The Role of Air Pollution Exposure and GSTM1-/GSTT1-Null Genotypes in Gestational Diabetes Mellitus Development: A Case–Control Study on Gene–Environment Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Participants
2.3. Data Collection
2.4. Air Pollution Exposure Assessment
2.5. Blood Sample Collection, DNA Extraction, and GSTM1 and GSTT1 Genotyping
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
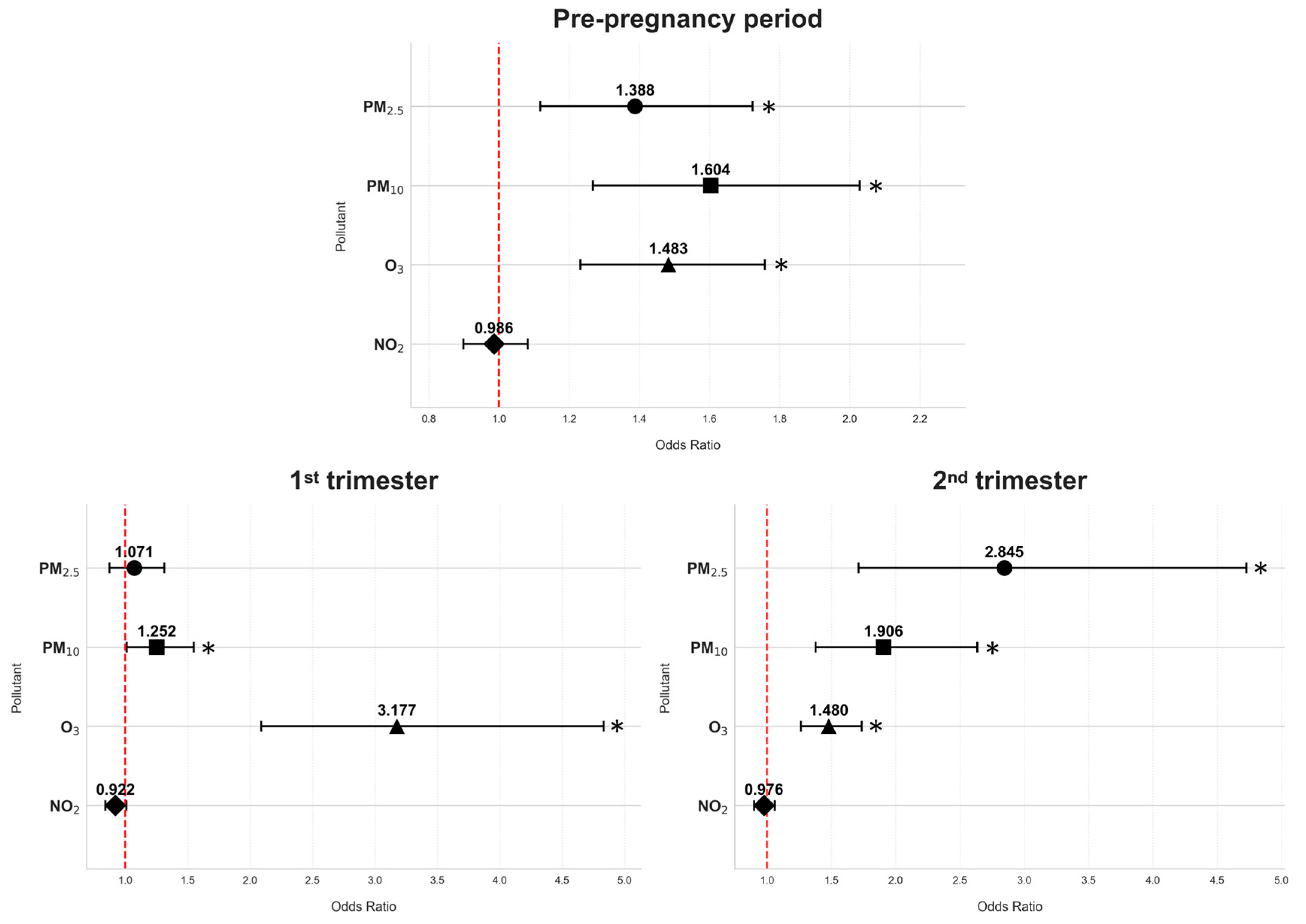
3.2. The Associations of Exposure to Air Pollutants with Gestational Diabetes Mellitus
3.3. The Associations Between GSTM1-Null and GSTT1-Null Genotypes and Gestational Diabetes Mellitus Risk
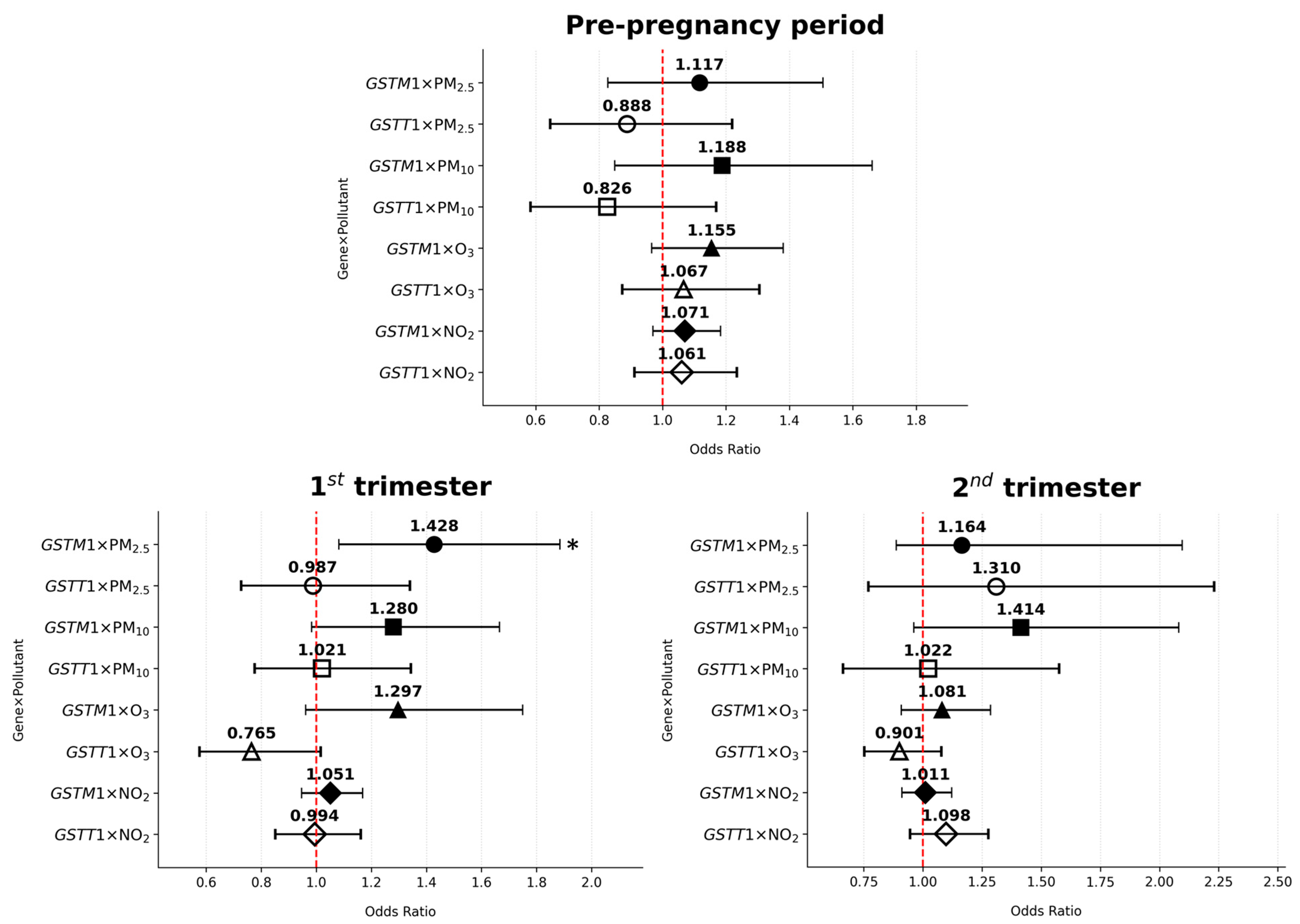
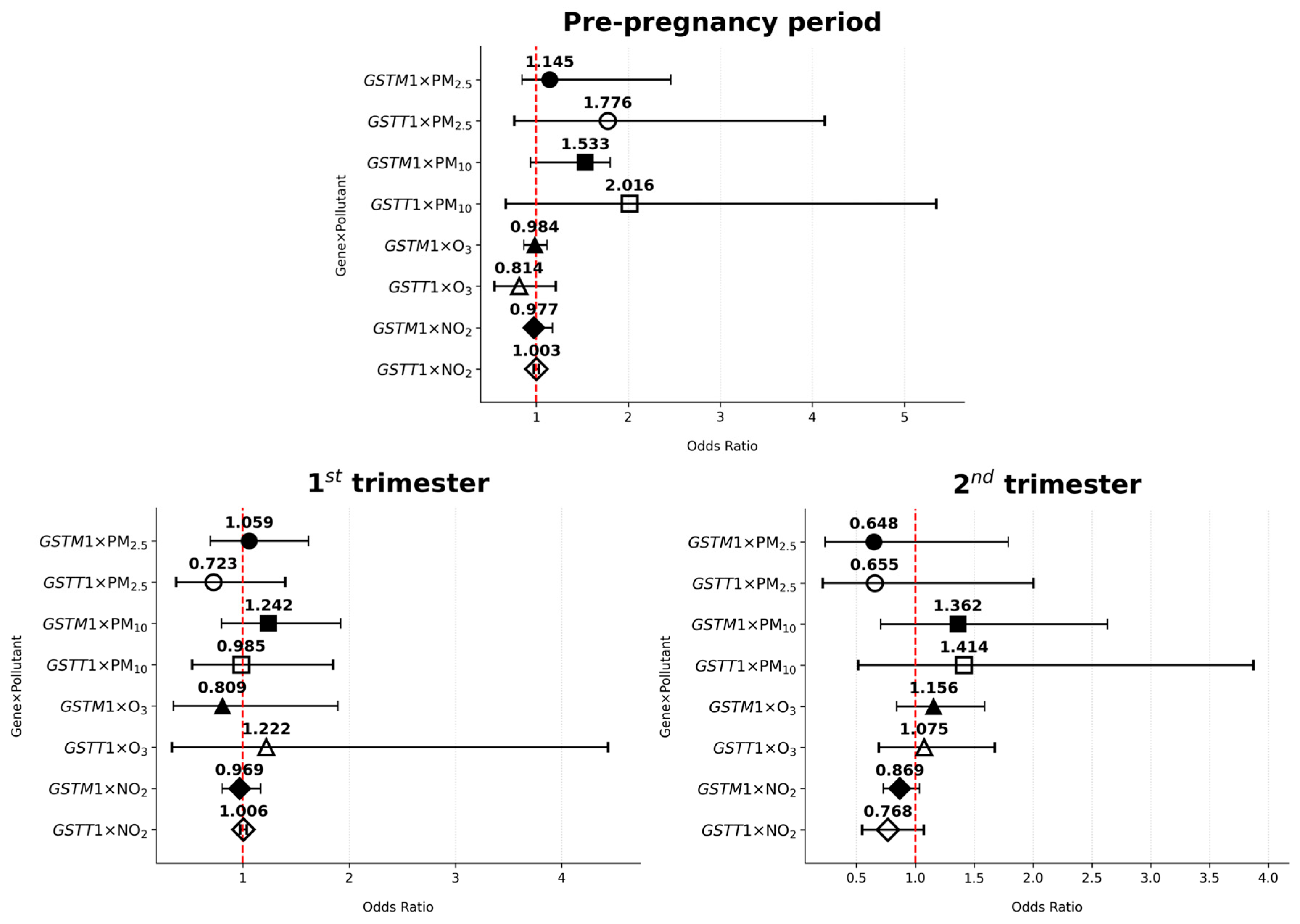
3.4. GSTM1/GSTT1–Air Pollutant Interaction and the Risk of Gestational Diabetes Mellitus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDM | Gestational diabetes mellitus |
| ROS | Reactive oxygen species |
| GSTs | Glutathione transferases |
| GSH | Glutathione |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| ARE | Antioxidant response element |
| GSTM1 | Glutathione S-transferase mu 1 |
| GSTT1 | Glutathione S-transferase theta 1 |
| T2DM | Diabetes mellitus type 2 |
References
- Baz, B.; Riveline, J.P.; Gautier, J.F. Endocrinology of Pregnancy: Gestational diabetes mellitus: Definition, aetiological and clinical aspects. Eur. J. Endocrinol. 2016, 174, R43–R51. [Google Scholar] [CrossRef] [PubMed]
- QuickStats: Percentage of Mothers with Gestational Diabetes,* by Maternal Age—National Vital Statistics System, United States, 2016 and 2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 16. [CrossRef] [PubMed]
- Paulo, M.S.; Abdo, N.M.; Bettencourt-Silva, R.; Al-Rifai, R.H. Gestational Diabetes Mellitus in Europe: A Systematic Review and Meta-Analysis of Prevalence Studies. Front. Endocrinol. 2021, 12, 691033. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef]
- Tobias, D.K.; Zhang, C.; Van Dam, R.M.; Bowers, K.; Hu, F.B. Physical Activity Before and During Pregnancy and Risk of Gestational Diabetes Mellitus. Diabetes Care 2011, 34, 223–229. [Google Scholar] [CrossRef]
- Zhang, C.; Tobias, D.K.; Chavarro, J.E.; Bao, W.; Wang, D.; Ley, S.H.; Hu, F.B. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: Prospective cohort study. BMJ 2014, 349, g5450. [Google Scholar] [CrossRef]
- Zhang, C.; Rawal, S.; Chong, Y.S. Risk factors for gestational diabetes: Is prevention possible? Diabetologia 2016, 59, 1385–1390. [Google Scholar] [CrossRef]
- Ray, G.W.; Zeng, Q.; Kusi, P.; Zhang, H.; Shao, T.; Yang, T.; Wei, Y.; Li, M.; Che, X.; Guo, R. Genetic and inflammatory factors underlying gestational diabetes mellitus: A review. Front. Endocrinol. 2024, 15, 1399694. [Google Scholar] [CrossRef]
- Yao, X.; Geng, S.; Zhu, L.; Jiang, H.; Wen, J. Environmental pollutants exposure and gestational diabetes mellitus: Evidence from epidemiological and experimental studies. Chemosphere 2023, 332, 138866. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, X.; Hu, S.; Wang, Y.; Liu, J. Association between short-term exposure to air pollution and respiratory diseases among children in China: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2022, 32, 2512–2532. [Google Scholar] [CrossRef] [PubMed]
- Moderato, L.; Aschieri, D.; Lazzeroni, D.; Rossi, L.; Biagi, A.; Binno, S.M.; Monello, A.; Pelizzoni, V.; Sticozzi, C.; Zanni, A.; et al. Air pollution and out-of-hospital cardiac arrest risk: A 7-year study from a highly polluted area. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Niu, Y.; Cai, J.; Lin, Z.; Liu, C.; Li, H.; Chen, C.; Song, W.; Zhao, Z.; Chen, R.; et al. Effects of Personal Short-Term Exposure to Ambient Ozone on Blood Pressure and Vascular Endothelial Function: A Mechanistic Study Based on DNA Methylation and Metabolomics. Environ. Sci. Technol. 2018, 52, 12774–12782. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; D’Angiulli, A. Air pollution and neurological diseases, current state highlights. Front. Neurosci. 2024, 18, 1351721. [Google Scholar] [CrossRef]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef]
- Lev Bar-Or, R.; Yuval; Twig, G.; Broday, D.M.; Keinan-Boker, L.; Paltiel, O.; Sinnreich, R.; Tzur, D.; Derazne, E.; Raz, R. Associations of Adolescence Exposure to Industrial Air Pollution with Cancer in Young Adults. Environ. Health 2023, 1, 53–62. [Google Scholar] [CrossRef]
- Pritchett, N.; Spangler, E.C.; Gray, G.M.; Livinski, A.A.; Sampson, J.N.; Dawsey, S.M.; Jones, R.R. Exposure to Outdoor Particulate Matter Air Pollution and Risk of Gastrointestinal Cancers in Adults: A Systematic Review and Meta-Analysis of Epidemiologic Evidence. Environ. Health Perspect. 2022, 130, 36001. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Xu, Z.; Guo, X.; Wu, S. Association between short-term exposure to ambient particulate air pollution and biomarkers of oxidative stress: A meta-analysis. Environ. Res. 2020, 191, 110105. [Google Scholar] [CrossRef]
- Møller, P.; Loft, S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ. Health Perspect. 2010, 118, 1126–1136. [Google Scholar] [CrossRef]
- Hu, C.Y.; Gao, X.; Fang, Y.; Jiang, W.; Huang, K.; Hua, X.G.; Yang, X.J.; Chen, H.B.; Jiang, Z.X.; Zhang, X.J. Human epidemiological evidence about the association between air pollution exposure and gestational diabetes mellitus: Systematic review and meta-analysis. Environ. Res. 2020, 180, 108843. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhou, J.B.; Luo, F.; Han, Y.; Heianza, Y.; Cardoso, M.A.; Qi, L. Air pollution and gestational diabetes mellitus: Evidence from cohort studies. BMJ Open Diabetes Res. Care 2020, 8, e000937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Q.; He, S.; Wu, K.; Ren, M.; Dong, H.; Di, J.; Yu, Z.; Huang, C. Ambient air pollution and gestational diabetes mellitus: A review of evidence from biological mechanisms to population epidemiology. Sci. Total Environ. 2020, 719, 137349. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef]
- Wang, S.C.; Huang, C.C.; Shen, C.H.; Lin, L.C.; Zhao, P.W.; Chen, S.Y.; Deng, Y.C.; Liu, Y.W. Gene Expression and DNA Methylation Status of Glutathione S-Transferase Mu1 and Mu5 in Urothelial Carcinoma. PLoS ONE 2016, 11, e0159102. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhai, Q.; Hai, J.; Wang, D.; Cao, M.; Zhang, Q. Association of GSTs polymorphisms with risk of gestational diabetes mellitus. Int. J. Clin. Exp. Pathol. 2015, 8, 15191–15197. [Google Scholar]
- Orhan, O.; Atalay, M.A.; Orhan, F.; Karkucak, M.; Centinkaya Demir, B.; Yakut, T.; Cengiz, C. Glutathione s-transferase m1 and t1 gene polymorphisms are not associated with increased risk of gestational diabetes mellitus development. West. Indian Med. J. 2014, 63, 300–306. [Google Scholar] [CrossRef][Green Version]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S19–S40. [Google Scholar] [CrossRef]
- Open Data Portal. Available online: https://data.gov.rs/sr/datasets/?organization=5858fc64cbe3c816ac018e61 (accessed on 18 February 2025). (In Serbian)
- Cleveland, W.S. Robust Locally Weighted Regression and Smoothing Scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia. Available online: https://www.hidmet.gov.rs/latin/meteorologija/klimatologija_godisnjaci.php (accessed on 27 February 2025). (In Serbian)
- Padula, A.M.; Yang, W.; Schultz, K.; Lee, C.; Lurmann, F.; Hammond, S.K.; Shaw, G.M. Gene-environment interactions between air pollution and biotransformation enzymes and risk of birth defects. Birth Defects Res. 2021, 113, 676–886. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Blanco, E.; Gómez-Moreno, F.J.; Díaz-Ramiro, E.; Fernández, J.; Coz, E.; Yagüe, C.; Román-Cascón, C.; Narros, A.; Borge, R.; Artíñano, B. Real-Time Measurements of Indoor–Outdoor Exchange of Gaseous and Particulate Atmospheric Pollutants in an Urban Area. Int. J. Environ. Res. Public Health 2023, 20, 6823. [Google Scholar] [CrossRef]
- Gaaib, J.; And, A.; Al-Assie, A. Simple salting–out method for genomic DNA extraction from whole blood. Tikrit J. Pure Sci. 2011, 16, 9–11. [Google Scholar]
- Jakovljevic, A.; Nikolic, N.; Carkic, J.; Beljic-Ivanovic, K.; Soldatovic, I.; Miletic, M.; Andric, M.; Milasin, J. Association of polymorphisms in TNF-α, IL-1β, GSTM and GSTT genes with apical periodontitis: Is there a link with herpesviral infection? Int. Endod. J. 2020, 53, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yuan, J.; Luo, Y.; Wang, J.; Li, Y. Association of air pollution and fine particulate matter (PM2.5) exposure with gestational diabetes: A systematic review and meta-analysis. Ann. Transl. Med. 2023, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Air Quality Guidelines: Global Update 2005. Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide; WHO Regional Office for Europe: Copenhagen, Denmark, 2006. Available online: https://www.who.int/publications/i/item/WHO-SDE-PHE-OEH-06.02 (accessed on 17 May 2025).
- Devlin, R.B.; Duncan, K.E.; Jardim, M.; Schmitt, M.T.; Rappold, A.G.; Diaz-Sanchez, D. Controlled Exposure of Healthy Young Volunteers to Ozone Causes Cardiovascular Effects. Circulation 2012, 126, 104–111. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef]
- Xuan Nguyen, K.; Bui Minh, T.; Dinh, H.T.; Viet Tran, T.; Dinh Le, T.; Phi Thi Nguyen, N.; Tran, T.T.H.; Hien Vu, T.; Ho Thi Nguyen, L.; Trung Nguyen, K.; et al. Low-Grade Inflammation in Gestational Diabetes Mellitus and Its Correlation with Maternal Insulin Resistance and Fetal Growth Indices. Int. J. Gen. Med. 2023, 16, 1429–1436. [Google Scholar] [CrossRef]
- Shamsad, A.; Gautam, T.; Singh, R.; Banerjee, M. Association of mRNA expression and polymorphism of antioxidant glutathione-S-transferase (GSTM1 and GSTT1) genes with the risk of Gestational Diabetes Mellitus (GDM). Gene 2024, 928, 148746. [Google Scholar] [CrossRef]
- Dłuski, D.F.; Wolińska, E.; Skrzypczak, M. Epigenetic Changes in Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 7649. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Paul, S.; Banerjee, M.; Patra, D.; Banerjee, P.; Ghoshal, N.; Bandyopadhyay, A.; Giri, A.K. Functional compensation of glutathione S-transferase M1 (GSTM1) null by another GST superfamily member, GSTM2. Sci. Rep. 2013, 3, 2704. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, Y.; Haile, Z.T.; Chertok, I.A. Association Between Prenatal Smoking and Gestational Diabetes Mellitus. Obstet. Gynecol. 2020, 135, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Ganjayi, M.S.; Yellanurkonda, P.; Basha, S.; Meriga, B. Role of glutathione S-transferases in detoxification of a polycyclic aromatic hydrocarbon, methylcholanthrene. Chem. Biol. Interact. 2018, 294, 81–90. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Human Health Effects of Polycyclic Aromatic Hydrocarbons as Ambient Air Pollutants: Report of the Working Group on Polycyclic Aromatic Hydrocarbons of the Joint Task Force on the Health Aspects of Air Pollution; WHO Regional Office for Europe: Copenhagen, Denmark, 2021. Available online: https://www.who.int/europe/publications/i/item/9789289056533 (accessed on 17 May 2025).
- Bowatte, G.; Lodge, C.J.; Perret, J.L.; Matheson, M.C.; Dharmage, S.C. Interactions of GST Polymorphisms in Air Pollution Exposure and Respiratory Diseases and Allergies. Curr. Allergy Asthma Rep. 2016, 16, 85. [Google Scholar] [CrossRef]
- Dai, X.; Bowatte, G.; Lowe, A.J.; Matheson, M.C.; Gurrin, L.C.; Burgess, J.A.; Dharmage, S.C.; Lodge, C.J. Do Glutathione S-Transferase Genes Modify the Link between Indoor Air Pollution and Asthma, Allergies, and Lung Function? A Systematic Review. Curr. Allergy Asthma Rep. 2018, 18, 20. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, Y.C. GSTM1, GSTT1, and GSTP1 Polymorphisms and Associations between Air Pollutants and Markers of Insulin Resistance in Elderly Koreans. Environ. Health Perspect. 2012, 120, 1378–1384. [Google Scholar] [CrossRef]
- Suh, Y.J.; Ha, E.H.; Park, H.; Kim, Y.J.; Kim, H.; Hong, Y.C. GSTM1 polymorphism along with PM10 exposure contributes to the risk of preterm delivery. Mutat. Res. 2008, 656, 62–77. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X.; Zhang, Y.; Jin, L.; Zhao, K.; Chen, J.; Shang, X.; Zhou, Y.; Yu, H. Genetic variations in IGF2BP2 and CAPN10 and their interaction with environmental factors increase gestational diabetes mellitus risk in Chinese women. Gene 2025, 941, 149226. [Google Scholar] [CrossRef]
- Huang, X.; Liang, W.; Yang, R.; Jin, L.; Zhao, K.; Chen, J.; Shang, X.; Zhou, Y.; Wang, X.; Yu, H. Variations in the LINGO2 and GLIS3 Genes and Gene-Environment Interactions Increase Gestational Diabetes Mellitus Risk in Chinese Women. Environ. Sci. Technol. 2024, 58, 11596–11605. [Google Scholar] [CrossRef]
- European Environment Agency. Europe’s Air Quality Status 2024; Briefing no. 06/2024; European Environment Agency: Copenhagen, Denmark, 2024. [Google Scholar] [CrossRef]


| Gene | Gene Accession Number | Primer Sequence |
|---|---|---|
| GSTM1 | NC_000001.11 | 5′-GTTGGGCTCAAATATACGGTGG-3′ 5′-GAACTCCCTGAAAAGCTAAAGC-3′ |
| GSTT1 | NT_187633.1 | 5′-TTCCTTACTGGTCCTCACATCT-3′ 5′-TCACCGGATCATGGCCAGCA-3′ |
| β-globin | NC_000011.10 | 5′-ACACAACTGTGTTCAACTAGC-3′ 5′-CAACTTCATCCACGTTCACC-3′ |
| Characteristic | Case Group (n = 133) | Control Group (n = 144) | Total (n = 277) | χ2/t Value | p Value |
|---|---|---|---|---|---|
| Age (years) n (%) | |||||
| <30 | 41 (30.8) | 48 (33.3) | 89 (32.1) | 0.199 * | 0.655 * |
| ≥30 | 92 (69.2) | 96 (66.7) | 188 (67.9) | ||
| Weight gain during pregnancy (kg) n (%) | |||||
| <16 | 94 (70.7) | 90 (62.5) | 184 (66.4) | 2.073 * | 0.150 * |
| ≥16 | 39 (29.3) | 54 (37.5) | 93 (33.6) | ||
| Parity n (%) | |||||
| 1 | 90 (67.7) | 78 (54.2) | 168 (61.0) | 5.305 * | 0.070 * |
| 2 | 34 (25.5) | 53 (36.8) | 78 (28.1) | ||
| ≥3 | 9 (6.8) | 13 (9.0) | 22 (7.9) | ||
| Smoking status n (%) | |||||
| No | 100 (75.2) | 101 (70.1) | 201 (72.6) | 1.940 * | 0.379 * |
| Yes | 10 (7.5) | 18 (12.5) | 28 (10.1) | ||
| Former smoker | 23 (17.3) | 25 (17.4) | 48 (17.3) | ||
| Pre-pregnancy BMI (kg/m2) Mean (SD) | 24.60 (4.67) | 22.60 (3.41) | 23.41 (4.09) | 3.580 † | <0.001 † |
| Case Group Mean (SD) | Control Group Mean (SD) | β * | p Value * | OR * | 95% CI * | |
|---|---|---|---|---|---|---|
| Non-smokers | N = 100 | N = 101 | ||||
| PM2.5 | ||||||
| Pre-pregnancy | 7.22 (1.98) | 6.51 (1.21) | 0.263 | <0.001 | 1.301 | 1.127–1.501 |
| 1st trimester | 6.56 (2.19) | 6.78 (1.23) | −0.103 | 0.126 | 0.902 | 0.791–1.029 |
| 2nd trimester | 5.01 (1.84) | 4.31 (0.75) | 0.471 | <0.001 | 1.602 | 1.296–1.980 |
| PM10 | ||||||
| Pre-pregnancy | 8.09 (1.95) | 6.84 (1.22) | 0.521 | <0.001 | 1.684 | 1.427–1.986 |
| 1st trimester | 7.51 (2.13) | 7.07 (1.36) | 0.127 | 0.050 | 1.136 | 1.000–1.291 |
| 2nd trimester | 6.70 (1.91) | 5.60 (0.97) | 0.575 | <0.001 | 1.777 | 1.470–2.148 |
| O3 | ||||||
| Pre-pregnancy | 7.88 (3.95) | 6.14 (2.02) | 0.209 | <0.001 | 1.232 | 1.130–1.343 |
| 1st trimester | 8.03 (3.69) | 4.79 (1.60) | 0.583 | <0.001 | 1.792 | 1.548–2.075 |
| 2nd trimester | 9.60 (3.72) | 7.06 (2.46) | 0.270 | <0.001 | 1.310 | 1.202–1.427 |
| NO2 | ||||||
| Pre-pregnancy | 31.78 (5.65) | 32.10 (3.45) | −0.017 | 0.492 | 0.983 | 0.937–1.032 |
| 1st trimester | 29.87 (5.12) | 31.10 (3.49) | −0.064 | 0.020 | 0.938 | 0.888–0.990 |
| 2nd trimester | 28.04 (4.97) | 27.94 (3.79) | 0.017 | 0.506 | 1.018 | 0.967–1.071 |
| Smokers/Former smokers | N = 33 | N = 43 | ||||
| PM2.5 | ||||||
| Pre-pregnancy | 7.87 (2.64) | 6.69 (1.37) | 0.328 | 0.003 | 1.388 | 1.118–1.723 |
| 1st trimester | 7.13 (2.45) | 6.82 (1.25) | 0.068 | 0.512 | 1.071 | 0.873–1.313 |
| 2nd trimester | 5.20 (1.46) | 4.34 (0.60) | 1.046 | <0.001 | 2.845 | 1.712–4.728 |
| PM10 | ||||||
| Pre-pregnancy | 8.57 (2.29) | 6.89 (1.47) | 0.473 | <0.001 | 1.604 | 1.268–2.029 |
| 1st trimester | 8.01 (2.19) | 7.20 (1.43) | 0.225 | 0.038 | 1.252 | 1.012–1.548 |
| 2nd trimester | 6.80 (1.31) | 5.68 (1.20) | 0.645 | <0.001 | 1.906 | 1.378–2.636 |
| O3 | ||||||
| Pre-pregnancy | 8.53 (3.15) | 6.25 (1.69) | 0.394 | <0.001 | 1.483 | 1.232–1.758 |
| 1st trimester | 8.41 (2.23) | 4.81 (1.52) | 1.156 | <0.001 | 3.177 | 2.090–4.831 |
| 2nd trimester | 10.48 (3.59) | 6.94 (2.44) | 0.392 | <0.001 | 1.480 | 1.262–1.736 |
| NO2 | ||||||
| Pre-pregnancy | 30.66 (3.51) | 31.78 (4.35) | −0.014 | 0.467 | 0.986 | 0.899–1.082 |
| 1st trimester | 28.58 (3.54) | 30.05 (4.25) | −0.082 | 0.081 | 0.922 | 0.841–1.010 |
| 2nd trimester | 26.50 (4.96) | 27.06 (4.23) | 0.025 | 0.559 | 0.976 | 0.899–1.059 |
| Genotype | Case Group N (%) | Control Group N (%) | β * | p Value * | OR * | 95% CI * |
|---|---|---|---|---|---|---|
| Non-smokers | N = 100 | N = 101 | ||||
| GSTM1 present | 50 (50.0) | 57 (56.4) | 0.192 | 0.514 | 1.212 | 0.681–2.156 |
| GSTM1-null genotype | 50 (50.0) | 44 (43.6) | ||||
| GSTT1 present | 80 (80.0) | 83 (82.2) | 0.217 | 0.558 | 1.242 | 0.601–2.567 |
| GSTT1-null genotype | 20 (20.0) | 18 (17.8) | ||||
| Smokers/Former smokers | N = 33 | N = 43 | ||||
| GSTM1 present | 18 (54.5) | 18 (41.9) | −0.456 | 0.350 | 0.634 | 0.243–1.651 |
| GSTM1-null genotype | 15 (45.5) | 25 (58.1) | ||||
| GSTT1 present | 25 (75.8) | 34 (79.1) | 0.020 | 0.973 | 1.020 | 0.322–3.227 |
| GSTT1-null genotype | 8 (24.2) | 9 (20.9) |
| Period | Pollutant | Interaction | β * | p Value * | OR * | 95% CI * |
|---|---|---|---|---|---|---|
| Non-smokers | ||||||
| Pre-pregnancy | PM2.5 | GSTM1 × PM2.5 | 0.110 | 0.469 | 1.117 | 0.828–1.506 |
| GSTT1 × PM2.5 | −0.119 | 0.464 | 0.888 | 0.646–1.220 | ||
| PM10 | GSTM1 × PM10 | 0.172 | 0.315 | 1.188 | 0.849–1.661 | |
| GSTT1 × PM10 | −0.191 | 0.280 | 0.826 | 0.584–1.169 | ||
| O3 | GSTM1 × O3 | 0.144 | 0.115 | 1.155 | 0.966–1.381 | |
| GSTT1 × O3 | 0.065 | 0.526 | 1.067 | 0.873–1.306 | ||
| NO2 | GSTM1 × NO2 | 0.069 | 0.175 | 1.071 | 0.970–1.183 | |
| GSTT1 × NO2 | 0.059 | 0.442 | 1.061 | 0.912–1.235 | ||
| 1st trimester | PM2.5 | GSTM1 × PM2.5 | 0.356 | 0.012 | 1.428 | 1.082–1.885 |
| GSTT1 × PM2.5 | −0.013 | 0.934 | 0.987 | 0.727–1.340 | ||
| PM10 | GSTM1 × PM10 | 0.247 | 0.067 | 1.280 | 0.983–1.666 | |
| GSTT1 × PM10 | 0.021 | 0.881 | 1.021 | 0.776–1.344 | ||
| O3 | GSTM1 × O3 | 0.260 | 0.090 | 1.297 | 0.961–1.750 | |
| GSTT1 × O3 | −0.268 | 0.064 | 0.765 | 0.576–1.016 | ||
| NO2 | GSTM1 × NO2 | 0.050 | 0.350 | 1.051 | 0.947–1.168 | |
| GSTT1 × NO2 | −0.006 | 0.940 | 0.994 | 0.851–1.161 | ||
| 2nd trimester | PM2.5 | GSTM1 × PM2.5 | 0.310 | 0.157 | 1.164 | 0.887–2.096 |
| GSTT1 × PM2.5 | 0.270 | 0.319 | 1.310 | 0.770–2.231 | ||
| PM10 | GSTM1 × PM10 | 0.347 | 0.079 | 1.414 | 0.961–2.082 | |
| GSTT1 × PM10 | 0.021 | 0.923 | 1.022 | 0.662–1.576 | ||
| O3 | GSTM1 × O3 | 0.077 | 0.383 | 1.081 | 0.908–1.286 | |
| GSTT1 × O3 | −0.105 | 0.255 | 0.901 | 0.752–1.078 | ||
| NO2 | GSTM1 × NO2 | 0.011 | 0.840 | 1.011 | 0.911–1.122 | |
| GSTT1 × NO2 | 0.093 | 0.221 | 1.098 | 0.945–1.276 | ||
| Smokers/Former smokers | ||||||
| Pre-pregnancy | PM2.5 | GSTM1 × PM2.5 | 0.432 | 0.069 | 1.145 | 0.847–2.464 |
| GSTT1 × PM2.5 | 0.574 | 0.183 | 1.776 | 0.763–4.136 | ||
| PM10 | GSTM1 × PM10 | 0.427 | 0.089 | 1.533 | 0.937–1.804 | |
| GSTT1 × PM10 | 0.701 | 0.159 | 2.016 | 0.670–5.348 | ||
| O3 | GSTM1 × O3 | −0.016 | 0.803 | 0.984 | 0.865–1.119 | |
| GSTT1 × O3 | −0.206 | 0.312 | 0.814 | 0.546–1.213 | ||
| NO2 | GSTM1 × NO2 | −0.023 | 0.809 | 0.977 | 0.911–1.177 | |
| GSTT1 × NO2 | −0.003 | 0.845 | 1.003 | 0.976–1.030 | ||
| 1st trimester | PM2.5 | GSTM1 × PM2.5 | 0.057 | 0.792 | 1.059 | 0.693–1.618 |
| GSTT1 × PM2.5 | −0.324 | 0.336 | 0.723 | 0.374–1.399 | ||
| PM10 | GSTM1 × PM10 | 0.206 | 0.332 | 1.242 | 0.801–1.922 | |
| GSTT1 × PM10 | −0.015 | 0.963 | 0.985 | 0.524–1.851 | ||
| O3 | GSTM1 × O3 | −0.212 | 0.625 | 0.809 | 0.346–1.893 | |
| GSTT1 × O3 | 0.200 | 0.761 | 1.222 | 0.336–4.436 | ||
| NO2 | GSTM1 × NO2 | −0.031 | 0.742 | 0.969 | 0.805–1.167 | |
| GSTT1 × NO2 | 0.006 | 0.708 | 1.006 | 0.977–1.035 | ||
| 2nd trimester | PM2.5 | GSTM1 × PM2.5 | −0.463 | 0.402 | 0.648 | 0.233–1.791 |
| GSTT1 × PM2.5 | −0.432 | 0.458 | 0.655 | 0.214–2.002 | ||
| PM10 | GSTM1 × PM10 | 0.309 | 0.358 | 1.362 | 0.705–2.633 | |
| GSTT1 × PM10 | 0.347 | 0.500 | 1.414 | 0.516–3.874 | ||
| O3 | GSTM1 × O3 | 0.145 | 0.369 | 1.156 | 0.842–1.588 | |
| GSTT1 × O3 | 0.072 | 0.750 | 1.075 | 0.689–1.676 | ||
| NO2 | GSTM1 × NO2 | −0.141 | 0.116 | 0.869 | 0.728–1.036 | |
| GSTT1 × NO2 | −0.264 | 0.121 | 0.768 | 0.550–1.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susa, A.; Davidovic, D.; Nikolic, N.; Sljivancanin Jakovljevic, T.; Kujundzic, V.; Mihajlovic, S.; Bogdanovic, L. The Role of Air Pollution Exposure and GSTM1-/GSTT1-Null Genotypes in Gestational Diabetes Mellitus Development: A Case–Control Study on Gene–Environment Interactions. Antioxidants 2025, 14, 652. https://doi.org/10.3390/antiox14060652
Susa A, Davidovic D, Nikolic N, Sljivancanin Jakovljevic T, Kujundzic V, Mihajlovic S, Bogdanovic L. The Role of Air Pollution Exposure and GSTM1-/GSTT1-Null Genotypes in Gestational Diabetes Mellitus Development: A Case–Control Study on Gene–Environment Interactions. Antioxidants. 2025; 14(6):652. https://doi.org/10.3390/antiox14060652
Chicago/Turabian StyleSusa, Ana, Dragana Davidovic, Nadja Nikolic, Tamara Sljivancanin Jakovljevic, Vera Kujundzic, Sladjana Mihajlovic, and Ljiljana Bogdanovic. 2025. "The Role of Air Pollution Exposure and GSTM1-/GSTT1-Null Genotypes in Gestational Diabetes Mellitus Development: A Case–Control Study on Gene–Environment Interactions" Antioxidants 14, no. 6: 652. https://doi.org/10.3390/antiox14060652
APA StyleSusa, A., Davidovic, D., Nikolic, N., Sljivancanin Jakovljevic, T., Kujundzic, V., Mihajlovic, S., & Bogdanovic, L. (2025). The Role of Air Pollution Exposure and GSTM1-/GSTT1-Null Genotypes in Gestational Diabetes Mellitus Development: A Case–Control Study on Gene–Environment Interactions. Antioxidants, 14(6), 652. https://doi.org/10.3390/antiox14060652







