Oleanolic Acid: A Promising Antioxidant—Sources, Mechanisms of Action, Therapeutic Potential, and Enhancement of Bioactivity
Abstract
1. Introduction
2. Natural Sources of Antioxidant OA
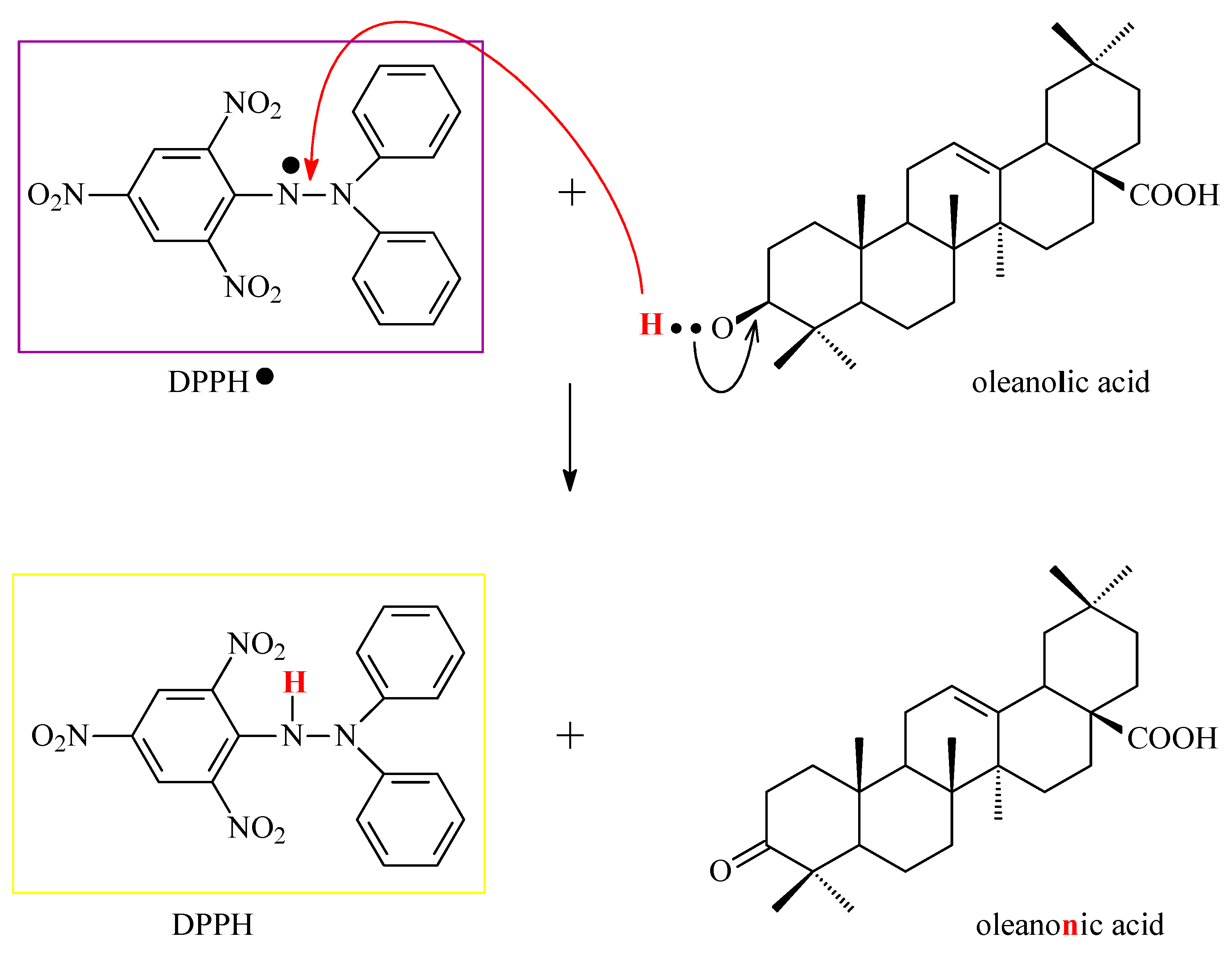
3. Research on the Mechanisms of OA Antioxidant Activity
4. How Is the Antioxidant Activity of OA Expressed: Antioxidant Activity of OA as a Method of Overcoming Other Diseases
4.1. Antihypoglycemic/Antidiabetic and Antioxidant Activity of OA
4.2. Neuroprotective and Antioxidant Activity of OA
4.3. Cardioprotective and Antioxidant Activity of OA
4.4. Hepatoprotective and Antioxidant Activity of OA
4.5. Nephroprotective and Antioxidant Activity of OA
4.6. Antiatherogenic and Antioxidant Activity of OA
4.7. Dermatoprotective and Antioxidant Activity of OA
5. Methods for Improving the Antioxidant Activity of OA
5.1. Microemulsions
5.2. Nanoemulsions
5.3. Nanoparticles
5.3.1. Self-Assembly Nanoparticles
5.3.2. Liposomal Nanoparticles
5.3.3. Nano-OA
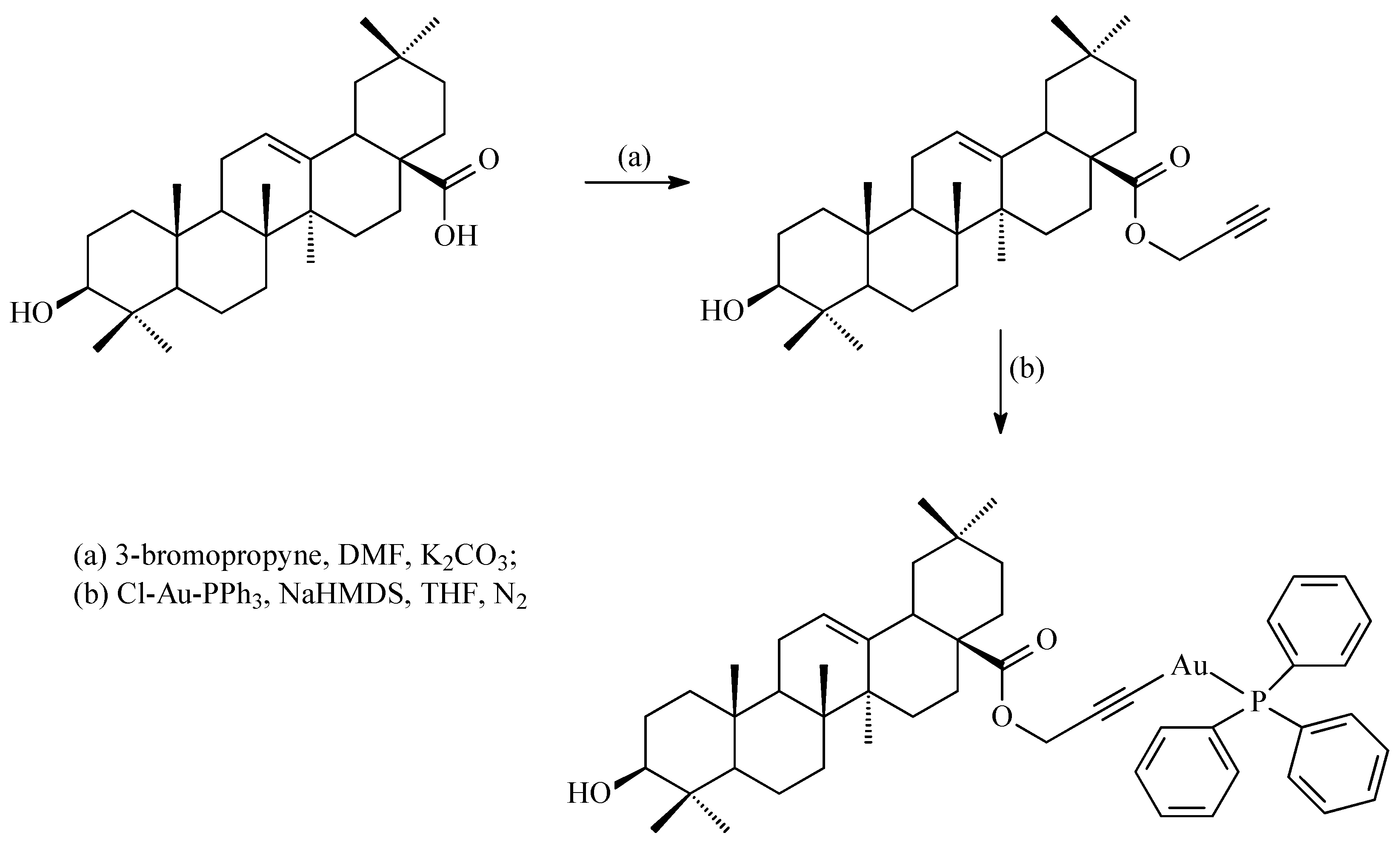
5.4. Gold(I) Complexes
5.5. Nanofibers
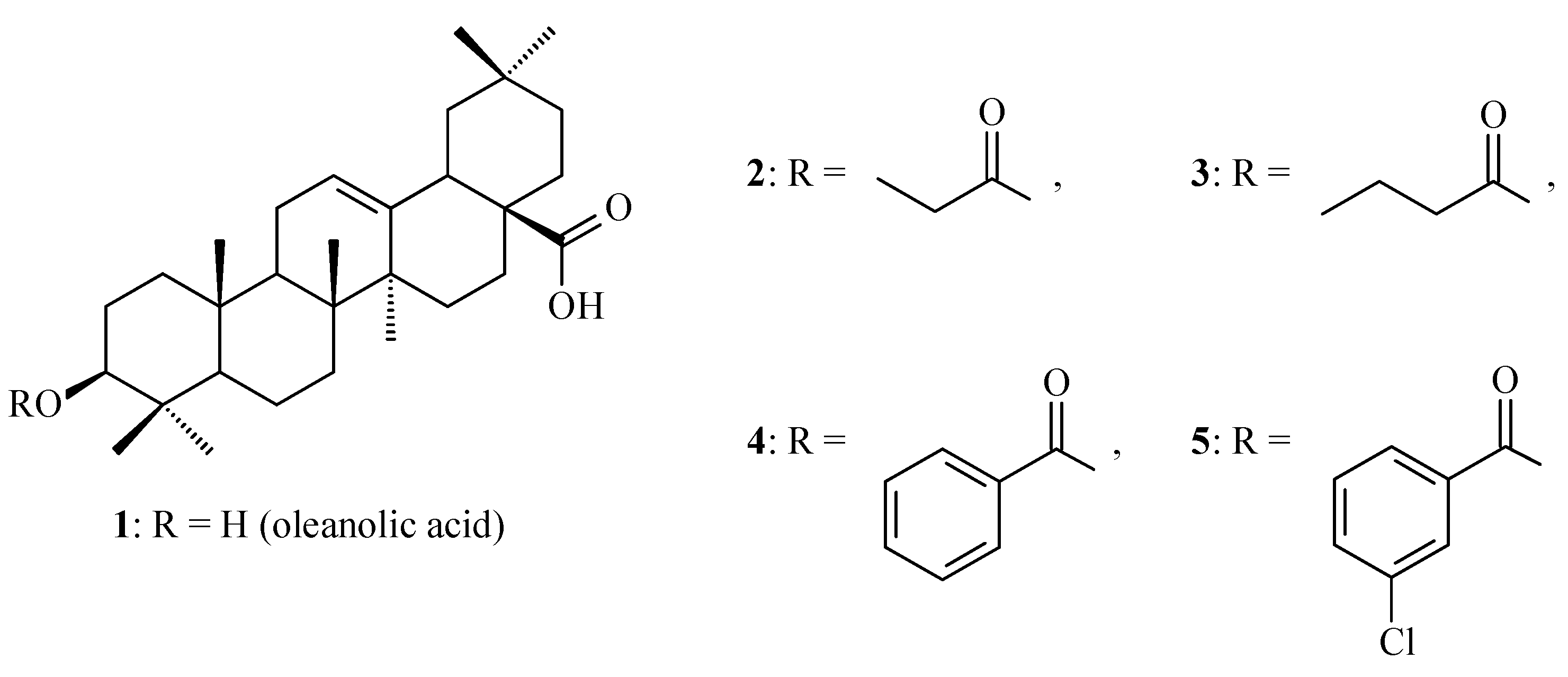
6. OA Derivatives as Antioxidant Agents
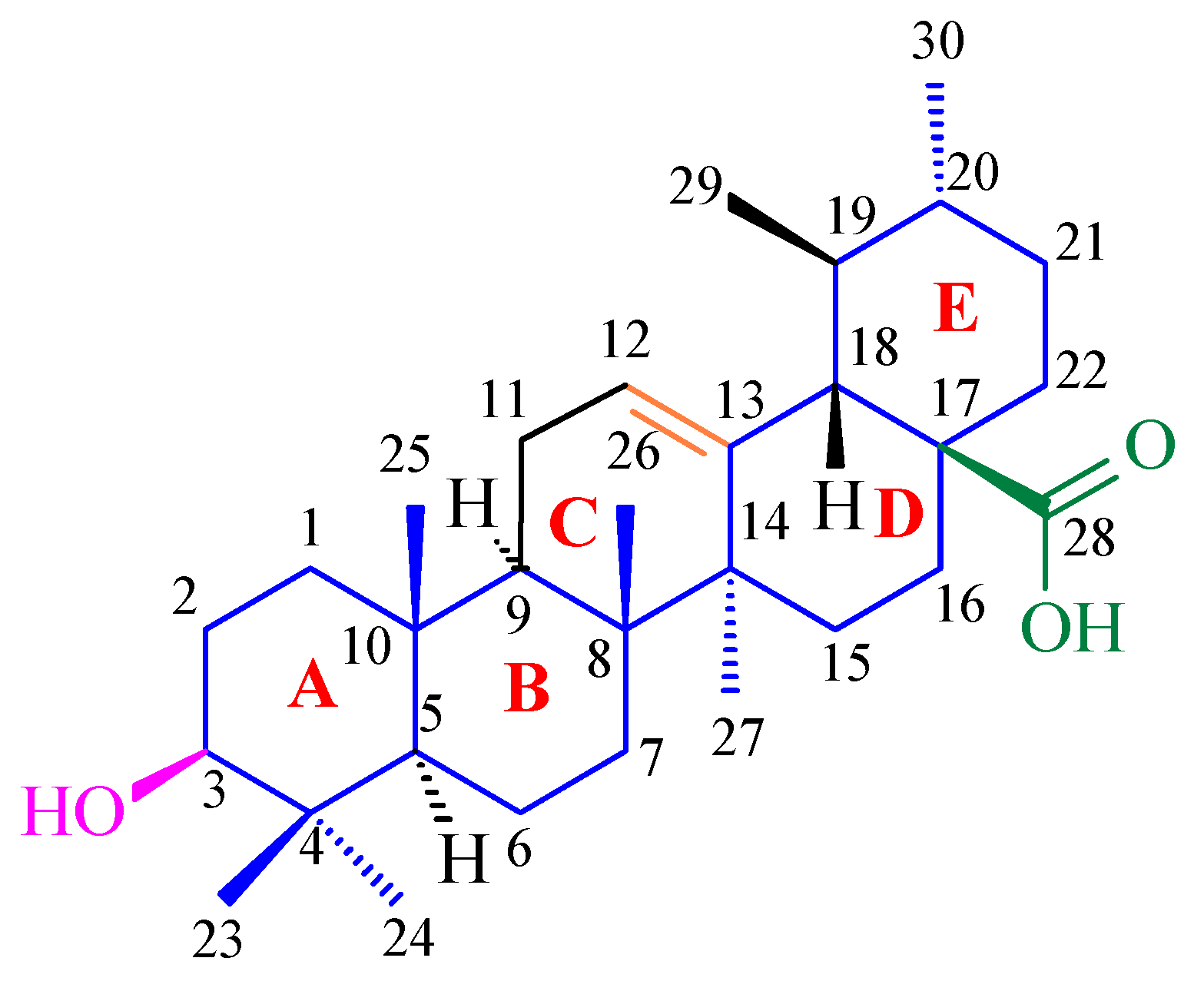
7. Structure–Activity Relationships of Oleanolic Acid and Antioxidant Activity
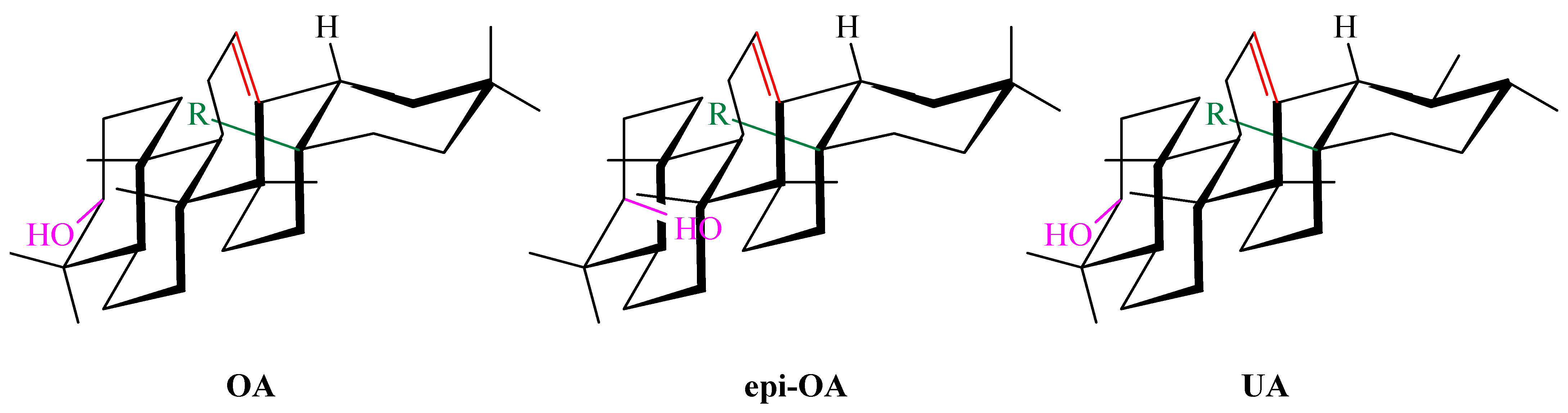
7.1. Stereocenters and Stereochemistry of Oleanolic Acid in Antioxidant Activity
7.2. Influence of Functional Groups on Antioxidant Activity
7.2.1. Influence of the C-3 Hydroxyl Group on Antioxidant Activity
7.2.2. Influence of the C-17 Carboxyl Group on Antioxidant Activity
7.2.3. Influence of the Olefinic Double Bond (C-12=C-13) on Antioxidant Activity
7.2.4. Influence of Additional Hydroxyl Group/Groups on Antioxidant Activity
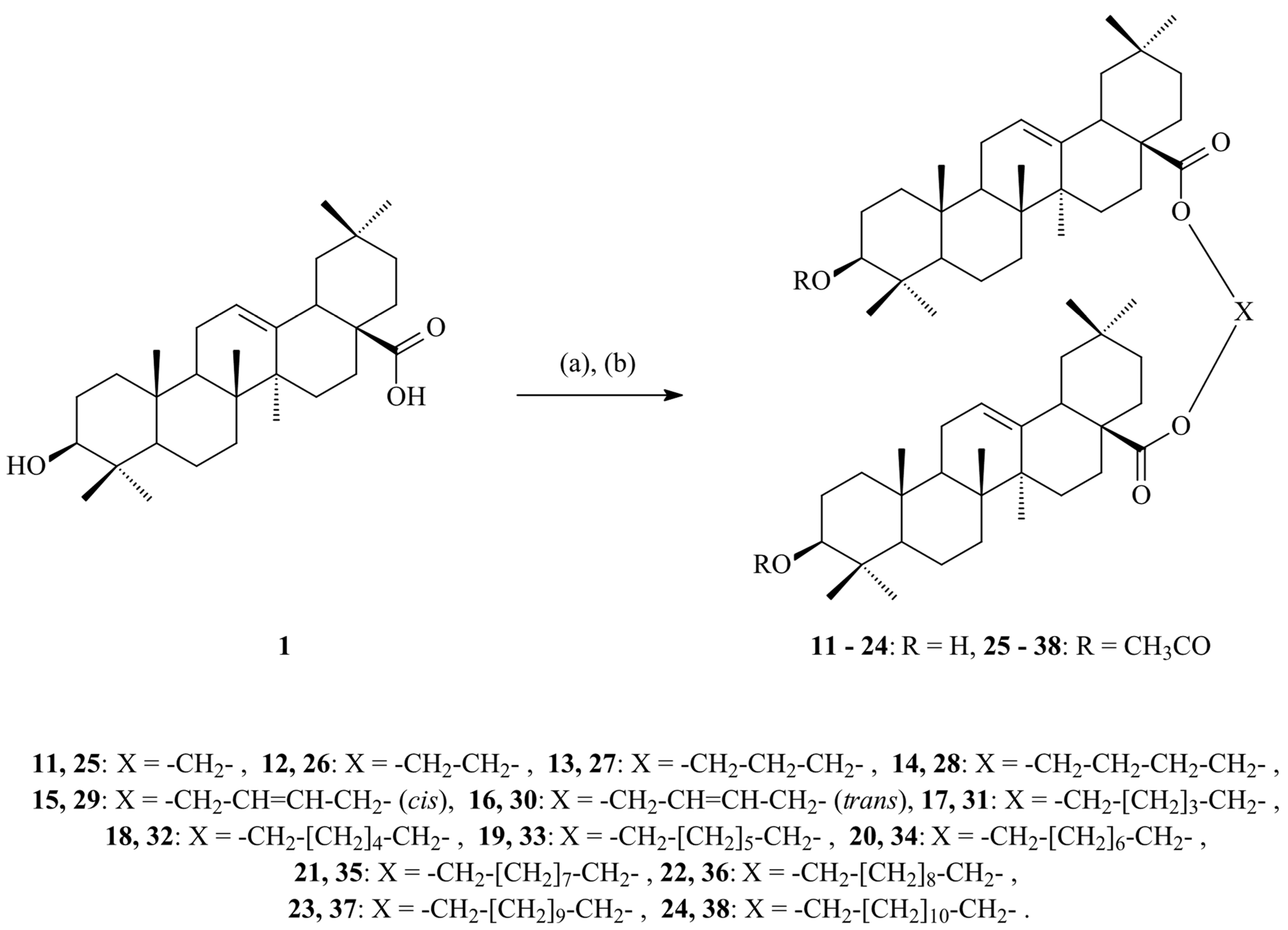
7.2.5. Influence of Dimerization on Antioxidant Activity
- (1)
- Dual radical-binding sites—a dimer essentially doubles the number of functional groups (e.g., two the C-3 hydroxyls) that are capable of quenching radicals or chelating metals;
- (2)
- Increased molecular size—this might localize the dimer in membranes or aqueous environments differently, potentially “shielding” oxidative targets more effectively;
- (3)
- Synergistic stabilization—a radical or electron could delocalize over the two linked OA units in some dimer structures, lowering the overall energy of the radical adduct.
8. Conclusions
- The deactivation of free radicals: OA can directly neutralize ROS and RNS, such as the hydroxyl radical (•OH), the superoxide anion radical (O2−•), and singlet oxygen (1O2 or 1ΔgO2). In addition, it acts as an electron or proton donor, which allows the transformation of free radicals into less reactive forms [5,23,25,29,31,32,37,41,43];
- The induction of antioxidant enzymes: OA triggers the activation of transcription factors, specifically Nrf2, which in turn regulate the expression of antioxidant enzymes, including SOD, CAT, and GSH-Px. Increased activity of these enzymes helps neutralize ROS and prevent oxidative damage [37,48,51,52,54,56,59,63,64,65,79];
- Inhibition of the activity of pro-oxidant enzymes: OA can inhibit the activity of ROS-generating enzymes such as NADPH-oxidase. It also acts as an inhibitor of certain MMPs, which generate reactive compounds during inflammatory processes [43];
- The inhibition of pro-inflammatory pathways: OA inhibits pro-inflammatory pathways such as NF-κB and MAPK, which are associated with the generation of ROS in inflammatory processes [37];
- The chelation of transition metal ions: OA binds metal ions such as iron, which catalyze Fenton reactions, leading to the generation of ROS [40].
9. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| −COO− | carboxylate anion |
| −COOH | carboxyl group |
| −OH | hydroxyl group |
| °C | degrees Celsius |
| µg/mL | microgram per milliliter (10−6 g per 10−3 L) |
| µg/L | microgram per liter (10−6 g per liter) |
| µmol | micromole (10−6 moles) |
| 1Δg O2 | singlet oxygen |
| 1O2 | singlet oxygen |
| 11-oxo-12-ene | a fragment of an oleanane molecule with a ketone group at C-11 and an unsaturated bond starting at C-12 atom |
| 17-COOH | carboxyl group located at the C-17 position of oleanane skeleton |
| 2α-OH | hydroxyl group located at the C-2 position of oleanane skeleton and at the α orientation |
| 3α-OH | hydroxyl group located at the C-3 position of oleanane skeleton and at the α orientation |
| 3β-OH | hydroxyl group located at the C-3 position of oleanane skeleton and at the β orientation |
| 3-Ac-OA | 3-acetyloleanolic acid |
| 3-oxo-OA | oleanonic acid, “3-oxooleanolic” acid (incorrect name) |
| 3-OH | hydroxyl group located at the C-3 position of oleanane skeleton |
| 3-Pht-OA | 3-phthaloyloleanolic acid |
| A2780 | ovarian cancer cells |
| AAE | Ascorbic Acid Equivalent |
| AAPH | (2,2′-azobis(2-amidinopropane) dihydrochloride) |
| ABTS | 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid |
| AcOADs | acetylated oleanolic acid dimers |
| AGEPs | advanced glycation end products |
| A-H | electron donor or proton donor |
| AhR | aryl hydrocarbon receptor |
| AKT/eNOS | protein kinase B/endothelial cell nitric oxide synthase |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AMPK | adenosine monophosphate-activated protein kinase |
| ARE | antioxidant response element |
| B16 | murine melanoma cell line |
| BA | betulinic acid |
| Bax | Bcl-2-associated protein x, B-cell lymphoma 2-associated protein x |
| BHT | butylated hydroxytoluene |
| Br-X-Br | α,ω-dihalogenoalkane or α,ω-dihalogenoalkene |
| BUN | blood urea nitrogen |
| C-12=C-13 | double bond between carbon atoms at the 12 and 13 positions of oleanane skeleton |
| C-2,3 | arrangement of two identical elements at the C-2 and C-3 position of the oleanane molecule |
| C-3 (C-5, etc.) | carbon atom at the position number 3 (number 5, etc.) of oleanane skeleton |
| C57BL/6 | common inbred strain of laboratory mouse |
| CAT | catalase |
| CCl4/NADPH | carbon tetrachloride/nicotinamide adenine dinucleotide phosphate |
| CHP | cumine hydroperoxide (correct name: cumene hydroperoxide) |
| CIRI | cerebral ischemia-reperfusion injury |
| CK-MB | creatine kinase-myocardial band |
| Cl-Au-PPh3 | chloro(triphenylphosphine)gold(I); correct abbreviation: (Ph3P)AuCl or Au(PPh3)Cl) |
| Co(II)/EDTA | cobalt(II) ions and ethylenediaminetetraacetic acid |
| COX-2 | Cyclooxygenase-2; prostaglandin-endoperoxide synthase 2 |
| Cu2+ | element copper in its +2 oxidation state |
| CYO1A1 | cytochrome P450, family 1, subfamily A, polypeptide 1 |
| DMAPP | dimethylallyl pyrophosphate |
| CML | carboxymethyl lysine |
| DMF | N,N-dimethylformamide |
| DN | Diabetic nephropathy |
| DNA | deoxyribonucleic acid |
| Dox | doxorubicin |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DPPH• | 1,1-diphenyl-2-picrylhydrazyl radical |
| DSS | Dahl-salt sensitive (rats) |
| e− | electron, electron donor |
| ECM | extracellular matrix |
| epi-OA | epi-oleanolic acid |
| e.g., | exempli gratia (Lat.), for example (Eng.) |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| ERS | endoplasmic reticulum stress |
| et al. | et alia (Lat.), and others (Eng.) |
| etc. | et cetera (Lat.), and so on (Eng.) |
| Fe2+ | element iron in its +2 oxidation state |
| Fe3+ | element iron in its +3 oxidation state |
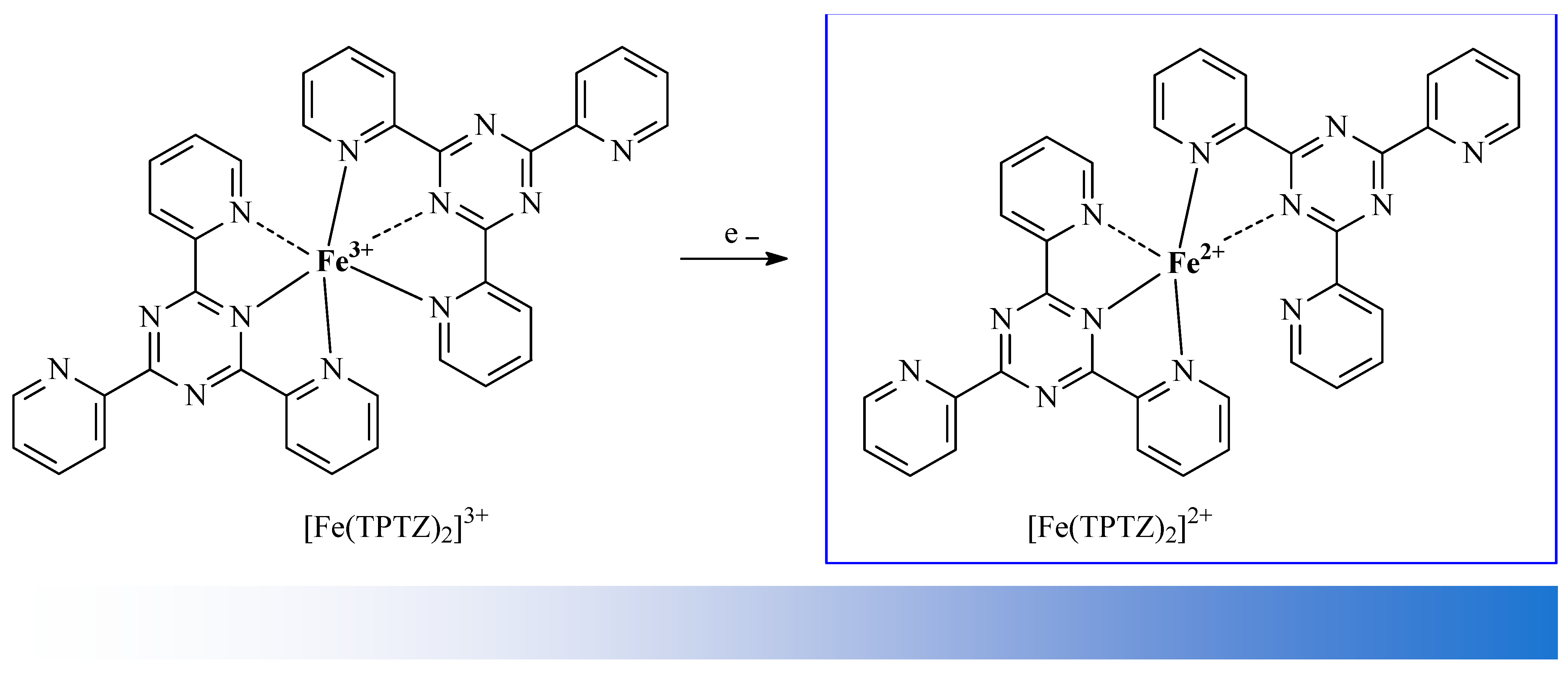
| [Fe(TPTZ)2]3+ | ferric-tripyridyltriazine |
| [Fe(TPTZ)2]3+ | reduced ferric-tripyridyltriazine |
| FRAP | Ferric Reducing Antioxidant Power |
| FTIR | Fourier transform infrared spectroscopy |
| CGLc | glutamate-cysteine ligase catalytic subunit |
| GA | glycyrrhetinic acid |
| GS | Granny Smith (apple variety) |
| GSH | glutathione in its reduced form |
| GSH/GSSG | reduced to oxidized glutathione |
| GSH-Px | glutathione peroxidase |
| H2O2 | hydrogen peroxide |
| HCT-116 | colon cancer cell line |
| HDL | high-density lipoprotein |
| HFF | high fat and fructose |
| HO-1 | heme oxygenase-1 |
| HPLC | High-Performance Liquid Chromatography |
| HUVEC | human umbilical vein endothelial cells |
| IC50 | minimum inhibitory concentration |
| i.e., | id est (Lat.), that is, it means (Eng.) |
| IFN-γ | interferon-γ |
| IL-1β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| IL-10 | interleukin 10 |
| iNOS | inducible nitric oxide synthase |
| IPP | isopentenyl pyrophosphate |
| I/R | ischemia/reperfusion |
| JAK-STAT | Janus kinase/signal transducers and activators of transcription |
| JNK | cellular Jun N-terminal kinase |
| K2CO3 | potassium carbonate |
| K562 | chronic myelogenous leukemia |
| Keap1 | Kelch-like ECH-associated protein 1 |
| KIM-1 | kidney injury molecule 1 |
| L1210 | mouse lymphocytic leukemia cell line |
| LC3-II | lipidated form of microtubule-associated protein 1 light chain |
| LDH | lactate dehydrogenase |
| LDL | low density lipoprotein |
| Lipo-OANPs | oleanolic acid loaded liposomal nanoparticles |
| LPS | lipopolysaccharides |
| LX-2 | human hepatic stellate cell line |
| MAPK | mitogen-activated protein kinase, MAP-kinase |
| MCF-10A | human breast epithelial cell line |
| MDA | malondialdehyde |
| MDA-MB-231 | triple negative human epithelial breast cancer cells |
| MEP | methylerythritol phosphate |
| MEs | microemulsions |
| ME-1, ME-2 | microemulsion no. 1, microemulsion no. 2 |
| mg | milligram (10−3 g) |
| mg/g | milligram per gram (10−3 g per gram) |
| mg/kg | milligram per kilogram (10−3 g per 103 g) |
| MPO | myeloperoxidase |
| miRNAs | key regulators of gene expression, play essential roles in the pathobiology of cancer |
| mmol | millimole (10−3 mole) |
| MMP1 | matrix metalloproteinase 1 |
| MMPs | matrix metalloproteinases |
| MPE | mango peel extract |
| MVA | mevalonic acid |
| MWCNTs/SPE | screen-printed electrode modified with multi-walled carbon nanotubes |
| N2 | nitrogen gas |
| NAC | N-acetylcysteine |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NaHMDS | sodium bis(trimethylsilyl)amide |
| nano-OA | nanoparticles of oleanolic acid |
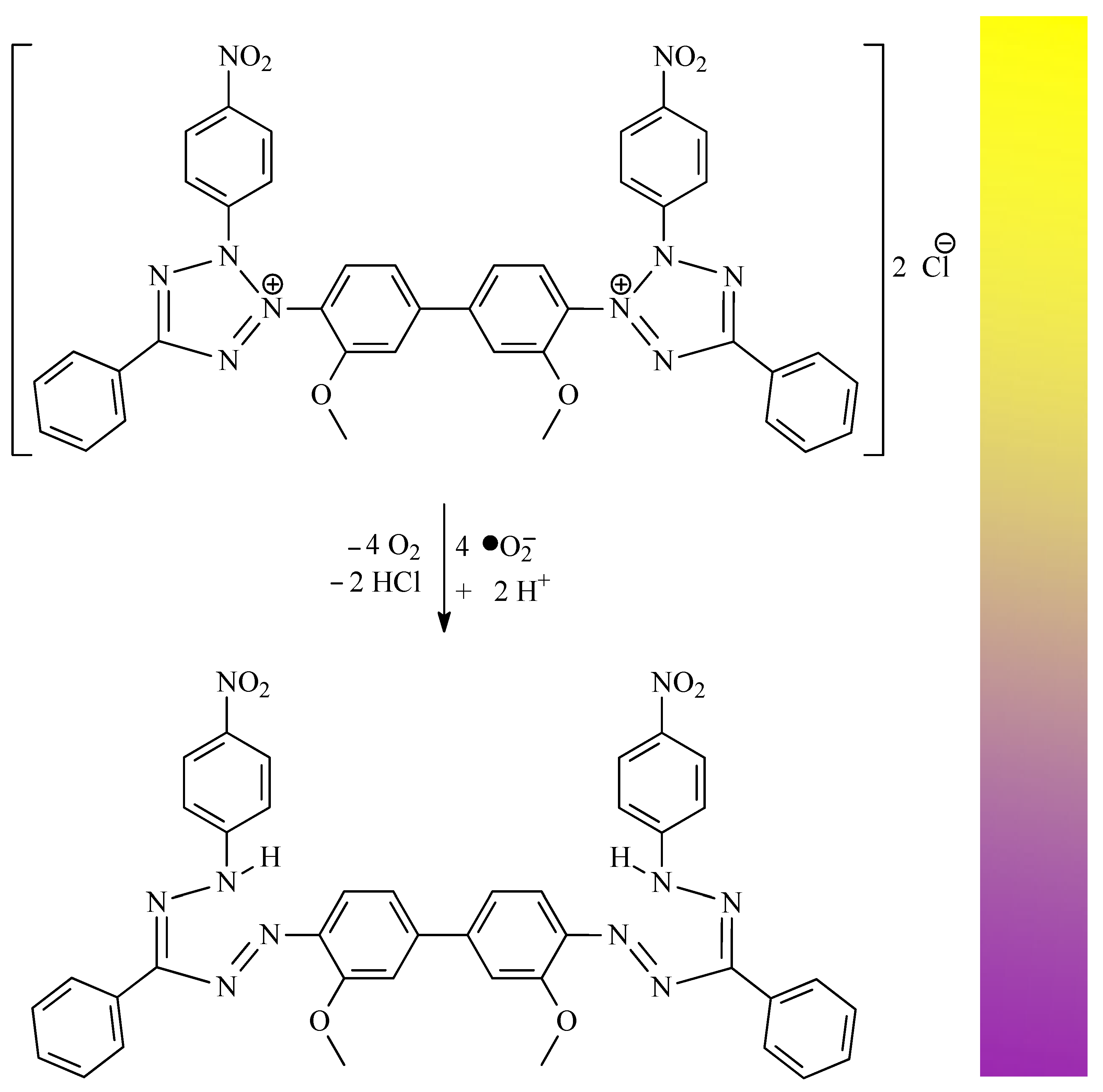
| NBT | nitroblue tetrazolium salt |
| n.d. | no data |
| N-EGR | non-enzymatic glycation reaction |
| NF-κB | nuclear factor kappa B |
| nm | nanometer (10−9 m) |
| nM | nanomole (10−9 moles) |
| NE | nanoemulsion |
| NO | nitric oxide |
| NOX | NADPH-oxidase |
| Nrf2 | nuclear erythroid 2-related transcription factor |
| NQO1 | quinone oxidoreductase |
| •O2− | superoxide radical |
| •OH | hydroxyl radical |
| OA | oleanolic acid |
| OADs | oleanolic acid dimers |
| OANF | oleanolic acid nanofibers |
| OANPs | oleanolic acid nanoparticles |
| ODG/R | oxygen and glucose deprivation and reoxygenation |
| ORAC | Oxygen Radical Absorbance Capacity |
| P12 | pheochromocytoma tumor of the rat adrenal medulla |
| p62 | stress-inducible protein |
| PAMPA | parallel artificial membrane permeability assay |
| pH | the negative decadic logarithm of the proton activity in the solution under scrutiny |
| PI3K/AKT | phosphatidylinositol 3-kinase and activated protein kinase |
| pKa | the negative decadic logarithm of acid dissociation constant |
| PM | particulate matter |
| PM2 | particulate matter with a diameter of 2.5 μm or less |
| PM10 | particulate matter with a diameter of 10 μm or less |
| PRX-1 | peroxidoxin 1 |
| QZG | human fetal liver cell line |
| RAW 264.7 | murine macrophages |
| RD | Red Delicious (apple variety) |
| RG | Royal Gala (apple variety) |
| ROS | reactive oxygen species |
| rpm | revolutions per minute |
| RSA | radical scavenging sctivity |
| SDH | succinate dehydrogenase |
| SGOT | serum glutamic-oxaloacetic transaminase |
| SGP | serum glutamic pyruvic transaminase |
| SHR | spontaneously hypertensive rats |
| SMAD | Suppressor of Mothers Against Decapentaplegic |
| SOD | superoxide dismutase |
| SRB | sulforhodamine-B |
| tBHP | tert-butyl hydroperoxide |
| TAC | total antioxidant capacity |
| TE | trolox equivalent |
| TEM | transmission electron microscopy |
| TGF-β/SMAD2/3 | transforming growth factor-β/SMAD 2 and SMAD 3 |
| TGR5 | Takeda G protein-coupled receptor 5 |
| THF | tetrahydrofurane |
| TNF-α | tumor necrosis factor alpha |
| Trx | thioredoxin |
| TrxR | thioredoxin reductase |
| UA | ursolic acid |
| UUO | unilateral ureteral obstruction |
| XRD | X-ray diffraction |
References
- Withers, S.T.; Keasling, J.D. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biotechnol. 2007, 73, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.F. A review on the presence of oleanolic acid in natural products. Nat. Proda Medica 2009, 2, 77–290. [Google Scholar]
- Cláudio, A.F.M.; Cognigni, A.; De Faria, E.L.; Silvestre, A.J.; Zirbs, R.; Freire, M.G.; Bica, K. Valorization of olive tree leaves: Extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids. Sep. Purif. Technol. 2018, 204, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT-Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Biltekin, S.İ.; Göğüş, F.; Yanık, D.K. Valorization of olive pomace: Extraction of maslinic and oleanolic by using closed vessel microwave extraction system. Waste Biomass Valor. 2022, 13, 1599–1608. [Google Scholar] [CrossRef]
- Alsoufi, A.S.M.; Staśkiewicz, K.; Markowski, M. Alterations in oleanolic acid and sterol content in marigold (Calendula officinalis) hairy root cultures in response to stimulation by selected phytohormones. Acta Physiol. Plant. 2021, 43, 44. [Google Scholar] [CrossRef]
- Soursouri, A.; Hosseini, S.M.; Fattahi, F. Biochemical analysis of European mistletoe (Viscum album L.) foliage and fruit settled on Persian ironwood (Parrotia persica C. A. Mey.) and hornbeam (Carpinus betulus L.). Biocatal. Agric. Biotechnol. 2019, 22, 101360. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Kim, Y.J.; Park, C.; Kim, J.K.; Park, S.U. Integrated analysis of transcriptome and metabolome and evaluation of antioxidant activities in Lavandula pubescens. Antioxidants 2021, 10, 1027. [Google Scholar] [CrossRef]
- da Silva Magedans, Y.V.; Phillips, M.A.; Fett-Neto, A.G. Production of plant bioactive triterpenoid saponins: From metabolites to genes and back. Phytochem. Rev. 2021, 20, 461–482. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, B.; Bhatia, A.; Wani, M.S.; Gupta, R.C. Intra-specific variability in anti-diabetic activity and UPLC quantification of oleanolic acid from two morphotypes and three cytotypes of Achyranthes aspera. J. Biol. Act. Prod. Nat. 2022, 12, 111–124. [Google Scholar] [CrossRef]
- Li, W.; Zeng, H.; Xu, M.; Huang, C.; Tao, L.; Li, J.; Zhang, T.; Chen, H.; Xia, J.; Li, C.; et al. Oleanolic acid improves obesity-related inflammation and insulin resistance by regulating macrophages activation. Front. Pharmacol. 2021, 12, 697483. [Google Scholar] [CrossRef] [PubMed]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and oleanolic acids: Plant metabolites with neuroprotective potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef]
- Cao, S.; Dong, X.L.; Ho, M.X.; Yu, W.X.; Wong, K.C.; Yao, X.S.; Wong, M.S. Oleanolic acid exerts osteoprotective effects and modulates vitamin D metabolism. Nutrients 2018, 10, 247. [Google Scholar] [CrossRef]
- Lee, W.; Yang, E.J.; Ku, S.K.; Song, K.S.; Bae, J.S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, K.; Xia, Y.; Mo, W.; Wang, F.; Dai, W.; Niu, P. The hepatoprotection by oleanolic acid preconditioning: Focusing on PPARα activation. PPAR Res. 2018, 2018, 3180396. [Google Scholar] [CrossRef]
- Carpio-Paucar, G.N.; Palo-Cardenas, A.I.; Rondón-Ortiz, A.N.; Pino-Figueroa, A.; Gonzales-Condori, E.G.; Villanueva-Salas, J.A. Cytotoxic activity of saponins and sapogenins isolated from Chenopodium quinoa Willd. in cancer cell lines. Scientifica 2022, 2023, 8846387. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Gülçin, I.; Elias, R.; Gepdiremen, A.; Boyer, L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur. Food Res. Technol. 2006, 223, 759–767. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kountouri, A.M.; Karathanos, V.T. Antioxidant properties of raisins (Vitis vinifera L.). J. Med. Food. 2009, 12, 1302–1309. [Google Scholar] [CrossRef]
- Sasikumar, K.; Dubey, V.; Ghosh, A.R. Oleanolic acid from black raisins, Vitis vinifera with antioxidant and antiproliferative potentials on HCT 116 colon cancer cell line. Braz. J. Pharm. Sci. 2020, 56, 17158. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. Oleanolic acid, a compound present in grapes and olives, protects against genotoxicity in human mammary epithelial cells. Molecules 2015, 20, 13670–13688. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Sasaki, G.; Tokuji, Y.; Kinoshita, M.; Naito, A.; Aida, K.; Ohnishi, M. Effect of dietary wine pomace extract and oleanolic acid on plasma lipids in rats fed high-fat diet and its DNA microarray analysis. J. Agric. Food Chem. 2008, 56, 12052–12058. [Google Scholar] [CrossRef]
- Bai, X.; Lai, T.; Zhou, T.; Li, Y.; Li, X.; Zhang, H. In vitro antioxidant activities of phenols and oleanolic acid from mango peel and their cytotoxic effect on A549 cell line. Molecules 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Chetty, K.; Reddy, L. Determination of the ursolic and OA oleanolic acids content with the antioxidant capacity in apple peel extract of various cultivars. Braz. J. Biol. 2022, 82, e258442. [Google Scholar] [CrossRef]
- Root, M.; Petroski, W.; Bechtold, K.; McCullen, L.; Lambiase, S.; Wilson, B.; Joyner, R. A simplified HPLC-UV method for the analysis of triterpenoid acids from heritage apples (Malus domestica) from western North Carolina, USA. Bioact. Compd. Health Dis. 2022, 5, 84–92. [Google Scholar] [CrossRef]
- Sharma, A.; Kathuria, D.; Kolita, B.; Gohain, A.; Das, A.K.; Bhardwaj, G.; Simal-Gandara, J. Greener approach for the isolation of oleanolic acid from Nepeta leucophylla Benth. Its derivatization and their molecular docking as antibacterial and antiviral agents. Heliyon 2023, 9, e18639. [Google Scholar] [CrossRef]
- Assimopoulou, A.; Zlatanos, S.; Papageorgiou, V. Antioxidant activity of natural resins and bioactive triterpenes in oil substrates. Food Chem. 2005, 92, 721–727. [Google Scholar] [CrossRef]
- Koca, I.; Tekguler, B.; Turkyilmaz, B. Bioactive properties of different parts of Vitis labrusca L. fruit. Comm. J. Biol. 2021, 5, 193–198. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Al-Yousef, H.M.; Alhowiriny, T.A.; Alam, P.; Hassan, W.H.B.; Amina, M.; Hussain, A.; Abdelaziz, S.; Abdallah, R.H. Concurrent analysis of bioactive triterpenes oleanolic acid and β-amyrin in antioxidant active fractions of Hibiscus calyphyllus, Hibiscus deflersii and Hibiscus micranthus grown in Saudi Arabia by applying validated HPTLC method. Saudi Pharm. J. 2018, 26, 266–273. [Google Scholar] [CrossRef]
- Adjei, S.; Amponsah, I.K.; Bekoe, S.O.; Harley, B.K.; Mensah, K.B.; Mensah, A.Y.; Baah, M.K.; Fosu-Mensah, G. Fruits of Vitex doniana sweet: Toxicity profile, anti-inflammatory and antioxidant activities, and quantification of one of its bioactive constituents oleanolic acid. Heliyon 2021, 7, 07910. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Baijnath, S.; Moodley, R.; Dwarka, D.; Naicker, T.; Mellem, J.; Govender, N. Antioxidant and angiotensin-converting enzyme inhibitory potential of South African traditional medicinal plants and plant-derived compounds. S. Afr. J. Bot. 2022, 152, 85–91. [Google Scholar] [CrossRef]
- Herodež, Š.S.; Hadolin, M.; Škerget, M.; Knez, Ž. Solvent extraction study of antioxidants from Balm (Melissa officinalis L.) leaves. Food Chem. 2003, 80, 275–282. [Google Scholar] [CrossRef]
- Balaei-Kahnamoei, M.; Saeedi, M.; Rastegari, A.; Ardekani, M.R.S.; Akbarzadeh, T.; Khanavi, M. Phytochemical analysis and evaluation of biological activity of Lawsonia inermis seeds related to Alzheimer’s disease. Evid.-Based Complement. Altern. Med. 2020, 2021, 5965061. [Google Scholar] [CrossRef]
- Xue, T.; Ruan, K.; Xu, H.; Liu, H.; Tang, Z.; Yang, Y.; Duan, J.; Sun, X.; Wang, M.; Song, Z. Effect of different drying methods on the drying characteristics, chemical properties and antioxidant capacity of Ziziphus jujuba var. Spinosa fruit. LWT-Food Sci. Technol. 2024, 196, 115873. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Barbosa, F.L.; Ehrenfried, C.A.; Rodrigues, T.E.B.; Salvador, M.J.; Zampronio, A.R.; Stefanello, M.E.A. Chemical constituents and anti-inflammatory, antinociceptive, and antioxidant activities of Salvia melissiflora aerial parts. Rev. Bras. Farmacogn. 2024, 34, 350–357. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.; Liu, R.; Chen, H.; Bai, H.; Liang, X.; Zhang, X.; Wang, Z.; Li, W.; Hai, C. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef]
- Roy, P.K.; Rashid, F.; Bragg, J.; Ibdah, J.A. Role of the JNK signal transduction pathway in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 200. [Google Scholar] [CrossRef]
- Lu, N.; Malemud, C.J. Extracellular signal-regulated kinase: A regulator of cell growth, inflammation, chondrocyte and bone cell receptor-mediated gene expression. Int. J. Mol. Sci. 2019, 20, 3792. [Google Scholar] [CrossRef]
- Senthilkumar, P.K.; Kandhavelu, M.; Reetha, D. Antioxidant properties of the oleanolic acid isolated from Cassia auriculata (Linn). J. Pharm. Res. Clin. Pract. 2014, 4, 30–36. [Google Scholar]
- Yang, H.; Ma, X.; Xiong, H.; Gao, J.; Li, X.; Gao, Y.; Zhang, Q. The electrochemical redox mechanism and antioxidant activity of oleanolic acid based on multi-walled carbon nanotuber screen-printing electrode. Int. J. Electrochem. Sci. 2016, 12, 770–781. [Google Scholar] [CrossRef]
- Available online: https://www.chemicalbook.com (accessed on 28 April 2025).
- Matysik-Woźniak, A.; Oaduch, R.; Maciejewski; Jünemann, A.G.; Rejdak, R. The impact of oleanolic and ursolic acid on corneal epithelial cells in vitro. Ophthalmol. J. 2016, 1, 124–132. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Ramirez-Acuńa, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Smith, S.M.; Fieschi, F. NADPH oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- De Stefani, C.; Vasarri, M.; Salvatici, M.C.; Grifoni, L.; Quintela, J.C.; Bilia, A.R.; Degl’Innocenti, D.; Bergonzi, M.C. Microemulsions enhance the in vitro antioxidant activity of oleanolic acid in RAW 264.7 Cells. Pharmaceutics 2022, 14, 2232. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ibrahim, H.S.; Rotimi, D.E.; Ogunlakin, A.D.; Ojo, A.B. Diabetes mellitus: From molecular mechanism to pathophysiology and pharmacology. Med. Nov. Technol. Devices 2023, 19, 100247. [Google Scholar] [CrossRef]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef]
- Gao, D.; Li, Q.; Li, Y.; Liu, Z.; Fan, Y.; Liu, Z.; Zhao, H.; Li, J.; Han, Z. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytother. Res. 2009, 23, 1257–1262. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef]
- Ding, H.; Ni, M.; Zhang, G.; Liao, Y.; Hu, X.; Zhang, Y.; Gong, D. The inhibition of oleanolic acid on protein non-enzymatic glycation. LWT-Food Sci. Technol. 2020, 125, 109253. [Google Scholar] [CrossRef]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic acid exerts a neuroprotective effect against microglial cell activation by modulating cytokine release and antioxidant defense systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef]
- Rong, Z.T.; Gong, X.J.; Sun, H.B.; Li, Y.M.; Ji, H. Protective effects of oleanolic acid on cerebral ischemic damage in vivo and H2O2-induced injury in vitro. Pharm. Biol. 2011, 49, 78–85. [Google Scholar] [CrossRef]
- Fujita, K.; Lazarovici, P.; Guroff, G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ. Health Perspect. 1989, 80, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Gu, S. Oleanolic acid improved inflammatory response and apoptosis of PC12 cells induced by OGD/R through downregulating miR-142-5p. Nat. Prod. Commun. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Zareifar, P.; Ahmed, H.M.; Ghaderi, P.; Farahmand, Y.; Rahnama, N.; Esbati, R.; Moradi, A.; Yazdani, O.; Sadeghipour, Y. MiR-142-3p/5p role in cancer: From epigenetic regulation to immunomodulation. Cell Biochem. Funct. 2024, 42, e3931. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, Z.; Zhang, Z.; Zhu, P.; Jiang, X.; Wang, Y.; Deng, Q.; Lam Yung, K.K.; Zhang, S. Oleanolic acid alleviates cerebral ischemia/reperfusion injury via regulation of the GSK-3β/HO-1 signaling pathway. Pharmaceuticals 2021, 15, 1. [Google Scholar] [CrossRef]
- Goyal, S.N.; Mahajan, U.B.; Chandrayan, G.; Kumawat, V.S.; Kamble, S.; Patil, P.; Agrawal, Y.O.; Patil, C.R.; Ojha, S. Protective effect of oleanolic acid on oxidative injury and cellular abnormalities in doxorubicin induced cardiac toxicity in rats. Am. J. Transl. Res. 2016, 15, 60–69. [Google Scholar]
- Yu, S.; Shang, P. A review of bioeffects of static magnetic field on rodent models. Prog. Biophys. Mol. Biol. 2013, 14, 14–24. [Google Scholar] [CrossRef]
- Fu, G.; Wang, J.; Cai, M.; Zhang, J.; Hu, H.; Dai, C.; Zheng, X.; Hu, Y.; Chen, K. Beneficial effects of oleanolic acid on hepatopancreas oxidative stress and intestinal microbiota structure of red swamp crayfish (Procambarus clarkia). Aquacult. Rep. 2023, 33, 101797. [Google Scholar] [CrossRef]
- Chung, S.; Yoon, H.E.; Kim, S.J.; Kim, S.J.; Koh, E.S.; Hong, Y.A.; Park, C.W.; Chang, Y.S.; Shin, S.J. Oleanolic acid attenuates renal fibrosis in mice with unilateral ureteral obstruction via facilitating nuclear translocation of Nrf2. Nutr. Metab. 2014, 11, 2. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, H.M.; Kang, J.S.; Lee, E.Y.; Yadav, D.; Kwon, M.H.; Kim, Y.M.; Kim, H.S.; Chung, C.H. Oleanolic acid and N-acetylcysteine ameliorate diabetic nephropathy through reduction of oxidative stress and endoplasmic reticulum stress in a type 2 diabetic rat model. Nephrol. Dial. Transpl. 2016, 31, 391–400. [Google Scholar] [CrossRef]
- Long, C.; Yang, J.; Yang, H.; Li, X.; Wang, G. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti-inflammatory, and anti-apoptotic activities. Mol. Med. Rep. 2016, 3, 469–4704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, J.; Cheng, B.; Lu, Q.; Chen, X. Oleanolic acid protects against oxidative stress-induced human umbilical vein endothelial cell injury by activating AKT/eNOS signaling. Mol. Med. Rep. 2018, 18, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Onat, D.; Brillon, D.; Colombo, P.C.; Schmidt, A.M. Human vascular endothelial cells: A model system for studying vascular inflammation in diabetes and atherosclerosis. Cur. Diab. Rep. 2011, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, G.; Vlaanderen, J.; Polidoro, S.; Gulliver, J.; Galassi, C.; Ranzi, A.; Krogh, V.; Grioni, S.; Agnoli, C.; Sacerdote, C.; et al. Oxidative stress and inflammation mediate the effect of air pollution on cardio- and cerebrovascular disease: A prospective study in nonsmokers. Environ. Mol. Mutagen. 2018, 59, 234–246. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.E.; Jang, H.S.; Hong, S.Y.; Lee, J.B.; Park, S.Y.; Hwang, J.S. Oleanolic acid protects the skin from particulate matter-induced aging. Biomol. Ther. 2021, 29, 220–226. [Google Scholar] [CrossRef]
- Castellano, J.M.; Perona, J.S. Oleanolic acid: Extraction, characterization and biological activity. Nutrients 2021, 14, 623. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Calpena, A.C.; Garduño-Ramírez, M.L.; Ortiz, R.; Melguizo, C.; Prados, J.C.; Clares, B. Nanoemulsion strategy for ursolic and oleanic acids isolates from Plumeria obtusa improves antioxidant and cytotoxic activity in melanoma cells. Anticancer Agents Med. Chem. 2018, 18, 847–853. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Zhu, X.; Zhao, Q.; Deng, G.; Li, Y.; Chen, Q. Improving anti-oxidant stress treatment of subarachnoid hemorrhage through self-assembled nanoparticles of oleanolic acid. Drug Deliv. 2024, 31, 2388735. [Google Scholar] [CrossRef]
- Yin, M.C.; Chan, K.C. Nonenzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, Y.; Wu, Y.; Lin, L.; Chen, C.; Chen, C.; Kuo, S. Reparative efficacy of liposome-encapsulated oleanolic acid against liver inflammation induced by fine ambient particulate matter and alcohol in mice. Pharmaceutics 2022, 14, 1108. [Google Scholar] [CrossRef]
- Dwiecki, K.; Przybył, K.; Dezor, D.; Bąkowska, E.; Rocha, S.M. Interactions of oleanolic acid, apigenin, rutin, resveratrol and ferulic acid with phosphatidylcholine lipid membranes—A spectroscopic and machine learning study. Appl. Sci. 2023, 13, 9362. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.; Jin, L.; Wang, Z.; Peng, J.; Liao, N.; Zhao, Y.; Zhang, J.; Pauluhn, J.; Hai, C.; et al. Nano-oleanolic acid alleviates metabolic dysfunctions in rats with high fat and fructose diet. Biomed. Pharmacother. 2018, 108, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Sun, Y.; Liu, Y.; Xu, Z.; Fan, R.; Liu, Z.; Liu, W. A gold(I) complex containing an oleanolic acid derivative as a potential anti-ovarian cancer agent via inhibiting TrxR and activating ROS-mediated ERS. Chem. Eur. J. 2020, 26, 7092–7108. [Google Scholar] [CrossRef]
- Fu, H.; Yen, F.L.; Huang, P.H.; Yang, C.Y.; Yen, C.H. Oleanolic acid nanofibers attenuated particulate matter-induced oxidative stress in keratinocytes. Antioxidants 2021, 10, 1411. [Google Scholar] [CrossRef]
- Silva, M.D.L.; David, J.P.; Silva, L.C.R.C.; Santos, R.A.F.; David, J.M.; Lima, L.S.; Reis, P.S.; Fontana, R. Bioactive oleanane, lupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef]
- Madlala, H.P.; Van Heerden, F.R.; Mubagwa, K.; Musabayane, C.T. Changes in renal function and oxidative status associated with the hypotensive effects of oleanolic acid and related synthetic derivatives in experimental animals. PLoS ONE 2015, 10, e0128192. [Google Scholar] [CrossRef]
- Günther, A.; Zalewski, P.; Sip, S.; Ruszkowski, P.; Bednarczyk-Cwynar, B. Oleanolic acid dimers with potential application in medicine—Design, synthesis, physico-chemical characteristics, cytotoxic and antioxidant activity. Int. J. Mol. Sci. 2024, 25, 6989. [Google Scholar] [CrossRef]
- Günther, A.; Zalewski, P.; Sip, S.; Bednarczyk-Cwynar, B. Exploring the potential of oleanolic acid dimers–cytostatic and antioxidant activities, molecular docking, and ADMETox profile. Molecules 2024, 29, 3623. [Google Scholar] [CrossRef]
- Günther, A.; Zalewski, P.; Sip, S.; Ruszkowski, P.; Bednarczyk-Cwynar, B. Acetylation of oleanolic acid dimers as a method of synthesis of powerful cytotoxic agents. Molecules 2024, 29, 4291. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Ropek, N.; Melillo, B.; Schreiber, S.L.; Cravatt, B.F.; Vinogradova, E.V. Stereochemical diversity as a source of discovery in chemical biology. Curr. Res. Chem. Biol. 2021, 2, 100028. [Google Scholar] [CrossRef]
- Ovesná, Z.; Kozics, K.; Slameňová, D. Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2006, 600, 131–137. [Google Scholar] [CrossRef]
- Jung, M.J.; Yoo, Y.C.; Lee, K.B.; Kim, J.B.; Song, K.S. Isolation of epi-oleanolic acid from Korean mistletoe and its apoptosis-lnducing activity in tumor cells. Arch. Pharm Res. 2004, 27, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Jannus, F.; Sainz, J.; Reyes-Zurita, F.J. Principal bioactive properties of oleanolic acid, its derivatives, and analogues. Molecules 2024, 29, 3291. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2021, 10, 132. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, M.; Wang, H.; Dong, X. Metal complexes or chelators with ROS regulation capacity: Promising candidates for cancer treatment. Molecules 2021, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687. [Google Scholar] [CrossRef]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; Van Heerden, F.R.; Musabayane, C.T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Ren. Fail. 2014, 36, 419–431. [Google Scholar] [CrossRef]
- Mokhtari, K.; Pérez-Jiménez, A.; García-Salguero, L.; Lupiáñez, J.A.; Rufino-Palomares, E.E. Unveiling the differential antioxidant activity of maslinic acid in murine melanoma cells and in rat embryonic healthy cells following treatment with hydrogen peroxide. Molecules 2020, 25, 4020. [Google Scholar] [CrossRef]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, H.; Yao, X.; Lv, J.; Wang, Q.; Zhao, Y.; Yang, S.; Xu, L.; Shi, Y.; Hu, J.; et al. Hederagenin reduces Aβ-induced oxidative damage, decreases Aβ deposition, and promotes cell survival by the P13K/Akt signaling pathway. J. Leukoc. Biol. 2025, 117, qiae124. [Google Scholar] [CrossRef] [PubMed]








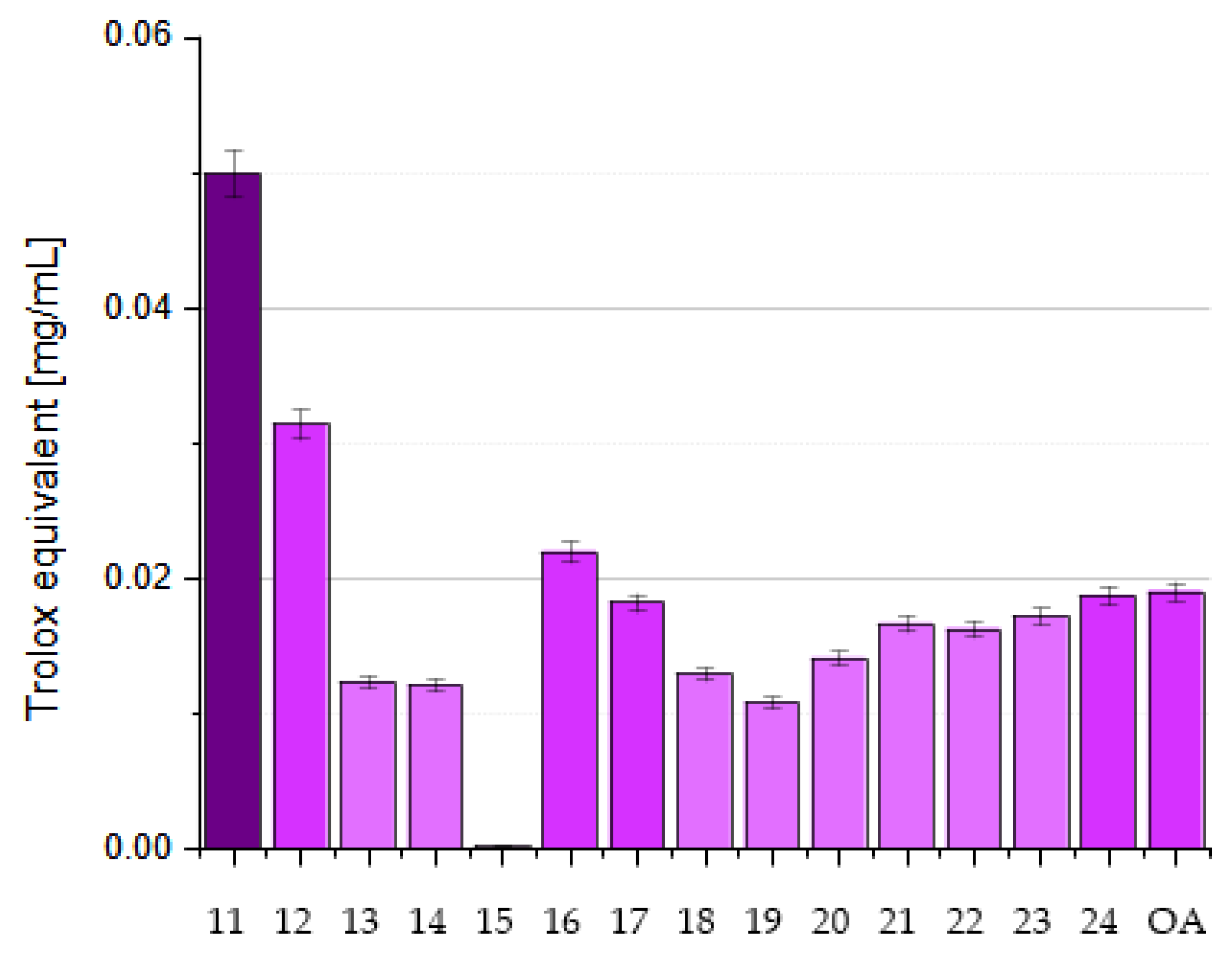
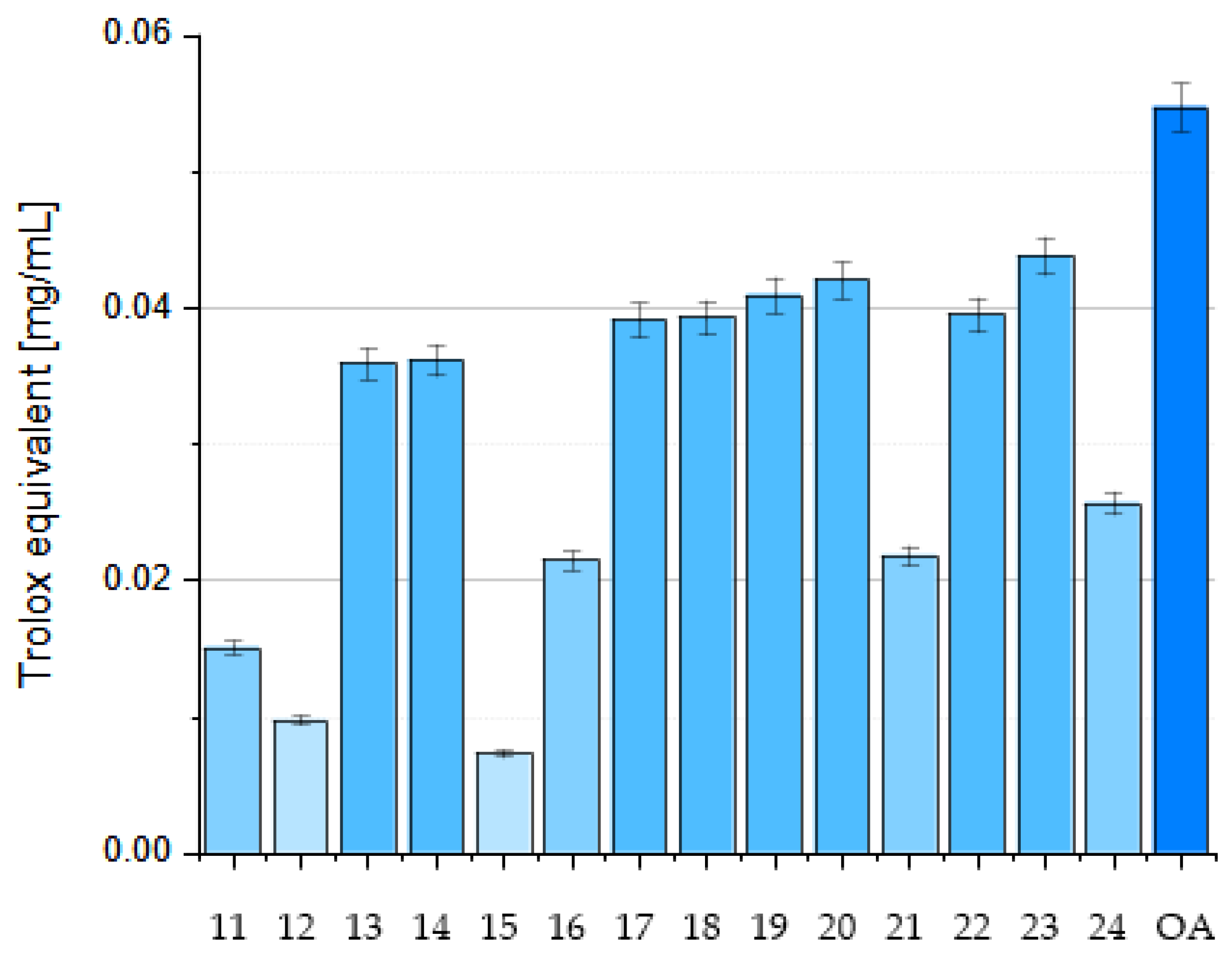
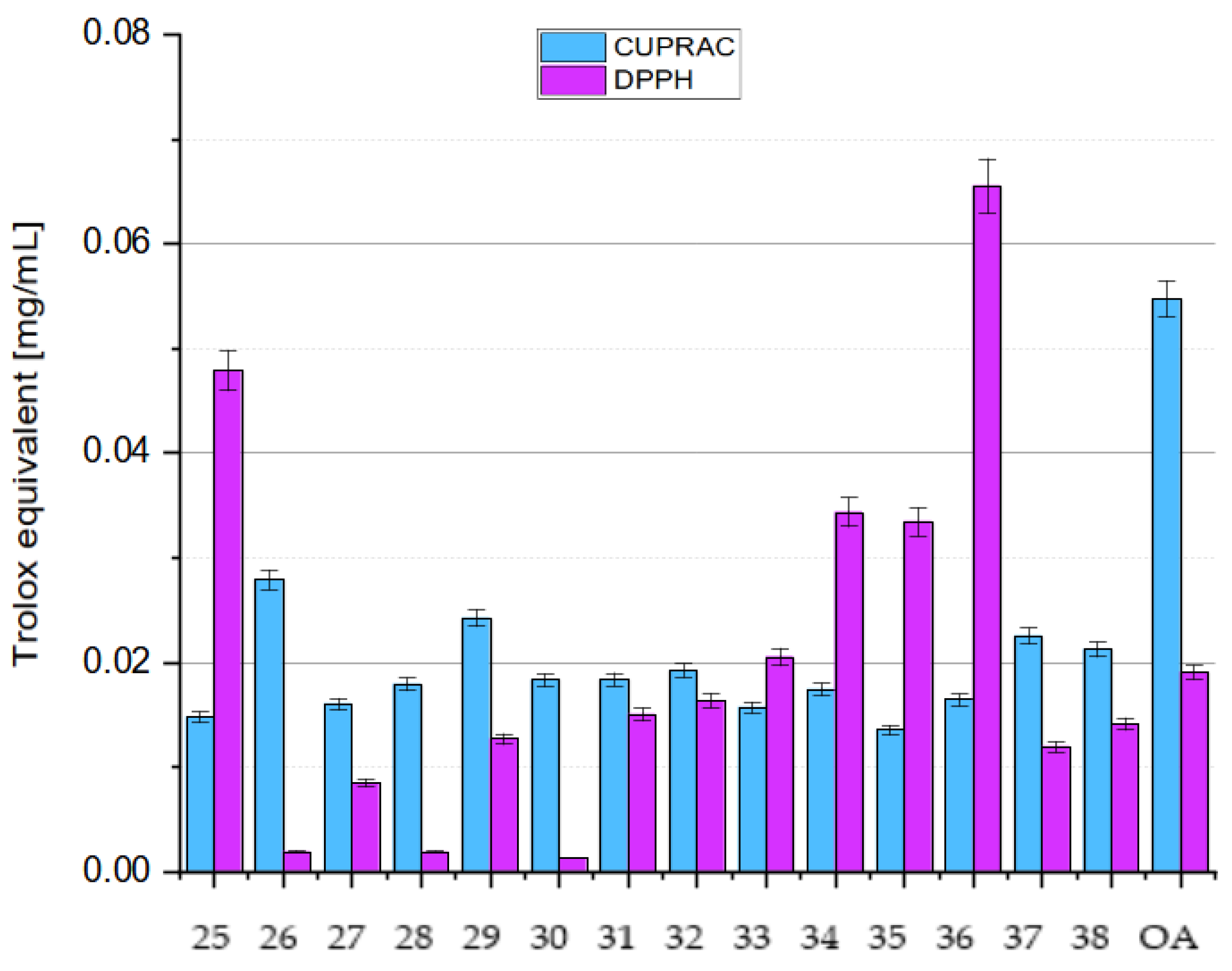
| OA Sources and Its Content | |||||
|---|---|---|---|---|---|
| Common Name | Latin Name | Part of the Plant | Content by Weight | Content by % | Reference |
| wine grapes | Vitis vinifera L. | fruits | 8.7 mg/g dry fruits | 0.87% | [21] |
| wine grapes | Vitis vinifera L. | fruits | n.d. for pure compound | - - - | [22] |
| olive | Olea europaea L. | fruits | n.d. for pure compound | - - - | [22] |
| Kiyomi oranges | Citrus reticulata Blanco × Citrus × aurantium L. | fruits | 110 mg/g dried fruit pomace | 11.0% | [23] |
| mango | Mangifera indica L. | peels | 0.003 mg/g | 0.0003% | [23] |
| apple | Malus domestica (Suckow) Borkh. | peels | 0.00011 g/mL | 0.011% | [25] |
| apple | Malus domestica (Suckow) Borkh. | peels | 3.6 mg/g dry peel weight | 0.36% | [26] |
| white leaved catmint | Nepeta leucophylla Benth. | herb | 1.1 mg/g dry herb | 0.11% | [27] |
| mastic tree | Pistacia lentiscus L. | resin | n.d. for pure compound | - - - | [28] |
| Indian frankincense | Boswellia serrata Roxb. Ex Colebr. | resin | n.d. for pure compound | - - - | [28] |
| common myrrh | Commiphora myrrh Nees | resin | n.d. for pure compound | - - - | [28] |
| wine grapes | Vitis vinifera L. | whole fruits | 0.178 mg/g | 0.018% | [29] |
| wine grapes | Vitis vinifera L. | fruit peels | 0.351 mg/g | 0.035% | [29] |
| wine grapes | Vitis vinifera L. | seeds | 0.042 mg/g | 0.004% | [29] |
| tiny flower hibiscus | Hibiscus micranthus L.f. | herb | up to 3.87 mg/g dry petroleum eter extract | 0.39% | [30] |
| hibiscus Roselle | Hibiscus deflersii Schweinf. ex Cufod. | herb | up to 0.41 mg/g dry petroleum eter extract | 0.04% | [30] |
| lemonyellow rosemallow | Hibiscus calyphyllus Cav. | herb | up to 1.21 mg/g dry petroleum eter extract | 0.12% | [30] |
| black plum | Vitex doniana Sweet | fruits | 90.24 mg/g methanolic extract | 0.02% | [31] |
| common olive | Olea europaea L. | fruits | 5.2 mg/g dry extract | 0.52% | [5] |
| mugwort, african wormwood | Artemisia afra Jacq. ex. Willd. | herb | n.d. for pure compound | - - - | [32] |
| horsewood tree | Clausena anisata (Willd.) Hook.f. ex. Benth | herb | n.d. for pure compound | - - - | [32] |
| dikbas, South African wild pear | Dombeya rotundifolia (Hochst.) Planch. | herb | n.d. for pure compound | - - - | [32] |
| morula, cider tree | Sclerocarya birrea (A.Rich.) Hochst. | herb | n.d. for pure compound | - - - | [32] |
| red currant tree | Searsia chirindensis (Baker f.) Moffett | herb | n.d. for pure compound | - - - | [32] |
| pepper-bark tree | Warburgia salutaris (Bertol. f.) Chiov. | herb | n.d. for pure compound | - - - | [32] |
| lemon balm | Melissa officinalis L. | leaves | 3.5 mg/g raw leaves | 0.35% | [33] |
| henna tree | Lawsonia inermis L. | seeds | n.d. for pure compound | - - - | [34] |
| Chinese jujube | Ziziphus jujuba Mill. | fruits | up to 0.308 mg/g | 0.031% | [35] |
| grape-scented sage | Salvia melissiflora Benth. | aerial parts | n.d. for pure compound | - - - | [36] |
| OA Antioxidant Activity | ||
|---|---|---|
| Assay | Results | Reference |
| DPPH free radical scavenging activity | IC50 = 61.5 µg/mL | [21] |
| DPPH free radical scavenging rate | 88.30% inhibition | [21] |
| DPPH free radical scavenging rate | 2.7% inhibition | [24] |
| ABTS free radical scavenging rate | 11.0% inhibition | [24] |
| FRAP free radical reducing rate | 1.2 µmol TE/kg DMP | [24] |
| DPPH free radical scavenging activity | n.d. for pure compound | [25] |
| DPPH free radical scavenging activity | 23.66% inhibition | [27] |
| total antioxidant capacity | 16.93 mg AAE/g of DPPH | [27] |
| increase of peroxide value | n.d. for pure compound | [28] |
| DPPH free radical scavenging activity | n.d. for pure compound | [29] |
| FRAP free radical reducing activity | n.d. for pure compound | [29] |
| DPPH free radical scavenging activity | n.d. for pure compound | [30] |
| DPPH free radical scavenging activity | IC50 = 2.80 µg/mL | [31] |
| DPPH free radical scavenging activity | 18.2% inhibition | [5] |
| FRAP total antioxidant capacity | 240.9 µmol | [5] |
| DPPH free radical scavenging activity | IC50 = 32.20 µg/mL | [32] |
| DPPH free radical scavenging activity | n.d. for pure compound | [33] |
| DPPH free radical scavenging activity | n.d. for pure compound | [34] |
| DPPH free radical scavenging activity | n.d. for pure compound | [35] |
| ABTS free radical scavenging rate | n.d. for pure compound | [35] |
| ORAC free radical absorbance activity | n.d. for pure compound | [36] |
| AAPH free radical scavenging activity | n.d. for pure compound | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, A.; Bednarczyk-Cwynar, B. Oleanolic Acid: A Promising Antioxidant—Sources, Mechanisms of Action, Therapeutic Potential, and Enhancement of Bioactivity. Antioxidants 2025, 14, 598. https://doi.org/10.3390/antiox14050598
Günther A, Bednarczyk-Cwynar B. Oleanolic Acid: A Promising Antioxidant—Sources, Mechanisms of Action, Therapeutic Potential, and Enhancement of Bioactivity. Antioxidants. 2025; 14(5):598. https://doi.org/10.3390/antiox14050598
Chicago/Turabian StyleGünther, Andrzej, and Barbara Bednarczyk-Cwynar. 2025. "Oleanolic Acid: A Promising Antioxidant—Sources, Mechanisms of Action, Therapeutic Potential, and Enhancement of Bioactivity" Antioxidants 14, no. 5: 598. https://doi.org/10.3390/antiox14050598
APA StyleGünther, A., & Bednarczyk-Cwynar, B. (2025). Oleanolic Acid: A Promising Antioxidant—Sources, Mechanisms of Action, Therapeutic Potential, and Enhancement of Bioactivity. Antioxidants, 14(5), 598. https://doi.org/10.3390/antiox14050598








