Abstract
Potentially toxic elements (PTE), such as cadmium (Cd), lead (Pb), and arsenic (As), threaten rice (Oryza sativa L.) crop productivity and pose significant risks to human health when they are present in soil. This review summarizes the current understanding of soil and rice contamination with As, Cd, and Pb to provide an in-depth understanding of the dynamics of these contaminants and the mechanisms regulating their flow from soil to plants. It focuses on the following aspects: (1) these metals’ geochemical distribution and speciation in soil–rice systems; (2) factors influencing the transformation, bioavailability, and uptake of these metals in paddy soils; (3) metal uptake, transport, translocation, and accumulation mechanisms in rice grains; and (4) the roles of transporters involved in metal uptake, transport, and accumulation in rice plants. Moreover, this review contributes to a clearer understanding of the environmental risks associated with these toxic metals in soil–rice ecosystems. Furthermore, it highlights the challenges in simultaneously managing the risks of As, Cd, and Pb contamination in rice. The study findings may help inspire innovative methods, biotechnological applications, and sustainable management strategies to mitigate the accumulation of As, Cd, and Pb in rice grains while effectively addressing multi-metal contamination in paddy soils.
1. Introduction
The contamination of agricultural soils with potentially toxic elements (PTE) is a serious global concern [1,2,3,4]. PTE primarily enter agricultural soil ecosystems via atmospheric deposition from mining and industrial activities; the application of synthetic fertilizers (e.g., phosphate fertilizers), farmyard manure, and agrochemicals (e.g., pesticides); the introduction of sewage sludge and wastewater; and irrigation using contaminated groundwater [2,5,6]. Among PTE, arsenic (As), cadmium (Cd), and lead (Pb) are of particular concern because they frequently occur in the environment, which increases the likelihood for human exposure [7]. In fact, the Agency for Harmful Substances and Disease Registry and the U.S. Environmental Protection Agency list these three toxic elements among the top 10 PTE [8,9]. Their toxicity levels are dose-dependent, and chronic exposure to these PTE through food consumption has been linked to various health risks, including cancer [10,11]. They are non-biodegradable, persistent, and devoid of biological functions once they enter the environment.
Moreover, these PTE significantly degrade soil health and fertility by disrupting soil ecosystem functions, negatively affecting natural soil microbial communities, and altering soil chemical and physical properties [12,13]. Upon entering the edible parts of crops, these PTE interfere with various physiological and biochemical processes, including nutrient uptake, membrane stability, photosynthesis (through decreased chlorophyll production), ion homeostasis, and lipid peroxidation, which generate reactive oxygen species, ultimately leading to decreased plant biomass and grain yield [14,15,16]. Furthermore, edible crops with elevated concentrations of PTE pose significant risks to consumers and are unsuitable for consumption because of compromised food quality and safety.
Therefore, the mechanism behind the transfer of PTE from the soil to edible plant parts is a critical research area because it is directly related to food safety and security, with potential implications for human health. For instance, rice, which contains both dietary calories and toxic metals [17], is the primary staple food for approximately half of the global human population [18]. Rice-based agroecosystems play a critical role in global food security. The uptake and accumulation of toxic elements in rice grains is directly related to food safety and public health. Flooded paddy soils, in particular, create unique chemical conditions that can increase the mobility and bioavailability of certain PTE, especially As. According to the World Health Organization (WHO), Food and Agricultural Organization (FAO), and European Commission legislation, the maximum allowable limits for total As, Cd, and Pb are 300 µg kg−1, 200–400 µg kg−1, and 100–200 µg kg−1, respectively [19,20,21]. Therefore, understanding the behavior of PTE in rice-growing environments and their uptake pathways and accumulation in rice is essential for developing effective strategies to ensure the safety and sustainability of rice production.
This review focuses on three PTE—As, Cd, and Pb—owing to their high potential transfer from soil to the food chain. These toxic elements exhibit distinct biogeochemical behaviors, complicating the development of remediation strategies that can simultaneously decrease their toxicity and accumulation in agricultural products. Previous studies have introduced a simple and cost-effective method (chemical immobilization technology with soil amendments) for reducing metal bioavailability [4,22,23], with detailed explanations of its mechanisms for the remediation of contaminated arable soils. However, a single technique is often insufficient for simultaneously and effectively decreasing the bioavailability and uptake of As, Cd, and Pb in plants. Therefore, understanding the mechanisms of their uptake and translocation mechanisms in plants is essential for developing effective strategies to decrease their risks in soil–rice systems.
This review summarizes and discusses the geochemical distribution and speciation of As, Cd, and Pb in the soil, their uptake and transportation mechanisms from the soil to plant roots, and their subsequent movement into aerial plant parts. Furthermore, this review provides an in-depth understanding of the dynamics of As, Cd, and Pb in the soil; their controlling factors; their interactions with other metals, cations, and anions; and the mechanisms regulating their flow from soil to plants.
2. Literature Search Strategy
This review is based on a comprehensive search of the published literature using several electronic databases, including PubMed, Scopus, Web of Science, and Google Scholar. The search was conducted using a combination of specific keywords and corresponding terms found in titles and/or abstracts: “arsenic”, “cadmium”, “lead”, “rice”, “paddy soil”, “metal uptake”, “bioavailability”, “speciation”, and “metal transporters”.
Articles were included if they focused on rice systems and examined the behavior, transformation, bioavailability, or uptake of these toxic elements (As, Cd, and Pb) in paddy soils and rice plants. The initial screening process involved removing duplicate records, followed by a review of titles, abstracts, and full texts to exclude studies that were not relevant to the objectives of this review. No formal inclusion or exclusion criteria or systematic data synthesis methods were applied. Instead, the goal was to provide a broad overview and a critical discussion of key findings and emerging trends in the literature.
3. Geochemical Distribution and Speciation of As, Cd, and Pb in Paddy Soils
3.1. As
Arsenic (As) has an atomic weight of 74.9 and a specific gravity of 5.73. This semi-metallic element exists in the +5, +3, 0, and −3 oxidation states, depending on the prevailing physiochemical conditions of the environment. In soils, As can occur in the form of inorganic species, such as arsenate oxyanions [As(V)] and arsenite [As(III)], as well as methylated organic species, such as monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), and trimethylarsinic acid (TMA) [24,25]. In contaminated soils, As exists primarily in two inorganic forms: As(V) and As(III). In addition, trace amounts of methylated organic As species, such as MMA and DMA, are also present in soils, often resulting from extensive pesticide, herbicide, and defoliant applications, as well as the microbial methylation of As [26,27]. Under aerobic conditions, As(V) is the predominant As species [28]. It can undergo hydrolysis and bind strongly to iron (oxy)hydroxides through inner-sphere bidentate and monodentate surface complexes [26]. However, under anaerobic conditions in flooded paddy soils, As(V) is reduced to As(III) through microbial activity. Moreover, As(III) is ten times more toxic and soluble than As(V) [29,30]. High concentrations of As(III) are dissolved in the soil solution under anaerobic conditions, leading to higher As concentrations in rice grains [29,31], as rice is typically cultivated under anaerobic conditions [32]. It was reported that As(V) accounts for approximately 73–96% of the total As content of aerobic soils [33], whereas As(III) accounts for approximately 87–94% of As in flooded soils. Although As(V) is less toxic than As(III), it is thermodynamically more stable under normal conditions and remains a major contaminant, owing to its toxicity and carcinogenicity to humans. The anthropogenic sources that contribute to elevated As concentrations in the soil include atmospheric deposition from metal mining; smelting; fossil fuel combustion; industrial dust and waste accumulation; the irrigation of wastewater and As-contaminated water originating from geogenic sources; and phosphate fertilizer, manure, pesticide, and herbicide application [34,35,36,37].
3.2. Cd
Cadmium (Cd) is a transition metal that has an atomic number of 48, an atomic weight of 112.4 g mol−1, and a density of 8.65 g cm−3. It predominantly exists as divalent Cd2+ ions and is mobilized under oxic and acidic conditions. Cd is more mobile and soluble than other toxic elements in the soil, making it readily available for plant uptake [38]. The behavior of Cd2+ in soil largely depends on the soil pH. At pH levels below 6.5, Cd2+ tends to form complexes with inorganic ligands (e.g., Cl−, SO42− etc.), while at pH levels above 6.5, it binds to iron oxides [39]. Additionally, a significant portion of Cd2+ binds to humic acids. In some soil types, CdCO3 is the dominant species; meanwhile, small quantities of Cd2+ sulfide species may also occur in other soil types. The primary sources of Cd2+ input into agricultural soils include the application of phosphate fertilizers, pesticides, wastewater irrigation, and biosolids, as well as the atmospheric deposition of dust and fumes from industrial activities, incineration, and mining [40,41,42,43,44].
3.3. Pb
Lead (Pb) has an atomic number of 82, an atomic weight of 207.19 g mol−1, and a specific gravity of 11.34 g cm−3. It primarily exists in the +2 oxidation state rather than in the +4 oxidation state. Pb2+ ranks second after As in terms of occurrence, human exposure potential, and toxicity. Inorganic Pb2+ is less mobile and soluble than As and Cd; therefore, rice absorbs minute concentration of Pb2+ [45,46]. Most of the absorbed Pb2+ ions are deposited in rice roots rather than being translocated to the aerial parts of rice. Pb2+ strongly binds to humic matter in organic soils and to iron oxides in mineral-rich soils. In flooded paddy soils, Pb2+ can bind to sulfur to form PbS precipitates; however, under oxidizing conditions, PbS releases Pb2+ ions. Pb2+ is released into agricultural soil ecosystems through various anthropogenic activities, including the use of leaded gasoline, fertilizers, and pesticides; the disposal of municipal solid waste; and the deposition of industrial dust and fumes [47].
4. Factors Influencing As, Cd, and Pb Distribution in Paddy Soils and Their Translocation in Rice
Not all PTE in the soil are available for uptake by rice; their availability depends on the type, concentration, and chemical forms of metals. Additionally, As, Cd, and Pb exhibit dynamic behaviors in paddy soils. Their bioavailability and uptake mechanisms are influenced by various factors, including soil physicochemical properties (e.g., soil pH and redox potential); plant root characteristics; As, Cd, and Pb forms and concentrations in the soil solution; environmental conditions; plant genotype; growth stages; soil amendment applications; and soil microbial dynamics [48,49,50,51]. Such a wide range of factors can significantly influence the bioavailability and uptake of toxic ions by rice, thereby affecting their accumulation in edible plant parts [52].
4.1. Soil pH
Soil pH significantly influences the concentrations of available As, Cd, and Pb in the soil, as well as their translocation to rice roots. As soil pH increases, the adsorption of cationic metals (Cd and Pb) tends to increase due to hydrolysis and the formation of MOH+ (M = Cd2+ or Pb2+), which, in turn, decreases their solubility and bioavailability. At high pH levels, several factors contribute to the decrease in bioavailable Cd and Pb in paddy soils: (1) an increase in negative charges on the soil surface, which enhances the adsorption of cationic metals (Cd and Pb); (2) the production of M(OH)+ precipitates due to hydrolysis; and (3) the formation of stable phases that bind to carbonate-bound, phosphate-bound, and Fe–Mn oxide-bound fractions [53]. It was also reported that increasing soil pH from 5.0 to 6.5 decreased the extractable phase of Cd by approximately 2.6 to 3 times [54]. In contrast, soil acidification increases the solubility of Cd, transforming it from a stable to a more bioavailable form [55]. Furthermore, Pb translocates to shoots when Pb ions cannot precipitate and are retained in plant cell walls due to low pH [56]. Conversely, oxyanionic As has the opposite solubility pattern. For example, the desorption of As increases with increasing soil pH, mainly because of proton consumption, which increases the number of anions on the exchange sites of the soil surface, along with both As(III) and As(V). It was reported that high soil pH (pH 8.5) increases negative surface charges [57], such as hydroxyl ions, thereby facilitating the desorption of As from Fe oxides. This, in turn, increases the mobilization of As at the root surface, leading to greater As accumulation in rice. Consequently, this condition enhances the desorption and mobilization of As at the root surface, ultimately increasing As accumulation in polished rice [58]. Therefore, the contrasting behaviors of oxyanionic As and cationic metals (Cd and Pb) in response to soil pH in paddy soils pose a significant challenge for simultaneously controlling the accumulation of these toxic elements in plants and rice.
4.2. Oxidation–Reduction Reactions (Redox Potential)
Redox potential is also a key driving factor that significantly and directly affects the solubility of As, Cd, and Pb in flooded paddy soils. Flooding and draining cycles during rice growing seasons cause substantial fluctuations in the redox potential, pH, and solubility of these toxic elements. The mobility and toxicity of As are highly dependent on the soil redox environment. The solubility of As increases dramatically as the redox potential decreases. For example, reduced redox potential facilitates the transformation of As(V) into As(III), which is more soluble and bioavailable than As(V) [59], because As(III) has a lower affinity for the soil solid phase. Conversely, As(V) readily adsorbs onto mineral components such as iron (hydr)oxides [60]. During drainage, As mobility decreases as As(V) becomes the predominant species [31].
By contrast, Cd and Pb are redox-insensitive toxic elements that do not change their oxidation states with respect to their solubility. However, the reductive dissolution of Fe and Mn hydr(oxide)s under decreasing redox potential in flooded soils can significantly increase the release and mobility of Cd and Pb through desorption reactions. Additionally, sulfide-rich conditions in reduced paddy soils promote the precipitation of Cd and Pb with sulfide minerals. For instance, the solubility of Cd decreases with lower redox potential due to the formation of CdS [61]. During drainage, increasing redox potential causes the dissolution of CdS [62] and the formation of water-soluble Cd sulfate (CdSO4), which, in turn, increases the solubility of Cd2+ in the soil solution, making it more available for rice roots to adsorb [63].
Sulfate reduction may also decrease the solubility of As, particularly in paddy soils that significantly produce Fe2+ under reducing conditions, which is likely due to the co-precipitation or sorption of As(III) by the newly formed FeS minerals [64]. Flooding conditions can also alter soil pH. Typically, soil pH in flooded paddy soils stabilizes within a neutral range. In flooded acidic soils, pH increases to a neutral level, owing to proton consumption during various reduction reactions. By contrast, alkaline soils tend to exhibit decreased soil pH due to CO2 accumulation during flooding [65]. The solubility of Cd and Pb decreases markedly as the redox potential decreases under flooded conditions because an increase in soil pH enhances the sorption of Cd2+ and Pb2+ ions.
Therefore, the solubility of As increases dramatically as the redox potential decreases, primarily because of the reduction of As(V) to As(III) and the attainment of neutral pH levels. When paddy soil is drained, the opposite processes occur rapidly, leading to increased Cd2+ and Pb2+ solubility and decreased As solubility [66,67,68].
4.3. Fe Plaque Formation and Radial O2 Loss
As rice is a semi-aquatic plant (moist or wet conditions), its roots have extensive longitudinal aerenchyma (a spongy tissue that forms spaces/air channels in plants) [69], which facilitates the transport of O2 from the shoots to the root tips, allowing it to thrive and extend its roots into flooded soils. Oxygen diffusion through the aerenchyma into the surrounding rhizosphere is referred to as radial O2 loss (ROL). ROL oxygenates the rhizosphere and promotes the oxidation of ferrous Fe (Fe2+) to ferric Fe (Fe3+). Consequently, yellow Fe (hydr) oxide precipitates, known as iron plaques, form on the root surface. These plaques primarily comprise amorphous or crystalline Fe (hydr)oxides [70]. As strong sorbents, Fe plaques hinder the uptake of As, Cd, and Pb by rice [70,71,72]. Several studies have reported that Fe plaques decrease metal concentrations in rice seedlings, with a negative correlation observed between As levels in rice grains and amorphous Fe oxide-bound As concentrations in the soil [73,74,75]. The role of Fe plaques in influencing As, Cd, and Pb concentrations varies depending on several factors, including the extent of plaque formation on the root surface, which differs among rice genotypes. In addition, Fe plaque formation is influenced by waterlogging conditions, O2 availability in the soil, the growth stages of rice plants, and the background concentration of Fe in paddy soils [76,77].
4.4. Rice Root Activity
As the first organ exposed to As, Cd, and Pb in the soil, rice roots serve as the first barrier against their toxicity. The root hairs and epidermal cells in the mature zone of the root tip absorb toxic metal ions from the soil. Root activity significantly influences the concentration and speciation of metal ions in the rhizosphere, thereby affecting the ability of roots to take up metal ions. To maintain the charge balance resulting from the absorption of nutrient ions, roots compensate by excreting H+ or OH− ions [78]. For instance, metal ions are exchanged with H+ and OH− ions that are produced during plant respiration and are adsorbed onto the surface of root epidermal cells [79]. Additionally, plant roots produce certain low-molecular-weight compounds and organic (e.g., dissolved organic C) or inorganic ligands (e.g., NO3− and Cl−) that form soluble metal–ligand complexes [80]. Consequently, these complexes enhance the mobility of metal ions and facilitate their entry into the root epidermis as chelates. Other factors, such as root surface area, mycorrhizal relationships in the rhizosphere, transpiration pull, and the extent of the root system, can influence the solubility of toxic metal ions in the soil. Moreover, rice cultivars may affect the solubility of metals in the rhizosphere and their accumulation in grains. Different rice cultivars exhibit varying capacities for As, Cd, and Pb uptake, transport, and accumulation in the reproductive tissues [81].
4.5. Interaction with Cations (Ca2+, Mg2+, K+, Na+, and Mn2+) and Anions (NO3−, SO42−, and PO42−)
The transport pathways of Cd and Pb chemically resemble those of other divalent cations. Consequently, the presence of competing cations, such as Ca2+, Mg2+, or K+, in the soil medium can alter the amount of Cd2+ or Pb2+ concentrations taken up by rice roots because they share the same transporter proteins [82]. Na+ has been reported to enhance Cd uptake by plants [83,84]. Conversely, other metallic elements, such as Ca2+, Mg2+, and K+, inhibit Cd2+ and Pb2+ uptake by blocking their transport into rice roots [85]. Other divalent metal ions, such as Mn2+, Fe2+, Zn2+, and Si, also enter rice root cells through ion channels and carrier proteins and share the same pathways as Cd and Pb. These cations can inhibit Cd and Pb uptake through competitive interactions with these channels [41,86,87,88,89,90]. For instance, the excessive release of Mn owing to the dissolution of Mn oxides inhibits Cd absorption by rice roots. Mn oxides, which have strong oxidation and adsorption capacities, can influence the availability of Cd by interfering with its adsorption onto other metal oxides [41,91].
By contrast, anionic As primarily interacts with elemental S in paddy soils, especially under reducing conditions in which reduced S species bind to arsenite [As(III)]. In flooded soils, sulfate is readily reduced to dissolved sulfide (S2−) by sulfate-reducing bacteria. It was also reported that reduced S2− immobilizes As through the formation of amorphous As–sulfide precipitates, such as AsS and As2S3 [92]. For instance, the high-sulfate treatment of rice plants has been reported to decrease the translocation of As from the roots to the shoots. Additionally, in the presence of Fe, S2− can bind with Fe(II) to form FeS minerals, such as Fe3S4 and FeS2, thereby enhancing the adsorption of As(III) and reducing its mobilization [93]. In paddy soils, N predominantly exists in the form of ammonium (NH4+), with lower concentrations of nitrate (NO3−). The release of O2 from rice roots into the rhizosphere can promote the microbial nitrification of NH4+ near the root surface [94]. NO3− concentrations can enhance the oxidation of As(III) and decrease its mobilization in paddy soils by increasing the abundance and activity of NO3−-dependent oxidizers [80,95]. The microbial oxidation of Fe(II) can occur simultaneously, forming insoluble Fe(III) precipitates that immobilize As(III) through co-precipitation and adsorption [96]. Furthermore, denitrification (NO3− reduction) and Fe(II) oxidation can occur concurrently, facilitated by specific microbes that enhance As(III) adsorption, thereby decreasing As (III) availability under flooded conditions [97,98].
5. As, Cd, and Pb Uptake and Transport Mechanisms in Rice
Understanding the uptake and transport mechanisms of As, Cd, and Pb is essential for producing tolerant rice varieties with low metal accumulation through breeding or genetic engineering. Various transporter proteins are critical in facilitating the transport of free As, Cd, and Pb ions from soil solutions into rice. Transporters and intracellular high-affinity binding sites regulate metal uptake across cell membranes. Several protein families, including heavy metal P-type ATPases (HMA), natural resistance-associated macrophage proteins (NRAMP), cation diffusion facilitators (CDF), Zn–Fe permease proteins (ZIP), and ATP-binding cassettes (ABC), have been implicated in metal ion transport in plants [99]. The transport of As, Cd, and Pb involves five major processes: (i) uptake by the roots; (ii) translocation to the xylem; (iii) transport to the shoot via the xylem; (iv) redistribution through intervascular transfer at the node; and (v) transport to rice grains from leaf blades via the phloem. Additionally, rice plants can absorb metal ions through their aerial parts, such as leaves. These aforementioned processes determine the accumulation of As, Cd, and Pb in rice grains [100].
Rice roots act as the primary entry point for metal ion uptake and serve as the first barrier to the absorption of toxic elements [101,102]. Metal ions adsorbed onto the surface of the root epidermis enter root cells via rhizosphere interactions, root membrane transporters, and ion channels. Once inside the root, metal ions move from the epidermis to the cortex, stele, and xylem for upward transport to the shoots. This movement occurs via two primary pathways: the apoplastic pathway (via extracellular spaces, including the cell wall, interstitial spaces, and conduit cavities) and the symplastic pathway (cell-to-cell movement mediated by polarized influx and efflux transporters) [103,104]. In the apoplastic pathway, Casparian strips act as barriers in the endodermis, limiting metal ion movement to the xylem. Meanwhile, the symplastic pathway is slower and involves intracellular flow through the plasmodesmata. Metal ions cross the endodermis, enter the stele, move into the parenchyma cells through the pericycle sheath, and finally enter the xylem [105]. During this process, a fraction of metal ions is sequestered into root cell vacuoles via specific transporter proteins before entering the xylem. Once in the xylem, metal ions are transported to aboveground plant parts, including leaves, stems, and nodes, driven by transpiration pull and root pressure. The efficiency of metal ion translocation depends on the sequestration capacity of the vacuole and efficiency of xylem loading. High levels of metal ion accumulation in rice grains are primarily associated with phloem transport, as the phloem redistributes metal ions within the aboveground parts of the plant [106,107]. Thus, metal ions absorbed by the roots are first translocated to the aboveground parts through the xylem and are subsequently transported to rice grains via the phloem [108,109]. The journey of As, Cd, and Pb from soil to rice grain is presented in Figure 1.
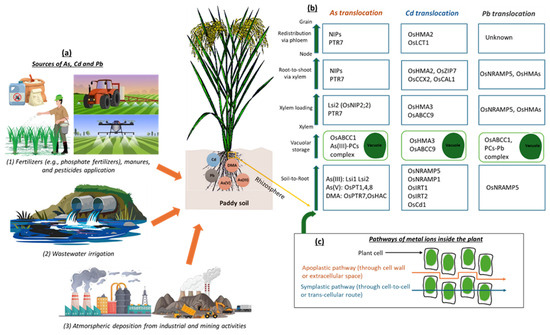
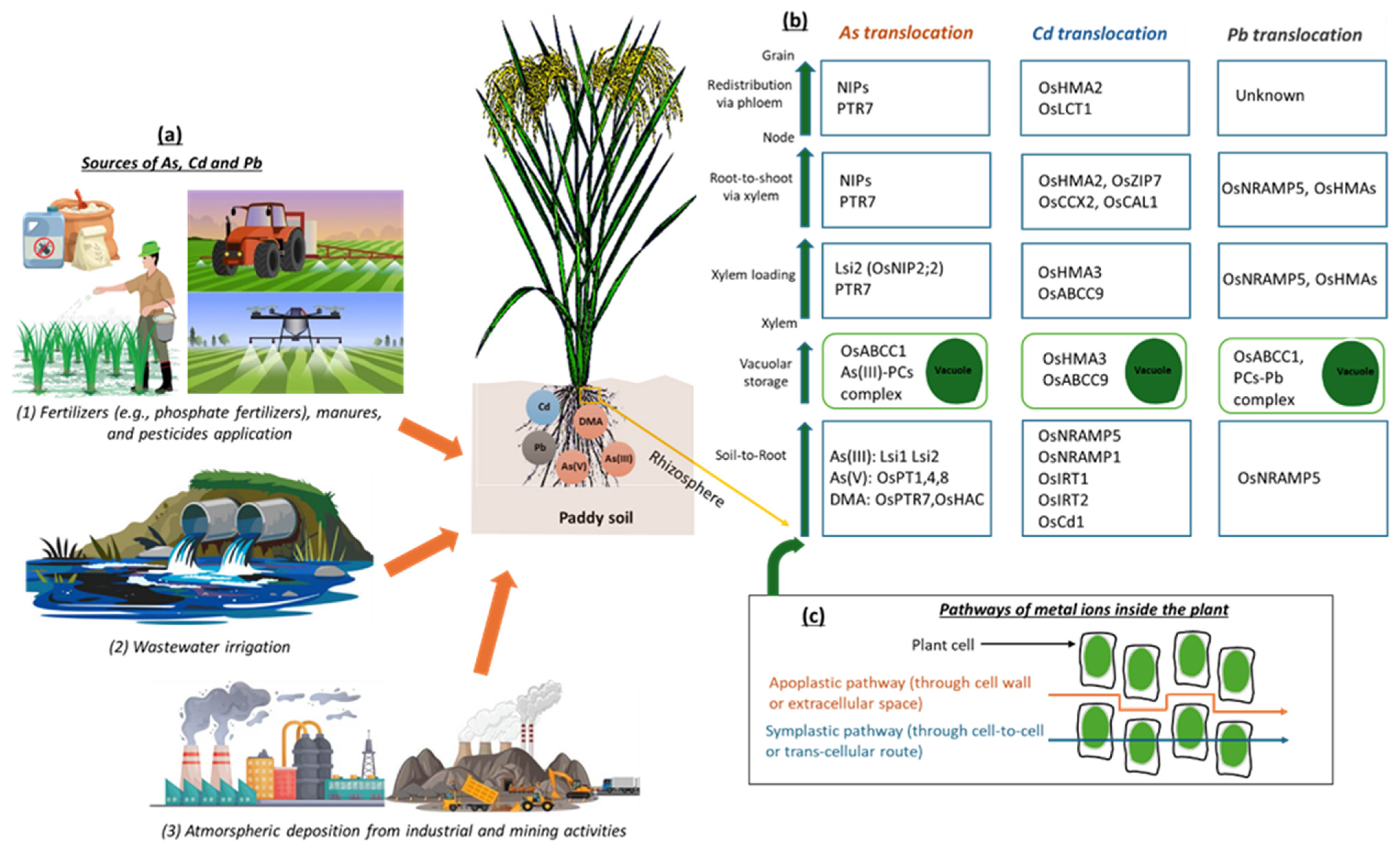
Figure 1.
Summary of the distribution and direction of As, Cd, and Pb transportation in soil–rice systems. (a) Possible anthropogenic sources of As, Cd, and Pb in paddy soil. (b) Summary of the contribution of transporter genes in As, Cd, and Pb translocation from the roots to the aboveground parts of rice and, finally, accumulation in the grain. (c) Pathways of As, Cd, and Pb transportation in rice plant first occurs through root absorption via apoplastic and symplastic routes. Once metal ions enter the root cells, they are sequestered in root vacuoles or transferred to the xylem and then transported upward via xylem loading, transferred from the root to the shoot via the xylem, and redistributed to the grain via the phloem after reaching the node.
5.1. As Transport in Soil–Rice Systems
Under flooded conditions, As(III) is the most dominant As species in the soil, and rice roots rapidly take up As(III), primarily through the influx transporter OsNIP2;1 [50,101,110]. OsNIP2;1, also known as Si influx transporter (Lsi1), primarily functions as a Si transporter and serves as the major pathway for As(III) entry into rice roots. Lsi1 is strongly expressed in rice roots, and mutations in this transporter significantly decrease As(III) influx into rice roots [110]. Lsi1 is localized in the plasma membrane on the distal side of both endodermal and exodermal cells, where Casparian strips occur. Although other NIP family members, such as OsNIP1;1, OsNIP2;2, and OsNIP3;1, are expressed at very low levels, they are unlikely to play a significant role in As(III) influx [50].
After entering the root cells, a portion of As(III) is detoxified via sequestering into vacuoles following complexation with thiol compounds, such as phytochelatins (PCs). This process occurs predominantly in the pericycle and endodermal cells of rice roots [71]. The transport of As(III)–PC complexes into vacuoles for further detoxification is catalyzed by Oryza sativa ABC transporter (OsABCC1) [111], which are crucial in limiting As translocation by sequestering As(III)–PC complexes into vacuoles, wherein As is stored and distributed in plants. As OsABCC1 is expressed in various plant parts, including the roots, leaves, and nodes, removing it will significantly increase As accumulation in rice grains [112].
While Lsi1 is responsible for As(III) uptake into rice roots, the Si efflux transporter (Lsi2) mediates As(III) translocation to the xylem [110,113]. Lsi2 is localized on the plasma membrane of exodermal and endodermal cells in the roots; however, unlike Lsi1, it is situated on the proximal side [114]. The pathway of As from the soil medium to the stele involves uptake through the aquaporin channel Lsi1 and efflux toward the stele mediated by Lsi2 [113,114]. Lsi2 mutation significantly decreases As accumulation in rice shoots by 66–75% [110]. Similarly, knocking out Lsi1 decreases As concentration in the straw, although it does not significantly affect the grains. By contrast, knocking out Lsi2 leads to a significant decrease in As accumulation in both the straw and grains compared to wild-type rice. These findings suggest that Lsi2 is more critical than Lsi1 in regulating As transport and accumulation in rice shoots and grains. However, Lsi2 disruption also impairs Si uptake, which negatively affects rice growth and can decrease grain yield by up to 60% [113].
When paddy soils are flooded, certain concentrations of As(V) are present due to the oxidation of As(III) coupled with the denitrification and release of oxygen by plant roots [99]. In this case, the As(V) species is taken up by rice roots through phosphate transporters, such as OsPT1, OsPT4, and OsTP8. Once inside the root cells, As(V) is rapidly reduced to As(III) via the activity of transporters such as OsHAC1;1, OsHAC1;2, and OsHAC4 [110]. The resulting As(III) is transported to the xylem sap via Lsi2. Consequently, As(III) is the primary form of As in plant cells [115,116]. Notably, the overexpression of OsHAC1;1, OsHAC1;2, and OsHAC4 significantly increases the efflux of As(III) from the root cells, thereby reducing As accumulation in rice.
Aside from inorganic As species, several methylated As species, including methylated As species such as MMA and DMA, are present in the soil. While rice plants lack the As methylation ability, methylated As species in rice are derived from the soil by microbial activity after soil flooding and additions of organic matter [117]. Although rice roots can absorb methylated As species mediated by certain transporters like Lsi1 [118], their uptake by rice plants is slower than that of As(III). However, organic As species are much easier and 10 times more efficiently translocated to the shoots and reproductive parts than inorganic As species via the xylem and phloem [119,120]. Additionally, DMA does not form complexes with PCs or become sequestered in vacuoles. Consequently, DMA remains highly mobile within plants [121]. The putative peptide transporter (OsPTR7) in rice has been identified as being involved in DMA accumulation in rice grains [122].
5.2. Cd Transport in Soil–Rice Systems
Cd is highly mobile in the soil solution, and the root uptake of Cd begins when Cd ions reach the root surface [123]. Rice roots primarily take up Cd ions in the forms of Cd2+ and CdCl−. The uptake of Cd2+ is mainly facilitated by the Mn transporter Oryza sativa NRAMP5 (OsNRAMP5) [79]. The NRAMP family, known as the transporters of divalent transition metals, is present across all living organisms [124]. OsNRAMP5 is predominantly expressed in rice roots and is polarly localized to the plasma membranes on the distal sides of both exodermal and endodermal cells [79]. The expression of OsNRAMP5 promotes Cd2+ uptake, leading to increased Cd accumulation in rice grains [125]. Hence, knocking out OsNRAMP5 significantly decreases Cd2+ uptake by rice roots and decreases Cd accumulation in shoots and grains [126]. Other transporters, such as OsNRAMP1, Oryza sativa iron-regulated transporters (OsIRT1 and OsIRT2), and OsCd1 (a member of a major facilitator family), are also involved in Cd uptake; however, their contributions are minor compared to those of OsNRAMP5 [127,128]. Studies have shown that OsIRT1 and OsIRT2 influence the root absorption of Cd [129], whereas OsCd1 also contributes to the uptake of Cd by rice roots [130].
After being transported into rice roots, a fraction of the Cd is sequestered into root vacuoles via the Oryza sativa heavy metal ATPase family protein (OsHMA3) [131]. Variations in the coding sequence of OsHMA3 across rice cultivars affect Cd sequestration and transportation, resulting in differences in Cd accumulation among cultivars [132]. OsHMA3, which is predominantly expressed in root cells, sequesters Cd in vacuoles and regulates its loading into the xylem [131]. Additionally, the ABC family member OsABCC9 contributes to Cd sequestration in root vacuoles [43,133]. As OsABCC9 is localized in the tonoplast of parenchyma cells, knocking it out results in increased Cd sensitivity in rice plants and elevated Cd levels in the xylem sap and grains [130,134].
Cd translocation from roots to shoots is facilitated by transporters such as OsHMA2 and OsZIP7. OsHMA2, which is localized in the plasma membrane of the root pericycle and parenchyma cells of vascular bundles in the nodes, is essential for delivering Cd from roots to developing tissues [135,136,137]. Hence, knocking out these genes decreases the inward flow of Cd [138]. Additionally, OsHMA2 is localized in the phloem of the vascular bundle and mediates Cd transport through the phloem into growing structures. Knocking out OsHMA2 significantly decreases Cd translocation to rice grains; moreover, it also disrupts Zn transport and affects plant growth [139]. Similarly, OsZIP7 is involved in xylem loading and transporting Cd upward to the phloem [137].
Node-expressed transporters such as OsCCX2 also mediate Cd translocation. OsCCX2, a plasma membrane-localized Ca2+ transporter, regulates Cd transport between roots and stems. Thus, knocking out this gene decreases Cd concentrations in rice grains [140]. Additionally, OsCAL1, a defensin-like protein expressed mainly in the root exodermis and xylem parenchyma cells, chelates Cd for secretion into the extracellular spaces, thereby reducing cytosolic Cd levels and facilitating root-to-shoot transport via xylem vessels [141]. Cd transport into rice grains occurs primarily via phloem transport, which is regulated by low-affinity cation transporters (OsHMA2 and OsLCT1). OsHMA2 is responsible for reloading Cd from parenchymal cells into the phloem of diffuse vascular bundles [125]. OsLCT1, a phloem plasma membrane protein localized in the uppermost node of the vascular bundle, is involved in Cd translocation through the phloem [142,143]. OsLCT1 expression increases significantly during grain maturity, which is a critical period for Cd accumulation in rice grains [142].
5.3. Pb Transport in Soil–Rice Systems
Rice roots serve as the initial point for facilitating the entry of Pb into the plant. Adsorbed Pb passively penetrates the roots and follows the water transport channels [51]. Once Pb is absorbed and passively accumulated in the roots, it utilizes the apoplastic pathway and is subsequently translocated through the vascular flow system. However, the specific transport mechanisms, including the roles of the transporter proteins responsible for stimulating Pb uptake in rice, remain unclear. Some studies have pointed out that plants can take up Pb via Ca2+-permeable channels [144]. Additionally, similar to divalent Cd2+, Pb2+ uptake is linked to the NRAMPs family of transporters in rice [90]. OsNRAMP5 has been identified as a major transporter for Pb2+ uptake, similar to its role in Mn2+ and Cd2+ uptake. Short-term hydroponic studies have shown that knocking out OsNRAMP5 Pb accumulation in rice grains by 50% significantly decreases its accumulation in roots and shoots. Moreover, studies have confirmed that the expression of transporter genes, including OsNRAMP5 and OsHMA6, is induced by Pb exposure in rice [145].
HMA facilitates metal detoxification. For example, OsHMA9 is predominantly expressed in vascular tissues (phloem and xylem) and contributes to Pb uptake and transport within and from these tissues [90]. Additionally, OsHMA2, OsHMA3, and OsHMA4 are implicated in Pb loading. These genes exhibit significantly higher expression in rice roots than in shoots and leaves, which likely explains increased Pb accumulation in the roots and decreased translocation to the shoots [146]. Furthermore, the ABCC1 transporter gene is expressed in rice, particularly in the roots of Pb-stressed plants, where it facilitates PC–Pb complex formation and vacuolar sequestration [146].
As Pb2+ is a divalent metal cation, it may also enter plant cells through channels or transporters associated with other divalent transition metals such as Cd, Co, Fe, Ni, and Zn. However, further studies are required to elucidate the precise mechanisms underlying Pb uptake in rice. Pb2+ is less mobile in the soil–rice system than Cd and As. Most Pb is retained in the root tissues, with only a small portion translocated to the aerial parts through transporters [147]. Several factors limit Pb transport from the roots to the aerial plant parts, including immobilization by negatively charged pectins in the cell wall [48,148,149], the precipitation of insoluble Pb salts in intercellular spaces [150], accumulation in the plasma membrane [151], and sequestration in the vacuoles of rhizodermal and cortical cells.
6. Key Challenges and Future Directions
Several studies have been conducted on the uptake, toxicity, and oxidative stress in rice exposed to high As, Cd, and Pb concentrations. Additionally, other researchers have attempted to develop various mitigation strategies, such as incorporating soil amendments to decrease metal bioavailability and limit plant uptake by modifying the soil environment, releasing essential plant nutrients, and developing rice varieties that can tolerate or avoid the accumulation of HM ions by enhancing or modifying transporter proteins. Numerous studies have demonstrated that agricultural soils are exposed to multiple PTE. Thus, the toxicological consequences of exposure to a mixture of metals differ significantly from those of exposure to individual metals. Therefore, the process and need for specific adaptations that simultaneously minimize metal uptake and translocation to the edible parts of rice are complicated. Information and research on developing new rice varieties that can tolerate multi-metal contamination is still lacking, which is one of the future challenges in mitigation strategies and research.
Metal(loid)s behave differently in the soil, affecting their availability, uptake, and transport to plants. In plants, the absorption of metal ions is facilitated by their respective transporters, and once inside the cells, different organelles utilize distinct transport proteins according to their metal needs and biological functions. Due to the chemical similarities between PTE such as As, Cd, and Pb and essential minerals, several transporters that manage nutrient uptake may also inadvertently allow for heavy metal uptake. Thus, creating or modifying transporter proteins that selectively exclude As, Cd, and Pb without interfering with the uptake of essential minerals is difficult.
Additionally, the bioavailability of As (metalloid), Cd, and Pb (metals) in flooded paddy soils varies widely because of factors such as pH, redox potential, organic matter content, and microbial interactions. A rice variety that is tolerant to metals in one type of soil may not perform well under different soil conditions. Therefore, specific soil and water management practices must be selected under metal-enriched conditions.
Effectively addressing multi-metal contamination in soils and preventing harmful translocation in plants may require a combination of genetic engineering, microbial support, and soil amendment strategies. Balancing HM tolerance with crop productivity and nutritional quality is a long-term goal, particularly under diverse environmental conditions. Developing new rice varieties that can tolerate multi-metal contamination presents significant challenges owing to the complexity of metal uptake and translocation pathways, environmental variability, and the need to maintain crop yield and quality. One major hurdle is designing transporter proteins that can exclude toxic metals, such As, Cd, and Pb, while allowing essential nutrients to enter the plant. Additionally, achieving tissue-specific exclusion, in which metals are sequestered in roots or shoots rather than in grains, requires advanced gene-editing techniques and detailed knowledge of regulatory networks. Multi-metal tolerance also demands balance; tolerance to one metal may increase sensitivity to others or decrease nutrient uptake, making it difficult to engineer effective broad-spectrum resistance. Furthermore, rigorous field trials across diverse environmental conditions are needed to validate laboratory results, whereas statutory approval and public acceptance of genetically modified or gene-edited rice can delay development. These complexities underscore the need for continued research, collaboration, and innovation in breeding and biotechnological approaches to develop safe, high-yield, and resilient rice varieties for growth in contaminated soils.
7. Conclusions
The contamination of paddy soils with As, Cd, and Pb, as well as the uptake and translocation of these PTE to rice, have raised significant environmental and food safety concerns. This review highlights the complex interactions among soil properties, metal speciation, and uptake and translocation mechanisms that govern the mobility and accumulation of As, Cd, and Pb in rice. While considerable progress has been made in understanding the uptake, translocation, and detoxification of these potentially toxic elements (PTE), managing multi-metal contamination remains a significant challenge. Future strategies should integrate soil and water management, the genetic improvement of rice varieties with selective metal exclusion or sequestration traits, and effective regulations to decrease the sources of these toxic elements to the rice ecosystems. Advancements in biotechnology and breeding approaches can offer promising tools for developing resilient and high-yielding rice varieties suited to contaminated environments, but sustained interdisciplinary efforts will be critical to ensure food safety and security under growing soil environmental pressure.
Author Contributions
Conceptualization: C.S.L. and H.-i.J.; methodology: C.S.L. and H.-i.J.; formal analysis: C.S.L., H.-i.J., M.-S.K., E.-J.L. and T.-G.L.; visualization: C.S.L.; writing—original draft: C.S.L.; writing—review and editing: H.-i.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ017441)”, RDA, South Korea.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources chemistry risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Hussain, B.; Ashraf, M.N.; Rahman, S.U.; Abbas, A.; Li, J.M.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef] [PubMed]
- Lwin, C.S.; Kim, Y.N.; Lee, M.; Jun, H.I.; Kim, K.R. In situ immobilization of potentially toxic elements in arable soil by adding soil amendments and the best ways to maximize their use efficiency. J. Soil. Sci. Plant Nutr. 2024, 24, 115–134. [Google Scholar] [CrossRef]
- Arao, T.; Ishikawa, S.; Murakami, M.; Abe, K.; Maejima, Y.; Makino, T. Heavy metal contamination of agricultural soil and countermeasures in Japan. Paddy Water Environ. 2010, 8, 247–257. [Google Scholar] [CrossRef]
- Hasnine, M.T.; Huda, M.E.; Khatun, R.; Saadat, A.H.M.; Ahasan, M.; Akter, S.; Uddin, M.F.; Monika, A.N.; Rahman, M.A.; Ohiduzzaman, M. Heavy metal contamination in agricultural soil at DEPZA, Bangladesh. Environ. Ecol. Res. 2017, 5, 510–516. [Google Scholar] [CrossRef]
- Wang, H.; Chen, P.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2020, 271, 129458. [Google Scholar] [CrossRef]
- Hunter, P. A toxic brew we cannot live without. Micronutrients give insights into the interplay between geochemistry and evolutionary biology. EMBO Rep. 2008, 9, 15–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koyama, H.; Kamogashira, T.; Yamasoba, T. Heavy Metal Exposure: Molecular Pathways, Clinical Implications, and Protective Strategies. Antioxidants 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, I.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Banik, C.; He, Z. The impact of heavy metal contamination on soil health. In Managing Soil Health for Sustainable Agriculture; Burleigh Dodds Science Publishing Ltd.: Cambridge, UK, 2018; Volume 2, pp. 63–95. [Google Scholar] [CrossRef]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, Z.; Gill, R.A. Lead toxicity in cereals: Mechanistic insight into toxicity, mode of action, and management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef] [PubMed]
- Alsherif, E.A.; Al-Shaikh, T.M.; Almaghrabi, O.; AbdElgawad, H. High Redox Status as the Basis for Heavy Metal Tolerance of Sesuvium portulacastrum L. Inhabiting Contaminated Soil in Jeddah, Saudi Arabia. Antioxidants 2022, 11, 19. [Google Scholar] [CrossRef]
- Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Exogenously-Sourced Salicylic Acid Imparts Resilience towards Arsenic Stress by Modulating Photosynthesis, Antioxidant Potential and Arsenic Sequestration in Brassica napus Plants. Antioxidants 2022, 11, 2010. [Google Scholar] [CrossRef]
- Sohn, E. Contamination: The toxic side of rice. Nature 2014, 514, S62–S63. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, e0177978. [Google Scholar] [CrossRef]
- Sombatjinda, P.; Sunphorka, S. Hongsibsong, Heavy metals contamination in paddy fields and rice crops in the vicinity of an industrial estate. Environ. Monit. Assess. 2021, 193, 1–11. [Google Scholar]
- FAO/WHO Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. Fifth Session. In Proceedings of the Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF (Prepared by Japan and the Netherlands) CF/5 INF/1, The Hague, The Netherlands, 21–25 March 2011; 89p. [Google Scholar]
- FAO/WHO Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme CODEX Committee on Contaminants in Foods. Eighth Session. In Proceedings of the Editorial Amendments to the General Standard for Contaminants and Toxins in Food and Feed. CX/CF 14/8/12, The Hague, The Netherlands, 31 March–4 April 2014. [Google Scholar]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil. Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Almaroai, Y.A.; Rajapaksha, A.U.; Huang, L.; Sung, J.K. Effects of carbon nanotube and biochar on bioavailability of Pb, Cu and Sb in multi-metal contaminated soil. Environ. Geochem. Health 2017, 39, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.S.; Sidhu, G.P.S. Arsenic acquisition, toxicity and tolerance in plants-From physiology to remediation: A review. Chemosphere 2021, 283, 131050. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, D.; Villada, A.; Cambell, R.C.J.; Sun, G.; Zhu, Y.G.; Feldmann, J.; et al. Geographical variation in total and inorganic As content of polished (white) rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rosen, B.P. The arsenic methylation cycle: How microbial communities adapted methylarsenicals for use as weapons in the continuing war for dominance. Front. Environ. Sci. 2020, 8, 43. [Google Scholar] [CrossRef]
- Takahashi, Y.; Minamikawa, R.; Hattori, K.H.; Kurishima, K.; Kihou, N.; Yuita, K. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol. 2004, 38, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; McGrath, S.P.; Meharg, A.A.; Zhao, F.J. Growing rice aerobically markedly decreases arsenic accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef]
- Amin, M.N.; Kaneco, S.; Kitagawa, T.; Begum, A.; Katsumata, H.; Suzuki, T.; Ohta, K. Removal of arsenic in aqueous solutions by adsorption onto waste rice husk. Ind. Eng. Chem. Res. 2006, 45, 8105–8110. [Google Scholar] [CrossRef]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced As shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef]
- Das, S.; Chou, L.M.; Jean, J.S.; Liu, C.C.; Yang, H.J. Water management impact on arsenic behaviour and rhizosphere bacterial communities and activities in a rice agro-ecosystem. Sci. Total Environ. 2016, 542, 442–452. [Google Scholar] [CrossRef]
- Basu, B.; Kundu, M.; Hedyatullah, M.; Kundu, C.K.; Bandyopadhyay, P.; Bhattacharya, K.; Sarkar, S. Mitigation of arsenic in rice through deficit irrigation in field and use of filtered water in kitchen. Int. J. Environ. Sci. Technol. 2015, 12, 2065–2070. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Soni, R.; Gupta, S.K.; Shukla, D.P. Mercury, arsenic, lead and cadmium in waters of the Singrauli coal mining and power plants industrial zone, Central East India. Environ. Monit. Assess. 2020, 192, 251. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.F. Arsenic contamination in Bangladesh—An overview. Agric. Ecosyst. Environ. 2006, 113, 1–16. [Google Scholar] [CrossRef]
- Brammer, H.; Ravenscroft, P. Arsenic in groundwater: A threat to sustainable agriculture in South and South-east Asia. Environ. Int. 2009, 35, 647–654. [Google Scholar] [CrossRef]
- Zou, M.; Zhou, S.; Zhou, Y.; Jia, Z.; Guo, T.; Wang, J. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, X.; Yu, X.; Zhang, H.; Li, Z.; Jin, D. Adsorption behaviors of Cd2+ on Fe2O3/MnO2 and the effects of coexisting ions under alkaline conditions. Chin. J. Geochem. 2010, 29, 197–203. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, M.; Wang, L.; Zhao, S.; Li, S.; Lei, X.; Qin, L.; Sun, X.; Chen, S. Manganese facilitates cadmium stabilization through physicochemical dynamics and amino acid accumulation in rice rhizosphere under flood-associated low pe+pH. J. Hazard. Mater. 2021, 416, 126079. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, Z.T.; Xu, Z.R.; Liu, R.X. Interactive effects of lanthanum and calcium on cadmium accumulation in wheat with special reference to TaNramp5 expression regulated by calmodulin. J. Agric. Food Chem. 2021, 69, 6870–6878. [Google Scholar] [CrossRef]
- Liu, H.B.; Qu, M.K.; Chen, J.; Guang, X.; Zhang, J.L.; Liu, M.S.; Kang, J.F.; Zhao, Y.; Huang, B. Heavy metal accumulation in the surrounding areas affected by mining in China: Spatial distribution patterns, risk assessment, and influencing factors. Sci. Total Environ. 2022, 825, 154004. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 26, 120. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.A.; Peralta-Videa, J.R.; Ellzey, J.T.; Ren, M.H.; Viveros, M.N.; Gardea-Torresdey, J.L. Effects of Glomus deserticola inoculation on prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ. Exp. Bot. 2010, 68, 139–148. [Google Scholar] [CrossRef]
- Ashraf, U.; Kanu, A.S.; Mo, Z.; Hussain, S.; Anjum, S.A.; Khan, I.; Abbas, R.N.; Tang, X. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies–A mini review. Environ. Sci. Pollut. Control Ser. 2015, 22, 18318–18332. [Google Scholar] [CrossRef]
- Zhao, F.J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil. 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Ashraf, U.; Mahmood, M.H.R.; Hussain, S.; Abbas, F.; Anjum, S.A.; Tang, X. Lead (Pb) distribution and accumulation in different plant parts and its associations with grain Pb contents in fragrant rice. Chemosphere 2020, 248, 126003. [Google Scholar] [CrossRef]
- Vega, F.; Andrade, M.; Covelo, E. Influence of soil properties on the sorption and retention of cadmium, copper and lead, separately and together, by 20 soil horizons: Comparison of linear regression and tree regression analyses. J. Hazard. Mater. 2010, 174, 522–533. [Google Scholar] [CrossRef]
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. CATENA 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Yang, X.; Wang, P.; McGrath, S.P.; Zhao, F.J. Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 2018, 207, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, B.; Song, Q.; Liu, X.; Xu, J.; Brookes, P.C. The identification of ‘hotspots’ of heavy metal pollution in soil-rice systems at a regional scale in eastern China. Sci. Total Environ. 2014, 472, 407–420. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Chatterjee, S.; Moogouei, R.; Gupta, D.K. Arsenic accumulation in rice and probable mitigation approaches: A review. Agronomy 2017, 7, 67. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Nakamura, T.; Dong, D.; Takahashi, Y.; Amachi, S.; Makino, T. Arsenic release from flooded paddy soils is influenced by speciation, Eh, pH, and iron dissolution. Chemosphere 2011, 83, 925–932. [Google Scholar] [CrossRef]
- Li, R.Y.; Stroud, J.L.; Ma, J.F.; McGrath, S.P.; Zhao, F.J. Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ. Sci. Technol. 2009, 43, 3778–3783. [Google Scholar] [CrossRef]
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Pandey, V.; Trivedi, P.K.; Chakrabarty, D.; Tripathi, R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 340. [Google Scholar] [CrossRef]
- Furuya, M.; Hashimoto, Y.; Yamaguchi, N. Time-course changes in speciation and solubility of cadmium in reduced and oxidized paddy soils. Soil. Sci. Soc. Am. J. 2016, 80, 870. [Google Scholar] [CrossRef]
- El-Naggar, A.; Shaheen, S.M.; Ok, Y.S.; Rinklebe, J. Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci. Total Environ. 2018, 624, 1059–1071. [Google Scholar] [CrossRef]
- Han, H.; Wang, Q.; He, L.; Sheng, X. Increased biomass and reduced rapeseed Cd accumulation of oilseed rape in the presence of Cd-immobilizing and polyamine-producing bacteria. J. Hazard. Mater. 2018, 353, 280–289. [Google Scholar] [CrossRef]
- Xu, X.; Wang, P.; Zhang, J.; Chen, C.; Wang, Z.M.; Kopittke, P.; Kretzschmar, R.; Zhao, F.J. Microbial sulfate reduction decreases arsenic mobilization in flooded paddy soils with high potential for microbial Fe reduction. Environ. Pollut. 2019, 251, 952–960. [Google Scholar] [CrossRef]
- Kirk, G.J.D. The Biogeochemistry of Submerged Soils; John Wiley and Sons: Chichester, UK, 2004. [Google Scholar]
- de Livera, J.; McLaughlin, M.J.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G. Cadmium solubility in paddy soils: Effects of soil oxidation, metal sulfides and competitive ions. Sci. Total Environ. 2011, 409, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Khaokaew, S.; Chaney, R.L.; Landrot, G.; Ginder-Vogel, M.; Sparks, D.L. Speciation and release kinetics of cadmium in an alkaline paddy soil under various flooding periods and draining conditions. Environ. Sci. Technol. 2011, 45, 4249–4255. [Google Scholar] [CrossRef]
- Fulda, B.; Voegelin, A.; Kretzschmar, R. Redox-controlled changes in cadmium solubility and solid-phase speciation in a paddy soil as affected by reducible sulfate and copper. Environ. Sci. Technol. 2013, 47, 12775–12783. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Geske, T.; Sauter, M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011, 190, 369–378. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhu, Y.G.; Hu, Y.; Williams, P.N.; Gault, A.G.; Meharg, A.A.; Charnock, J.M.; Smith, F.A. Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ. Sci. Technol. 2006, 40, 5730–5736. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Schroder, M.; Wu, Z.; Martin, B.G.H.; Hawes, C.R.; McGrath, S.P.; Hawkesford, M.J.; Ma, J.F.; Zhao, F.J.; Grovenor, C.R.M. High-resolution secondary ion mass spectrometry reveals the contrasting subcellular distribution of arsenic and silicon in rice roots. Plant Physiol. 2011, 156, 913–924. [Google Scholar] [CrossRef]
- Liu, J.; Leng, X.; Wang, M.; Zhu, Z.; Dai, Q. Iron plaque formation on roots of different rice cultivars and the relation with lead uptake. Ecotoxicol. Environ. Saf. 2011, 74, 1304–1309. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhu, Y.G.; Smith, F.A.; Smith, S.E. Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? J. Exp. Bot. 2004, 55, 1707–1713. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhu, Y.G.; Smith, F.A. Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil. 2005, 277, 127–138. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, F.B.; Liu, C.S.; Huang, W.; Liu, T.-X.; Yu, W.-M. Iron redox cycling coupled to transformation and immobilization of heavy metals: Implications for paddy rice safety in the red soil of South China. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 279–317. [Google Scholar]
- Colmer, T.D.; Cox, M.C.H.; Voesenek, L.A.C.J. Root aeration in rice (Oryza sativa), evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatization. New Phytol. 2006, 170, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, Z.; Zhang, Y.; Xie, X.; Li, Y.; Shen, X. Uptake and accumulation characteristics of arsenic and iron plaque in rice at different growth stages. Commun. Soil. Sci. Plant Anal. 2015, 46, 2509–2522. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil. 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, J.; Pan, Y.; Hu, Y.; Zhang, J.; Shakouri, M.; Chen, N.; Chernikov, R.; Wiens, E.; Jia, Y. Effects of nitrate concentrations on As(III) immobilization via new ferric arsenite hydroxynitrate precipitates. Geoderma 2023, 432, 116423. [Google Scholar] [CrossRef]
- He, Y.B.; Huang, D.Y.; Zhu, Q.H.; Wang, S.; Liu, S.L.; He, H.B.; Zhu, H.H.; Xu, C. A three-season field study on the in situ remediation of Cd-contaminated paddy soil using lime, two industrial by-products, and a low-Cd-accumulation rice cultivar. Ecotoxicol. Environ. Saf. 2017, 136, 135–141. [Google Scholar]
- Zhang, Y.C.; Fan, J.J.; Fu, M.L.; Ok, Y.S.; Hou, Y.; Cai, C. Adsorption antagonism and synergy of arsenate [As(V)] and cadmium(II) onto Fe-modified rice straw biochars. Environ. Geochem. Health 2019, 41, 1755–1766. [Google Scholar] [CrossRef]
- Manousaki, E.; Kadukova, J.; Papadantonakis, N.; Kalogerakis, N. Phytoextraction and phytoexcretion of Cd by the leaves of Tamarix smyrnensis growing on contaminated non-saline and saline soils. Environ. Res. 2008, 106, 326–332. [Google Scholar] [CrossRef]
- Chai, M.W.; Shi, F.C.; Li, R.L.; Liu, F.C.; Qiu, G.Y.; Liu, L.M. Effect of NaCl on growth and Cd accumulation of halophyte Spartina alterniflora under CdCl2 stress. S. Afr. J. Bot. 2013, 85, 63–69. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Yang, Y.Y.; Lee, Y. Pb and Cd uptake in rice roots. Physiol. Plant 2002, 116, 368–372. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. Role of the iron transporter osnramp1 in cadmium uptake and accumulation in rice. Plant Signal. Behav. 2011, 6, 1813–1816. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Xia, J.X.; Ma, J.F. OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol. 2011, 157, 1832–1840. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Zou, J.; Zhou, H.; Zeng, Q.; Liao, B. Effects of calcium magnesium phosphate fertilizer on Cd bioavailability in soil and Cd contents in rice. Acta Sci. Circumst. 2017, 37, 2322–2330. [Google Scholar] [CrossRef]
- Shao, J.F.; Che, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. Silicon reduces cadmium accumulation by suppressing expression of transporter genes involved in cadmium uptake and translocation in rice. J. Exp. Bot. 2017, 68, 5641–5651. [Google Scholar] [CrossRef]
- Chang, J.D.; Gao, W.; Wang, P.; Zhao, F. J OsNRAMP5 is a major transporter for lead uptake in rice. Environ. Sci. Technol. 2022, 56, 17481–17490. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.-M.; Gu, Y.; Kopittke, P.M.; Zhao, F.J.; Wang, P. Iron–manganese oxy(hydr)oxides, rather than oxidation of sulfides, determine mobilization of Cd during soil drainage in paddy soil systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.D.; Johnston, S.G.; Planer-Friedrich, B. Coupling of arsenic mobility to sulfur transformations during microbial sulfate reduction in the presence and absence of humic acid. Chem. Geol. 2013, 343, 12–24. [Google Scholar] [CrossRef]
- Wang, H.Y.; Göttlicher, J.; Byrne, J.M.; Guo, H.M.; Benning, L.G.; Norra, S. Vertical redox zones of Fe–S–As coupled mineralogy in the sediments of Hetao Basin–constraints for groundwater As contamination. J. Hazard. Mater. 2020, 408, 124924. [Google Scholar] [CrossRef]
- Srivastava, S.; Pathare, V.S.; Sounderajan, S.; Suprasanna, P. Nitrogen supply influences arsenic accumulation and stress responses of rice (Oryza sativa L.) seedlings. J. Hazard. Mater. 2019, 367, 599–606. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Xu, W.; Huang, K.; Tang, Z.; Zhao, F.-J. Nitrate stimulates anaerobic microbial arsenite oxidation in paddy soils. Environ. Sci. Technol. 2017, 51, 4377–4386. [Google Scholar] [CrossRef]
- Hohmann, C.; Winkler, E.; Morin, G.; Kappler, A. Anaerobic Fe(II)-oxidizing bacteria show as resistance and immobilize as during Fe(III) mineral precipitation. Environ. Sci. Technol. 2010, 44, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Kent, D.B.; Repert, D.A.; Böhlke, J.K. Anoxic nitrate reduction coupled with iron oxidation and attenuation of dissolved arsenic and phosphate in a sand and gravel aquifer. Geochim. Cosmochim. Acta 2017, 196, 102–120. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physicochemical, and biological factors-a review. Water Res. 2018, 140, 403–414. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Fujiwara, T. Rice breaks ground for cadmium-free cereals. Curr. Opin. Plant Biol. 2013, 16, 328–334. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Yang, H.; Pian, R.; Wang, J.; Wu, A.-M. The Uptake, Transfer, and Detoxification of Cadmium in Plants and Its Exogenous Effects. Cells 2024, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zhu, Y.C.; Yu, L.J.; Yang, M.; Zou, X.; Yin, C.X.; Lin, Y.J. Research advances in cadmium uptake, transport and resistance in rice (Oryza sativa L.). Cells 2022, 11, 569. [Google Scholar] [CrossRef]
- White, P. Ion uptake mechanisms of individual cells and roots: Short-distance transport. In Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 41–43. [Google Scholar]
- Barberon, M.; Geldner, N. Radial transport of nutrients: The plant root as a polarized epithelium. Plant Physiol. 2014, 166, 528–537. [Google Scholar] [CrossRef]
- Cai, H.; Huang, S.; Che, J.; Yamaji, N.; Ma, J.F. The tonoplast-localized transporter oshma3 plays an important role in maintaining Zn homeostasis in rice. J. Exp. Bot. 2019, 70, 2717–2725. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef]
- Kutrowska, A.; Szelag, M. Low-molecular weight organic acids and peptides involved in the long-distance transport of trace metals. Acta Physiol. Plant 2014, 36, 1957–1968. [Google Scholar] [CrossRef]
- Tao, J.; Lu, L. Advances in genes-encoding transporters for cadmium uptake, translocation, and accumulation in plants. Toxics 2022, 10, 411. [Google Scholar] [CrossRef]
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The journey of arsenic from soil to grain in rice. Front. Plant Sci. 2017, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Nati. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef]
- Song, W.Y.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.S.; Ma, J.F. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Kuramata, M.; Abe, T.; Takagi, H.; Ozawa, K.; Ishikawa, S. Phytochelatin synthase OsPCS1 plays a crucial role in reducing arsenic levels in rice grains. Plant J. 2017, 91, 840–848. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, I.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Shi, S.; Wang, T.; Chen, Z.; Tang, Z.; Wu, Z.; Salt, D.E.; Chao, D.Y.; Zhao, F.J. OsHAC1;1 and OsHAC1;2 function as arsenate reductases and regulate arsenic accumulation. Plant Physiol. 2016, 172, 1708–1719. [Google Scholar] [CrossRef]
- Xu, J.; Shi, S.; Wang, L.; Tang, Z.; Lv, T.; Zhu, X.; Ding, X.; Wang, Y.; Zhao, F.J.; Wu, Z. OsHAC4 is critical for arsenate tolerance and regulates arsenic accumulation in rice. New Phytol. 2017, 215, 1090–1101. [Google Scholar] [CrossRef]
- Zhao, F.J.; Zhu, Y.G.; Meharg, A.A. Methylated arsenic species in rice: Geographical variation, origin, and uptake mechanisms. Environ. Sci. Technol. 2013, 47, 3957–3966. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Ago, Y.; Liu, W.J.; Mitani, N.; Feldmann, J.; McGrath, S.P.; Ma, J.F.; Zhao, F.-J. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009, 150, 2071–2080. [Google Scholar] [CrossRef]
- Carey, A.M.; Scheckel, K.G.; Lombi, E.; Newville, M.; Choi, Y.; Norton, G.J.; Charnock, J.M.; Feldmann, J.; Price, A.H.; Meharg, A.A. Grain unloading of arsenic species in rice. Plant Physiol. 2010, 152, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lomax, C.; Liu, W.J.; Wu, L.; Xue, K.; Xiong, J.; Zhou, J.; McGrath, S.P.; Meharg, A.A.; Miller, A.J.; Zhao, F.J. Methylated arsenic species in plants originate from soil microorganisms. New Phytol. 2012, 193, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, Y.; Wang, P.; Zhao, F.J. Phytotoxicity and detoxification mechanism differ among inorganic and methylated arsenic species in Arabidopsis thaliana. Plant Soil. 2016, 401, 243–257. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, Y.; Chen, F.; Ji, Y.; Zhao, F.-J. OsPTR7 (OsNPF8. 1), a putative peptide transporter in rice, is involved in dimethylarsenate accumulation in rice grain. Plant Cell Physiol. 2017, 58, 904–913. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.C.; Tam, N.F.Y.; Ye, Z. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J. Environ. Sci. 2019, 75, 296–306. [Google Scholar] [CrossRef]
- Bozzi, A.T.; Gaudet, R. Molecular mechanism of Nramp-family transition metal transport. J. Mol. Biol. 2021, 433, 166991. [Google Scholar] [CrossRef]
- Ma, J.F.; Shen, R.F.; Shao, J.F. Transport of cadmium from soil to grain in cereal crops: A review. Pedosphere 2021, 31, 3–10. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Zhang, L.; Hu, J.; Zhang, X.; Lu, K.; Dong, H.; Wang, D.; Zhao, F.J.; Huang, C.F.; et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 2014, 65, 4849–4861. [Google Scholar] [CrossRef]
- Lee, S.; An, G. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ. 2009, 32, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.; Song, S.; Shu, Q.; Huang, J. Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N. K Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil. Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Moons, A. Ospdr9, which encodes a PDR-type ABC transporter, is induced by heavy metals, hypoxic stress and redox perturbations in rice roots. FEBS Lett. 2003, 553, 370–376. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sui, F.Q.; Zhao, D.K.; Zhu, H.T.; Gong, Y.; Tang, Z.; Huang, X.Y.; Zhang, G.; Zhao, F.J. Map-based colonising of a new total loss-of-function allele of OsHMA3 causes high cadmium accumulation in rice grain. J. Exp. Bot. 2019, 70, 2857–2871. [Google Scholar] [CrossRef]
- Yang, G.Z.; Fu, S.; Huang, J.J.; Li, L.Y.; Long, Y.; Wei, Q.X.; Wang, Z.G.; Chen, Z.W.; Xia, J. The tonoplast-localized transporter OsABCC9 is involved in cadmium tolerance and accumulation in rice. Plant Sci. 2021, 307, 110894–110902. [Google Scholar] [CrossRef]
- Oda, K.; Otani, M.; Uraguchi, S.; Akihiro, T.; Fujiwara, T. Rice ABCG43 is Cd inducible and confers Cd tolerance on yeast. Biosci. Biotechnol. Biochem. 2011, 75, 1211–1213. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Hu, H.; Shaheen, S.M.; Zhong, H.; Tack, F.M.G.; Wu, M.J.; Li, Y.F.; Gao, Y.; Rinklebe, J.; et al. Speciation, transportation, and pathways of cadmium in soil-rice systems: A review on the environmental implications and remediation approaches for food safety. Environ. Int. 2021, 156, 106749. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Feng Ma, J. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Takemoto, Y.; Miyaji, T.; Mitani-Ueno, N.; Yoshida, K.T.; Ma, J.F. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 2017, 541, 92–95. [Google Scholar] [CrossRef]
- Hao, X.; Zeng, M.; Wang, J.; Zeng, Z.; Dai, J.; Xie, Z.; Yang, Y.; Tian, L.; Chen, L.; Li, D. A node-expressed transporter OsCCX2 is involved in grain cadmium accumulation of rice Front. Plant Sci. 2018, 9, 476. [Google Scholar] [CrossRef]
- Luo, J.S.; Huang, J.; Zeng, D.L.; Peng, J.S.; Zhang, G.B.; Ma, H.L.; Guan, Y.; Yi, H.Y.; Fu, Y.L.; Han, B.; et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Kamiya, T.; Clemens, S.; Fujiwara, T. Characterization of OsLCT1, a cadmium transporter from indica rice (Oryza sativa). Physiol. Plant 2014, 151, 339–347. [Google Scholar] [CrossRef]
- Moon, J.Y.; Belloeil, C.; Ianna, M.L.; Shin, R. Arabidopsis cngc family members contribute to heavy metal ion uptake in plants. Int. J. Mol. Sci. 2019, 20, 413. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, F.; Shahid, M.J.; Zhong, M.; Zia, M.A.; Alomrani, S.O.; Liu, J.; Sun, L.; Ali, S.; Liu, X.; Shahid, M.Q. Alleviated lead toxicity in rice plant by co-augmented action of genome doubling and TiO2 nanoparticles on gene expression, cytological and physiological changes. Sci. Total Environ. 2024, 911, 168709. [Google Scholar] [CrossRef]
- Salavati, J.; Fallah, H.; Niknejad, Y.; Barari Tari, D. Methyl jasmonate ameliorates lead toxicity in Oryza sativa by modulating chlorophyll metabolism, antioxidative capacity and metal translocation. Physiol. Mol. Biol. Plants 2021, 27, 1089–1104. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Flores, L.C. Plant mediated detoxification of mercury and lead. Arab. J. Chem. 2017, 10 (Suppl. 2), S2335–S2342. [Google Scholar] [CrossRef]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Asher, C.J.; Kopittke, R.A.; Menzies, N.W. Toxic effects of Pb2+ on growth of cowpea (Vigna unguiculata). Environ. Pollut. 2007, 150, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.E.R.; Auchterlonie, G.J.; Webb, R.I.; Wood, B. Uptake and localisation of lead in the root system of Brassica juncea. Environ. Pollut. 2008, 153, 323–332. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 2010, 10, 40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).