Investigation of the Effects of Salinity Exposure on Immune Defense, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Measurement of Antioxidant Enzyme Activity
2.3. Histological Observation
2.4. Transcriptome Profiling Analysis
2.5. qPCR Analysis
2.6. Statistical Analysis
3. Results
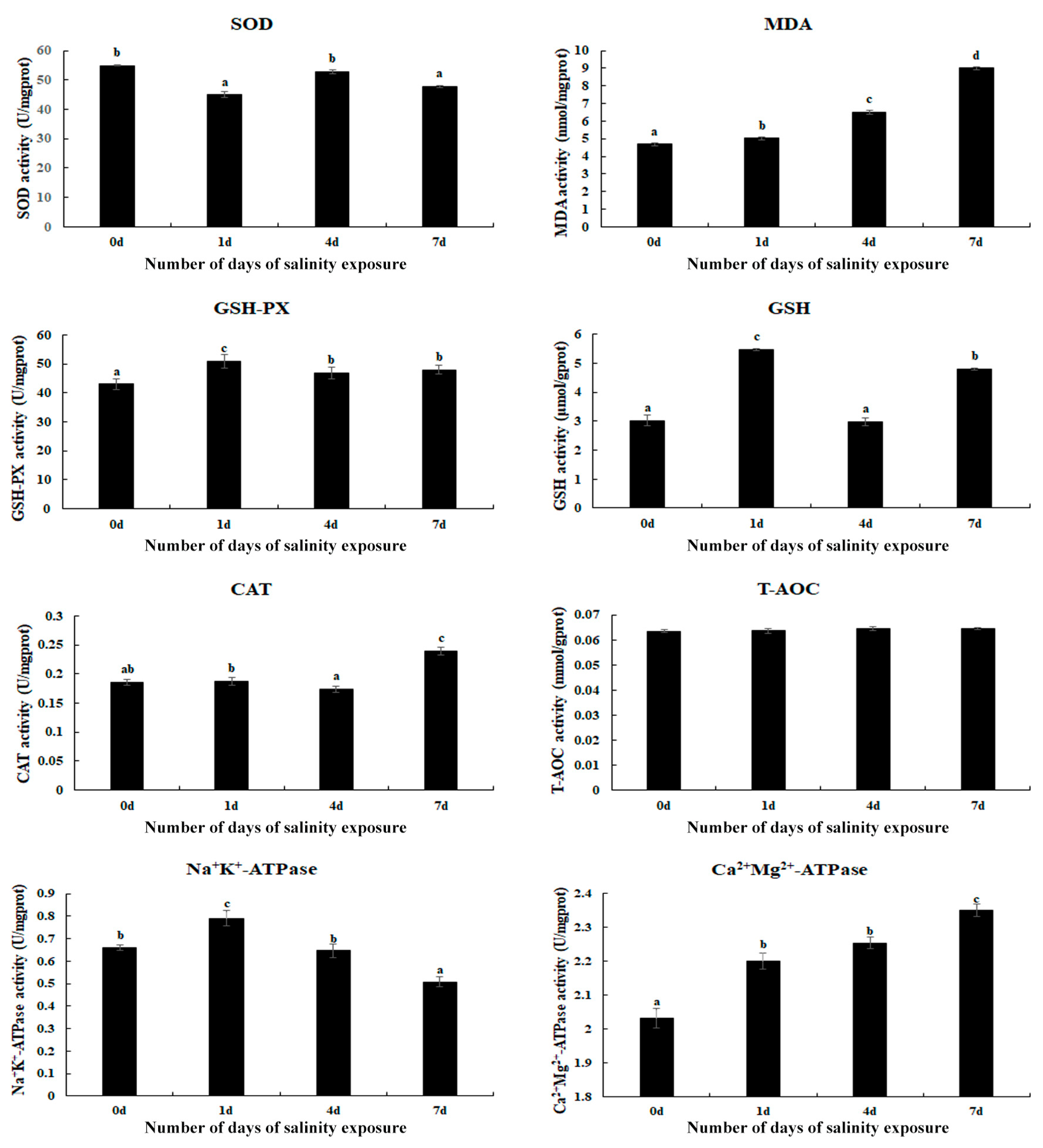
3.1. Measurement of Antioxidant Enzymes in Gills After Saline Treatment
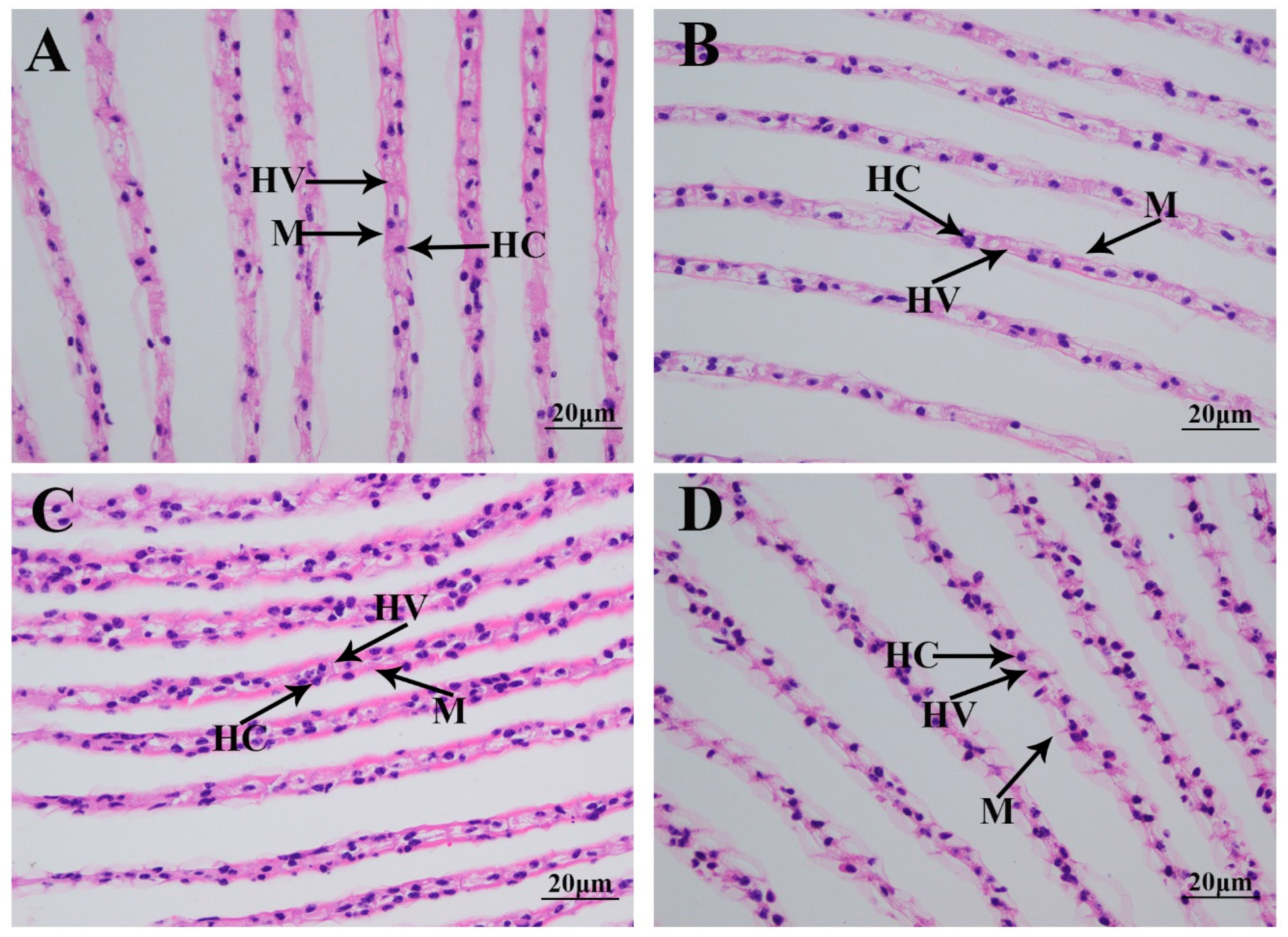
3.2. Morphological Changes in Gills Caused by the Saline Treatment
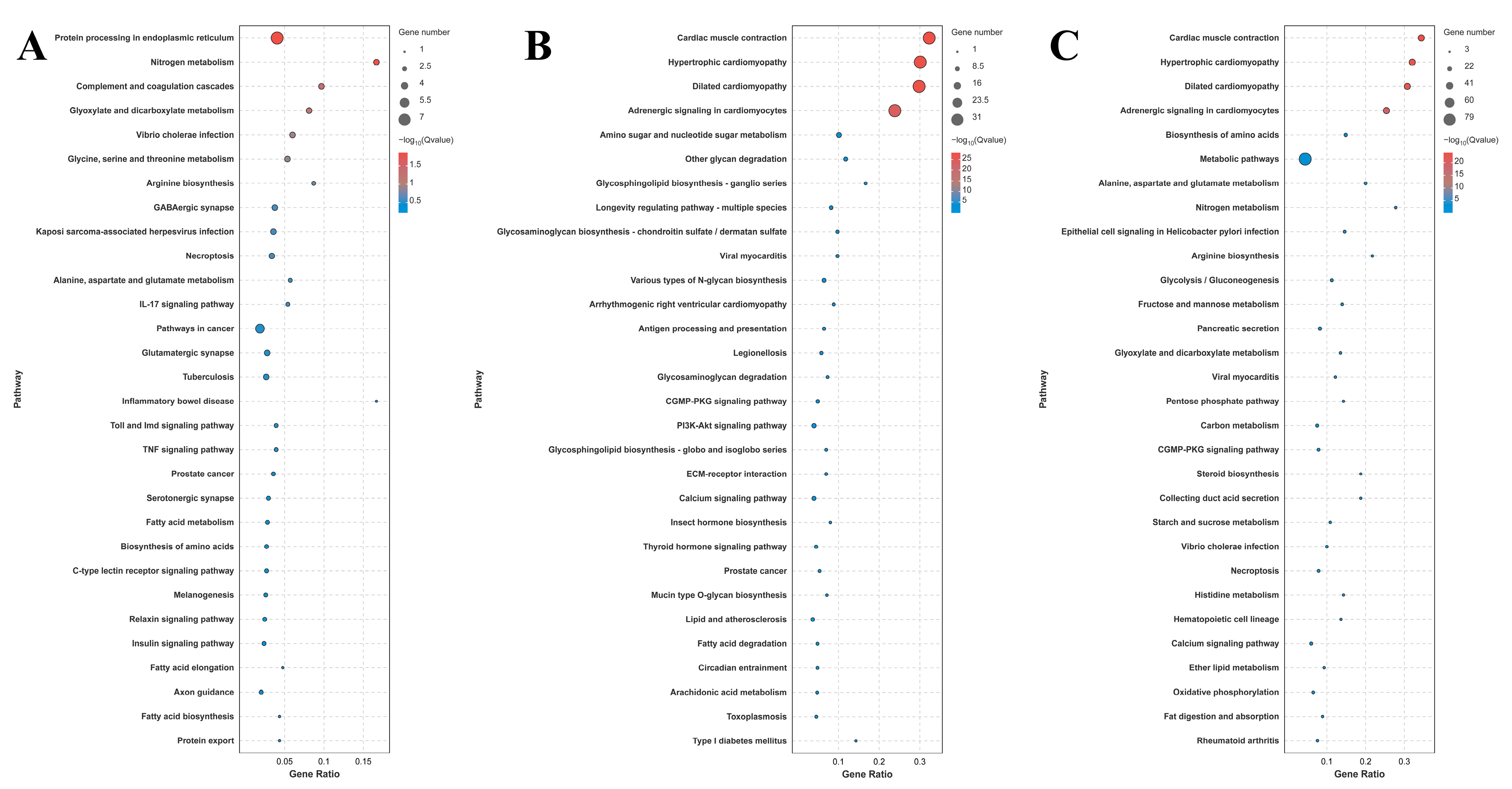
3.3. Transcriptome Profiling Analysis
3.4. Identification of Candidate Genes Involved in the Regulation of Saline Acclimation
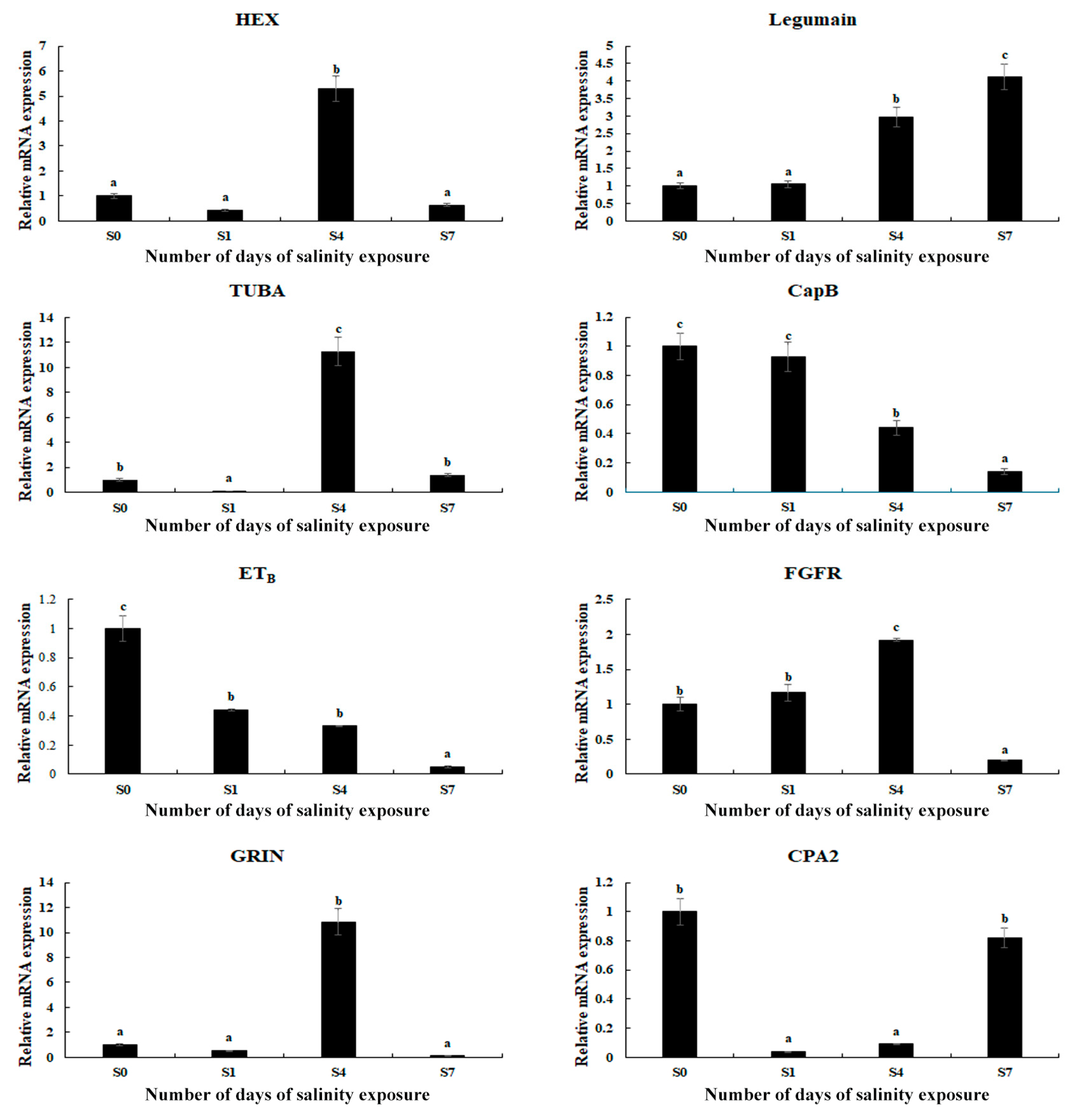
3.5. qPCR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, L.Q.; Ren, B.; Chang, Y.M.; Tang, R.; Zhang, L.M. Inland brackish (alkaline-saline) water resources and fisheries utilization in China. Chin. Fish. Econ. 2013, 31, 138–145. [Google Scholar]
- Liu, Y.X.; Fang, H.; Lai, Q.F.; Liang, L.Q. The current state and development strategy for China’s saline-alkaline fisheries. Strateg. Stud. CAE 2016, 18, 74–78. [Google Scholar]
- Derry, A.M.; Prepas, E.E.; Herbert, P.D.N. A composition of zooplankton communities in saline lakewater with variable anion composition. Hydrobiologia 2003, 505, 199–215. [Google Scholar] [CrossRef]
- Weber-Scannell, P.K.; Duffy, L.K. Effects of total dissolved solids on aquatic organisms; a review of literature and recommendation for salmonid species. Am. J. Environ. Sci. 2007, 3, 1–6. [Google Scholar]
- Wang, J.L.; Huang, X.J.; Zhong, T.Y.; Chen, Z.G. Review on sustainable utilization of salt-affected land. Acta Geogr. Sin. 2011, 66, 673–684. [Google Scholar]
- Xu, W.; Geng, L.W.; Jiang, H.F.; Tong, G.X. A review of development and utilization of fish culture in saline-alkaline water. Chin. J. Fish. 2015, 28, 44–47. [Google Scholar]
- Florkin, M.S.E. Isosmotic intracellular regulation. In Molecular Approaches to Ecology; Florkin, M.S.E., Ed.; Academic Press: Cambridge, MA, USA, 1969; pp. 89–111. [Google Scholar]
- Amado, E.M.; Souza-Bastos, L.R.; Vidal, E.A.G.; Leite, T.S.; Freire, C.A. Different abilities to regulate tissue hydration upon osmotic challenge in vitro, in the cephalopods Octopus vulgaris and O. insularis. Mar. Freshw. Behav. Physiol. 2015, 48, 205–211. [Google Scholar] [CrossRef]
- Keith, C.; Kevin, S. Volume regulation and osmosensing in animal cells. In Osmotic and Ionic Regulation; CRC Press: Boca Raton, FL, USA, 2008; pp. 37–67. [Google Scholar]
- Castille, F.L.L.A. The effect of salinity on the osmotic, sodium and chloride concentrations in the hemolymph of euryhaline shrimp of the genus Penaeus. Comp. Biochem. Physiol. Part A Physiol. 1981, 68A, 75–80. [Google Scholar] [CrossRef]
- Ferraris, R.P.; Parado-Estepa, E.; de Jesus, E.G.; Ladja, J.M. Osmotic and chloride regulation in the hemolymph of the tiger prawn Penaeus monodon during molting in various salinities. Mar. Biol. 1987, 95, 377–385. [Google Scholar] [CrossRef]
- Jaffer, Y.; Saraswathy, R.; Ishfaq, M.; Antony, J.; Bundela, D.; Sharma, P. Effect of low salinity on the growth and survival of juvenile pacific white shrimp, Penaeus vannamei: A revival. Aquaculture 2020, 515, 734561. [Google Scholar] [CrossRef]
- Williams, A.B. The influence of temperature on osmotic regulation in two species of estuarine shrimps (Penaeus). Biol. Bull. 1960, 119, 560–571. [Google Scholar] [CrossRef]
- Anger, K. Neotropical macrobrachium (Caridea: Palaemonidae): On the biology, origin, and radiation of freshwater-invading shrimp. J. Crustac. Biol. 2013, 33, 151–183. [Google Scholar] [CrossRef]
- Frolová, P.; Horká, I.; Ďurĭs, Z. Molecular phylogeny and historical biogeography of marine palaemonid shrimps (Palaemonidae: Palaemonella–Cuapetes group). Sci. Rep. 2022, 12, 15237. [Google Scholar] [CrossRef] [PubMed]
- Wilder, M.N.; Ikuta, K.; Atmomarsono, M.; Hatta, T.; Komuro, K. Changes in osmotic and ionic concentrations in the hemolymph of Macrobrachium rosenbergii exposed to varying salinities and correlation to ionic and crystalline composition of the cuticle. Comp. Biochem. Physiol. A 1998, 119, 941–950. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, M.; Li, Y.M.; Wu, D.L.; Liu, Z.Q.; Jiang, Q.C.; Zhao, Y.L. Effects of salinity acclimation on the growth performance, osmoregulation and energy metabolism of the oriental river prawn, Macrobrachium nipponense (De Haan). Aquac. Res. 2019, 50, 685–693. [Google Scholar] [CrossRef]
- Chand, B.K.; Trivedi, R.K.; Dubey, S.K.; Rout, S.K.; Beg, M.M.; Das, U.K. Effect of salinity on survival and growth of giant freshwater prawn Macrobrachium rosenbergii (de Man). Aquac. Rep. 2015, 2, 26–33. [Google Scholar] [CrossRef]
- Ye, L.; Jiang, S.; Zhu, X.; Yang, Q.; Wen, W.; Wu, K. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture 2009, 290, 140–144. [Google Scholar] [CrossRef]
- Torres, G.; Giménez, L.; Anger, K. Growth, tolerance to low-salinity, and osmoregulation in decapod crustacean larvae. Aquat. Biol. 2011, 12, 249–260. [Google Scholar] [CrossRef]
- Zhang, X.L.; Cui, L.F.; Li, S.M.; Liu, X.Z.; Han, X.; Jiang, K.Y.; Bureau of Fisheries, Ministry of Agriculture of the People’s Republic of China. Fisheries Economic Statistics. In China Fishery Yearbook; Beijing China Agricultural: Beijing, China, 2020; p. 24. [Google Scholar]
- Cheng, X.; Li, J.L.; Feng, J.B.; Nie, S.Z.; Fan, Y.P. Salinity tolerance of juvenile prawn Macrobrachium nipponense. J. Dalian Fish. Uni. 2008, 23, 315–317. [Google Scholar]
- Wang, W.; Gan, L.; Zhang, D.; Mu, F. Effects of salinity on survival and growth of Pseudobagrus vachelli. Fish. Sci. Technol. Inf. 2004, 31, 121–124. [Google Scholar]
- Li, H.; Zhou, W.; Gao, H.; Zhang, G. Joint Toxicity of salinity and alkalinity to Procambarus clarkii. J. Aquac. 2006, 27, 1–4. [Google Scholar]
- Iko, R.; Gao, Z.; Jiang, S.; Xiong, Y.; Zhang, W.; Qiao, H.; Jin, S.; Fu, H. Genetic diversity and population structure of Macrobrachium nipponense populations in the saline-alkaline regions of China. Animals 2025, 15, 158. [Google Scholar] [CrossRef]
- Ma, X.K.; Liu, X.Z.; Wen, H.S.; Xu, Y.J.; Zhang, L.J. Histological observation on gonadal sex differentiation in Cynoglossus semilaevis Günther. Mar. Fish Res. 2006, 27, 55–61. [Google Scholar]
- Bumin, S.; Liu, Z.Z.; Li, S.Q. Histological studies on ovarian development in Scylla serrata. J. Fish. China. 1991, 15, 96–103. [Google Scholar]
- Jin, S.B.; Fu, H.T.; Zhou, Q.; Sun, S.M.; Jiang, S.F.; Xiong, Y.W.; Gong, Y.S.; Qiao, H.; Zhang, W.Y. Transcriptome analysis of androgenic gland for discovery of novel genes from the oriental river prawn, Macrobrachium nipponense, using Illumina Hiseq 2000. PLoS ONE 2013, 8, e76840. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.B.; Fu, Y.; Hu, Y.N.; Fu, H.T.; Jiang, S.F.; Xiong, Y.W.; Qiao, H.; Zhang, W.Y.; Gong, Y.S. Identification of candidate genes from androgenic gland in Macrobrachium nipponense regulated by eyestalk ablation. Sci. Rep. 2021, 11, 1985. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Itoh, M. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, 480–484. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Zwinderman, A.H. On the benjamini–hochberg method. Ann. Statist. 2006, 34, 1827–1849. [Google Scholar] [CrossRef]
- Jin, S.B.; Hu, Y.N.; Fu, H.T.; Jiang, S.F.; Xiong, Y.W.; Qiao, H.; Zhang, W.Y.; Gong, Y.S.; Wu, Y. Potential functions of gem-associated protein 2-like isoform X1 in the oriental river prawn Macrobrachium nipponense: Cloning, qPCR, in situ hybridization, and RNAi analysis. Int. J. Mol. Sci. 2019, 20, 3995. [Google Scholar] [CrossRef]
- Jin, S.B.; Hu, Y.N.; Fu, H.T.; Jiang, S.F.; Xiong, Y.W.; Qiao, H.; Zhang, W.Y.; Gong, Y.S.; Wu, Y. Identification and characterization of the succinate dehydrogenase complex iron sulfur subunit B gene in the oriental river prawn Macrobrachium nipponense. Front. Genet. 2021, 12, 698318. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.N.; Fu, H.T.; Qiao, H.; Sun, S.M.; Zhang, W.Y.; Jin, S.B.; Jiang, S.F.; Gong, Y.S.; Xiong, Y.W.; Wu, Y. Validation and evaluation of reference genes for quantitative real-time PCR in Macrobrachium nipponense. Int. J. Mol. Sci. 2018, 19, 2258. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Phys. C 2010, 151, 142–151. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.C. Effects of pH, Temperature and salinity on immune parameters of the freshwater prawn Macrobrachium Rosenbergii. Fish Shellfish Immunol. 2000, 10, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.Q.; Jiang, L.X.; Miao, J.J. Effects of Salinity and pH on Immune Parameters of the White Shrimp Litopenaeus vannamei. J. Shellfish Res. 2005, 24, 1223–1228. [Google Scholar]
- Liu, Y.; Wang, W.N.; Wang, A.L.; Wang, J.M.; Sun, R.Y. Effects of dietary vitamin E supplementation on antioxidant enzyme activities in Litopenaeus vannamei (Boone, 1931) exposed to acute salinity changes. Aquaculture 2007, 265, 351–358. [Google Scholar] [CrossRef]
- Choi, C.Y.; An, K.W.; An, M.I. Molecular characterization and mRNA expression of glutathione peroxidase and glutathione S-transferase during osmotic stress in olive flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. A 2008, 149, 330337. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Gebicka, L.; Didik, J. Catalytic scavenging of peroxynitrite by catalase. J. Inorg. Biochem. 2009, 103, 1375–1379. [Google Scholar] [CrossRef]
- Brunelli, L.; Yermilov, V.; Beckman, J.S. Modulation of catalase peroxidatic and catalatic activity by nitric oxide. Free Radic. Biol. Med. 2001, 30, 709–714. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Arif, M.; Arif, F.; Arora, N.; Straub, K.D. Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biol. 2017, 12, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.H.; Borg, L.A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, A.M.; Heck, D.E.; Mariano, T.M.; Mishin, V.; Laskin, D.L.; Laskin, J.D. Characterization of the oxidase activity in mammalian catalase. J. Biol. Chem. 2005, 280, 35372–35381. [Google Scholar] [CrossRef]
- Flik, G.; Verbost, P.M.; Wendelaar, B.S.E. Calcium transport processes in fishes. Fish Physiol. 1995, 14, 317–342. [Google Scholar]
- Pérez-Reyes, E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol. Rev. 2003, 83, 117–161. [Google Scholar] [CrossRef]
- Carmona, R.; Garcia-Gallego, M.; Sanz, A. Chloride cells and pavement cells in gill epithelia of Acipenser naccarii: Ultrastructural modifications in seawater-acclimated specimens. J. Fish Biol. 2004, 64, 553–566. [Google Scholar] [CrossRef]
- Hou, J.L.; Chen, L.Q.; Zhuang, P.; Zhang, L.Z.; Tian, H.J.; Wang, W.; Yan, W.G. Structural changes of chloride cells in gills epithelia of juvenile Acipenser sinensis acclimated to various salinities. J. Fish. China 2006, 30, 316–322. [Google Scholar]
- Wang, X.J.; Zhang, X.M.; Jiang, M. Salinity stress on the ultrastructure of gill, head kidney and spleen of rockfish (Sebastes schlegeli). Period. Ocean Uni. China 2006, 36, 85–90. [Google Scholar]
- Galat, D.L.; Post, G.; Keefe, T.J.; Bouck, G.R. Historical changes in the gill, kidney and liver of Labhontan cutthroat trout (Salmoclarki henshawi) living in lakes of different salinity-alkalinity. J. Fish Biol. 1998, 27, 533–552. [Google Scholar] [CrossRef]
- Ma, L.; Lian, Y.T.; Li, S.Y.; Fahim, A.M.; Hou, X.F.; Liu, L.J.; Pu, Y.Y.; Yang, G.; Wang, W.T.; Wu, J.Y.; et al. Integrated transcriptome and metabolome analysis revealed molecular regulatory mechanism of saline-alkali stress tolerance and identified bHLH142 in winter rapeseed (Brassica rapa). Int. J. Biol. Macromol. 2025, 295, 139542. [Google Scholar] [CrossRef]
- Huang, R.S.; Tao, Y.F.; Jiang, B.J.; Badran, M.F.; Zhu, J.; Hua, J.X.; Wang, Q.C.; Lu, S.Q.; Saleh, M.H.L.; Aboueleila, R.H.M.; et al. Integrated gill transcriptome and biochemical indices analyses reveal that acute salinity stress induces oxidative stress and immune and metabolic disorders in Red Tilapia (Oreochromis spp.). Aquaculture 2025, 599, 742108. [Google Scholar] [CrossRef]
- Yuan, C.; Zhou, K.Q.; Pan, X.H.; Lin, Y.; Qin, J.Q.; Wang, D.P.; Chen, Z.; Du, X.S.; Huang, Y. Comparative transcriptome analysis reveals potential regulatory mechanisms in response to changes in physiological functions in Oreochromis aureus under salinity stress. Aquacul. Rep. 2025, 40, 102608. [Google Scholar] [CrossRef]
- Wang, R.X.; Bu, Y.K.; Xing, K.F.; Yuan, L.B.; Wu, Z.X.; Sun, Y.Y.; Zhang, J.Q. Integrated analysis of transcriptome and metabolome reveals chronic low salinity stress responses in the muscle of Exopalaemon carinicauda. Comp. Biochem. Phys. D 2024, 52, 101340. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Xu, K.; Jin, Y.; Bian, C.; Sun, S. Transcriptome analysis to study the molecular response in the gill and hepatopancreas tissues of Macrobrachium nipponense to salinity acclimation. Front. Physiol. 2022, 13, 926885. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Zoncu, R. The lysosome as a regulatory hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Sango, K.; Mcdonald, M.P.; Crawley, J.N.; Mack, M.L.; Tifft, C.J.; Skop, E.; Starr, C.M.; Hoffmann, A.; Sandhoff, K.; Suzuki, K.; et al. Mice lacking both subunits of lysosomal β–hexosaminidase display gangliosidosis and mucopolysaccharidosis. Nat. Genet. 1996, 14, 348–352. [Google Scholar] [CrossRef]
- Sandhoff, K.; Harzer, K. Gangliosides and gangliosidoses: Principles of molecular and metabolic pathogenesis. J. Neurosci. 2013, 33, 10195–10208. [Google Scholar] [CrossRef]
- Trasler, J.; Saberi, F.; Somani, I.H.; Adamali, H.I.; Huang, J.Q.; Fortunato, S.R.; Ritter, G.; Gu, M.; Aebersold, R.; Gravel, R.A.; et al. Characterization of the testis and epididymis in mouse models of human Tay Sachs and Sandhoff diseases and partial determination of accumulated gangliosides. Endocrinology 1998, 139, 3280–3288. [Google Scholar] [CrossRef]
- Bell, R.A.; Al-Khalaf, M.; Megeney, L.A. The beneficial role of proteolysis in skeletal muscle growth and stress adaptation (in eng). Skelet. Muscle 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Carbone, J.W. Assessment of skeletal muscle proteolysis and the regulatory response to nutrition and exercise. IUBMB Life 2014, 66, 478–484. [Google Scholar] [CrossRef]
- Solberg, R.; Lunde, N.N.; Forbord, K.M.; Okla, M.; Kassem, M.; Jafari, A. The mammalian cysteine protease legumain in Health and disease. Int. J. Mol. Sci. 2022, 23, 15983. [Google Scholar] [CrossRef]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef]
- Akhmanova, A.; Steinmetz, M.O. Control of microtubule organization and dynamics: Two ends in the limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef]
- Janke, C.; Bulinski, J.C. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2011, 12, 773–786. [Google Scholar] [CrossRef]
- Lopes, D.; Maiato, H. The tubulin code in mitosis and cancer. Cells 2020, 9, 2356. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xie, Q.; Wu, X.; Liu, W.; Li, D.F.; Li, C.; Zhao, W.X.; Chen, L.L.; Zheng, Z.; Li, G.M. Tension of plus-end tracking protein Clip170 confers directionality and aggressiveness during breast cancer migration. Cell Death Dis. 2022, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, J.; Parato, J.; Sharma, A.; Soleilhac, J.M.; Qu, X.; Tein, E.; Sproul, A.; Andrieux, A.; Goldberg, Y.; Moutin, M.J.; et al. Crosstalk between acetylation and the tyrosination/detyrosination cycle of α-tubulin in Alzheimer’s disease. Front. Cell Dev. Biol. 2022, 10, 926914. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Amrute-Nayak, M.; Allgeyer, E.; Li, X. MARK4 controls ischaemic heart failure through microtubule detyrosination. Nature 2021, 594, 560–565. [Google Scholar] [CrossRef]
- Margulies, K.B.; Prosser, B.L. Tubulin detyrosination: An emerging therapeutic target in hypertrophic cardiomyopathy. Circ. Heart Fail 2021, 14, e008006. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Kumar, V.; Bhatt, D.N.; Irfan, M.; Datta, A. N-acetylglucosamine sensing and metabolic engineering for attenuating human and plant pathogens. Bioengineering 2022, 9, 64. [Google Scholar] [CrossRef]
- Ghosh, S.; Rao, K.H.; Sengupta, M.; Bhattacharya, S.K.; Datta, A. Two gene clusters co-ordinate for a functional N-acetylglucosamine catabolic pathway in Vibrio cholerae. Mol. Microbiol. 2011, 80, 1549–1560. [Google Scholar] [CrossRef]
- Corfield, A.P.; Berry, M. Glycan variation and evolution in the eukaryotes. Trends Biochem. Sci. 2015, 40, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.H.; Jiang, Z.; Fu, X. Chitin and chitin-based biomaterials: A review of advances in processing and food applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef]
- Adrangi, S.; Faramarzi, M.A. From bacteria to human: A journey into the world of chitinases. Biotechnol. Adv. 2013, 31, 1786–1795. [Google Scholar] [CrossRef]
- Hamid, R.; Khan, M.A.; Ahmad, M.; Ahmad, M.M.; Abdin, M.Z.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21–29. [Google Scholar]
- Boot, R.G.; Renkema, G.H.; Strijland, A.; van Zonneveld, A.J.; Aerts, J.M.F.G. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J. Biol. Chem. 1995, 270, 26252–26256. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Jiang, S.F.; Xiong, Y.W.; Fu, H.T.; Sun, S.M.; Qiao, H.; Zhang, W.Y.; Jiang, F.W.; Jin, S.B.; Gong, Y.S. Six chitinases from oriental river prawn Macrobrachium nipponense: cDNA characterization, classification and mRNA expression during post-embryonic development and moulting cycle. Comp. Biochem. Phys. B 2014, 167, 30–40. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc. Natl. Acad. Sci. USA 2008, 105, 6650–6655. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, N.; Tewari, R.; Hoondal, G.S. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006, 71, 773–782. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Feil, R.; Kleppisch, T.; Schlossmann, J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol. Rev. 2006, 86, 1–23. [Google Scholar] [CrossRef]
- Tsai, E.J.; Kass, D.A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 2009, 122, 216–238. [Google Scholar] [CrossRef]
- Deguchi, A.; Thompson, W.J.; Weinstein, I.B. Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res. 2004, 64, 3966–3973. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Morgan, A.J.; Davis, L.C.; Wagner, S.K.T.Y.; Lewis, A.M.; Parrington, J.; Churchill, G.C.; Galione, A. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J. Cell Biol. 2013, 200, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.R.; Brock, T.A. Endothelin and Calcium Signaling; Pollock, D.M., Highsmith, R.F., Eds.; Endothelin Receptors and Signaling Mechanisms; Springer: Berlin/Heidelberg, Germany, 1998; pp. 131–146. [Google Scholar]
- Arai, H.; Hori, S.; Aramori, I.; Ohkubo, H.; Nakanishi, S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 1990, 348, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Yanagisawa, M.; Takuwa, Y.; Miyazaki, H.; Kimura, S.; Goto, K.; Masaki, T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 1990, 348, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Sokolovsky, M. Endothelin receptor subtypes and their role in transmembrane signaling mechanisms. Pharmacol. Ther. 1995, 68, 435–471. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L.; Drucker, D.J. Structure-function of the glucagon receptor family of G protein-coupled receptors: The glucagon, GIP, GLP-1, and GLP-2 receptors. Recept. Channels 2002, 8, 179–188. [Google Scholar] [CrossRef]
- Brubaker, P.L. The glucagon-like peptides: Pleiotropic regulators of nutrient homeostasis. Ann. N. Y. Acad. Sci. 2010, 1070, 10–26. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Suzuki, S.; Takiguchi, S.; Sato, N.; Kanai, S.; Kawanami, T.; Yoshida, Y.; Miyasaka, K.; Takata, Y.; Funakoshi, A.; Noda, T. Importance of CCK-A receptor for gallbladder contraction and pancreatic secretion: A study in CCK-A receptor knockout mice. Jpn. J. Physiol. 2001, 51, 585–590. [Google Scholar] [CrossRef]
- Shoji, E.; Okumura, T.; Onodera, S.; Takahashi, N.; Harada, K.; Kohgo, Y. Gastric emptying in OLETF rats not expressing CCK-A receptor gene. Dig. Dis. Sci. 1997, 42, 915–919. [Google Scholar] [CrossRef]
- Varga, G.; Balint, A.; Burghardt, B.; D’Amato, M. Involvement of endogenous CCK and CCK1 receptors in colonic motor function. Br. J. Pharmacol. 2004, 141, 1275–1284. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 59, 3041–3051. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Efficiency (%) |
|---|---|---|
| HEX | F: CCTTCTTCGGTGCTCGTCAT | 102.34 |
| R: GGCGCATCAGGTCTTCCTTA | ||
| Legumain | F: TCACTGAACCCAAACCCAGG | 96.13 |
| R: CCCAATTCCTTCCATGGCCT | ||
| TUBA | F: ACCTTCTTCAGCGAATCGGG | 97.88 |
| R: TTGTTAGCCGCGTCCTCTTT | ||
| CapB | F: ATTCCCGAATGCGAGCATCA | 92.36 |
| R: CCTCAACGGGGCCATTAGTC | ||
| EDNRB | F: ATCACCCACATGGCGTTCTT | 104.64 |
| R: CCTCGTTCGGTGGCTCTTTA | ||
| FGFR | F: CAGGCTTCAGGTTCTGAGGG | 98.59 |
| R: CCAACTGGAGCGTCACTCTT | ||
| GRIN | F: AGACGCCATCCAAGTGACAG | 105.23 |
| R: ATTCGGTCTCCGCTCGAATC | ||
| CPA2 | F: GTATCAAGTCCTACGCCGGG | 98.83 |
| R: TGAACACCTGACGTACCTGC | ||
| EIF | F: CATGGATGTACCTGTGGTGAAAC | 101.39 |
| R: CATGGATGTACCTGTGGTGAAAC |
| Gene | Accession Number | Metabolic Pathway | Log (Flod Change) | ||
|---|---|---|---|---|---|
| S0 vs. S1 | S1 vs. S4 | S4 vs. S7 | |||
| Sphingomyelin phosphodiesterase (SMPD) | ncbi_135213205 | Lysosome | −3.2 | 5.3 | |
| Hexosaminidase (HEX) | ncbi_135212715 | Lysosome, amino sugar, and nucleotide sugar metabolism | 3.6 | −3.8 | |
| Legumain | ncbi_135217338 | Lysosome | 1.4 | 1.7 | |
| Tubulin alpha (TUBA) | ncbi_135200421 | Apoptosis | 9.9 | −9.9 | |
| Cathepsin B (CapB) | MSTRG.22967 | Apoptosis | −1.2 | −1.5 | |
| Chitinase | ncbi_135197868 | Amino sugar and nucleotide sugar metabolism | 8.7 | 3.2 | |
| Endothelin receptor type B (ETB) | ncbi_135219635 | cGMP-PKG signaling pathway, calcium signaling pathway | −2.1 | −3.3 | |
| Solute carrier family 8 (SLC8A) | ncbi_135203101 | cGMP-PKG signaling pathway, calcium signaling pathway | 8.6 | −11.8 | |
| Myosin heavy chain 6/7 (MYH) | ncbi_135196459 | cGMP-PKG signaling pathway | 12.2 | −11.3 | |
| Cholecystokinin A receptor (CCKAR) | ncbi_135216887 | Calcium signaling pathway, pancreatic secretion | 3.3 | −3.5 | |
| Fibroblast growth factor receptor 1 (FGFR) | ncbi_135226152 | Calcium signaling pathway | 1.8 | −3.0 | |
| Ryanodine receptor 2 (RyR2) | ncbi_135205294 | Calcium signaling pathway, pancreatic secretion | 5.4 | −7.5 | |
| Glutamate receptor ionotropic (GRIN) | ncbi_135225876 | Calcium signaling pathway | 9.0 | −9.0 | |
| Carboxypeptidase A2 (CPA2) | ncbi_135221766 | Pancreatic secretion | −4.5 | 4.2 | |
| Solute carrier family 12 (SLC12A2) | ncbi_135195635 | Pancreatic secretion | −2.3 | 1.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Zhou, R.; Fu, H.; Zhang, W.; Qiao, H.; Xiong, Y.; Jiang, S. Investigation of the Effects of Salinity Exposure on Immune Defense, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense. Antioxidants 2025, 14, 655. https://doi.org/10.3390/antiox14060655
Jin S, Zhou R, Fu H, Zhang W, Qiao H, Xiong Y, Jiang S. Investigation of the Effects of Salinity Exposure on Immune Defense, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense. Antioxidants. 2025; 14(6):655. https://doi.org/10.3390/antiox14060655
Chicago/Turabian StyleJin, Shubo, Rong Zhou, Hongtuo Fu, Wenyi Zhang, Hui Qiao, Yiwei Xiong, and Sufei Jiang. 2025. "Investigation of the Effects of Salinity Exposure on Immune Defense, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense" Antioxidants 14, no. 6: 655. https://doi.org/10.3390/antiox14060655
APA StyleJin, S., Zhou, R., Fu, H., Zhang, W., Qiao, H., Xiong, Y., & Jiang, S. (2025). Investigation of the Effects of Salinity Exposure on Immune Defense, Morphology, and Gene Expression in the Gills of Macrobrachium nipponense. Antioxidants, 14(6), 655. https://doi.org/10.3390/antiox14060655








