, M.; Hoover, K.; Siemiatycki, J.; Abrahamowicz, M.; White, W. Reanalysis of the Harvard Six Cities Study, part I: Validation and replication. Inhal. Toxicol. 2005, 17, 335–342. [Google Scholar] [CrossRef]
- Cao, J.J.; Chow, J.C.; Lee, F.S.C.; Watson, J.G. Evolution of PM2.5 Measurements and Standards in the US and Future Perspectives for China. Aerosol Air Qual. Res. 2013, 13, 1197–1211. [Google Scholar] [CrossRef]
- Zanobetti, A.; Franklin, M.; Koutrakis, P.; Schwartz, J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ. Health 2009, 8, 58. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, P.; Xia, X.; Wang, L.; Li, X. The underlying mechanism of PM2.5-induced ischemic stroke. Environ. Pollut. 2022, 310, 119827. [Google Scholar] [CrossRef]
- Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. PM2.5 exposure in association with AD-related neuropathology and cognitive outcomes. Environ. Pollut. 2022, 292, 118320. [Google Scholar] [CrossRef]
- Balmes, J.R.; Fine, J.M.; Sheppard, D. Symptomatic bronchoconstriction after short-term inhalation of sulfur dioxide. Am. Rev. Respir. Dis. 1987, 136, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, J. Evaluation of biological significance of nitrogen oxides exposure. Tokai J. Exp. Clin. Med. 1985, 10, 348–353. [Google Scholar]
- Suvarapu, L.N.; Baek, S.O. Determination of heavy metals in the ambient atmosphere. Toxicol. Ind. Health 2017, 33, 79–96. [Google Scholar] [CrossRef]
- Jiang, S.L.; Zhang, Y.; Yu, G.Y.; Han, Z.M.; Zhao, J.R.; Zhang, T.L.; Zheng, M. Source-resolved atmospheric metal emissions, concentrations, and deposition fluxes into the East Asian seas. Atmos. Chem. Phys. 2024, 24, 8363–8381. [Google Scholar] [CrossRef]
- Tian, H.Z.; Zhu, C.Y.; Gao, J.J.; Cheng, K.; Hao, J.M.; Wang, K.; Hua, S.B.; Wang, Y.; Zhou, J.R. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: Historical trend, spatial distribution, uncertainties, and control policies. Atmos. Chem. Phys. 2015, 15, 10127–10147. [Google Scholar] [CrossRef]
- Markiv, B.; Exposito, A.; Ruiz-Azcona, L.; Santibanez, M.; Fernandez-Olmo, I. Environmental exposure to manganese and health risk assessment from personal sampling near an industrial source of airborne manganese. Environ. Res. 2023, 224, 115478. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Huang, P.; Blomberg, E.; Odnevall, I. Unravelling the Mechanistic Understanding of Metal Nanoparticle-Induced Reactive Oxygen Species Formation: Insights from a Cu Nanoparticle Study. Chem. Res. Toxicol. 2023, 36, 1891–1900. [Google Scholar] [CrossRef]
- Huang, X.; Moir, R.D.; Tanzi, R.E.; Bush, A.I.; Rogers, J.T. Redox-active metals, oxidative stress, and Alzheimer’s disease pathology. Ann. NY Acad. Sci. 2004, 1012, 153–163. [Google Scholar] [CrossRef]
- Fiedler, A.T.; Fischer, A.A. Oxygen activation by mononuclear Mn, Co, and Ni centers in biology and synthetic complexes. J. Biol. Inorg. Chem. 2017, 22, 407–424. [Google Scholar] [CrossRef]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media-A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Yang, L.S. Characteristics of Ambient Black Carbon Mass and Size-Resolved Particle Number Concentrations during Corn Straw Open-Field Burning Episode Observations at a Rural Site in Southern Taiwan. Int. J. Environ. Res. Public Health 2016, 13, 688. [Google Scholar] [CrossRef]
- Geng, F.; Hua, J.; Mu, Z.; Peng, L.; Xu, X.; Chen, R.; Kan, H. Differentiating the associations of black carbon and fine particle with daily mortality in a Chinese city. Environ. Res. 2013, 120, 27–32. [Google Scholar] [CrossRef]
- Sharma, S.; Chandra, M.; Harsha Kota, S. Four year long simulation of carbonaceous aerosols in India: Seasonality, sources and associated health effects. Environ. Res. 2022, 213, 113676. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, R.D.; Badali, K.; Liggio, J.; Li, S.M.; Abbatt, J.P. Filterable redox cycling activity: A comparison between diesel exhaust particles and secondary organic aerosol constituents. Environ. Sci. Technol. 2013, 47, 3362–3369. [Google Scholar] [CrossRef]
- Niranjan, R.; Thakur, A.K. The Toxicological Mechanisms of Environmental Soot (Black Carbon) and Carbon Black: Focus on Oxidative Stress and Inflammatory Pathways. Front. Immunol. 2017, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.T.; Xia, K.; Pan, L.; Zhang, J.C.; Luo, Y.; Zhang, Y.; Cui, Z.F.; El-Sayed, N.N.; Aldalbahi, A.; Chen, N.; et al. Autophagy and lysosomal dysfunction: A new insight into mechanism of synergistic pulmonary toxicity of carbon black-metal ions co-exposure. Carbon 2017, 111, 322–333. [Google Scholar] [CrossRef]
- Liu, D.; Allan, J.D.; Young, D.E.; Coe, H.; Beddows, D.; Fleming, Z.L.; Flynn, M.J.; Gallagher, M.W.; Harrison, R.M.; Lee, J.; et al. Size distribution, mixing state and source apportionment of black carbon aerosol in London during wintertime. Atmos. Chem. Phys. 2014, 14, 10061–10084. [Google Scholar] [CrossRef]
- Li, R.; Han, Y.; Wang, L.; Shang, Y.; Chen, Y. Differences in oxidative potential of black carbon from three combustion emission sources in China. J. Environ. Manage 2019, 240, 57–65. [Google Scholar] [CrossRef]
- Li, X.; Tan, M.; Wu, B.; Wang, J.; Ma, J.; Chen, B.; Chu, C. Redox Oscillation-Driven Production of Reactive Oxygen Species from Black Carbon. Environ. Sci. Technol. 2024, 58, 21210–21217. [Google Scholar] [CrossRef]
- Verma, N.; Pink, M.; Schmitz-Spanke, S. A new perspective on calmodulin-regulated calcium and ROS homeostasis upon carbon black nanoparticle exposure. Arch. Toxicol. 2021, 95, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, H.; Shang, J.; Yuan, L.; Zhang, Y.; Wang, L.; Zhang, W.; Luan, X.; Hu, G.; Chu, H.; et al. Ozonized carbon black induces mitochondrial dysfunction and DNA damage. Environ. Toxicol. 2017, 32, 944–955. [Google Scholar] [CrossRef]
- Goldstein, A.H.; Galbally, I.E. Known and unknown organic constituents in the Earth’ s atmosphere. Environ. Sci. Technol. 2007, 41, 1514–1521. [Google Scholar] [CrossRef]
- Vondracek, J.; Machala, M. The Role of Metabolism in Toxicity of Polycyclic Aromatic Hydrocarbons and their Non-genotoxic Modes of Action. Curr. Drug Metab. 2021, 22, 584–595. [Google Scholar] [CrossRef]
- Bukowska, B.; Duchnowicz, P. Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress. Molecules 2022, 27, 1379. [Google Scholar] [CrossRef]
- Jin, Y.; Miao, W.; Lin, X.; Pan, X.; Ye, Y.; Xu, M.; Fu, Z. Acute exposure to 3-methylcholanthrene induces hepatic oxidative stress via activation of the Nrf2/ARE signaling pathway in mice. Environ. Toxicol. 2014, 29, 1399–1408. [Google Scholar] [CrossRef]
- Ishihara, Y.; Haarmann-Stemmann, T.; Kado, N.Y.; Vogel, C.F.A. Interleukin 33 Expression Induced by Aryl Hydrocarbon Receptor in Macrophages. Toxicol. Sci. 2019, 170, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fujikawa, M.; Oguro, A.; Itoh, K.; Vogel, C.F.A.; Ishihara, Y. Involvement of the Microglial Aryl Hydrocarbon Receptor in Neuroinflammation and Vasogenic Edema after Ischemic Stroke. Cells 2021, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Boerboom, A.M.; Baechle, C.; El-Bahay, C.; Kahl, R.; Degen, G.H.; Abel, J. Regulation of prostaglandin endoperoxide H synthase-2 induction by dioxin in rat hepatocytes: Possible c-Src-mediated pathway. Carcinogenesis 2000, 21, 2267–2274. [Google Scholar] [CrossRef]
- Vogel, C.F.; Sciullo, E.; Wong, P.; Kuzmicky, P.; Kado, N.; Matsumura, F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ. Health Perspect. 2005, 113, 1536–1541. [Google Scholar] [CrossRef]
- Sutter, T.R.; Guzman, K.; Dold, K.M.; Greenlee, W.F. Targets for dioxin: Genes for plasminogen activator inhibitor-2 and interleukin-1 beta. Science 1991, 254, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, B.D.; Beischlag, T.V.; Dinatale, B.C.; Ramadoss, P.; Perdew, G.H. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008, 68, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Li, W.; Wu, D.; Miller, J.K.; Sweeney, C.; Lazennec, G.; Fujisawa, Y.; Matsumura, F. Interaction of aryl hydrocarbon receptor and NF-kappaB subunit RelB in breast cancer is associated with interleukin-8 overexpression. Arch. Biochem. Biophys. 2011, 512, 78–86. [Google Scholar] [CrossRef]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef]
- Lee, J.S.; Cella, M.; McDonald, K.G.; Garlanda, C.; Kennedy, G.D.; Nukaya, M.; Mantovani, A.; Kopan, R.; Bradfield, C.A.; Newberry, R.D.; et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2011, 13, 144–151. [Google Scholar] [CrossRef]
- Lecureur, V.; Ferrec, E.L.; N’Diaye, M.; Vee, M.L.; Gardyn, C.; Gilot, D.; Fardel, O. ERK-dependent induction of TNFalpha expression by the environmental contaminant benzo(a)pyrene in primary human macrophages. FEBS Lett. 2005, 579, 1904–1910. [Google Scholar] [CrossRef]
- Ishihara, N.; Okuda, T.; Hagino, H.; Oguro, A.; Tani, Y.; Okochi, H.; Tokoro, C.; Fujii-Kuriyama, Y.; Itoh, K.; Vogel, C.F.A.; et al. Involvement of polycyclic aromatic hydrocarbons and endotoxin in macrophage expression of interleukin-33 induced by exposure to particulate matter. J. Toxicol. Sci. 2022, 47, 201–210. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lane, D.A.; Kim, Y.P. Formation of polyaromatic hydrocarbon (PAH)-quinones during the gas phase reactions of PAHs with the OH radical in the atmosphere. Environ. Chem. 2015, 12, 307–315. [Google Scholar] [CrossRef]
- Cho, A.K.; Di Stefano, E.; You, Y.; Rodriguez, C.E.; Schmitz, D.A.; Kumagai, Y.; Miguel, A.H.; Eiguren-Fernandez, A.; Kobayashi, T.; Avol, E.; et al. Determination of four quinones in diesel exhaust particles, SRM 1649a, an atmospheric PM. Aerosol. Sci. Tech. 2004, 38, 68–81. [Google Scholar] [CrossRef]
- Eiguren-Fernandez, A.; Miguel, A.H.; Di Stefano, E.; Schmitz, D.A.; Cho, A.K.; Thurairatnam, S.; Avol, E.L.; Froines, J.R. Atmospheric distribution of gas- and particle-phase quinones in Southern California. Aerosol. Sci. Tech. 2008, 42, 854–861. [Google Scholar] [CrossRef]
- Ishihara, Y.; Shiba, D.; Shimamoto, N. Enhancement of DMNQ-induced hepatocyte toxicity by cytochrome P450 inhibition. Toxicol. Appl. Pharmacol. 2006, 214, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Ishii, S.; Sakai, Y.; Yamamura, N.; Onishi, Y.; Shimamoto, N. Crucial role of cytochrome P450 in hepatotoxicity induced by 2,3-dimethoxy-1,4-naphthoquinone in rats. J. Appl. Toxicol. 2011, 31, 173–178. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Ma, L.; Gao, T.; Wang, Y. Environmental profiles, hazard identification, and toxicological hallmarks of emerging tire rubber-related contaminants 6PPD and 6PPD-quinone. Environ. Int. 2024, 187, 108677. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yu, X.; Zhu, M.; Wu, Z.; Fu, Z.; Chen, J. Distinct Species-Specific and Toxigenic Metabolic Profiles for 6PPD and 6PPD Quinone by P450 Enzymes: Insights from In Vitro and In Silico Studies. Environ. Sci. Technol. 2024, 58, 14994–15004. [Google Scholar] [CrossRef] [PubMed]
- Le, V.G.; Nguyen, M.K.; Nguyen, H.L.; Lin, C.; Hadi, M.; Hung, N.T.Q.; Hoang, H.G.; Nguyen, K.N.; Tran, H.T.; Hou, D.; et al. A comprehensive review of micro- and nano-plastics in the atmosphere: Occurrence, fate, toxicity, and strategies for risk reduction. Sci. Total Environ. 2023, 904, 166649. [Google Scholar] [CrossRef]
- Luo, D.; Chu, X.; Wu, Y.; Wang, Z.; Liao, Z.; Ji, X.; Ju, J.; Yang, B.; Chen, Z.; Dahlgren, R.; et al. Micro- and nano-plastics in the atmosphere: A review of occurrence, properties and human health risks. J. Hazard. Mater. 2024, 465, 133412. [Google Scholar] [CrossRef]
- Fu, Y.M.; Pang, Q.T.; Ga, S.L.Z.; Wu, P.P.; Wang, Y.J.; Mao, M.; Yuan, Z.; Xu, X.R.; Liu, K.; Wang, X.H.; et al. Modeling atmospheric microplastic cycle by GEOS- Chem: An optimized estimation by a global dataset suggests likely 50 times lower ocean emissions. One Earth 2023, 6, 705–714. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, S.; Wang, Y.; Song, Z.; Xie, L.; Shang, X.; Fu, H.; Yang, X.; Wang, H.; Wu, M.; et al. Quantification and Characterization of Fine Plastic Particles as Considerable Components in Atmospheric Fine Particles. Environ. Sci. Technol. 2024, 58, 4691–4703. [Google Scholar] [CrossRef]
- Hu, M.; Palic, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Shi, X.; Li, Y.; Yu, Y.; Sun, Z.; Duan, J. Size-dependent toxicity of polystyrene microplastics on the gastrointestinal tract: Oxidative stress related-DNA damage and potential carcinogenicity. Sci. Total Environ. 2024, 912, 169514. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Gedik, K.; Gaga, E.O. Atmospheric micro (nano) plastics: Future growing concerns for human health. Air. Qual. Atmos. Health 2023, 16, 233–262. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Wang, Y.; Qu, C.; Yi, W.; Yang, J.; Pan, H.; Zhang, J.; Chen, C.; Bai, C.; Zhou, P.K.; et al. Exposure to polystyrene nanoplastics induces abnormal activation of innate immunity via the cGAS-STING pathway. Ecotoxicol. Environ. Saf. 2024, 275, 116255. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ding, P.; Li, X.; Huang, C.; Li, X.; Chen, X.; Zhang, L.; Qi, J. Environmentally persistent free radicals on photoaged microplastics from disposable plastic cups induce the oxidative stress-associated toxicity. J. Hazard. Mater. 2024, 464, 132990. [Google Scholar] [CrossRef]
- Ishihara, Y.; Kajino, M.; Iwamoto, Y.; Nakane, T.; Nabetani, Y.; Okuda, T.; Kono, M.; Okochi, H. Impact of artificial sunlight aging on the respiratory effects of polyethylene terephthalate microplastics through degradation-mediated terephthalic acid release in male mice. Toxicol. Sci. 2025, 203, 242–252. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef]
- Wei, M.; Liu, H.; Chen, J.; Xu, C.; Li, J.; Xu, P.; Sun, Z. Effects of aerosol pollution on PM(2.5)-associated bacteria in typical inland and coastal cities of northern China during the winter heating season. Environ. Pollut. 2020, 262, 114188. [Google Scholar] [CrossRef]
- Wei, M.; Li, M.; Xu, C.; Xu, P.; Liu, H. Pollution characteristics of bioaerosols in PM2.5 during the winter heating season in a coastal city of northern China. Environ. Sci. Pollut. Res. Int. 2020, 27, 27750–27761. [Google Scholar] [CrossRef]
- Minakami, R.; Sumimotoa, H. Phagocytosis-coupled activation of the superoxide-producing phagocyte oxidase, a member of the NADPH oxidase (nox) family. Int. J. Hematol. 2006, 84, 193–198. [Google Scholar] [CrossRef]
- Samadi, S.; Rietbroek, N.N.; Dwars, R.M.; Jamshidifard, A.R.; Heederik, D.J.; Wouters, I.M. Endotoxin and beta-(1 --> 3)-glucan exposure in poultry and ruminant clinics. J. Environ. Monit. 2011, 13, 3254–3261. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Oberdorster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J.; et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef]
- Lewis, J.; Bench, G.; Myers, O.; Tinner, B.; Staines, W.; Barr, E.; Divine, K.K.; Barrington, W.; Karlsson, J. Trigeminal uptake and clearance of inhaled manganese chloride in rats and mice. Neurotoxicology 2005, 26, 113–123. [Google Scholar] [CrossRef]
- Adams, R.J.; Bray, D. Rapid transport of foreign particles microinjected into crab axons. Nature 1983, 303, 718–720. [Google Scholar] [CrossRef]
- Maher, B.A.; Ahmed, I.A.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.; Torres-Jardon, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [PubMed]
- Vanbrabant, K.; Van Dam, D.; Bongaerts, E.; Vermeiren, Y.; Bove, H.; Hellings, N.; Ameloot, M.; Plusquin, M.; De Deyn, P.P.; Nawrot, T.S. Accumulation of Ambient Black Carbon Particles Within Key Memory-Related Brain Regions. JAMA Netw. Open 2024, 7, e245678. [Google Scholar] [CrossRef]
- Amato-Lourenco, L.F.; Dantas, K.C.; Junior, G.R.; Paes, V.R.; Ando, R.A.; de Oliveira Freitas, R.; da Costa, O.; Rabelo, R.S.; Soares Bispo, K.C.; Carvalho-Oliveira, R.; et al. Microplastics in the Olfactory Bulb of the Human Brain. JAMA Netw. Open 2024, 7, e2440018. [Google Scholar] [CrossRef]
- Garcia, G.J.; Schroeter, J.D.; Kimbell, J.S. Olfactory deposition of inhaled nanoparticles in humans. Inhal. Toxicol. 2015, 27, 394–403. [Google Scholar] [CrossRef]
- Tsuda, A.; Henry, F.S. Editorial: The effect of heterogeneity of the network of alveolar wall tissue on airflow, interstitial flow and lung biology. Front. Netw. Physiol. 2023, 3, 1272172. [Google Scholar] [CrossRef]
- Jain, P.; Nishiguchi, A.; Linz, G.; Wessling, M.; Ludwig, A.; Rossaint, R.; Moller, M.; Singh, S. Reconstruction of Ultra-thin Alveolar-capillary Basement Membrane Mimics. Adv. Biol. 2021, 5, e2000427. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E. Structural basis for some permeability properties of the air--blood barrier. Fed. Proc. 1978, 37, 2471–2478. [Google Scholar] [PubMed]
- Furuyama, A.; Kanno, S.; Kobayashi, T.; Hirano, S. Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch. Toxicol. 2009, 83, 429–437. [Google Scholar] [CrossRef]
- Serra, N.D.; Sundaram, M.V. Transcytosis in the development and morphogenesis of epithelial tissues. EMBO J. 2021, 40, e106163. [Google Scholar] [CrossRef] [PubMed]
- Detampel, P.; Tehranian, S.; Mukherjee, P.; Foret, M.; Fuerstenhaupt, T.; Darbandi, A.; Bogari, N.; Hlasny, M.; Jeje, A.; Olszewski, M.A.; et al. Caveolin-initiated macropinocytosis is required for efficient silica nanoparticles’ transcytosis across the alveolar epithelial barrier. Sci. Rep. 2022, 12, 9474. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Xie, Q.; Wu, D.; Cao, J.; Chen, H.; Gao, M.; Zheng, H.; Liu, X.; Jiang, J.; et al. Using Chicken Embryos to Identify the Key Determinants of Nanoparticles for the Crossing of Air-Blood Barriers. Anal. Chem. 2023, 95, 6009–6019. [Google Scholar] [CrossRef]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schurch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Im Hof, V.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef]
- Rasking, L.; Koshy, P.; Bongaerts, E.; Bove, H.; Ameloot, M.; Plusquin, M.; De Vusser, K.; Nawrot, T.S. Ambient black carbon reaches the kidneys. Environ. Int. 2023, 177, 107997. [Google Scholar] [CrossRef]
- Van Pee, T.; Hogervorst, J.; Dockx, Y.; Witters, K.; Thijs, S.; Wang, C.; Bongaerts, E.; Van Hamme, J.D.; Vangronsveld, J.; Ameloot, M.; et al. Accumulation of Black Carbon Particles in Placenta, Cord Blood, and Childhood Urine in Association with the Intestinal Microbiome Diversity and Composition in Four- to Six-Year-Old Children in the ENVIRONAGE Birth Cohort. Environ. Health Perspect. 2023, 131, 17010. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ejazi, S.A.; Louisthelmy, R.; Maisel, K. Mechanisms of Nanoparticle Transport across Intestinal Tissue: An Oral Delivery Perspective. ACS Nano 2023, 17, 13044–13061. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Seo, E.U.; Hwang, K.S.; Kim, H.; Choi, J.; Kim, H.N. Evaluation of size-dependent uptake, transport and cytotoxicity of polystyrene microplastic in a blood-brain barrier (BBB) model. Nano Converg. 2024, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Kopatz, V.; Wen, K.; Kovacs, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Holloczki, O.; Kenner, L. Micro- and Nanoplastics Breach the Blood-Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef]
- Beltran-Velasco, A.I.; Clemente-Suarez, V.J. Impact of Peripheral Inflammation on Blood-Brain Barrier Dysfunction and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 2440. [Google Scholar] [CrossRef]
- Chen, I.C.; Hsiao, I.L.; Lin, H.C.; Wu, C.H.; Chuang, C.Y.; Huang, Y.J. Influence of silver and titanium dioxide nanoparticles on in vitro blood-brain barrier permeability. Environ. Toxicol. Pharmacol. 2016, 47, 108–118. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Schall, K.; Smith, D.; Pandya, R.; Persidsky, Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J. Neurochem. 2007, 101, 566–576. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Cummins, P.M. The blood-brain barrier endothelium: A target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015, 43, 702–706. [Google Scholar] [CrossRef]
- Pustulka, S.M.; Ling, K.; Pish, S.L.; Champion, J.A. Protein Nanoparticle Charge and Hydrophobicity Govern Protein Corona and Macrophage Uptake. ACS Appl. Mater. Interfaces 2020, 12, 48284–48295. [Google Scholar] [CrossRef]
- Davis, D.L.; Bell, M.L.; Fletcher, T. A look back at the London smog of 1952 and the half century since. Environ. Health Perspect. 2002, 110, A734–A735. [Google Scholar] [CrossRef]
- Yorifuji, T.; Kashima, S.; Suryadhi, M.A.H.; Abudureyimu, K. Acute exposure to sulfur dioxide and mortality: Historical data from Yokkaichi, Japan. Arch. Environ. Occup. Health 2019, 74, 271–278. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Gaston, B.; Hunt, J. Acid stress in the pathology of asthma. J. Allergy Clin. Immunol. 2004, 113, 610–619. [Google Scholar] [CrossRef]
- Ichimonji, I.; Tomura, H.; Mogi, C.; Sato, K.; Aoki, H.; Hisada, T.; Dobashi, K.; Ishizuka, T.; Mori, M.; Okajima, F. Extracellular acidification stimulates IL-6 production and Ca2+ mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L567–L577. [Google Scholar] [CrossRef]
- Sun, Y.W.; El-Bayoumy, K.; Aliaga, C.; Awad, A.S.; Gowda, K.; Amin, S.; Chen, K.M. Tissue Distribution, Excretion and Pharmacokinetics of the Environmental Pollutant Dibenzo[def,p]chrysene in Mice. Chem. Res. Toxicol. 2015, 28, 1427–1433. [Google Scholar] [CrossRef]
- Jin, X.; Hua, Q.; Liu, Y.; Wu, Z.; Xu, D.; Ren, Q.; Zhao, W.; Guo, X. Organ and tissue-specific distribution of selected polycyclic aromatic hydrocarbons (PAHs) in ApoE-KO mouse. Environ. Pollut. 2021, 286, 117219. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Tohyama, C. Embryonic and Postnatal Expression of Aryl Hydrocarbon Receptor mRNA in Mouse Brain. Front. Neuroanat. 2017, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yang, Z.; Shi, R.; Luo, C.; Zhang, Z. Expression of aryl hydrocarbon receptor in rat brain lesions following traumatic brain injury. Diagn. Pathol. 2016, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Garrison, P.M.; Tullis, K.; Aarts, J.M.; Brouwer, A.; Giesy, J.P.; Denison, M.S. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam. Appl. Toxicol. 1996, 30, 194–203. [Google Scholar] [CrossRef]
- Pieterse, B.; Felzel, E.; Winter, R.; van der Burg, B.; Brouwer, A. PAH-CALUX, an optimized bioassay for AhR-mediated hazard identification of polycyclic aromatic hydrocarbons (PAHs) as individual compounds and in complex mixtures. Environ. Sci. Technol. 2013, 47, 11651–11659. [Google Scholar] [CrossRef]
- Chepelev, N.L.; Long, A.S.; Bowers, W.J.; Gagne, R.; Williams, A.; Kuo, B.; Phillips, D.H.; Arlt, V.M.; White, P.A.; Yauk, C.L. Transcriptional profiling of the mouse hippocampus supports an NMDAR-mediated neurotoxic mode of action for benzo[a]pyrene. Environ. Mol. Mutagen. 2016, 57, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Braidy, N.; Vijayan, K.R.; Subash, S.; Guillemin, G.J. Excitotoxicity in the pathogenesis of autism. Neurotox. Res. 2013, 23, 393–400. [Google Scholar] [CrossRef]
- Belousova, M.A.; Tokareva, O.G.; Gorodetskaya, E.A.; Kalenikova, E.I.; Medvedev, O.S. Neuroprotective Effectiveness of Intravenous Ubiquinone in Rat Model of Irreversible Cerebral Ischemia. Bull. Exp. Biol. Med. 2016, 161, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.L.; Vadlamani, N.L.; Pontani, R.B.; Mule, S.J. Some physicochemical and pharmacological properties of morphine-2,3-quinone, the morphine metabolite in the rat brain. J. Pharm. Pharmacol. 1974, 26, 990–992. [Google Scholar] [CrossRef]
- Ma, C.S.; Liu, Y.X.; Han, B.; Bai, M.; Li, D.L.; Meng, S.C.; Zhang, L.Y.; Duan, M.Y.; He, M.T. Long-Term Exposure to Tire-Derived 6-PPD Quinone Causes Neurotoxicity and Neuroinflammation via Inhibition of HTR2A in C57BL/6 Mice. Environ. Sci. Technol. 2025, 59, 1542–1552. [Google Scholar] [CrossRef]
- Bissonnette, E.Y.; Lauzon-Joset, J.F.; Debley, J.S.; Ziegler, S.F. Cross-Talk Between Alveolar Macrophages and Lung Epithelial Cells is Essential to Maintain Lung Homeostasis. Front. Immunol. 2020, 11, 583042. [Google Scholar] [CrossRef]
- Galanos, C.; Luderitz, O.; Rietschel, E.T.; Westphal, O.; Brade, H.; Brade, L.; Freudenberg, M.; Schade, U.; Imoto, M.; Yoshimura, H.; et al. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 1985, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Milici, A.; Talavera, K. TRP Channels as Cellular Targets of Particulate Matter. Int. J. Mol. Sci. 2021, 22, 2783. [Google Scholar] [CrossRef]
- Lv, H.; Yue, J.; Chen, Z.; Chai, S.; Cao, X.; Zhan, J.; Ji, Z.; Zhang, H.; Dong, R.; Lai, K. Effect of transient receptor potential vanilloid-1 on cough hypersensitivity induced by particulate matter 2.5. Life Sci. 2016, 151, 157–166. [Google Scholar] [CrossRef]
- Gu, Y.; Sheng, F.; Gao, M.; Zhang, L.; Hao, S.; Chen, S.; Chen, R.; Xu, Y.; Wu, D.; Han, Y.; et al. Acute and continuous exposure of airborne fine particulate matter (PM2.5): Diverse outer blood-retinal barrier damages and disease susceptibilities. Part. Fibre Toxicol. 2023, 20, 50. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Liu, L.; Wang, Q.; Zeng, J.; Chen, C. PM2.5 exposure perturbs lung microbiome and its metabolic profile in mice. Sci. Total Environ. 2020, 721, 137432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fang, J.; Tang, S.; Deng, F.; Liu, X.; Shen, Y.; Liu, Y.; Kong, F.; Du, Y.; Cui, L.; et al. PM2.5 and Serum Metabolome and Insulin Resistance, Potential Mediation by the Gut Microbiome: A Population-Based Panel Study of Older Adults in China. Environ. Health Perspect. 2022, 130, 27007. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of gaba (gamma-Aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Rothwell, J. Meet the brain neurophysiology. Int. Rev. Neurobiol. 2009, 86, 51–65. [Google Scholar] [CrossRef]
- Peters, D.G.; Connor, J.R. Introduction to cells comprising the nervous system. Adv. Neurobiol. 2014, 9, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Velasco, C.A.; Herbert, G.W.; Lucas, S.N.; Sanchez, B.N.; Cerrato, J.M.; Spilde, M.; Li, Q.Z.; Campen, M.J.; Zychowski, K.E. Mine-site derived particulate matter exposure exacerbates neurological and pulmonary inflammatory outcomes in an autoimmune mouse model. J. Toxicol. Environ. Health A 2021, 84, 503–517. [Google Scholar] [CrossRef]
- Ishihara, Y.; Takemoto, T.; Itoh, K.; Ishida, A.; Yamazaki, T. Dual role of superoxide dismutase 2 induced in activated microglia: Oxidative stress tolerance and convergence of inflammatory responses. J. Biol. Chem. 2015, 290, 22805–22817. [Google Scholar] [CrossRef]
- Choi, J.; Zheng, Q.; Katz, H.E.; Guilarte, T.R. Silica-based nanoparticle uptake and cellular response by primary microglia. Environ. Health Perspect. 2010, 118, 589–595. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, D.; Kim, H.Y.; Jeong, S.W.; Lee, S.G.; Kim, H.C.; Lee, Y.J.; Kwon, M.K.; Hwang, J.S.; Han, J.E.; et al. Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, F.; Yang, Y.; Yang, L.; Wu, Q.; Sun, H.; An, Z.; Li, J.; Wu, H.; Song, J.; et al. PM2.5 exposure upregulates pro-inflammatory protein expression in human microglial cells via oxidant stress and TLR4/NF-kappaB pathway. Ecotoxicol. Environ. Saf. 2024, 277, 116386. [Google Scholar] [CrossRef]
- Wang, B.R.; Shi, J.Q.; Ge, N.N.; Ou, Z.; Tian, Y.Y.; Jiang, T.; Zhou, J.S.; Xu, J.; Zhang, Y.D. PM2.5 exposure aggravates oligomeric amyloid beta-induced neuronal injury and promotes NLRP3 inflammasome activation in an in vitro model of Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Itoh, K.; Ishida, A.; Yamazaki, T. Selective estrogen-receptor modulators suppress microglial activation and neuronal cell death via an estrogen receptor-dependent pathway. J. Steroid. Biochem. Mol. Biol. 2015, 145, 85–93. [Google Scholar] [CrossRef]
- Han, B.; Li, X.; Ai, R.S.; Deng, S.Y.; Ye, Z.Q.; Deng, X.; Ma, W.; Xiao, S.; Wang, J.Z.; Wang, L.M.; et al. Atmospheric particulate matter aggravates cns demyelination through involvement of TLR-4/NF-kB signaling and microglial activation. Elife 2022, 11, e72247. [Google Scholar] [CrossRef] [PubMed]
- Rahmatinia, M.; Mohseni-Bandpei, A.; Khodagholi, F.; Abdollahifar, M.A.; Amouei Torkmahalleh, M.; Hassani Moghaddam, M.; Hopke, P.K.; Ghavimehr, E.; Bazzazpour, S.; Shahsavani, A. Exposure to different PM2.5 extracts induces gliosis and changes behavior in male rats similar to autism spectrum disorders features. Environ. Pollut. 2024, 340, 122804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pei, Y.; Sun, Y.; Yang, X.; Liang, J.; Yin, Z.; Liu, Q.S.; Zhou, Q.; Jiang, G. AhR Agonistic Components in Urban Particulate Matter Regulate Astrocytic Activation and Function. Environ. Sci. Technol. 2024, 58, 4571–4580. [Google Scholar] [CrossRef]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Rentschler, K.M.; Kodavanti, U.P. Mechanistic insights regarding neuropsychiatric and neuropathologic impacts of air pollution. Crit. Rev. Toxicol. 2024, 54, 953–980. [Google Scholar] [CrossRef]
- Marchetti, C. Role of calcium channels in heavy metal toxicity. ISRN Toxicol. 2013, 2013, 184360. [Google Scholar] [CrossRef]
- Frye, R.E.; Cakir, J.; Rose, S.; Delhey, L.; Bennuri, S.C.; Tippett, M.; Palmer, R.F.; Austin, C.; Curtin, P.; Arora, M. Early life metal exposure dysregulates cellular bioenergetics in children with regressive autism spectrum disorder. Transl. Psychiatry 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, Y.; Yin, J.; Zhu, Q.; Liao, C.; Jiang, G. Neurotoxicities induced by micro/nanoplastics: A review focusing on the risks of neurological diseases. J. Hazard. Mater. 2024, 469, 134054. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tan, S.; Xie, D.; Li, H.; Chen, H.; Dang, Y.; Xiang, M. Photoaged microplastics induce neurotoxicity associated with damage to serotonergic, glutamatergic, dopaminergic, and GABAergic neuronal systems in Caenorhabditis elegans. Sci. Total Environ. 2023, 900, 165874. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef]
- Nolt, M.; Connor, J. Implications of Iron in Ferroptosis, Necroptosis, and Pyroptosis as Potential Players in TBI Morbidity and Mortality. ASN Neuro. 2024, 16, 2394352. [Google Scholar] [CrossRef]
- Morris, D.R.; Levenson, C.W. Neurotoxicity of Zinc. Adv. Neurobiol. 2017, 18, 303–312. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gale, J.R.; Reynolds, I.J.; Weiss, J.H.; Aizenman, E. The Multifaceted Roles of Zinc in Neuronal Mitochondrial Dysfunction. Biomedicines 2021, 9, 489. [Google Scholar] [CrossRef]
- Koh, J.Y. Zinc and disease of the brain. Mol. Neurobiol. 2001, 24, 99–106. [Google Scholar] [CrossRef]
- Ciubotariu, D.; Nechifor, M.; Dimitriu, G. Chromium picolinate reduces morphine-dependence in rats, while increasing brain serotonin levels. J. Trace Elem. Med. Biol. 2018, 50, 676–683. [Google Scholar] [CrossRef]
- Fatima, R.; Akhtar, K.; Hossain, M.M.; Ahmad, R. Chromium oxide nanoparticle-induced biochemical and histopathological alterations in the kidneys and brain of Wistar rats. Toxicol. Ind. Health 2017, 33, 911–921. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Tang, Z.; Li, Y.; Hu, L.; Pan, J. The Effects of Copper on Brain Microvascular Endothelial Cells and Claudin Via Apoptosis and Oxidative Stress. Biol. Trace Elem. Res. 2016, 174, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Skalny, A.V.; Lu, R.; Martins, A.C.; Tizabi, Y.; Nekhoroshev, S.V.; Santamaria, A.; Sinitskiy, A.I.; Tinkov, A.A. Mitochondrial pathways of copper neurotoxicity: Focus on mitochondrial dynamics and mitophagy. Front. Mol. Neurosci. 2024, 17, 1504802. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, Y.; Zhao, C.; Zhang, H.; Pu, Y.; Yin, L. Copper induces oxidative stress and apoptosis of hippocampal neuron via pCREB/BDNF/ and Nrf2/HO-1/NQO1 pathway. J. Appl. Toxicol. 2022, 42, 694–705. [Google Scholar] [CrossRef]
- Tuschl, K.; Mills, P.B.; Clayton, P.T. Manganese and the brain. Int. Rev. Neurobiol. 2013, 110, 277–312. [Google Scholar] [CrossRef] [PubMed]
- Nyarko-Danquah, I.; Pajarillo, E.; Digman, A.; Soliman, K.F.A.; Aschner, M.; Lee, E. Manganese Accumulation in the Brain via Various Transporters and Its Neurotoxicity Mechanisms. Molecules 2020, 25, 5880. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, T.; Xue, J.; Yu, P.; Zhang, J.; Wang, J. Nanosized carbon black exposure induces neural injury: Effects on nicotinamide adenine dinucleotide phosphate oxidases and endoplasmic reticulum stress. J. Appl. Toxicol. 2019, 39, 1108–1117. [Google Scholar] [CrossRef]
- Kim, Y.K.; Eom, Y.; Yoon, H.; Lee, Y.; Lee, S.H. Benzo[a]pyrene represses synaptic vesicle exocytosis by inhibiting P/Q-type calcium channels in hippocampal neurons. Ecotoxicol. Environ. Saf. 2023, 263, 115301. [Google Scholar] [CrossRef]
- Abd El Naby, W.S.H.; Zong, C.; Fergany, A.; Ekuban, F.A.; Ahmed, S.; Reda, Y.; Sato, H.; Ichihara, S.; Kubota, N.; Yanagita, S.; et al. Exposure to Benzo[a]pyrene Decreases Noradrenergic and Serotonergic Axons in Hippocampus of Mouse Brain. Int. J. Mol. Sci. 2023, 24, 9895. [Google Scholar] [CrossRef]
- Liang, X.; Tang, Y.; Duan, L.; Cheng, S.; Luo, L.; Cao, X.; Tu, B. Adverse effect of sub-chronic exposure to benzo(a)pyrene and protective effect of butylated hydroxyanisole on learning and memory ability in male Sprague-Dawley rat. J. Toxicol. Sci. 2014, 39, 739–748. [Google Scholar] [CrossRef]
- Xu, P.; Liu, B.; Chen, H.; Wang, H.; Guo, X.; Yuan, J. PAHs as environmental pollutants and their neurotoxic effects. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 283, 109975. [Google Scholar] [CrossRef]
- Nikmanesh, Y.; Mohammadi, M.J.; Yousefi, H.; Mansourimoghadam, S.; Taherian, M. The effect of long-term exposure to toxic air pollutants on the increased risk of malignant brain tumors. Rev. Environ. Health 2023, 38, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Li, D.L.; Wang, F.; Wang, J.P.; He, M.T. Neurotoxicity from long-term exposure to 6-PPDQ: Recent advances. Ecotoxicol. Environ. Saf. 2024, 282, 116689. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Marino, M.; Vandenberg, L.N.; Szychlinska, M.A.; Lamparelli, E.P.; Scalia, F.; Rocca, N.D.; D’Auria, R.; Giovanna Pastorino, G.M.; Porta, G.D.; et al. PLASTAMINATION: Outcomes on the Central Nervous System and Reproduction. Curr. Neuropharmacol. 2024, 22, 1870–1898. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, D.; Wan, Z.; Wei, Z.; Chen, Z.; Wang, Y.; Han, X.; Chen, Y. Exposure to different surface-modified polystyrene nanoparticles caused anxiety, depression, and social deficit in mice via damaging mitochondria in neurons. Sci. Total Environ. 2024, 919, 170739. [Google Scholar] [CrossRef]
- Prust, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Farmen, K.; Tofino-Vian, M.; Iovino, F. Neuronal Damage and Neuroinflammation, a Bridge Between Bacterial Meningitis and Neurodegenerative Diseases. Front. Cell. Neurosci. 2021, 15, 680858. [Google Scholar] [CrossRef]
- Peng, X.; Luo, Z.; He, S.; Zhang, L.; Li, Y. Blood-Brain Barrier Disruption by Lipopolysaccharide and Sepsis-Associated Encephalopathy. Front. Cell. Infect. Microbiol. 2021, 11, 768108. [Google Scholar] [CrossRef]
- Harding, C.F.; Pytte, C.L.; Page, K.G.; Ryberg, K.J.; Normand, E.; Remigio, G.J.; DeStefano, R.A.; Morris, D.B.; Voronina, J.; Lopez, A.; et al. Mold inhalation causes innate immune activation, neural, cognitive and emotional dysfunction. Brain Behav. Immun. 2020, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.; Carpentier, J.; Simonin, O.; Gosset, P.; Platel, A.; Happillon, M.; Alleman, L.Y.; Perdrix, E.; Riffault, V.; Chassat, T.; et al. Oxidative stress and inflammation induced by air pollution-derived PM2.5 persist in the lungs of mice after cessation of their sub-chronic exposure. Environ. Int. 2023, 181, 108248. [Google Scholar] [CrossRef]
- Li, R.; Kou, X.; Xie, L.; Cheng, F.; Geng, H. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ. Sci. Pollut. Res. Int. 2015, 22, 20167–20176. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef] [PubMed]
- Martinez Leo, E.E.; Segura Campos, M.R. Systemic Oxidative Stress: A key Point in Neurodegeneration—A Review. J. Nutr. Health Aging 2019, 23, 694–699. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of Oxidative Stress in Blood-Brain Barrier Disruption and Neurodegenerative Diseases. Antioxidants 2024, 13, 1462. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, N.; Tanaka, M.; Namba, K.; Kawano, S.; Nishimura, S.; Nezu, N.; Nakane, T.; Oguro, A.; Okuda, T.; Itoh, K.; et al. Long-term exposure to urban particulate matter exacerbates mortality after ischemic stroke in mice. J. Toxicol. Sci. 2025, 50, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, H.A.; Lucero, J.; Guyot, A.C.; Herbert, L.M.; McDonald, J.D.; Mabondzo, A.; Lund, A.K. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part. Fibre Toxicol. 2013, 10, 62. [Google Scholar] [CrossRef]
- Nunez, Y.; Boehme, A.K.; Li, M.; Goldsmith, J.; Weisskopf, M.G.; Re, D.B.; Navas-Acien, A.; van Donkelaar, A.; Martin, R.V.; Kioumourtzoglou, M.A. Parkinson’s disease aggravation in association with fine particle components in New York State. Environ. Res. 2021, 201, 111554. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Steenland, K.; Liu, P.; van Donkelaar, A.; Martin, R.V.; Chang, H.H.; Caudle, W.M.; Schwartz, J.; Koutrakis, P.; et al. Long-term effects of PM(2.5) components on incident dementia in the northeastern United States. Innovation 2022, 3, 100208. [Google Scholar] [CrossRef]
- Chiang, K.L.; Lee, J.Y.; Chang, Y.M.; Kuo, F.C.; Huang, C.Y. The effect of weather, air pollution and seasonality on the number of patient visits for epileptic seizures: A population-based time-series study. Epilepsy Behav. 2021, 115, 107487. [Google Scholar] [CrossRef]
- Ying, N.; Tang, Y.; Wang, D.; Fan, J.F.; Zhao, Z.D.; Xue, Z.G.; Liu, Y. Detecting atmospheric oxidation in the PM and ozone multilayer complex network. Environ. Res. Lett. 2024, 19, 104072. [Google Scholar] [CrossRef]
- Li, J.; Liang, L.; Lyu, B.; Cai, Y.S.; Zuo, Y.; Su, J.; Tong, Z. Double trouble: The interaction of PM2.5 and O3 on respiratory hospital admissions. Environ. Pollut. 2023, 338, 122665. [Google Scholar] [CrossRef]
- Szyszkowicz, M.; Stieb, D.M.; Rowe, B.H. Air pollution and daily ED visits for migraine and headache in Edmonton, Canada. Am. J. Emerg. Med. 2009, 27, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Tsai, S.S.; Yang, C.Y. Association between Fine Particulate Air Pollution and Daily Clinic Visits for Migraine in a Subtropical City: Taipei, Taiwan. Int. J. Environ. Res. Public Health 2015, 12, 4697–4708. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Fang, J.; Mittleman, M.A.; Kapral, M.K.; Wellenius, G.A.; Investigators of the Registry of Canadian Stroke Network. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology 2011, 22, 422–431. [Google Scholar] [CrossRef]
- Chiu, H.F.; Yang, C.Y. Short-term effects of fine particulate air pollution on ischemic stroke occurrence: A case-crossover study. J. Toxicol. Environ. Health A 2013, 76, 1188–1197. [Google Scholar] [CrossRef]
- Wing, J.J.; Adar, S.D.; Sanchez, B.N.; Morgenstern, L.B.; Smith, M.A.; Lisabeth, L.D. Ethnic differences in ambient air pollution and risk of acute ischemic stroke. Environ. Res. 2015, 143, 62–67. [Google Scholar] [CrossRef]
- Rhinehart, Z.J.; Kinnee, E.; Essien, U.R.; Saul, M.; Guhl, E.; Clougherty, J.E.; Magnani, J.W. Association of Fine Particulate Matter and Risk of Stroke in Patients With Atrial Fibrillation. JAMA Netw. Open 2020, 3, e2011760. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Zhao, Z.; Xiang, X.; Li, M.; Juan, J.; Song, J.; Cao, Y.; Wang, X.; Chen, L.; et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: A nationwide time-series analysis. PLoS Med. 2018, 15, e1002668. [Google Scholar] [CrossRef]
- Zhang, H.W.; Kok, V.C.; Chuang, S.C.; Tseng, C.H.; Lin, C.T.; Li, T.C.; Sung, F.C.; Wen, C.P.; Hsiung, C.A.; Hsu, C.Y. Long-term ambient hydrocarbons exposure and incidence of ischemic stroke. PLoS ONE 2019, 14, e0225363. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Li, C.Y.; Huang, W.J.; Yu, H.L.; Yang, C.C.; Lu, M.C.; Lang, H.C.; Yan, Y.H. Short-, Mid-, and Long-Term Associations Between PM2.5 and Stroke Incidence in Taiwan. J. Occup. Environ. Med. 2021, 63, 742–751. [Google Scholar] [CrossRef]
- Wang, M.; Han, Y.; Wang, C.J.; Xue, T.; Gu, H.Q.; Yang, K.X.; Liu, H.Y.; Cao, M.; Meng, X.; Jiang, Y.; et al. Short-term effect of PM2.5 on stroke in susceptible populations: A case-crossover study. Int. J. Stroke 2023, 18, 312–321. [Google Scholar] [CrossRef]
- Nzwalo, H.; Guilherme, P.; Nogueira, J.; Felix, C.; Andre, A.; Teles, J.; Mouzinho, M.; Ferreira, F.; Marreiros, A.; Logallo, N.; et al. Fine particulate air pollution and occurrence of spontaneous intracerebral hemorrhage in an area of low air pollution. Clin. Neurol. Neurosurg. 2019, 176, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Sohn, J.; Han, M.; Kang, D.R.; Choi, Y.J.; Kim, H.C.; Suh, I.; Kim, C.; Shin, D.C. Long-term Effects of Cumulative Average PM2.5 Exposure on the Risk of Hemorrhagic Stroke. Epidemiology 2019, 30 (Suppl. 1), S90–S98. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Xia, T.; Qian, Y.; Lu, H.; Cai, R.; Wang, C. Association Between Fine Particulate Matter and Fatal Hemorrhagic Stroke Incidence: A Time Stratified Case-Crossover Study in Shanghai, China. J. Occup. Environ. Med. 2020, 62, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.H.; Park, S.H.; Lim, D.J.; Park, D.H. The relationship between air pollutant levels and aneurysmal subarachnoid hemorrhage. Medicine 2022, 101, e30373. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lin, Y.L.; Chang, W.T.; Lee, C.T.; Chan, C.C. Increasing emergency room visits for stroke by elevated levels of fine particulate constituents. Sci. Total Environ. 2014, 473–474, 446–450. [Google Scholar] [CrossRef]
- Hoffmann, B.; Weinmayr, G.; Hennig, F.; Fuks, K.; Moebus, S.; Weimar, C.; Dragano, N.; Hermann, D.M.; Kalsch, H.; Mahabadi, A.A.; et al. Air quality, stroke, and coronary events: Results of the Heinz Nixdorf Recall Study from the Ruhr Region. Dtsch. Arztebl. Int. 2015, 112, 195–201. [Google Scholar] [CrossRef][Green Version]
- Lin, H.; Tao, J.; Du, Y.; Liu, T.; Qian, Z.; Tian, L.; Di, Q.; Zeng, W.; Xiao, J.; Guo, L.; et al. Differentiating the effects of characteristics of PM pollution on mortality from ischemic and hemorrhagic strokes. Int. J. Hyg. Environ. Health 2016, 219, 204–211. [Google Scholar] [CrossRef]
- Crichton, S.; Barratt, B.; Spiridou, A.; Hoang, U.; Liang, S.F.; Kovalchuk, Y.; Beevers, S.D.; Kelly, F.J.; Delaney, B.; Wolfe, C.D. Associations between exhaust and non-exhaust particulate matter and stroke incidence by stroke subtype in South London. Sci. Total Environ. 2016, 568, 278–284. [Google Scholar] [CrossRef]
- Guan, T.; Xue, T.; Liu, Y.; Zheng, Y.; Fan, S.; He, K.; Zhang, Q. Differential Susceptibility in Ambient Particle-Related Risk of First-Ever Stroke: Findings From a National Case-Crossover Study. Am. J. Epidemiol. 2018, 187, 1001–1009. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, Y.; Wang, L.; Wei, Y.; Lu, R.; Xia, J.; Chai, B.; Liang, X. Ambient fine particulate pollution and daily morbidity of stroke in Chengdu, China. PLoS ONE 2018, 13, e0206836. [Google Scholar] [CrossRef]
- Luo, L.; Dai, Y.; Zhang, F.; Chen, M.; Chen, F.; Qing, F. Time series analysis of ambient air pollution effects on dynamic stroke mortality. Int. J. Health Plann. Manage 2020, 35, 79–103. [Google Scholar] [CrossRef] [PubMed]
- Dirgawati, M.; Hinwood, A.; Nedkoff, L.; Hankey, G.J.; Yeap, B.B.; Flicker, L.; Nieuwenhuijsen, M.; Brunekreef, B.; Heyworth, J. Long-term Exposure to Low Air Pollutant Concentrations and the Relationship with All-Cause Mortality and Stroke in Older Men. Epidemiology 2019, 30 (Suppl. 1), S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Gaines, B.; Kloog, I.; Zucker, I.; Ifergane, G.; Novack, V.; Libruder, C.; Hershkovitz, Y.; Sheffield, P.E.; Yitshak-Sade, M. Particulate Air Pollution Exposure and Stroke among Adults in Israel. Int. J. Environ. Res. Public Health 2023, 20, 1482. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.H.; Sorensen, M.; Hvidtfeldt, U.A.; Brandt, J.; Frohn, L.M.; Ketzel, M.; Christensen, J.H.; Im, U.; Raaschou-Nielsen, O. ‘Source-specific’ air pollution and risk of stroke in Denmark. Int. J. Epidemiol. 2023, 52, 727–737. [Google Scholar] [CrossRef]
- Zanobetti, A.; Dominici, F.; Wang, Y.; Schwartz, J.D. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ. Health 2014, 13, 38. [Google Scholar] [CrossRef]
- Palacios, N.; Fitzgerald, K.C.; Hart, J.E.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. Particulate matter and risk of Parkinson disease in a large prospective study of women. Environ. Health 2014, 13, 80. [Google Scholar] [CrossRef]
- Kirrane, E.F.; Bowman, C.; Davis, J.A.; Hoppin, J.A.; Blair, A.; Chen, H.; Patel, M.M.; Sandler, D.P.; Tanner, C.M.; Vinikoor-Imler, L.; et al. Associations of Ozone and PM2.5 Concentrations With Parkinson’s Disease Among Participants in the Agricultural Health Study. J. Occup. Environ. Med. 2015, 57, 509–517. [Google Scholar] [CrossRef]
- Liu, R.; Young, M.T.; Chen, J.C.; Kaufman, J.D.; Chen, H. Ambient Air Pollution Exposures and Risk of Parkinson Disease. Environ. Health Perspect. 2016, 124, 1759–1765. [Google Scholar] [CrossRef]
- Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Brook, J.R.; Copes, R.; Tu, K.; Goldberg, M.S.; Villeneuve, P.J.; et al. Effects of ambient air pollution on incident Parkinson’s disease in Ontario, 2001 to 2013: A population-based cohort study. Int. J. Epidemiol. 2018, 47, 2038–2048. [Google Scholar] [CrossRef]
- Jo, S.; Kim, Y.J.; Park, K.W.; Hwang, Y.S.; Lee, S.H.; Kim, B.J.; Chung, S.J. Association of NO2 and Other Air Pollution Exposures With the Risk of Parkinson Disease. JAMA Neurol. 2021, 78, 800–808. [Google Scholar] [CrossRef]
- Luo, C.W.; Kuan, Y.H.; Chen, W.Y.; Chen, C.J.; Lin, F.C.; Tsai, S.C. Association between PM2.5 exposure and risk of Parkinson’s disease in individuals with chronic obstructive pulmonary disease in Taiwan: A nested case-control study. Epidemiol. Health 2023, 45, e2023094. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yuan, Y.; White, A.J.; Li, C.; Luo, Z.; D’Aloisio, A.A.; Huang, X.; Kaufman, J.D.; Sandler, D.P.; Chen, H. Air Pollutants and Risk of Parkinson’s Disease among Women in the Sister Study. Environ. Health Perspect. 2024, 132, 17001. [Google Scholar] [CrossRef]
- Krzyzanowski, B.; Mullan, A.F.; Turcano, P.; Camerucci, E.; Bower, J.H.; Savica, R. Air Pollution and Parkinson Disease in a Population-Based Study. JAMA Netw. Open 2024, 7, e2433602. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.; Culqui, D.; Carmona, R.; Ortiz, C.; Diaz, J. Short-term association between environmental factors and hospital admissions due to dementia in Madrid. Environ. Res. 2017, 152, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Ljungman, P.L.S.; Eneroth, K.; Bellander, T.; Rizzuto, D. Association Between Cardiovascular Disease and Long-term Exposure to Air Pollution With the Risk of Dementia. JAMA Neurol. 2020, 77, 801–809. [Google Scholar] [CrossRef]
- Grande, G.; Wu, J.; Ljungman, P.L.S.; Stafoggia, M.; Bellander, T.; Rizzuto, D. Long-Term Exposure to PM2.5 and Cognitive Decline: A Longitudinal Population-Based Study. J. Alzheimers. Dis. 2021, 80, 591–599. [Google Scholar] [CrossRef]
- Wang, X.; Younan, D.; Millstein, J.; Petkus, A.J.; Garcia, E.; Beavers, D.P.; Espeland, M.A.; Chui, H.C.; Resnick, S.M.; Gatz, M.; et al. Association of improved air quality with lower dementia risk in older women. Proc. Natl. Acad. Sci. USA 2022, 119, e2107833119. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Zhou, T.; Wang, M.; Heianza, Y.; Qi, L. Long-term exposure to low-level air pollution, genetic susceptibility and risk of dementia. Int. J. Epidemiol. 2023, 52, 738–748. [Google Scholar] [CrossRef]
- de Crom, T.O.E.; Ginos, B.N.R.; Oudin, A.; Ikram, M.K.; Voortman, T.; Ikram, M.A. Air Pollution and the Risk of Dementia: The Rotterdam Study. J. Alzheimers. Dis. 2023, 91, 603–613. [Google Scholar] [CrossRef]
- Zhang, B.; Weuve, J.; Langa, K.M.; D’Souza, J.; Szpiro, A.; Faul, J.; Mendes de Leon, C.; Gao, J.; Kaufman, J.D.; Sheppard, L.; et al. Comparison of Particulate Air Pollution From Different Emission Sources and Incident Dementia in the US. JAMA Intern. Med. 2023, 183, 1080–1089. [Google Scholar] [CrossRef]
- Elser, H.; Frankland, T.B.; Chen, C.; Tartof, S.Y.; Mayeda, E.R.; Lee, G.S.; Northrop, A.J.; Torres, J.M.; Benmarhnia, T.; Casey, J.A. Wildfire Smoke Exposure and Incident Dementia. JAMA Neurol. 2025, 82, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, Y.; Bartell, S.M.; Corrada, M.M.; Manson, S.M.; O’Connell, J.; Jiang, L. Potential Effects of Long-Term Exposure to Air Pollution on Dementia: A Longitudinal Analysis in American Indians Aged 55 Years and Older. Int. J. Environ. Res. Public Health 2024, 21, 128. [Google Scholar] [CrossRef]
- Oudin, A.; Segersson, D.; Adolfsson, R.; Forsberg, B. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS ONE 2018, 13, e0198283. [Google Scholar] [CrossRef]
- Smargiassi, A.; Sidi, E.A.L.; Robert, L.E.; Plante, C.; Haddad, M.; Gamache, P.; Burnett, R.; Goudreau, S.; Liu, L.; Fournier, M.; et al. Exposure to ambient air pollutants and the onset of dementia in Quebec, Canada. Environ. Res. 2020, 190, 109870. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.M.; Blanco, M.N.; Li, G.; Adar, S.D.; Carone, M.; Szpiro, A.A.; Kaufman, J.D.; Larson, T.V.; Larson, E.B.; Crane, P.K.; et al. Fine Particulate Matter and Dementia Incidence in the Adult Changes in Thought Study. Environ. Health Perspect. 2021, 129, 87001. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, Q.; Wang, Y.; Hao, H.; Zhang, H.; Schwartz, J.; Amini, H.; van Donkelaar, A.; Martin, R.V.; Steenland, K.; et al. Incident dementia and long-term exposure to constituents of fine particle air pollution: A national cohort study in the United States. Proc. Natl. Acad. Sci. USA 2023, 120, e2211282119. [Google Scholar] [CrossRef]
- Shi, L.; Steenland, K.; Li, H.; Liu, P.; Zhang, Y.; Lyles, R.H.; Requia, W.J.; Ilango, S.D.; Chang, H.H.; Wingo, T.; et al. A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat. Commun. 2021, 12, 6754. [Google Scholar] [CrossRef]
- Jung, C.R.; Lin, Y.T.; Hwang, B.F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. J. Alzheimers. Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef]
- Li, R.L.; Ho, Y.C.; Luo, C.W.; Lee, S.S.; Kuan, Y.H. Influence of PM2.5 Exposure Level on the Association between Alzheimer’s Disease and Allergic Rhinitis: A National Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 3357. [Google Scholar] [CrossRef]
- Younan, D.; Petkus, A.J.; Widaman, K.F.; Wang, X.; Casanova, R.; Espeland, M.A.; Gatz, M.; Henderson, V.W.; Manson, J.E.; Rapp, S.R.; et al. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 2020, 143, 289–302. [Google Scholar] [CrossRef]
- Shaffer, R.M.; Li, G.; Adar, S.D.; Dirk Keene, C.; Latimer, C.S.; Crane, P.K.; Larson, E.B.; Kaufman, J.D.; Carone, M.; Sheppard, L. Fine Particulate Matter and Markers of Alzheimer’s Disease Neuropathology at Autopsy in a Community-Based Cohort. J. Alzheimers. Dis. 2021, 79, 1761–1773. [Google Scholar] [CrossRef]
- Yang, L.; Wan, W.; Yu, C.; Xuan, C.; Zheng, P.; Yan, J. Associations between PM(2.5) exposure and Alzheimer’s Disease prevalence Among elderly in eastern China. Environ. Health 2022, 21, 119. [Google Scholar] [CrossRef]
- Alhasan, D.M.; Larson, G.; Lohman, M.C.; Cai, B.; LaPorte, F.B.; Miller, M.C.; Jackson, W.B., 2nd; MacNell, N.S.; Hirsch, J.A.; Jackson, C.L. Features of the Physical and Social Neighborhood Environment and Neighborhood-Level Alzheimer’s Disease and Related Dementia in South Carolina. Environ. Health Perspect. 2024, 132, 27013. [Google Scholar] [CrossRef]
- Park, S.Y.; Han, J.; Kim, S.H.; Suk, H.W.; Park, J.E.; Lee, D.Y. Impact of Long-Term Exposure to Air Pollution on Cognitive Decline in Older Adults Without Dementia. J. Alzheimers. Dis. 2022, 86, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.Y.; Huang, L.Y.; Cheng, G.R.; Liu, D.; Hu, F.F.; Zhang, J.J.; Han, G.B.; Liu, X.C.; Wang, J.Y.; Zhou, J.; et al. Association Between Long-Term Exposure to Ambient Air Pollution and the Risk of Mild Cognitive Impairment in a Chinese Urban Area: A Case-Control Study. J. Alzheimers. Dis. 2024, 98, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Su, B.; Cui, F.P.; Li, D.; Ma, Y.; Xing, M.; Tang, L.; Wang, J.; Tian, Y.; Zheng, X. Long-Term Exposure to PM2.5 Constituents, Genetic Susceptibility, and Incident Dementia: A Prospective Cohort Study among 0.2 Million Older Adults. Environ. Sci. Technol. 2025, 59, 4493–4504. [Google Scholar] [CrossRef]
- Rhew, S.H.; Kravchenko, J.; Lyerly, H.K. Exposure to low-dose ambient fine particulate matter PM2.5 and Alzheimer’s disease, non-Alzheimer’s dementia, and Parkinson’s disease in North Carolina. PLoS ONE 2021, 16, e0253253. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.T.; Johansen, M.S.; Ravnskjaer, L.; Andersen, K.K.; Brauner, E.V.; Loft, S.; Ketzel, M.; Becker, T.; Brandt, J.; Hertel, O.; et al. Long-term exposure to ambient air pollution and incidence of brain tumours: The Danish Nurse Cohort. Neurotoxicology 2016, 55, 122–130. [Google Scholar] [CrossRef]
- Weichenthal, S.; Olaniyan, T.; Christidis, T.; Lavigne, E.; Hatzopoulou, M.; Van Ryswyk, K.; Tjepkema, M.; Burnett, R. Within-city Spatial Variations in Ambient Ultrafine Particle Concentrations and Incident Brain Tumors in Adults. Epidemiology 2020, 31, 177–183. [Google Scholar] [CrossRef]
- Xu, C.; Fan, Y.N.; Kan, H.D.; Chen, R.J.; Liu, J.H.; Li, Y.F.; Zhang, Y.; Ji, A.L.; Cai, T.J. The Novel Relationship between Urban Air Pollution and Epilepsy: A Time Series Study. PLoS ONE 2016, 11, e0161992. [Google Scholar] [CrossRef]
- Bergamaschi, R.; Monti, M.C.; Trivelli, L.; Mallucci, G.; Gerosa, L.; Pisoni, E.; Montomoli, C. PM(2.5) exposure as a risk factor for multiple sclerosis. An ecological study with a Bayesian mapping approach. Environ. Sci. Pollut. Res. Int. 2021, 28, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Scartezzini, A.; Tateo, F.; Perini, P.; Benacchio, L.; Ermani, M.; Ferro, A.; Cadaldini, M.; Piccinno, M.G.; Colledan, L.; Freddi, N.; et al. Association of Multiple Sclerosis with PM2.5 levels. Further evidence from the highly polluted area of Padua Province, Italy. Mult. Scler. Relat. Disord. 2021, 48, 102677. [Google Scholar] [CrossRef]
- Kazemi Moghadam, V.; Dickerson, A.S.; Shahedi, F.; Bazrafshan, E.; Seyedhasani, S.N.; Sarmadi, M. Association of the global distribution of multiple sclerosis with ultraviolet radiation and air pollution: An ecological study based on GBD data. Environ. Sci. Pollut. Res. Int. 2021, 28, 17802–17811. [Google Scholar] [CrossRef] [PubMed]
- Teekaput, C.; Rachbundit, C.; Wantaneeyawong, C.; Teekaput, K.; Thiankhaw, K. Impact of air pollution on the clinical exacerbation of central demyelinating disease: A 10-year data from the Northern Thailand MS and NMOSD registry. Mult. Scler. Relat. Disord. 2025, 94, 106266. [Google Scholar] [CrossRef]
- Worthington, M.A.; Petkova, E.; Freudenreich, O.; Cather, C.; Holt, D.; Bello, I.; Diminich, E.; Tang, Y.; Ardekani, B.A.; Zeng, B.; et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr. Res. 2020, 218, 63–69. [Google Scholar] [CrossRef]
- Gladka, A.; Zatonski, T.; Rymaszewska, J. Association between the long-term exposure to air pollution and depression. Adv. Clin. Exp. Med. 2022, 31, 1139–1152. [Google Scholar] [CrossRef]
- Lawrence, W.R.; Yang, M.; Zhang, C.; Liu, R.Q.; Lin, S.; Wang, S.Q.; Liu, Y.; Ma, H.; Chen, D.H.; Zeng, X.W.; et al. Association between long-term exposure to air pollution and sleep disorder in Chinese children: The Seven Northeastern Cities study. Sleep 2018, 41, zsy122. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Longcore, T.; Benbow, J.; Chung, N.T.; Chau, K.; Wang, S.S.; Lacey, J.V.; Franklin, M. Environmental Influences on Sleep in the California Teachers Study Cohort. Am. J. Epidemiol. 2022, 191, 1532–1539. [Google Scholar] [CrossRef]
- Cai, J.; Shen, Y.; Zhao, Y.; Meng, X.; Niu, Y.; Chen, R.; Quan, G.; Li, H.; Groeger, J.A.; Du, W.; et al. Early-Life Exposure to PM2.5 and Sleep Disturbances in Preschoolers from 551 Cities of China. Am. J. Respir. Crit. Care Med. 2023, 207, 602–612. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, M.S.; Kim, D.J.; Uhm, T.W.; Yi, S.B.; Han, J.H.; Lee, I.W. Effects of meteorological factor and air pollution on sudden sensorineural hearing loss using the health claims data in Busan, Republic of Korea. Am. J. Otolaryngol. 2019, 40, 393–399. [Google Scholar] [CrossRef]
- Cheng, C.G.; Chen, Y.H.; Yen, S.Y.; Lin, H.C.; Lin, H.C.; Chou, K.R.; Cheng, C.A. Air Pollution Exposure and the Relative Risk of Sudden Sensorineural Hearing Loss in Taipei. Int. J. Environ. Res. Public Health 2022, 19, 6144. [Google Scholar] [CrossRef] [PubMed]
- Guarneros, M.; Lopez-Rivera, C.; Gonsebatt, M.E.; Alcaraz-Zubeldia, M.; Hummel, T.; Schriever, V.A.; Valdez, B.; Hudson, R. Metal-containing Particulate Matter and Associated Reduced Olfactory Identification Ability in Children from an Area of High Atmospheric Exposure in Mexico City. Chem. Senses 2020, 45, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, I.A.; Rizzuto, D.; Grande, G.; Bellander, T.; Laukka, E.J. Environmental Air Pollution and Olfactory Decline in Aging. Environ. Health Perspect. 2022, 130, 27005. [Google Scholar] [CrossRef]
- Zhang, Z.; Rowan, N.R.; Pinto, J.M.; London, N.R.; Lane, A.P.; Biswal, S.; Ramanathan, M., Jr. Exposure to Particulate Matter Air Pollution and Anosmia. JAMA Netw. Open 2021, 4, e2111606. [Google Scholar] [CrossRef] [PubMed]
- Nunez, Y.; Balalian, A.; Parks, R.M.; He, M.Z.; Hansen, J.; Raaschou-Nielsen, O.; Ketzel, M.; Khan, J.; Brandt, J.; Vermeulen, R.; et al. Exploring Relevant Time Windows in the Association Between PM2.5 Exposure and Amyotrophic Lateral Sclerosis: A Case-Control Study in Denmark. Am. J. Epidemiol. 2023, 192, 1499–1508. [Google Scholar] [CrossRef]
- Lee, K.S.; Min, W.K.; Choi, Y.J.; Jin, S.; Park, K.H.; Kim, S. The Effect of Maternal Exposure to Air Pollutants and Heavy Metals during Pregnancy on the Risk of Neurological Disorders Using the National Health Insurance Claims Data of South Korea. Medicina 2023, 59, 951. [Google Scholar] [CrossRef]
- Chuang, B.R.; Lee, C.C.; Lin, Y.T.; Jung, C.R.; Chen, M.L.; Hwang, B.F. Association between preconception and early pregnancy exposure to fine particulate matter and nervous system anomalies: A nested case-control study. Eur. J. Epidemiol. 2025, 40, 71–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Talarico, R.; Qiu, X.; Schwartz, J.; Fell, D.B.; Oskoui, M.; Lavigne, E.; Messerlian, C. Prenatal Exposure to Ambient Air Pollution and Cerebral Palsy. JAMA Netw. Open 2024, 7, e2420717. [Google Scholar] [CrossRef]
- Chang, Y.T.; Jung, C.R.; Chang, Y.C.; Chuang, B.R.; Chen, M.L.; Hwang, B.F. Prenatal and postnatal exposure to PM2.5 and the risk of tic disorders. Paediatr. Perinat. Epidemiol. 2023, 37, 191–200. [Google Scholar] [CrossRef]
- Che, L.; Li, Y.; Gan, C. Effect of short-term exposure to ambient air particulate matter on incidence of delirium in a surgical population. Sci. Rep. 2017, 7, 15461. [Google Scholar] [CrossRef]
- Wilker, E.H.; Preis, S.R.; Beiser, A.S.; Wolf, P.A.; Au, R.; Kloog, I.; Li, W.; Schwartz, J.; Koutrakis, P.; DeCarli, C.; et al. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke 2015, 46, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Wang, X.; Wellenius, G.A.; Serre, M.L.; Driscoll, I.; Casanova, R.; McArdle, J.J.; Manson, J.E.; Chui, H.C.; Espeland, M.A. Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Ann. Neurol. 2015, 78, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Wellenius, G.A.; Boyle, L.D.; Wilker, E.H.; Sorond, F.A.; Coull, B.A.; Koutrakis, P.; Mittleman, M.A.; Lipsitz, L.A. Ambient fine particulate matter alters cerebral hemodynamics in the elderly. Stroke 2013, 44, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
| Classification | Components | Action | Reference |
|---|---|---|---|
| Water-soluble ions | Sulfur oxides | (Do not affect the brain) | |
| Nitrogen oxides | (Do not affect the brain) | ||
| Metals | Fe | ROS generation | [144] |
| Ferroptosis | [145] | ||
| Zn | Excitotoxicity | [146] | |
| Mitochondrial dysfunction | [147] | ||
| Oxidative stress | [148] | ||
| Neuroinflammation | [148] | ||
| Cr | Neurotransmitter disruption | [149] | |
| Oxidative stress | [150] | ||
| Cu | BBB disruption | [151] | |
| Mitochondrial dysfunction | [152] | ||
| Oxidative stress | [153] | ||
| Mn | Impaired neurotransmission | [154] | |
| Oxidative stress | [155] | ||
| Neuroinflammation | [155] | ||
| Carbons | Black carbon | ER stress | [156] |
| Oxidative stress | [156] | ||
| PAHs | Benzo[a]pyrene | Synaptic dysfunction | [157] |
| Neuroinflammation | [158] | ||
| Oxidative stress | [159] | ||
| DNA methylation | [160] | ||
| Tumorigenesis | [161] | ||
| Benzo[k]fluoranthene | Oxidative stress | [160] | |
| DNA methylation | [160] | ||
| Quinones | 1,2-naphthoquinone | (No literature) | |
| 9,10-phenanthrenequinone | (No literature) | ||
| 6PPDQ | Oxidative stress | [162] | |
| Neuroinflammation | [162] | ||
| Apoptosis | [162] | ||
| Plastics | MPs/NPs | Neuroinflammation | [163] |
| Oxidative stress | [163] | ||
| Mitochondrial dysfunction | [164] | ||
| Neurotransmitter disruption | [165] | ||
| Bioaerosols | Bacteria | BBB disruption | [166] |
| LPS | BBB disruption | [167] | |
| Mold | Innate immune activation | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, Y.; Tanaka, M.; Nezu, N.; Ishihara, N.; Oguro, A.; Vogel, C.F.A. Pathways to the Brain: Impact of Fine Particulate Matter Components on the Central Nervous System. Antioxidants 2025, 14, 730. https://doi.org/10.3390/antiox14060730
Ishihara Y, Tanaka M, Nezu N, Ishihara N, Oguro A, Vogel CFA. Pathways to the Brain: Impact of Fine Particulate Matter Components on the Central Nervous System. Antioxidants. 2025; 14(6):730. https://doi.org/10.3390/antiox14060730
Chicago/Turabian StyleIshihara, Yasuhiro, Miki Tanaka, Naoyuki Nezu, Nami Ishihara, Ami Oguro, and Christoph F. A. Vogel. 2025. "Pathways to the Brain: Impact of Fine Particulate Matter Components on the Central Nervous System" Antioxidants 14, no. 6: 730. https://doi.org/10.3390/antiox14060730
APA StyleIshihara, Y., Tanaka, M., Nezu, N., Ishihara, N., Oguro, A., & Vogel, C. F. A. (2025). Pathways to the Brain: Impact of Fine Particulate Matter Components on the Central Nervous System. Antioxidants, 14(6), 730. https://doi.org/10.3390/antiox14060730






