Impact of Diquat on the Intestinal Health and the Composition and Function of the Gut Microbiome
Abstract
1. Introduction
2. Toxic Effects of DQ on the Intestine and the Gut Barrier
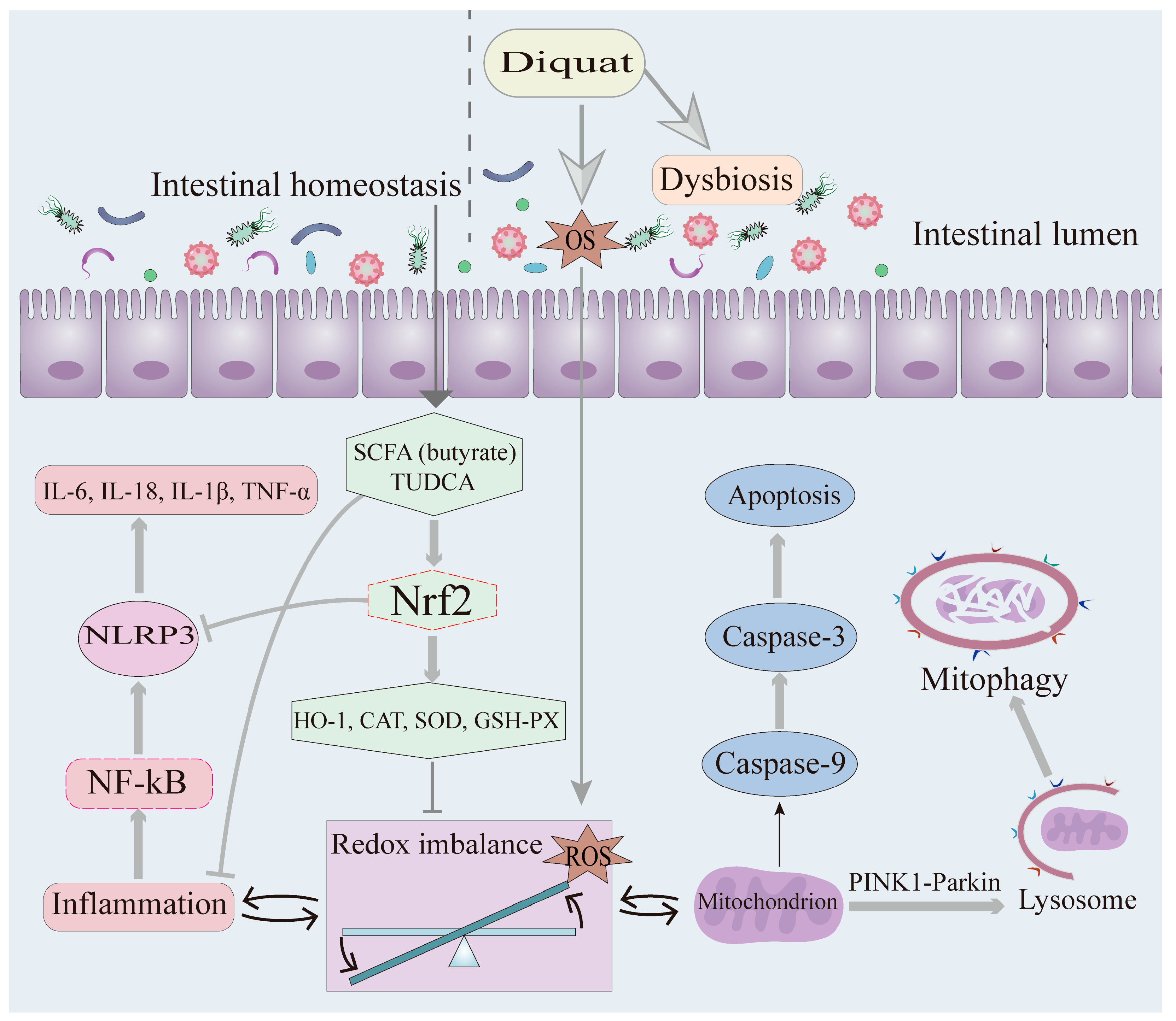
2.1. Oxidative Stress and Inflammation
2.2. The Role of OS and Inflammation in DQ-Induced Intestinal Injury
2.3. Mitophagy
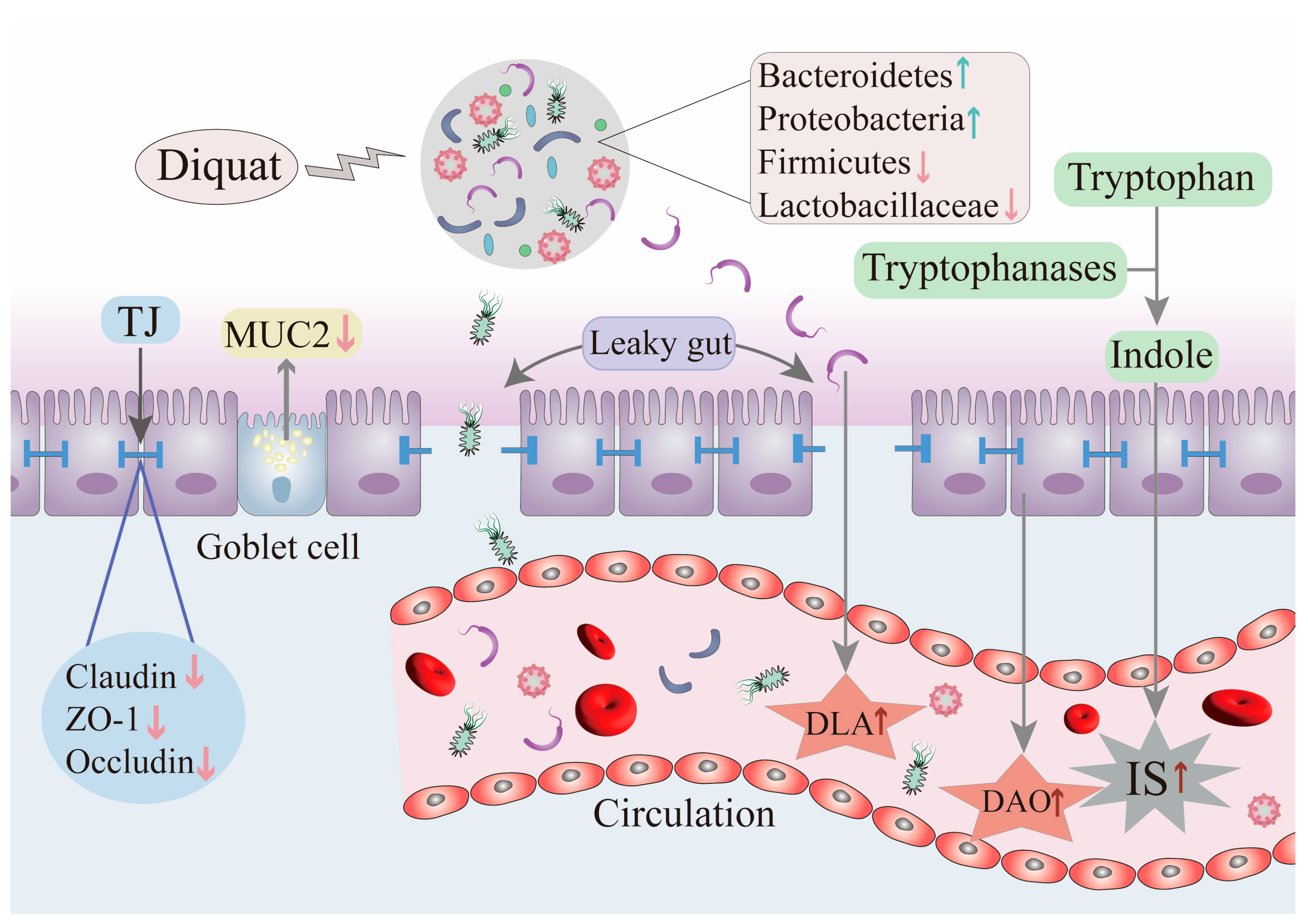
2.4. Impact of DQ on Intestinal Barrier Integrity
3. Effects of DQ on the Composition and Function of the Intestinal Microbiome
4. Impact of DQ on Metabolites
5. Several Therapies to Alleviate the Toxic Effects of DQ
6. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | Bile acid |
| CAT | Catalase |
| CUR | Curcumin |
| CD | Crypt depth |
| CKD | Chronic kidney disease |
| DAO | Diamine oxidase |
| DQ | Diquat |
| DLA | D-lactate |
| DEGs | Differentially expressed genes |
| EVs | Extracellular vesicles |
| EUPS | Eucommia ulmoides polysaccharide |
| F/B | Firmicutes/Bacteroidetes |
| GSH-PX | Glutathione peroxidase |
| HCA | Hyocholic acid |
| HDCA | Hyodeoxycholic acid |
| HT | Hydroxytyrosol |
| HO-1 | Heme oxygenase-1 |
| Ht | Hydrogenobacter thermophilus strain |
| IECs | Intestinal epithelial cells |
| IS | Indoxyl sulfate |
| LAB | Lactic acid bacteria |
| LAB-EVs | Lactic acid bacteria derived extracellular vesicles |
| LPS | Lipopolysaccharides |
| MDA | Malondialdehyde |
| MUC2 | Mucin 2 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NLRP3 | NLR family pyrin domain containing 3 |
| NF-κB | Nuclear factor kappa-B |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| OS | Oxidative stress |
| PAE | Periplaneta americana extract |
| PBA | Primary bile acid |
| PQ | Paraquat |
| QG | Quercetagetin |
| RNS | Reactive nitrogen species |
| RES | Resveratrol |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SBA | Secondary bile acid |
| SOD | Superoxide dismutase |
| SeNPs | Selenium nanoparticles |
| TUDCA | Tauroursodeoxycholic acid |
| TMAO | Trimethylamine-N-oxide |
| TJs | Tight junctions |
| TDO | Tryptophan 2,3-dioxygenase |
| VH | Villus height |
| ZO-1 | Zonula occludens-1 |
References
- Zhou, J.-N.; Lu, Y.-Q. Lethal Diquat Poisoning Manifests as Acute Central Nervous System Injury and Circulatory Failure: A Retrospective Cohort Study of 50 Cases. eClinicalMedicine 2022, 52, 101609. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, F.; Wang, L. Imaging Findings and Toxicological Mechanisms of Nervous System Injury Caused by Diquat. Mol. Neurobiol. 2024, 61, 9272–9283. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, N.; Carvalho, F.; Dinis-Oliveira, R. Human and Experimental Toxicology of Diquat Poisoning: Toxicokinetics, Mechanisms of Toxicity, Clinical Features, and Treatment. Hum. Exp. Toxicol. 2018, 37, 1131–1160. [Google Scholar] [CrossRef]
- Pateiro-Moure, M.; Martínez-Carballo, E.; Arias-Estévez, M.; Simal-Gándara, J. Determination of Quaternary Ammonium Herbicides in Soils. Comparison of Digestion, Shaking and Microwave-Assisted Extractions. J. Chromatogr. A 2008, 1196–1197, 110–116. [Google Scholar] [CrossRef]
- Pizzutti, I.R.; Vela, G.M.E.; de Kok, A.; Scholten, J.M.; Dias, J.V.; Cardoso, C.D.; Concenço, G.; Vivian, R. Determination of Paraquat and Diquat: LC-MS Method Optimization and Validation. Food Chem. 2016, 209, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Su, Y.; Lin, R.; Lin, F.; Shang, P.; Hussain, R.; Shi, D. Effects of Acute Diquat Poisoning on Liver Mitochondrial Apoptosis and Autophagy in Ducks. Front. Vet. Sci. 2021, 8, 727766. [Google Scholar] [CrossRef]
- Rose, M.S.; Crabtree, H.C.; Fletcher, K.; Wyatt, I. Biochemical Effects of Diquat and Paraquat. Disturbance of the Control of Corticosteroid Synthesis in Rat Adrenal and Subsequent Effects on the Control of Liver Glycogen Utilization. Biochem. J. 1974, 138, 437–443. [Google Scholar] [CrossRef]
- Li, W.; Wei, H.; Li, N.; Li, S.; Liu, Y.; Liu, R.; Zou, W.; Hu, P.; Zhang, Z.; Wang, C. Rapid Identification and Quantification of Diquat in Biological Fluids within 30 s Using a Portable Raman Spectrometer. Biosens. Bioelectron. 2023, 225, 115083. [Google Scholar] [CrossRef]
- Wageed, M.; Mahdy, H.M.; Kalaba, M.H.; Kelany, M.A.; Soliman, M. Development of LC-MS/MS Analytical Method for the Rapid Determination of Diquat in Water and Beverages. Food Chem. 2024, 438, 137869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, H.; Qian, J.; Sun, L.; Zong, C.; Zhang, J.; Yuan, B. High Expression of 3-Hydroxy-3-Methylglutaryl-CoA Synthase 2 (HMGCS2) Associated with Diquat-Induced Damage. Ecotoxicol. Environ. Saf. 2024, 281, 116623. [Google Scholar] [CrossRef]
- Koch, R.E.; Hill, G.E. An Assessment of Techniques to Manipulate Oxidative Stress in Animals. Funct. Ecol. 2017, 31, 9–21. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Lin, Q.; Yu, M.; Lu, J.; Feng, J.; Hu, C. Effects of Curcumin on Mitochondrial Function, Endoplasmic Reticulum Stress, and Mitochondria-Associated Endoplasmic Reticulum Membranes in the Jejunum of Oxidative Stress Piglets. J. Agric. Food Chem. 2022, 70, 8974–8985. [Google Scholar] [CrossRef]
- Yu, G.; Jian, T.; Cui, S.; Shi, L.; Kan, B.; Jian, X. Acute Diquat Poisoning Resulting in Toxic Encephalopathy: A Report of Three Cases. Clin. Toxicol. 2022, 60, 647–650. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Li, J.; Mao, Z.; Qian, J.; Zong, C.; Sun, H.; Yuan, B. Multi-Omics Analyses Reveal the Mechanisms of Early Stage Kidney Toxicity by Diquat. Toxics 2023, 11, 184. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, L.; Cao, W.; Fang, T.; Jia, G.; Chen, X.; Zhao, H.; Wu, C.; Wang, J. Changes in the Metabolome of Rats after Exposure to Arginine and N-Carbamylglutamate in Combination with Diquat, a Compound That Causes Oxidative Stress, Assessed by 1H NMR Spectroscopy. Food Funct. 2016, 7, 964–974. [Google Scholar] [CrossRef]
- Sharma, T.; Natesh, N.S.; Pothuraju, R.; Batra, S.K.; Rachagani, S. Gut Microbiota: A Non-Target Victim of Pesticide-Induced Toxicity. Gut Microbes 2023, 15, 2187578. [Google Scholar] [CrossRef]
- Sosnowski, K.; Przybyłkowski, A. Ethanol-Induced Changes to the Gut Microbiome Compromise the Intestinal Homeostasis: A Review. Gut Microbes 2024, 16, 2393272. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine Improves Gut Barrier Integrity and Gut Microbiota Function in Diet-Induced Obese Mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Triantos, C.; Maroulis, I.; Gogos, C. The Role of the Gut Barrier Function in Health and Disease. Gastroenterol. Res. 2018, 11, 261–263. [Google Scholar] [CrossRef]
- Huo, J.; Wu, Z.; Sun, W.; Wang, Z.; Wu, J.; Huang, M.; Wang, B.; Sun, B. Protective Effects of Natural Polysaccharides on Intestinal Barrier Injury: A Review. J. Agric. Food Chem. 2022, 70, 711–735. [Google Scholar] [CrossRef]
- Song, D.; Cheng, Y.; Li, X.; Wang, F.; Lu, Z.; Xiao, X.; Wang, Y. Biogenic Nanoselenium Particles Effectively Attenuate Oxidative Stress-Induced Intestinal Epithelial Barrier Injury by Activating the Nrf2 Antioxidant Pathway. ACS Appl. Mater. Interfaces 2017, 9, 14724–14740. [Google Scholar] [CrossRef]
- Willott, E.; Balda, M.S.; Fanning, A.S.; Jameson, B.; Van Itallie, C.; Anderson, J.M. The Tight Junction Protein ZO-1 Is Homologous to the Drosophila Discs-Large Tumor Suppressor Protein of Septate Junctions. Proc. Natl. Acad. Sci. USA 1993, 90, 7834–7838. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota-Liver Axis in Hepatic Disease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef]
- Coutry, N.; Gasmi, I.; Herbert, F.; Jay, P. Mechanisms of Intestinal Dysbiosis: New Insights into Tuft Cell Functions. Gut Microbes 2024, 16, 2379624. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The Gut Microbiota and Host Health: A New Clinical Frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Wong, C.C.; Yu, J. Gut Microbiota in Colorectal Cancer Development and Therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef]
- Mancin, L.; Wu, G.D.; Paoli, A. Gut Microbiota–Bile Acid–Skeletal Muscle Axis. Trends Microbiol. 2023, 31, 254–269. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Wu, S.; Ren, Z.; Liu, G.; Wu, J. Synergistic Protective Effect of Konjac Mannan Oligosaccharides and Bacillus Subtilis on Intestinal Epithelial Barrier Dysfunction in Caco-2 Cell Model and Mice Model of Lipopolysaccharide Stimulation. Front. Immunol. 2021, 12, 696148. [Google Scholar] [CrossRef]
- Vergauwen, H.; Prims, S.; Degroote, J.; Wang, W.; Casteleyn, C.; van Cruchten, S.; de Smet, S.; Michiels, J.; van Ginneken, C. In Vitro Investigation of Six Antioxidants for Pig Diets. Antioxidants 2016, 5, 41. [Google Scholar] [CrossRef]
- Tian, J.; Jiang, Q.; Bao, X.; Yang, F.; Li, Y.; Sun, H.; Yao, K.; Yin, Y. Plant-Derived Squalene Supplementation Improves Growth Performance and Alleviates Acute Oxidative Stress-Induced Growth Retardation and Intestinal Damage in Piglets. Anim. Nutr. 2023, 15, 386–398. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress—The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxidative Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are Antioxidants Helpful for Disease Prevention? Res. Pharm. Sci. 2010, 5, 1–8. [Google Scholar]
- Li, W.; Liu, C.; Yang, Q.; Zhou, Y.; Liu, M.; Shan, H. Oxidative Stress and Antioxidant Imbalance in Ovulation Disorder in Patients with Polycystic Ovary Syndrome. Front. Nutr. 2022, 9, 1018674. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Lightfoot, T.J.; Skibola, C.F.; Smith, A.G.; Forrest, M.S.; Adamson, P.J.; Morgan, G.J.; Bracci, P.M.; Roman, E.; Smith, M.T.; Holly, E.A. Polymorphisms in the Oxidative Stress Genes, Superoxide Dismutase, Glutathione Peroxidase and Catalase and Risk of Non-Hodgkin’s Lymphoma. Haematologica 2006, 91, 1222–1227. [Google Scholar]
- Halliwell, B. Biochemistry of Oxidative Stress. Biochem. Soc. Trans. 2007, 35 Pt 5, 1147–1150. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Liu, S.; Zhuang, Y.; Yang, H.; Li, Y.; Chen, S.; Wang, L.; Yin, L.; Yao, Y.; et al. Tannic Acid Modulates Intestinal Barrier Functions Associated with Intestinal Morphology, Antioxidative Activity, and Intestinal Tight Junction in a Diquat-Induced Mouse Model. RSC Adv. 2019, 9, 31988–31998. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The Intestinal Barrier as an Emerging Target in the Toxicological Assessment of Mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, W.; Yin, J.; Xia, S.; Jiang, Y.; Ge, Q.; Liu, J.; Wang, M.; Hou, Z.; Bai, Y.; et al. Biomineralized ZIF-8 Encapsulating SOD from Hydrogenobacter Thermophilus: Maintaining Activity in the Intestine and Alleviating Intestinal Oxidative Stress. Small 2024, 20, 2402812. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Dietary Carcinogens and Anticarcinogens. Oxygen Radicals and Degenerative Diseases. Science 1983, 221, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Dou, X.; Yan, S.; Zhang, B.; Xu, C. Biogenic Selenium Nanoparticles Synthesized by Lactobacillus Casei ATCC 393 Alleviate Diquat-Induced Intestinal Barrier Dysfunction in C57BL/6 Mice through Their Antioxidant Activity. Food Funct. 2020, 11, 3020–3031. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin Ameliorates Oxidative Stress-Induced Intestinal Barrier Injury and Mitochondrial Damage by Promoting Parkin Dependent Mitophagy through AMPK-TFEB Signal Pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, M.; Chao, X.; Zhang, C.; Yang, H.; Chen, J.; Zhao, C.; Zhou, B. Acidifiers Attenuate Diquat-Induced Oxidative Stress and Inflammatory Responses by Regulating NF-κB/MAPK/COX-2 Pathways in IPEC-J2 Cells. Antioxidants 2022, 11, 2002. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhao, S.; Xu, W.; Li, Y.; Zhao, P.; Wang, D.; Cheng, H.; Ke, Y.; Zhang, X. Oxidative Stress-Induced FABP5 S-Glutathionylation Protects against Acute Lung Injury by Suppressing Inflammation in Macrophages. Nat. Commun. 2021, 12, 7094. [Google Scholar] [CrossRef]
- Ghelli Luserna di Rora’, A.; Iacobucci, I.; Martinelli, G. The Cell Cycle Checkpoint Inhibitors in the Treatment of Leukemias. J. Hematol. Oncol. 2017, 10, 77. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, Dietary and Postprandial Oxidative Stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef]
- Awad, W.A.; Aschenbach, J.R.; Setyabudi, F.M.C.S.; Razzazi-Fazeli, E.; Böhm, J.; Zentek, J. In Vitro Effects of Deoxynivalenol on Small Intestinal D-Glucose Uptake and Absorption of Deoxynivalenol across the Isolated Jejunal Epithelium of Laying Hens. Poult. Sci. 2007, 86, 15–20. [Google Scholar] [CrossRef]
- Zhao, G.-P.; Wang, X.-Y.; Li, J.-W.; Wang, R.; Ren, F.-Z.; Pang, G.-F.; Li, Y.-X. Imidacloprid Increases Intestinal Permeability by Disrupting Tight Junctions. Ecotoxicol. Env. Saf. 2021, 222, 112476. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric Oxide and Superoxide in Inflammation and Immune Regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Lorenzen, I.; Mullen, L.; Bekeschus, S.; Hanschmann, E.-M. Redox Regulation of Inflammatory Processes Is Enzymatically Controlled. Oxidative Med. Cell Longev. 2017, 2017, 8459402. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, S.; Zhang, Q.; Shen, Z.; Feng, J.; Hong, Q.; Lu, J.; Xie, F.; Peng, Y.; Hu, C. Dietary Tributyrin Attenuates Intestinal Inflammation, Enhances Mitochondrial Function, and Induces Mitophagy in Piglets Challenged with Diquat. J. Agric. Food Chem. 2019, 67, 1409–1417. [Google Scholar] [CrossRef]
- Wang, P.; Song, C.-Y.; Lu, X.; Zhou, J.-N.; Lin, L.-Y.; Li, T.; Zhang, Q.; Lu, Y.-Q. Diquat Exacerbates Oxidative Stress and Neuroinflammation by Blocking the Autophagic Flux of Microglia in the Hippocampus. Ecotoxicol. Environ. Saf. 2024, 286, 117188. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Yadav, U.C.S.; Reddy, A.B.M.; Saxena, A.; Tammali, R.; Shoeb, M.; Ansari, N.H.; Bhatnagar, A.; Petrash, M.J.; Srivastava, S.; et al. Aldose Reductase Inhibition Suppresses Oxidative Stress-Induced Inflammatory Disorders. Chem. Biol. Interact. 2011, 191, 330–338. [Google Scholar] [CrossRef]
- Wei, H.; Chen, G.; Wang, R.-J.; Peng, J. Oregano Essential Oil Decreased Susceptibility to Oxidative Stress-Induced Dysfunction of Intestinal Epithelial Barrier in Rats. J. Funct. Food. 2015, 18, 1191–1199. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Ding, B.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Wu, X.; Yin, Y.; Wu, G. Dietary Alpha-Ketoglutarate Supplementation Ameliorates Intestinal Injury in Lipopolysaccharide-Challenged Piglets. Amino Acids 2010, 39, 555–564. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, W.; Liu, G.; Fang, T.; Wu, X.; Jia, G.; Chen, X.; Zhao, H.; Wang, J.; Wu, C.; et al. Arginine, N-Carbamylglutamate, and Glutamine Exert Protective Effects against Oxidative Stress in Rat Intestine. Anim. Nutr. 2016, 2, 242–248. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Q.; Li, P.; Shi, P.; Liu, C.; Wang, W.; Huang, K.; Yin, Y.; Huang, P. The Water Extract of Ampelopsis Grossedentata Alleviates Oxidative Stress and Intestinal Inflammation. Antioxidants 2023, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Liu, Z.; Yin, J.; Gao, J.; He, L.; Wang, C.; Hou, R.; He, X.; Wang, G.; Li, T.; et al. D-Galactose Induces Chronic Oxidative Stress and Alters Gut Microbiota in Weaned Piglets. Front. Physiol. 2021, 12, 634283. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Cheng, Y.; Su, W.; Wang, C.; Lu, Z.; Jin, M.; Wang, F.; Wang, Y. Pediococcus Pentosaceus ZJUAF-4 Relieves Oxidative Stress and Restores the Gut Microbiota in Diquat-Induced Intestinal Injury. Appl. Microbiol. Biotechnol. 2021, 105, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jia, H.; Li, J.; Yu, M.; Yang, Y.; Tian, D.; Zhang, H.; Zou, Z. Cecal Gut Microbiota and Metabolites Might Contribute to the Severity of Acute Myocardial Ischemia by Impacting the Intestinal Permeability, Oxidative Stress, and Energy Metabolism. Front. Microbiol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Houde, V.; Grenier, D.; Chandad, F. Protective Effects of Grape Seed Proanthocyanidins against Oxidative Stress Induced by Lipopolysaccharides of Periodontopathogens. J. Periodontol. 2006, 77, 1371–1379. [Google Scholar] [CrossRef]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The Immune System Bridges the Gut Microbiota with Systemic Energy Homeostasis: Focus on TLRs, Mucosal Barrier, and SCFAs. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Lee, H.H.; Molla, M.N.; Cantor, C.R.; Collins, J.J. Bacterial Charity Work Leads to Population-Wide Resistance. Nature 2010, 467, 82–85. [Google Scholar] [CrossRef]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary Grape Seed Proanthocyanidins (GSPs) Improve Weaned Intestinal Microbiota and Mucosal Barrier Using a Piglet Model. Oncotarget 2016, 7, 80313–80326. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-Oxide Induces Inflammation and Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells via Activating ROS-TXNIP-NLRP3 Inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Vašíček, O.; Lojek, A.; Číž, M. Serotonin and Its Metabolites Reduce Oxidative Stress in Murine RAW264.7 Macrophages and Prevent Inflammation. J. Physiol. Biochem. 2020, 76, 49–60. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as Potential Antioxidants: A Systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Park, A.; Koh, H.C. NF-κB/mTOR-Mediated Autophagy Can Regulate Diquat-Induced Apoptosis. Arch. Toxicol. 2019, 93, 1239–1253. [Google Scholar] [CrossRef]
- Wen, C.; Li, F.; Guo, Q.; Zhang, L.; Duan, Y.; Wang, W.; Li, J.; He, S.; Chen, W.; Yin, Y. Protective Effects of Taurine against Muscle Damage Induced by Diquat in 35 Days Weaned Piglets. J. Anim. Sci. Biotechnol. 2020, 11, 56. [Google Scholar] [CrossRef]
- Osburn, W.O.; Wakabayashi, N.; Misra, V.; Nilles, T.; Biswal, S.; Trush, M.A.; Kensler, T.W. Nrf2 Regulates an Adaptive Response Protecting against Oxidative Damage Following Diquat-Mediated Formation of Superoxide Anion. Arch. Biochem. Biophys. 2006, 454, 7–15. [Google Scholar] [CrossRef]
- Zha, A.; Yan, J.; Li, J.; Wang, J.; Qi, M.; Liao, P.; Chun, G.; Yin, Y. Melatonin Increased Antioxidant Capacity to Ameliorate Growth Retardation and Intestinal Epithelial Barrier Dysfunction in Diquat-Challenged Piglets. J. Sci. Food Agric. 2024, 104, 2262–2271. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and Oxidative Stress: A Review. Med. Sci. Monit. 2004, 10, RA141–RA147. [Google Scholar]
- Xie, L.-W.; Cai, S.; Zhao, T.-S.; Li, M.; Tian, Y. Green Tea Derivative (-)-Epigallocatechin-3-Gallate (EGCG) Confers Protection against Ionizing Radiation-Induced Intestinal Epithelial Cell Death Both in Vitro and in Vivo. Free Radic. Biol. Med. 2020, 161, 175–186. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The Mitochondrially Targeted Antioxidant MitoQ Protects the Intestinal Barrier by Ameliorating Mitochondrial DNA Damage via the Nrf2/ARE Signaling Pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Zhu, Q.; Liu, H.; Wang, J.; Lu, W. Melatonin Inhibits the Apoptosis of Rooster Leydig Cells by Suppressing Oxidative Stress via AKT-Nrf2 Pathway Activation. Free Radic. Biol. Med. 2020, 160, 1–12. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Yuan, J.; Meng, H.; Liu, Y.; Wang, L.; Zhu, Q.; Wang, Z.; Liu, H.; Zhang, K.; Zhao, J.; Li, W.; et al. Bacillus Amyloliquefaciens Attenuates the Intestinal Permeability, Oxidative Stress and Endoplasmic Reticulum Stress: Transcriptome and Microbiome Analyses in Weaned Piglets. Front. Microbiol. 2024, 15, 1362487. [Google Scholar] [CrossRef]
- Cao, S.; Wu, H.; Wang, C.; Zhang, Q.; Jiao, L.; Lin, F.; Hu, C.H. Diquat-Induced Oxidative Stress Increases Intestinal Permeability, Impairs Mitochondrial Function, and Triggers Mitophagy in Piglets. J. Anim. Sci. 2018, 96, 1795–1805. [Google Scholar] [CrossRef]
- Yin, J.; Liu, M.; Ren, W.; Duan, J.; Yang, G.; Zhao, Y.; Fang, R.; Chen, L.; Li, T.; Yin, Y. Effects of Dietary Supplementation with Glutamate and Aspartate on Diquat-Induced Oxidative Stress in Piglets. PLoS ONE 2015, 10, e0122893. [Google Scholar] [CrossRef]
- Lv, M.; Yu, B.; Mao, X.B.; Zheng, P.; He, J.; Chen, D.W. Responses of Growth Performance and Tryptophan Metabolism to Oxidative Stress Induced by Diquat in Weaned Pigs. Animal 2012, 6, 928–934. [Google Scholar] [CrossRef]
- Zheng, P.; Yu, B.; Lv, M.; Chen, D. Effects of Oxidative Stress Induced by Diquat on Arginine Metabolism of Postweaning Pigs. Asian-Australasions J. Anim. Sci. 2010, 23, 98–105. [Google Scholar] [CrossRef]
- Bafana, A.; Dutt, S.; Kumar, S.; Ahuja, P.S. Superoxide Dismutase: An Industrial Perspective. Crit. Rev. Biotechnol. 2011, 31, 65–76. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.-Y.; Kim, J.H.; Kang, J.-E.; Park, M.H.; Kim, E.-J.; et al. SOD1 Suppresses Pro-Inflammatory Immune Responses by Protecting against Oxidative Stress in Colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Liu, P.; Luo, G.; Dodson, M.; Schmidlin, C.J.; Wei, Y.; Kerimoglu, B.; Ooi, A.; Chapman, E.; Garcia, J.G.; Zhang, D.D. The NRF2-LOC344887 Signaling Axis Suppresses Pulmonary Fibrosis. Redox Biol. 2021, 38, 101766. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Si, X.; Jin, Y.; Jiang, D.; Dai, Z.; Wu, Z. Quercetin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Nutrients 2021, 13, 375. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, Toxicity, and Clinical Applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Wen, X.; Tang, S.; Wan, F.; Zhong, R.; Chen, L.; Zhang, H. The PI3K/Akt-Nrf2 Signaling Pathway and Mitophagy Synergistically Mediate Hydroxytyrosol to Alleviate Intestinal Oxidative Damage. Int. J. Biol. Sci. 2024, 20, 4258–4276. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, Characteristics and Antioxidant Activity of Polysaccharides and Proteins-Capped Selenium Nanoparticles Synthesized by Lactobacillus Casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The Role of Mitochondria in NLRP3 Inflammasome Activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, X.; Pi, S.; Chang, J.; Dou, X.; Yan, S.; Song, X.; Chen, Y.; Zeng, X.; Zhu, L.; et al. Dietary Supplementation with Biogenic Selenium Nanoparticles Alleviate Oxidative Stress-Induced Intestinal Barrier Dysfunction. npj Sci. Food 2022, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Hussain, T.; Tan, B.; Liu, Y.; Ji, P.; Yin, Y. The Evaluation of Antioxidant and Anti-Inflammatory Effects of Eucommia Ulmoides Flavones Using Diquat-Challenged Piglet Models. Oxidative Med. Cell Longev. 2017, 2017, 8140962. [Google Scholar] [CrossRef]
- Ma, T.Y.; Boivin, M.A.; Ye, D.; Pedram, A.; Said, H.M. Mechanism of TNF-α Modulation of Caco-2 Intestinal Epithelial Tight Junction Barrier: Role of Myosin Light-Chain Kinase Protein Expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G422–G430. [Google Scholar] [CrossRef]
- Al-Sadi, R.M.; Ma, T.Y. IL-1beta Causes an Increase in Intestinal Epithelial Tight Junction Permeability. J. Immunol. 2007, 178, 4641–4649. [Google Scholar] [CrossRef]
- Chen, K.; Tang, Y.; Lan, L.; Li, M.; Lu, Z. Autophagy Mediated FTH1 Degradation Activates Gasdermin E Dependent Pyroptosis Contributing to Diquat Induced Kidney Injury. Food Chem. Toxicol. 2024, 184, 114411. [Google Scholar] [CrossRef]
- Wen, X.; Tang, L.; Zhong, R.; Liu, L.; Chen, L.; Zhang, H. Role of Mitophagy in Regulating Intestinal Oxidative Damage. Antioxidants 2023, 12, 480. [Google Scholar] [CrossRef]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.B.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a Source of Reactive Oxygen and Nitrogen Species: From Molecular Mechanisms to Human Health. Antioxid. Redox Signal. 2013, 18, 2029–2074. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-Induced ROS Release: An Update and Review. Biochim. Biophys. Acta 2006, 1757, 509–517. [Google Scholar] [CrossRef]
- Sweetman, E.; Kleffmann, T.; Edgar, C.; de Lange, M.; Vallings, R.; Tate, W. A SWATH-MS Analysis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Peripheral Blood Mononuclear Cell Proteomes Reveals Mitochondrial Dysfunction. J. Transl. Med. 2020, 18, 365. [Google Scholar] [CrossRef]
- De Gaetano, A.; Gibellini, L.; Zanini, G.; Nasi, M.; Cossarizza, A.; Pinti, M. Mitophagy and Oxidative Stress: The Role of Aging. Antioxidants 2021, 10, 794. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Ji, S.; Jia, P.; Chen, Y.; Li, Y.; Wang, T. Resveratrol and Its Derivative Pterostilbene Attenuate Oxidative Stress-Induced Intestinal Injury by Improving Mitochondrial Redox Homeostasis and Function via SIRT1 Signaling. Free Radic. Biol. Med. 2021, 177, 1–14. [Google Scholar] [CrossRef]
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host Mitochondria Influence Gut Microbiome Diversity: A Role for ROS. Sci. Signal. 2019, 12, eaaw3159. [Google Scholar] [CrossRef]
- Ballard, J.W.O.; Towarnicki, S.G. Mitochondria, the Gut Microbiome and ROS. Cell. Signal. 2020, 75, 109737. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Mark Evers, B.; Chung, D.H. Oxidative Stress-Induced Intestinal Epithelial Cell Apoptosis Is Mediated by P38 MAPK. Biochem. Biophys. Res. Commun. 2006, 350, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wei, Y.; Hua, H.; Jing, X.; Zhu, H.; Xiao, K.; Zhao, J.; Liu, Y. Polyphenols Sourced from Ilex Latifolia Thunb. Relieve Intestinal Injury via Modulating Ferroptosis in Weanling Piglets under Oxidative Stress. Antioxidants 2022, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Alan, P.; Vandevoorde, K.R.; Joshi, B.; Cardoen, B.; Gao, G.; Mohammadzadeh, Y.; Hamarneh, G.; Nabi, I.R. Basal Gp78-Dependent Mitophagy Promotes Mitochondrial Health and Limits Mitochondrial ROS. Cell. Mol. Life Sci. 2022, 79, 565. [Google Scholar] [CrossRef]
- Saita, S.; Shirane, M.; Nakayama, K.I. Selective Escape of Proteins from the Mitochondria during Mitophagy. Nat. Commun. 2013, 4, 1410. [Google Scholar] [CrossRef]
- Kim, I.; Lemasters, J.J. Mitophagy Selectively Degrades Individual Damaged Mitochondria after Photoirradiation. Antioxid. Redox Signal. 2011, 14, 1919–1928. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, P.; Zhou, C.; Hu, Y.; Duan, S.; Gu, M.; Jiang, D.; Wang, Y.; Deng, Z.; Chen, J.; et al. Oxidative Stress-Induced Mitophagy Is Suppressed by the miR-106b-93-25 Cluster in a Protective Manner. Cell Death Dis. 2021, 12, 209. [Google Scholar] [CrossRef]
- Wang, Y.; Nartiss, Y.; Steipe, B.; McQuibban, G.A.; Kim, P.K. ROS-Induced Mitochondrial Depolarization Initiates PARK2/PARKIN-Dependent Mitochondrial Degradation by Autophagy. Autophagy 2012, 8, 1462–1476. [Google Scholar] [CrossRef]
- Chen, T.; Kim, C.Y.; Kaur, A.; Lamothe, L.; Shaikh, M.; Keshavarzian, A.; Hamaker, B.R. Dietary Fibre-Based SCFA Mixtures Promote Both Protection and Repair of Intestinal Epithelial Barrier Function in a Caco-2 Cell Model. Food Funct. 2017, 8, 1166–1173. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Yang, J.; Pei, G.; Sun, X.; Xiao, Y.; Miao, C.; Zhou, L.; Wang, B.; Yang, L.; Yu, M.; Zhang, Z.-S.; et al. RhoB Affects Colitis through Modulating Cell Signaling and Intestinal Microbiome. Microbiome 2022, 10, 149. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The Interplay between Gut Microbiota, Short-Chain Fatty Acids, and Implications for Host Health and Disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Gonçalves, P.; Gregório, I.; Catarino, T.A.; Martel, F. The Effect of Oxidative Stress upon the Intestinal Epithelial Uptake of Butyrate. Eur. J. Pharmacol. 2013, 699, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fang, Z.; Che, L.; Xu, S.; Wu, D.; Wu, C.; Wu, X. Use of Sodium Butyrate as an Alternative to Dietary Fiber: Effects on the Embryonic Development and Anti-Oxidative Capacity of Rats. PLoS ONE 2014, 9, e97838. [Google Scholar] [CrossRef]
- Bai, Y.; Mansell, T.J. Production and Sensing of Butyrate in a Probiotic Escherichia coli Strain. Int. J. Mol. Sci. 2020, 21, 3615. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Sun, J.; Gong, Q.; Ma, H.; Kan, X.; Cao, Y.; Wang, J.; Fu, S. Butyrate Alleviates Oxidative Stress by Regulating NRF2 Nuclear Accumulation and H3K9/14 Acetylation via GPR109A in Bovine Mammary Epithelial Cells and Mammary Glands. Free Radic. Biol. Med. 2020, 152, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Jia, Y.; Yang, S.; Zhao, N.; Hu, Y.; Hong, J.; Gao, S.; Zhao, R. Sodium Butyrate Protects against High-Fat Diet-Induced Oxidative Stress in Rat Liver by Promoting Expression of Nuclear Factor E2-Related Factor 2. Br. J. Nutr. 2019, 122, 400–410. [Google Scholar] [CrossRef]
- Dicks, L.M.T. How Important Are Fatty Acids in Human Health and Can They Be Used in Treating Diseases? Gut Microbes 2024, 16, 2420765. [Google Scholar] [CrossRef]
- Nielsen, D.S.G.; Jensen, B.B.; Theil, P.K.; Nielsen, T.S.; Knudsen, K.E.B.; Purup, S. Effect of Butyrate and Fermentation Products on Epithelial Integrity in a Mucus-Secreting Human Colon Cell Line. J. Funct. Food. 2018, 40, 9–17. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review Article: The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Galvez, J.; Rodríguez-Cabezas, M.E.; Zarzuelo, A. Effects of Dietary Fiber on Inflammatory Bowel Disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, G.M. Effect of Sodium Butyrate on Autophagy and Apoptosis in Chinese Hamster Ovary Cells. Biotechnol. Prog. 2012, 28, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F.; et al. Butyrate Enhances Mitochondrial Function during Oxidative Stress in Cell Lines from Boys with Autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Gou, F.; Lin, Q.; Tu, X.; Zhu, J.; Li, X.; Chen, S.; Hu, C. Hesperidin Alleviated Intestinal Barrier Injury, Mitochondrial Dysfunction, and Disorder of Endoplasmic Reticulum Mitochondria Contact Sites under Oxidative Stress. J. Agric. Food Chem. 2024, 72, 16276–16286. [Google Scholar] [CrossRef]
- Wijtten, P.J.A.; van der Meulen, J.; Verstegen, M.W.A. Intestinal Barrier Function and Absorption in Pigs after Weaning: A Review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Tang, X.; Ma, W.; Zhan, W.; Wang, X.; Dong, H.; Zhao, H.; Yang, L.; Ji, C.; Han, Q.; Ji, C.; et al. Internal Biliary Drainage Superior to External Biliary Drainage in Improving Gut Mucosa Barrier Because of Goblet Cells and Mucin-2 up-Regulation. Biosci. Rep. 2018, 38, BSR20171241. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Wen, X.; Wan, F.; Zhong, R.; Chen, L.; Zhang, H. Hydroxytyrosol Alleviates Intestinal Oxidative Stress by Regulating Bile Acid Metabolism in a Piglet Model. Int. J. Mol. Sci. 2024, 25, 5590. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The Inner of the Two Muc2 Mucin-Dependent Mucus Layers in Colon Is Devoid of Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Van Der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Rao, R. Oxidative Stress-Induced Disruption of Epithelial and Endothelial Tight Junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, X.; Wang, X.; Wu, H.; Zhu, H.; Hou, Y.; Dai, B.; Liu, X.; Liu, Y. Glutamate Alleviates Intestinal Injury, Maintains mTOR and Suppresses TLR4 and NOD Signaling Pathways in Weanling Pigs Challenged with Lipopolysaccharide. Sci. Rep. 2018, 8, 15124. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic Acid Improves Intestinal Barrier Functions by Suppressing Mucosa Inflammation and Improving Antioxidant Capacity in Weaned Pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miao, L.; Yao, Y.; Wu, W.; Wu, X.; Gong, C.; Qiu, L.; Chen, J. Dexmedetomidine Ameliorate CLP-Induced Rat Intestinal Injury via Inhibition of Inflammation. Mediat. Inflamm. 2015, 2015, 918361. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, L.; Gan, Z.; Xiong, H.; Yu, C.; Du, H.; Wang, Y. High Therapeutic Efficacy of Cathelicidin-WA against Postweaning Diarrhea via Inhibiting Inflammation and Enhancing Epithelial Barrier in the Intestine. Sci. Rep. 2016, 6, 25679. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Huang, Z.; Yan, H.; et al. Chlorogenic Acid Attenuates Oxidative Stress-Induced Intestinal Mucosa Disruption in Weaned Pigs. Front. Vet. Sci. 2022, 9, 806253. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Anestis, V.; Filioussis, G.; Papanastasiou, D.K.; Bartzanas, T.; Papaioannou, N.; Tzora, A.; Skoufos, I. Effects of Protease Addition and Replacement of Soybean Meal by Corn Gluten Meal on the Growth of Broilers and on the Environmental Performances of a Broiler Production System in Greece. PLoS ONE 2017, 12, e0169511. [Google Scholar] [CrossRef]
- Elkouby-Naor, L.; Ben-Yosef, T. Functions of Claudin Tight Junction Proteins and Their Complex Interactions in Various Physiological Systems. Int. Rev. Cell Mol. Biol. 2010, 279, 1–32. [Google Scholar] [CrossRef]
- Apostolova, D.; Apostolov, G.; Moten, D.; Batsalova, T.; Dzhambazov, B. Claudin-12: Guardian of the Tissue Barrier or Friend of Tumor Cells. Tissue Barriers 2024, 12, 2387408. [Google Scholar] [CrossRef]
- Zheng, P.; Yu, B.; He, J.; Yu, J.; Mao, X.; Luo, Y.; Luo, J.; Huang, Z.; Tian, G.; Zeng, Q.; et al. Arginine Metabolism and Its Protective Effects on Intestinal Health and Functions in Weaned Piglets under Oxidative Stress Induced by Diquat. Br. J. Nutr. 2017, 117, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Wu, Z.; Ji, Y.; Wang, B.; Dai, Z.; Wu, G. L-Glutamate Enhances Barrier and Antioxidative Functions in Intestinal Porcine Epithelial Cells. J. Nutr. 2015, 145, 2258–2264. [Google Scholar] [CrossRef]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and Vitamin E Together Improve Intestinal Epithelial Barrier Function and Alleviate Oxidative Stress in Heat-Stressed Pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Guo, Q.; Wang, W.; Duan, Y.; Zhang, L.; Li, J.; He, S.; Chen, W.; Li, F. Taurine Alleviates Intestinal Injury by Mediating Tight Junction Barriers in Diquat-Challenged Piglet Models. Front. Physiol. 2020, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Duckworth, C.A.; Watson, A.J.M.; Frey, M.R.; Miguel, J.C.; Burkitt, M.D.; Sutton, R.; Hughes, K.R.; Hall, L.J.; Caamaño, J.H.; et al. A Mouse Model of Pathological Small Intestinal Epithelial Cell Apoptosis and Shedding Induced by Systemic Administration of Lipopolysaccharide. Dis. Model. Mech. 2013, 6, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Song, D.; Zong, X.; Zhang, H.; Wang, T.; Yi, H.; Luan, C.; Wang, Y. Antimicrobial Peptide Cathelicidin-BF Prevents Intestinal Barrier Dysfunction in a Mouse Model of Endotoxemia. Int. Immunopharmacol. 2015, 25, 141–147. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, X.; Han, F.; Jiang, Q.; Rong, Y.; Song, D.; Wang, Y. Cathelicidin-BF, a Novel Antimicrobial Peptide from Bungarus Fasciatus, Attenuates Disease in a Dextran Sulfate Sodium Model of Colitis. Mol. Pharm. 2015, 12, 1648–1661. [Google Scholar] [CrossRef]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl2 Interacting Protein, Mediates Cytochrome c Release from Mitochondria in Response to Activation of Cell Surface Death Receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef]
- Zhou, N.; Tian, Y.; Liu, W.; Tu, B.; Gu, T.; Xu, W.; Zou, K.; Lu, L. Effects of Quercetin and Coated Sodium Butyrate Dietary Supplementation in Diquat-Challenged Pullets. Anim. Biosci. 2022, 35, 1434–1443. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Ning, M.-X.; Chen, D.-K.; Ma, W.-T. Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef]
- Kulkarni, D.H.; Rusconi, B.; Floyd, A.N.; Joyce, E.L.; Talati, K.B.; Kousik, H.; Alleyne, D.; Harris, D.L.; Garnica, L.; McDonough, R.; et al. Gut Microbiota Induces Weight Gain and Inflammation in the Gut and Adipose Tissue Independent of Manipulations in Diet, Genetics, and Immune Development. Gut Microbes 2023, 15, 2284240. [Google Scholar] [CrossRef]
- Strati, F.; Pujolassos, M.; Burrello, C.; Giuffrè, M.R.; Lattanzi, G.; Caprioli, F.; Troisi, J.; Facciotti, F. Antibiotic-Associated Dysbiosis Affects the Ability of the Gut Microbiota to Control Intestinal Inflammation upon Fecal Microbiota Transplantation in Experimental Colitis Models. Microbiome 2021, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The Role of the Gut Microbiome in Systemic Inflammatory Disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Samul, D.; Worsztynowicz, P.; Leja, K.; Grajek, W. Beneficial and Harmful Roles of Bacteria from the Clostridium Genus. Acta Biochim. Pol. 2013, 60, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Jones-Hall, Y.L.; Kozik, A.; Nakatsu, C. Ablation of Tumor Necrosis Factor Is Associated with Decreased Inflammation and Alterations of the Microbiota in a Mouse Model of Inflammatory Bowel Disease. PLoS ONE 2015, 10, e0119441. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Tang, L.; Zhou, Y.; Wang, Q.; Gong, L.; Ni, J.; Li, W. Probiotic Bacillus Alleviates Oxidative Stress-Induced Liver Injury by Modulating Gut-Liver Axis in a Rat Model. Antioxidants 2022, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial Diversity and Community Composition of Caecal Microbiota in Commercial and Indigenous Indian Chickens Determined Using 16s rDNA Amplicon Sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Tian, Y.; Zhang, M.; Cheng, K.; Zhang, X.; Zhou, M.; Hui, M.; Zhang, Y. Extraction, Characterization, and Antioxidant Activity of Eucommia Ulmoides Polysaccharides. Molecules 2024, 29, 4793. [Google Scholar] [CrossRef]
- Wang, H.; Ren, P.; Mang, L.; Shen, N.; Chen, J.; Zhang, Y. In Vitro Fermentation of Novel Microwave-Synthesized Non-Digestible Oligosaccharides and Their Impact on the Composition and Metabolites of Human Gut Microbiota. J. Funct. Food. 2019, 55, 156–166. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Fu, Q.; Tan, Z.; Shi, L.; Xun, W. Resveratrol Attenuates Diquat-Induced Oxidative Stress by Regulating Gut Microbiota and Metabolome Characteristics in Piglets. Front. Microbiol. 2021, 12, 695155. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, B.; Chen, D.; Zheng, P.; Luo, Y.; Huang, Z.; Luo, J.; Mao, X.; Yu, J.; He, J. Changes of Porcine Gut Microbiota in Response to Dietary Chlorogenic Acid Supplementation. Appl. Microbiol. Biotechnol. 2019, 103, 8157–8168. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhong, R.; Zhang, S.; Wang, M.; Wen, X.; Yi, B.; Zhao, Y.; Chen, L.; Zhang, H. Hydroxytyrosol Attenuates Diquat-Induced Oxidative Stress by Activating Nrf2 Pathway and Modulating Colonic Microbiota in Mice. J. Nutr. Biochem. 2023, 113, 109256. [Google Scholar] [CrossRef]
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.-X.; He, J.; Wu, S. Dietary Protocatechuic Acid Ameliorates Inflammation and Up-Regulates Intestinal Tight Junction Proteins by Modulating Gut Microbiota in LPS-Challenged Piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Li, S.; Fang, B.; Duan, W.; Zhang, J.; Song, J.; Wang, M. Vinegar Extract Ameliorates Alcohol-Induced Liver Damage Associated with the Modulation of Gut Microbiota in Mice. Food Funct. 2020, 11, 2898–2909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Li, Q.-M.; Zha, X.-Q.; Luo, J.-P. Dendrobium Fimbriatum Hook Polysaccharide Ameliorates Dextran-Sodium-Sulfate-Induced Colitis in Mice via Improving Intestinal Barrier Function, Modulating Intestinal Microbiota, and Reducing Oxidative Stress and Inflammatory Responses. Food Funct. 2022, 13, 143–160. [Google Scholar] [CrossRef]
- Yang, S.; Huo, M.; Su, Z.; Wang, F.; Zhang, Y.; Zhong, C.; Shi, Y. The Impact of Dietary Supplementation of Quercetagetin on Growth, Antioxidant Capacity, and Gut Microbiota of Diquat-Challenged Broilers. Front. Microbiol. 2024, 15, 1453145. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Fu, P. Gut Microbiota-Derived Short-Chain Fatty Acids and Kidney Diseases. Drug Des. Dev. Ther. 2017, 11, 3531–3542. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Zhu, X.; Sun, X.; Xiao, J.; Li, D.; Cui, Y.; Wang, C.; Shi, Y. Response of Gut Microbiota to Dietary Fiber and Metabolic Interaction with SCFAs in Piglets. Front. Microbiol. 2018, 9, 2344. [Google Scholar] [CrossRef]
- Mateos, I.; Combes, S.; Pascal, G.; Cauquil, L.; Barilly, C.; Cossalter, A.-M.; Laffitte, J.; Botti, S.; Pinton, P.; Oswald, I.P. Fumonisin-Exposure Impairs Age-Related Ecological Succession of Bacterial Species in Weaned Pig Gut Microbiota. Toxins 2018, 10, 230. [Google Scholar] [CrossRef]
- Hung, D.-Y.; Cheng, Y.-H.; Chen, W.-J.; Hua, K.-F.; Pietruszka, A.; Dybus, A.; Lin, C.-S.; Yu, Y.-H. Bacillus Licheniformis-Fermented Products Reduce Diarrhea Incidence and Alter the Fecal Microbiota Community in Weaning Piglets. Animals 2019, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Gaukroger, C.H.; Stewart, C.J.; Edwards, S.A.; Walshaw, J.; Adams, I.P.; Kyriazakis, I. Changes in Faecal Microbiota Profiles Associated with Performance and Birthweight of Piglets. Front. Microbiol. 2020, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zheng, W.; Lu, M.; Yang, X.; Xue, Y.; Yao, W. A Controlled Heat Stress during Late Gestation Affects Thermoregulation, Productive Performance, and Metabolite Profiles of Primiparous Sow. J. Therm. Biol. 2019, 81, 33–40. [Google Scholar] [CrossRef]
- Vezza, T.; Abad-Jiménez, Z.; Marti-Cabrera, M.; Rocha, M.; Víctor, V.M. Microbiota-Mitochondria Inter-Talk: A Potential Therapeutic Strategy in Obesity and Type 2 Diabetes. Antioxidants 2020, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Walsh, L.; Hill, C.; Ross, R.P. Impact of Glyphosate (RoundupTM) on the Composition and Functionality of the Gut Microbiome. Gut Microbes 2023, 15, 2263935. [Google Scholar] [CrossRef]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic Significance of Lactobacillus Strains: A Comprehensive Review on Health Impacts, Research Gaps, and Future Prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small Metabolites, Possible Big Changes: A Microbiota-Centered View of Non-Alcoholic Fatty Liver Disease. Gut 2019, 68, 359–370. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Feng, Z.; Jia, C.; Lin, X.; Hao, H.; Li, S.; Li, F.; Cui, Q.; Chen, Y.; Wu, F.; Xiao, X. The Inhibition of Enterocyte Proliferation by Lithocholic Acid Exacerbates Necrotizing Enterocolitis through Downregulating the Wnt/β-Catenin Signalling Pathway. Cell Prolif. 2022, 55, e13228. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Li, M.; He, T.; Guo, S.; Zhu, L.; Tan, J.; Wang, B. Novel Approaches in IBD Therapy: Targeting the Gut Microbiota-Bile Acid Axis. Gut Microbes 2024, 16, 2356284. [Google Scholar] [CrossRef]
- Kriaa, A.; Mariaule, V.; Jablaoui, A.; Rhimi, S.; Mkaouar, H.; Hernandez, J.; Korkmaz, B.; Lesner, A.; Maguin, E.; Aghdassi, A.; et al. Bile Acids: Key Players in Inflammatory Bowel Diseases? Cells 2022, 11, 901. [Google Scholar] [CrossRef]
- Lin, M.; Li, L.; Zhang, Y.; Zheng, L.; Xu, M.; Rong, R.; Zhu, T. Baicalin Ameliorates H2O2 Induced Cytotoxicity in HK-2 Cells through the Inhibition of ER Stress and the Activation of Nrf2 Signaling. Int. J. Mol. Sci. 2014, 15, 12507–12522. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Zhou, Y.; Tan, Y.; Feng, W.; Peng, C. Interactions between Gut Microbiota and Polyphenols: A Mechanistic and Metabolomic Review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef]
- Xie, F.; Yang, W.; Xing, M.; Zhang, H.; Ai, L. Natural Polyphenols-Gut Microbiota Interactions and Effects on Glycolipid Metabolism via Polyphenols-Gut-Brain Axis: A State-of-the-Art Review. Trends Food Sci. Technol. 2023, 140, 104171. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Li, R.; Zhang, X.; Chen, T.; Mei, F.; Liu, R.; Chen, M.; Ge, Y.; Hu, H.; et al. Gut Microbial Metabolite Hyodeoxycholic Acid Targets the TLR4/MD2 Complex to Attenuate Inflammation and Protect against Sepsis. Mol. Ther. 2023, 31, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Paumgartner, G.; Beuers, U. Ursodeoxycholic Acid in Cholestatic Liver Disease: Mechanisms of Action and Therapeutic Use Revisited. Hepatology 2002, 36, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M. Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Vettorazzi, J.F.; Rosa, L.R.O.; Carneiro, E.M.; Barbosa, H.C.L. The Bile Acid TUDCA and Neurodegenerative Disorders: An Overview. Life Sci. 2021, 272, 119252. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible Carbohydrates, Butyrate, and Butyrate-Producing Bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S130–S152. [Google Scholar] [CrossRef]
- Mao, X.; Lv, M.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Wang, Q.; Chen, D. The Effect of Dietary Tryptophan Levels on Oxidative Stress of Liver Induced by Diquat in Weaned Piglets. J. Anim. Sci. Biotechnol. 2014, 5, 49. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Devlin, A.S.; Marcobal, A.; Dodd, D.; Nayfach, S.; Plummer, N.; Meyer, T.; Pollard, K.S.; Sonnenburg, J.L.; Fischbach, M.A. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe 2016, 20, 709–715. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I Interferons and Microbial Metabolites of Tryptophan Modulate Astrocyte Activity and Central Nervous System Inflammation via the Aryl Hydrocarbon Receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. PLoS ONE 2013, 8, e80604. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Shimizu, H. Indoxyl Sulfate Induces Nephrovascular Senescence. J. Ren. Nutr. 2012, 22, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Britan, A.; Maffre, V.; Tone, S.; Drevet, J.R. Quantitative and Spatial Differences in the Expression of Tryptophan-Metabolizing Enzymes in Mouse Epididymis. Cell Tissue Res. 2006, 324, 301–310. [Google Scholar] [CrossRef]
- Dairam, A.; Antunes, E.M.; Saravanan, K.S.; Daya, S. Non-Steroidal Anti-Inflammatory Agents, Tolmetin and Sulindac, Inhibit Liver Tryptophan 2,3-Dioxygenase Activity and Alter Brain Neurotransmitter Levels. Life Sci. 2006, 79, 2269–2274. [Google Scholar] [CrossRef]
- Kraal, L.; Abubucker, S.; Kota, K.; Fischbach, M.A.; Mitreva, M. The Prevalence of Species and Strains in the Human Microbiome: A Resource for Experimental Efforts. PLoS ONE 2014, 9, e97279. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From Gut Microbiota to Host Appetite: Gut Microbiota-Derived Metabolites as Key Regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018, 19, 995. [Google Scholar] [CrossRef]
- Waldman, M.; Peterson, S.J.; Arad, M.; Hochhauser, E. The Role of 20-HETE in Cardiovascular Diseases and Its Risk Factors. Prostaglandins Other Lipid Mediat. 2016, 125, 108–117. [Google Scholar] [CrossRef]
- Nambo-Venegas, R.; Valdez-Vargas, C.; Cisneros, B.; Palacios-González, B.; Vela-Amieva, M.; Ibarra-González, I.; Cerecedo-Zapata, C.M.; Martínez-Cruz, E.; Cortés, H.; Reyes-Grajeda, J.P.; et al. Altered Plasma Acylcarnitines and Amino Acids Profile in Spinocerebellar Ataxia Type 7. Biomolecules 2020, 10, 390. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Luo, L.; Zhang, G.-Z.; Li, Z.; Bai, F.-J.; Shi, Y.-Q.; Yang, H.-S. Effect of Dietary L-Malic Acid Supplementation on Growth, Feed Utilization and Digestive Function of Juvenile GIFT Tilapia Oreochromis Niloticus (Linnaeus, 1758). J. Appl. Ichthyol. 2016, 32, 1118–1123. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, Characterization, and Assessment of Lactic Acid Bacteria toward Their Selection as Poultry Probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef]
- Kong, Y.; Olejar, K.J.; On, S.L.W.; Chelikani, V. The Potential of Lactobacillus Spp. for Modulating Oxidative Stress in the Gastrointestinal Tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, Y.; Pan, D.; Cao, J.; Sun, Y.; Zeng, X. Associations of Gut Microbiota with Heat Stress-Induced Changes of Growth, Fat Deposition, Intestinal Morphology, and Antioxidant Capacity in Ducks. Front. Microbiol. 2019, 10, 903. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, V.; Flórez, M.J.V. The Gastrointestinal Microbiome and Its Association with the Control of Pathogens in Broiler Chicken Production: A Review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Li, M.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Chen, W.; Cui, S. Lactic Acid Bacteria Derived Extracellular Vesicles: Emerging Bioactive Nanoparticles in Modulating Host Health. Gut Microbes 2024, 16, 2427311. [Google Scholar] [CrossRef]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-Derived Extracellular Vesicles as a Promising Cell-Free Therapeutic Tool for Wound Healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- Feng, S.; Liu, Y.; Xu, J.; Fan, J.; Li, J.; Wu, Z.; Sun, Y.; Xiong, W. Three Strains of Lactobacillus Derived from Piglets Alleviated Intestinal Oxidative Stress Induced by Diquat through Extracellular Vesicles. Nutrients 2023, 15, 4198. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, J.; Xi, W.; Wang, Z.; Liu, H.; Zhang, K.; Zhao, J.; Wang, Y. Lactiplantibacillus Plantarum Ameliorated Morphological Damage and Barrier Dysfunction and Reduced Apoptosis and Ferroptosis in the Jejunum of Oxidatively Stressed Piglets. Animals 2024, 14, 3335. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Jaeger, L.A.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Meininger, C.J.; Spencer, T.E.; Yin, Y.-L. Important Roles for the Arginine Family of Amino Acids in Swine Nutrition and Production. Livest. Sci. 2007, 112, 8–22. [Google Scholar] [CrossRef]
- Zheng, P.; Yu, B.; He, J.; Tian, G.; Luo, Y.; Mao, X.; Zhang, K.; Che, L.; Chen, D. Protective Effects of Dietary Arginine Supplementation against Oxidative Stress in Weaned Piglets. Br. J. Nutr. 2013, 109, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.L.; Santos, R.G.C.; Generoso, S.V.; Arantes, R.M.E.; Correia, M.I.T.D.; Cardoso, V.N. Pretreatment with Arginine Preserves Intestinal Barrier Integrity and Reduces Bacterial Translocation in Mice. Nutrition 2010, 26, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Bitterman, J.L.; Chung, J.H. Metabolic Effects of Resveratrol: Addressing the Controversies. Cell Mol. Life Sci. 2015, 72, 1473–1488. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary Resveratrol Supplementation Improves Meat Quality of Finishing Pigs through Changing Muscle Fiber Characteristics and Antioxidative Status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant Effects of Resveratrol in the Cardiovascular System. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Chen, D.W.; He, J.; Zheng, P.; Yu, J.; Mao, X.B.; Huang, Z.Q.; Luo, Y.H.; Luo, J.Q.; Yu, B. Long-Term Dietary Resveratrol Supplementation Decreased Serum Lipids Levels, Improved Intramuscular Fat Content, and Changed the Expression of Several Lipid Metabolism-Related miRNAs and Genes in Growing-Finishing Pigs1. J. Anim. Sci. 2019, 97, 1745–1756. [Google Scholar] [CrossRef]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A Dietary Flavonoid with Diverse Anticancer Properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Li, F.; Lang, F.; Zhang, H.; Xu, L.; Wang, Y.; Zhai, C.; Hao, E. Apigenin Alleviates Endotoxin-Induced Myocardial Toxicity by Modulating Inflammation, Oxidative Stress, and Autophagy. Oxidative Med. Cell Longev. 2017, 2017, 2302896. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Pérez-Sánchez, A.; Ruiz-Torres, V.; Martínez-Tébar, A.; Castillo, J.; Herranz-López, M.; Barrajón-Catalán, E. Antioxidant and Photoprotective Activity of Apigenin and Its Potassium Salt Derivative in Human Keratinocytes and Absorption in Caco-2 Cell Monolayers. Int. J. Mol. Sci. 2019, 20, 2148. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, Y.; Zou, X.; Zhang, J.; Zheng, Z.; Wang, Z. Antioxidant Apigenin Relieves Age-Related Muscle Atrophy by Inhibiting Oxidative Stress and Hyperactive Mitophagy and Apoptosis in Skeletal Muscle of Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2081–2088. [Google Scholar] [CrossRef]
- Zhou, N.; Tian, Y.; Liu, W.; Tu, B.; Xu, W.; Gu, T.; Zou, K.; Lu, L. Protective Effects of Resveratrol and Apigenin Dietary Supplementation on Serum Antioxidative Parameters and mRNAs Expression in the Small Intestines of Diquat-Challenged Pullets. Front. Vet. Sci. 2022, 9, 850769. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, M.; Tang, Z.; Wang, F.; Han, S.; Liu, S.; Chen, B. Curcumin Alleviates Cecal Oxidative Injury in Diquat-Induced Broilers by Regulating the Nrf2/ARE Pathway and Microflora. Poult. Sci. 2024, 103, 103651. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.; Hou, C.; Chen, L.; Zhang, Y.; Li, J. Effects of Pomegranate Peel Polyphenols Combined with Inulin on Gut Microbiota and Serum Metabolites of High-Fat-Induced Obesity Rats. J. Agric. Food Chem. 2023, 71, 5733–5744. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, D.M.; Grakoui, A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 2020, 159, 849–863. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H. Gut Bacteroides Species in Health and Disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Durant, L.; Stentz, R.; Noble, A.; Brooks, J.; Gicheva, N.; Reddi, D.; O’Connor, M.J.; Hoyles, L.; McCartney, A.L.; Man, R.; et al. Bacteroides Thetaiotaomicron-Derived Outer Membrane Vesicles Promote Regulatory Dendritic Cell Responses in Health but Not in Inflammatory Bowel Disease. Microbiome 2020, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.-M.; Van de Wiele, T. Butyrate-Producing Clostridium Cluster XIVa Species Specifically Colonize Mucins in an in Vitro Gut Model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef]
- Gong, X.; Wu, M.; Jiang, Y.; Wang, H. Effects of Different Temperatures and pH Values on Volatile Fatty Acids Production during Codigestion of Food Waste and Thermal-Hydrolysed Sewage Sludge and Subsequent Volatile Fatty Acids for Polyhydroxyalkanoates Production. Bioresour. Technol. 2021, 333, 125149. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, L.; Li, Y.; Xia, G.; Chen, C.; Zhang, Y. Bamboo-Shaving Polysaccharide Protects against High-Diet Induced Obesity and Modulates the Gut Microbiota of Mice. J. Funct. Food. 2018, 49, 20–31. [Google Scholar] [CrossRef]
- Verstreken, I.; Laleman, W.; Wauters, G.; Verhaegen, J. Desulfovibrio Desulfuricans Bacteremia in an Immunocompromised Host with a Liver Graft and Ulcerative Colitis. J. Clin. Microbiol. 2012, 50, 199–201. [Google Scholar] [CrossRef]
- Qiao, L.; Dou, X.; Song, X.; Chang, J.; Pi, S.; Zhang, X.; Zhu, L.; Zeng, X.; Xu, C. Protective Effect of Biogenic Selenium Nanoparticles against Diquat-Induced Acute Toxicity via Regulation of Gut Microbiota and Its Metabolites. Food Chem. Toxicol. 2022, 170, 113480. [Google Scholar] [CrossRef]
- Bollag, W.B. Regulation of Aldosterone Synthesis and Secretion. Compr. Physiol. 2014, 4, 1017–1055. [Google Scholar] [CrossRef]
- Dominko, K.; Đikić, D. Glutathionylation: A Regulatory Role of Glutathione in Physiological Processes. Arch. Ind. Hyg. Toxicol. 2018, 69, 1–24. [Google Scholar] [CrossRef]
- Li, Q.J.; Wang, Z.G.; Liu, Q.; Xie, Y.; Hu, H.L. [Research Status of Periplaneta Americana with Analyses and Prospects of Key Issues]. China J. Chin. Mater. Med. 2018, 43, 1507–1516. [Google Scholar] [CrossRef]
- Nguyen, T.; Chen, X.; Chai, J.; Li, R.; Han, X.; Chen, X.; Liu, S.; Chen, M.; Xu, X. Antipyretic, Anti-Inflammatory and Analgesic Activities of Periplaneta Americana Extract and Underlying Mechanisms. Biomed. Pharmacother. 2020, 123, 109753. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xu, S.; Chen, L.; Deng, Y.; Feng, J. Periplaneta Americana Extract Pretreatment Alleviates Oxidative Stress and Inflammation and Increases the Abundance of Gut Akkermansia Muciniphila in Diquat-Induced Mice. Antioxidants 2022, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, M.E.; Valdivia, R.H. Akkermansia in the Gastrointestinal Tract as a Modifier of Human Health. Gut Microbes 2024, 16, 2406379. [Google Scholar] [CrossRef]
- Hagi, T.; Belzer, C. The Interaction of Akkermansia Muciniphila with Host-Derived Substances, Bacteria and Diets. Appl. Microbiol. Biotechnol. 2021, 105, 4833–4841. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia Muciniphila Is a Promising Probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia Muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, M.; Shao, F.; Guan, G.; Huang, B.; Tan, B.; Yin, Y. N-Acetyl-L-Cysteine Protects the Enterocyte against Oxidative Damage by Modulation of Mitochondrial Function. Mediat. Inflamm. 2016, 2016, 8364279. [Google Scholar] [CrossRef]
- Biasi, F.; Deiana, M.; Guina, T.; Gamba, P.; Leonarduzzi, G.; Poli, G. Wine Consumption and Intestinal Redox Homeostasis. Redox Biol. 2014, 2, 795–802. [Google Scholar] [CrossRef]
- Wang, S.; Mu, L.; Yu, C.; He, Y.; Hu, X.; Jiao, Y.; Xu, Z.; You, S.; Liu, S.-L.; Bao, H. Microbial Collaborations and Conflicts: Unraveling Interactions in the Gut Ecosystem. Gut Microbes 2024, 16, 2296603. [Google Scholar] [CrossRef]
- Cho, N.A.; Strayer, K.; Dobson, B.; McDonald, B. Pathogenesis and Therapeutic Opportunities of Gut Microbiome Dysbiosis in Critical Illness. Gut Microbes 2024, 16, 2351478. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Changes (DQ) | Intervention | Changes (Intervention) | Ref. |
|---|---|---|---|---|
| C57BL/6 mice | Allobaculum, Providencia, Escherichia-Shigella, Bacteroidetes, proteobacteria↑; Firmicutes↓ | Periplaneta americana extract (PAE) | Akkermansia muciniphila↑; Bacteroidetes↓ | [262] |
| Weaned piglets | HCA, HDCA, TUDCA↓ | Hydroxytyrosol | HCA, HDCA, TUDCA↑ | [137] |
| ICR mice | Firmicutes↓; Bacteroidetes↑ | Hydroxytyrosol | Firmicutes, Lactobacillus↑; Bacteroidetes↓; butyrate↑; glycerophospholipid metabolism, pentose, glucuronate interconversions↓ | [173] |
| C57BL/6J mice | Bacteroidota, Coriobacteriia, Enterorhabdus↓; Escherichia–Shigella↑ | L. delbrueckii, L. amylovorus, and L. salivarius EVs | Enterococcus↓; Parasutterella, Bifidobacterium, Erysipelatoclostridium↑ | [230] |
| Mice | Firmicutes↓ | Eucommia ulmoides polysaccharide (EUPS) | Firmicutes, Ligilactobacillus↑; Helicobacter↓ | [168] |
| WOD168 broilers | Lactobacillus, Alistipes↓ | Quercetagetin (QG) | Lactobacillus, Alistipes↑ | [178] |
| Weaned piglets | Firmicutes, Actinobacteria, Ruminococcaceae UCG-005, Eubacterium coprostanoligenes↑ | Resveratrol (RES) | Clostridium sensu stricto 1, Lachnospiraceae↑ | [171] |
| C57BL/6 mice | Selenium nanoparticles (SeNPs) | Akkermansia, Muribaculaceae, Bacteroides, Parabacteroides↑ | [257] | |
| C57BL/6 mice | Bacteroides, Helicobacter↑; Firmicutes, Pediococcus, Enterococcus, Dubosiella↓ | Pediococcus pentosaceus ZJUAF-4; VC | ZJUAF-4 reversed these changes induced by DQ and the reversed abilities of ZJUAF-4 seemed to be higher than those of VC | [63] |
| Cobb broilers | Lactobacillaceae, Victivallis, Bacillus↓; Saccharopolyspora_hir-suta, Staphylococcus_succinus↑ | Curcumin (CUR) | Lactobacillaceae, Ruminococcaceae_Clostridium↑; Saccharopolyspora_hirsuta, Staphylococcus_succinus↓; acetate, total SCFAs↑ | [246] |
| C57BL/6J mice | Hydrogenobacter thermophilus strain (HtSOD) | Dubosiella, Alistipes↑ | [42] | |
| Weaned piglets | Acidobacteria↓; Turicibacteraceae, Clostridium, Turicibacter↑ | Bacillus amyloliquefaciens SC06 | Ruminococcaceae, Clostridium↓; Pasteurellaceae, Lactobacillus, Actinobacillus↑ | [81] |
| SD rats | g_Lactobacillus, p_Firmicutes, g_Akkermansia, p_Verrucomicrobia↓; p_Proteobacteria, g_Escherichia, s_Escherichiacoli↑ | Bacillus SC06 | g_Anaerofilum, s_Bacteroides uniformis↑; s_Oscillospira guilliermondil↓ | [165] |
| C57BL/6 mice | SeNPs | Bacteroidetes, Clostridium_XlVa↑; Verrucomicrobia, Desulfovibrio↓; total SCFAs, butyrate, isobutyrate, valerate, isovalerate↑ | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Tang, Q.; Liu, Y.-C.; Wang, L.-J.; Chai, Y.-F. Impact of Diquat on the Intestinal Health and the Composition and Function of the Gut Microbiome. Antioxidants 2025, 14, 721. https://doi.org/10.3390/antiox14060721
He J, Tang Q, Liu Y-C, Wang L-J, Chai Y-F. Impact of Diquat on the Intestinal Health and the Composition and Function of the Gut Microbiome. Antioxidants. 2025; 14(6):721. https://doi.org/10.3390/antiox14060721
Chicago/Turabian StyleHe, Jiao, Qing Tang, Yan-Cun Liu, Li-Jun Wang, and Yan-Fen Chai. 2025. "Impact of Diquat on the Intestinal Health and the Composition and Function of the Gut Microbiome" Antioxidants 14, no. 6: 721. https://doi.org/10.3390/antiox14060721
APA StyleHe, J., Tang, Q., Liu, Y.-C., Wang, L.-J., & Chai, Y.-F. (2025). Impact of Diquat on the Intestinal Health and the Composition and Function of the Gut Microbiome. Antioxidants, 14(6), 721. https://doi.org/10.3390/antiox14060721







