Characteristics of Intestinal Microbiota and Host Gene Regulation in Coilia nasus Responding to Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Stress Experiment
2.2. Intestinal Transcriptome Sequencing
2.3. Metagenomic Sequencing of Intestinal Microbiota
2.4. Metabolites of Intestinal Microbiota
2.5. Detection of Intestinal Physiological Indexes
2.6. Real-Time Fluorescence Quantitative PCR for Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of Transport Stress on Intestinal Inflammation and Antioxidant Indexes in Coilia nasus
3.2. Transcriptomic Analysis of Intestine in C. nasus Under Transport Stress
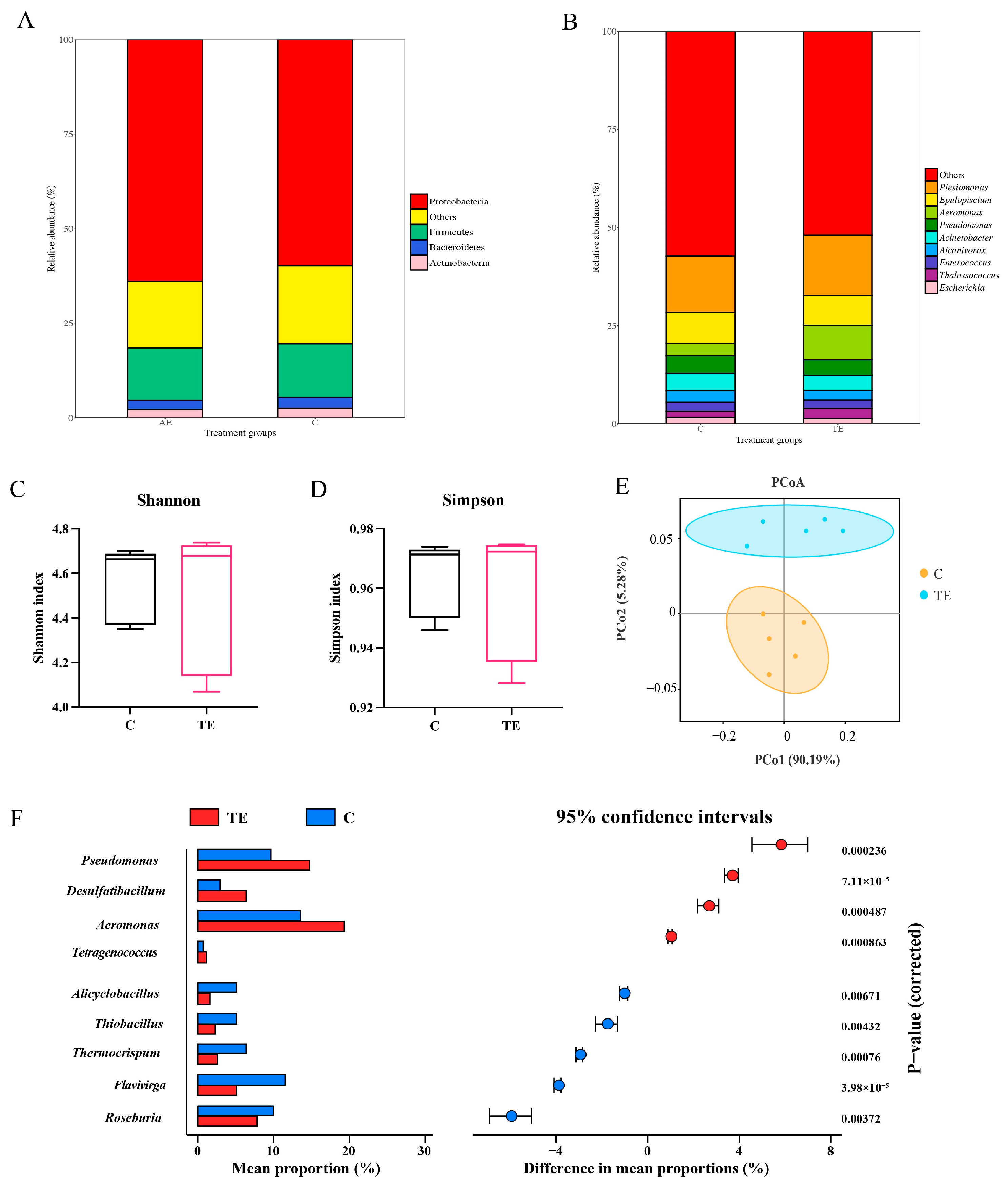
3.3. Changes of Intestinal Microbial Communities in C. nasus Under Transport Stress
3.4. Metabolic Alterations of Intestinal Microbiota in C. nasus Under Transport Stress
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jia, Y.; Gao, Y.; Wan, J.; Gao, Y.; Li, J.; Guan, C. Altered Physiological Response and Gill Histology in Black Rockfish, Sebastes Schlegelii, during Progressive Hypoxia and Reoxygenation. Fish Physiol. Biochem. 2021, 47, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.S.; Medland, J.E.; Mackey, E.; Edwards, L.L.; Bagley, K.D.; DeWilde, M.P.; Williams, K.J.; Moeser, A.J. Early Weaning Stress Induces Chronic Functional Diarrhea, Intestinal Barrier Defects, and Increased Mast Cell Activity in a Porcine Model of Early Life Adversity. Neurogastroenterol. Motil. 2017, 29, e13118. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jiang, R.; Liu, T.; Wei, Y.; Li, S.; Yan, Y. Bta-miR-378 Promote the Differentiation of Bovine Skeletal Muscle-Derived Satellite Cells. Gene 2018, 668, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.A.; Baffy, N. Modulation of the Gut Microbiota: A Focus on Treatments for Irritable Bowel Syndrome. Postgrad. Med. 2017, 129, 872–888. [Google Scholar] [CrossRef]
- Zeng, G.; Li, J.; Wang, Y.; Su, J.; Lu, Z.; Zhang, F.; Ding, W. Polystyrene Microplastic-Induced Oxidative Stress Triggers Intestinal Barrier Dysfunction via the NF-ΚB/NLRP3/IL-1β/MCLK Pathway. Environ. Pollut. 2024, 345, 123473. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z.; Li, W.; Xu, L.; Yang, M.; Ma, Y.; Liu, H.; Qian, H.; Wang, W. Copper–Luteolin Nanocomplexes for Mediating Multifaceted Regulation of Oxidative Stress, Intestinal Barrier, and Gut Microbiota in Inflammatory Bowel Disease. Bioact. Mater. 2025, 46, 118–133. [Google Scholar] [CrossRef]
- Wen, X.; Tang, L.; Zhong, R.; Liu, L.; Chen, L.; Zhang, H. Role of Mitophagy in Regulating Intestinal Oxidative Damage. Antioxidants 2023, 12, 480. [Google Scholar] [CrossRef]
- Sun, M.; Li, Q.; Zou, Z.; Liu, J.; Gu, Z.; Li, L. The Mechanisms behind Heatstroke-Induced Intestinal Damage. Cell Death Discov. 2024, 10, 455. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.; Chu, W. Neurotoxicity and Intestinal Microbiota Dysbiosis Induced by Per- and Polyfluoroalkyl Substances in Crucian Carp (Carassius auratus). J. Hazard. Mater. 2024, 478, 135611. [Google Scholar] [CrossRef]
- Luo, Q.; Qian, R.; Qiu, Z.; Yamamoto, F.Y.; Du, Y.; Lin, X.; Zhao, J.; Xu, Q. Dietary α-Ketoglutarate Alleviates Glycinin and β-Conglycinin Induced Damage in the Intestine of Mirror Carp (Cyprinus carpio). Front. Immunol. 2023, 14, 1140012. [Google Scholar] [CrossRef]
- Ahrendt, C.; Perez-Venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M.; Pulgar, J.; Galbán-Malagón, C. Microplastic Ingestion Cause Intestinal Lesions in the Intertidal Fish Girella Laevifrons. Mar. Pollut. Bull. 2020, 151, 110795. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Sun, Y.X.; Dong, H.B.; Zhan, A.J.; Wang, W.H.; Duan, Y.F.; Xie, M.; Liu, Q.S.; Li, H.; Zhang, J.S. Protection of Teprenone against Hypoxia and Reoxygenation Stress in Stomach and Intestine of Lateolabrax maculatus. Fish Physiol. Biochem. 2020, 46, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Reytor, D.; Jaña, V.; Pavez, L.; Navarrete, P.; García, K. Accessory Toxins of Vibriopathogens and Their Role in Epithelial Disruption during Infection. Front. Microbiol. 2018, 9, 2248. [Google Scholar] [CrossRef]
- Cao, Q.; Lin, L.; Xu, T.; Lu, Y.; Zhang, C.; Yue, K.; Huang, S.; Dong, H.; Jian, F. Aflatoxin B1 Alters Meat Quality Associated with Oxidative Stress, Inflammation, and Gut-Microbiota in Sheep. Ecotoxicol. Environ. Saf. 2021, 225, 112754. [Google Scholar] [CrossRef] [PubMed]
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Yang, M.; Yang, F.; Wang, G.; Liu, D.; Li, X.; Yang, L.; Wang, Z. Copper-Induced Oxidative Stress, Transcriptome Changes, Intestinal Microbiota, and Histopathology of Common Carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2022, 246, 114136. [Google Scholar] [CrossRef] [PubMed]
- Hieu, D.Q.; Hang, B.T.B.; Lokesh, J.; Garigliany, M.-M.; Huong, D.T.T.; Yen, D.T.; Liem, P.T.; Tam, B.M.; Hai, D.M.; Son, V.N.; et al. Salinity Significantly Affects Intestinal Microbiota and Gene Expression in Striped Catfish Juveniles. Appl. Microbiol. Biotechnol. 2022, 106, 3245–3264. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Huang, C.; Gao, C.; Wang, B.; He, J.; Yan, Y. Dietary Berberine against Intestinal Oxidative Stress, Inflammation Response, and Microbiota Disturbance Caused by Chronic Copper Exposure in Freshwater Grouper (Acrossocheilus fasciatus). Fish Shellfish. Immunol. 2023, 139, 108910. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Quan, J.; Sun, J.; Lu, J.; Zhao, G. Dietary Nano-Selenium Alleviates Heat Stress-Induced Intestinal Damage through Affecting Intestinal Antioxidant Capacity and Microbiota in Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish. Immunol. 2023, 133, 108537. [Google Scholar] [CrossRef]
- Du, F.; Xu, G.; Nie, Z.; Xu, P.; Gu, R. Transcriptome Analysis Gene Expression in the Liver of Coilia nasus during the Stress Response. BMC Genom. 2014, 15, 558. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Nie, Z.; Wang, J.; Sun, Y.; Xu, G. Integration of Metagenome and Metabolome Analysis Reveals the Correlation of Gut Microbiota, Oxidative Stress, and Inflammation in Coilia nasus under Air Exposure Stress and Salinity Mitigation. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101175. [Google Scholar] [CrossRef]
- Gao, J.; Mang, Q.; Liu, Y.; Sun, Y.; Xu, G. Integrated mRNA and miRNA Analysis Reveals the Regulatory Network of Oxidative Stress and Inflammation in Coilia nasus Brains during Air Exposure and Salinity Mitigation. BMC Genom. 2024, 25, 446. [Google Scholar] [CrossRef] [PubMed]
- Mang, Q.; Gao, J.; Li, Q.; Sun, Y.; Xu, G.; Xu, P. Metagenomic Insight into the Effect of Probiotics on Nitrogen Cycle in the Coilia nasus Aquaculture Pond Water. Microorganisms 2024, 12, 627. [Google Scholar] [CrossRef]
- Mang, Q.; Gao, J.; Li, Q.; Sun, Y.; Xu, G.; Xu, P. Probiotics Enhance Coilia nasus Growth Performance and Nutritional Value by Regulating Glucolipid Metabolism via the Gut–Liver Axis. Int. J. Mol. Sci. 2024, 25, 12196. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Sun, X.; Li, J.; Zhang, R.; Jiao, Y.; Wang, R.; Song, W.; Zhao, J. Characteristics and Candidate Genes Associated with Excellent Stalk Strength in Maize (Zea mays L.). Front. Plant Sci. 2022, 13, 957566. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Yousefi, M.; Hoseinifar, S.H.; Van Doan, H. Cytokines’ Gene Expression, Humoral Immune and Biochemical Responses of Common Carp (Cyprinus carpio, Linnaeus, 1758) to Transportation Density and Recovery in Brackish Water. Aquaculture 2019, 504, 13–21. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, W.; Tao, Y.; Li, Y.; Lu, S.; Xu, P.; Qiang, J. Transport Stress Induces Oxidative Stress and Immune Response in Juvenile Largemouth Bass (Micropterus salmoides): Analysis of Oxidative and Immunological Parameters and the Gut Microbiome. Antioxidants 2023, 12, 157. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D. Transport Stress Changes Blood Biochemistry, Antioxidant Defense System, and Hepatic HSPs mRNA Expressions of Channel Catfish Ictalurus punctatus. Front. Physiol. 2018, 9, 1628. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, Y.; Liu, W.; Zhou, Y. Evaluation of Zinc-Bearing Palygorskite Effects on the Growth, Immunity, Antioxidant Capability, and Resistance to Transport Stress in Blunt Snout Bream (Megalobrama amblycephala). Aquaculture 2021, 532, 735963. [Google Scholar] [CrossRef]
- Ren, Y.; Men, X.; Yu, Y.; Li, B.; Zhou, Y.; Zhao, C. Effects of Transportation Stress on Antioxidation, Immunity Capacity and Hypoxia Tolerance of Rainbow Trout (Oncorhynchus mykiss). Aquac. Rep. 2022, 22, 100940. [Google Scholar] [CrossRef]
- Gao, F.; Shi, X.; Pei, C.; Zhao, X.; Zhu, L.; Zhang, J.; Li, L.; Li, C.; Kong, X. The Role of Ferroptosis in Fish Inflammation. Rev. Aquac. 2023, 15, 318–332. [Google Scholar] [CrossRef]
- Tang, Z.; Ju, Y.; Dai, X.; Ni, N.; Liu, Y.; Zhang, D.; Gao, H.; Sun, H.; Zhang, J.; Gu, P. HO-1-Mediated Ferroptosis as a Target for Protection against Retinal Pigment Epithelium Degeneration. Redox Biol. 2021, 43, 101971. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Autophagy Promotes Ferroptosis by Degradation of Ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Hao, L.; Mi, J.; Song, L.; Guo, Y.; Li, Y.; Yin, Y.; Zhang, C. SLC40A1 Mediates Ferroptosis and Cognitive Dysfunction in Type 1 Diabetes. Neuroscience 2021, 463, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z. Iron and Oxidizing Species in Oxidative Stress and Alzheimer’s Disease. Aging Med. 2019, 2, 82–87. [Google Scholar] [CrossRef]
- Sokol, K.H.; Lee, C.J.; Rogers, T.J.; Waldhart, A.; Ellis, A.E.; Madireddy, S.; Daniels, S.R.; House, R.R.J.; Ye, X.; Olesnavich, M.; et al. Lipid Availability Influences Ferroptosis Sensitivity in Cancer Cells by Regulating Polyunsaturated Fatty Acid Trafficking. Cell Chem. Biol. 2025, 32, 408–422.e6. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting Glutathione Metabolism: Partner in Crime in Anticancer Therapy. Nutrients 2019, 11, 1926. [Google Scholar] [CrossRef]
- Bao, H.; Wang, Y.; Xiong, H.; Xia, Y.; Cui, Z.; Liu, L. Mechanism of Iron Ion Homeostasis in Intestinal Immunity and Gut Microbiota Remodeling. Int. J. Mol. Sci. 2024, 25, 727. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, X.; Liu, J.; Mo, Y.; Guo, X.; Qiu, Y.; Liu, Y.; Ma, X.; Wang, Y.; Xiong, Y. Therapeutic Material Basis and Underling Mechanisms of Shaoyao Decoction-Exerted Alleviation Effects of Colitis Based on GPX4-Regulated Ferroptosis in Epithelial Cells. Chin. Med. 2022, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Role of Gastrointestinal Microbiota in Fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Hassenrück, C.; Reinwald, H.; Kunzmann, A.; Tiedemann, I.; Gärdes, A. Effects of Thermal Stress on the Gut Microbiome of Juvenile Milkfish (Chanos chanos). Microorganisms 2021, 9, 5. [Google Scholar] [CrossRef]
- Li, X.; Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas Aeruginosa: A Typical Biofilm Forming Pathogen and an Emerging but Underestimated Pathogen in Food Processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef]
- Semwal, A.; Kumar, A.; Kumar, N. A Review on Pathogenicity of Aeromonas Hydrophila and Their Mitigation through Medicinal Herbs in Aquaculture. Heliyon 2023, 9, e14088. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The Role of Short-Chain Fatty Acids in Intestinal Barrier Function, Inflammation, Oxidative Stress, and Colonic Carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- He, X.; Long, Q.; Zhong, Y.; Zhang, Y.; Qian, B.; Huang, S.; Chang, L.; Qi, Z.; Li, L.; Wang, X.; et al. Short-Chain Fatty Acids Regulate Erastin-Induced Cardiomyocyte Ferroptosis and Ferroptosis-Related Genes. Front. Pharmacol. 2024, 15, 1409321. [Google Scholar] [CrossRef]




| Name | log2FC | p-Value | VIP | Regulated |
|---|---|---|---|---|
| 3-indoleacetic acid | −0.46297269 | 0.005879618 | 1.454613619 | down |
| indole-5-acetic acid | −1.654871937 | 0.006137787 | 1.388295277 | down |
| indole-1-propanoic acid | −0.658598224 | 0.037968101 | 1.084461372 | down |
| indole-3-carboxylic acid | −0.178969357 | 0.030991001 | 1.086786359 | down |
| cholic acid | −0.440879157 | 0.000378487 | 1.821650945 | down |
| propanoic acid | −5.600468707 | 1.14 × 10−10 | 2.886101393 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhu, H.; Gao, J.; Xu, G. Characteristics of Intestinal Microbiota and Host Gene Regulation in Coilia nasus Responding to Stress. Antioxidants 2025, 14, 626. https://doi.org/10.3390/antiox14060626
Gao J, Zhu H, Gao J, Xu G. Characteristics of Intestinal Microbiota and Host Gene Regulation in Coilia nasus Responding to Stress. Antioxidants. 2025; 14(6):626. https://doi.org/10.3390/antiox14060626
Chicago/Turabian StyleGao, Jun, Haojun Zhu, Jiancao Gao, and Gangchun Xu. 2025. "Characteristics of Intestinal Microbiota and Host Gene Regulation in Coilia nasus Responding to Stress" Antioxidants 14, no. 6: 626. https://doi.org/10.3390/antiox14060626
APA StyleGao, J., Zhu, H., Gao, J., & Xu, G. (2025). Characteristics of Intestinal Microbiota and Host Gene Regulation in Coilia nasus Responding to Stress. Antioxidants, 14(6), 626. https://doi.org/10.3390/antiox14060626






