Reactive Oxygen Species in Plants: Metabolism, Signaling, and Oxidative Modifications
Abstract
1. Introduction
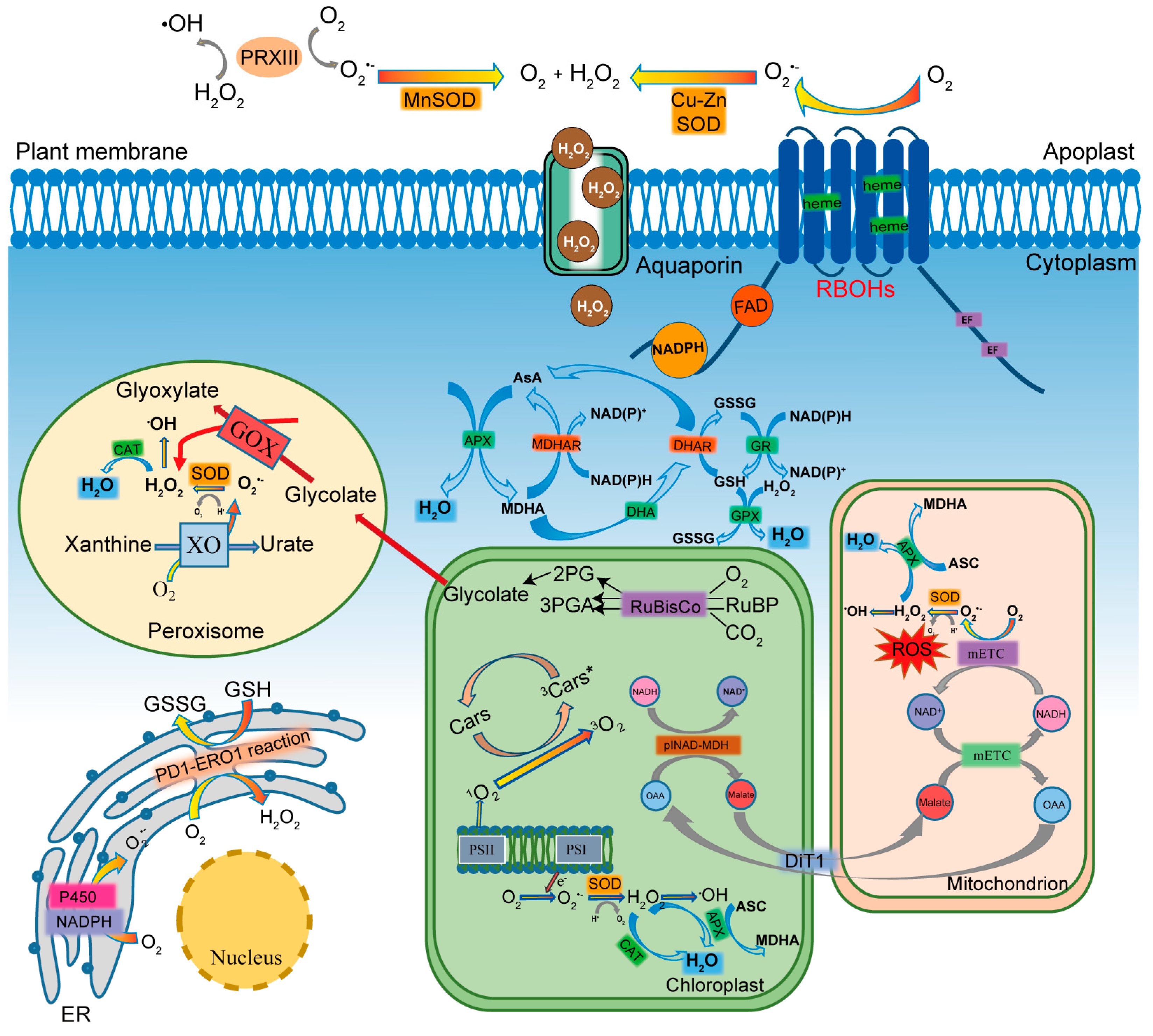
2. ROS Metabolism
2.1. Types of ROS
2.2. Generation of ROS
2.3. Scavenging of ROS
3. ROS Signaling Pathways
3.1. ROS Sensing
3.2. ROS Transport
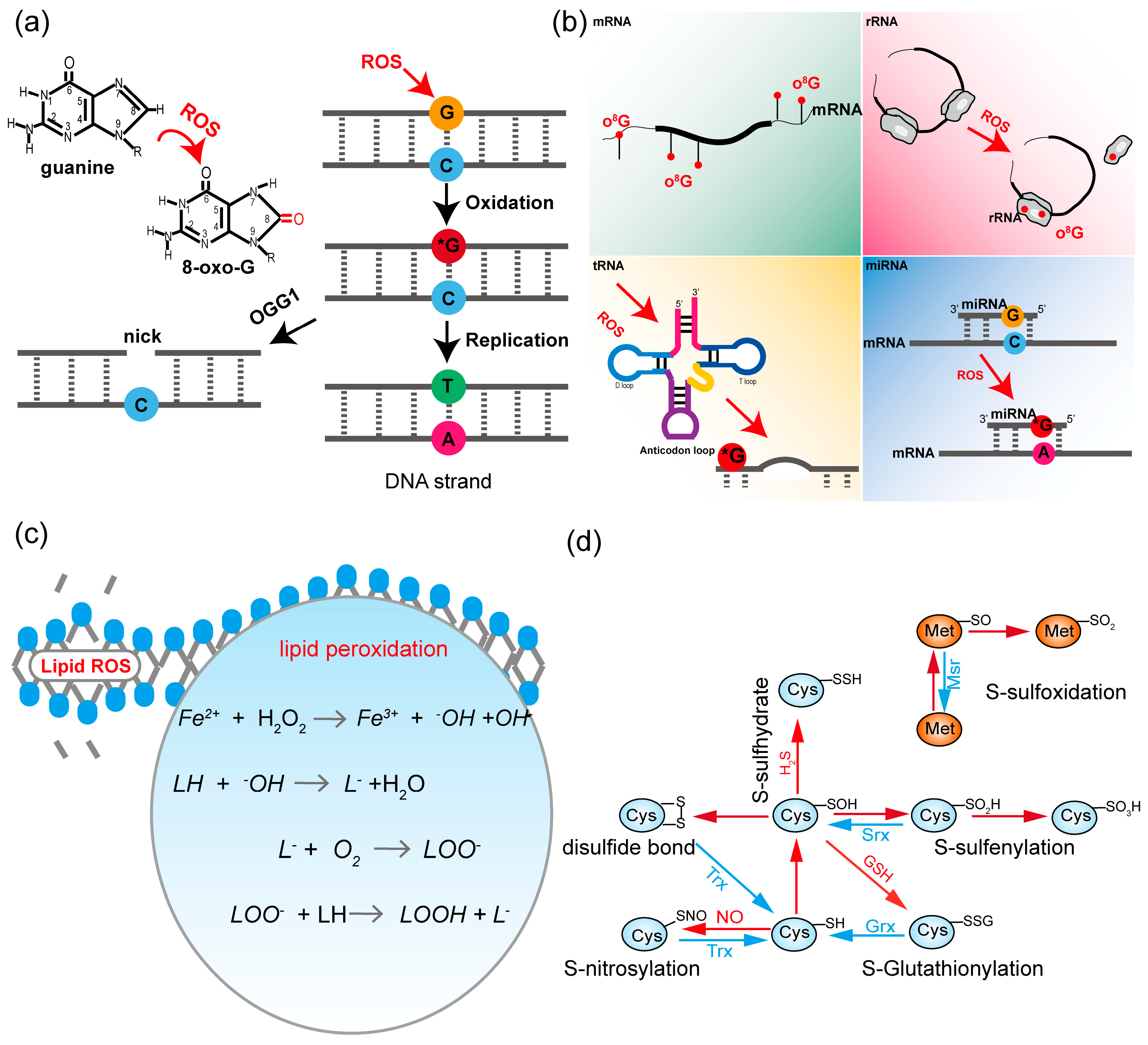
4. ROS-Mediated Oxidative Modifications
4.1. Nucleic Acids
4.2. Lipids
4.3. Proteins
4.3.1. ROS-Mediated Oxidative Modifications
4.3.2. Lipid-Peroxidation-Derived Reactive Carbonyl Species (RCS) Mediated Oxidative Modifications
4.3.3. ROS-RNS-Mediated Protein Modifications
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Williams, C.E.; Nemacheck, J.A.; Wang, H.; Subramanyam, S.; Zheng, C.; Chen, M.S. Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol. 2010, 152, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef]
- Camejo, D.; Guzman-Cedeno, A.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Gadjev, I.; Stone, J.M.; Gechev, T.S. Programmed cell death in plants: New insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell Mol. Biol. 2008, 270, 87–144. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Parihar, P.; Singh, S.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Reactive oxygen species signaling and stomatal movement: Current updates and future perspectives. Redox Biol. 2017, 11, 213–218. [Google Scholar] [CrossRef]
- Papamichos-Chronakis, M.; Peterson, C.L. Chromatin and the genome integrity network. Nat. Rev. Genet. 2013, 14, 62–75. [Google Scholar] [CrossRef]
- Tuteja, N.; Ahmad, P.; Panda, B.B.; Tuteja, R. Genotoxic stress in plants: Shedding light on DNA damage, repair and DNA repair helicases. Mutat. Res. 2009, 681, 134–149. [Google Scholar] [CrossRef]
- Roldan-Arjona, T.; Ariza, R.R. Repair and tolerance of oxidative DNA damage in plants. Mutat. Res. 2009, 681, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha Ambuj, B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Filkowski, J.; Smith, K.; Kovalchuk, O. Reactive oxygen species stimulate homologous recombination in plants. Plant Cell Environ. 2003, 26, 1531–1539. [Google Scholar] [CrossRef]
- Su, C.; Zhao, H.; Zhao, Y.; Ji, H.; Wang, Y.; Zhi, L.; Li, X. RUG3 and ATM synergistically regulate the alternative splicing of mitochondrial nad2 and the DNA damage response in Arabidopsis thaliana. Sci. Rep. 2017, 7, 43897. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early events in plant abiotic stress signaling: Interplay between calcium, reactive oxygen species and phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- Wang, C.; Deng, Y.; Liu, Z.; Liao, W. Hydrogen sulfide in plants: Crosstalk with other signal molecules in response to abiotic stresses. Int. J. Mol. Sci. 2021, 22, 12068. [Google Scholar] [CrossRef]
- Jacques, S.; Ghesquiere, B.; Van Breusegem, F.; Gevaert, K. Plant proteins under oxidative attack. Proteomics 2013, 13, 932–940. [Google Scholar] [CrossRef]
- Huang, J.; Willems, P.; Wei, B.; Tian, C.; Ferreira, R.B.; Bodra, N.; Martinez Gache, S.A.; Wahni, K.; Liu, K.; Vertommen, D.; et al. Mining for protein S-sulfenylation in Arabidopsis uncovers redox-sensitive sites. Proc. Natl. Acad. Sci. USA 2019, 116, 21256–21261. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Zuo, J. Protein S-Nitrosylation in plants: Current progresses and challenges. J. Integr. Plant Biol. 2019, 61, 1206–1223. [Google Scholar] [CrossRef]
- Kalinina, E.; Novichkova, M. Glutathione in protein redox modulation through S-Glutathionylation and S-Nitrosylation. Molecules 2021, 26, 435. [Google Scholar] [CrossRef]
- Daly, N.L.; Clark, R.J.; Craik, D.J. Disulfide folding pathways of cystine knot proteins. Tying the knot within the circular backbone of the cyclotides. J. Biol. Chem. 2003, 278, 6314–6322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, Y.Q. Role of reactive oxygen species in regulating epigenetic modifications. Cell Signal. 2025, 125, 111502. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Mhmb, B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Zhang, M.; Jiao, S.; Guo, Y.; Jiang, T. The role of reactive oxygen species in plant-virus interactions. Plant Cell Rep. 2024, 43, 197. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; He, S.; Wu, S.; Yang, C. Singlet oxygen: Properties, generation, detection, and environmental applications. J. Hazard. Mater. 2024, 461, 132538. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Dong, Z.; Wu, H.; Tian, Z.; Zhao, Z. Redox regulation of plant stem cell fate. EMBO J. 2017, 36, 2844–2855. [Google Scholar] [CrossRef]
- Li, S. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef]
- Gad, M.; Karen, S.; Rachel, T.; Diego, C.; Miguel, A.T.; Vladimir, S.; Jeffery, L.D.; Ron, M. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef]
- Ling, H.; Sun, H.; Lu, L.; Zhang, J.; Liao, L.; Wang, J.; Zhang, X.; Lan, Y.; Li, R.; Lu, W.; et al. Sustainable photocatalytic hydrogen peroxide production over octonary high-entropy oxide. Nat. Commun. 2024, 15, 9505. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Daudi, A.; Finch, P.; Butt, V.S.; Whitelegge, J.P.; Souda, P.; Ausubel, F.M.; Bolwell, G.P. A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 2012, 158, 2013–2027. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Ranocha, P.; Kasulin, L.; Fusari, C.M.; Servi, L.; Aptekmann, A.A.; Gabarain, V.B.; Peralta, J.M.; Borassi, C.; Marzol, E.; et al. Apoplastic class III peroxidases PRX62 and PRX69 promote Arabidopsis root hair growth at low temperature. Nat. Commun. 2022, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Kaya, H.; Hiraoka, G.; Yumoto, F.; Kimura, S.; Kadota, Y.; Hishinuma, H.; Senzaki, E.; Yamagoe, S.; Nagata, K.; et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 2008, 283, 8885–8892. [Google Scholar] [CrossRef]
- Oda, T.; Hashimoto, H.; Kuwabara, N.; Akashi, S.; Hayashi, K.; Kojima, C.; Wong, H.L.; Kawasaki, T.; Shimamoto, K.; Sato, M.; et al. Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J. Biol. Chem. 2010, 285, 1435–1445. [Google Scholar] [CrossRef]
- Torres, M.A.; Onouchi, H.; Hamada, S.; Machida, C.; Hammond-Kosack, K.E.; Jones, J.D. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 1998, 14, 365–370. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Yu, L.; Zhou, Z.; Liang, X.; Liu, Z.; Cai, G.; Gao, L.; Zhang, X.; Wang, Y.; et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 2014, 15, 329–338. [Google Scholar] [CrossRef]
- Li, P.; Zhao, L.; Qi, F.; Nmps, H.; Li, Q.; Zhang, D.; Lin, F.; Shang-Guan, K.; Liang, Y. The receptor-like cytoplasmic kinase RIPK regulates broad-spectrum ROS signaling in multiple layers of plant immune system. Mol. Plant 2021, 14, 1652–1667. [Google Scholar] [CrossRef]
- Rao, S.; Zhou, Z.; Miao, P.; Bi, G.; Hu, M.; Wu, Y.; Feng, F.; Zhang, X.; Zhou, J.M. Roles of receptor-like cytoplasmic kinase VII members in pattern-triggered immune signaling. Plant Physiol. 2018, 177, 1679–1690. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef] [PubMed]
- Drerup, M.M.; Schlucking, K.; Hashimoto, K.; Manishankar, P.; Steinhorst, L.; Kuchitsu, K.; Kudla, J. The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant 2013, 6, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Chen, Q.F.; Liao, K.; Zhou, D.M.; Yang, Y.C.; He, M.; Yu, L.J.; Guo, D.-Y.; Xiao, S.; Xie, R.-H.; et al. The calcium-dependent protein kinase CPK16 regulates hypoxia-induced ROS production by phosphorylating the NADPH oxidase RBOHD in Arabidopsis. Plant Cell 2024, 36, 3451–3466. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, J.W.; Ai, Y.F.; Shangguan, K.K.; Li, P.; Lin, F.C.; Liang, Y. DGK5β-derived phosphatidic acid regulates ROS production in plant immunity by stabilizing NADPH oxidase. Cell Host Microbe 2024, 32, 425–440.e7. [Google Scholar] [CrossRef]
- Menzel, W.; Stenzel, I.; Helbig, L.M.; Krishnamoorthy, P.; Neumann, S.; Eschen-Lippold, L.; Heilmann, M.; Lee, J.; Heilmann, I. A PAMP-triggered MAPK cascade inhibits phosphatidylinositol 4,5-bisphosphate production by PIP5K6 in Arabidopsis thaliana. New Phytol. 2019, 224, 833–847. [Google Scholar] [CrossRef]
- Yun, B.W.; Feechan, A.; Yin, M.H.; Saidi, N.B.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.-G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based Modification of Cysteine Desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Lee, J.; Hanh Nguyen, H.; Park, Y.; Lin, J.; Hwang, I. Spatial regulation of RBOHD via AtECA4-mediated recycling and clathrin-mediated endocytosis contributes to ROS accumulation during salt stress response but not flg22-induced immune response. Plant J. 2022, 109, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Lukan, T.; Pompe-Novak, M.; Baebler, S.; Tusek-Znidaric, M.; Kladnik, A.; Kriznik, M.; Blejec, A.; Zagorščak, M.; Stare, K.; Dušak, B.; et al. Precision transcriptomics of viral foci reveals the spatial regulation of immune-signaling genes and identifies RBOHD as an important player in the incompatible interaction between potato virus Y and potato. Plant J. 2020, 104, 645–661. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef]
- Janku, M.; Luhova, L.; Petrivalsky, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2018, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Edreva, A. Generation and scavenging of reactive oxygen species in chloroplasts: A submolecular approach. Agric. Ecosyst. Environ. 2005, 106, 119–133. [Google Scholar] [CrossRef]
- Mehler, A.H. Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch. Biochem. Biophys. 1951, 34, 339–351. [Google Scholar] [CrossRef]
- Cleland, R.E.; Grace, S.C. Voltammetric detection of superoxide production by photosystem II. FEBS Lett. 1999, 457, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997, 17, 3–8. [Google Scholar] [CrossRef]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; St Clair, D.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta 2012, 1822, 794–814. [Google Scholar] [CrossRef]
- Schwarzlander, M.; Finkemeier, I. Mitochondrial energy and redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2122–2144. [Google Scholar] [CrossRef]
- Del Rio, L.A.; Lopez-Huertas, E. ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The applications and mechanisms of superoxide dismutase in medicine, food, and cosmetics. Antioxiddants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Glorieux, C.; Zamocky, M.; Sandoval, J.M.; Verrax, J.; Calderon, P.B. Regulation of catalase expression in healthy and cancerous cells. Free Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuine, S.; Triantaphylides, C.; Ravanat, J.L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Dcga, P.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]
- Chae, H.B.; Kim, M.G.; Kang, C.H.; Park, J.H.; Lee, E.S.; Lee, S.U.; Chi, Y.H.; Paeng, S.K.; Bin Bae, S.; Wi, S.D.; et al. Redox sensor QSOX1 regulates plant immunity by targeting GSNOR to modulate ROS generation. Mol. Plant 2021, 14, 1312–1327. [Google Scholar] [CrossRef]
- Bi, G.; Hu, M.; Fu, L.; Zhang, X.; Zuo, J.; Li, J.; Yang, J.; Zhou, J.M. The cytosolic thiol peroxidase PRXIIB is an intracellular sensor for H(2)O(2) that regulates plant immunity through a redox relay. Nat. Plants 2022, 8, 1160–1175. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Fang, Y.; Yin, J.; He, M.; Wei, Y.; Zhang, J.; Yong, S.; Cha, J.; Song, L.; Zhu, X.; et al. Rice transcription factor bHLH25 confers resistance to multiple diseases by sensing H(2)O(2). Cell Res. 2025, 35, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Bienert, G.P.; Moller, A.L.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183065. [Google Scholar] [CrossRef] [PubMed]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef]
- Hooijmaijers, C.; Rhee, J.Y.; Kwak, K.J.; Chung, G.C.; Horie, T.; Katsuhara, M.; Kang, H. Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J. Plant Res. 2012, 125, 147–153. [Google Scholar] [CrossRef]
- Tian, S.; Wang, X.; Li, P.; Wang, H.; Ji, H.; Xie, J.; Qiu, Q.; Shen, D.; Dong, H. Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef]
- Grondin, A.; Rodrigues, O.; Verdoucq, L.; Merlot, S.; Leonhardt, N.; Maurel, C. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 2015, 27, 1945–1954. [Google Scholar] [CrossRef]
- Mubarakshina Borisova, M.M.; Kozuleva, M.A.; Rudenko, N.N.; Naydov, I.A.; Klenina, I.B.; Ivanov, B.N. Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim. Biophys. Acta 2012, 1817, 1314–1321. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Kobayashi, Y.; Nakano, Y.; Iuchi, S.; Kobayashi, M.; Sahoo, L.; Koyama, H. Genome-wide association study reveals that the aquaporin NIP1;1 contributes to variation in hydrogen peroxide sensitivity in Arabidopsis thaliana. Mol. Plant 2017, 10, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, H.; Li, N.; Wei, N.; Tian, Y.; Peng, J.; Chen, X.; Zhang, L.; Zhang, M.; Dong, H. Aquaporin OsPIP2;2 links the H2O2 signal and a membrane-anchored transcription factor to promote plant defense. Plant Physiol. 2022, 188, 2325–2341. [Google Scholar] [CrossRef]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef]
- Prak, S.; Hem, S.; Boudet, J.; Viennois, G.; Sommerer, N.; Rossignol, M.; Maurel, C.; Santoni, V. Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: Role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol. Cell Proteom. 2008, 7, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Sahr, T.; Adam, T.; Fizames, C.; Maurel, C.; Santoni, V. O-carboxyl- and N-methyltransferases active on plant aquaporins. Plant Cell Physiol. 2010, 51, 2092–2104. [Google Scholar] [CrossRef]
- Santoni, V.; Verdoucq, L.; Sommerer, N.; Vinh, J.; Pflieger, D.; Maurel, C. Methylation of aquaporins in plant plasma membrane. Biochem. J. 2006, 400, 189–197. [Google Scholar] [CrossRef]
- Wudick, M.M.; Li, X.; Valentini, V.; Geldner, N.; Chory, J.; Lin, J.; Maurel, C.; Luu, D.T. Subcellular redistribution of root aquaporins induced by hydrogen peroxide. Mol. Plant 2015, 8, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Gasparutto, D.; Ravanat, J.L. Oxidative damage to DNA: Formation, measurement and biochemical features. Mutat. Res. 2003, 531, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.; Peak, J.; Park, J.; Ahn, S.H.; Cho, Y.K.; Jeong, Y.; Lee, H.S.; Lee, J.; Ignatova, E.; Lee, S.E.; et al. Widespread 8-oxoguanine modifications of miRNA seeds differentially regulate redox-dependent cancer development. Nat. Cell Biol. 2023, 25, 1369–1383. [Google Scholar] [CrossRef]
- Seok, H.; Lee, H.; Lee, S.; Ahn, S.H.; Lee, H.S.; Kim, G.D.; Peak, J.; Park, J.; Cho, Y.K.; Jeong, Y.; et al. Position-specific oxidation of miR-1 encodes cardiac hypertrophy. Nature 2020, 584, 279–285. [Google Scholar] [CrossRef]
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf-Bouteau, H.; Bailly, C. Targeted mRNA oxidation regulates sunflower seed dormancy alleviation during dry after-ripening. Plant Cell 2011, 23, 2196–2208. [Google Scholar] [CrossRef]
- Koh, E.; Cohen, D.; Brandis, A.; Fluhr, R. Attenuation of cytosolic translation by RNA oxidation is involved in singlet oxygen-mediated transcriptomic responses. Plant Cell Environ. 2021, 44, 3597–3615. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Papolu, P.K.; Satish, L.; Vinod, K.K.; Wei, Q.; Sharma, A.; Emamverdian, A.; Zou, L.H.; Zhou, M. Redox status of the plant cell determines epigenetic modifications under abiotic stress conditions and during developmental processes. J. Adv. Res. 2022, 42, 99–116. [Google Scholar] [CrossRef]
- Berglund, T.; Wallstrom, A.; Nguyen, T.V.; Laurell, C.; Ohlsson, A.B. Nicotinamide; antioxidative and DNA hypomethylation effects in plant cells. Plant Physiol. Biochem. 2017, 118, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.E.; Spalding, J.W.; Virgil, K.M.; Faircloth, R.S.; Humble, M.C.; Lacks, G.D.; Tennant, R.W. Induction of transgene expression in Tg.AC(v-Ha-ras) transgenic mice concomitant with DNA hypomethylation. Mol. Carcinog. 1998, 21, 244–250. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, H.; Wei, M.; Tang, Y.; Xia, Y.; Chen, Y.; Shen, Z.; Chen, C. Reactive oxygen species partly mediate DNA methylation in responses to different heavy metals in pokeweed. Front. Plant Sci. 2022, 13, 845108. [Google Scholar] [CrossRef] [PubMed]
- Causevic, A.; Gentil, M.V.; Delaunay, A.; El-Soud, W.A.; Garcia, Z.; Pannetier, C.; Brignolas, F.; Hagège, D.; Maury, S. Relationship between DNA methylation and histone acetylation levels, cell redox and cell differentiation states in sugarbeet lines. Planta 2006, 224, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet molecular oxygen reactions with nucleic acids, lipids, and proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef]
- Dogra, V.; Li, M.; Singh, S.; Li, M.; Kim, C. Oxidative post-translational modification of EXECUTER1 is required for singlet oxygen sensing in plastids. Nat. Commun. 2019, 10, 2834. [Google Scholar] [CrossRef]
- Schulte, L.; Mao, J.; Reitz, J.; Sreeramulu, S.; Kudlinzki, D.; Hodirnau, V.V.; Meier-Credo, J.; Saxena, K.; Buhr, F.; Langer, J.D.; et al. Cysteine oxidation and disulfide formation in the ribosomal exit tunnel. Nat. Commun. 2020, 11, 5569. [Google Scholar] [CrossRef]
- Rehder, D.S.; Borges, C.R. Cysteine sulfenic acid as an intermediate in disulfide bond formation and nonenzymatic protein folding. Biochemistry 2010, 49, 7748–7755. [Google Scholar] [CrossRef]
- Woo, H.A.; Kang, S.W.; Kim, H.K.; Yang, K.S.; Chae, H.Z.; Rhee, S.G. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. J. Biol. Chem. 2003, 278, 47361–47364. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakano, M.; Kato, S.; Yoshihara, D.; Ookawara, T.; Eguchi, H.; Taniguchi, N.; Suzuki, K. Oxidative modification to cysteine sulfonic acid of Cys111 in human copper-zinc superoxide dismutase. J. Biol. Chem. 2007, 282, 35933–35944. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Rossi, R.; Colombo, R.; Milzani, A. Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic. Biol. Med. 2003, 34, 23–32. [Google Scholar] [CrossRef]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Prokaeva, T.; McComb, M.E.; Connors, L.H.; Skinner, M.; Costello, C.E. Identification of S-sulfonation and S-thiolation of a novel transthyretin Phe33Cys variant from a patient diagnosed with familial transthyretin amyloidosis. Protein Sci. 2003, 12, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Lindermayr, C.; Sell, S.; Muller, B.; Leister, D.; Durner, J. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 2010, 22, 2894–2907. [Google Scholar] [CrossRef]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. 2008. Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, M.; Qin, Z.; Lv, H.; Wang, M.; Zhang, Z.; Zhou, W.; Zhao, N.; Li, X.; Han, C.; et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Zhang, W.; Zhi, W.; Qiao, H.; Huang, J.; Li, S.; Lu, Q.; Wang, N.; Li, Q.; Zhou, Q.; Sun, J.; et al. H2O2-dependent oxidation of the transcription factor GmNTL1 promotes salt tolerance in soybean. Plant Cell 2023, 36, 112–135. [Google Scholar] [CrossRef]
- Ji, E.; Hu, S.; Lu, Q.; Zhang, M.; Jiang, M. Hydrogen peroxide positively regulates ABA signaling via oxidative modification of the C2H2-type zinc finger protein ZFP36 in rice. Plant Physiol. Biochem. 2024, 213, 108844. [Google Scholar] [CrossRef]
- Cao, L.; Karapetyan, S.; Yoo, H.; Chen, T.; Mwimba, M.; Zhang, X.; Dong, X. H(2)O(2) sulfenylates CHE, linking local infection to the establishment of systemic acquired resistance. Science 2024, 385, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mu, J.; Chen, L.; Feng, J.; Hu, J.; Li, L.; Zhou, J.M.; Zuo, J. S-nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol. 2015, 167, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jia, P.F.; Wang, W.; Chen, L.; Gong, X.; Lin, H.; Wu, R.; Yang, W.-C.; Li, H.-J.; Zuo, J.; et al. S-sulfenylation-mediated inhibition of the GSNOR1 activity regulates ovule development in Arabidopsis. J. Genet. Genom. 2025, in press. [Google Scholar] [CrossRef]
- Moskovitz, J. Roles of methionine suldfoxide reductases in antioxidant defense, protein regulation and survival. Curr. Pharm. Des. 2005, 11, 1451–1457. [Google Scholar] [CrossRef]
- Marondedze, C.; Turek, I.; Parrott, B.; Thomas, L.; Jankovic, B.; Lilley, K.S.; Gehring, C. Structural and functional characteristics of cGMP-dependent methionine oxidation in Arabidopsis thaliana proteins. Cell Commun. Signal 2013, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.; Ghesquiere, B.; De Bock, P.J.; Demol, H.; Wahni, K.; Willems, P.; Messens, J.; Van Breusegem, F.; Gevaert, K. Protein methionine sulfoxide dynamics in Arabidopsis thaliana under oxidative stress. Mol. Cell Proteom. 2015, 14, 1217–1229. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L.; Wu, C.; Shan, W.; Cai, D.; Lin, Z.; Wei, W.; Chen, J.; Lu, W.; Kuang, J. Methionine oxidation and reduction of the ethylene signaling component MaEIL9 are involved in banana fruit ripening. J. Integr. Plant Biol. 2023, 65, 150–166. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Ali, M.; Liu, J.; Fu, X.; Song, Y.; Ding, J.; Li, X.; Ji, N.; Zhang, X. Interaction of methionine sulfoxide reductase B5 with SlMYC2 stimulates the transcription of MeJA-mediated autophagy-related genes in tomato fruit. Hortic. Res. 2023, 10, uhad012. [Google Scholar] [CrossRef]
- Yan, H.; Jiang, G.; Wu, F.; Li, Z.; Xiao, L.; Jiang, Y.; Duan, X. Sulfoxidation regulation of transcription factor NAC42 influences its functions in relation to stress-induced fruit ripening in banana. J. Exp. Bot. 2021, 72, 682–699. [Google Scholar] [CrossRef]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid peroxidation derived reactive carbonyl species in free and conjugated forms as an index of lipid peroxidation: Limits and perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef]
- Biswas, M.S.; Mano, J. Reactive carbonyl species activate caspase-3-like protease to initiate programmed cell death in plants. Plant Cell Physiol. 2016, 57, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.H.; Nakashima, Y.; Nakamura, T.; Nakamura, Y.; Hirai, Y.; Hoque, M.A.; Rhaman, M.S.; Munemasa, S.; Mano, J.; Murata, Y. Involvement of reactive carbonyl species in inhibition of germination and seedling growth by salt stress in rice. Biosci. Biotechnol. Biochem. 2025. [Google Scholar] [CrossRef]
- Sultana, M.S.; Sakurai, C.; Biswas, M.S.; Szabados, L.; Mano, J. Accumulation of reactive carbonyl species in roots as the primary cause of salt stress-induced growth retardation of Arabidopsis thaliana. Physiol. Plant. 2024, 176, 14198. [Google Scholar] [CrossRef]
- Guo, J.B.; Sun, W.; Liu, H.Y.; Chi, J.L.; Odiba, A.S.; Li, G.C.; Jin, L.P.; Xin, C.H. Aldehyde dehydrogenase plays crucial roles in response to lower temperature stress in Solanum tuberosum and Nicotiana benthamiana. Plant Sci. 2020, 297, 110525. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, S.R.; An, G. Rice aldehyde dehydrogenase7 is needed for seed maturation and viability. Plant Physiol. 2009, 149, 905–915. [Google Scholar] [CrossRef]
- Islam, M.; Ye, W.X.; Akter, F.; Rhaman, M.S.; Matsushima, D.; Munemasa, S.; Okuma, E.; Nakamura, Y.; Biswas, S.; Mano, J.; et al. Reactive carbonyl species mediate methyl jasmonate-induced stomatal closure. Plant Cell Physiol. 2020, 61, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Ye, W.X.; Matsushima, D.; Munemasa, S.; Okuma, E.; Nakamura, Y.; Biswas, M.S.; Mano, J.; Murata, Y. Reactive carbonyl species mediate ABA signaling in guard cells. Plant Cell Physiol. 2016, 57, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Debska, K.; Bogatek, R.; Gniazdowska, A. Protein carbonylation and its role in physiological processes in plants. Postep. Biochem. 2012, 58, 34–43. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23214127 (accessed on 19 May 2025).
- Viedma-Poyatos, A.; Gonzalez-Jimenez, P.; Langlois, O.; Company-Marin, I.; Spickett, C.M.; Perez-Sala, D. Protein lipoxidation: Basic concepts and emerging roles. Antioxidants 2021, 10, 295. [Google Scholar] [CrossRef]
- Mano, J.; Nagata, M.; Okamura, S.; Shiraya, T.; Mitsui, T. Identification of oxidatively modified proteins in salt-stressed Arabidopsis: A carbonyl-targeted proteomics approach. Plant Cell Physiol. 2014, 55, 1233–1244. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Kim, A.; Peñuelas, M.; Ihling, C.; Griesser, E.; Hoffmann, R.; Fedorova, M.; Frolov, A.; Becana, M. Protein carbonylation and glycation in legume nodules. Plant Physiol. 2018, 177, 1510–1528. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, B.K.; Askerlund, P.; Bykova, N.V.; Egsgaard, H.; Moller, I.M. Identification of oxidised proteins in the matrix of rice leaf mitochondria by immunoprecipitation and two-dimensional liquid chromatography-tandem mass spectrometry. Phytochemistry 2004, 65, 1839–1851. [Google Scholar] [CrossRef]
- Fangue-Yapseu, G.Y.; Tola, A.J.; Missihoun, T.D. Proteome-wide analysis of hydrogen peroxide-induced protein carbonylation in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 1049681. [Google Scholar] [CrossRef] [PubMed]
- Turkan, I. ROS and RNS: Key signalling molecules in plants. J. Exp. Bot. 2018, 69, 3313–3315. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) in plants- maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Y.J.; Jia, L.X.; Chu, H.Y.; Zhou, S.; Chen, K.M.; Wu, D.; Zhao, L.Q. Hydrogen peroxide acts upstream of nitric oxide in the heat shock pathway in Arabidopsis seedlings. Plant Physiol. 2014, 164, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Jiang, M.Y.; Zhang, J.H.; Ding, H.D.; Xu, S.C.; Hu, X.L.; Tan, M.P. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007, 175, 36–50. [Google Scholar] [CrossRef]
- Lin, A.H.; Wang, Y.Q.; Tang, J.Y.; Xue, P.; Li, C.L.; Liu, L.C.; Hu, B.; Yang, F.Q.; Loake, G.J.; Chu, C.C. Nitric oxide and protein-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012, 158, 451–464. [Google Scholar] [CrossRef]
- Cui, B.M.; Pan, Q.N.; Cui, W.Q.; Wang, Y.Q.; Loake, V.I.P.; Yuan, S.G.; Liu, F.Q.; Loake, G.J. S-nitrosylation of a receptor-like cytoplasmic kinase regulates plant immunity. Sci. Adv. 2024, 10, eadk3126. [Google Scholar] [CrossRef]
| oxiPTM | Description | |
|---|---|---|
| ROS | S-nitrosylation of Cys | NPR1 Cys: nuclear import and oligomerization |
| S-sulfenylation of Cys | GSNOR1 Cys284: enzymatic activity inhibition | |
| BZR1 Cys63: transcriptional activity | ||
| Oxidative modifications of Cys | GmNTL1 Cys247: activation of GmRbohB, GmCHX1/GmSALT3, GmNHX1 gene expression | |
| ZFP36 Cys32: enhanced expression and activity of antioxidant enzyme genes upon ABA treatment | ||
| CHE Cys residue: promotes binding to ICS1 promoter | ||
| Sulfoxidation of Met | Methionine sulfoxidation of MaNAC42, MaEIL9, and SlMYC2 decrease their DNA-binding activity and transcription activity | |
| bHLH25 Met256: transcriptional activity | ||
| RNS | S-nitrosylation of Cys | BIK1 Cys80: stability, activity, and flg22-induced ROS production. |
| AtRBOHD Cys890: abolishment of reactive oxygen intermediates synthesis ability | ||
| APX1 Cys32: ROS-scavenging activity | ||
| MYB30 Cys49: transcriptional activity enhancement and PYL4 interaction disruption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Chen, J.-P.; Wang, X.-W.; Li, P. Reactive Oxygen Species in Plants: Metabolism, Signaling, and Oxidative Modifications. Antioxidants 2025, 14, 617. https://doi.org/10.3390/antiox14060617
Zheng C, Chen J-P, Wang X-W, Li P. Reactive Oxygen Species in Plants: Metabolism, Signaling, and Oxidative Modifications. Antioxidants. 2025; 14(6):617. https://doi.org/10.3390/antiox14060617
Chicago/Turabian StyleZheng, Chao, Jian-Ping Chen, Xiao-Wei Wang, and Ping Li. 2025. "Reactive Oxygen Species in Plants: Metabolism, Signaling, and Oxidative Modifications" Antioxidants 14, no. 6: 617. https://doi.org/10.3390/antiox14060617
APA StyleZheng, C., Chen, J.-P., Wang, X.-W., & Li, P. (2025). Reactive Oxygen Species in Plants: Metabolism, Signaling, and Oxidative Modifications. Antioxidants, 14(6), 617. https://doi.org/10.3390/antiox14060617








