Manganese Porphyrin Treatment Improves Redox Status Caused by Acute Compressive Spinal Cord Trauma
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Synthesis and Characterization of Manganese Porphyrins (MnPs)
2.3. Acute Compressive Spinal Cord Trauma Model
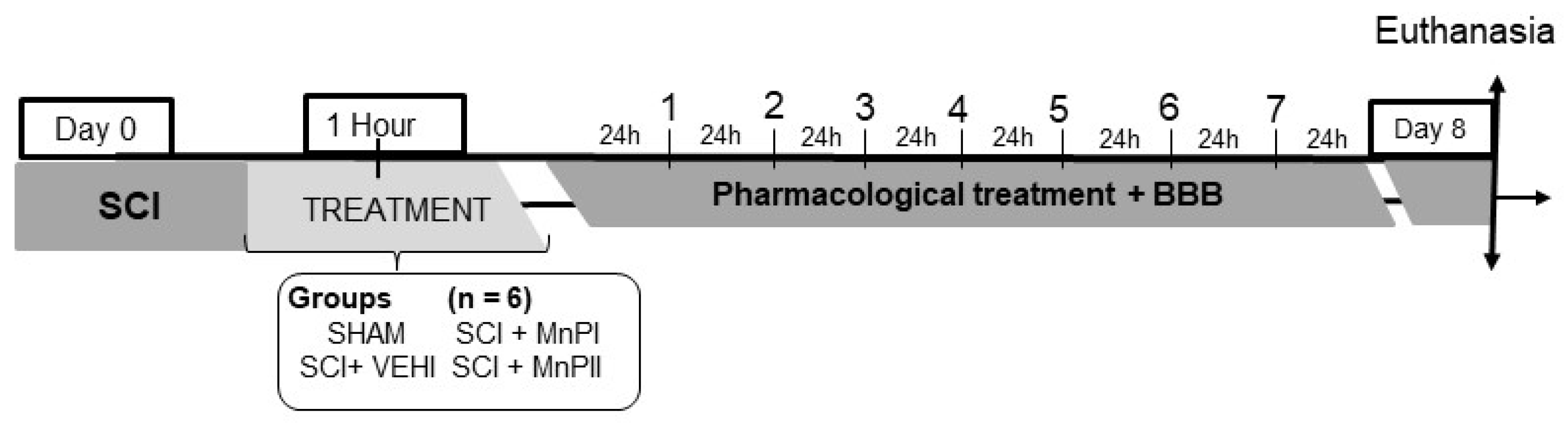
2.4. Experimental Design
- Negative control (SHAM)—The subjects underwent dorsal laminectomy at T13 without spinal cord injury. One hour post-procedure, 0.9% NaCl solution (0.15 mL/100 g) was administered intraperitoneally.
- Positive control (SCI + VEHI)—The subjects underwent T13 dorsal laminectomy and spinal cord injury. One hour post-injury, 0.9% saline solution (0.15 mL/100 g) was administered intraperitoneally.
- Manganese Porphyrin I (SCI + MnPI)—The subjects underwent a dorsal laminectomy at the T13 vertebra, followed by spinal cord injury. One hour post-injury, MnPI—MnTE-2-PyP]5+—was administered intraperitoneally at a dose of 0.1 mg/kg/day. The dose was defined according to the Hambright [26], Batinic-Haberle [22], Rebouças [23], Rebouças [24], Pinto [25], and Cordeiro [21] studies.
- Manganese porphyrin II (SCI + MnPII)—The subjects underwent dorsal laminectomy at T13 and spinal cord injury. One hour after the injury, MnP II—[MnT(5-Br-3-E-Py)P]5+—(0.1 mg/Kg/day) was administered via IP every 24 h for seven days. The dose was defined according to the Cordeiro [21] study.
2.5. Motor Skills Assessment
2.6. Necropsy and Material Collection
2.7. Immunohistochemistry (IHC)
2.8. Real-Time Polymerase Chain Reaction (RT-qPCR)
2.9. Evaluation of the Enzymatic Activity of SOD and Catalase
2.10. Statistical Analysis
3. Results
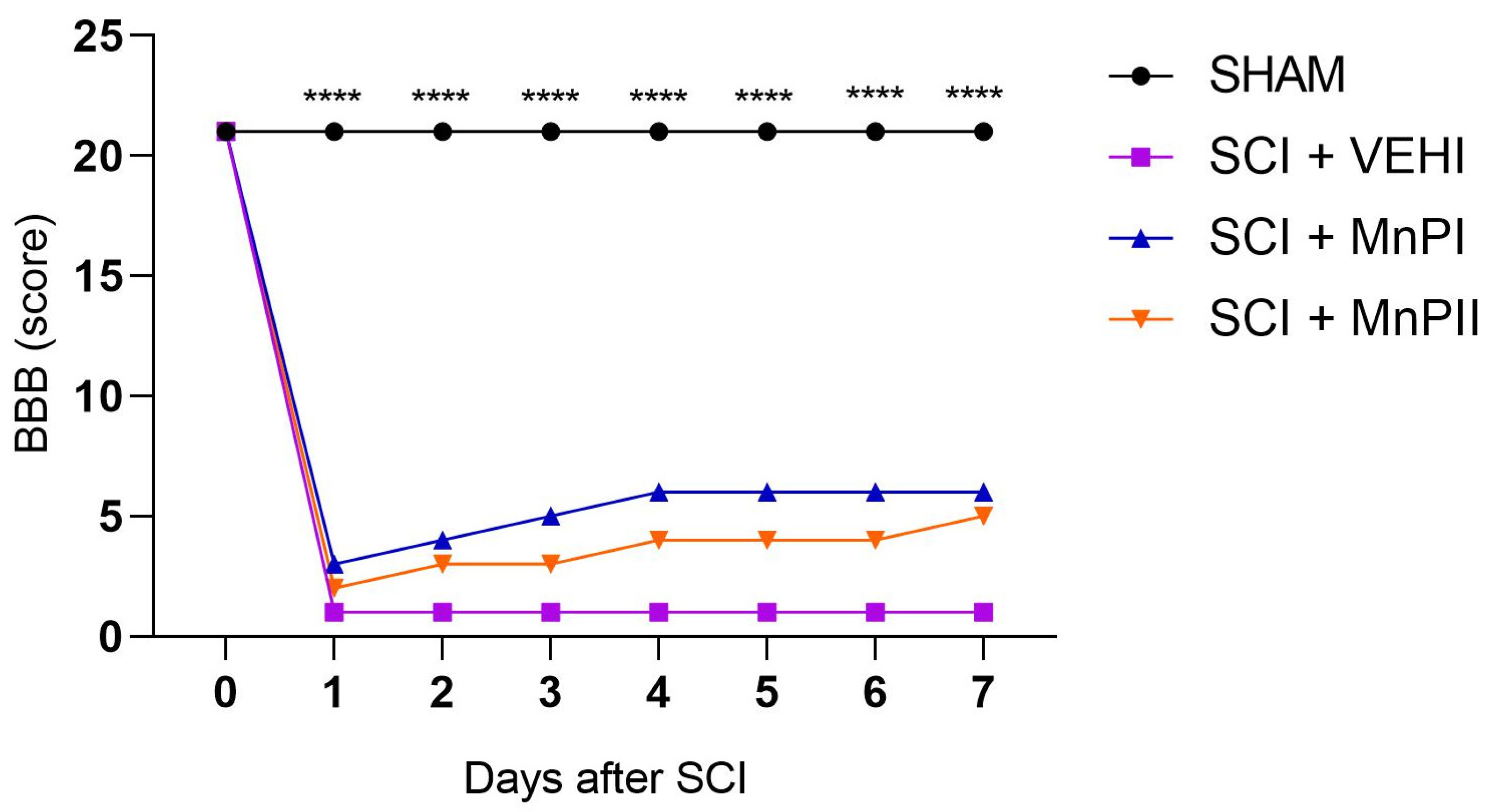
3.1. Motor Capacity Assessment
3.2. Anatomopathological Evaluation of the Spinal Cord
3.3. MnP Treatment Mitigates Oxidative Damage by Inhibiting the Spinal Elevation of 8-OHdG, MDA, and HIF1α in Rats Following Spinal Cord Trauma
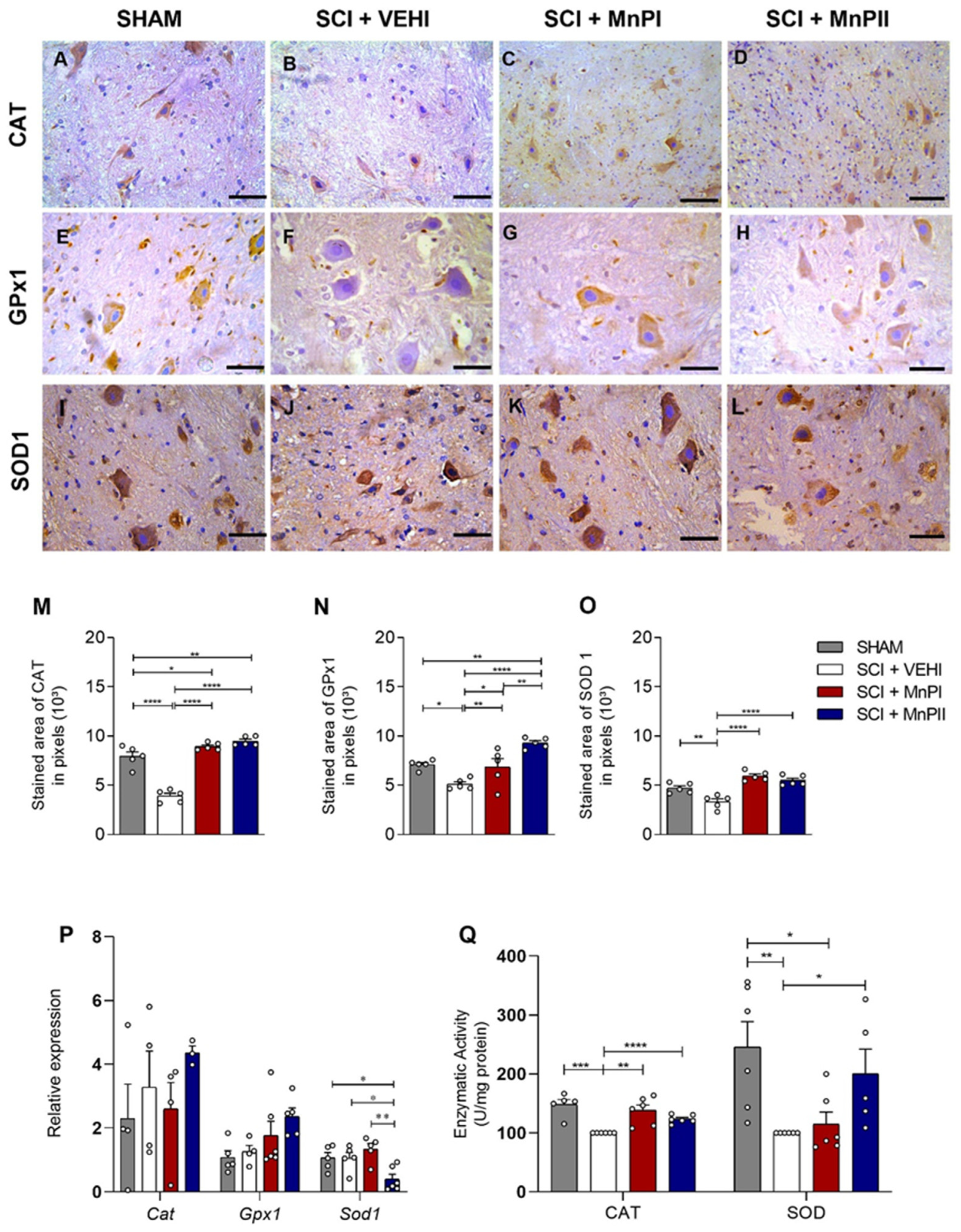
3.4. MnP Treatment Enhances Antioxidant Enzyme Protein Expression and Activity in Rats Following Spinal Cord Trauma
3.5. MnP Treatment Inhibits the Elevation of Unfolded Protein Response (UPR) Mediators in Rats Following Spinal Cord Trauma
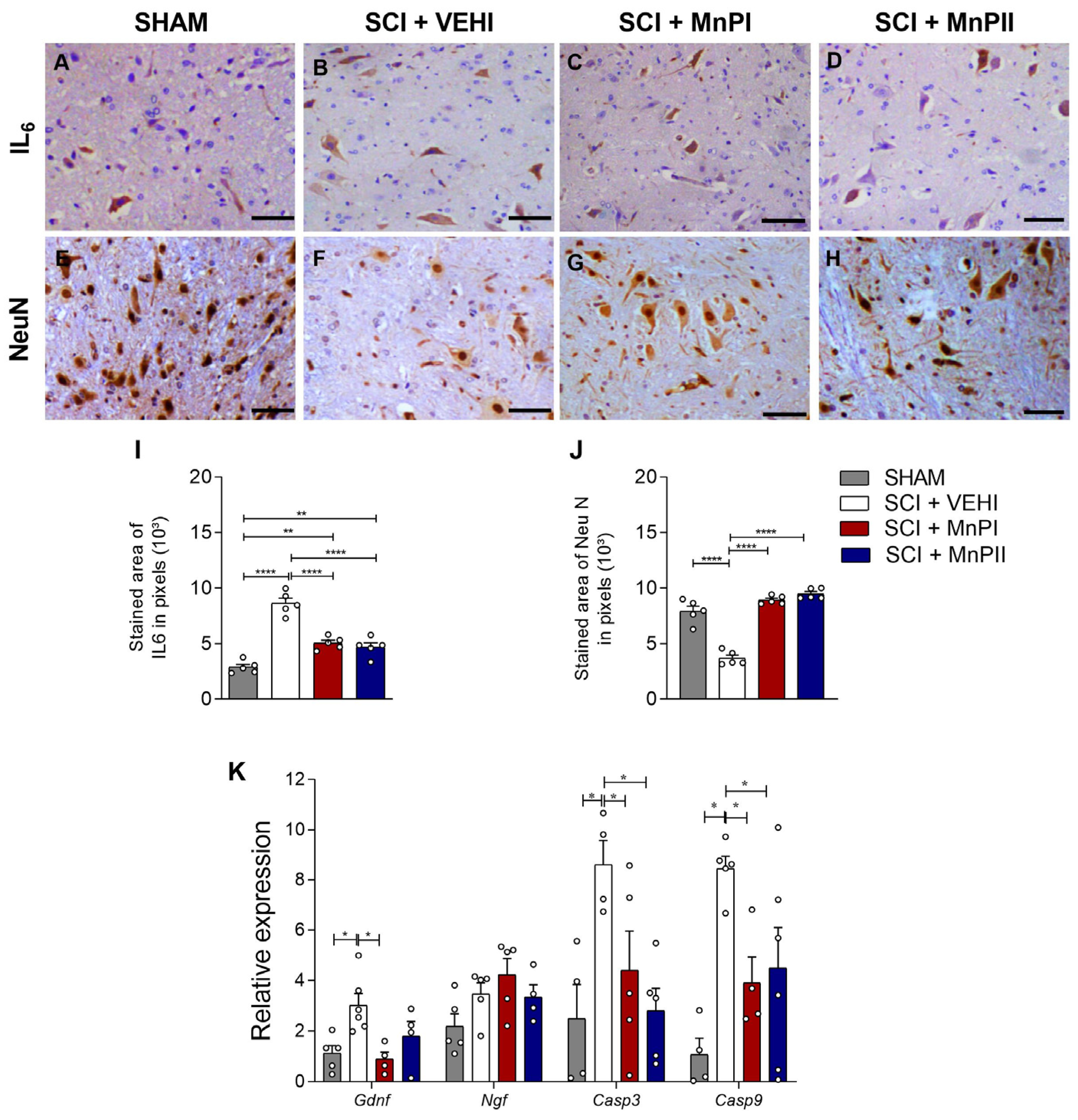
3.6. MnP Treatment Changes IL-6, NeuN, Gdnf, Casp 3, and Casp 9 Expression in Rats Following Spinal Cord Trauma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hu, R.; Zhou, J.; Lou, C.; Luo, C.; Lin, J.; Wang, X.; Li, X.; Bian, X.; Li, Y.; Wan, Q.; et al. Glial scar and neuroregeneration: Histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J. Neurosurg. Spine 2010, 13, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 20, 7533. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.; Lanz, O.; Shell, L. Acute spinal cord trauma: Mechanisms and clinical syndromes. Vet. Med. 2000, 95, 846–849. [Google Scholar]
- Khorasanizadeh, M.; Yousefifard, M.; Eskian, M.; Lu, Y.; Chalangari, M.; Harrop, J.S.; Jazayeri, S.B.; Seyedpour, S.; Khodaei, B.; Hosseini, M.; et al. Neurological recovery following traumatic spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. 2019, 30, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Danner, S.M.; Mayr, W. Neurocontrol of Movement in Humans With Spinal Cord Injury. Artif. Organs 2015, 39, 823–833. [Google Scholar] [CrossRef]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar] [CrossRef]
- Kundi, S.; Bicknell, R.; Ahmed, Z. The role of angiogenic and wound-healing factors after spinal cord injury in mammals. Neurosci. Res. 2013, 76, 1–9. [Google Scholar] [CrossRef]
- Thuret, S.; Moon, L.D.F.; Gage, F.H. Therapeutic interventions after spinal Cord injury. Nature 2006, 7, 628–643. [Google Scholar] [CrossRef]
- Ng, M.T.L.; Stammers, A.T.; Kwon, B.K. Vascular disruption and the role of angiogenic proteins after spinal cord injury. Transl. Stroke Res. 2011, 2, 474–491. [Google Scholar] [CrossRef]
- Lanz, O.; Bergman, R.; Shell, L. Initial assessment of patients with spinal cord trauma. Vet. Med. 2000, 95, 851–854. [Google Scholar]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Spasojevic, I.; Tse, H.; Tovmasyan, A.; Rajic, Z.; Clair, D.; Vujaskovic, Z.; Dewhirst, M.W.; Piganelli, J.D. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids 2012, 42, 95–113. [Google Scholar] [CrossRef]
- Ratcliffe, P.; Koivunen, P.; Myllyharju, J.; Ragoussis, J.; Bovée, J.V.; Batinic-Haberle, I.; Vinatier, C.; Trichet, V.; Robriquet, F.; Oliver, L.; et al. Update on hypoxia-inducible factors and hydroxylases in oxygen regulatory pathways: From physiology to therapeutics. Hypoxia 2017, 5, 11–20. [Google Scholar] [CrossRef]
- Sheng, H.; Spasojevic, I.; Warner, D.S.; Batinic-Haberle, I. Mouse spinal cord compression injury is ameliorated by intrathecal cationic manganese(III) porphyrin catalytic antioxidant therapy. Neurosci. Lett. 2004, 366, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Celic, T.; Spanjol, J.; Bobinac, M.; Tovmasyan, A.; Vukelic, I.; Reboucas, J.S.; Batinic-Haberle, I.; Bobinac, D. Mn porphyrin-based SOD mimic, MnTnHex-2-PyP(5+), and non-SOD mimic, MnTBAP(3-), suppressed rat spinal cord ischemia/reperfusion injury via NF-kappaB pathways. Free Radic. Res. 2014, 48, 1426–1442. [Google Scholar] [CrossRef]
- Liu, D.; Shan, Y.; Valluru, L.; Bao, F. Mn (III) tetrakis (4-benzoic acid) porphyrin scavenges reactive species, reduces oxidative stress, and improves functional recovery after experimental spinal cord injury in rats: Comparison with methylprednisolone. BMC Neurosci. 2013, 14, 23. [Google Scholar] [CrossRef]
- Valluru, L.; Diao, Y.; Hachmeister, J.E.; Liu, D. Mn (III) tetrakis (4-benzoic acid) porphyrin protects against neuronal and glial oxidative stress and death after spinal cord injury CNS. Neurol. Disord. Drug Targets 2012, 11, 774–790. [Google Scholar] [CrossRef]
- Sheng, H.; Warner, D.S. Metalloporphyrin in CNS Injuries. In Redox-Active Therapeutics; Batinic-Haberle, I., Reboucas, J.S., Spasojevic, I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Chapter 24. [Google Scholar] [CrossRef]
- Cordeiro, J.M.A.; Cardoso, L.C.; Reis, B.S.; Nascimento, A.E.J.; Oliveira, E.S.; Barbosa, E.M.; Macedo, I.O.; Mendonça, L.D.; Sarmento-Neto, J.F.; Pinho, C.S.; et al. Manganese porphyrin-based treatment improves fetal-placental development and protects against oxidative damage and NLRP3 inflammasome activation in a rat maternal hypothyroidism model. Redox Biol. 2024, 74, 103238. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Spasojevic, I.P.; Hambright, L.; Benov, A.; Crumbliss, L.; Fridovich, I. Relationship among redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vivo and in vitro superoxide dismutating activities of manganese(III) and iron(III) water-soluble porphyrins. Inorg. Chem. 1999, 38, 4011–4022. [Google Scholar] [CrossRef]
- Rebouças, J.S.; Kos, I.; Vujaskovic, Z.; Batinic-Haberle, I. Determination of residual manganese in Mn porphyrin-based superoxide dismutase (SOD) and peroxynitrite reductase mimics. J. Pharm. Biomed. Anal. 2009, 50, 1088. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, J.S.; Spasojevic, I.; Batini’C-Haberle, I. Quality of potent Mn porphyrin based SOD mimics and peroxynitrite scavengers for pre-clinical mechanistic/therapeutic purposes. J. Pharm. Biomed. Anal. 2008, 48, 1046. [Google Scholar] [CrossRef]
- Pinto, V.H.A.; Carvalho Da-Silva, D.; Santos, J.L.M.S.; Weitner, T.; Fonseca, M.G.; Yoshida, M.I.; Idemori, Y.M.; Batini’C-Haberle, I.; Rebouças, J.S. Thermal stability of the prototypical Mn porphyrin-based superoxide dismutase mimic and potent oxidative-stress redox modulator Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl) porphyrin chloride, MnTE-2-PyP5+. J. Pharm. Biomed. Anal. 2013, 73, 29. [Google Scholar] [CrossRef]
- Hambright, P.; Adeyemo, A.; Shamim, A.; Lemelle, S.; Lavallee, D.K.; Miller, D.; White, A. [[4,4′4″,4‴-Porphyrin-5,10,15, 20-tetrayltetrakis(1-methylpyridiniumato](2-)]- indium(III) pentaperchlorate. Inorg. Synth. 1985, 23, 55–59. [Google Scholar] [CrossRef]
- Khan, M.; Griebel, R.; Rozdilsky, B.; Politis, M. Hemorrhagic changes in experimental spinal cord injury models. Can. J. Neurol. sciences. Le J. Can. Des Sci. Neurol. 1985, 12, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Maternal thyroid dysfunction affects placental profile of inflammatory mediators and the intrauterine trophoblast migration kinetics. Reproduction 2014, 147, 803–816. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Method 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and Placental Development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef]
- Francisqueti-Ferron, F.V.; Ferron, A.J.T.; Garcia, J.L.; Almeida, S.; Ccv, D.E.; Costa, M.R.; Gregolin, C.S.; Moreto, F.; Ferreira, A.L.A.; Minatel, I.O.; et al. Basic Concepts on the Role of Nuclear Factor Erythroid-Derived 2-Like 2 (Nrf2) in Age-Related Diseases. Int. J. Mol. Sci. 2019, 20, 3208. [Google Scholar] [CrossRef]
- Muir, G.D.; Webb, A.A. Assessment of behavioural recovery following spinal cord injury in rats. Eur. J. Neurosci. 2000, 12, 3079–3086. [Google Scholar] [CrossRef]
- Agrawal, G.; Thakor, N.V.; All, A.H. Evoked potential versus behavior to detect minor insult to the spinal cord in a rat model. J. Clin. Neurosci. 2009, 16, 1052–1055. [Google Scholar] [CrossRef]
- Sedy, J.; Urdzikova, L.; Jendelova, P.; Sykova, E. Methods for behavioral testing of spinal cord injured rats. Neurosci. Biobehav. Rev. 2008, 32, 550–580. [Google Scholar] [CrossRef]
- Liang, W.; Han, Q.; Jin, W.; Xiao, Z.; Huang, J.; Ni, H.; Chen, B.; Kong, J.; Wu, J.; Dai, J. The promotion of neurological recovery in the rat spinal cord crushed injury model by collagen-binding BDNF. Biomaterials 2010, 31, 8634–8641. [Google Scholar] [CrossRef]
- Mao, L.; Wang, H.; Wang, X.; Liao, H.; Zhao, X. Transcription factor Nrf2 protects the spinal cord from inflammation produced by spinal cord injury. J. Surg. Res. 2011, 170, e105–e115. [Google Scholar] [CrossRef]
- Jin, W.; Ni, H.; Hou, X.; Ming, X.; Wang, J.; Yuan, B.; Zhu, T.; Jiang, J.; Wang, H.; Liang, W. Tert-butylhydroquinone protects the spinal cord against inflammatory response produced by spinal cord injury. Ann. Clin. Lab. Sci. 2014, 44, 151–157. [Google Scholar] [PubMed]
- Krupa, P.; Stepankova, K.; Kwok, J.C.; Fawcett, J.W.; Cimermanova, V.; Jendelova, P.; Machova Urdzikova, L. New Model of Ventral Spinal Cord Lesion Induced by Balloon Compression in Rats. Biomedicines 2020, 8, 477. [Google Scholar] [CrossRef]
- Sun, Y.L.; Li, G.; Zheng, Z.; Yao, M.; Cui, J.W.; Liu, S.F.; Zhou, L.Y.; Sng, K.S.; Cui, X.J.; Wang, Y.J. A Neuronal Apoptosis Model induced by Spinal Cord Compression in Rat. J. Vis. Exp. JoVE 2021, 172, e62604. [Google Scholar] [CrossRef]
- Silva, C.M.O. Efeito da Prednisona em Lesão Medular Aguda Experimental em Ratos (Rattus novergicus). Master’s Thesis (Mestrado em Ciência Animal), Escola de Veterinária-Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2016; p. 46f. [Google Scholar]
- Torres, B.B.J. Efeitos do Dantrolene Sódico em Ratos Adultos com Trauma medular Agudo Experimental. Master’s Thesis (Mestrado em Ciência Animal), Escola de Veterinária-Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2008; p. 84f. [Google Scholar]
- Caldeira, F.M.C. Células Tronco Mesenquimais Indiferenciadas no Tratamento do Trauma Medular Espinhal de Ratos Lewis. Ph.D. Thesis (Doutorado em Ciência Animal), Escola de Veterinária-Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2011. [Google Scholar]
- Fukushima, F.B. Avaliação do Efeito Neuroprotetor do Propofol e do Etomidato em Ratos Submetidos ao Trauma Medular Espinhal. Ph.D. Thesis (Doutorado em Ciência Animal), Escola de Veterinária-Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2012; p. 85f. [Google Scholar]
- Oliveira, K.M. Efeitos de Diferentes Doses de ω-Conotoxina MVIIC no Tratamento de Ratos Submetidos ao Trauma Medular Agudo Compressivo. Master’s Thesis (Mestrado em Ciência Animal), Escola de Veterinária-Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2012; p. 61f. [Google Scholar]
- Urbaniak, S.K.; Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine (8-oxodG) and 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) as a Potential Biomarker for Gestational Diabetes Mellitus (GDM) Development. Molecules 2020, 25, 202. [Google Scholar] [CrossRef]
- Varija, D.; Kumar, K.; Reddy, K.; Reddy, V. Prolonged constriction of sciatic nerve affecting oxidative stressors & antioxidant enzymes in rat. Indian J. Med. Res. 2009, 129, 587–592. [Google Scholar]
- Fatima, G.; Sharma, V.P.; Das, S.K.; Mahdi, A.A. Oxidative stress and antioxidative parameters in patients with spinal cord injury: Implications in the pathogenesis of disease. Spinal Cord. 2015, 53, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Patel, S.P.; Vanrooyen, J.L.; Sullivan, P.G.; Rabchevsky, A.G. Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol. 2016, 8, 59–67. [Google Scholar] [CrossRef]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; Derakhshan, P.; et al. Animal models of spinal cord injury: A systematic review. Spinal Cord. 2017, 55, 714–721. [Google Scholar] [CrossRef]
- Bains, M.; Hall, E. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta 2012, 1822, 675–684. [Google Scholar] [CrossRef]
- Rabbani, Z.N.; Spasojevic, I.; Zhang, X.; Moeller, B.J.; Haberle, S.; Vasquez-Vivar, J.; Dewhirst, M.W.; Vujaskovic, Z.; Batinic-Haberle, I. Antiangiogenic action of redox modulating Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin, MnTE-2-PyP(5+), via suppression of oxidative stress in a mouse model of breast tumor. Free Radic. Biol. Med. 2009, 47, 992–1004. [Google Scholar] [CrossRef]
- Gauter-Fleckenstein, B.; Fleckenstein, K.; Owzar, K.; Jiang, C.; Rebouças, J.S.; Batinic-Haberle, I.; Vujaskovic, Z. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic. Biol. Med. 2010, 48, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Drugs: Differential Effects as Cancer Therapeutics and Protectors of Normal Tissue Against Oxidative Injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sidor, A.; O’Rourke, B. Compartment-specific Control of Reactive Oxygen Species Scavenging by Antioxidant Pathway Enzymes. J. Biol. Chem. 2016, 291, 11185–11197. [Google Scholar] [CrossRef]
- Watanabe, K.; Shibuya, S.; Ozawa, Y.; Nojiri, H.; Izuo, N.; Yokote, K.; Shimizu, T. Superoxide dismutase 1 loss disturbs intracellular redox signaling, resulting in global age-related pathological changes. BioMed Res. Int. 2014, 2014, 140165. [Google Scholar] [CrossRef]
- Shrishrimal, S.; Chatterjee, A.; Kosmacek, E.A.; Davis, P.J.; Mcdonald, J.T.; Oberley-Deegan, R.E. Manganese porphyrin, MnTE-2-PyP, treatment protects the prostate from radiation-induced fibrosis (RIF) by activating the NRF2 signaling pathway and enhancing SOD2 and sirtuin activity. Free Radic. Biol. Med. 2020, 152, 255–270. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kosmacek, E.A.; Shrishrimal, S.; Mcdonald, J.T.; Oberley-Deegan, R.E. MnTE-2-PyP, a manganese porphyrin, reduces cytotoxicity caused by irradiation in a diabetic environment through the induction of endogenous antioxidant defenses. Redox Biol. 2020, 34, 101542. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.Y.; Guo, F.; Lu, J.Q.; Cao, Y.Z.; Wang, T.C.; Yang, Q.; Xia, Q. MnTM-4-PyP Modulates Endogenous Antioxidant Responses and Protects Primary Cortical Neurons against Oxidative Stress. CNS Neurosci. Ther. 2015, 21, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hardin, P.E.; Hall, J.C.; Rosbash, M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc. Natl. Acad. Sci. USA 1992, 89, 11711–11715. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Gao, K.; Zhou, Z.; Wang, C.; Shen, Z.; Guo, Y.; Li, Z.; Wan, Z.; Liu, C.; et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience 2017, 348, 241–251. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, D.; Song, C.; Wang, R.; Dong, X. MnTMPyP inhibits paraquat-induced pulmonary epithelial-like cell injury by inhibiting oxidative stress. J. Toxicol. Sci. 2018, 43, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Harding, H.P. Protein-Folding Homeostasis in the Endoplasmic Reticulum an Nutritional Regulation. Cold Spring Harb. Perspect. Biol. 2012, 4, 013177. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Bayr, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef]
- Bolisetty, S.; Jaimes, E. Mitochondria and Reactive Oxygen Species: Physiology and Pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344. [Google Scholar] [CrossRef]
- Penas, C.; Guzmán, M.S.; Verdú, E.; Forés, J.; Navarro, X.; Casas, C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J. Neurochem. 2007, 102, 1242–1255. [Google Scholar] [CrossRef]
- Ohri, S.S.; Maddie, M.A.; Zhao, Y.; Qiu, M.S.; Hetman, M.; Whittemore, S.R. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia 2011, 59, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhang, X.; Wang, Z.G.; Shi, H.X.; Wu, F.Z.; Lin, B.B.; Xu, X.L.; Wang, X.J.; Fu, X.B.; Li, Z.Y.; et al. Exogenous basic fibroblast growth factor inhibits ER stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci. Ther. 2013, 19, 20–29. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.J.; Wu, Y.P.; Chen, W.S. Inflammation & apoptosis in spinal cord injury. Indian J. Med. Res. 2012, 135, 287–296. [Google Scholar]
- Mullen, R.J.; Buck, C.R.; Smith, A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 1992, 116, 201–211. [Google Scholar] [CrossRef]
- Shin, S.W.; Choi, C.; Lee, G.H.; Son, A.; Kim, S.H.; Park, H.C.; Batinic-Haberle, I.; Park, W. Mechanism of the Antitumor and Radiosensitizing Effects of a Manganese Porphyrin, MnHex-2-PyP. Antioxid. Redox Signal. 2017, 27, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Hattori, H.; Sasaki, T.; Gotoh, J.; Hamada, J.; Fukuuchi, Y. Subcellular localization of a promoter and an inhibitor of apoptosis (Smac/DIABLO and XIAP) during brain ischemia/reperfusion. Neuroreport 2002, 13, 1985–1988. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.P.; Singh, A.K.; Siddiqui, M.A.; Kumar, V.; Tripathi, V.K.; Khanna, V.K.; Yadav, S.; Jain, S.K.; Pant, A.B. Caspase cascade regulated mitochondria mediated apoptosis in monocrotophos exposed PC12 cells. Chem. Res. Toxicol. 2010, 23, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signaling pathway. Nat. Rev. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Wong, I.; Liao, H.; Bai, X.; Zaknic, A.; Zhong, J.; Guan, Y.; Li, H.Y.; Wang, Y.J.; Zhou, X.F. ProBDNF inhibits infiltration of ED1+ macrophages after spinal cord injury. Brain Behav. Immun. 2010, 24, 585–597. [Google Scholar] [CrossRef]



| Gene | Sequence (5->3) | Access No. |
| Grp78 | Forward: TGAAGGGGAGCGTCTGATTG Reverse: TCATTCCAAGTGCGTCCGAT | NM_013083.2 |
| Chop | Forward: TGGCACAGCTTGCTGAAGAG Reverse: TCAGGCGCTCGATTTCCT | NM_001109986.1 |
| Perk | Forward: GGCTGGTGAGGGATGGTAAA Reverse: TTGGCTGTGTAACTTGTGTCATC | NM_031599.2 |
| Ho1 | Forward: CAGCATACGTAAAGCGTCTCCA Reverse:CATGGCCTTCTGCGCAATCTTCTT | NM_012580.2 |
| Hifα | Forward: AGCAATTCTCCAAGCCCTCC Reverse: TTCATCAGTGGTGGCAGTTG | NM_024359.1 |
| Nrf2 | Forward: CCCATTGAGGGCTGTGATCT Reverse: GCCTTCAGTGTGCTTCTGGTT | NM_031789.2 |
| Catalase | Forward: CTGACTGACGCGATTGCCTA Reverse: GTGGTCAGGACATCGGGTTT | NM_012520.2 |
| Gpx1 | Forward: GCGCTACAGCGGATTTTTGA Reverse: GAAGGCATACACGGTGGACT | NM_030826.4 |
| Sod1 | Forward: GAAAGGACGGTGTGGCCAAT Reverse: CTCGTGGACCACCATAGTACGT | NM_017050.1 |
| Gdnf | Forward: CAAGGTAGGCCAGGCATGTT Reverse: CACACCGTTTAGCGGAAT | NM_001401780.1 |
| Ngf | Forward: CCTGGAGCCGAAGGGGA Reverse: CACTGAGGTGGAGCTTGGGTC | NM_001277055.1 |
| Caps3 | Forward: GAGCTTGGAACGCGAAGAAA Reverse: AGTCCATCGACTTGCTTCCA | NM_012922.2 |
| Casp9 | Forward: TCCCCACTGATCAAGTCTCCT Reverse: CCAGGCTCACTTAGCAAGGAA | NM_031632.2 |
| Gapdh | Forward: GCGCTACAGCGGATTTTTGA Reverse: GAAGGCATACACGGTGGACT | NM_031797.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niella, R.V.; Corrêa, J.M.X.; Marques, C.S.d.C.; Silva, Á.J.C.; Santos, L.C.; Oliveira, I.S.d.; DeFreitas-Silva, G.; Rebouças, J.S.; Silva, J.F.; de Lavor, M.S.L. Manganese Porphyrin Treatment Improves Redox Status Caused by Acute Compressive Spinal Cord Trauma. Antioxidants 2025, 14, 587. https://doi.org/10.3390/antiox14050587
Niella RV, Corrêa JMX, Marques CSdC, Silva ÁJC, Santos LC, Oliveira ISd, DeFreitas-Silva G, Rebouças JS, Silva JF, de Lavor MSL. Manganese Porphyrin Treatment Improves Redox Status Caused by Acute Compressive Spinal Cord Trauma. Antioxidants. 2025; 14(5):587. https://doi.org/10.3390/antiox14050587
Chicago/Turabian StyleNiella, Raquel Vieira, Janaína Maria Xavier Corrêa, Claire Souza da Costa Marques, Álvaro José Chávez Silva, Luciano Cardoso Santos, Iago Santos de Oliveira, Gilson DeFreitas-Silva, Júlio Santos Rebouças, Juneo Freitas Silva, and Mário Sérgio Lima de Lavor. 2025. "Manganese Porphyrin Treatment Improves Redox Status Caused by Acute Compressive Spinal Cord Trauma" Antioxidants 14, no. 5: 587. https://doi.org/10.3390/antiox14050587
APA StyleNiella, R. V., Corrêa, J. M. X., Marques, C. S. d. C., Silva, Á. J. C., Santos, L. C., Oliveira, I. S. d., DeFreitas-Silva, G., Rebouças, J. S., Silva, J. F., & de Lavor, M. S. L. (2025). Manganese Porphyrin Treatment Improves Redox Status Caused by Acute Compressive Spinal Cord Trauma. Antioxidants, 14(5), 587. https://doi.org/10.3390/antiox14050587







