The Biological Consequences of the Knockout of Genes Involved in the Synthesis and Metabolism of H2S in Drosophila melanogaster
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila melanogaster Strains
2.2. Detection of H2S Production Level in Fly Samples
2.3. Lifespan Analysis
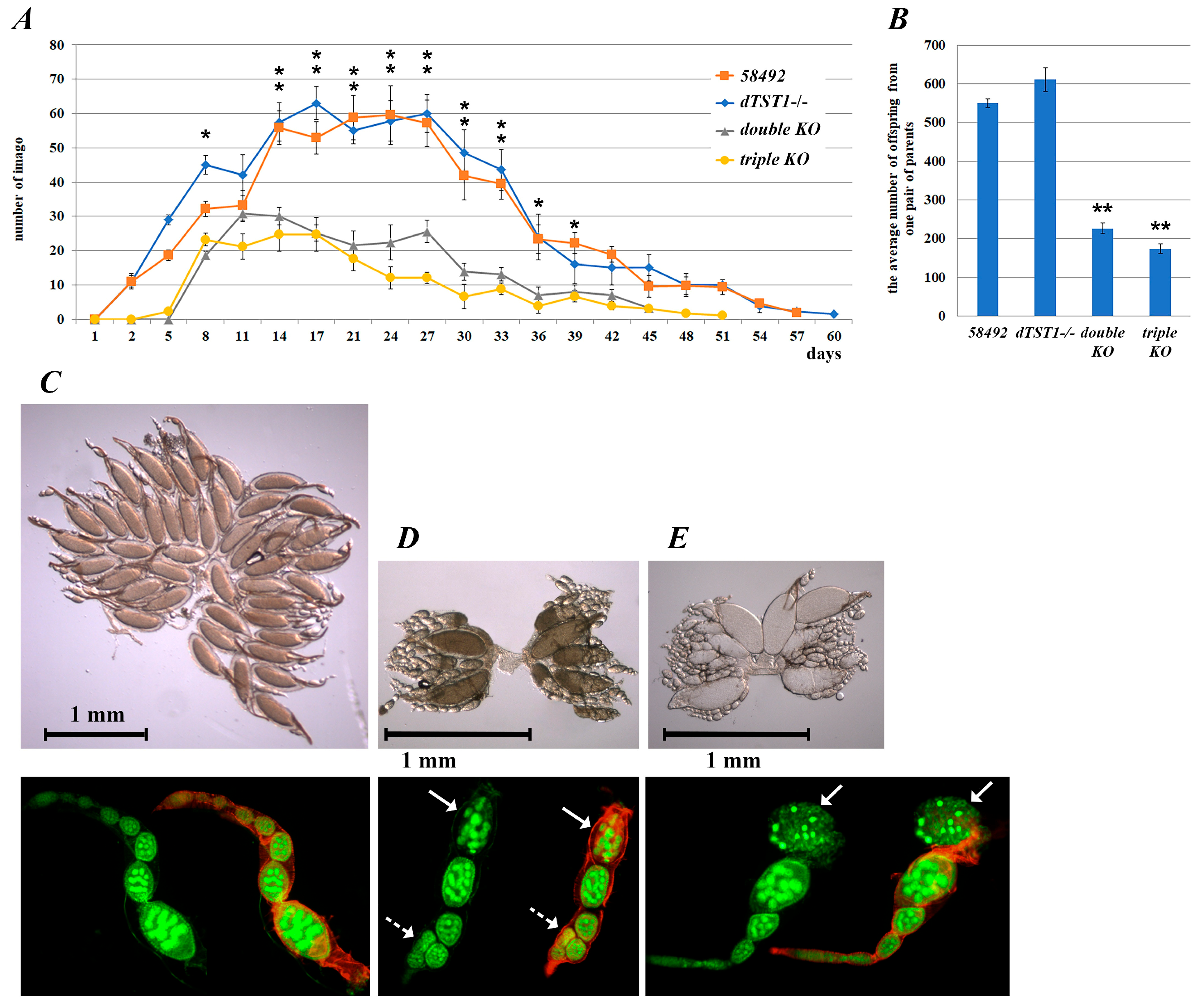
2.4. Analysis of the Reproductive Parameters
2.5. Fluorescent Staining of the Ovaries
2.6. The Generation of Triple-Knockout Flies (cbs, cse, and dtst1)
2.7. RNA Extraction and Quantitative Real-Time PCR
2.8. RNA-Seq Library Preparation and Transcriptomic Analysis
2.9. Malpighian Tubule Dissection and Staining
3. Results
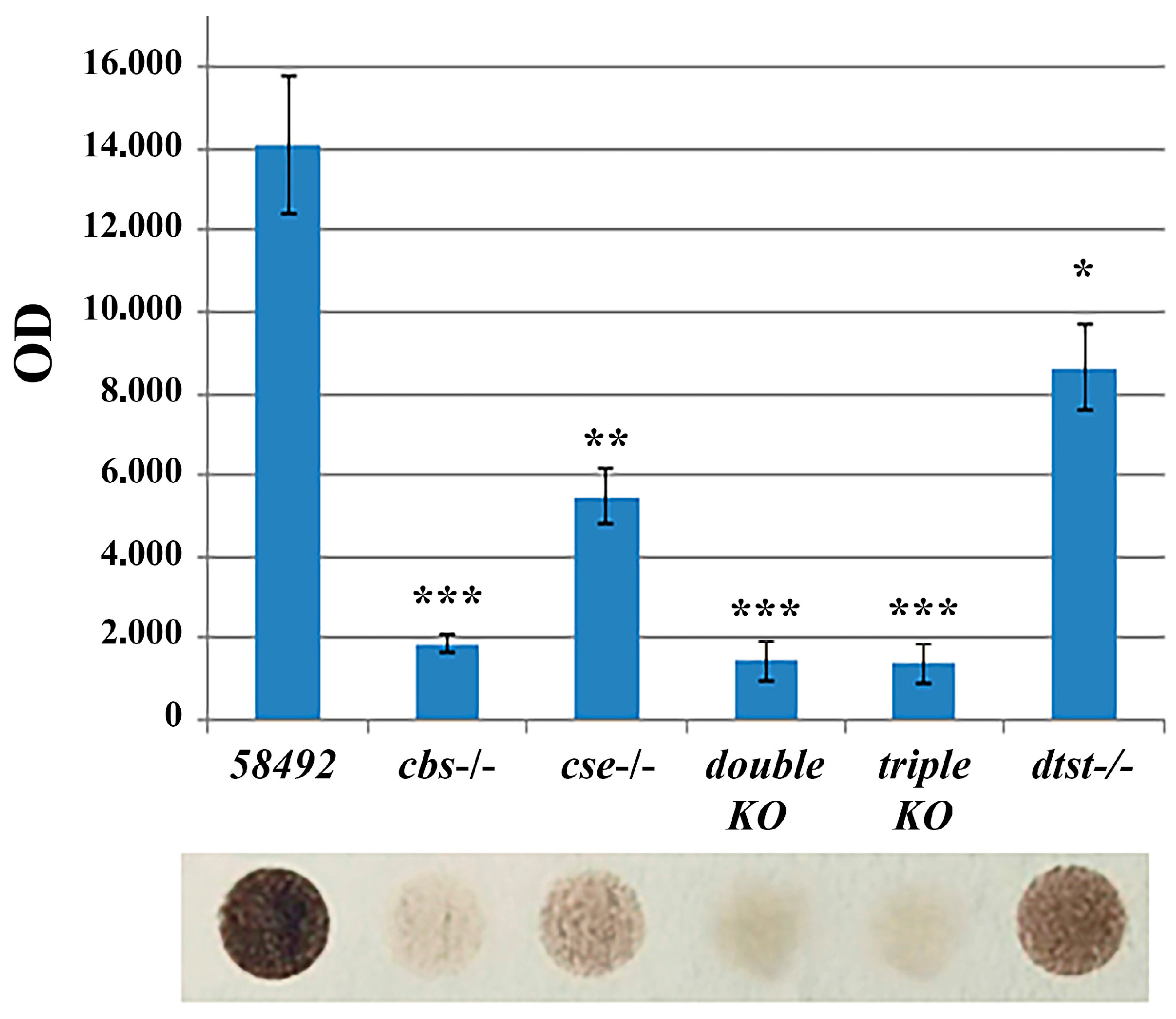
3.1. H2S Production Is Drastically Reduced in Double- and Triple-Knockout Strains
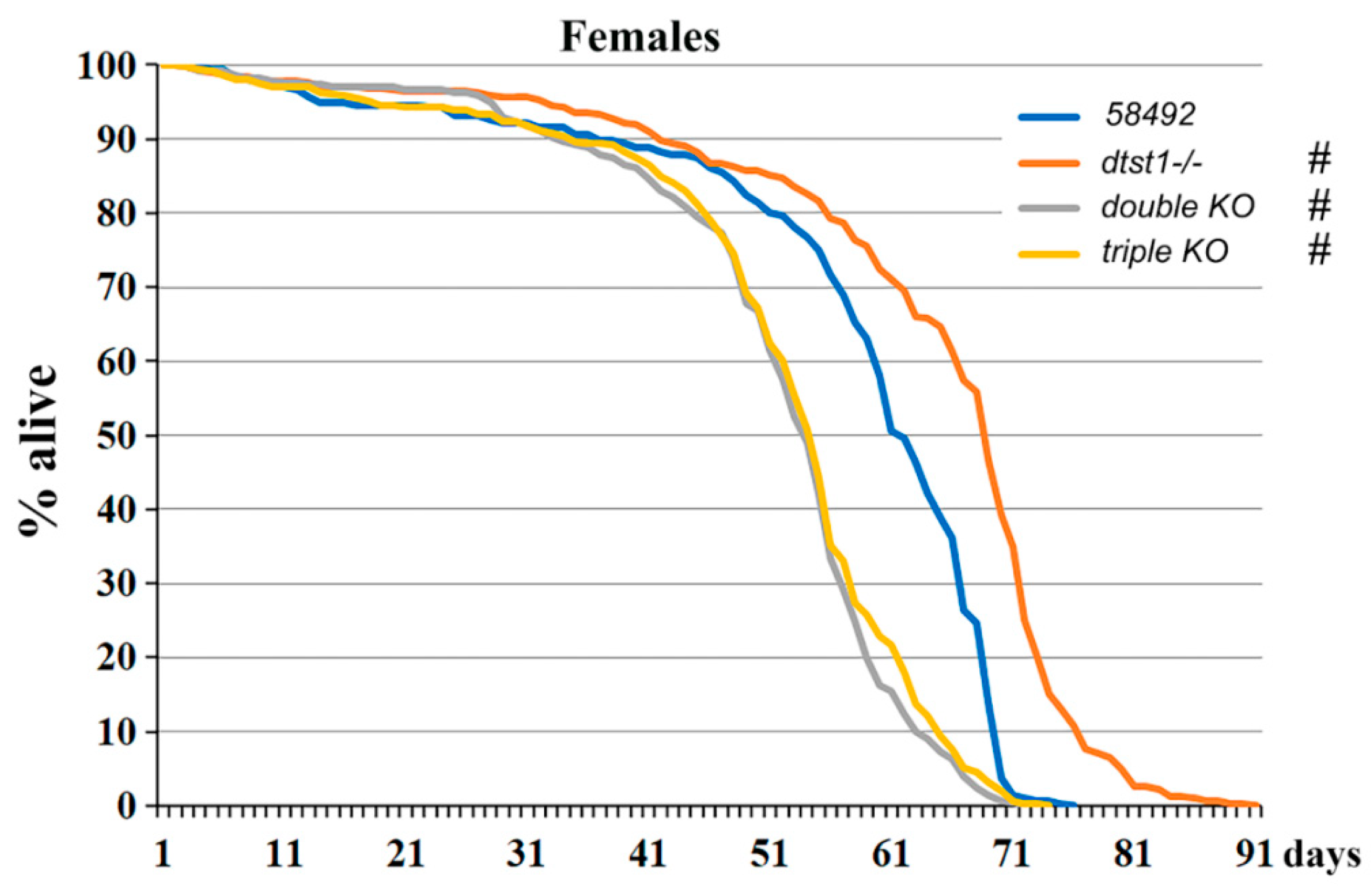
3.2. Genes Involved in H2S Synthesis Affect the Lifespan of Drosophila Females
3.3. Fecundity Changes in the Knockout Strains
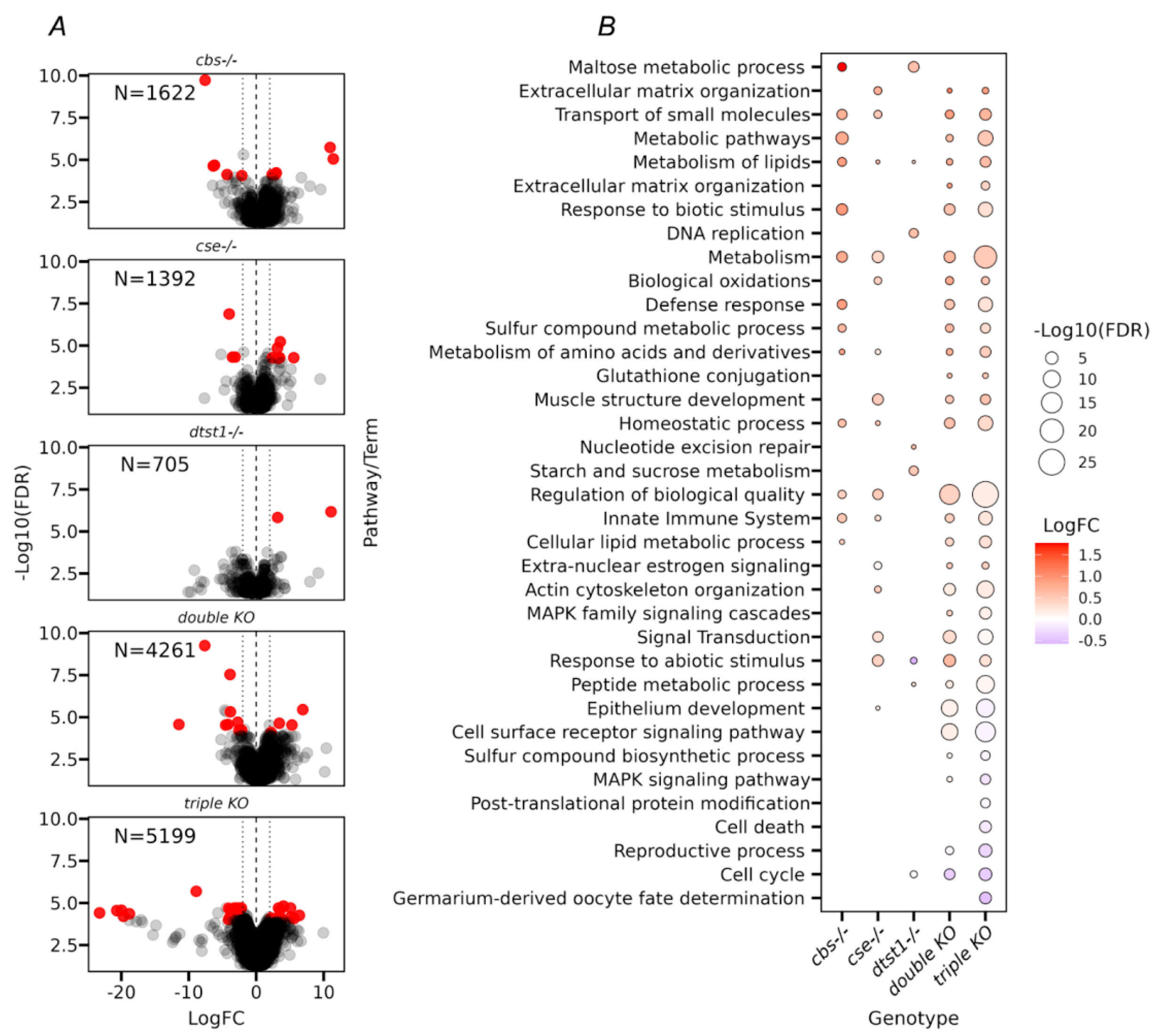
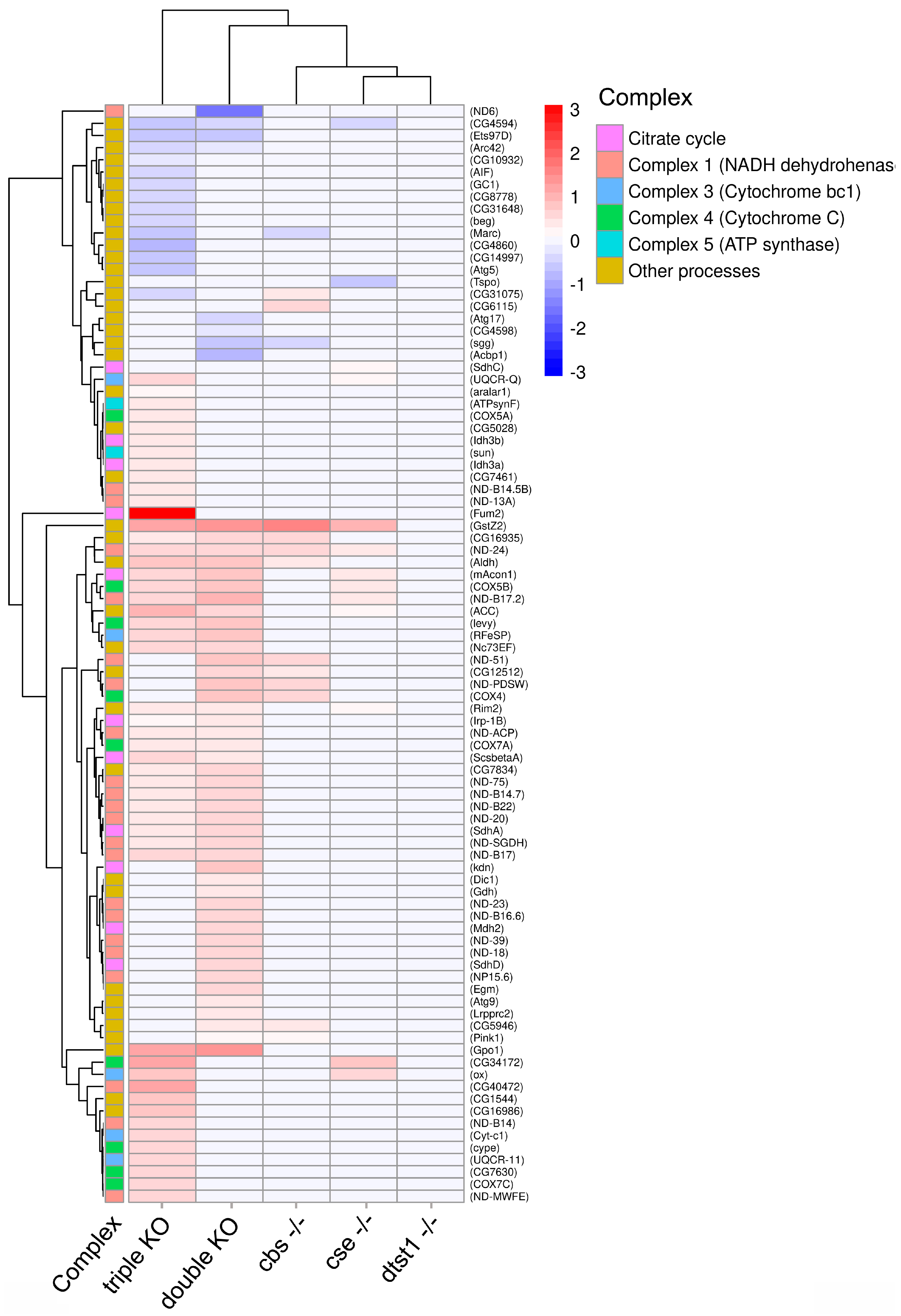
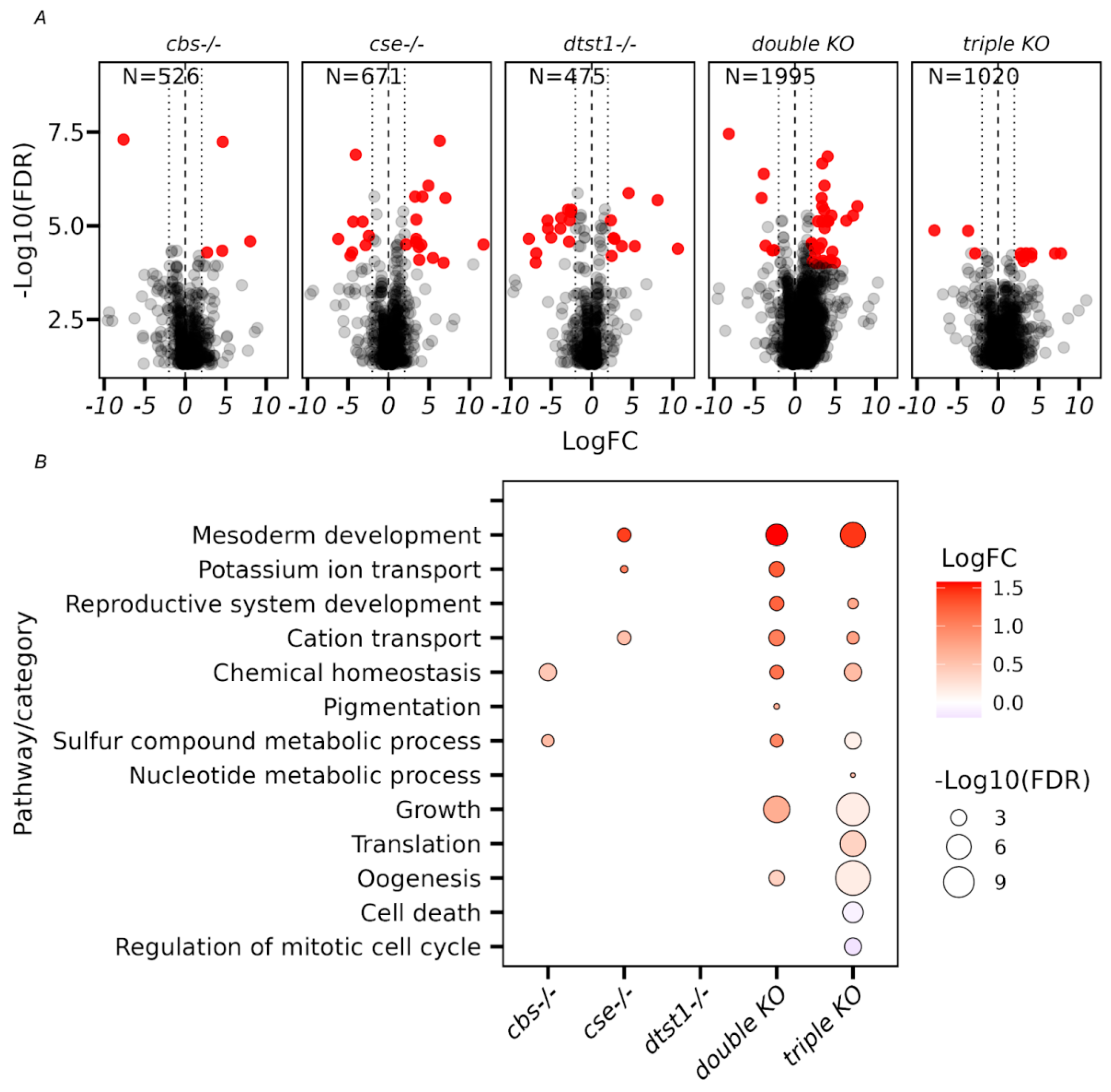
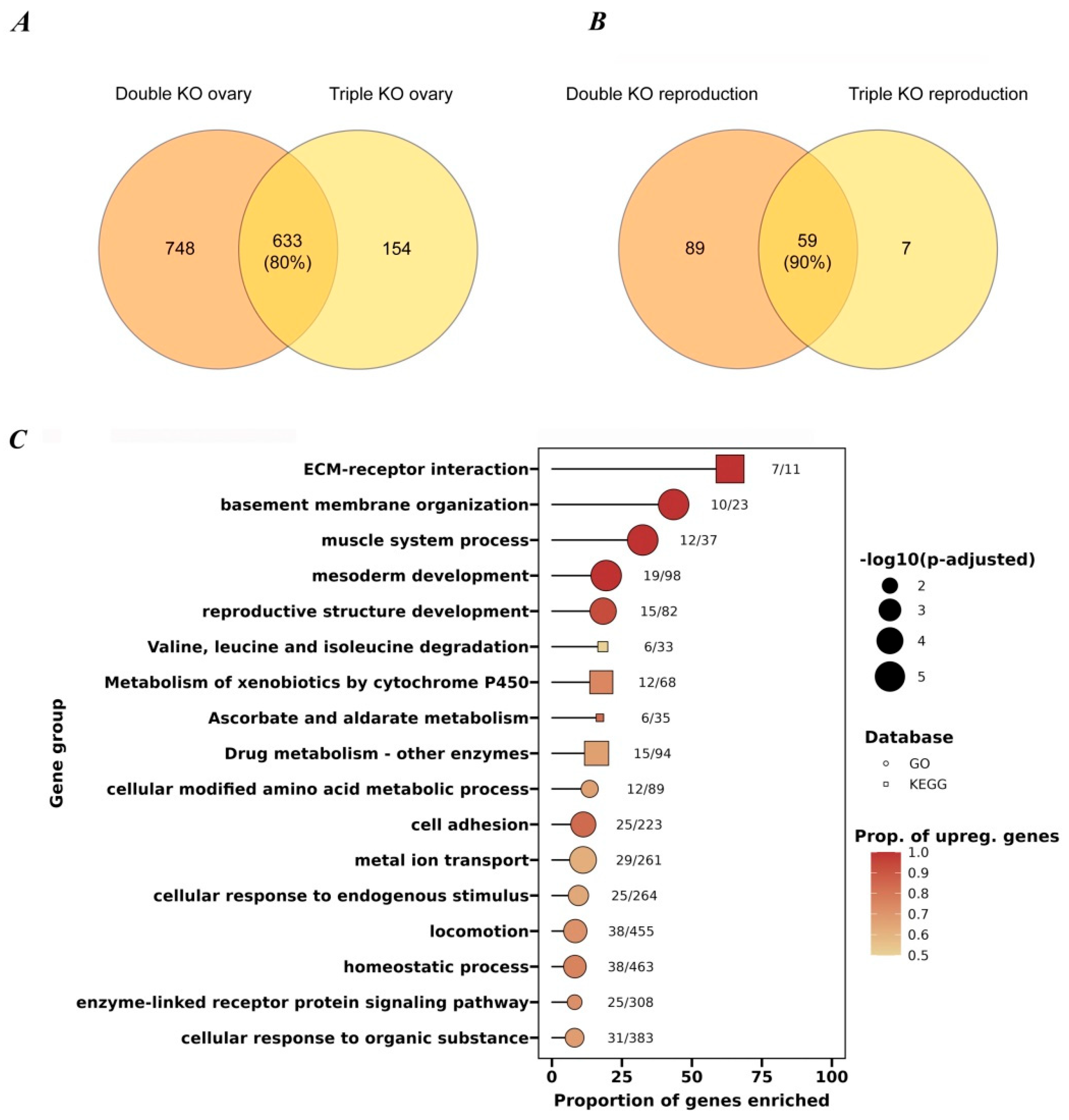
3.4. Transcriptome Analysis of Ovaries from the Studied KO Strains
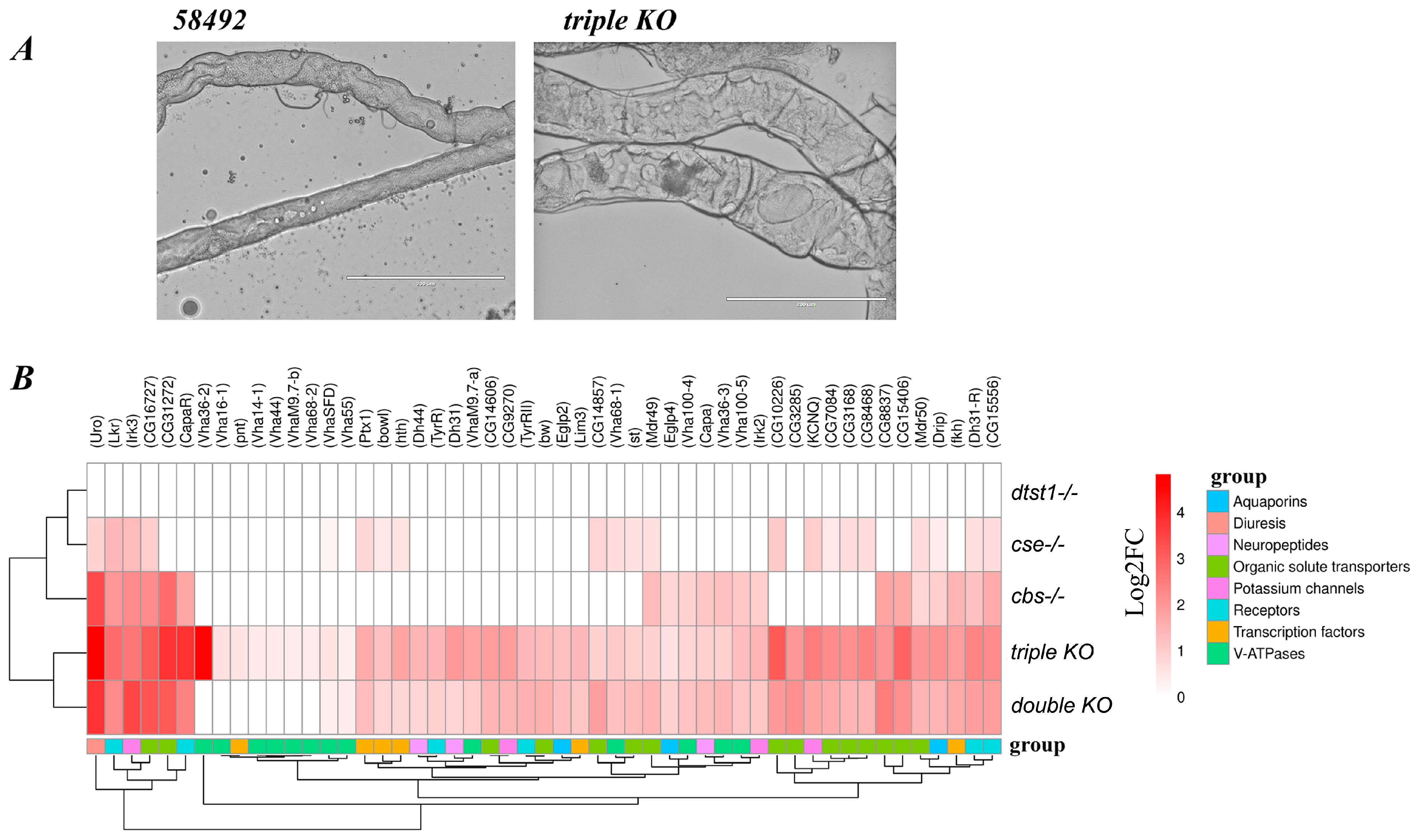
3.5. Changes in Malpighian Tubule Structure and Function in Double- and Triple-Knockout Strains
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mesfin, F.M.; Hunter, C.E.; Olson, K.R.; Shelley, W.C.; Brokaw, J.P.; Manohar, K.; Markel, T.A. Recent Development of the Molecular and Cellular Mechanisms of Hydrogen Sulfide Gasotransmitter. Antioxidants 2022, 11, 1788. [Google Scholar] [CrossRef]
- Wang, R. Hydrogen sulfide: The third gasotransmitter in biology and medicine. Antioxid. Redox Signal. 2010, 12, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Moore, P.K.; Zhu, Y.Z. H2S biosynthesis and catabolism: New insights from molecular studies. Cell. Mol. Life Sci. 2017, 74, 1391–1412. [Google Scholar] [CrossRef]
- Li, L.; Moore, P.K. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol. Sci. 2008, 29, 84–90. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay, and signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Singh, R.P.; Banerjee, N. Exploring the Influence of Celebrity Credibility on Brand Attitude, Advertisement Attituoxidative stress de and Purchase Intention. Glob. Bus. Rev. 2018, 19, 1622–1639. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.; Wen, J.Y.; Chen, Z.W. 3-Mercaptopyruvate sulfurtransferase/hydrogen sulfide protects cerebral endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury via mitoprotection and inhibition of the RhoA/ROCK pathway. Am. J. Physiol.-Cell Physiol. 2020, 319, C720–C733. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B. Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Deleon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R592–R603. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Sawa, T.; Kitajima, N.; Ono, K.; Inoue, H.; Ihara, H.; Motohashi, H.; Yamamoto, M.; Suematsu, M.; Kurose, H.; et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012, 8, 714–724. [Google Scholar] [CrossRef]
- Yadav, P.K.; Martinov, M.; Vitvitsky, V.; Seravalli, J.; Wedmann, R.; Filipovic, M.R.; Banerjee, R. Biosynthesis and Reactivity of Cysteine Persulfides in Signaling. J. Am. Chem. Soc. 2016, 138, 289–299. [Google Scholar] [CrossRef]
- Zainol Abidin, Q.H.; Ida, T.; Morita, M.; Matsunaga, T.; Nishimura, A.; Jung, M.; Hassan, N.; Takata, T.; Ishii, I.; Kruger, W.; et al. Synthesis of Sulfides and Persulfides Is Not Impeded by Disruption of Three Canonical Enzymes in Sulfur Metabolism. Antioxidants 2023, 12, 868. [Google Scholar] [CrossRef]
- Carballal, S.; Banerjee, R. Overview of cysteine metabolism. In Redox Chemistry and Biology of Thiols; Elsevier: Amsterdam, The Netherlands, 2022; pp. 423–450. [Google Scholar]
- Li, Z.; Polhemus, D.J.; Lefer, D.J. Evolution of Hydrogen Sulfide Therapeutics to Treat Cardiovascular Disease. Circ. Res. 2018, 123, 590–600. [Google Scholar] [CrossRef]
- Scammahorn, J.J.; Nguyen, I.T.N.; Bos, E.M.; Van Goor, H.; Joles, J.A. Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Antioxidants 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, H.; Guo, N. Protective effect of hydrogen sulfide on the kidney (Review). Mol. Med. Rep. 2021, 24, 696. [Google Scholar] [CrossRef]
- Kabil, O.; Motl, N.; Banerjee, R. H2S and its role in redox signaling. Biochim. Biophys. Acta 2014, 1844, 1355–1366. [Google Scholar] [CrossRef]
- Pilsova, A.; Pilsova, Z.; Klusackova, B.; Zelenkova, N.; Chmelikova, E.; Postlerova, P.; Sedmikova, M. Hydrogen sulfide and its role in female reproduction. Front. Vet. Sci. 2024, 11, 1378435. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, R.; Zhong, Q.; Song, Y.; Feng, X. Regulatory effects of hydrogen sulfide on the female reproductive system. Eur. J. Pharmacol. 2024, 963, 176265. [Google Scholar] [CrossRef] [PubMed]
- Zatsepina, O.G.; Chuvakova, L.N.; Nikitina, E.A.; Rezvykh, A.P.; Zakluta, A.S.; Sarantseva, S.V.; Surina, N.V.; Ksenofontov, A.L.; Baratova, L.A.; Shilova, V.Y.; et al. Genes Responsible for H2S Production and Metabolism Are Involved in Learning and Memory in Drosophila melanogaster. Biomolecules 2022, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Shilova, V.; Zatsepina, O.; Zakluta, A.; Karpov, D.; Chuvakova, L.; Garbuz, D.; Evge’ev, M. Age-dependent expression profiles of two adaptogenic systems and thermotolerance in Drosophila melanogaster. Cell Stress Chaperones 2020, 25, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, M.V.; Zakluta, A.S.; Zemskaya, N.V.; Guvatova, Z.G.; Shilova, V.Y.; Yakovleva, D.V.; Gorbunova, A.A.; Koval, L.A.; Ulyasheva, N.S.; Evgen’ev, M.B.; et al. Deletions of the cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) genes, involved in the control of hydrogen sulfide biosynthesis, significantly affect lifespan and fitness components of Drosophila melanogaster. Mech. Ageing Dev. 2022, 203, 111656. [Google Scholar] [CrossRef]
- Zatsepina, O.; Karpov, D.; Chuvakova, L.; Rezvykh, A.; Funikov, S.; Sorokina, S.; Zakluta, A.; Garbuz, D.; Shilova, V.; Evgen’ev, M. Genome-wide transcriptional effects of deletions of sulphur metabolism genes in Drosophila melanogaster. Redox Biol. 2020, 36, 101654. [Google Scholar] [CrossRef]
- Mathew, N.D.; Schlipalius, D.I.; Ebert, P.R. Sulfurous gases as biological messengers and toxins: Comparative genetics of their metabolism in model organisms. J. Toxicol. 2011, 2011, 394970. [Google Scholar] [CrossRef]
- Rao, S.P.; Dobariya, P.; Bellamkonda, H.; More, S.S. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants 2023, 12, 603. [Google Scholar] [CrossRef]
- Cipollone, R.; Ascenzi, P.; Visca, P. Common themes and variations in the rhodanese superfamily. IUBMB Life 2007, 59, 51–59. [Google Scholar] [CrossRef]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef]
- Nagahara, N.; Okazaki, T.; Nishino, T. Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking similarity in active site amino acid sequence and the increase in the mercaptopyruvate sulfurtransferase activity of rhodanese by site-directed mutagenesis. J. Biol. Chem. 1995, 270, 16230–16235. [Google Scholar] [CrossRef]
- Kruithof, P.D.; Lunev, S.; Aguilar Lozano, S.P.; de Assis Batista, F.; Al-Dahmani, Z.M.; Joles, J.A.; Dolga, A.M.; Groves, M.R.; van Goor, H. Unraveling the role of thiosulfate sulfurtransferase in metabolic diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165716. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Motl, N.; Akey, D.L.; Sakamoto, N.; Fearon, E.R.; Smith, J.L.; Banerjee, R. Thiosulfate sulfurtransferase-like domain-containing 1 protein interacts with thioredoxin. J. Biol. Chem. 2018, 293, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Melideo, S.L.; Jackson, M.R.; Jorns, M.S. Biosynthesis of a central intermediate in hydrogen sulfide metabolism by a novel human sulfurtransferase and its yeast ortholog. Biochemistry 2014, 53, 4739–4753. [Google Scholar] [CrossRef]
- Ansar, M.; Thu, L.T.A.; Hung, C.S.; Su, C.-M.; Huang, M.-H.; Liao, L.-M.; Chung, Y.-M.; Lin, R.-K. Promoter hypomethylation and overexpression of TSTD1 mediate poor treatment response in breast cancer. Front. Oncol. 2022, 12, 1004261. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Feng, Z.; Liu, J. Integrative Analyses Reveal. Cells 2021, 10, 2976. [Google Scholar] [CrossRef]
- Hine, C.; Mitchell, J.R. Endpoint or Kinetic Measurement of Hydrogen Sulfide Production Capacity in Tissue Extracts. Bio-Protocol 2017, 7, e2382. [Google Scholar] [CrossRef]
- Shaposhnikov, M.V.; Zemskaya, N.V.; Koval, L.A.; Schegoleva, E.V.; Yakovleva, D.V.; Ulyasheva, N.S.; Gorbunova, A.A.; Minnikhanova, N.R.; Moskalev, A.A. Geroprotective potential of genetic and pharmacological interventions to endogenous hydrogen sulfide synthesis in Drosophila melanogaster. Biogerontology 2021, 22, 197–214. [Google Scholar] [CrossRef]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.S.; Lee, S.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Redden, D.T.; Weindruch, R.; Allison, D.B. Statistical methods for testing effects on “maximum lifespan”. Mech. Ageing Dev. 2004, 125, 629–632. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, G.S.; Dmitriev, A.A.; Kudryavtseva, A.V.; Shargunov, A.V.; Karpov, D.S.; Uroshlev, L.A.; Melnikova, N.V.; Blinov, V.M.; Poverennaya, E.V.; Archakov, A.I.; et al. PPLine: An Automated Pipeline for SNP, SAP, and Splice Variant Detection in the Context of Proteogenomics. J. Proteome Res. 2015, 14, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Zhu, R.; Wang, F.; Cheng, Y.; Liu, Y. Three Differential Expression Analysis Methods for RNA Sequencing: Limma, EdgeR, DESeq2. J. Vis. Exp. 2021, 175, e62528. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Olson, K.R. H2S and polysulfide metabolism: Conventional and unconventional pathways. Biochem. Pharmacol. 2018, 149, 77–90. [Google Scholar] [CrossRef]
- Braccia, D.J.; Jiang, X.; Pop, M.; Hall, A.B. The Capacity to Produce Hydrogen Sulfide. Front. Microbiol. 2021, 12, 705583. [Google Scholar] [CrossRef]
- Research responsibility. Nature 1985, 316, 380. [CrossRef]
- Parkhitko, A.A.; Filine, E.; Mohr, S.E.; Moskalev, A.; Perrimon, N. Targeting metabolic pathways for extension of lifespan and healthspan across multiple species. Ageing Res. Rev. 2020, 64, 101188. [Google Scholar] [CrossRef]
- Kansara, K.; Gupta, S.S. DNA Damage, repair, and maintenance of telomere length: Role of nutritional supplements. In Mutagenicity: Assays and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 287–307. [Google Scholar] [CrossRef]
- Schellnegger, M.; Hofmann, E.; Carnieletto, M.; Kamolz, L.P. Unlocking longevity: The role of telomeres and its targeting interventions. Front. Aging 2024, 5, 1339317. [Google Scholar] [CrossRef] [PubMed]
- Strilbytska, O.; Strutynska, T.; Semaniuk, U.; Burdyliyk, N.; Lushchak, O. Dietary sucrose defines lifespan and metabolism in Drosophila. Ukr. Biochem. J. 2020, 92, 5. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef]
- Huang, D.; Jing, G.; Zhu, S. Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants 2023, 12, 1644. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Wei, Y.H. Mitochondria and aging. Adv. Exp. Med. Biol. 2012, 942, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Hanson, F.B.; Ferris, F.R. A quantitative study of fecundity in Drosophila melanogaster. J. Exp. Zool. 1929, 54, 485–506. [Google Scholar] [CrossRef]
- Töpfer, U.; Guerra Santillán, K.Y.; Fischer-Friedrich, E.; Dahmann, C. Distinct contributions of ECM proteins to basement membrane mechanical properties in Drosophila. Development 2022, 149, dev200456. [Google Scholar] [CrossRef]
- Cohen, E.; Sawyer, J.K.; Peterson, N.G.; Dow, J.A.T.; Fox, D.T. Physiology, Development, and Disease Modeling in the Drosophila Excretory System. Genetics 2020, 214, 235–264. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Davies, S.A. The Drosophila melanogaster malpighian tubule. Adv. Insect Physiol. 2001, 28, 455–464. [Google Scholar]
- Dow, J.A. Insights into the Malpighian tubule from functional genomics. J. Exp. Biol. 2009, 212 Pt 3, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.A.T.; Davies, S.A.; SoöZEN, M.A. Fluid Secretion by the Drosophila Malpighian Tubule1. Am. Zool. 2015, 38, 450–460. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Simons, M.; Romero, M.F. Drosophila melanogaster: A simple genetic model of kidney structure, function and disease. Nat. Rev. Nephrol. 2022, 18, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kean, L.; Yang, J.; Allan, A.K.; Davies, S.A.; Herzyk, P.; Dow, J.A. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004, 5, R69. [Google Scholar] [CrossRef]
- Coast, G.M.; Webster, S.G.; Schegg, K.M.; Tobe, S.S.; Schooley, D.A. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J. Exp. Biol. 2001, 204 Pt 10, 1795–1804. [Google Scholar] [CrossRef]
- Dates, J.; Kolosov, D. Voltage-gated ion channels as novel regulators of epithelial ion transport in the osmoregulatory organs of insects. Front. Insect Sci. 2024, 4, 1385895. [Google Scholar] [CrossRef]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef]
- Kimura, H. Signalling by hydrogen sulfide and polysulfides via protein S-sulfuration. Br. J. Pharmacol. 2020, 177, 720–733. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Tsuji, J.; Fu, S.C.; Tomii, K.; Horton, P.; Imai, K. MitoFates: Improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol. Cell. Proteom. 2015, 14, 1113–1126. [Google Scholar] [CrossRef]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Casadio, R. TPpred3 detects and discriminates mitochondrial and chloroplastic targeting peptides in eukaryotic proteins. Bioinformatics 2015, 31, 3269–3275. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Salvatore, M.; Emanuelsson, O.; Winther, O.; von Heijne, G.; Elofsson, A.; Nielsen, H. Detecting sequence signals in targeting peptides using deep learning. Life Sci. Alliance 2019, 2, e201900429. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Tanaka, M.; Tanaka, Y.; Ito, T. Novel Characterization of Antioxidant Enzyme, 3-Mercaptopyruvate Sulfurtransferase-Knockout Mice: Overexpression of the Evolutionarily-Related Enzyme Rhodanese. Antioxidants 2019, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Buonvino, S.; Arciero, I.; Melino, S. Thiosulfate-Cyanide Sulfurtransferase a Mitochondrial Essential Enzyme: From Cell Metabolism to the Biotechnological Applications. Int. J. Mol. Sci. 2022, 23, 8452. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Kamińska, M.; Kaminski, K.; Wróbel, M. The Expression and Activity of Rhodanese, 3-Mercaptopyruvate Sulfurtransferase, Cystathionine γ-Lyase in the Most Frequently Chosen Cellular Research Models. Biomolecules 2021, 11, 1859. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide and polysulfides as signaling molecules. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 131–159. [Google Scholar] [CrossRef]
- Wen, J.; Wang, Y.; Yuan, M.; Huang, Z.; Zou, Q.; Pu, Y.; Zhao, B.; Cai, Z. Role of mismatch repair in aging. Int. J. Biol. Sci. 2021, 17, 3923–3935. [Google Scholar] [CrossRef]
- Hall, B.S.; Barnett, Y.A.; Crofts, J.J.; Chuzhanova, N. Identification of novel genes associated with longevity in Drosophila melanogaster—A computational approach. Aging 2019, 11, 11244–11267. [Google Scholar] [CrossRef]
- Inomata, N.; Takahasi, K.R.; Koga, N. Association between duplicated maltase genes and the transcriptional regulation for the carbohydrate changes in Drosophila melanogaster. Gene 2019, 686, 141–145. [Google Scholar] [CrossRef]
- Zuhra, K.; Petrosino, M.; Janickova, L.; Petric, J.; Ascenção, K.; Vignane, T.; Khalaf, M.; Philipp, T.M.; Ravani, S.; Anand, A.; et al. Regulation of mammalian cellular metabolism by endogenous cyanide production. Nat. Metab. 2025, 7, 531–555. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Pang, J.; Xue, W.; Cui, Y.; Jiang, N.; Zhi, W.; Shi, H.; Horuzsko, A.; Pace, B.S.; Zhu, X. Transsulfuration pathway activation attenuates oxidative stress and ferroptosis in sickle primary erythroblasts and transgenic mice. Commun. Biol. 2025, 8, 15. [Google Scholar] [CrossRef]
- Liu, M.; Barnes, V.L.; Pile, L.A. Disruption of Methionine Metabolism in Drosophila melanogaster Impacts Histone Methylation and Results in Loss of Viability. G3 2015, 6, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Parkhitko, A.A.; Jouandin, P.; Mohr, S.E.; Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 2019, 18, e13034. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Moskovitz, J.; Levine, R.L. Oxidation of methionine residues of proteins: Biological consequences. Antioxid. Redox Signal. 2003, 5, 577–582. [Google Scholar] [CrossRef]
- Deng, H. Multiple roles of Nrf2-Keap1 signaling: Regulation of development and xenobiotic response using distinct mechanisms. Fly 2014, 8, 7–12. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef]
- Hochmuth, C.E.; Biteau, B.; Bohmann, D.; Jasper, H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 2011, 8, 188–199. [Google Scholar] [CrossRef] [PubMed]
- The PLOS Genetics Editors. Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells. PLoS Genet. 2015, 11, e1005377. [Google Scholar] [CrossRef]
- Salinas, A.E.; Wong, M.G. Glutathione S-transferases—A review. Curr. Med. Chem. 1999, 6, 279–309. [Google Scholar] [CrossRef]
- Koirala, B.K.S.; Moural, T.; Zhu, F. Functional and Structural Diversity of Insect Glutathione S-transferases in Xenobiotic Adaptation. Int. J. Biol. Sci. 2022, 18, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Dimunová, D.; Matoušková, P.; Podlipná, R.; Boušová, I.; Skálová, L. The role of UDP-glycosyltransferases in xenobioticresistance. Drug Metab. Rev. 2022, 54, 282–298. [Google Scholar] [CrossRef]
- Ahn, S.J.; Marygold, S.J. The UDP-Glycosyltransferase Family in Drosophila melanogaster: Nomenclature Update, Gene Expression and Phylogenetic Analysis. Front. Physiol. 2021, 12, 648481. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Robert, K.; Nehmé, J.; Bourdon, E.; Pivert, G.; Friguet, B.; Delcayre, C.; Delabar, J.; Janel, N. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology 2005, 128, 1405–1415. [Google Scholar] [CrossRef]
- Outinen, P.A.; Sood, S.K.; Pfeifer, S.I.; Pamidi, S.; Podor, T.J.; Li, J.; Weitz, J.I.; Austin, R.C. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood 1999, 94, 959–967. [Google Scholar] [CrossRef]
- Farina, P.; Bedini, S.; Conti, B. Multiple Functions of Malpighian Tubules in Insects: A Review. Insects 2022, 13, 1001. [Google Scholar] [CrossRef]
- Lobb, I.; Sonke, E.; Aboalsamh, G.; Sener, A. Hydrogen sulphide and the kidney: Important roles in renal physiology and pathogenesis and treatment of kidney injury and disease. Nitric Oxide 2015, 46, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Daborn, P.J.; Yen, J.L.; Bogwitz, M.R.; Le Goff, G.; Feil, E.; Jeffers, S.; Tijet, N.; Perry, T.; Heckel, D.; Batterham, P.; et al. A single p450 allele associated with insecticide resistance in Drosophila. Science 2002, 297, 2253–2256. [Google Scholar] [CrossRef]
- Joussen, N.; Schuphan, I.; Schmidt, B. Metabolism of methoxychlor by the P450-monooxygenase CYP6G1 involved in insecticide resistance of Drosophila melanogaster after expression in cell cultures of Nicotiana tabacum. Chem. Biodivers. 2010, 7, 722–735. [Google Scholar] [CrossRef]
- Seong, K.M.; Sun, W.; Clark, J.M.; Pittendrigh, B.R. Splice form variant and amino acid changes in MDR49 confers DDT resistance in transgenic Drosophila. Sci. Rep. 2016, 6, 23355. [Google Scholar] [CrossRef] [PubMed]
- Denecke, S.; Bảo Lương, H.N.; Koidou, V.; Kalogeridi, M.; Socratous, R.; Howe, S.; Vogelsang, K.; Nauen, R.; Batterham, P.; Geibel, S.; et al. Characterization of a novel pesticide transporter and P-glycoprotein orthologues in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220625. [Google Scholar] [CrossRef] [PubMed]
- Kalantaridou, S.N.; Makrigiannakis, A.; Zoumakis, E.; Chrousos, G.P. Stress and the female reproductive system. J. Reprod. Immunol. 2004, 62, 61–68. [Google Scholar] [CrossRef]
- Marshall, K.E.; Sinclair, B.J. Repeated stress exposure results in a survival-reproduction trade-off in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2010, 277, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Belles, X.; Piulachs, M.D. Ecdysone signalling and ovarian development in insects: From stem cells to ovarian follicle formation. Biochim. Biophys. Acta 2015, 1849, 181–186. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sheng, Z.; Sun, Z.; Palli, S.R. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 429–439. [Google Scholar] [CrossRef]
- Meiselman, M.R.; Kingan, T.G.; Adams, M.E. Stress-induced reproductive arrest in Drosophila occurs through ETH deficiency-mediated suppression of oogenesis and ovulation. BMC Biol. 2018, 16, 18. [Google Scholar] [CrossRef]
- Guzmán, M.A.; Navarro, M.A.; Carnicer, R.; Sarría, A.J.; Acín, S.; Arnal, C.; Muniesa, P.; Surra, J.C.; Arbonés-Mainar, J.M.; Maeda, N.; et al. Cystathionine beta-synthase is essential for female reproductive function. Hum. Mol. Genet. 2006, 15, 3168–3176. [Google Scholar] [CrossRef]
- Gu, M.; Wang, Y.; Yu, Y. Ovarian fibrosis: Molecular mechanisms and potential therapeutic targets. J. Ovarian Res. 2024, 17, 139. [Google Scholar] [CrossRef]
- Van De Bor, V.; Loreau, V.; Malbouyres, M.; Cerezo, D.; Placenti, A.; Ruggiero, F.; Noselli, S. A dynamic and mosaic basement membrane controls cell intercalation in Drosophila ovaries. Development 2021, 148, dev195511. [Google Scholar] [CrossRef]
- Daley, W.P.; Yamada, K.M. ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Curr. Opin. Genet. Dev. 2013, 23, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, M.A.; Sherwood, D.R. An active role for basement membrane assembly and modification in tissue sculpting. J. Cell Sci. 2015, 128, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lewis, W.; Page-McCaw, A. Basement membrane mechanics shape development: Lessons from the fly. Matrix Biol. 2019, 75–76, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Shahid, A.; Bhatia, M. Hydrogen Sulfide: A Versatile Molecule and Therapeutic Target in Health and Diseases. Biomolecules 2024, 14, 1145. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilova, V.Y.; Garbuz, D.G.; Chuvakova, L.N.; Rezvykh, A.P.; Funikov, S.Y.; Davletshin, A.I.; Sorokina, S.Y.; Nikitina, E.A.; Gorenskaya, O.; Evgen’ev, M.B.; et al. The Biological Consequences of the Knockout of Genes Involved in the Synthesis and Metabolism of H2S in Drosophila melanogaster. Antioxidants 2025, 14, 693. https://doi.org/10.3390/antiox14060693
Shilova VY, Garbuz DG, Chuvakova LN, Rezvykh AP, Funikov SY, Davletshin AI, Sorokina SY, Nikitina EA, Gorenskaya O, Evgen’ev MB, et al. The Biological Consequences of the Knockout of Genes Involved in the Synthesis and Metabolism of H2S in Drosophila melanogaster. Antioxidants. 2025; 14(6):693. https://doi.org/10.3390/antiox14060693
Chicago/Turabian StyleShilova, Victoria Y., David G. Garbuz, Lyubov N. Chuvakova, Alexander P. Rezvykh, Sergei Y. Funikov, Artem I. Davletshin, Svetlana Y. Sorokina, Ekaterina A. Nikitina, Olga Gorenskaya, Michael B. Evgen’ev, and et al. 2025. "The Biological Consequences of the Knockout of Genes Involved in the Synthesis and Metabolism of H2S in Drosophila melanogaster" Antioxidants 14, no. 6: 693. https://doi.org/10.3390/antiox14060693
APA StyleShilova, V. Y., Garbuz, D. G., Chuvakova, L. N., Rezvykh, A. P., Funikov, S. Y., Davletshin, A. I., Sorokina, S. Y., Nikitina, E. A., Gorenskaya, O., Evgen’ev, M. B., & Zatsepina, O. G. (2025). The Biological Consequences of the Knockout of Genes Involved in the Synthesis and Metabolism of H2S in Drosophila melanogaster. Antioxidants, 14(6), 693. https://doi.org/10.3390/antiox14060693







