Prolonged In Vivo Chemogenetic Generation of Hydrogen Peroxide by Endothelial Cells Induces Cardiac Remodelling and Vascular Dysfunction
Abstract
1. Introduction
2. Materials and Methods
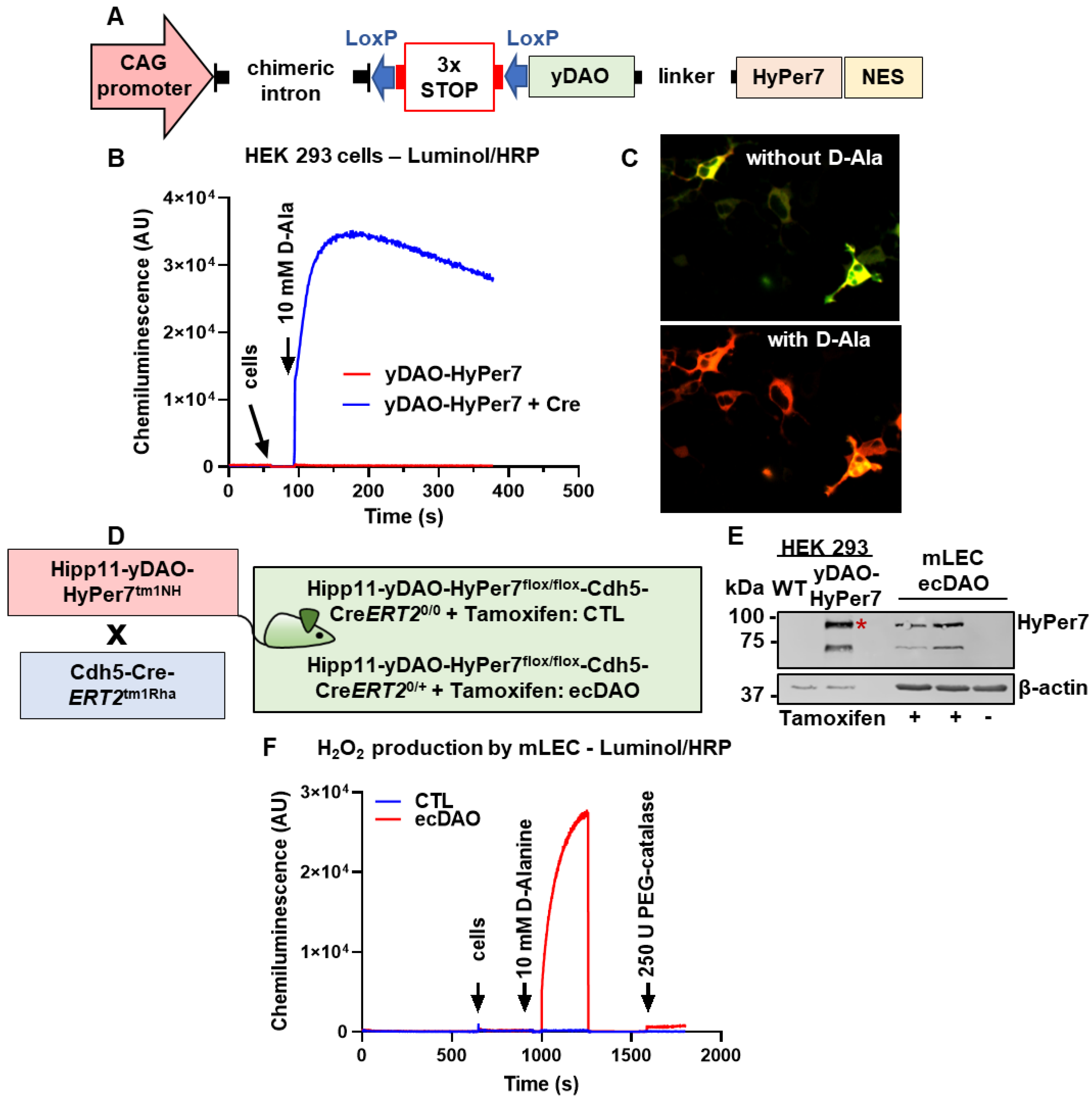
2.1. Generation of yDAO-HyPer7 Construct
2.2. Chemiluminescence Assays
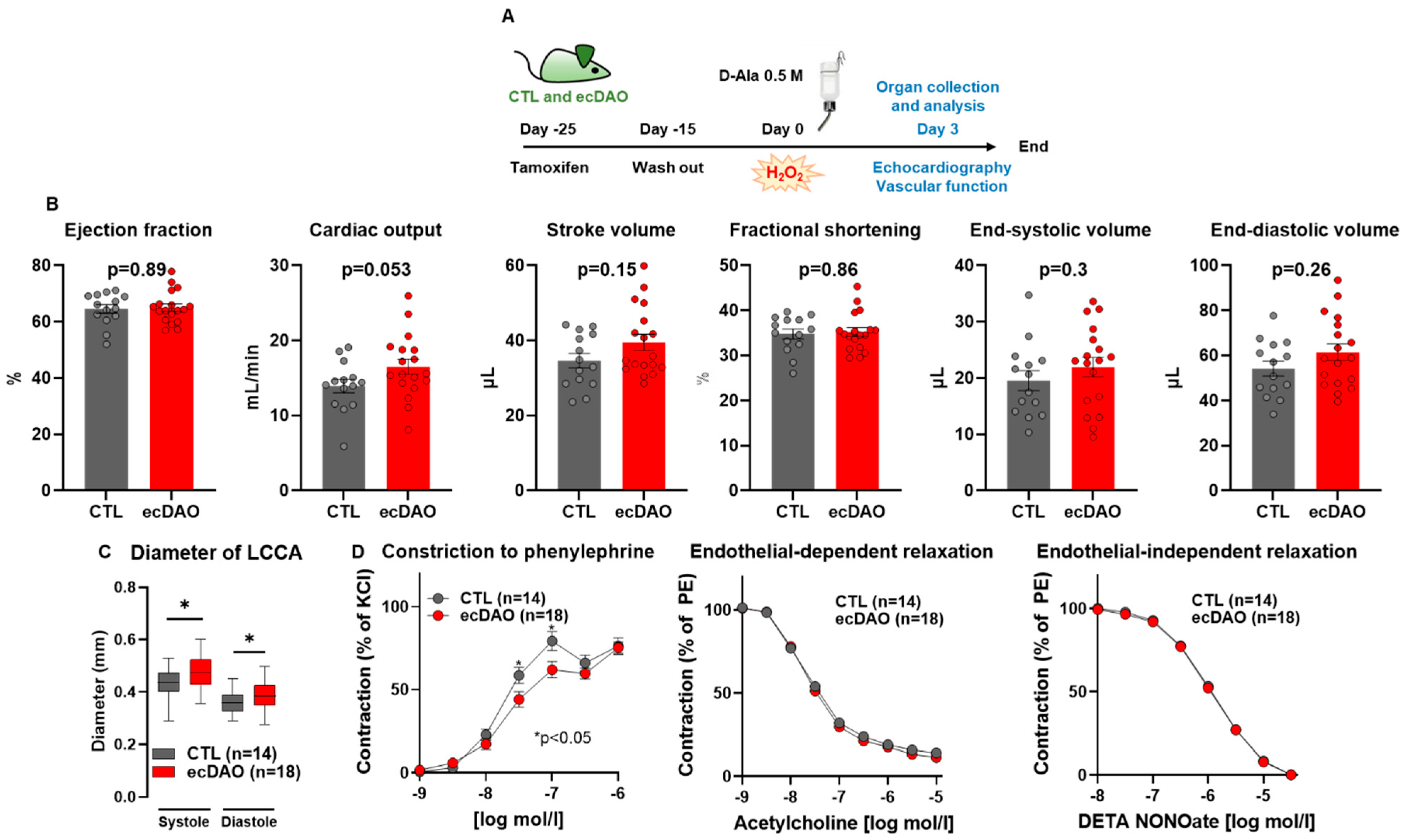
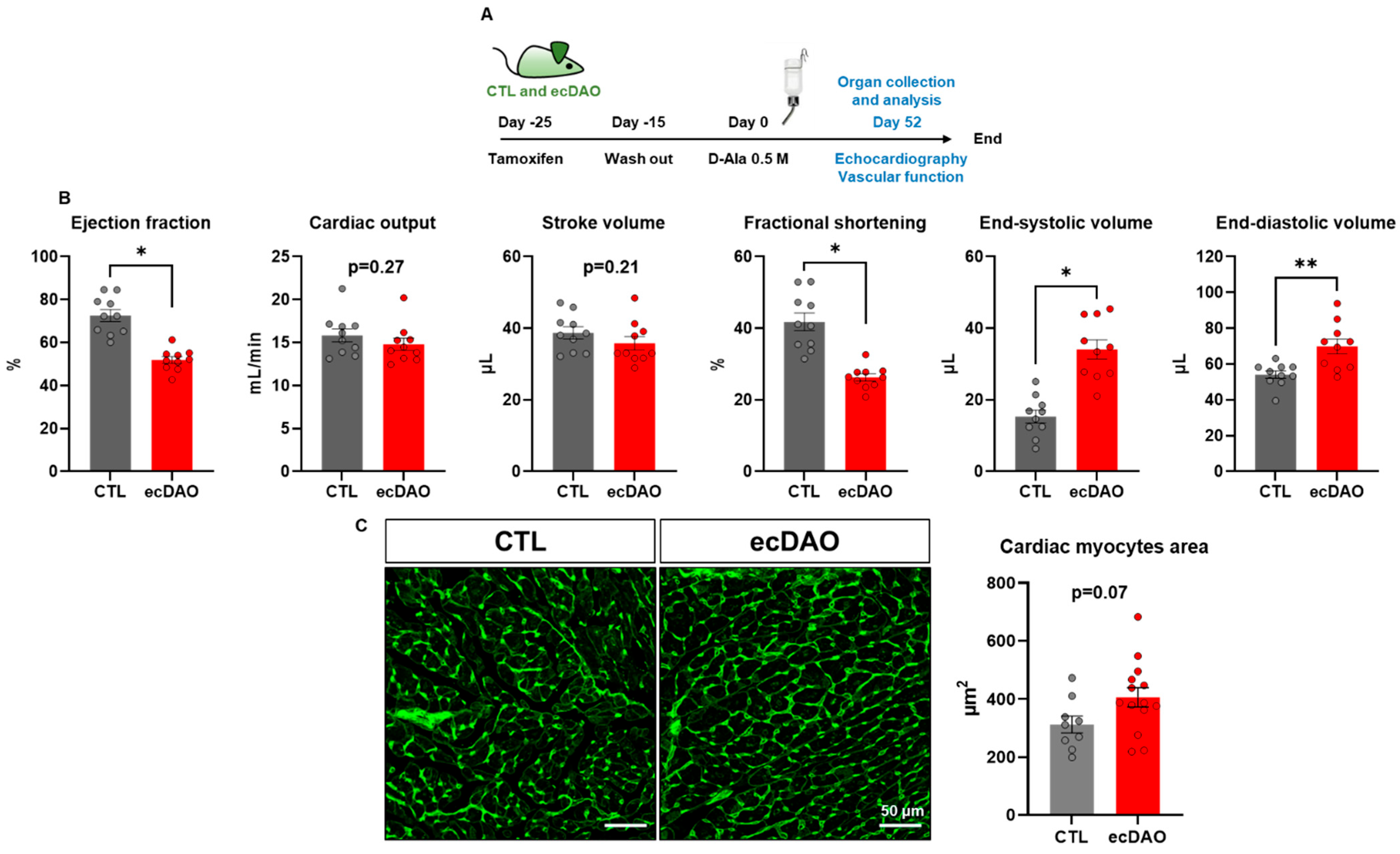
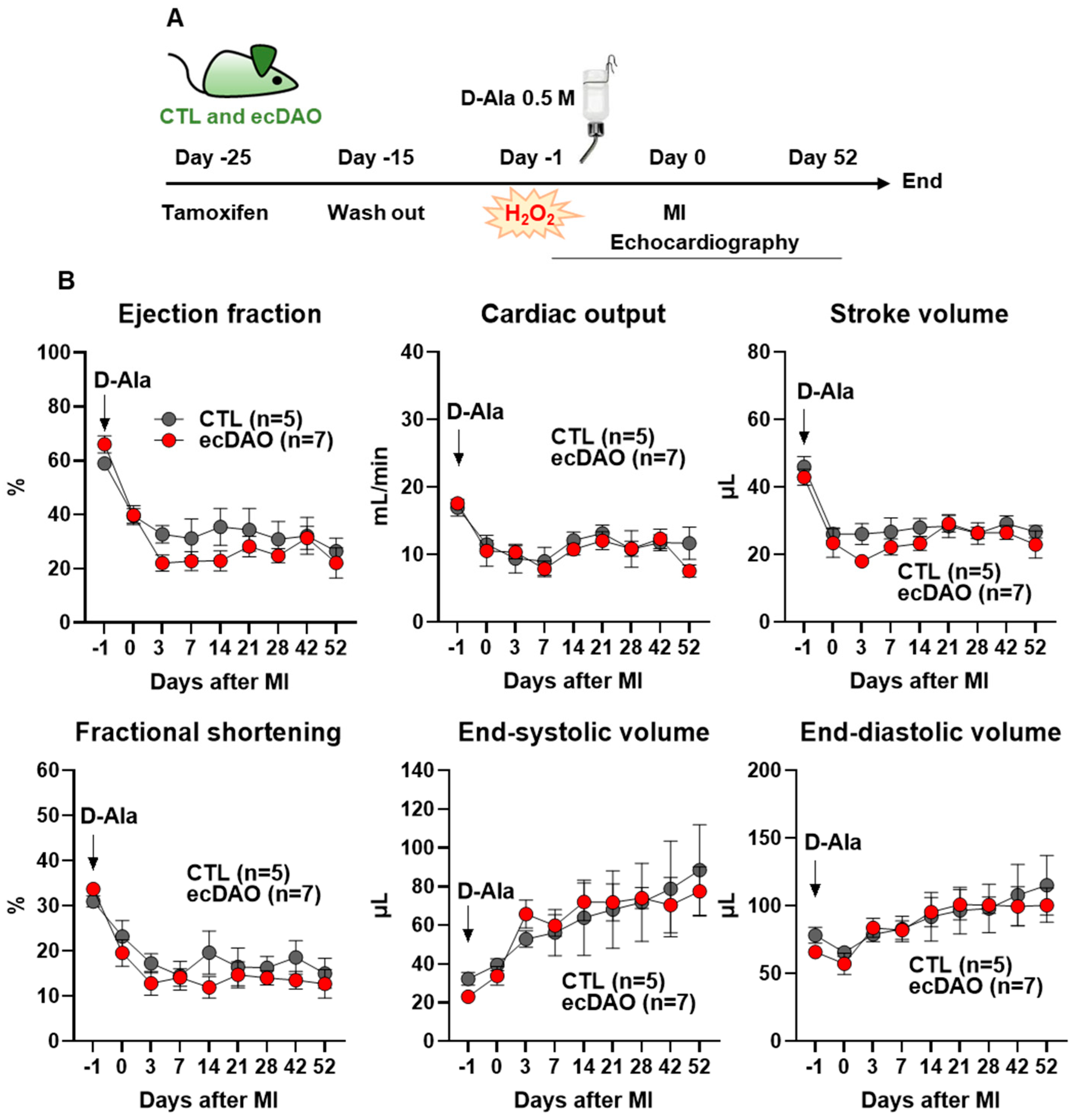
2.3. Animal Procedure
2.4. Minimally Invasive Myocardial Infarction
2.5. Vascular Reactivity Measurements
2.6. Isolation of Mouse Lung Endothelial Cells
2.7. Western Blot Analysis
2.8. Echocardiography
2.9. Histology
2.10. En Face Immunofluorescence
2.11. Statistics
3. Results
3.1. Generation of an Inducible, Endothelial Cell-Specific Knock-In Mouse of DAO
3.2. Acute Chemogenetic Generation of H2O2 by Endothelial Cells Decreases Vascular Tone
3.3. Prolonged Chemogenetic Generation of H2O2 by Endothelial Cells Induces Cardiovascular Dysfunction in Mice
3.4. Prolonged Generation of H2O2 by Endothelial Cells Does Not Affect EC Integrity but Increases Protein Oxidation
3.5. In Vivo Chemogenetic Generation of H2O2 in Endothelial Cells Does Not Affect the Outcome After Myocardial Infarction (MI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Kang, D.H.; Lee, D.J.; Kim, J.; Lee, J.Y.; Kim, H.-W.; Kwon, K.; Taylor, W.R.; Jo, H.; Kang, S.W. Vascular injury involves the overoxidation of peroxiredoxin type II and is recovered by the peroxiredoxin activity mimetic that induces reendothelialization. Circulation 2013, 128, 834–844. [Google Scholar] [CrossRef]
- Lin, S.-L.; Yeh, J.-L.; Tsai, P.-C.; Chang, T.-H.; Huang, W.-C.; Lee, S.-T.; Wassler, M.; Geng, Y.-J.; Sulistyowati, E. Inhibition of Neointima Hyperplasia, Inflammation, and Reactive Oxygen Species in Balloon-Injured Arteries by HVJ Envelope Vector-Mediated Delivery of Superoxide Dismutase Gene. Transl. Stroke Res. 2019, 10, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Rafea, R.; Siragusa, M.; Fleming, I. The Ever-Expanding Influence of the Endothelial Nitric Oxide Synthase. Basic Clin. Pharmacol. Toxicol. 2025, 136, e70029. [Google Scholar] [CrossRef]
- Waldeck-Weiermair, M.; Yadav, S.; Spyropoulos, F.; Krüger, C.; Pandey, A.K.; Michel, T. Dissecting in vivo and in vitro redox responses using chemogenetics. Free Radic. Biol. Med. 2021, 177, 360–369. [Google Scholar] [CrossRef]
- Das, A.A.; Waldeck-Weiermair, M.; Yadav, S.; Spyropoulos, F.; Pandey, A.; Dutta, T.; Covington, T.A.; Michel, T. Differential aortic aneurysm formation provoked by chemogenetic oxidative stress. J. Clin. Investig. 2025, 135, e188743. [Google Scholar] [CrossRef]
- Yadav, S.; Waldeck-Weiermair, M.; Spyropoulos, F.; Bronson, R.; Pandey, A.K.; Das, A.A.; Sisti, A.C.; Covington, T.A.; Thulabandu, V.; Caplan, S.; et al. Sensory ataxia and cardiac hypertrophy caused by neurovascular oxidative stress in chemogenetic transgenic mouse lines. Nat. Commun. 2023, 14, 3094. [Google Scholar] [CrossRef]
- Erdogan, Y.C.; Altun, H.Y.; Secilmis, M.; Ata, B.N.; Sevimli, G.; Cokluk, Z.; Zaki, A.G.; Sezen, S.; Caglar, T.A.; Sevgen, İ.; et al. Complexities of the chemogenetic toolkit: Differential mDAAO activation by d-amino substrates and subcellular targeting. Free Radic. Biol. Med. 2021, 177, 132–142. [Google Scholar] [CrossRef]
- Saeedi Saravi, S.S.; Eroglu, E.; Waldeck-Weiermair, M.; Sorrentino, A.; Steinhorn, B.; Belousov, V.; Michel, T. Differential endothelial signaling responses elicited by chemogenetic H2O2 synthesis. Redox Biol. 2020, 36, 101605. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, B.; Eroglu, E.; Michel, T. Chemogenetic Approaches to Probe Redox Pathways: Implications for Cardiovascular Pharmacology and Toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, F.; Sorrentino, A.; van der Reest, J.; Yang, P.; Waldeck-Weiermair, M.; Steinhorn, B.; Eroglu, E.; Saeedi Saravi, S.S.; Yu, P.; Haigis, M.; et al. Metabolomic and transcriptomic signatures of chemogenetic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H451–H465. [Google Scholar] [CrossRef]
- Sorrentino, A.; Steinhorn, B.; Troncone, L.; Saravi, S.S.S.; Badole, S.; Eroglu, E.; Kijewski, M.F.; Divakaran, S.; Di Carli, M.; Michel, T. Reversal of heart failure in a chemogenetic model of persistent cardiac redox stress. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H617–H626. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, B.; Sorrentino, A.; Badole, S.; Bogdanova, Y.; Belousov, V.; Michel, T. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 2018, 9, 4044. [Google Scholar] [CrossRef]
- Spyropoulos, F.; Das, A.A.; Waldeck-Weiermair, M.; Yadav, S.; Pandey, A.K.; Guo, R.; Covington, T.A.; Thulabandu, V.; Kosmas, K.; Steinhorn, B.; et al. Adult and neonatal models of chemogenetic heart failure caused by oxidative stress. J. Clin. Investig. 2024, 134, e178251. [Google Scholar] [CrossRef]
- ATCC 26217. Available online: https://www.atcc.org/products/26217 (accessed on 8 June 2025).
- Pak, V.V.; Ezeriņa, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Martínez Gache, S.A.; et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab. 2020, 31, 642–653.e6. [Google Scholar] [CrossRef]
- Browning, J.; Rooney, M.; Hams, E.; Takahashi, S.; Mizuno, S.; Sugiyama, F.; Fallon, P.G.; Kelly, V.P. Highly efficient CRISPR-targeting of the murine Hipp11 intergenic region supports inducible human transgene expression. Mol. Biol. Rep. 2020, 47, 1491–1498. [Google Scholar] [CrossRef]
- Wang, Y.; Nakayama, M.; Pitulescu, M.E.; Schmidt, T.S.; Bochenek, M.L.; Sakakibara, A.; Adams, S.; Davy, A.; Deutsch, U.; Lüthi, U.; et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010, 465, 483–486. [Google Scholar] [CrossRef]
- Sicklinger, F.; Zhang, Y.; Lavine, K.J.; Simon, N.; Bucher, V.; Jugold, M.; Lehmann, L.; Konstandin, M.H.; Katus, H.A.; Leuschner, F. A Minimal-Invasive Approach for Standardized Induction of Myocardial Infarction in Mice. Circ. Res. 2020, 127, 1214–1216. [Google Scholar] [CrossRef]
- Malacarne, P.F.; Ratiu, C.; Gajos-Draus, A.; Müller, N.; Lopez, M.; Pflüger-Müller, B.; Ding, X.; Warwick, T.; Oo, J.; Siragusa, M.; et al. Loss of Endothelial Cytochrome P450 Reductase Induces Vascular Dysfunction in Mice. Hypertension 2022, 79, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Paldino, A.; Falcão-Pires, I.; Daskalopoulos, E.P.; Dal Ferro, M.; Vodret, S.; Lesizza, P.; Cannatà, A.; Miranda-Silva, D.; Lourenço, A.P.; et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: A position paper of the ESC Working Group on Myocardial Function. Cardiovasc. Res. 2021, 117, 43–59. [Google Scholar] [CrossRef]
- Lucchesi, P.A.; Belmadani, S.; Matrougui, K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J. Hypertens. 2005, 23, 571–579. [Google Scholar] [CrossRef]
- Wagner, E.; Luche, S.; Penna, L.; Chevallet, M.; van Dorsselaer, A.; Leize-Wagner, E.; Rabilloud, T. A method for detection of overoxidation of cysteines: Peroxiredoxins are oxidized in vivo at the active-site cysteine during oxidative stress. Biochem. J. 2002, 366, 777–785. [Google Scholar] [CrossRef][Green Version]
- Portillo-Ledesma, S.; Randall, L.M.; Parsonage, D.; Dalla Rizza, J.; Karplus, P.A.; Poole, L.B.; Denicola, A.; Ferrer-Sueta, G. Differential Kinetics of Two-Cysteine Peroxiredoxin Disulfide Formation Reveal a Novel Model for Peroxide Sensing. Biochemistry 2018, 57, 3416–3424. [Google Scholar] [CrossRef]
- Carofino, B.L.; Justice, M.J. Tissue-Specific Regulation of Oncogene Expression Using Cre-Inducible ROSA26 Knock-In Transgenic Mice. Curr. Protoc. Mouse Biol. 2015, 5, 187–204. [Google Scholar] [CrossRef]
- Burgoyne, J.R.; Madhani, M.; Cuello, F.; Charles, R.L.; Brennan, J.P.; Schröder, E.; Browning, D.D.; Eaton, P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 2007, 317, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, J.R.; Rudyk, O.; Cho, H.-J.; Prysyazhna, O.; Hathaway, N.; Weeks, A.; Evans, R.; Ng, T.; Schröder, K.; Brandes, R.P.; et al. Deficient angiogenesis in redox-dead Cys17Ser PKARIα knock-in mice. Nat. Commun. 2015, 6, 7920. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.; Wei, Q.; Xia, Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: Co-commitment and interplay of Akt and AMPK. J. Biol. Chem. 2008, 283, 25256–25263. [Google Scholar] [CrossRef]
- Drummond, G.R.; Cai, H.; Davis, M.E.; Ramasamy, S.; Harrison, D.G. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ. Res. 2000, 86, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Murdoch, C.E.; Wang, M.; Santos, C.X.; Zhang, M.; Alom-Ruiz, S.; Anilkumar, N.; Ouattara, A.; Cave, A.C.; Walker, S.J.; et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.K.; Murray, T.V.A.; Prysyazhna, O.; Martin, D.; Burgoyne, J.R.; Santos, C.; Eaton, P.; Shah, A.M.; Brewer, A.C. Transcriptional Regulation of Cystathionine-γ-Lyase in Endothelial Cells by NADPH Oxidase 4-Dependent Signaling. J. Biol. Chem. 2016, 291, 1774–1788. [Google Scholar] [CrossRef]
- Suvorava, T.; Lauer, N.; Kumpf, S.; Jacob, R.; Meyer, W.; Kojda, G. Endogenous vascular hydrogen peroxide regulates arteriolar tension in vivo. Circulation 2005, 112, 2487–2495. [Google Scholar] [CrossRef]
- Witting, P.K.; Rayner, B.S.; Wu, B.-J.; Ellis, N.A.; Stocker, R. Hydrogen peroxide promotes endothelial dysfunction by stimulating multiple sources of superoxide anion radical production and decreasing nitric oxide bioavailability. Cell. Physiol. Biochem. 2007, 20, 255–268. [Google Scholar] [CrossRef]
- Faraci, F.M. Hydrogen peroxide: Watery fuel for change in vascular biology. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1931–1933. [Google Scholar] [CrossRef]
- Thakali, K.; Davenport, L.; Fink, G.D.; Watts, S.W. Pleiotropic effects of hydrogen peroxide in arteries and veins from normotensive and hypertensive rats. Hypertension 2006, 47, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.R.; Chen, K.; Keaney, J.F. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 2002, 277, 6017–6024. [Google Scholar] [CrossRef]
- Cai, H.; Davis, M.E.; Drummond, G.R.; Harrison, D.G. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1571–1576. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183065. [Google Scholar] [CrossRef] [PubMed]

| Name | Host | Manufacturer | Catalogue Number |
|---|---|---|---|
| β-Actin | Mouse | Sigma-Aldrich Taufkirchen, Germany | A1978 |
| GFP | Rabbit | Cell Signaling Technology, Leiden, Netherlands | 2956 |
| Prx-SO3 | Rabbit | Abcam, Cambridge, UK | Ab16830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, M.; Herrle, N.; Amirmiran, B.; Malacarne, P.F.; Werkhäuser, J.; Chatterjee, S.; Kader, C.; Jurisch, V.; Wen, X.; Gheisari, M.; et al. Prolonged In Vivo Chemogenetic Generation of Hydrogen Peroxide by Endothelial Cells Induces Cardiac Remodelling and Vascular Dysfunction. Antioxidants 2025, 14, 705. https://doi.org/10.3390/antiox14060705
Lopez M, Herrle N, Amirmiran B, Malacarne PF, Werkhäuser J, Chatterjee S, Kader C, Jurisch V, Wen X, Gheisari M, et al. Prolonged In Vivo Chemogenetic Generation of Hydrogen Peroxide by Endothelial Cells Induces Cardiac Remodelling and Vascular Dysfunction. Antioxidants. 2025; 14(6):705. https://doi.org/10.3390/antiox14060705
Chicago/Turabian StyleLopez, Melina, Niklas Herrle, Bardia Amirmiran, Pedro F. Malacarne, Julia Werkhäuser, Souradeep Chatterjee, Carine Kader, Victoria Jurisch, Xin Wen, Maedeh Gheisari, and et al. 2025. "Prolonged In Vivo Chemogenetic Generation of Hydrogen Peroxide by Endothelial Cells Induces Cardiac Remodelling and Vascular Dysfunction" Antioxidants 14, no. 6: 705. https://doi.org/10.3390/antiox14060705
APA StyleLopez, M., Herrle, N., Amirmiran, B., Malacarne, P. F., Werkhäuser, J., Chatterjee, S., Kader, C., Jurisch, V., Wen, X., Gheisari, M., Schäfer, K., Münch, C., Leuschner, F., Gilsbach, R., Rezende, F., & Brandes, R. P. (2025). Prolonged In Vivo Chemogenetic Generation of Hydrogen Peroxide by Endothelial Cells Induces Cardiac Remodelling and Vascular Dysfunction. Antioxidants, 14(6), 705. https://doi.org/10.3390/antiox14060705







