1. Introduction
The Chinese soft-shelled turtle (
Pelodiscus sinensis) stands as one of the most highly prized species in East and Southeast Asia, finding extensive use in food production, traditional medicine, and the pet trade [
1]. In recent years, it has emerged as a significant economic component of China’s aquaculture industry, with an annual production exceeding 400,000 tons in 2023, while the output value has exceeded CNY 20 billion (China Fishery Statistical Yearbook 2024) [
2]. However, the sustainable and healthy development of
P. sinensis aquaculture faces significant challenges due to frequent disease outbreaks over the past few decades [
3]. Studies have indicated that the suboptimal health state caused by the deteriorating aquaculture environments, imbalanced feed nutrition, and drug misuse constitutes key contributing factors to immune suppression and pathogen susceptibility in
P. sinensis [
4,
5,
6]. Notably, the liver—a vital organ integrating functions in nutrient metabolism, drug detoxification, and immune regulation [
7]—plays a pivotal role in modulating the suboptimal health status of
P. sinensis [
8]. Its functional integrity shows significant correlations, with both compromised growth performance and heightened disease susceptibility in this species [
5,
9,
10,
11]. Hence, strategies aimed at improving hepatic health and immune resilience in
P. sinensis are of significant importance for optimizing aquaculture sustainability.
Assessment of hepatic health status in animals constitutes a complex systematic engineering project [
12] involving a multi-scale approach, from molecular to organ levels. Molecular indicators include gene expression related to metabolism, detoxification, and immunity, along with antioxidant enzyme activities and lipid peroxidation markers [
13]. Cellular assessments focus on hepatocyte morphology and serum biomarkers [
14]. Organ-level indicators encompass macroscopic liver characteristics, such as color [
15] and size [
16]. Hepatic health is also influenced by its bidirectional relationship with intestinal function, affecting overall resistance to pathogens in aquatic species [
17]. This integrated approach provides a comprehensive assessment of liver health in animals. However, a comprehensive assessment of liver health is only the first step; developing targeted intervention strategies is even more critical.
D-glucuronolactone (DGL), a naturally occurring compound, is ubiquitously present in living organisms and chemically defined as D-(+)-glucofuranurono-6,3-lactone [
18,
19]. In animal physiology, DGL undergoes hepatic metabolism primarily via hydrolysis into glucuronic acid [
18], a bioactive metabolite with multifaceted physiological functions including detoxification, antioxidant activity, and anti-inflammatory properties [
19,
20]. Consequently, DGL has been extensively employed in clinical settings for managing liver disorders, ranging from chronic and acute hepatitis to cirrhosis and other liver diseases, with demonstrated efficacy in mitigating drug-induced liver injury incidence [
21,
22,
23].
The current literature on the application of DGL as a sole feed additive in animal nutrition is rather limited and predominantly focuses on poultry. In laying hens, dietary supplementation with 140 mg kg
−1 DGL has been shown to enhance hepatic enzyme activity, ovarian follicle development, and eggshell quality during peak production periods [
24]. A patented formulation further suggests that 280 mg kg
−1 DGL reduces hepatic lipid deposition in hens, potentially through the modulation of fatty acid metabolism [
25]. By contrast, research in aquatic species is scarce, with only one study demonstrating hepatoprotective effects in teleost fish: Shi et al. reported that 200 mg kg
−1 DGL improved lipid metabolism, antioxidant capacity, and immune function in tilapia (
Oreochromis niloticus), highlighting its potential for aquatic applications [
26].
As an aquatic reptile,
P. sinensis exhibits distinct physiological and metabolic characteristics compared to teleost fish, including seasonal hibernation behavior with hepatic lipid accumulation serving as the primary energy reserve during dormancy [
27]; bimodal respiration utilizing pulmonary ventilation supplemented by cutaneous and buccopharyngeal gas exchange during prolonged submergence [
28]; hepatic expression of complete ornithine–urea cycle enzymes enabling ureotelism [
29], contrasting with the predominant ammoniotelic excretion in most fish species; and unique urea excretion through oral mucosa rather than conventional reptilian cloacal pathways [
30]. These traits suggest that the effect of DGL on detoxification, lipid metabolism, and antioxidant defense in
P. sinensis may differ mechanistically from those observed in fish.
Therefore, this study aimed to evaluate the effects of dietary DGL supplementation on growth, liver and gut health, antioxidant status, gene expression, and disease resistance in
P. sinensis. The findings of this study will serve as a scientific basis for the application of DGL in aquaculture reptiles, with a particular focus on hepatoprotective strategies in intensive aquatic farming systems. Given that liver health is a critical issue in fish aquaculture [
31], the insights from this study could potentially inform strategies for mitigating liver disorders in other fish species as well.
2. Materials and Methods
2.1. Ethic
This study was approved by the Animal Experimental Ethical Inspection of Laboratory Animal Center, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (NO. YFI2024liuwei03).
2.2. Experimental Diets
This study utilized a single-factor experimental design to assess the effects of dietary DGL supplementation. The feed formula was designed according to the national standard of the People’s Republic of China (GB/T 32140-2015 formula feed for soft-shelled turtle
P. sinensis) [
32]. The primary raw materials included fish meal, blood meal, soybean meal, expanded soybean, corn gluten meal, and α-starch. Based on previously reported efficacious levels in
O. niloticus (200 mg kg
−1) [
26] and poultry studies (up to 280 mg kg
−1) [
25], experimental diets were formulated by supplementing with 0 (control, C), 200 (G200), and 400 (G400) mg kg
−1 pharmaceutical-grade DGL (purity ≥ 98%, Zhucheng Haotian Pharmaceutical Co., Ltd., Zhucheng, China). The former employs a conservative dosing strategy, while the latter explores the potential for dose optimization. Experimental diets were created by supplementing with 0 (Control, C), 200 (G200), and 400 (G400) mg kg
−1 pharmaceutical-grade DGL (purity ≥ 98%, Zhucheng Haotian Pharmaceutical Co., Ltd., Zhucheng, China). Diet formulation and proximate composition are presented in
Table 1.
2.3. Experimental Soft-Shelled Turtle and Culture Management
The male
P. sinensis used in the experiment was obtained from Xijia Agricultural Development Co., Ltd. in Bengbu, Anhui Province, China. After being transported back to the base, the experimental turtles were temporarily cultured in a recirculating water aquaculture system for three weeks to acclimate to the environmental conditions. During this accumulation period, they were fed the C diet. Following acclimatization, 180 healthy turtles with vigorous vitality, intact skin, and uniform sizes (average weight: 110.77 ± 1.70 g) were selected. These turtles were randomly assigned to 9 tanks with the original holding system, with 20 turtles per tank (L × W × H: 100 cm × 100 cm × 35 cm). The tanks were randomly allocated into three treatment groups, each receiving one of the three experimental diets described in
Section 2.2. Each dietary treatment was replicated in three tanks. The feeding trial lasted for 8 weeks. During this period, turtles were fed twice daily (at 08:00 and 17:00) at a feeding rate of 2–3% of body weight. After 30 min, any uneaten feed was collected, and feed intake was calculated based on the feed dissolution rate. Throughout the experiment, the water temperature was maintained at 28–31 °C, ammonia nitrogen was less than 0.5 mg kg
−1, nitrite was less than 0.1 mg kg
−1, and the pH was between 6.5 and 7.2.
For further molecular investigations, approximately 0.1 g of liver, intestinal, and muscle tissues were collected from three additional turtles per tank, flash-frozen in liquid nitrogen at −80 °C for future research purposes. Subsequently, all remaining tissues were combined and stored at −20 °C for body composition analysis. Finally, the remaining turtles in each tank were counted, and their body weights were recorded.
2.4. Sample Collection
After the feeding experiment, three turtles per tank were randomly selected and euthanized for morphometric measurements and biochemical analyses. Each turtle was rapidly measured on an ice-cooled surface within 2 min to determine trunk length (longest carapace point), trunk width (widest carapace point), trunk height (highest carapace point), and body weight. Following decapitation, blood samples were collected and centrifuged at 3000 rpm (4 °C, 10 min) to separate serum, which was then stored at −80 °C for biochemical and antioxidant analyses. The liver was carefully dissected and weighed. Portions of liver and intestinal tissues at the same site were fixed in 4% paraformaldehyde for histological analysis, while parallel samples were homogenized (1:9 w/v) in ice-cold 0.9% NaCl, centrifuged (4000 rpm, 4 °C, 10 min), and the supernatant stored at −80 °C for antioxidant and digestive enzyme assays. To assess nutritional composition, the remaining liver and leg muscle tissues from each tank were pooled and stored at −20 °C. Additionally, liver and carapace/plastron regions were analyzed for L*, a*, and b* color values using a WSC-1B colorimeter (Shanghai Yidian Scientific Instrument Co., Ltd., Shanghai, China).
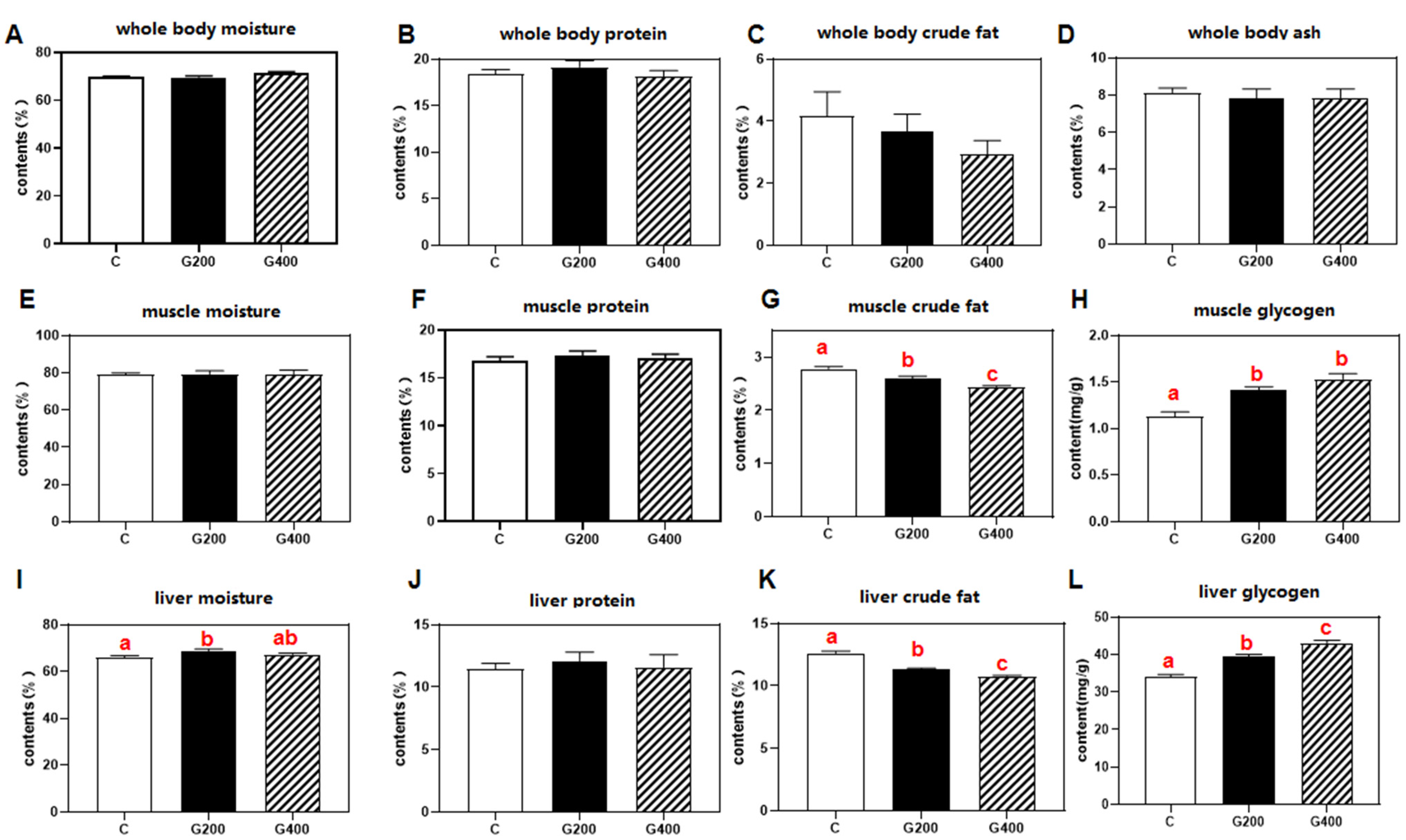
2.5. Proximate Composition Analysis
The moisture, crude protein, crude fat, and ash contents of the collected samples were determined according to the national standard of the People’s Republic of China: GB/T 5009.3-2003 (Determination of Moisture in Foods) [
34], GB/T 5009.5-2003 (Determination of Protein in Foods) [
35], GB/T 5009.6-2003 (Determination of Fat in Foods) [
36], and GB/T 5009.4-2003 (Determination of Ash in Foods) [
37]. Briefly, moisture content was measured by drying samples at 105 °C for 4 h until a constant weight was achieved. Crude protein was analyzed using a fully automated Kjeldahl nitrogen analyzer (Model K-360, BÜCHI Instrument, Flawil, Switzerland), with protein content calculated as nitrogen content 6.25. Crude fat was extracted via the Soxhlet petroleum ether extraction, and ash content was determined by incinerating samples at 550 °C for 12 h in a muffle furnace.
2.6. Serum Biochemical, Antioxidant, and Digestive Enzyme Assays
Serum biochemical indices were analyzed using Sysmex reagent kits (Sysmex Wuxi Co., Ltd., Wuxi, China) and a Sysmex-800 fully automated biochemistry analyzer (Sysmex Infosystems, Kobe, Japan). The measured parameters included: aspartate aminotransferase (AST, determined by the UV malate dehydrogenase method, code: 290505, 290506), alanine aminotransferase (ALT, determined by the UV lactate dehydrogenase method, code: 290503, 290504), glucose (GLU, measured using the hexokinase method, code: 290523), total cholesterol (TCHO, assayed by the CHOD-PAP method, code: 290536, 290537), triacylglycerols (TG, assayed by the GPO-PAP method, code: 80943, 80944), high-density lipoprotein cholesterol (HDLC, measured using the direct method, code: 290544), low-density lipoprotein cholesterol (LDLC, also measured using the direct method, code: 84106, 84107), total protein (TP, determined by the Biuret method, code: 290518), albumin (ALB, assayed using the bromocresol green method, code: 290515), and alkaline phosphatase (ALP, measured using the AMP buffer method, code: 290501, 290502).
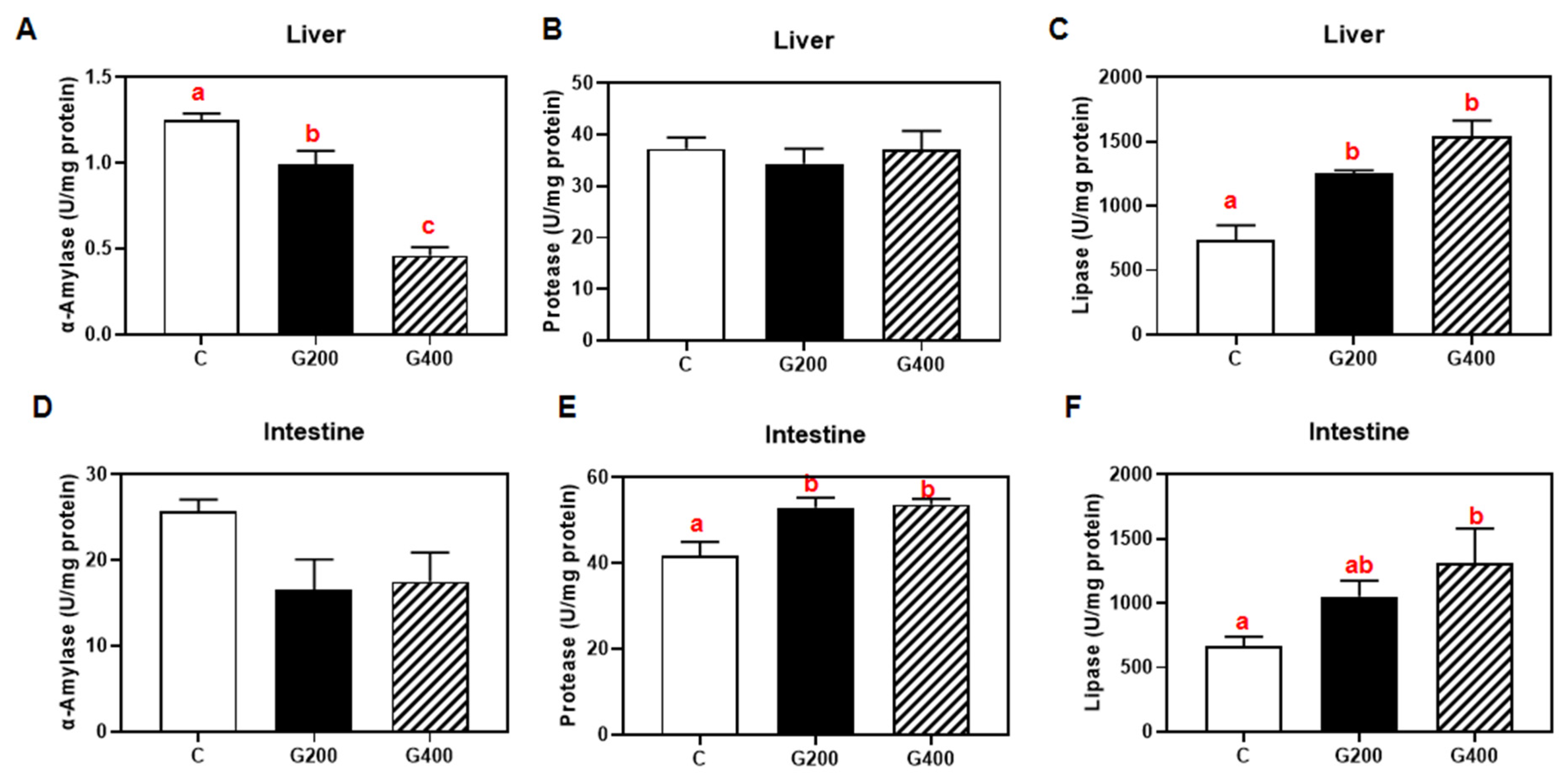
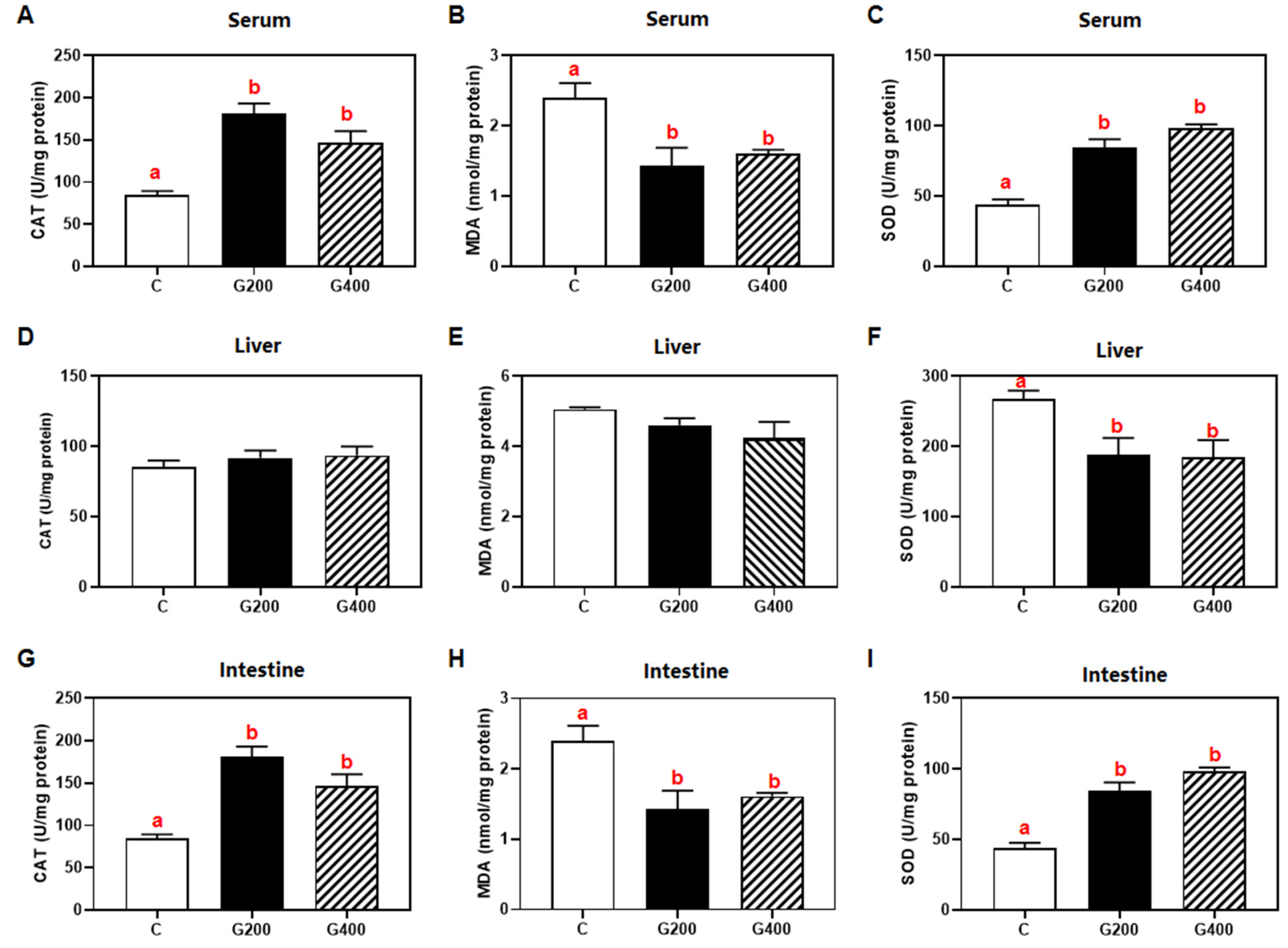
Superoxide dismutase (SOD, code: A001-1-2) and catalase (CAT, code: A007-1-1) activities, malondialdehyde (MDA, code: A003-1-2) content in serum, liver, and intestine, serum lipopolysaccharide (LPS, code: H255-1-2), and lipase (code: A054-1-1), protease (code: A080-1-1), and α-amylase (code: C016-1-1) activities in hepatic and intestinal tissues were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocols. All enzymatic activities and metabolic concentrations in tissues were normalized to total protein content, which was determined using the Bradford method, with bovine serum albumin (BSA) as the standard [
38].
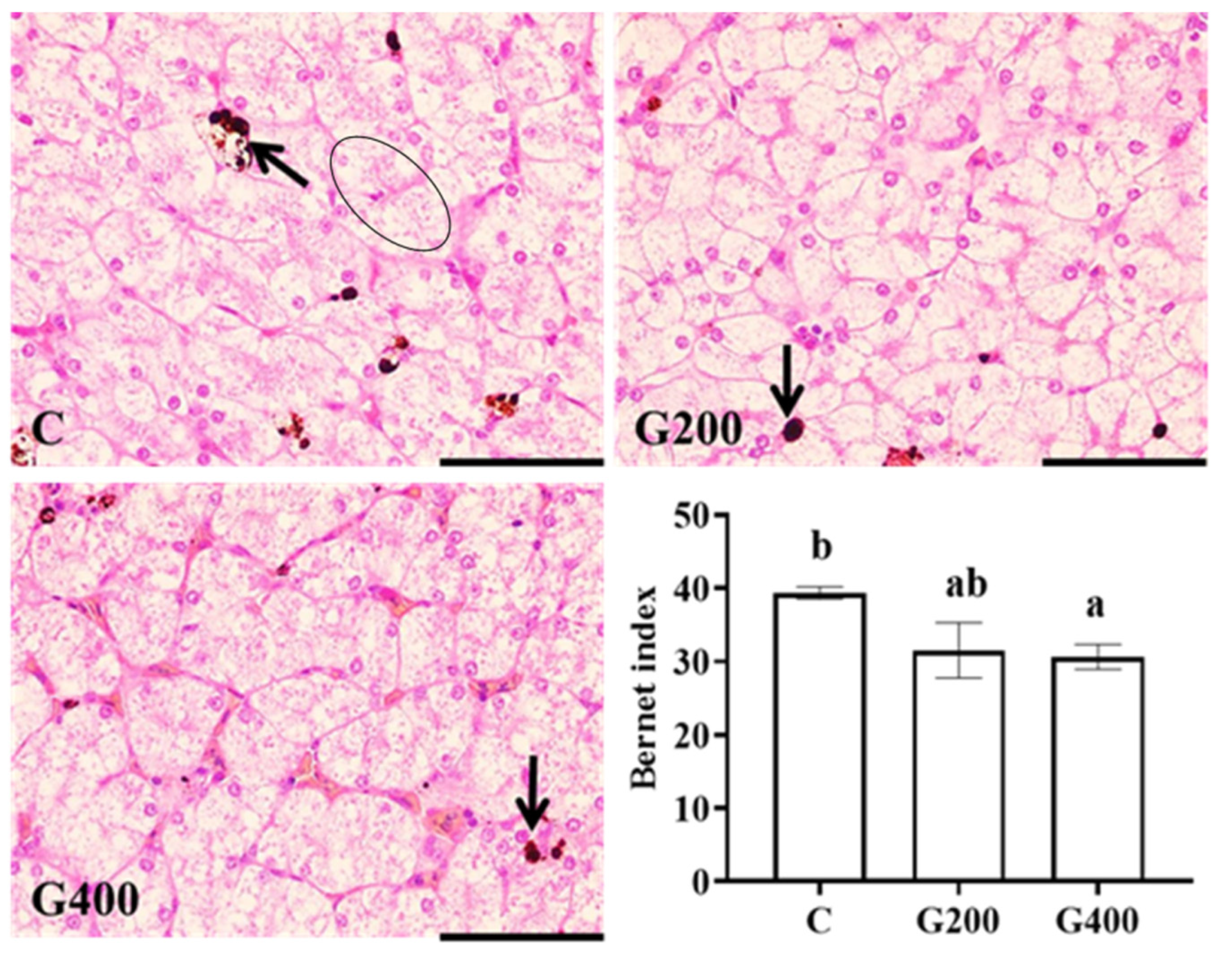
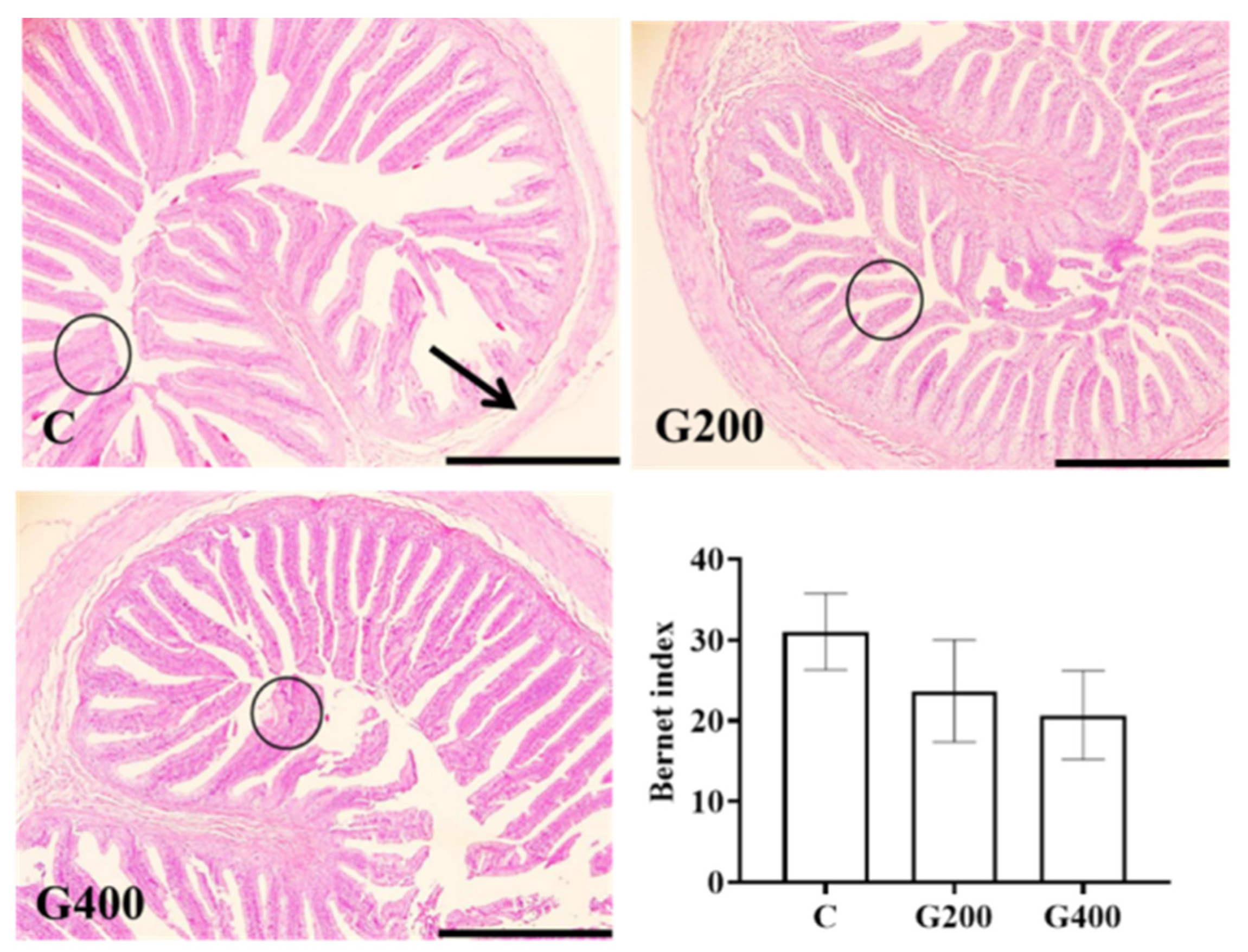
2.7. Liver and Intestinal Histology
Liver and intestinal tissues fixed with 4% paraformaldehyde (24 h) were processed using standard histological techniques to generate semi-continuous sections (5 μm thickness), which were then stained with hematoxylin and eosin. Images were acquired using an OLYMPUS CX41 microscope coupled with an OLYMPUS DP73 digital camera (Tokyo, Japan). Four representative fields per tissue sample were captured per turtle, following methodologies described by [
39,
40]. Histopathological evaluation was performed using semi-quantitative criteria outlined in
Table 2.
2.8. Real-Time PCR Analysis
Total RNA was extracted from liver, muscle, and intestine, and subsequently purified using the VeZol reagent kit (Code: R411-01, Vazyme Biotechnology Co., Ltd., Nanjing, China). To eliminate potential genomic DNA contamination, the extracted RNA was treated with RNA-free DNase. The reverse transcriptase cDNA was synthesis using a reverse transcriptase cDNA synthesis kit (Code: R223-01, Vazyme Biotechnology Co., Ltd.) following the manufacturer’s instructions.
Quantitative real-time PCR (RT-qPCR) was performed on a CFX96TM real-time PCR detection system (Bio-Rad, Hercules, CA, USA). Each 20 μL reaction contained 10 μL 2× SYBR
® Premix Ex Taq
TM II (code: Q311-02, Vazyme Biotechnology Co., Ltd.), 7.8 μL sterilized double-distilled water (ddH
2O), 1 μL diluted cDNA (1:10), and 0.6 μL each of forward and reverse primer (10 μM). The RT-qPCR program consisted of an initial activation step at 95 °C for 30 s, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 15 s at 60 °C. The amplification efficiency was between 95% and 105%, with the standard curve correlation coefficient exceeding 0.98. β-actin was used as the internal gene for normalization. Following PCR, a melting curve analysis was conducted to confirm the specificity of the amplified products. Relative quantification of target gene expression was calculated using the 2
−ΔΔCt method. Primer sequences for qPCR are provided in
Table 3.
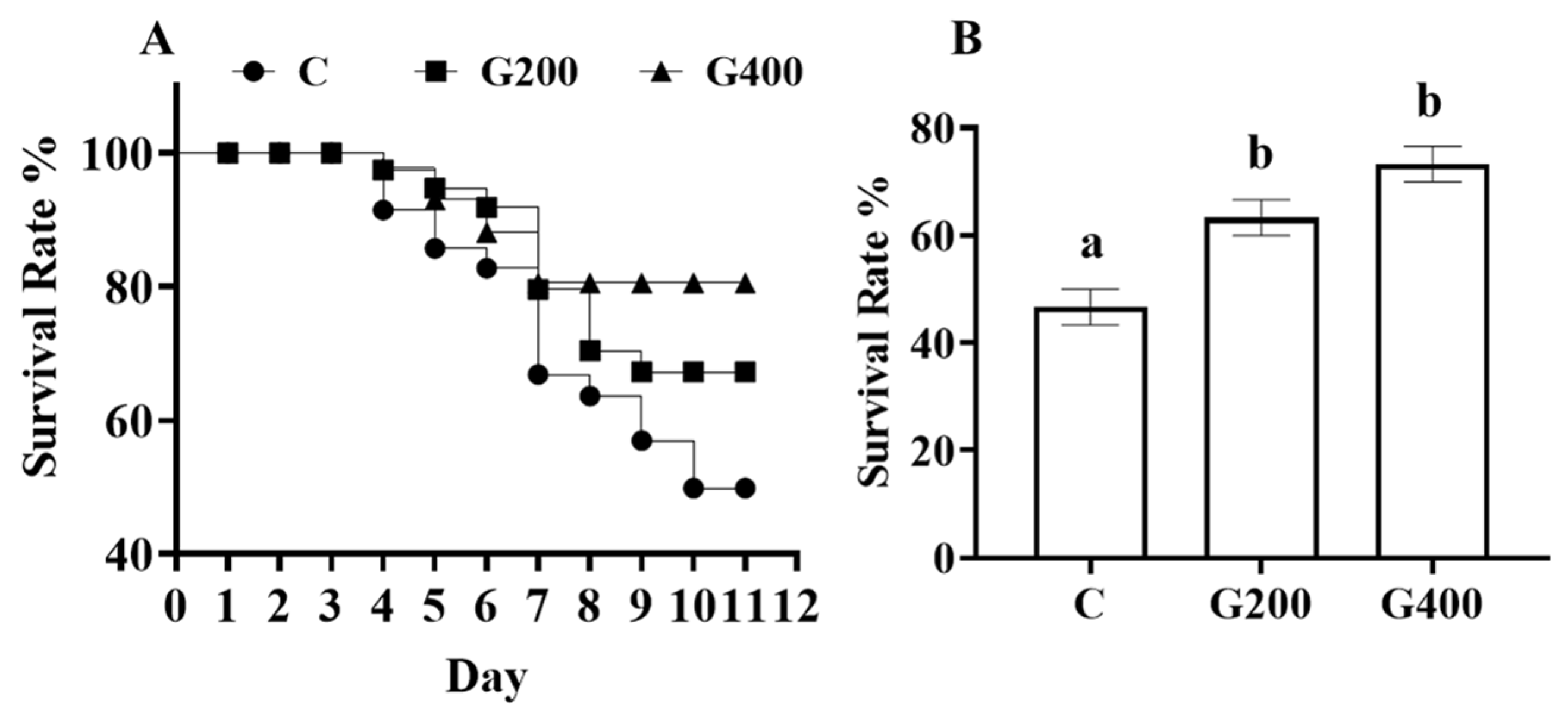
2.9. Challenge Test
The challenge test was conducted using the
Aeromonas hydrophila T3 strain [
43,
44] which was originally isolated from
P. sinensis. The experiment followed the protocol established by Zhang et al. [
44]. For the challenge test, 10 turtles per tank were randomly selected and marked dorsally with a non-invasive identifier. The selected turtles were immersion-challenged with a bacterial suspension at 1.35 × 10⁶ CFU mL
−1. Daily mortality rates were recorded throughout the 10-day challenge period, while routine feeding protocols were maintained unchanged.
2.10. Calculation of Performance Parameters and Statistical Analysis
The following parameters were calculated using standard equations:
Survival rate (SR, %) = 100 × final number/initial number
Weight gain rate (WGR, %) = 100 × [final mean weight (g) − initial mean weight (g)]/initial mean weight (g)
Specific growth rate (SGR, % day−1) = 100 × [ln (final mean weight (g)) − ln (initial mean weight (g))]/rearing period (days)
Feed conversion ratio (FCR) = dry feed intake (g)/[final weight (g) + dead weight (g) − initial weight (g))]
Feed intake (FI, % body weight day−1) = 100 × total feed intake (g)/[(final weight (g) + dead weight (g) + initial weight (g))]/2 × rearing period days]
Hepatosomatic index (HSI, %) = 100 × liver weight (g)/body weight (g)
Condition factor (CF, g cm−3) = 100 × body weight (g)/[body length (cm)]3
All statistical analyses were conducted using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Experimental data are presented as mean ± standard error (SEM) based on three independent replicates. One-way analysis of variance (ANOVA) was used for intergroup comparisons, followed by Tukey’s post hoc test for multiple comparisons. Statistical significance was determined at p < 0.05. Data visualization was created with GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA).
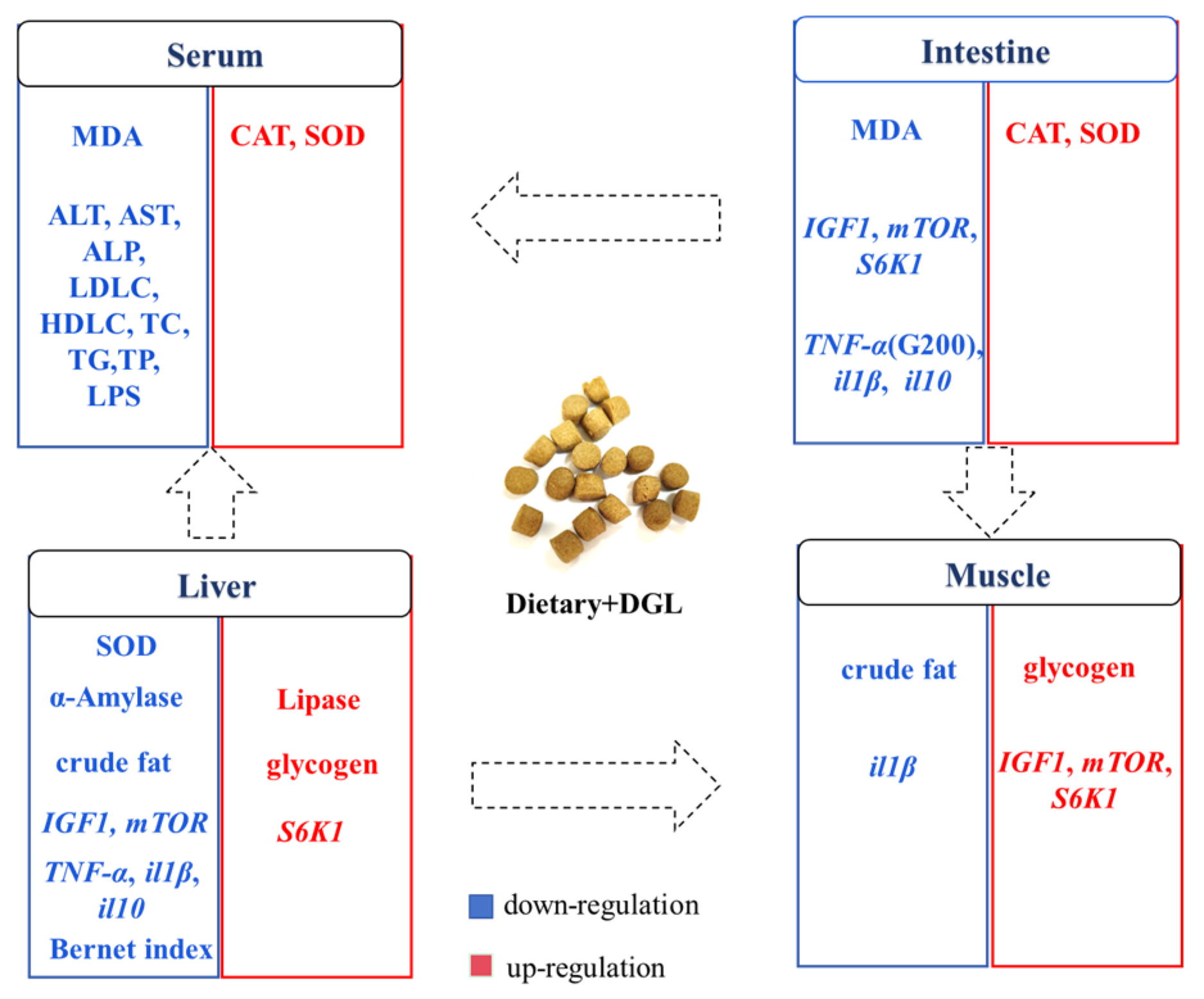
5. Conclusions
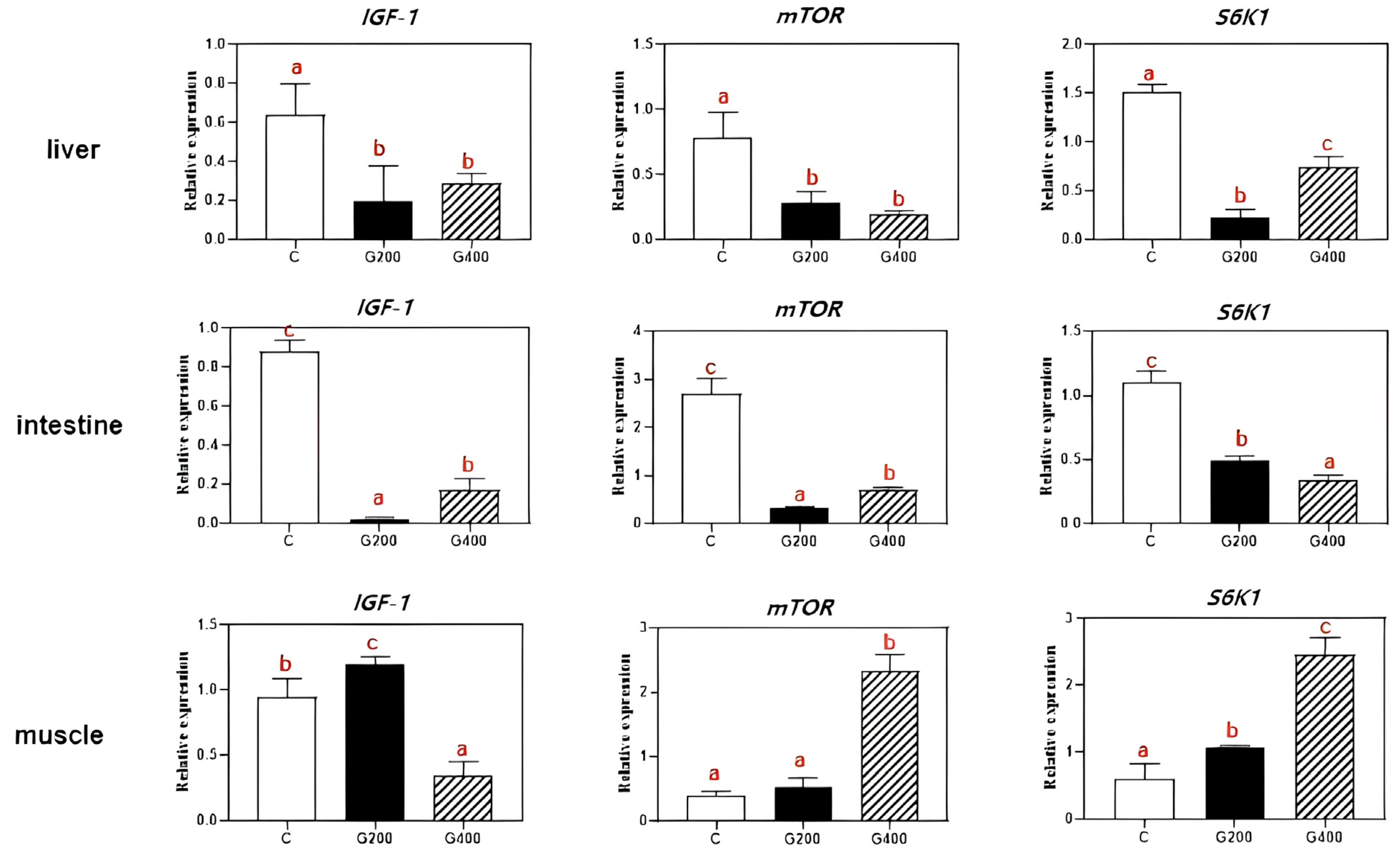
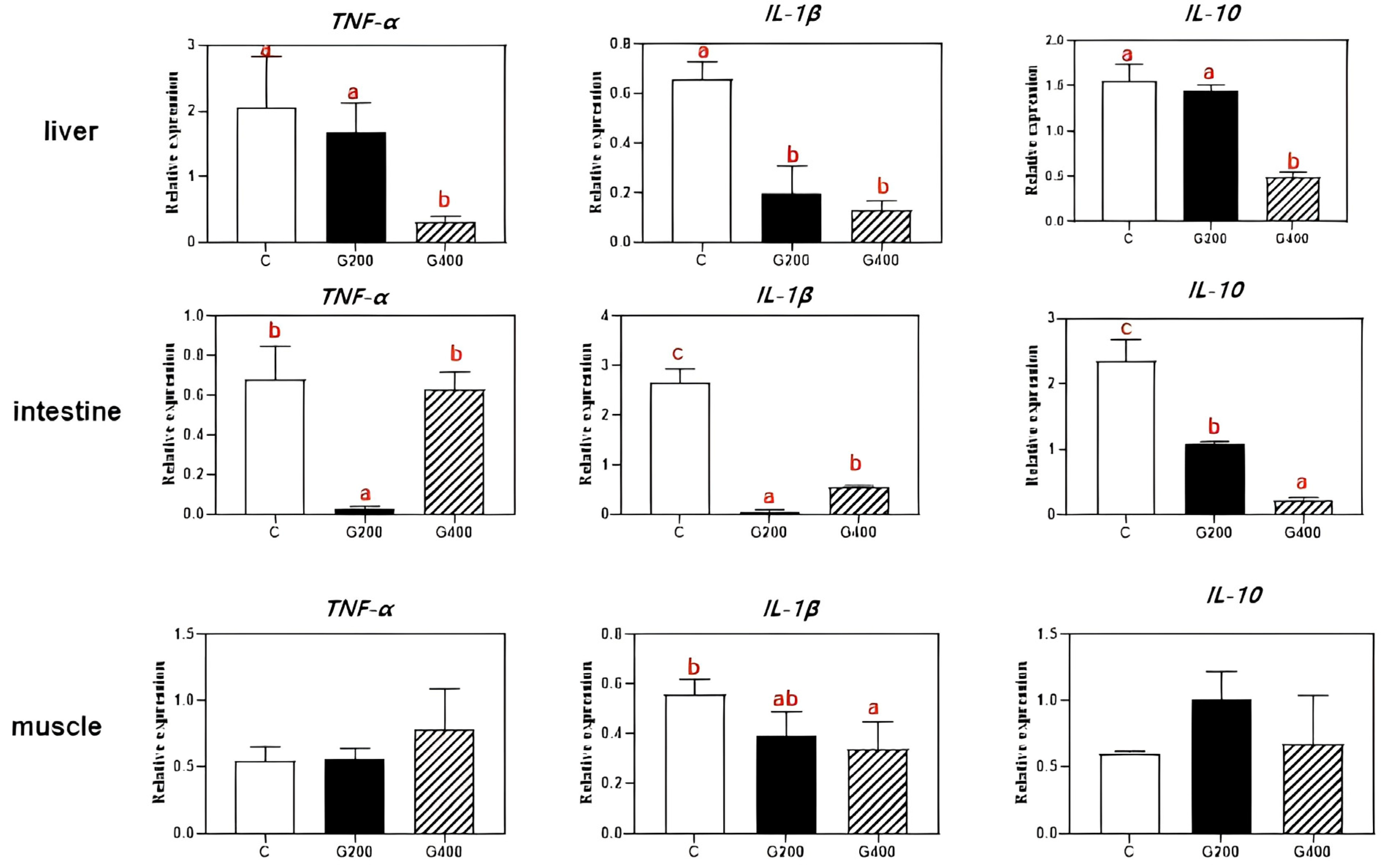
Our study represents the inaugural demonstration that dietary supplementation with 200–400 mg kg
−1 of DGL significantly enhances the growth performance and feed efficiency of
P. sinensis. This beneficial effect can be attributed to the activation of the IGF1/mTOR/S6K1 signaling pathway in muscle tissue, which stimulates protein synthesis and myofiber growth. Additionally, DGL optimizes carbohydrate metabolism by suppressing hepatic amylase activity and boosting glycogen storage. Remarkably, DGL exhibits robust antioxidant and anti-inflammatory properties, as manifested by elevated serum and intestinal SOD/CAT activity, reduced oxidative stress (evidenced by lower MDA levels), and a dose-dependent decrease in pro-inflammatory cytokines (TNF-α, IL-1β) in tissues. The hepatoprotective effects of DGL are particularly noteworthy, including the mitigation of hepatic lipid accumulation, reduction in serum ALT/AST levels, and restoration of liver color. Importantly, DGL supplementation at 200–400 mg kg
−1 significantly enhances the resistance of
P. sinensis to
A. hydrophila infection. Collectively, these findings suggest that DGL holds great promise as a functional feed additive for promoting sustainable aquaculture practices (
Figure 9). Indeed, many studies on feed additives for aquatic animals have observed dose-dependent effects, where low doses promote growth and physiological performance, while high doses inhibit or have no effect [
75,
76,
77]. In our trial, the highest dose was only designed to reach 400 mg kg
−1, and whether a dose-dependent effect exists remains to be determined. In the future, we will continue to explore the impact of higher doses of DGL on aquatic animals.