Reactive Oxidative Species in Carotid Body Chemoreception: Their Role in Oxygen Sensing and Cardiorespiratory Alterations Induced by Chronic Intermittent Hypoxia
Abstract
1. Introduction
1.1. The Carotid Body Chemoreceptors
1.2. The Oxygen-Sensing Mechanisms in the Carotid Body
1.3. Contribution of the Carotid Body to the Pathological Consequences of Autonomic-Related Diseases
2. The Carotid Body Oxygen Sensing Process and ROS
2.1. The Mitochondria to Membrane Signaling Model for O2 Sensing in the Carotid Body
2.2. Unique Higher Oxygen Sensitivity of the Carotid Body Chemoreceptor Cells
3. Chronic Intermittent Hypoxia Enhanced Carotid Body Chemosensory Discharges in Preclinical Models of Obstructive Sleep Apnea
3.1. Obstructive Sleep Apnea
3.2. Chronic Intermittent Hypoxia Produces Carotid Body Chemosensory Potentiation
4. Carotid Body Chemosensory Potentiation Induced by CIH: The Role of Oxidative Stress
Oxidative Stress Induced by Chronic Intermittent Hypoxia the Carotid Body and the Chemoreflex Neural Pathway
5. Carotid Body Chemosensory Potentiation Induced by CIH: Role of Pro-Inflammatory Molecules
Oxidative Stress and Inflammation in the Carotid Body and the Chemoreflex Neural Pathway Induced by Chronic Intermittent Hypoxia
6. Chronic Intermittent Hypoxia-Induced Activation of the Carotid Body Chemosensory Pathway
6.1. Chronic Intermittent Hypoxia Activates Neurons and Glial Cells in the Nucleus of the Tractus Solitarius
6.2. Role of Nucleus of the Tractus Solitarius Resident Glial Cells on the Inflammation Induced by Chronic Intermittent Hypoxia
6.3. Crucial Role of the Carotid Body in the Glial Cell Activation and Inflammation Elicited in the Nucleus of the Tractus Solitarius by Chronic Intermittent Hypoxia
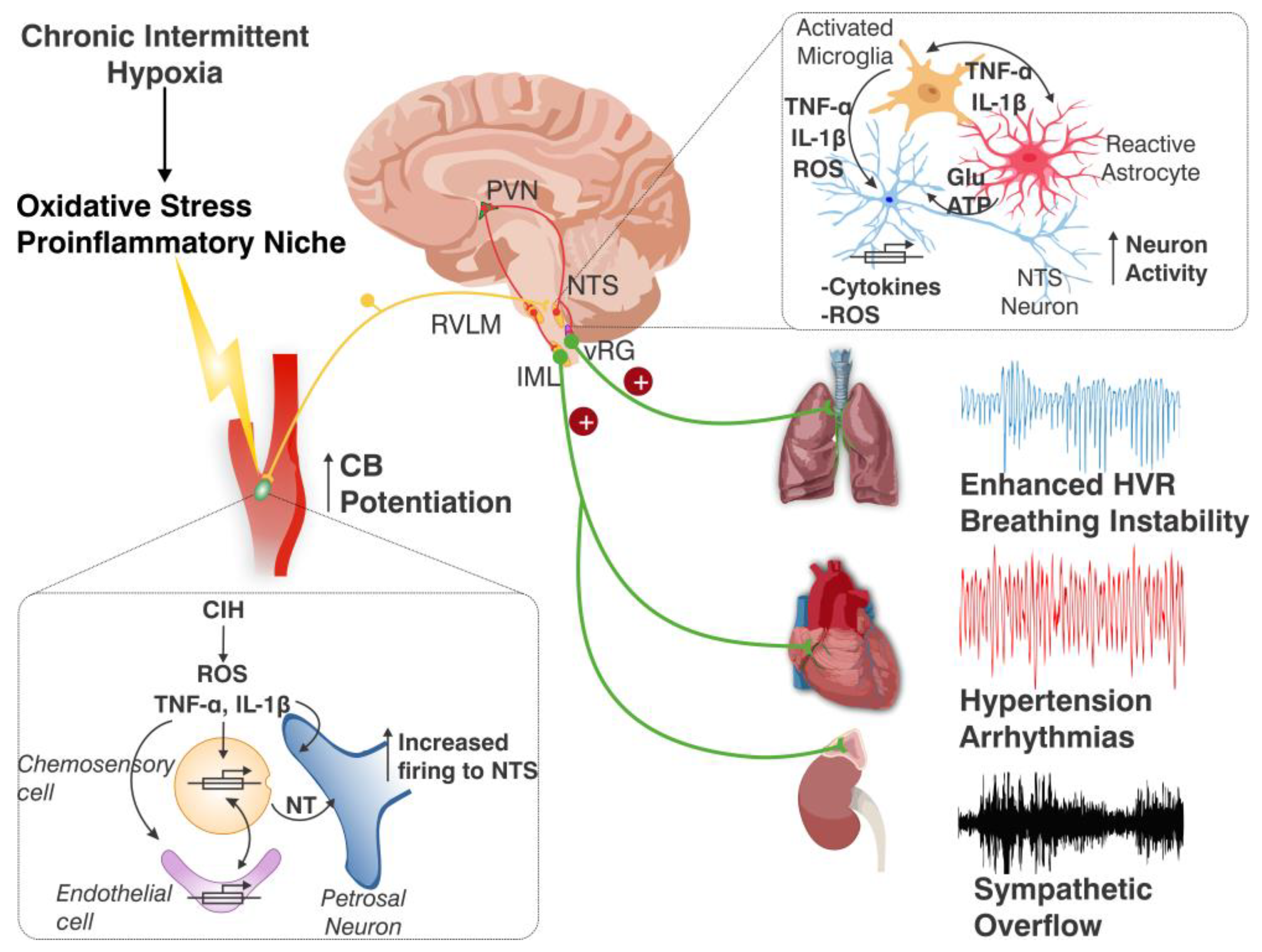
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Prabhakar, N.R.; Peng, Y.J.; Kumar, G.K.; Nanduri, J. Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr. Physiol. 2015, 5, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Zera, T.; Moraes, D.J.A.; da Silva, M.P.; Fisher, J.P.; Paton, J.F.R. The logic of carotid body connectivity to the brain. Physiology 2019, 34, 264–282. [Google Scholar] [CrossRef]
- Iturriaga, R.; Del Rio, R.; Alcayaga, J. Carotid Body Inflammation: Role in Hypoxia and in the Anti-inflammatory Reflex. Physiology 2021, 37, 128–140. [Google Scholar] [CrossRef]
- Kline, D.D. Chronic intermittent hypoxia affects integration of sensory input by neurons in the nucleus tractus solitarii. Respir. Physiol. Neurobiol. 2010, 174, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zoccal, D.B.; Furuya, W.I.; Bassi, M.; Colombari, D.S.A.; Colombari, E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front. Physiol. 2014, 5, 238. [Google Scholar] [CrossRef]
- Moreira, T.S.; Takakura, A.C.; Falquetto, B.; Ramirez, J.M.; Oliveira, L.M.; Silva, P.E.; Araujo, E.V. Neuroanatomical and neurochemical organization of brainstem and forebrain circuits involved in breathing regulation. J. Neurophysiol. 2025, 133, 1116–1137. [Google Scholar] [CrossRef]
- Iturriaga, R.; Alcayaga, J. Neurotransmission in the carotid body: Transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res. Rev. 2004, 47, 46–53. [Google Scholar] [CrossRef]
- Nurse, C.A. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp. Physiol. 2010, 95, 657–667. [Google Scholar] [CrossRef]
- Moya, E.A.; Alcayaga, J.; Iturriaga, R. NO modulation of carotid body chemoreception in health and disease. Respir. Physiol. Neurobiol. 2012, 184, 158–164. [Google Scholar] [CrossRef]
- Fung, M.L. Expressions of angiotensin and cytokine receptors in the paracrine signaling of the carotid body in hypoxia and sleep apnea. Respir. Physiol. Neurobiol. 2015, 209, 6–12. [Google Scholar] [CrossRef]
- Conde, S.V.; Martins, F.O.; Sacramento, J.F. Carotid body interoception in health and disease. Auton. Neurosci. Basic Clin. 2024, 255, 103207. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; González, S.; Rey, S.; Cortés, P.P.; Maisey, K.R.; Reyes, E.P.; Larraín, C.; Zapata, P. Lipopolysaccharide-induced carotid body inflammation in cats: Functional manifestations, histopathology and involvement of tumour necrosis factor-alpha. Exp. Physiol. 2008, 93, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.; Larraín, C.; Reyes, P.; Fernández, R. Immunosensory signalling by carotid body chemoreceptors. Respir. Physiol. Neurobiol. 2011, 178, 370–374. [Google Scholar] [CrossRef]
- Iturriaga, R. Carotid Body Ablation: A New Target to Address Central Autonomic Dysfunction. Curr. Hypertens. Rep. 2018, 20, 53. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; De Caro, R.; Di Giulio, C. Inflammatory and immunomodulatory mechanisms in the carotid body. Respir. Physiol. Neurobiol. 2013, 187, 31–40. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Tortorella, C.; Macchi, V.; De Caro, R.; Porzionato, A. Growth Factors in the Carotid Body—An Update. Int. J. Mol. Sci. 2020, 21, 7267. [Google Scholar] [CrossRef]
- Moreno-Domínguez, A.; Ortega-Sáenz, P.; Gao, L.; Colinas, O.; García-Flores, P.; Bonilla-Henao, V.; Aragonés, J.; Hüttemann, M.; Grossman, L.I.; Weissmann, N.; et al. Acute O2 sensing through HIF2α-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci. Signal. 2020, 13, eaay9452. [Google Scholar] [CrossRef]
- Mills, E.; Jöbsis, F.F. Simultaneous Measurement of Cytochrome a3 Reduction and Chemoreceptor Afferent Activity in the Carotid Body. Nature 1970, 225, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R.; Biscoe, T.J. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J. Physiol. 1992, 450, 13–31. [Google Scholar] [CrossRef]
- Mulligan, E.; Lahiri, S. Separation of carotid body chemoreceptor responses to O2 and CO2 by oligomycin and by antimycin A. Am. J. Physiol. 1982, 242, C200–C206. [Google Scholar] [CrossRef]
- López-Barneo, J.; López-López, J.R.; Ureña, J.; González, C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science 1988, 241, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Buckler, K.J. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J. Physiol. 1997, 498, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Arias-Mayenco, I.; González-Rodríguez, P.; Torres-Torrelo, H.; Gao, L.; Fernández-Agüera, M.C.; Bonilla-Henao, V.; Ortega-Sáenz, P.; López-Barneo, J. Acute O2 Sensing: Role of Coenzyme QH2/Q Ratio and Mitochondrial ROS Compartmentalization. Cell Metab. 2018, 28, 145–158.E4. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; González-Rodríguez, P.; Ortega-Sáenz, P.; López-Barneo, J. Redox signaling in acute oxygen sensing. Redox Biol. 2017, 12, 908–915. [Google Scholar] [CrossRef]
- Gao, L.; Ortega-Sáenz, P.; Moreno-Domínguez, A.; López-Barneo, J. Mitochondrial Redox Signaling in O2-Sensing Chemoreceptor Cells. Antioxid. Redox Signal. 2022, 37, 274–289. [Google Scholar] [CrossRef]
- Buckler, K.J.; Turner, P.J. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J. Physiol. 2013, 591, 3549–3563. [Google Scholar] [CrossRef]
- Varas, R.; Wyatt, C.N.; Buckler, K.J. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J. Physiol. 2007, 583, 521–536. [Google Scholar] [CrossRef]
- Abdala, A.P.; McBryde, F.D.; Marina, N.; Hendy, E.B.; Engelman, Z.J.; Fudim, M.; Sobotka, P.A.; Gourine, A.V.; Paton, J.F. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J. Physiol. 2012, 590, 4269–4277. [Google Scholar] [CrossRef]
- Díaz, H.S.; Toledo, C.; Andrade, D.C.; Marcus, N.J.; Del Rio, R. Neuroinflammation in heart failure: New insights for an old disease. J. Physiol. 2020, 598, 33–59. [Google Scholar] [CrossRef]
- Conde, S.V.; Ribeiro, M.J.; Melo, B.F.; Guarino, M.P.; Sacramento, J.F. Insulin resistance: A new consequence of altered carotid body chemoreflex? J. Physiol. 2017, 595, 31–41. [Google Scholar] [CrossRef]
- Lazarov, N.E.; Atanasova, D.Y. Carotid Body Dysfunction and Mechanisms of Disease. In Morphofunctional and Neurochemical Aspects of the Mammalian Carotid Body; Springer Nature: Cham, Switzerland, 2023; Volume 237, pp. 123–138. [Google Scholar] [CrossRef]
- Żera, T.; Paleczny, B.; Siński, M.; Conde, S.V.; Narkiewicz, K.; Ponikowski, P.; Paton, J.F.R.; Niewiński, P. Translating physiology of the arterial chemoreflex into novel therapeutic interventions targeting carotid bodies in cardiometabolic disorders. J. Physiol. 2025, 603, 2487–2516. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.D.; Li, Y.L. Carotid body function in heart failure. Respir. Physiol. Neurobiol. 2007, 157, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Moya, E.A.; Del Rio, R. Carotid body potentiation induced by intermittent hypoxia: Implications for cardiorespiratory changes induced by sleep apnoea. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1197–1204. [Google Scholar] [CrossRef]
- McBryde, F.D.; Abdala, A.P.; Hendy, E.B.; Pijacka, W.; Marvar, P.; Moraes, D.J.; Sobotka, P.A.; Paton, J.F. The carotid body is a putative therapeutic target for the treatment of neurogenic hypertension. Nat. Commun. 2013, 4, 2395. [Google Scholar] [CrossRef]
- Kim, L.J.; Polotsky, V.Y. Carotid Body and Metabolic Syndrome: Mechanisms and Potential Therapeutic Targets. Int. J. Mol. Sci. 2020, 21, 5117. [Google Scholar] [CrossRef]
- Peng, Y.-J.; Prabhakar, N.R. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J. Appl. Physiol. 2003, 94, 2342–2349. [Google Scholar] [CrossRef]
- Peng, Y.J.; Yuan, G.; Khan, S.; Nanduri, J.; Makarenko, V.V.; Reddy, V.D.; Vasavda, C.; Kumar, G.K.; Semenza, G.L.; Prabhakar, N.R. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J. Physiol. 2014, 592, 3841–3858. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R. Carotid body contribution to the physio-pathological consequences of intermittent hypoxia: Role of nitro-oxidative stress and inflammation. J. Physiol. 2023, 601, 5495–5507. [Google Scholar] [CrossRef]
- Oyarce, M.P.; Iturriaga, R. Proinflammatory Cytokines in the Nucleus of the Solitary Tract of Hypertensive Rats Exposed to Chronic Intermittent Hypoxia. In Arterial Chemoreceptors; Springer International Publishing: Cham, Switzerland, 2018; pp. 69–74. [Google Scholar]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Carotid body hypersensitivity in intermittent hypoxia and obtructive sleep apnoea. J. Physiol. 2023, 601, 5481–5494. [Google Scholar] [CrossRef]
- Fernández-Agüera, M.C.; Gao, L.; González-Rodríguez, P.; Pintado, C.O.; Arias-Mayenco, I.; García-Flores, P.; García-Pergañeda, A.; Pascual, A.; Ortega-Sáenz, P.; López-Barneo, J. Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015, 22, 825–837. [Google Scholar] [CrossRef]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Arias-Mayenco, I.; Ortega-Sáenz, P.; López-Barneo, J. Using redox-sensitive fluorescent probes to record real-time reactive oxygen species production in cells from mouse carotid body slices. STAR Protoc. 2021, 2, 100535. [Google Scholar] [CrossRef] [PubMed]
- Papreck, J.R.; Martin, E.A.; Lazzarini, P.; Kang, D.; Kim, D. Modulation of K2P3.1 (TASK-1), K2P9.1 (TASK-3), and TASK-1/3 heteromer by reactive oxygen species. Pflug. Arch. Eur. J. Physiol. 2012, 464, 471–480. [Google Scholar] [CrossRef]
- Wilson, D.F.; Rumsey, W.L.; Green, T.J.; Vanderkooi, J.M. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J. Biol. Chem. 1988, 263, 2712–2718. [Google Scholar] [CrossRef]
- Rumsey, W.L.; Iturriaga, R.; Spergel, D.; Lahiri, S.; Wilson, D.F. Optical measurements of the dependence of chemoreception on oxygen pressure in the cat carotid body. Am. J. Physiol. 1991, 261, C614–C622. [Google Scholar] [CrossRef]
- Lahiri, S.; Rumsey, W.L.; Wilson, D.F.; Iturriaga, R. Contribution of in vivo microvascular PO2 in the cat carotid body chemotransduction. J. Appl. Physiol. 1993, 75, 1035–1043. [Google Scholar] [CrossRef]
- Holmes, A.P.; Ray, C.J.; Coney, A.M.; Kumar, P. Is Carotid Body Physiological O2 Sensitivity Determined by a Unique Mitochondrial Phenotype? Front. Physiol. 2018, 9, 562. [Google Scholar] [CrossRef]
- Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; Hla, K.M. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar] [PubMed]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death: A Scientific Statement From the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation 2008, 118, 1080–1111. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet. Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Idiaquez, J.; Santos, I.; Santin, J.; Del Rio, R.; Iturriaga, R. Neurobehavioral and autonomic alterations in adults with obstructive sleep apnea. Sleep Med. 2014, 15, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.E.; Matenchuk, B.A.; Vucenovic, A.; Sivak, A.; Davenport, M.H.; Steinback, C.D. Influence of Obstructive Sleep Apnea Severity on Muscle Sympathetic Nerve Activity and Blood Pressure: A Systematic Review and Meta-Analysis. Hypertension 2022, 79, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.C.; Martinez, D.; Gus, M.; de Abreu-Silva, E.O.; Bertoluci, C.; Dutra, I.; Branchi, T.; Moreira, L.B.; Fuchs, S.C.; de Oliveira, A.C.T.; et al. Obstructive Sleep Apnea and Resistant Hypertension: A Case-Control Study. Chest 2007, 132, 1858–1862. [Google Scholar] [CrossRef]
- Brown, J.; Yazdi, F.; Jodari-Karimi, M.; Owen, J.G.; Reisin, E. Obstructive Sleep Apnea and Hypertension: Updates to a Critical Relationship. Curr. Hypertens. Rep. 2022, 24, 173–184. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef]
- Fletcher, E.C.; Lesske, J.; Behm, R.; Miller, C.C., 3rd; Stauss, H.; Unger, T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J. Appl. Physiol. 1992, 72, 1978–1984. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef]
- Harki, O.; Boete, Q.; Pépin, J.-L.; Arnaud, C.; Belaidi, E.; Faury, G.; Khouri, C.; Briançon-Marjollet, A. Intermittent hypoxia-related alterations in vascular structure and function: A systematic review and meta-analysis of rodent data. Eur. Respir. J. 2022, 59, 2100866. [Google Scholar] [CrossRef]
- Fernandes, J.L.; Martins, F.O.; Olea, E.; Prieto-Lloret, J.; Braga, P.C.; Sacramento, J.F.; Sequeira, C.O.; Negrinho, A.P.; Pereira, S.A.; Alves, M.G.; et al. Chronic Intermittent Hypoxia-Induced Dysmetabolism Is Associated with Hepatic Oxidative Stress, Mitochondrial Dysfunction and Inflammation. Antioxidants 2023, 12, 1910. [Google Scholar] [CrossRef]
- Del Rio, R.; Moya, E.A.; Iturriaga, R. Carotid body potentiation during chronic intermittent hypoxia: Implication for hypertension. Front. Physiol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.B.J.; Yuan, Z.F.; Lin, Y.S.; Lin, Y.-N.; Li, W.-S.; Yang, C.C.H.; Lai, C.J. Reactive oxygen species are the cause of the enhanced cardiorespiratory response induced by intermittent hypoxia in conscious rats. Respir. Physiol. Neurobiol. 2011, 175, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Narkiewicz, K.; van de Borne, P.J.H.; Pesek, C.A.; Dyken, M.E.; Montano, N.; Somers, V.K. Selective Potentiation of Peripheral Chemoreflex Sensitivity in Obstructive Sleep Apnea. Circulation 1999, 99, 1183–1189. [Google Scholar] [CrossRef]
- Rey, S.; Tarvainen, M.P.; Karjalainen, P.A.; Iturriaga, R. Dynamic time-varying analysis of heart rate and blood pressure variability in cats exposed to short-term chronic intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R28–R37. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Rey, S.; Alcayaga, J.; Del Rio, R. Chronic Intermittent Hypoxia Enhances Carotid Body Chemosensory Responses to Acute Hypoxia. In The Arterial Chemoreceptors; Springer: Boston, MA, USA, 2006; pp. 227–232; discussion 351–229. [Google Scholar] [CrossRef]
- Peng, Y.J.; Nanduri, J.; Yuan, G.; Wang, N.; Deneris, E.; Pendyala, S.; Natarajan, V.; Kumar, G.K.; Prabhakar, N.R. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J. Neurosci. 2009, 29, 4903–4910. [Google Scholar] [CrossRef]
- Del Rio, R.; Andrade David, C.; Lucero, C.; Arias, P.; Iturriaga, R. Carotid Body Ablation Abrogates Hypertension and Autonomic Alterations Induced by Intermittent Hypoxia in Rats. Hypertension 2016, 68, 436–445. [Google Scholar] [CrossRef]
- Pereyra, K.; Diaz-Jara, E.; Bernal-Santander, I.; Vicencio, S.; Del Rio, R.; Iturriaga, R. Carotid bodies mediate glial cell activation and neuroinflammation in the NTS following long-term intermittent hypoxia: Role in cardiorespiratory dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2025, 328, L357–L371. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef]
- Thompson, L.; Werthammer, J.W.; Gozal, D. Apnea of Prematurity and Oxidative Stress: Potential Implications. Antioxidants 2024, 13, 1304. [Google Scholar] [CrossRef]
- Moya, E.A.; Arias, P.; Varela, C.; Oyarce, M.P.; Del Rio, R.; Iturriaga, R. Intermittent Hypoxia-Induced Carotid Body Chemosensory Potentiation and Hypertension Are Critically Dependent on Peroxynitrite Formation. Oxid. Med. Cell. Longev. 2016, 2016, 9802136. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Yuan, G.; Nanduri, J. Reactive oxygen radicals and gaseous transmitters in carotid body activation by intermittent hypoxia. Cell Tissue Res. 2018, 372, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Rocher, A.; Aaronson, P.I. Inflammation, nitro-oxidative stress and altered autonomic outflow in obstructive sleep apnoea: An assault on homeostasis. J. Physiol. 2023, 601, 5465–5466. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, R.; Moya, E.A.; Iturriaga, R. Carotid body and cardiorespiratory alterations in intermittent hypoxia: The oxidative link. Eur. Respir. J. 2010, 36, 143–150. [Google Scholar] [CrossRef]
- Marcus, N.J.; Li, Y.L.; Bird, C.E.; Schultz, H.D.; Morgan, B.J. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: Role of the angiotensin II type 1 receptor. Respir. Physiol. Neurobiol. 2010, 171, 36–45. [Google Scholar] [CrossRef]
- Troncoso Brindeiro, C.M.; da Silva, A.Q.; Allahdadi, K.J.; Youngblood, V.; Kanagy, N.L. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2971–H2976. [Google Scholar] [CrossRef]
- Krause, B.J.; Casanello, P.; Dias, A.C.; Arias, P.; Velarde, V.; Arenas, G.A.; Preite, M.D.; Iturriaga, R. Chronic Intermittent Hypoxia-Induced Vascular Dysfunction in Rats is Reverted by N-Acetylcysteine Supplementation and Arginase Inhibition. Front. Physiol. 2018, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Pena, E.; El Alam, S.; Gonzalez, C.; Cortés, I.; Aguilera, D.; Flores, K.; Arriaza, K. Astaxanthin Supplementation Effects in Right Ventricle of Rats Exposed to Chronic Intermittent Hypobaric Hypoxia. Antioxidants 2024, 13, 1269. [Google Scholar] [CrossRef]
- Iturriaga, R.; Moya, E.A.; Del Rio, R. Inflammation and oxidative stress during intermittent hypoxia: The impact on chemoreception. Exp. Physiol. 2015, 100, 149–155. [Google Scholar] [CrossRef]
- Morgan, B.J.; Bates, M.L.; Rio, R.D.; Wang, Z.; Dopp, J.M. Oxidative stress augments chemoreflex sensitivity in rats exposed to chronic intermittent hypoxia. Respir. Physiol. Neurobiol. 2016, 234, 47–59. [Google Scholar] [CrossRef]
- Semenza, G.L.; Prabhakar, N.R. The role of hypoxia-inducible factors in carotid body (patho) physiology. J. Physiol. 2018, 596, 2977–2983. [Google Scholar] [CrossRef]
- Nanduri, J.; Peng, Y.J.; Wang, N.; Khan, S.A.; Semenza, G.L.; Kumar, G.K.; Prabhakar, N.R. Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long-term intermittent hypoxia. J. Physiol. 2017, 595, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.K.; Peng, Y.J.; Nanduri, J.; Prabhakar, N.R. Carotid Body Chemoreflex Mediates Intermittent Hypoxia-Induced Oxidative Stress in the Adrenal Medulla. In Arterial Chemoreceptors in Physiology and Pathophysiology; Springer: Cham, Switzerland, 2015; pp. 195–199. [Google Scholar] [CrossRef]
- Moya, E.A.; Arias, P.; Iturriaga, R. Nitration of MnSOD in the Carotid Body and Adrenal Gland Induced by Chronic Intermittent Hypoxia. J. Histochem. Cytochem. 2018, 66, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, R.; Muñoz, C.; Arias, P.; Court, F.A.; Moya, E.A.; Iturriaga, R. Chronic intermittent hypoxia-induced vascular enlargement and VEGF upregulation in the rat carotid body is not prevented by antioxidant treatment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L702–L711. [Google Scholar] [CrossRef]
- Rey, S.; Del Rio, R.; Iturriaga, R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res. 2006, 1086, 152–159. [Google Scholar] [CrossRef]
- Pawar, A.; Nanduri, J.; Yuan, G.; Khan, S.A.; Wang, N.; Kumar, G.K.; Prabhakar, N.R. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R735–R742. [Google Scholar] [CrossRef]
- Lam, S.Y.; Liu, Y.; Ng, K.M.; Liong, E.C.; Tipoe, G.L.; Leung, P.S.; Fung, M.L. Upregulation of a local renin-angiotensin system in the rat carotid body during chronic intermittent hypoxia. Exp. Physiol. 2014, 99, 220–231. [Google Scholar] [CrossRef]
- Del Rio, R.; Moya, E.A.; Iturriaga, R. Differential expression of pro-inflammatory cytokines, endothelin-1 and nitric oxide synthases in the rat carotid body exposed to intermittent hypoxia. Brain Res. 2011, 1395, 74–85. [Google Scholar] [CrossRef]
- Lam, S.Y.; Liu, Y.; Ng, K.M.; Lau, C.F.; Liong, E.C.; Tipoe, G.L.; Fung, M.L. Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways. Histochem. Cell Biol. 2012, 137, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, R.; Moya, E.A.; Parga, M.J.; Madrid, C.; Iturriaga, R. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur. Respir. J. 2012, 39, 1492–1500. [Google Scholar] [CrossRef]
- Popa, D.; Fu, Z.; Go, A.; Powell, F.L. Ibuprofen blocks time-dependent increases in hypoxic ventilation in rats. Respir. Physiol. Neurobiol. 2011, 178, 381–386. [Google Scholar] [CrossRef]
- Kishi, T. Clarification of hypertension mechanisms provided by the research of central circulatory regulation. Hypertens. Res. Hypertens. 2023, 46, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Davis, C.W.; Fenik, P.; Zhan, G.; Hsu, Y.J.; Pratico, D.; Gow, A. Long-term intermittent hypoxia in mice: Protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004, 27, 194–201. [Google Scholar] [CrossRef]
- Ramanathan, L.; Gozal, D.; Siegel, J.M. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J. Neurochem. 2005, 93, 47–52. [Google Scholar] [CrossRef]
- MacFarlane, P.M.; Wilkerson, J.E.; Lovett-Barr, M.R.; Mitchell, G.S. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir. Physiol. Neurobiol. 2008, 164, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, Y.J.; Kakall, Z.; Farnham, M.M.J.; Pilowsky, P.M. Intermittent hypoxia-induced cardiorespiratory long-term facilitation: A new role for microglia. Respir. Physiol. Neurobiol. 2016, 226, 30–38. [Google Scholar] [CrossRef]
- Bhagavan, H.; Wei, A.D.; Oliveira, L.M.; Aldinger, K.A.; Ramirez, J.M. Chronic intermittent hypoxia elicits distinct transcriptomic responses among neurons and oligodendrocytes within the brainstem of mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 326, L698–L712. [Google Scholar] [CrossRef] [PubMed]
- El Amine, B.; Fournier, J.; Minoves, M.; Baillieul, S.; Roche, F.; Perek, N.; Pépin, J.L.; Tamisier, R.; Khouri, C.; Rome, C.; et al. Cerebral oxidative stress, inflammation and apoptosis induced by intermittent hypoxia: A systematic review and meta-analysis of rodent data. Eur. Respir. Rev. 2024, 33, 240162. [Google Scholar] [CrossRef]
- Bathina, C.S.; Rajulapati, A.; Franzke, M.; Yamamoto, K.; Cunningham, J.T.; Mifflin, S. Knockdown of tyrosine hydroxylase in the nucleus of the solitary tract reduces elevated blood pressure during chronic intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1031–R1039. [Google Scholar] [CrossRef]
- Wu, Q.; Cunningham, J.T.; Mifflin, S. Transcription factor ΔFosB acts within the nucleus of the solitary tract to increase mean arterial pressure during exposures to intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H270–H277. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.M.; Hu, K.; Zhou, X.F.; Tang, S. Sensory plasticity of carotid body is correlated with oxidative stress in paraventricular nucleus during chronic intermittent hypoxia. J. Cell. Physiol. 2019, 234, 13534–13543. [Google Scholar] [CrossRef]
- de Paula, P.M.; Tolstykh, G.; Mifflin, S. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am. J. physiology. Regul. Integr. Comp. Physiol. 2007, 292, R2259–R2265. [Google Scholar] [CrossRef]
- Kline, D.D.; Wang, S.; Kunze, D.L. TRPV1 channels contribute to spontaneous glutamate release in nucleus tractus solitarii following chronic intermittent hypoxia. J. Neurophysiol. 2019, 121, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.M.; Farnham, M.M.J.; Kakall, Z.; Kim, S.J.; Nedoboy, P.E.; Pilowsky, P.M. Glia and central cardiorespiratory pathology. Auton. Neurosci. Basic Clin. 2018, 214, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Lamy, C.M. Nucleus of Tractus Solitarius Astrocytes as Homeostatic Integrators. J. Neurosci. 2012, 32, 2579–2581. [Google Scholar] [CrossRef]
- Accorsi-Mendonça, D.; Almado, C.E.; Bonagamba, L.G.; Castania, J.A.; Moraes, D.J.; Machado, B.H. Enhanced Firing in NTS Induced by Short-Term Sustained Hypoxia Is Modulated by Glia-Neuron Interaction. J. Neurosci. 2015, 35, 6903–6917. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Ellacott, K.L.J. Astrocytes in the nucleus of the solitary tract: Contributions to neural circuits controlling physiology. Physiol. Behav. 2020, 223, 112982. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.R.; de Deus, J.L.; Cazuza, R.A.; Mota, C.M.D.; da Silva, L.E.V.; Borges, G.S.; Batalhão, M.E.; Cárnio, E.C.; Branco, L.G.S. Neuroinflammation in the NTS is associated with changes in cardiovascular reflexes during systemic inflammation. J. Neuroinflamm. 2019, 16, 125. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lin, Y.T.; Chen, H.H.; Ho, W.Y.; Sun, G.C.; Hsiao, M.; Lu, P.J.; Cheng, P.W.; Tseng, C.J. CX3CR1-microglia mediates neuroinflammation and blood pressure regulation in the nucleus tractus solitarii of fructose-induced hypertensive rats. J. Neuroinflamm. 2020, 17, 185. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Feng, J.; Cao, J.; Chen, B. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: The potential roles played by microglia. Neuropsychiatr. Dis. Treat. 2013, 9, 1077–1086. [Google Scholar] [CrossRef]
- Tadmouri, A.; Champagnat, J.; Morin-Surun, M.P. Activation of microglia and astrocytes in the nucleus tractus solitarius during ventilatory acclimatization to 10% hypoxia in unanesthetized mice. J. Neurosci. Res. 2014, 92, 627–633. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yuan, C.; Eubank, W.; Franzke, M.; Mifflin, S. Astrocytes in NTS after exposure to chronic intermittent hypoxia. FASEB J. 2012, 26, 898.10. [Google Scholar] [CrossRef]
- Costa, K.M.; Moraes, D.J.; Machado, B.H. Acute inhibition of glial cells in the NTS does not affect respiratory and sympathetic activities in rats exposed to chronic intermittent hypoxia. Brain Res. 2013, 1496, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho, C.R.; Gonçalves, C.M.; Takakura, A.C.; Mulkey, D.K.; Moreira, T.S. Fluorocitrate-mediated depolarization of astrocytes in the retrotrapezoid nucleus stimulates breathing. J. Neurophysiol. 2017, 118, 1690–1697. [Google Scholar] [CrossRef]
- Martinez, D.; Rogers, R.C.; Hasser, E.M.; Hermann, G.E.; Kline, D.D. Loss of excitatory amino acid transporter restraint following chronic intermittent hypoxia contributes to synaptic alterations in nucleus tractus solitarii. J. Neurophysiol. 2020, 123, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, K.; Las Heras, A.; Toledo, C.; Díaz-Jara, E.; Iturriaga, R.; Del Rio, R. Chemogenetic inhibition of NTS astrocytes normalizes cardiac autonomic control and ameliorate hypertension during chronic intermittent hypoxia. Biol. Res. 2023, 56, 57. [Google Scholar] [CrossRef]
- Garcia, A.J., 3rd; Viemari, J.C.; Khuu, M.A. Respiratory rhythm generation, hypoxia, and oxidative stress-Implications for development. Respir. Physiol. Neurobiol. 2019, 270, 103259. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iturriaga, R.; Diaz, H.S. Reactive Oxidative Species in Carotid Body Chemoreception: Their Role in Oxygen Sensing and Cardiorespiratory Alterations Induced by Chronic Intermittent Hypoxia. Antioxidants 2025, 14, 675. https://doi.org/10.3390/antiox14060675
Iturriaga R, Diaz HS. Reactive Oxidative Species in Carotid Body Chemoreception: Their Role in Oxygen Sensing and Cardiorespiratory Alterations Induced by Chronic Intermittent Hypoxia. Antioxidants. 2025; 14(6):675. https://doi.org/10.3390/antiox14060675
Chicago/Turabian StyleIturriaga, Rodrigo, and Hugo S. Diaz. 2025. "Reactive Oxidative Species in Carotid Body Chemoreception: Their Role in Oxygen Sensing and Cardiorespiratory Alterations Induced by Chronic Intermittent Hypoxia" Antioxidants 14, no. 6: 675. https://doi.org/10.3390/antiox14060675
APA StyleIturriaga, R., & Diaz, H. S. (2025). Reactive Oxidative Species in Carotid Body Chemoreception: Their Role in Oxygen Sensing and Cardiorespiratory Alterations Induced by Chronic Intermittent Hypoxia. Antioxidants, 14(6), 675. https://doi.org/10.3390/antiox14060675






