Tea Polyphenols Mitigate Radiation-Induced Ferroptosis and Intestinal Injury by Targeting the Nrf2/HO-1/GPX4 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. Animals
2.3. UPLC-Q-TOF/MS Conditions for Ingredient Identification in TP
2.4. Cell Viability Assay and Radiation Source
2.5. Calcein AM Staining
2.6. Oxidative Stress Assay
2.7. Transmission Electron Microscopy (TEM)
2.8. Flow Cytometry for Detecting ROS and Fe2+
2.9. Immunofluorescence
2.10. Western Blotting
2.11. Co-Immunoprecipitation (Co-IP)
2.12. Transfection
2.13. Molecular Docking
2.14. Biolayer Interference Analysis
2.15. Analysis of TP’s Active Components and Their Corresponding Targets
2.16. Construction of the ’Active Ingredient–Target’ Network and Enrichment Analyses
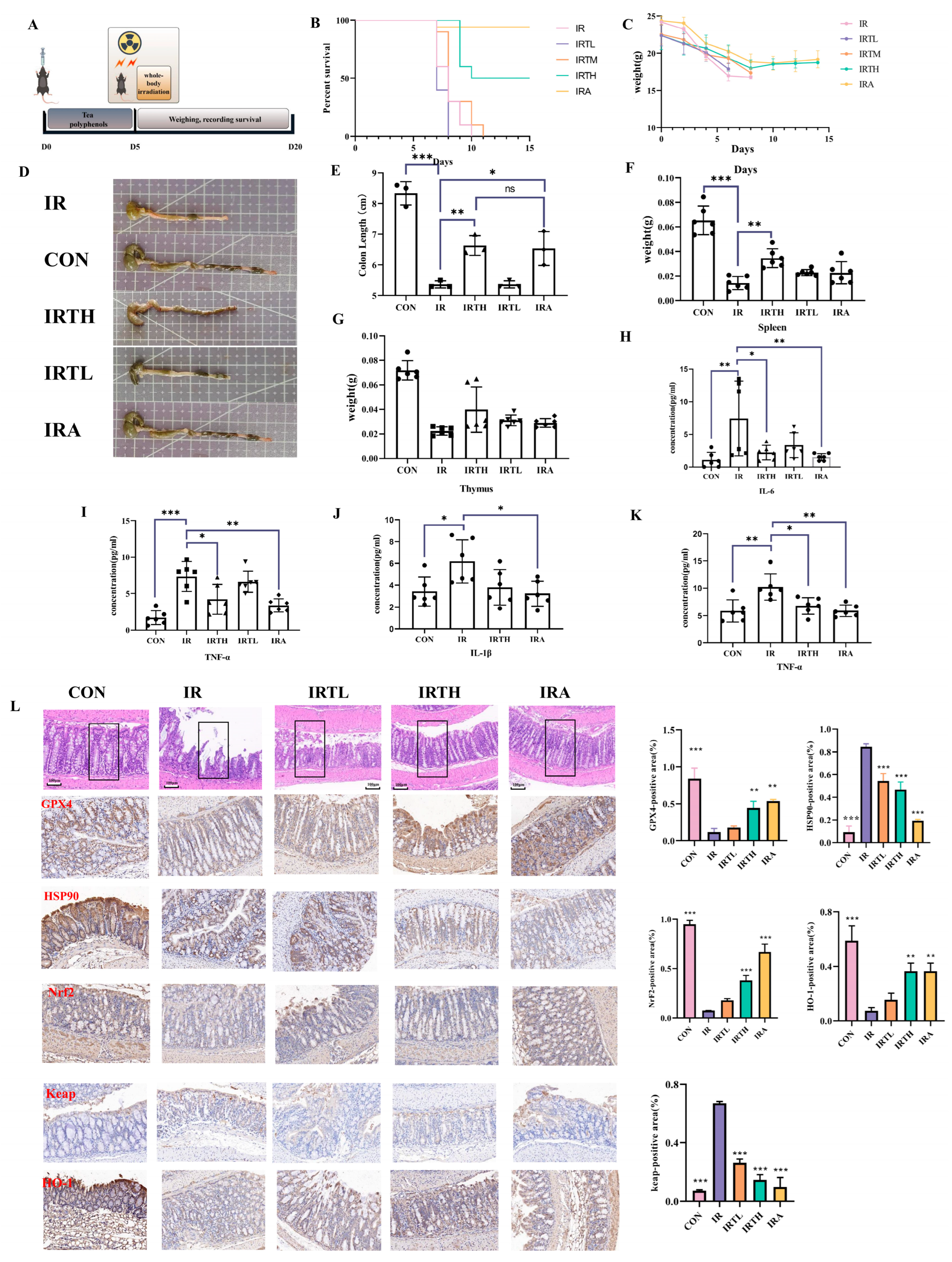
2.17. Animal Grouping and Irradiation
2.18. Hematoxylin–Eosin (HE) Staining and Immunohistochemistry (IHC)
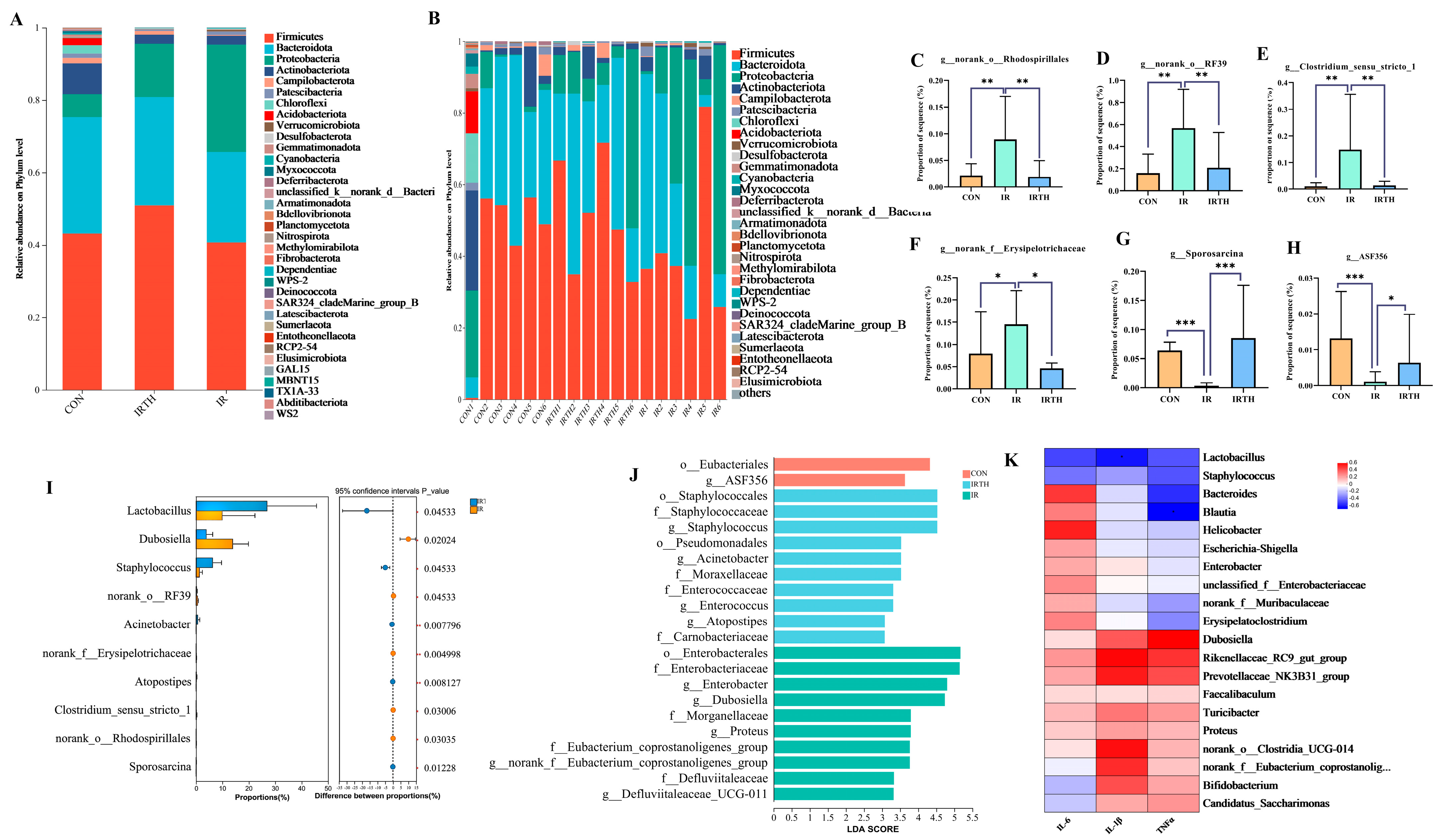
2.19. Fecal Sample Collection and Analysis Through 16S rRNA Gene Sequencing
2.20. Non-Targeted Metabolomics Determination of Mouse Serum
2.21. Measurement of Cytokine Levels
2.22. Statistical Analysis
3. Results
3.1. Components Identifying in TP
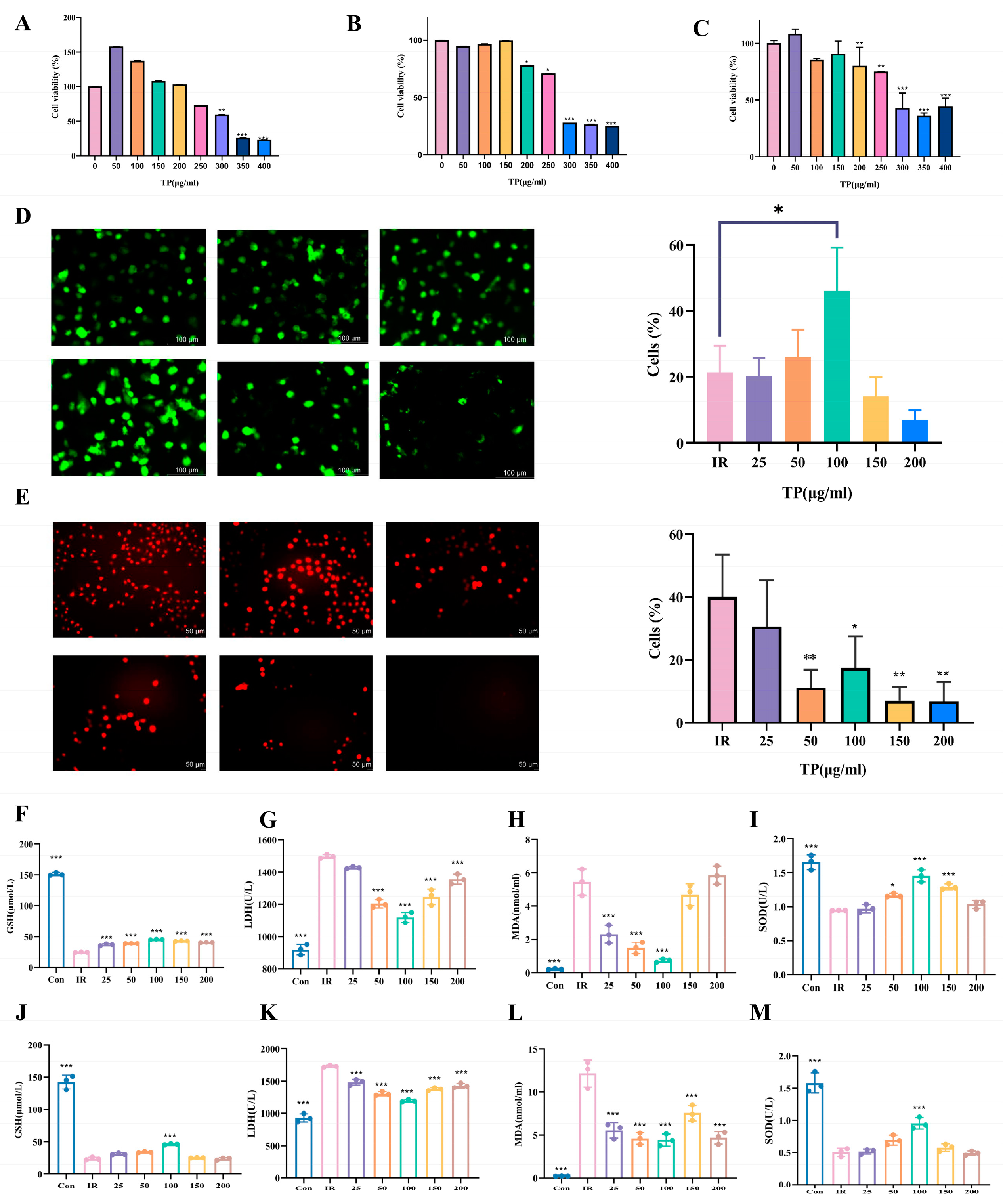
3.2. TP Markedly Enhanced HIEC-6 Cell Proliferation and Mitigated Oxidative Damage Following IR Exposure
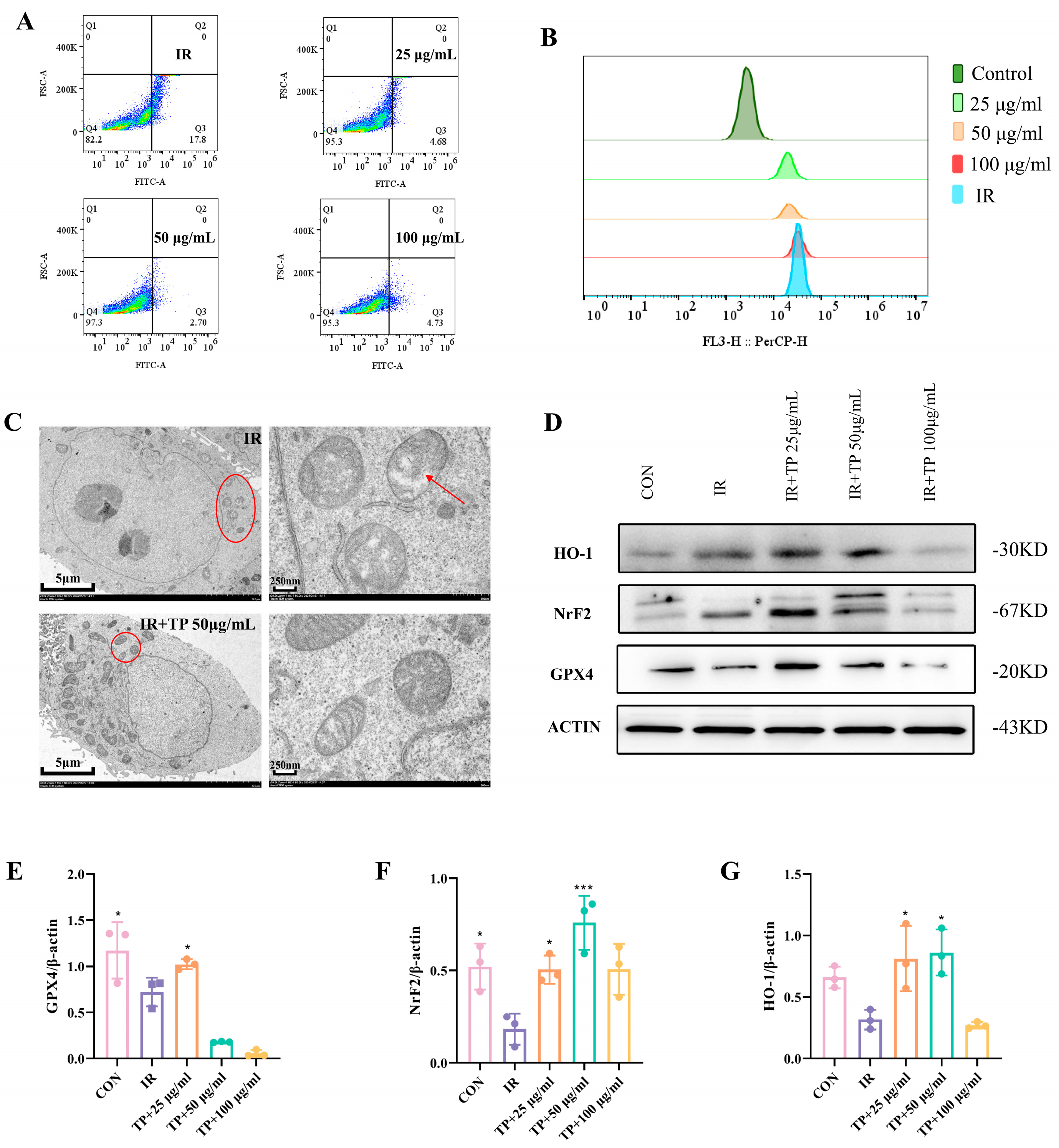
3.3. TP Reversed the Irradiation-Induced Ferroptosis of ICE-6
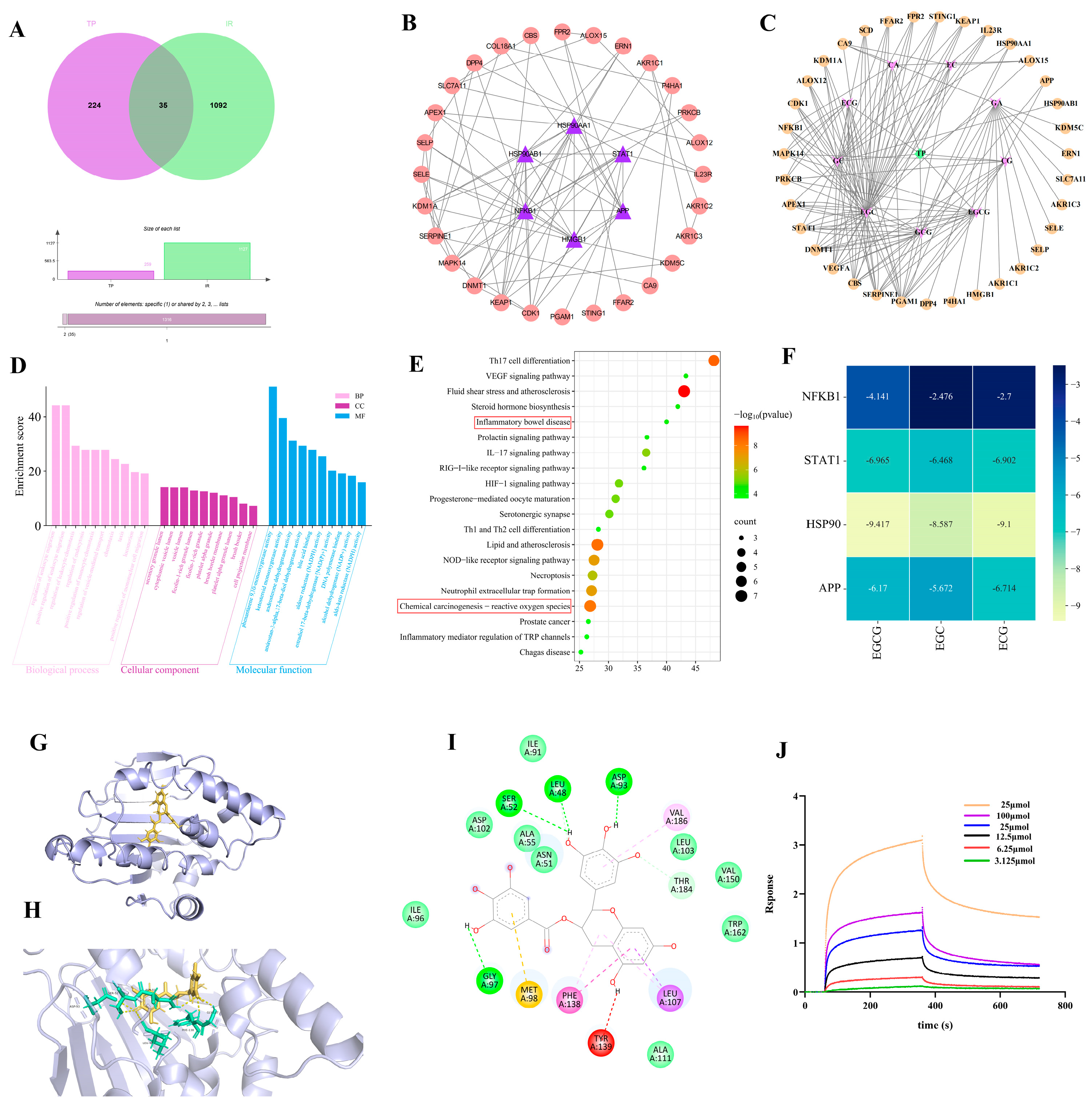
3.4. Network Pharmacological Target Prediction
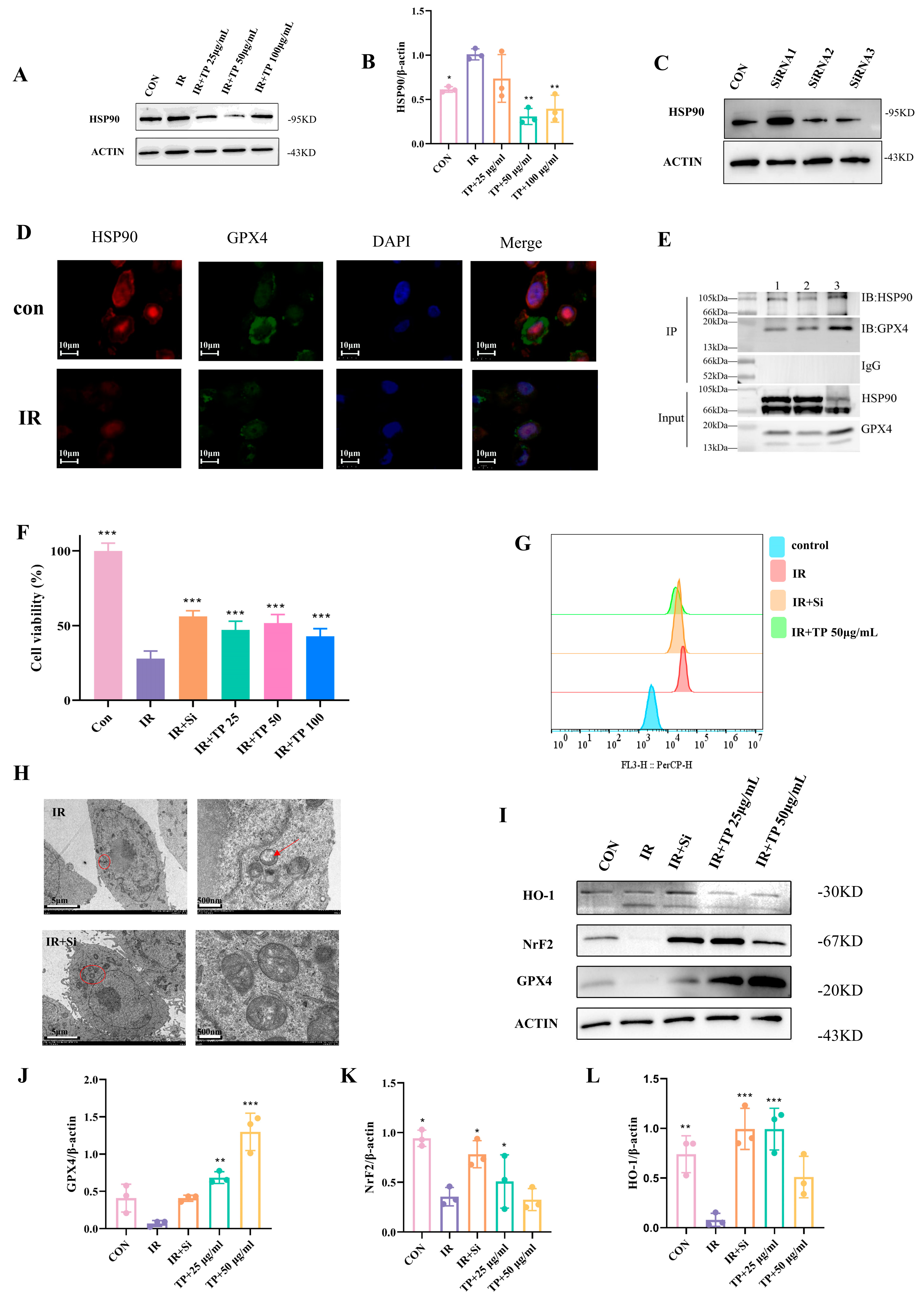
3.5. TP Reduce Ferroptosis in IEC-6 Cells Post-Radiation by Targeting HSP90
3.6. TP Alleviated Radiation-Induced Intestinal Damage in Mice
3.7. Effects of TP on Gut Microbiota in Radiation-Exposed Mice
3.8. Gut Microbiota Community Analysis
3.9. Serum UPLC-MS Untargeted Metabolomics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronyms | Definition | Acronyms | Definition |
| RIII | Radiation-induced intestinal injury | HO-1 | Heme oxygenase 1 |
| TP | Tea polyphenols | HE | Hematoxylin–eosin |
| BLI | Biolayer interference | IHC | Immunohistochemistry |
| HSP90 | Heat Shock Protein 90 | UPLC-Q-TOF/MS | Ultra-performance liquid chromatography quadrupole-time-flight mass spectrometry |
| GPX4 | Glutathione peroxidase 4 | PCoA | Principal Coordinates Analysis |
| IR | Ionizing radiation | PCA | Principal Component Analysis |
| ROS | Reactive oxygen species | LEfSe | Linear discriminant analysis effect size |
| FDA | Food and Drug Administration | PLSDA | Partial least-squares discriminant analysis |
| GSH | Glutathione | TNF-α | Tumor necrosis factor- α |
| MDA | Malondialdehyde | IL-1β | Interleukin-1 |
| SOD | Superoxide dismutase | IL-6 | Interleukin-6 |
| LDH | Lactate dehydrogenase | TCM | Traditional Chinese Medicine |
| EGC | Epigallocatechin | DCFH-DA | Dichlorofluorescin diacetate |
| EC | Epicatech | Keap1 | Kelch-like ECH-associated protein 1 |
| ECG | Epicatechin gallate | TCM | Traditional Chinese Medicines |
| EGCG | Epigallocatechin gallate | KEGG | Kyoto Encyclopedia of Genes and Genomes |
| Nrf2 | Nuclear respiratory factor 2 | LDA | Linear discriminant analysis |
References
- Jian, Y.; Zhang, D.; Liu, M.; Wang, Y.; Xu, Z.X. The Impact of Gut Microbiota on Radiation-Induced Enteritis. Front. Cell. Infect. Microbiol. 2021, 11, 586392. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.Y.; Wang, J.; Ding, Q.Q.; Chen, W.; Xu, X.K.; Wei, X.T.; Lv, Y.H.; Wei, Y.P.; Feng, Y.; Zu, X.P. Potential role of gut microbiota and its metabolites in radiation-induced intestinal damage. Ecotoxicol. Environ. Saf. 2022, 248, 114341. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, H.; Sun, W.; Wang, H.; Wang, H. Radiation-Induced Intestinal Injury: Molecular Mechanisms and Therapeutic Status. DNA Cell Biol. 2024, 43, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Venkidesh, B.S.; Mumbrekar, K.D. Bacterial supplementation in mitigation of radiation-induced gastrointestinal damage. Life Sci. 2024, 353, 122921. [Google Scholar] [CrossRef]
- Su, J.; Bian, C.; Zheng, Z.; Wang, H.; Meng, L.; Xin, Y.; Jiang, X. Cooperation effects of radiation and ferroptosis on tumor suppression and radiation injury. Front. Cell Dev. Biol. 2022, 10, 951116. [Google Scholar] [CrossRef]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J. Radiation enteropathy—Pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Leng, J.; Tan, J.; Zhao, Y.; Xie, S.; Zhao, S.; Yan, X.; Zhu, L.; Luo, J.; Kong, L.; et al. Discovery of Novel Potent Covalent Glutathione Peroxidase 4 Inhibitors as Highly Selective Ferroptosis Inducers for the Treatment of Triple-Negative Breast Cancer. J. Med. Chem. 2023, 66, 10036–10059. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.D.; Ma, M.Q.; Liang, Y.; Cai, Y.B.; Zhu, Z.X.; Xu, T.; Zhu, L.; Ren, K. The mechanisms of ferroptosis and its role in atherosclerosis. Biomed. Pharmacother. = Biomed. Pharmacother. 2024, 171, 116112. [Google Scholar] [CrossRef]
- Liang, D.; Feng, Y.; Zandkarimi, F.; Wang, H.; Zhang, Z.; Kim, J.; Cai, Y.; Gu, W.; Stockwell, B.R.; Jiang, X. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 2023, 186, 2748–2764.e22. [Google Scholar] [CrossRef]
- Xu, C.; Wu, M.; Yu, W.; Xie, D.; Wang, Q.; Chen, B.; Xi, Y.; Yu, L.; Yan, Y.; Yamamoto, T.; et al. High Uric Acid Orchestrates Ferroptosis to Promote Cardiomyopathy Via ROS-GPX4 Signaling. Antioxid. Redox Signal. 2024, 41, 1134–1149. [Google Scholar] [CrossRef]
- Chen, C.; Wang, D.; Yu, Y.; Zhao, T.; Min, N.; Wu, Y.; Kang, L.; Zhao, Y.; Du, L.; Zhang, M.; et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021, 12, 65. [Google Scholar] [CrossRef]
- Elsahly, N. Effect of radiotherapy on the gut microbiome in pediatric cancer patients: A pilot study. PeerJ 2020, 7, e7683. [Google Scholar]
- Zha, X.; Liu, X.; Wei, M.; Huang, H.; Cao, J.; Liu, S.; Bian, X.; Zhang, Y.; Xiao, F.; Xie, Y.; et al. Microbiota-derived lysophosphatidylcholine alleviates Alzheimer’s disease pathology via suppressing ferroptosis. Cell Metab. 2025, 37, 169–186.e9. [Google Scholar] [CrossRef]
- Trisha, A.T.; Shakil, M.H.; Talukdar, S.; Rovina, K.; Huda, N.; Zzaman, W. Tea Polyphenols and Their Preventive Measures against Cancer: Current Trends and Directions. Foods 2022, 11, 3349. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, L.; Han, P.; Zhang, H.; Wang, F.; Meng, Z.; Gan, H.; Wu, Z.; Sun, W.; Chen, C.; et al. Blocking TRAIL-DR5 signaling pathway with soluble death receptor 5 fusion protein mitigates radiation-induced injury. Front. Pharmacol. 2023, 14, 1171293. [Google Scholar] [CrossRef]
- Sun, H.; Yang, L.; Li, M.X.; Fang, H.; Zhang, A.H.; Song, Q.; Liu, X.Y.; Su, J.; Yu, M.D.; Makino, T.; et al. UPLC-G2Si-HDMS untargeted metabolomics for identification of metabolic targets of Yin-Chen-Hao-Tang used as a therapeutic agent of dampness-heat jaundice syndrome. J. Chromatogr. B 2018, 1081–1082, 41–50. [Google Scholar] [CrossRef]
- Tian, X.; Guo, J.; Gu, C.; Wang, H.; Wang, D.; Liao, Y.; Zhu, S.; Zhao, M.; Gu, Z. Ergothioneine-Sodium Hyaluronate Dressing: A Promising Approach for Protecting against Radiation-Induced Skin Injury. ACS Appl. Mater. Interfaces 2024, 16, 29917–29929. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Yang, M.; Wang, Y.; Yu, K.N.; Wu, L.; Han, W. Ferroptosis triggered by STAT1- IRF1-ACSL4 pathway was involved in radiation-induced intestinal injury. Redox Biol. 2023, 66, 102857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zheng, C.; Yang, C.; Zhang, R.; Wang, A.; Feng, J.; Hu, X.; Chang, S.; Zhang, H. Ferroptosis, a new form of cell death defined after radiation exposure. Int. J. Radiat. Biol. 2022, 98, 1201–1209. [Google Scholar] [CrossRef]
- Wang, X.; Wei, T.; Luo, J.; Lang, K.; Song, Y.; Ning, X.; Chao, Y.; Gu, Z.; Wang, L.; Chen, C.; et al. Iron Overload-Dependent Ferroptosis Aggravates LPS-Induced Acute Lung Injury by Impairing Mitochondrial Function. Inflammation 2024, 47, 2013–2026. [Google Scholar] [CrossRef]
- Mao, B.; Guo, W.; Cui, S.; Zhang, Q.; Zhao, J.; Tang, X.; Zhang, H.J.F.S.; Wellness, H. Blautia producta displays potential probiotic properties against dextran sulfate sodium-induced colitis in mice. Food Sci. Hum. Wellness 2024, 13, 709–720. [Google Scholar] [CrossRef]
- Jahng, J.W.S.; Little, M.P.; No, H.J.; Loo, B.W., Jr.; Wu, J.C. Consequences of ionizing radiation exposure to the cardiovascular system. Nat. Rev. Cardiol. 2024, 21, 880–898. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Cui, P.; Zhou, S.; Qiu, L.; Huang, H.; Wang, C.; Wang, J. Advances of Amifostine in Radiation Protection: Administration and Delivery. Mol. Pharm. 2023, 20, 5383–5395. [Google Scholar] [CrossRef]
- Lyamzaev, K.G.; Panteleeva, A.A.; Simonyan, R.A.; Avetisyan, A.V.; Chernyak, B.V. The critical role of mitochondrial lipid peroxidation in ferroptosis: Insights from recent studies. Biophys. Rev. 2023, 15, 875–885. [Google Scholar] [CrossRef]
- Yang, P.; Li, J.; Zhang, T.; Ren, Y.; Zhang, Q.; Liu, R.; Li, H.; Hua, J.; Wang, W.A.; Wang, J.; et al. Ionizing radiation-induced mitophagy promotes ferroptosis by increasing intracellular free fatty acids. Cell Death Differ. 2023, 30, 2432–2445. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hu, W.; Qian, D.; Bai, X.; He, H.; Li, L.; Sun, S. The Mechanisms of Ferroptosis Under Hypoxia. Cell. Mol. Neurobiol. 2023, 43, 3329–3341. [Google Scholar] [CrossRef]
- Begum, N.; Rajendra Prasad, N.; Kanimozhi, G.; Agilan, B. Apigenin prevents gamma radiation-induced gastrointestinal damages by modulating inflammatory and apoptotic signalling mediators. Nat. Prod. Res. 2022, 36, 1631–1635. [Google Scholar] [CrossRef]
- Babini, G.; Ugolini, M.; Morini, J.; Baiocco, G.; Mariotti, L.; de Fatis, P.T.; Liotta, M.; Ottolenghi, A. Investigation of radiation-induced multilayered signalling response of the inflammatory pathway. Radiat. Prot. Dosim. 2015, 166, 157–160. [Google Scholar] [CrossRef]
- Ning, X.; Zhao, W.; Wu, Q.; Wang, C.; Liang, S. Therapeutic potential of dihydroartemisinin in mitigating radiation-induced lung injury: Inhibition of ferroptosis through Nrf2/HO-1 pathways in mice. Immun. Inflamm. Dis. 2024, 12, e1175. [Google Scholar] [CrossRef]
- Fu, H.; He, J.; Li, C.; Chang, H. Theaflavin-3,3’-Digallate Protects Liver and Kidney Functions in Diabetic Rats by Up-Regulating Circ-ITCH and Nrf2 Signaling Pathway. J. Agric. Food Chem. 2024, 72, 14630–14639. [Google Scholar] [CrossRef]
- Huang, Q.; Ren, Y.; Yuan, P.; Huang, M.; Liu, G.; Shi, Y.; Jia, G.; Chen, M. Targeting the AMPK/Nrf2 Pathway: A Novel Therapeutic Approach for Acute Lung Injury. J. Inflamm. Res. 2024, 17, 4683–4700. [Google Scholar] [CrossRef] [PubMed]
- Sethi, P.; Mehan, S.; Khan, Z.; Maurya, P.K.; Kumar, N.; Kumar, A.; Tiwari, A.; Sharma, T.; Das Gupta, G.; Narula, A.S.; et al. The SIRT-1/Nrf2/HO-1 axis: Guardians of neuronal health in neurological disorders. Behav. Brain Res. 2025, 476, 115280. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Yang, G.; Huang, H.; Zhou, Y.; Hu, X.; Lu, Q.; Guo, P.; Hou, J.; Cao, L.; Tian, F.; et al. Ubiquitin-Like Modifier Activating Enzyme 1 as a Novel Diagnostic and Prognostic Indicator That Correlates with Ferroptosis and the Malignant Phenotypes of Liver Cancer Cells. Front. Oncol. 2020, 10, 592413. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhao, F.; Li, H.; Li, L.; Yang, Y.; Liu, F. HSP90 mediates the connection of multiple programmed cell death in diseases. Cell Death Dis. 2022, 13, 929. [Google Scholar] [CrossRef]
- Wu, Z.; Geng, Y.; Lu, X.; Shi, Y.; Wu, G.; Zhang, M.; Shan, B.; Pan, H.; Yuan, J. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl. Acad. Sci. USA 2019, 116, 2996–3005. [Google Scholar] [CrossRef]
- Cheng, L.; Zhu, M.; Xu, X.; Li, X.; Yao, Y.; Liu, C.; He, K. AMPD3 promotes doxorubicin-induced cardiomyopathy through HSP90α-mediated ferroptosis. iScience 2024, 27, 111005. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Liu, R.; Zhao, G. HSP90 Inhibition Attenuated Isoflurane-Induced Neurotoxicity in Mice and Human Neuroglioma Cells. Neurochem. Res. 2024, 49, 706–717. [Google Scholar] [CrossRef]
- Aceros, H.; Der Sarkissian, S.; Borie, M.; Stevens, L.M.; Mansour, S.; Noiseux, N. Celastrol-type HSP90 modulators allow for potent cardioprotective effects. Life Sci. 2019, 227, 8–19. [Google Scholar] [CrossRef]
- Wen, J.J.; Li, M.Z.; Chen, C.H.; Hong, T.; Yang, J.R.; Huang, X.J.; Geng, F.; Hu, J.L.; Nie, S.P. Tea polyphenol and epigallocatechin gallate ameliorate hyperlipidemia via regulating liver metabolism and remodeling gut microbiota. Food Chem. 2023, 404 Pt A, 134591. [Google Scholar] [CrossRef]
- Prysyazhna, O.; Wolhuter, K.; Switzer, C.; Santos, C.; Yang, X.; Lynham, S.; Shah, A.M.; Eaton, P.; Burgoyne, J.R. Blood Pressure-Lowering by the Antioxidant Resveratrol Is Counterintuitively Mediated by Oxidation of cGMP-Dependent Protein Kinase. Circulation 2019, 140, 126–137. [Google Scholar] [CrossRef]
- Purbey, P.K.; Scumpia, P.O.; Kim, P.J.; Tong, A.J.; Iwamoto, K.S.; McBride, W.H.; Smale, S.T. Defined Sensing Mechanisms and Signaling Pathways Contribute to the Global Inflammatory Gene Expression Output Elicited by Ionizing Radiation. Immunity 2017, 47, 421–434.e3. [Google Scholar] [CrossRef]
- Moraitis, I.; Guiu, J.; Rubert, J. Gut microbiota controlling radiation-induced enteritis and intestinal regeneration. Trends Endocrinol. Metab. TEM 2023, 34, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, D.Y.; Zhong, Q.; Tan, F.; Li, W.; Liao, W.; Zhao, X. Lactobacillus fermentum HFY06 reduced CCl(4)-induced hepatic damage in Kunming mice. RSC Adv. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic Effects of Inflammation on Vitamin A and Carotenoids in Humans and Animal Models. Adv. Nutr. 2017, 8, 197–212. [Google Scholar] [CrossRef]
- Geuking, M.B.; Köller, Y.; Rupp, S.; McCoy, K.D. The interplay between the gut microbiota and the immune system. Gut Microbes 2014, 5, 411–418. [Google Scholar] [CrossRef]


| No. | tR (min) | MS 1 (m/z) | Formula | Error (ppm) | MS 2 (m/z) | Compounds |
|---|---|---|---|---|---|---|
| 1 | 1.33 | 305.06652 [M-H]− | C15H14O7 | −2.3 | 109,125,137,169,177,289 | Epigallocatehin |
| 2 | 0.65 | 305.06597 [M-H]− | C15H14O7 | −0.5 | 125,269 | Gallocatehin |
| 3 | 2.13 | 289.07148 [M-H]− | C15H14O6 | −1 | 124,125,137,271 | Cianidanol |
| 4 | 0.85 | 289.07088 [M-H]− | C15H14O6 | −3 | 109,123,151 | Epicatehin |
| 5 | 2.7 | 273.07625 [M-H]− | C15H14O5 | −2.2 | 125,137,255 | Epiafzelechin |
| 6 | 1.33 | 457.07745 [M-H]− | C22H18O11 | −3.2 | 125,169,305,331,413 | Epigallocatehin gallate |
| 7 | 0.81 | 441.08234 [M-H]− | C22H18O10 | −0.9 | 124,125,145,169,289 | Catechin gallate |
| 8 | 0.69 | 441.08222 [M-H]− | C22H18O10 | −1.1 | 169,287 | Epicatehin gallate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Li, L.; Wu, H.; Gan, H.; Wu, Z.; Gu, R.; Zhu, X.; Liu, S.; Meng, Z.; Dou, G. Tea Polyphenols Mitigate Radiation-Induced Ferroptosis and Intestinal Injury by Targeting the Nrf2/HO-1/GPX4 Signaling Pathway. Antioxidants 2025, 14, 580. https://doi.org/10.3390/antiox14050580
Li R, Li L, Wu H, Gan H, Wu Z, Gu R, Zhu X, Liu S, Meng Z, Dou G. Tea Polyphenols Mitigate Radiation-Induced Ferroptosis and Intestinal Injury by Targeting the Nrf2/HO-1/GPX4 Signaling Pathway. Antioxidants. 2025; 14(5):580. https://doi.org/10.3390/antiox14050580
Chicago/Turabian StyleLi, Runtian, Lintao Li, Haiyang Wu, Hui Gan, Zhuona Wu, Ruolan Gu, Xiaoxia Zhu, Shuchen Liu, Zhiyun Meng, and Guifang Dou. 2025. "Tea Polyphenols Mitigate Radiation-Induced Ferroptosis and Intestinal Injury by Targeting the Nrf2/HO-1/GPX4 Signaling Pathway" Antioxidants 14, no. 5: 580. https://doi.org/10.3390/antiox14050580
APA StyleLi, R., Li, L., Wu, H., Gan, H., Wu, Z., Gu, R., Zhu, X., Liu, S., Meng, Z., & Dou, G. (2025). Tea Polyphenols Mitigate Radiation-Induced Ferroptosis and Intestinal Injury by Targeting the Nrf2/HO-1/GPX4 Signaling Pathway. Antioxidants, 14(5), 580. https://doi.org/10.3390/antiox14050580






