Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation
Abstract
1. Introduction
2. Materials and Methods
3. Results
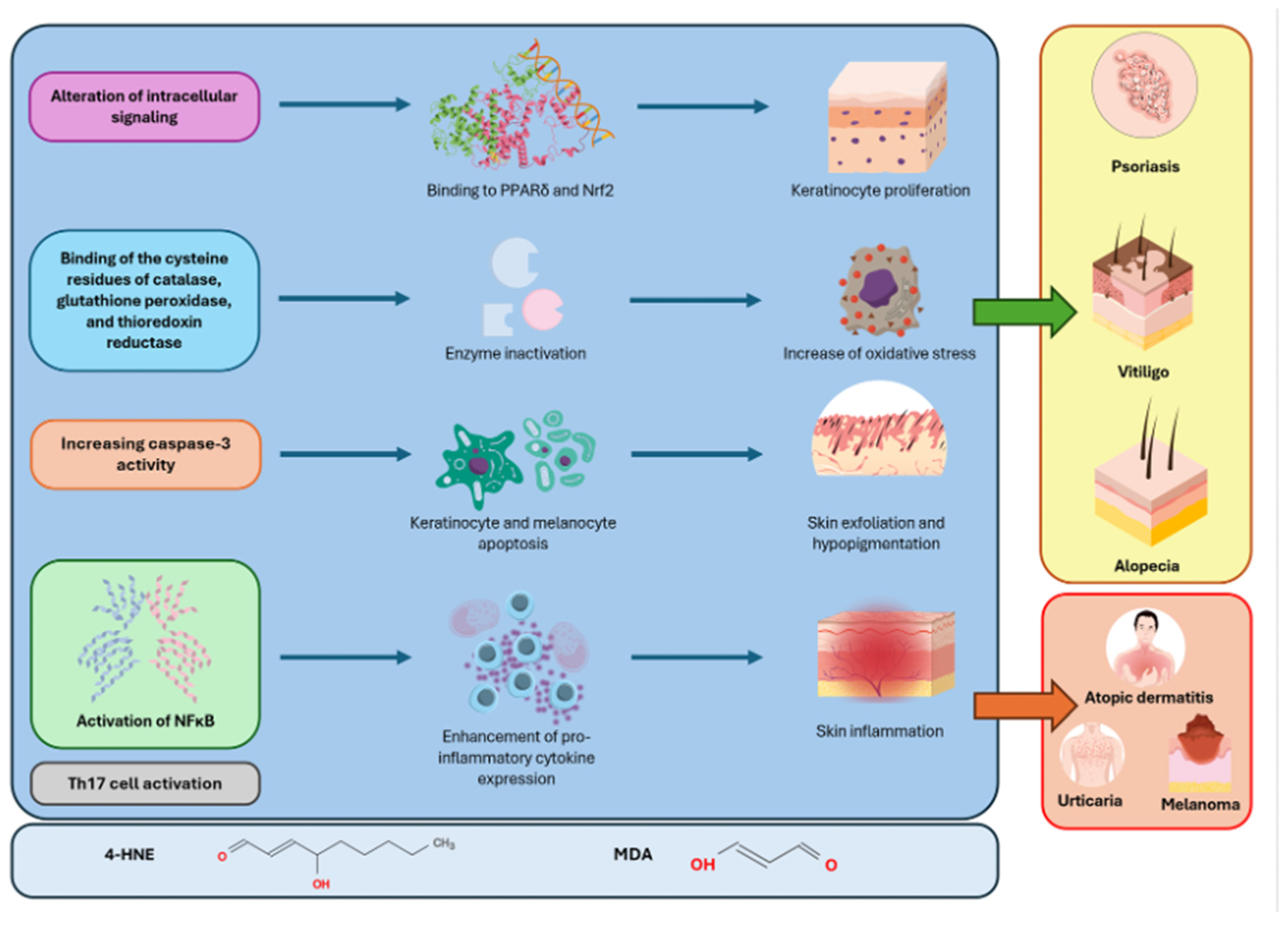
3.1. Psoriasis
3.2. Atopic Dermatitis
3.3. Urticaria
3.4. Vitiligo
3.5. Alopecia Areata
3.6. Pemphigus
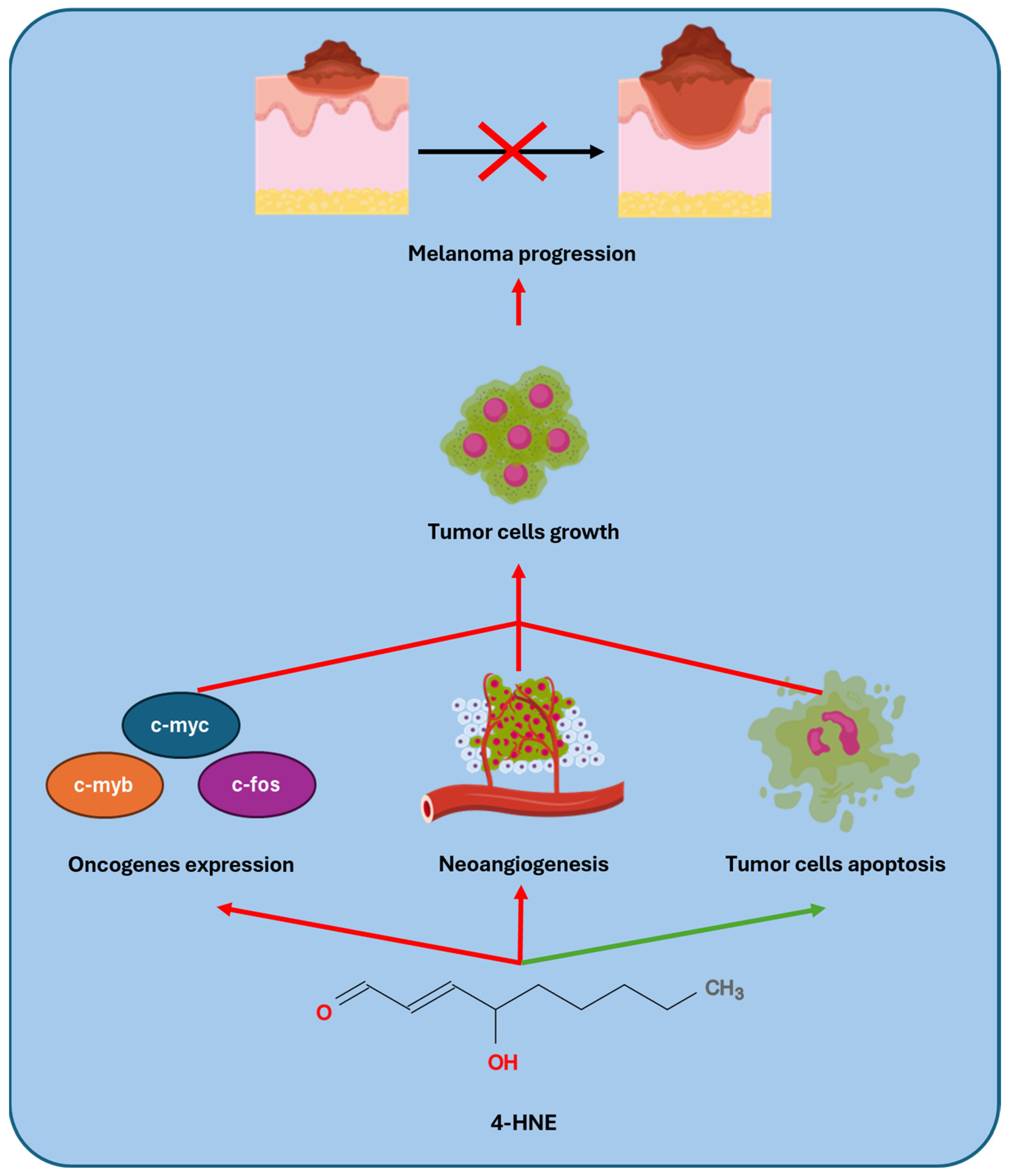
3.7. Melanoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danieli, M.G.; Antonelli, E.; Piga, M.A.; Cozzi, M.F.; Allegra, A.; Gangemi, S. Oxidative Stress, Mitochondrial Dysfunction, and Respiratory Chain Enzyme Defects in Inflammatory Myopathies. Autoimmun. Rev. 2023, 22, 103308. [Google Scholar] [CrossRef] [PubMed]
- Catalá, A.; Díaz, M. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front. Physiol. 2016, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Turini, M.E.; DuBois, R.N. Cyclooxygenase-2: A Therapeutic Target. Annu. Rev. Med. 2002, 53, 35–57. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Pope, L.E.; Dixon, S.J. Regulation of Ferroptosis by Lipid Metabolism. Trends Cell Biol. 2023, 33, 1077–1087. [Google Scholar] [CrossRef]
- Repetto, M.; Semprine, J.; Boveris, A.; Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. In Lipid Peroxidation; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H.; Dianzani, M.U.; Poli, G.; Slater, T.F. Separation and Characterization of the Aldehydic Products of Lipid Peroxidation Stimulated by ADP-Fe2+ in Rat Liver Microsomes. Biochem. J. 1982, 208, 129–140. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A Review of Recent Studies on Malondialdehyde as Toxic Molecule and Biological Marker of Oxidative Stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible Mutagens Derived from Lipids and Lipid Precursors. Mutat. Res. 1990, 238, 223–233. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 Promotes Ferroptosis of Astrocytes by Oxidative Stress-Induced Lipid Peroxidation via the Impairment of Mitochondrial Metabolism in Alzheimer’s Diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Pan, M.H.; Sun, J.; Wang, M.; Huang, Z.H.; Wang, G.; Wang, R.; Gong, H.B.; Huang, R.T.; Huang, F.; et al. Membrane Phospholipid Peroxidation Promotes Loss of Dopaminergic Neurons in Psychological Stress-Induced Parkinson’s Disease Susceptibility. Aging Cell 2023, 22, e13970. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological Markers of Oxidative Stress: Applications to Cardiovascular Research and Practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Jaganjac, M.; Tirosh, O.; Cohen, G.; Sasson, S.; Zarkovic, N. Reactive Aldehydes—Second Messengers of Free Radicals in Diabetes Mellitus. Free Radic. Res. 2013, 47 (Suppl. 1), 39–48. [Google Scholar] [CrossRef]
- Merendino, R.A.; Salvo, F.; Saija, A.; Di Pasquale, G.; Tomaino, A.; Minciullo, P.L.; Fraccica, G.; Gangemi, S. Malondialdehyde in Benign Prostate Hypertrophy: A Useful Marker? Mediat. Inflamm. 2003, 12, 127–128. [Google Scholar] [CrossRef]
- Morabito, F.; Cristani, M.; Saija, A.; Stelitano, C.; Callea, V.; Tomaino, A.; Minciullo, P.L.; Gangemi, S. Lipid Peroxidation and Protein Oxidation in Patients Affected by Hodgkin’s Lymphoma. Mediat. Inflamm. 2004, 13, 381–383. [Google Scholar] [CrossRef]
- Xiao, L.; Xian, M.; Zhang, C.; Guo, Q.; Yi, Q. Lipid Peroxidation of Immune Cells in Cancer. Front. Immunol. 2024, 14, 1322746. [Google Scholar] [CrossRef]
- Woodby, B.; Penta, K.; Pecorelli, A.; Lila, M.A.; Valacchi, G. Skin Health from the Inside Out. Annu. Rev. Food Sci. Technol. 2020, 11, 235–254. [Google Scholar] [CrossRef]
- Danieli, M.G.; Casciaro, M.; Paladini, A.; Bartolucci, M.; Sordoni, M.; Shoenfeld, Y.; Gangemi, S. Exposome: Epigenetics and Autoimmune Diseases. Autoimmun. Rev. 2024, 23, 103584. [Google Scholar] [CrossRef]
- Ron-Doitch, S.; Kohen, R. The Cutaneous Physiological Redox: Essential to Maintain but Difficult to Define. Antioxidants 2020, 9, 942. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.; Wieczorek, T.; Drabińska, N.; Gould, O.; Osborne, A.; De Lacy Costello, B. A Mechanistic Study and Review of Volatile Products from Peroxidation of Unsaturated Fatty Acids: An Aid to Understanding the Origins of Volatile Organic Compounds from the Human Body. J. Breath Res. 2020, 14, 034001. [Google Scholar] [CrossRef]
- Pelle, E.; Miranda, E.P.; Fthenakis, C.; Mammone, T.; Marenus, K.; Maes, D. Cigarette Smoke-Induced Lipid Peroxidation in Human Skin and Its Inhibition by Topically Applied Antioxidants. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 63–68. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant Activity, Lipid Peroxidation and Skin Diseases. What’s New. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Minzaghi, D.; Pavel, P.; Kremslehner, C.; Gruber, F.; Oberreiter, S.; Hagenbuchner, J.; Del Frari, B.; Blunder, S.; Gruber, R.; Dubrac, S. Excessive Production of Hydrogen Peroxide in Mitochondria Contributes to Atopic Dermatitis. J. Investig. Dermatol. 2023, 143, 1906–1918.e8. [Google Scholar] [CrossRef]
- Niki, E. Lipid Oxidation in the Skin. Free Radic. Res. 2015, 49, 827–834. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Macca, L.; Li Pomi, F.; Ingrasciotta, Y.; Morrone, P.; Trifirò, G.; Guarneri, C. Hidradenitis Suppurativa and Psoriasis: The Odd Couple. Front. Med. 2023, 10, 1208817. [Google Scholar] [CrossRef]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef]
- Liu, L.; Lian, N.; Shi, L.; Hao, Z.; Chen, K. Ferroptosis: Mechanism and Connections with Cutaneous Diseases. Front. Cell Dev. Biol. 2023, 10, 1079548. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Yang, L.; Yang, Y.; Xu, J. Inhibition of Keratinocyte Ferroptosis Suppresses Psoriatic Inflammation. Cell Death Dis. 2021, 12, 1009. [Google Scholar] [CrossRef]
- Kökçam, I.; Naziroǧlu, M. Antioxidants and Lipid Peroxidation Status in the Blood of Patients with Psoriasis. Clin. Chim. Acta 1999, 289, 23–31. [Google Scholar] [CrossRef]
- Wroński, A.; Wójcik, P. Impact of ROS-Dependent Lipid Metabolism on Psoriasis Pathophysiology. Int. J. Mol. Sci. 2022, 23, 2137. [Google Scholar] [CrossRef]
- Bilski, R.; Kupczyk, D.; Woźniak, A. Oxidative Imbalance in Psoriasis with an Emphasis on Psoriatic Arthritis: Therapeutic Antioxidant Targets. Molecules 2024, 29, 5460. [Google Scholar] [CrossRef]
- Sunitha, S.; Rajappa, M.; Thappa, D.M.; Chandrashekar, L.; Munisamy, M.; Revathy, G.; Priyadarssini, M. Comprehensive Lipid Tetrad Index, Atherogenic Index and Lipid Peroxidation: Surrogate Markers for Increased Cardiovascular Risk in Psoriasis. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Ciamporcero, E.S.; Daga, M.; Ullio, C.; Arcaro, A.; Cetrangolo, G.P.; Ferretti, C.; Dianzani, C.; Lepore, A.; et al. Role of 4-Hydroxynonenal-Protein Adducts in Human Diseases. Antioxid. Redox Signal. 2015, 22, 1681–1702. [Google Scholar] [CrossRef]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Ambrożewicz, E.; Skrzydlewska, E. The Proteomic Profile of Keratinocytes and Lymphocytes in Psoriatic Patients. Proteom. Clin. Appl. 2019, 13, 1800119. [Google Scholar] [CrossRef]
- Wójcik, P.; Biernacki, M.; Wroński, A.; Łuczaj, W.; Waeg, G.; Žarković, N.; Skrzydlewska, E. Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis. Int. J. Mol. Sci. 2019, 20, 4249. [Google Scholar] [CrossRef]
- Karabowicz, P.; Wroński, A.; Ostrowska, H.; Waeg, G.; Zarkovic, N.; Skrzydlewska, E. Reduced Proteasome Activity and Enhanced Autophagy in Blood Cells of Psoriatic Patients. Int. J. Mol. Sci. 2020, 21, 7608. [Google Scholar] [CrossRef]
- Bivik Eding, C.; Köhler, I.; Verma, D.; Sjögren, F.; Bamberg, C.; Karsten, S.; Pham, T.; Scobie, M.; Helleday, T.; Warpman Berglund, U.; et al. MTH1 Inhibitors for the Treatment of Psoriasis. J. Investig. Dermatol. 2021, 141, 2037–2048.e4. [Google Scholar] [CrossRef] [PubMed]
- Blunder, S.; Pavel, P.; Minzaghi, D.; Dubrac, S. PPARdelta in Affected Atopic Dermatitis and Psoriasis: A Possible Role in Metabolic Reprograming. Int. J. Mol. Sci. 2021, 22, 7354. [Google Scholar] [CrossRef]
- Sauerland, M.B.; Davies, M.J. Electrophile versus Oxidant Modification of Cysteine Residues: Kinetics as a Key Driver of Protein Modification. Arch. Biochem. Biophys. 2022, 727, 109344. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Heck, D.E.; Mishin, V.; Black, A.T.; Shakarjian, M.P.; Kong, A.N.T.; Laskin, D.L.; Laskin, J.D. Modulation of Keratinocyte Expression of Antioxidants by 4-Hydroxynonenal, a Lipid Peroxidation End Product. Toxicol. Appl. Pharmacol. 2014, 275, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, X.; Cui, T.; Dang, E.; Wang, G. Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17. J. Investig. Dermatol. 2017, 137, 2168–2176. [Google Scholar] [CrossRef]
- Awasthi, Y.C.; Sharma, R.; Cheng, J.Z.; Yang, Y.; Sharma, A.; Singhal, S.S.; Awasthi, S. Role of 4-Hydroxynonenal in Stress-Mediated Apoptosis Signaling. Mol. Asp. Med. 2003, 24, 219–230. [Google Scholar] [CrossRef]
- Liu, W.; Kato, M.; Akhand, A.A.; Hayakawa, A.; Suzuki, H.; Miyata, T.; Kurokawa, K.; Hotta, Y.; Ishikawa, N.; Nakashima, I. 4-Hydroxynonenal Induces a Cellular Redox Status-Related Activation of the Caspase Cascade for Apoptotic Cell Death. J. Cell Sci. 2000, 113 Pt 4, 635–641. [Google Scholar] [CrossRef]
- Wójcik, P.; Gȩgotek, A.; Wroński, A.; Jastrzab, A.; Zebrowska, A.; Skrzydlewska, E. Effect of Redox Imbalance on Protein Modifications in Lymphocytes of Psoriatic Patients. J. Biochem. 2020, 167, 323–331. [Google Scholar] [CrossRef]
- Jové, M.; Mota-Martorell, N.; Pamplona, R.; Pradas, I.; Martín-Gari, M.; Ayala, V. The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef]
- Iversen, L.; Kragballe, K.; Ziboh, V.A. Significance of Leukotriene-A4 Hydrolase in the Pathogenesis of Psoriasis. Skin Pharmacol. 1997, 10, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chen, Y.L.; Ng, S.W.; Cain, D.; Etherington, R.; Hardman, C.; Ogg, G. Phospholipase Activity of Acyloxyacyl Hydrolase Induces IL-22-Producing CD1a-Autoreactive T Cells in Individuals with Psoriasis. Eur. J. Immunol. 2022, 52, 511–524. [Google Scholar] [CrossRef]
- Ahn, C.; Huang, W. Clinical Presentation of Atopic Dermatitis. Adv. Exp. Med. Biol. 2017, 1027, 39–46. [Google Scholar] [CrossRef]
- Peng, W.; Novak, N. Pathogenesis of Atopic Dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.K. Oxidative Stress in Atopic Dermatitis. Oxid. Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef] [PubMed]
- Antille, C.; Sorg, O.; Lübbe, J.; Saurat, J.H. Decreased Oxidative State in Non-Lesional Skin of Atopic Dermatitis. Dermatology 2002, 204, 69–71. [Google Scholar] [CrossRef]
- Niwa, Y.; Sumi, H.; Kawahira, K.; Terashima, T.; Nakamura, T.; Akamatsu, H. Protein Oxidative Damage in the Stratum Corneum: Evidence for a Link between Environmental Oxidants and the Changing Prevalence and Nature of Atopic Dermatitis in Japan. Br. J. Dermatol. 2003, 149, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-Hydroxy-2-Nonenal in Pollution-Induced Skin Damage. Biofactors 2019, 45, 536–547. [Google Scholar] [CrossRef]
- Wakamatsu, T.H.; Dogru, M.; Ayako, I.; Takano, Y.; Matsumoto, Y.; Ibrahim, O.M.A.; Okada, N.; Satake, Y.; Fukagawa, K.; Shimazaki, J.; et al. Evaluation of Lipid Oxidative Stress Status and Inflammation in Atopic Ocular Surface Disease. Mol. Vis. 2010, 16, 2465. [Google Scholar]
- Sivaranjani, N.; Venkata Rao, S.; Rajeev, G. Role of Reactive Oxygen Species and Antioxidants in Atopic Dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef]
- Amin, M.N.; Liza, K.F.; Sarwar, M.S.; Ahmed, J.; Adnan, M.T.; Chowdhury, M.I.; Hossain, M.Z.; Islam, M.S. Effect of Lipid Peroxidation, Antioxidants, Macro Minerals and Trace Elements on Eczema. Arch. Dermatol. Res. 2015, 307, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Yoneda, K.; Maeda, R.; Munehiro, A.; Fujita, N.; Yokoi, I.; Moriue, J.; Moriue, T.; Kosaka, H.; Kubota, Y. Urinary Biomarker of Oxidative Stress in Patients with Psoriasis Vulgaris and Atopic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Uysal, P.; Avcil, S.; Abas, B.İ.; Yenisey, Ç. Evaluation of Oxidant-Antioxidant Balance in Children with Atopic Dermatitis: A Case-Control Study. Am. J. Clin. Dermatol. 2016, 17, 527–537. [Google Scholar] [CrossRef]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The International EAACI/GA2LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis, and Management of Urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The Role and Relevance of Mast Cells in Urticaria. Immunol. Rev. 2018, 282, 232–247. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Riso, G.; Di Salvo, E.; Casciaro, M.; Giuffrida, R.; Minciullo, P.L.; Guarneri, F.; Nettis, E.; Gangemi, S. Oxidative Stress Involvement in Urticaria. J. Biol. Regul. Homeost. Agents 2020, 34, 675–678. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Brzoza, Z.; Polaniak, R.; Rogala, B.; Birkner, E. Markers of Antioxidant Defence System and Lipid Peroxidation in Peripheral Blood of Female Patients with Chronic Idiopathic Urticaria. Arch. Dermatol. Res. 2007, 298, 499–503. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Brzoza, Z.; Rogala, B.; Polaniak, R.; Birkner, E. Antioxidant Enzyme Activity and Malondialdehyde Concentration in the Plasma and Erythrocytes of Patients with Urticaria Induced by Nonsteroidal Anti-Inflammatory Drugs. J. Investig. Allergol. Clin. Immunol. 2008, 18, 372–375. [Google Scholar]
- Sagdic, A.; Sener, O.; Bulucu, F.; Karadurmus, N.; Yamanel, L.; Tasci, C.; Naharci, I.; Ocal, R.; Aydin, A. Oxidative Stress Status in Patients with Chronic Idiopathic Urticaria. Allergol. Immunopathol. 2011, 39, 150–153. [Google Scholar] [CrossRef]
- Rajappa, M.; Chandrashekar, L.; Sundar, I.; Munisamy, M.; Ananthanarayanan, P.H.; Thappa, D.M.; Toi, P.C. Platelet Oxidative Stress and Systemic Inflammation in Chronic Spontaneous Urticaria. Clin. Chem. Lab. Med. 2013, 51, 1789–1794. [Google Scholar] [CrossRef]
- Verma, P.; Bhattacharya, S.N.; Banerjee, B.D.; Khanna, N. Oxidative Stress and Leukocyte Migration Inhibition Response in Cutaneous Adverse Drug Reactions. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 664. [Google Scholar] [CrossRef]
- Kalkan, G.; Seçkin, H.Y.; Duygu, F.; Akbaş, A.; Özyurt, H.; Şahin, M. Oxidative Stress Status in Patients with Acute Urticaria. Cutan. Ocul. Toxicol. 2014, 33, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Focus on Pathogenesis and Its Therapeutic Implications. J. Dermatol. 2021, 48, 252–270. [Google Scholar] [CrossRef]
- Białczyk, A.; Wełniak, A.; Kamińska, B.; Czajkowski, R. Oxidative Stress and Potential Antioxidant Therapies in Vitiligo: A Narrative Review. Mol. Diagn. Ther. 2023, 27, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.H.; Wu, Y.; Li, L.; Cai, Y.F.; Liu, M.; Gao, X.H.; Chen, H.D. Meta-Analysis of the Association between Vitiligo and the Level of Superoxide Dismutase or Malondialdehyde. Clin. Exp. Dermatol. 2017, 42, 21–29. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as Instigators and Victims of Oxidative Stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Li, C. Mechanisms of Melanocyte Death in Vitiligo. Med. Res. Rev. 2021, 41, 1138–1166. [Google Scholar] [CrossRef]
- Chang, W.L.; Ko, C.H. The Role of Oxidative Stress in Vitiligo: An Update on Its Pathogenesis and Therapeutic Implications. Cells 2023, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Negre-Salvayre, A.; Guerby, P.; Gayral, S.; Laffargue, M.; Salvayre, R. Role of Reactive Oxygen Species in Atherosclerosis: Lessons from Murine Genetic Models. Free Radic. Biol. Med. 2020, 149, 8–22. [Google Scholar] [CrossRef]
- He, S.; Xu, J.; Wu, J. The Promising Role of Chemokines in Vitiligo: From Oxidative Stress to the Autoimmune Response. Oxid. Med. Cell. Longev. 2022, 2022, 8796735. [Google Scholar] [CrossRef]
- Wagner, R.Y.; Luciani, F.; Cario-André, M.; Rubod, A.; Petit, V.; Benzekri, L.; Ezzedine, K.; Lepreux, S.; Steingrimsson, E.; Taieb, A.; et al. Altered E-Cadherin Levels and Distribution in Melanocytes Precede Clinical Manifestations of Vitiligo. J. Investig. Dermatol. 2015, 135, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Li, K.; Liu, L.; Zhang, Y.; Zhou, Z.; Li, C.; Gao, T. Heme Oxygenase-1 Protects Human Melanocytes from H2O2-Induced Oxidative Stress via the Nrf2-ARE Pathway. J. Investig. Dermatol. 2011, 131, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, I.; Batcioglu, K.; Karatas, F.; Hazneci, E.; Genc, M. Comparison of Plasma Malondialdehyde, Glutathione, Glutathione Peroxidase, Hydroxyproline and Selenium Levels in Patients with Vitiligo and Healthy Controls. Indian J. Dermatol. 2008, 53, 106–110. [Google Scholar] [CrossRef]
- Yildirim, M.; Baysal, V.; Inaloz, H.S.; Can, M. The Role of Oxidants and Antioxidants in Generalized Vitiligo at Tissue Level. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, S.; Ribero, S.; Cucci, M.A.; Grattarola, M.; Monge, C.; Dianzani, C.; Barrera, G.; Muzio, G. Oxidative Stress-Related Mechanisms in Melanoma and in the Acquired Resistance to Targeted Therapies. Antioxidants 2021, 10, 1942. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Y.; Wei, G.; Mao, H.; Liu, R.; He, Y. Damage-Associated Molecular Patterns in Vitiligo: Igniter Fuse from Oxidative Stress to Melanocyte Loss. Redox Rep. 2022, 27, 193–199. [Google Scholar] [CrossRef]
- Mosenson, J.A.; Flood, K.; Klarquist, J.; Eby, J.M.; Koshoffer, A.; Boissy, R.E.; Overbeck, A.; Tung, R.C.; Le Poole, I.C. Preferential Secretion of Inducible HSP70 by Vitiligo Melanocytes under Stress. Pigment Cell Melanoma Res. 2014, 27, 209–220. [Google Scholar] [CrossRef]
- Koca, R.; Armutcu, F.; Altinyazar, H.C.; Gürel, A. Oxidant-Antioxidant Enzymes and Lipid Peroxidation in Generalized Vitiligo. Clin. Exp. Dermatol. 2004, 29, 406–409. [Google Scholar] [CrossRef]
- Khan, R.; Satyam, A.; Gupta, S.; Sharma, V.K.; Sharma, A. Circulatory Levels of Antioxidants and Lipid Peroxidation in Indian Patients with Generalized and Localized Vitiligo. Arch. Dermatol. Res. 2009, 301, 731–737. [Google Scholar] [CrossRef]
- Dammak, I.; Boudaya, S.; Ben Abdallah, F.; Turki, H.; Attia, H.; Hentati, B. Antioxidant Enzymes and Lipid Peroxidation at the Tissue Level in Patients with Stable and Active Vitiligo. Int. J. Dermatol. 2009, 48, 476–480. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia Areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Sun, Z.; Li, Y.M.; Xu, H. Oxidative Stress and Alopecia Areata. Front. Med. 2023, 10, 1181572. [Google Scholar] [CrossRef] [PubMed]
- Yenin, J.Z.; Serarslan, G.; Yönden, Z.; Ulutaş, K.T. Investigation of Oxidative Stress in Patients with Alopecia Areata and Its Relationship with Disease Severity, Duration, Recurrence and Pattern. Clin. Exp. Dermatol. 2015, 40, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Bakry, O.A.; Elshazly, R.M.A.; Shoeib, M.A.M.; Gooda, A. Oxidative Stress in Alopecia Areata: A Case-Control Study. Am. J. Clin. Dermatol. 2014, 15, 57–64. [Google Scholar] [CrossRef]
- Abdel Fattah, N.S.A.; Ebrahim, A.A.; El Okda, E.S. Lipid Peroxidation/Antioxidant Activity in Patients with Alopecia Areata. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 403–408. [Google Scholar] [CrossRef]
- Koca, R.; Armutcu, F.; Altinyazar, H.C.; Gürel, A. Evaluation of Lipid Peroxidation, Oxidant/Antioxidant Status, and Serum Nitric Oxide Levels in Alopecia Areata. Med. Sci. Monit. 2005, 11, CR296–CR299. [Google Scholar]
- Akar, A.; Arca, E.; Erbil, H.; Akay, C.; Sayal, A.; Gür, A.R. Antioxidant Enzymes and Lipid Peroxidation in the Scalp of Patients with Alopecia Areata. J. Dermatol. Sci. 2002, 29, 85–90. [Google Scholar] [CrossRef]
- Malik, A.M.; Tupchong, S.; Huang, S.; Are, A.; Hsu, S.; Motaparthi, K. An Updated Review of Pemphigus Diseases. Medicina 2021, 57, 1080. [Google Scholar] [CrossRef]
- Yesilova, Y.; Ucmak, D.; Selek, S.; Dertlioǧlu, S.B.; Sula, B.; Bozkus, F.; Turan, E. Oxidative Stress Index May Play a Key Role in Patients with Pemphigus Vulgaris. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 465–467. [Google Scholar] [CrossRef]
- Naziroğlu, M.; Kökçam, I.; Şimşek, H.; Karakilçk, A.Z. Lipid Peroxidation and Antioxidants in Plasma and Red Blood Cells from Patients with Pemphigus Vulgaris. J. Basic Clin. Physiol. Pharmacol. 2003, 14, 31–42. [Google Scholar] [CrossRef]
- Abida, O.; Ben Mansour, R.; Gargouri, B.; Ben Ayed, M.; Masmoudi, A.; Turki, H.; Masmoudi, H.; Lassoued, S. Catalase and Lipid Peroxidation Values in Serum of Tunisian Patients with Pemphigus Vulgaris and Foliaceus. Biol. Trace Elem. Res. 2012, 150, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, M.H.; Djalali, M.; Daneshpazhooh, M.; Zarei, M.; Eshraghian, M.R.; Derakhshanian, H.; Chams-Davatchi, C. Evaluation of Antioxidant Enzyme Activity and Antioxidant Capacity in Patients with Newly Diagnosed Pemphigus Vulgaris. Clin. Exp. Dermatol. 2015, 40, 313–317. [Google Scholar] [CrossRef]
- Abida, O.; Gargouri, B.; Ben Mansour, R.; Mseddi-Djemal, M.; Masmoudi, A.; Ben Ayed, M.; Abdelmoula, M.; Turki, H.; Lassoued, S.; Masmoudi, H. Biomarkers of Oxidative Stress in Epidermis of Tunisian Pemphigus Foliaceus Patients. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e271–e275. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.L.; Seminario-Vidal, L.; Ronceros, G.; Ramos, W.; Tello, M.; Ortega-Loayza, A.G. Oxidative Stress in Patients with Endemic Pemphigus Foliaceus and Healthy Subjects with Anti-Desmoglein 1 Antibodies. An. Bras. Dermatol. 2018, 93, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, K.; Kazimierczak, U.; Kolenda, T. Oxidative Stress in Melanogenesis and Melanoma Development. Contemp. Oncol. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Li Pomi, F.; Vadalà, R.; Costa, R.; Cicero, N.; Gangemi, S. The Role of Nutraceuticals and Functional Foods in Skin Cancer: Mechanisms and Therapeutic Potential. Foods 2023, 12, 2629. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Tonacci, A.; Bertino, L.; Casciaro, M.; Borgia, F.; Gangemi, S. The Role of Oxidative Stress in the Biology of Melanoma: A Systematic Review. Pathol. Res. Pract. 2019, 215, 21–28. [Google Scholar] [CrossRef]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative Stress in Malignant Melanoma and Non-Melanoma Skin Cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef]
- Woźniak, A.; Drewa, G.; Woźniak, B.; Schachtschabel, D.O. Activity of Antioxidant Enzymes and Concentration of Lipid Peroxidation Products in Selected Tissues of Mice of Different Ages, Both Healthy and Melanoma-Bearing. Z. Gerontol. Geriatr. 2004, 37, 184–189. [Google Scholar] [CrossRef]
- Bisevac, J.P.; Djukic, M.; Stanojevic, I.; Stevanovic, I.; Mijuskovic, Z.; Djuric, A.; Gobeljic, B.; Banovic, T.; Vojvodic, D. Association Between Oxidative Stress and Melanoma Progression. J. Med. Biochem. 2018, 37, 12–20. [Google Scholar] [CrossRef]
- Blendea, A.; Serban, I.L.; Brănisteanu, D.C.; Brănisteanu, D. Evaluation of Immunostaining for 4-Hydroxy-2-Nonenal Receptors in Cutaneous Malignant Melanoma Immunohistochemical Study of 55 Cases. J. Mol. Biomark. Diagn. 2017, 8, 6. [Google Scholar] [CrossRef]
- Shoeb, M.; Ansari, N.; Srivastava, S.; Ramana, K. 4-Hydroxynonenal in the Pathogenesis and Progression of Human Diseases. Curr. Med. Chem. 2014, 21, 230–237. [Google Scholar] [CrossRef]
- Gasparovic, A.C.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. Cancer Growth Regulation by 4-Hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 226–234. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, T.; Grube, R.; Wutte, A.; Zarkovic, N.; Schaur, R.J. 4-Hydroxynonenal Modifies the Effects of Serum Growth Factors on the Expression of the c-Fos Proto-Oncogene and the Proliferation of HeLa Carcinoma Cells. Free Radic. Biol. Med. 1998, 25, 42–49. [Google Scholar] [CrossRef]
- Kreuzer, T.; Žarković, N.; Grube, R.; Schaur, R.J. Inhibition of HeLa Cell Proliferation by 4-Hydroxynonenal Is Associated with Enhanced Expression of the c-Fos Oncogene. Cancer Biother. Radiopharm. 1997, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Serra, A.; Ferretti, C.; Fazio, V.M.; Saglio, G.; Dianzani, M.U. 4-Hydroxynonenal Specifically Inhibits c-Myb but Does Not Affect c-Fos Expressions in HL-60 Cells. Biochem. Biophys. Res. Commun. 1996, 227, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Martinotti, S.; Fazio, V.; Manzari, V.; Paradisi, L.; Parola, M.; Frati, L.; Dianzani, M.U. Effect of 4-Hydroxynonenal on c-Myc Expression. Toxicol. Pathol. 1987, 15, 238–240. [Google Scholar] [CrossRef]
- Zarkovic, N.; Tillian, M.H.; Schaur, J.; Waeg, G.; Jurin, M.; Esterbauer, H. Inhibition of Melanoma B16-F10 Growth by Lipid Peroxidation Product 4-Hydroxynonenal. Cancer Biother. 1995, 10, 153–156. [Google Scholar] [CrossRef]
- Zarkovic, N.; Jörg Schaur, R.; Puhl, H.; Jurin, M.; Esterbauer, H. Mutual Dependence of Growth Modifying Effects of 4-Hydroxynonenal and Fetal Calf Serum in Vitro. Free Radic. Biol. Med. 1994, 16, 877–884. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Daga, M.; Ciamporcero, E.; Toaldo, C.; Pettazzoni, P.; Osella-Abate, S.; Novelli, M.; Minelli, R.; Bisazza, A.; Gamba, P.; et al. Improved Anti-Tumoral Therapeutic Efficacy of 4-Hydroxynonenal Incorporated in Novel Lipid Nanocapsules in 2D and 3D Models. J. Biomed. Nanotechnol. 2015, 11, 2169–2185. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, S.; Ciamporcero, E.; Pettazzoni, P.; Osella-Abate, S.; Novelli, M.; Toaldo, C.; Husse, M.; Daga, M.; Minelli, R.; Bisazza, A.; et al. The Inclusion Complex of 4-Hydroxynonenal with a Polymeric Derivative of β-Cyclodextrin Enhances the Antitumoral Efficacy of the Aldehyde in Several Tumor Cell Lines and in a Three-Dimensional Human Melanoma Model. Free Radic. Biol. Med. 2013, 65, 765–777. [Google Scholar] [CrossRef]
- Lu, B.; Chen, X.B.; Ying, M.D.; He, Q.J.; Cao, J.; Yang, B. The Role of Ferroptosis in Cancer Development and Treatment Response. Front. Pharmacol. 2018, 8, 992. [Google Scholar] [CrossRef] [PubMed]
- Ta, N.; Jiang, X.; Zhang, Y.; Wang, H. Ferroptosis as a Promising Therapeutic Strategy for Melanoma. Front. Pharmacol. 2023, 14, 1252567. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Atalay, S.; Rogowska-Wrzesińska, A.; Skrzydlewska, E. The Effect of Cannabidiol on UV-Induced Changes in Intracellular Signaling of 3D-Cultured Skin Keratinocytes. Int. J. Mol. Sci. 2021, 22, 1501. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Skrzydlewska, E. Protective Effects of Cannabidiol on the Membrane Proteome of UVB-Irradiated Keratinocytes. Antioxidants 2021, 10, 402. [Google Scholar] [CrossRef]
- Vincenzi, C.; Tosti, A. Efficacy and Tolerability of a Shampoo Containing Broad-Spectrum Cannabidiol in the Treatment of Scalp Inflammation in Patients with Mild to Moderate Scalp Psoriasis or Seborrheic Dermatitis. Skin Appendage Disord. 2020, 6, 355–361. [Google Scholar] [CrossRef]
- Alesci, A.; Lauriano, E.R.; Fumia, A.; Irrera, N.; Mastrantonio, E.; Vaccaro, M.; Gangemi, S.; Santini, A.; Cicero, N.; Pergolizzi, S. Relationship between Immune Cells, Depression, Stress, and Psoriasis: Could the Use of Natural Products Be Helpful? Molecules 2022, 27, 1953. [Google Scholar] [CrossRef]
- Khorsandi, K.; Esfahani, H.S.; Ghamsari, S.K.; Lakhshehei, P. Targeting Ferroptosis in Melanoma: Cancer Therapeutics. Cell Commun. Signal. 2023, 21, 337. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Y.; Mao, C.; Liu, S.; Xiao, D.; Huang, J.; Tao, Y. Emerging Mechanisms and Targeted Therapy of Ferroptosis in Cancer. Mol. Ther. 2021, 29, 2185–2208. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Ren, Z.; Li, Y.; Zou, W.; Chen, J.; Wang, H. Overcoming Cancer Chemotherapy Resistance by the Induction of Ferroptosis. Drug Resist. Updates 2023, 66, 100916. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. Targeting Ferroptosis as a Vulnerability in Cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Tao, R.; Li, Y.; Gong, S.; Zhang, Q.; Zhu, Z. Unveiling Intricating Roles and Mechanisms of Ferroptosis in Melanoma. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189234. [Google Scholar] [CrossRef]
- Habib, E.; Linher-Melville, K.; Lin, H.X.; Singh, G. Expression of XCT and Activity of System Xc- Are Regulated by NRF2 in Human Breast Cancer Cells in Response to Oxidative Stress. Redox Biol. 2015, 5, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kästle, M.; Grimm, S.; Nagel, R.; Breusing, N.; Grune, T. Combination of PDT and Inhibitor Treatment Affects Melanoma Cells and Spares Keratinocytes. Free Radic. Biol. Med. 2011, 50, 305–312. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The Role of Apoptosis in Response to Photodynamic Therapy: What, Where, Why, and How. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef]
- Baldea, I.; Olteanu, D.E.; Bolfa, P.; Ion, R.M.; Decea, N.; Cenariu, M.; Banciu, M.; Sesarman, A.V.; Filip, A.G. Efficiency of Photodynamic Therapy on WM35 Melanoma with Synthetic Porphyrins: Role of Chemical Structure, Intracellular Targeting and Antioxidant Defense. J. Photochem. Photobiol. B 2015, 151, 142–152. [Google Scholar] [CrossRef]
- Pantic, I.; Paunovic, J.; Pejic, S.; Drakulic, D.; Todorovic, A.; Stankovic, S.; Vucevic, D.; Cumic, J.; Radosavljevic, T. Artificial Intelligence Approaches to the Biochemistry of Oxidative Stress: Current State of the Art. Chem. Biol. Interact. 2022, 358, 109888. [Google Scholar] [CrossRef]
- Davidovic, L.M.; Laketic, D.; Cumic, J.; Jordanova, E.; Pantic, I. Application of Artificial Intelligence for Detection of Chemico-Biological Interactions Associated with Oxidative Stress and DNA Damage. Chem. Biol. Interact. 2021, 345, 109533. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; Durand, T.; Oger, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Assessment of Lipid Peroxidation and Artificial Neural Network Models in Early Alzheimer Disease Diagnosis. Clin. Biochem. 2019, 72, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Yuan, W.; Jiang, R.; Zhan, Z.; Zhang, L.; Xu, X.; Qian, Y.; Yang, W.; Zhang, Z. Machine Learning-Based Integration Identifies the Ferroptosis Hub Genes in Nonalcoholic Steatohepatitis. Lipids Health Dis. 2024, 23, 23. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Skin Disease | Molecules | Results |
|---|---|---|---|
| Gęgotek et al. [39], 2019 | Psoriasis | 4-HNE | The proteomic approach showed an increase in the level of 4-HNE protein adducts. Among inactivated proteins, many are involved in the antioxidant system. |
| Wójcik et al. [40], 2019 | Psoriasis | 4-HNE | Patients with psoriasis show elevated levels of 4-HNE-adducts and an alteration in lipid metabolism with an enhancement in mediators that modulate the immune system in mononuclear cells. |
| Blunder et al. [43], 2021 | Psoriasis, AD | General LPO products | LPO products bind to PPARδ, promoting keratinocyte differentiation and exacerbating epidermal exfoliation. |
| Yang et al. [46], 2017 | Psoriasis | 4-HNE | 4-HNE regulates Nrf2 by binding to cysteine residues of its inhibitor, Keap1. The excessive activation of Nrf2 promotes keratinocyte proliferation, thus contributing to the development of skin lesions. |
| Niwa et al. [58], 2003 | AD | 4-HNE | In an immunohistochemical analysis of skin samples from AD subjects, anti-4-HNE antibodies are more intensely distributed on the superficial layers, areas where OS is more expressed. These findings suggest that 4-HNE increases OS in patients with AD. |
| Wang et al. [87], 2022 | Vitiligo | LPO | OS and LPO products promote the release of DAMPs from keratinocytes and melanocytes in the skin, inducing immune responses. |
| Koca et al. [89], 2004 | Vitiligo | MDA | Serum MDA levels in vitiligo patients are significantly increased compared to healthy controls. This leads to increased OS and damage to the melanocyte cell membrane. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li Pomi, F.; Gammeri, L.; Borgia, F.; Di Gioacchino, M.; Gangemi, S. Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation. Antioxidants 2025, 14, 555. https://doi.org/10.3390/antiox14050555
Li Pomi F, Gammeri L, Borgia F, Di Gioacchino M, Gangemi S. Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation. Antioxidants. 2025; 14(5):555. https://doi.org/10.3390/antiox14050555
Chicago/Turabian StyleLi Pomi, Federica, Luca Gammeri, Francesco Borgia, Mario Di Gioacchino, and Sebastiano Gangemi. 2025. "Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation" Antioxidants 14, no. 5: 555. https://doi.org/10.3390/antiox14050555
APA StyleLi Pomi, F., Gammeri, L., Borgia, F., Di Gioacchino, M., & Gangemi, S. (2025). Oxidative Stress and Skin Diseases: The Role of Lipid Peroxidation. Antioxidants, 14(5), 555. https://doi.org/10.3390/antiox14050555










