Natural Products from the Mediterranean Area as Wound Healing Agents—In Vitro Studies: A Systematic Review
Abstract
1. Introduction
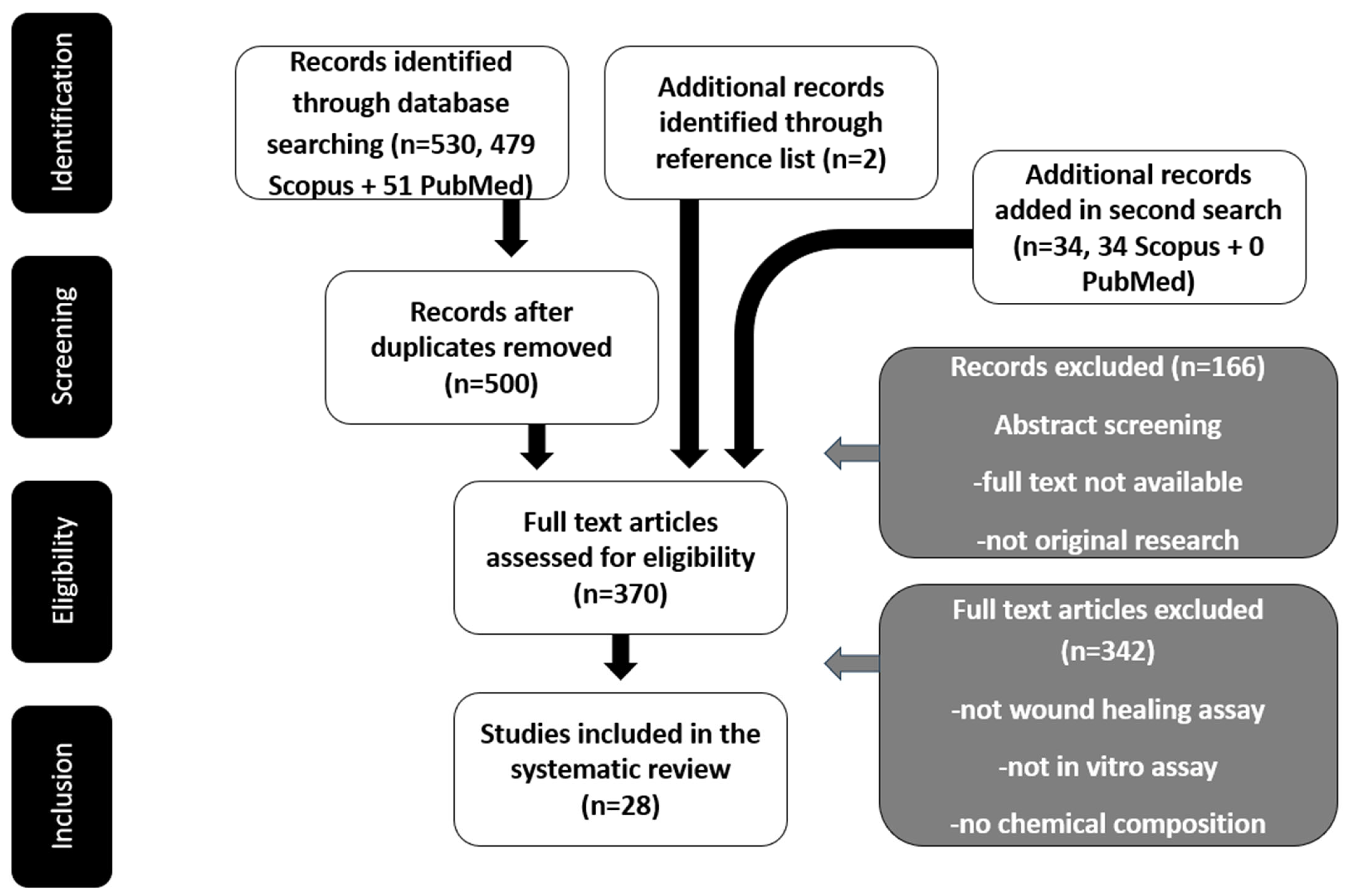
2. Methodology
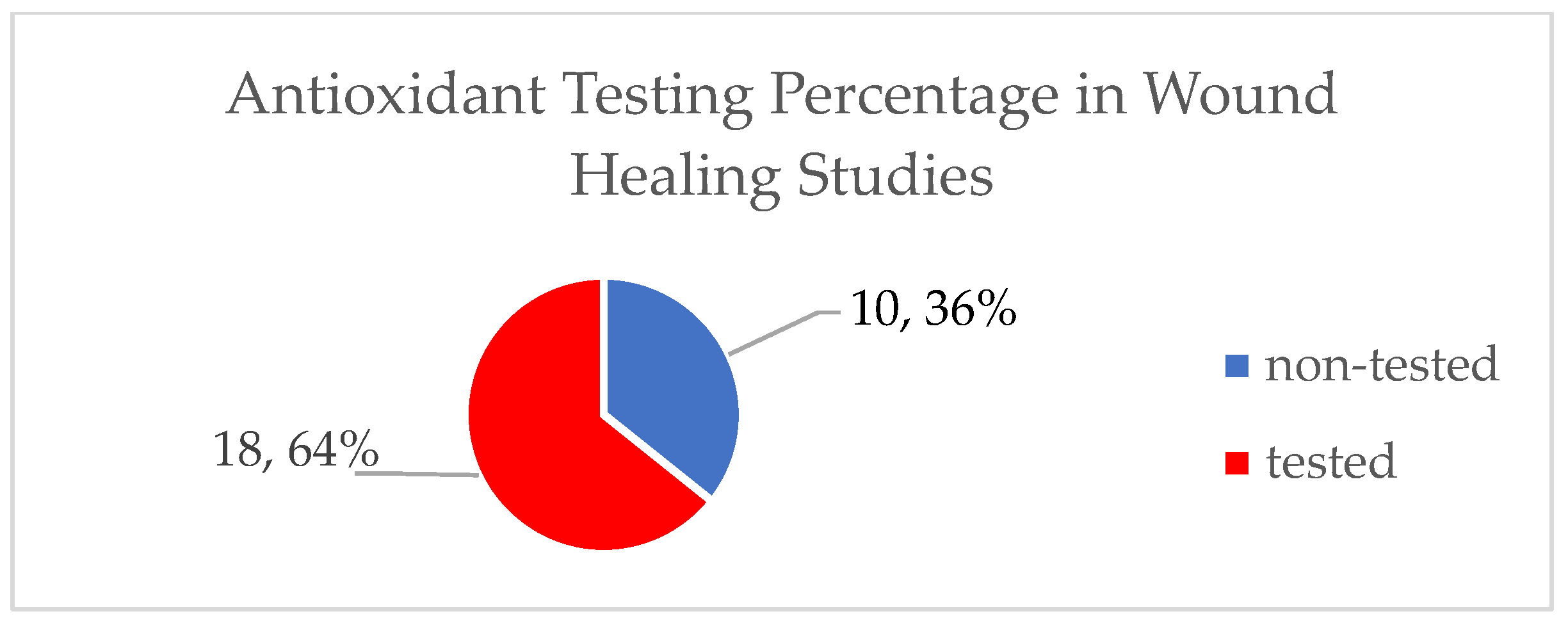
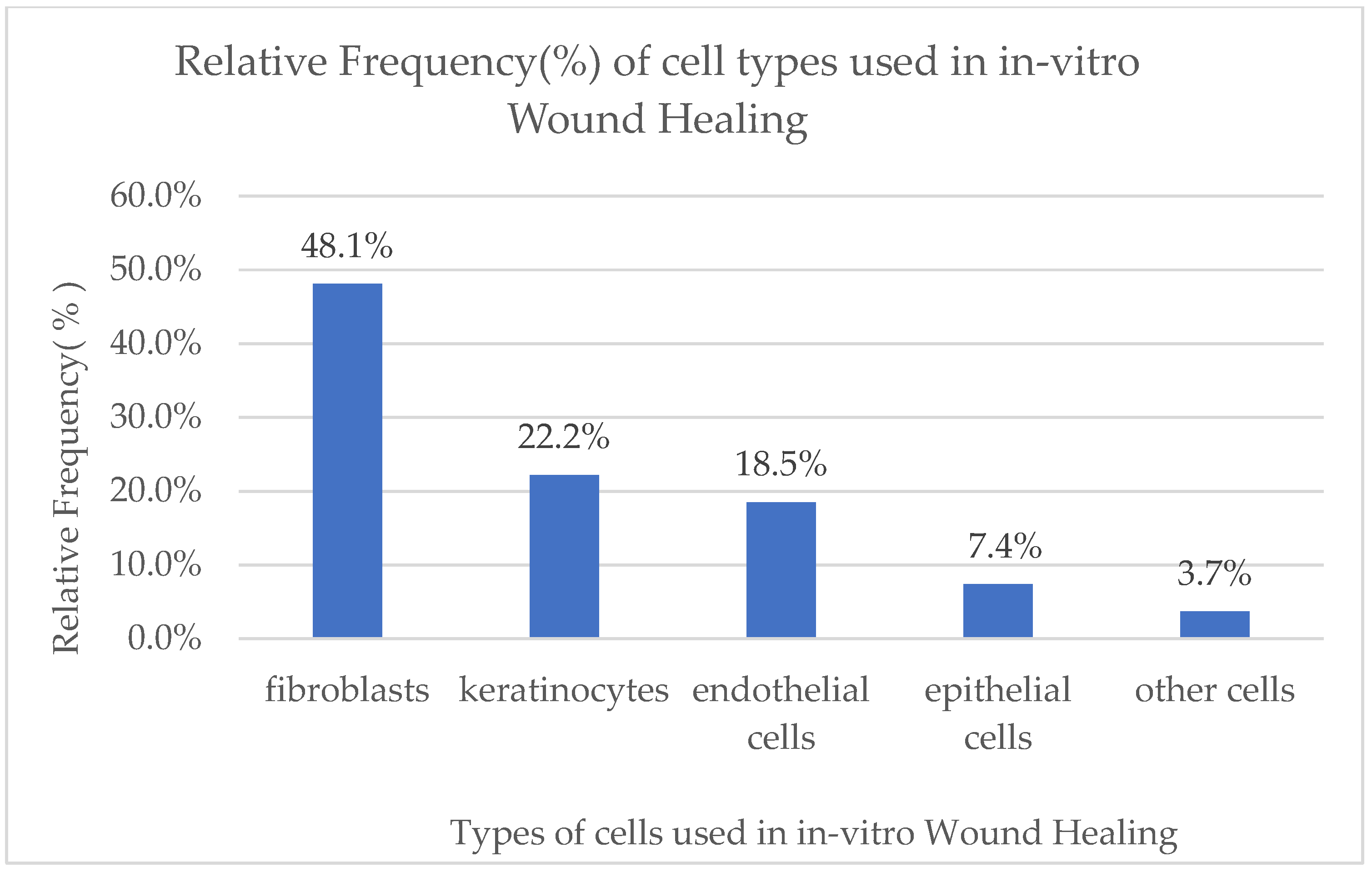
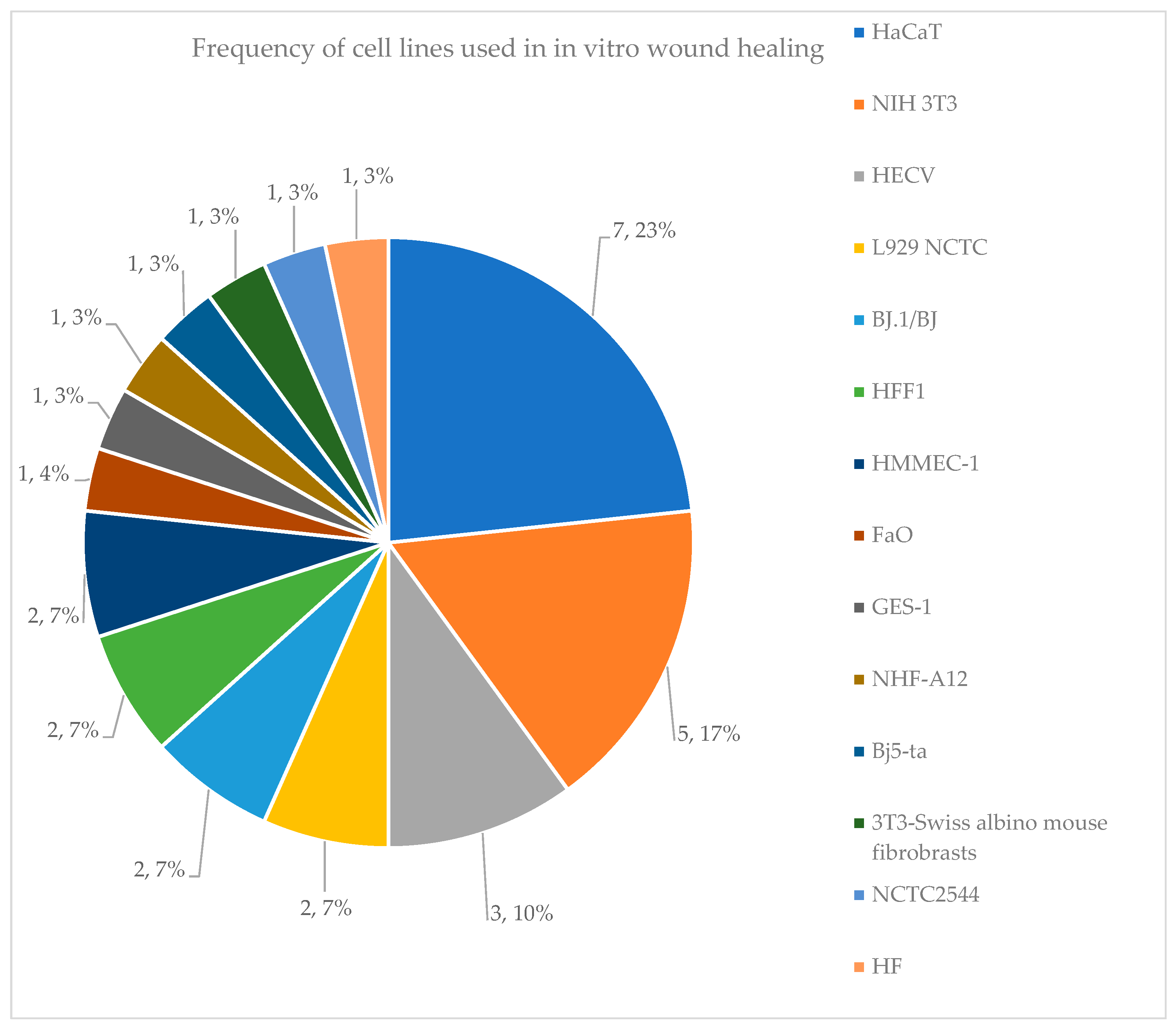
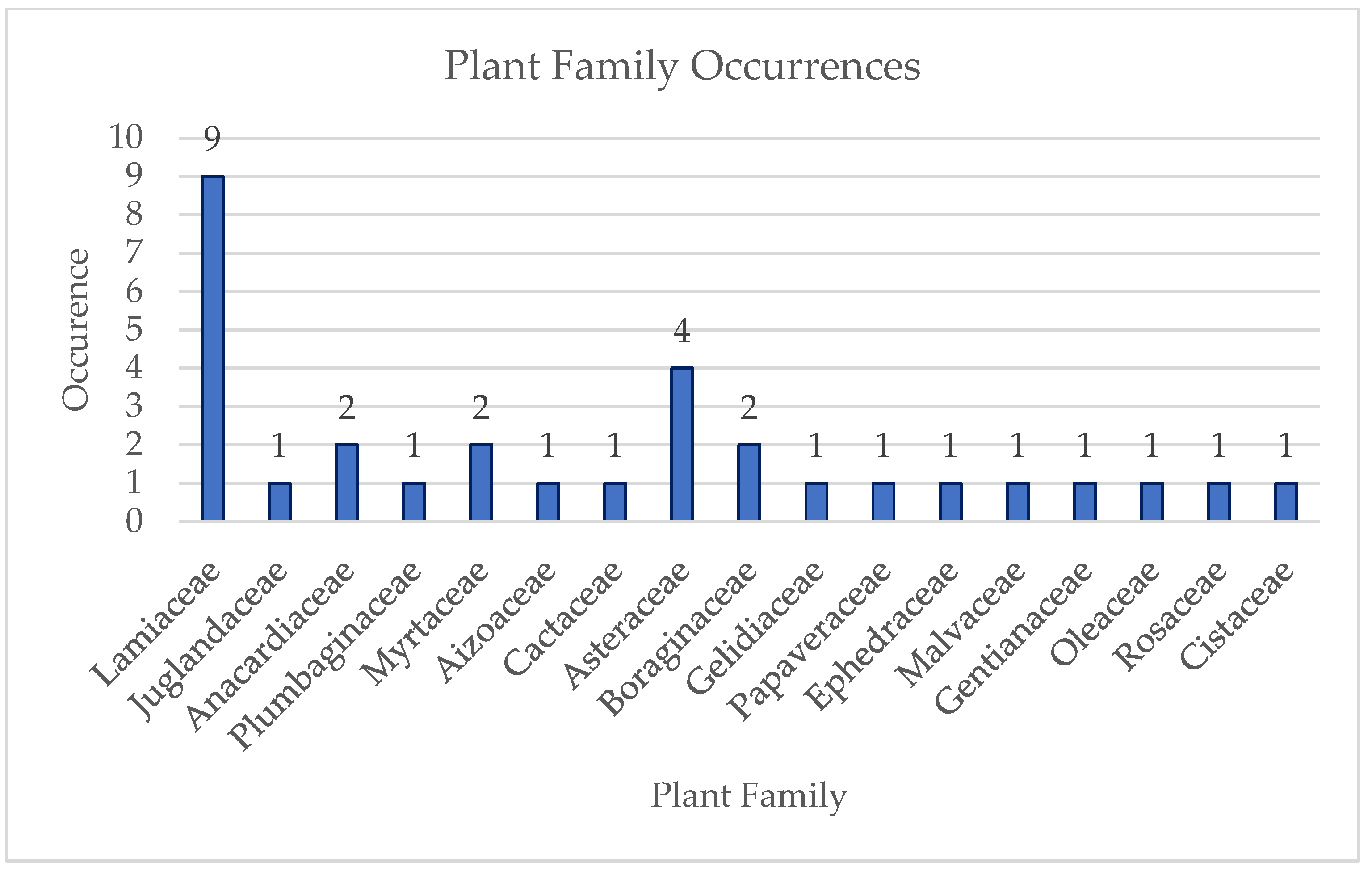
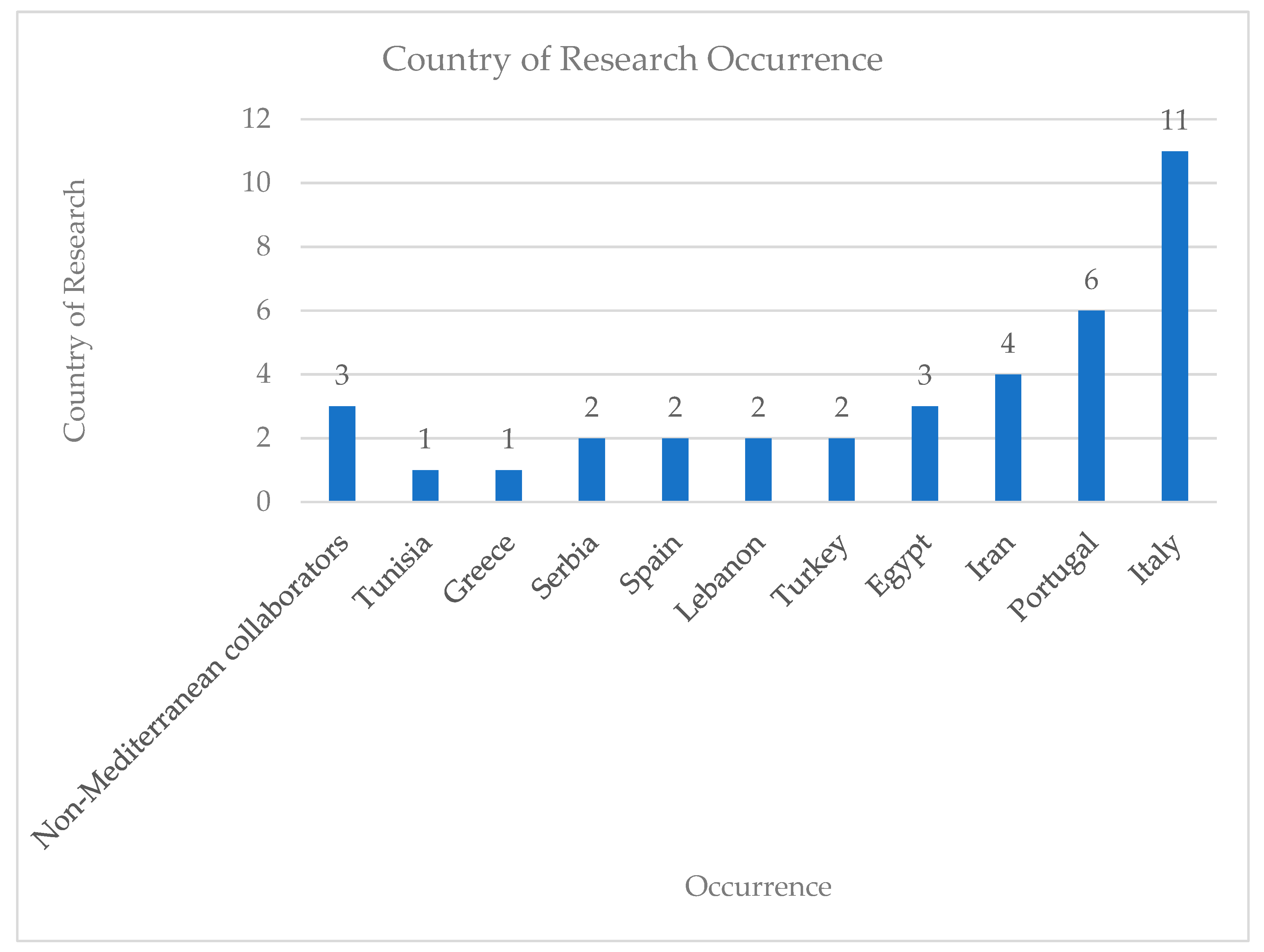
3. Results
3.1. Natural Products with Wound Healing Activity: Chemical Composition and Biological Activity
3.2. Calendula arvensis, Lavandula stoechas, and Helichrysum italicum Essential Oils
3.3. Carpobrotus edulis (L.) N.E.Br. Extract
3.4. Centaurium spicatum (L.) Fritch Extract
3.5. Bioactive Films Based on Chitosan and Cynara cardunculus Leaf Extracts
3.6. Extracts from Ephedra foeminea Forssk Fruits
3.7. Eucalyptus globulus Leaf EO and Extract
3.8. Fumaria parviflora
3.9. Gelidium corneum Extracts
3.10. Helichrysum italicum Hydrolate
3.11. Juglans regia Leaves
3.12. Lavandula austroapenina (Lamiaceae)
3.13. Limonium pruinosum (L.) Extracts
3.14. Malva sylvestris Extracts
3.15. Olive Mill Wastewater Extracts
3.16. Onosma dichroantha Boiss Root Extracts
3.17. Egyptian Opuntia ficus-indica Seed Oils
3.18. Oregano (Origanum vulgare L.) Essential Oil
3.19. Phlomis Rigida Labill. Extracts
3.20. Pistachia vera L. Hull Extract
3.21. Butter Oil (Ghee) Enrichment with Rosemary and Clove Plants
3.22. Active Metabolites from Salvia officinalis L.
3.23. Santolina rosmarinifolia L. Essential Oil
3.24. Ethanolic Extract of Sarcopoterium spinosum Fruits
3.25. Thymbra spicata L. Extracts
3.26. Thymus mastichina (L.) L. and Cistus ladanifer L.
3.27. Thymus sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas Extracts
| No. | Natural Complex Mixtures | Cell Lines of In Vitro Wound Healing | Wound Closure Rate | Ref. |
|---|---|---|---|---|
| 1 | Calendula arvensis L. (C), Helichrysum italicum (Roth) Don subsp. Microphyllum (Willd.) Nym. (H), Lavandula stoechas L. Post-distillation Waste Extracts (L) | HFF1 | (-) Control1: 16.2% ± 2.5% 24 h | [16] |
| (-) Control1: 20.4% ± 0.4% 48 h | ||||
| (-) Control1: 21% ± 0.0% 72 h | ||||
| C: 21.3% ± 1.68% (1 μL/mL), 20.3% ± 2.9% (5 μL/mL), 20.1% ± 2.5% (10 μL/mL) 24 h | ||||
| C: 26.1% ± 0.8% (1 μL/mL), 22.8% ± 0.4% (5 μL/mL), 23.1% ± 0.6% (10 μL/mL) 48 h | ||||
| C: 27.2% ± 0.0% (1 μL/mL), 23.8% ± 0.0% (5 μL/mL), 24.1% ± 0.0% (10 μL/mL) 72 h | ||||
| L: 21.7% ± 3% (1 μL/mL), 16.7% ± 1.3% (5 μL/mL), 14.1% ± 3.2% (10 μL/mL) 24 h | ||||
| L: 27.4% ± 1.9% (1 μL/mL), 21.7% ± 1.7% (5 μL/mL), 21% ± 3.6% (10 μL/mL) 48 h | ||||
| L: 29.2% ± 0.5% (1 μL/mL), 21.1% ± 0.6% (5 μL/mL), 23.85% ± 0.6% (10 μL/mL) 72 h | ||||
| H: 14.2% ± 3.4% (1 μL/mL), 17.1% ± 3% (5 μL/mL), 14.4% ± 1.9% (10 μL/mL) 24 h | ||||
| H: 19.3% ± 0.2% (1 μL/mL), 22.8% ± 0.3% (5 μL/mL), 16% ± 0.0% (10 μL/mL) 48 h | ||||
| H: 19.9% ± 0.0% (1 μL/mL), 23.6% ± 0.0% (5 μL/mL), 16.4% ± 0.0% (10 μL/mL) 72 h | ||||
| (-) Control2: 24.9% ± 1.8% 24 h | ||||
| (-) Control2: 30.6% ± 2.1% 48 h | ||||
| (-) Control2: 31.8% ± 0.0% 72 h | ||||
| CLH: 27.6% ± 1.6% (1 μL/mL), 21.7% ± 2.7% (5 μL/mL), 19.5% ± 4.2% (10 μL/mL) 24 h | ||||
| CLH: 31% ± 0.0% (1 μL/mL), 29.2% ± 0.9% (5 μL/mL), 27.2% ± 2.5% (10 μL/mL) 48 h | ||||
| CLH: CLOSED (1 μL/mL), 31.2% ± 0.0% (5 μL/mL), 32.3% ± 1.7% (10 μL/mL) 72 h | ||||
| CL: 24.2% ± 3.8% (1 μL/mL), 18.4% ± 3.1% (5 μL/mL), 14.1% ± 1.9% (10 μL/mL) 24 h | ||||
| CL: 29.8% ± 0.8% (1 μL/mL), 27.4% ± 1.9% (5 μL/mL), 22.6% ± 2.2% (10 μL/mL) 48 h | ||||
| CL: 30.4% ± 0.0% (1 μL/mL), 30.1% ± 0.2% (5 μL/mL), 28% ± 1.6% (10 μL/mL) 72 h | ||||
| CH: 23.8% ± 2% (1 μL/mL), 20.8% ± 2.4% (5 μL/mL), 17.6% ± 2.4% (10 μL/mL) 24 h | ||||
| CH: 30.6% ± 0.0% (1 μL/mL), 32.1% ± 0.6% (5 μL/mL), 30% ± 1.5% (10 μL/mL) 48 h | ||||
| CH: CLOSED (1 μL/mL), 33% ± 0.0% (5 μL/mL), 32.6% ± 0.0% (10 μL/mL) 72 h | ||||
| LH: 24.7% ± 1.3% (1 μL/mL), 20.8% ± 2.4% (5 μL/mL), 18.6% ± 1.5% (10 μL/mL) 24 h | ||||
| LH: 30.9% ± 0.7% (1 μL/mL), 28% ± 0.9% (5 μL/mL), 24.2% ± 1.9% (10 μL/mL) 48 h | ||||
| LH: 31.7% ± 0.0% (1 μL/mL), 30.2% ± 0.2% (5 μL/mL), 28.7% ± 1.1% (10 μL/mL) 72 h | ||||
| 2 | Carpobrotus edulis L. N.E.Br. Extract (CAE) | HaCaT | (-) Control: ~38% | [17] |
| CAE: 83% 24 h (500 μg/mL) | ||||
| (+) Control-Alantoin: 100% 24 h (50 μg/mL) | ||||
| 3 | Centaurium spicatum (L.) Fritch extracts | HaCaT | Control: 0.083 ± 0.008% 48 h | [18] |
| Cs1: 10.210 ± 1.110% 48 h (400 μg/mL) | ||||
| Cs2: 14.230 ± 1.070% 48 h (400 μg/mL) | ||||
| Cs3: 41.980 ± 2.130% 48 h (400 μg/mL) | ||||
| Cs4: 27.660 ± 1.990% 48 h (400 μg/mL) | ||||
| Cs5: 15.130 ± 2.150% 48 h (400 μg/mL) | ||||
| Cs6: 8.510 ± 0.950% 48 h (400 μg/mL) | ||||
| Cs7: 9.930 ± 0.170% 48 h (400 μg/mL) | ||||
| Cs8: 6.210 ± 0.910% 48 h (400 μg/mL) | ||||
| Cs9: 7.580 ± 1.710% 48 h (400 μg/mL) | ||||
| 4 | Cynara cardunculus Ultrasonic Assisted Extraction Pulsed Ethanolic Extract (EtPUAE) in Chitosan Films (CF) (CF + EtPUAE) | Bj5-ta | (-) Control: 13% ± 3% 7 h | [20] |
| CF: 17% ± 9% 7 h | ||||
| CF + 1% EtPUAE: 16% ± 6% 7 h | ||||
| (-) Control: 78% ± 6% 24 h | ||||
| CF: 72% ± 8% 24 h | ||||
| CF + 1% EtPUAE: 85% ± 9% 24 h | ||||
| 5 | Ephedra foeminea Forssk fruits Ethanol (EE), Methanol/Water (EMW), Hexane (Ehex), Ethyl Acetate/Water (Epoly) extracts | HECV | (-) Control = 66% 24 h | [23] |
| Epoly = 75% 24 h (25 μg/mL) | ||||
| Epoly = 77% 24 h (50 μg/mL) | ||||
| 6 | Eucalyptus globulus leaf Essential Oil (EO), Hydrodistillation Residual Water Extract (HRW) | NIH 3T3 | No significant difference compared to control but slight increase in wound healing | [26] |
| 7 | Fumaria parviflora Isoquinoline Alkaloids | HF | (-) Control: 47% 24 h, 62.6% 48 h, 72% 72 h | [28] |
| 9: 67% 24 h, 86% 48 h, 94% 72 h (1 μg/mL) | ||||
| 15: 67% 24 h, 83.8% 48 h, 92.4% 72 h (1 μg/mL) | ||||
| 12: 64% 24 h, 83% 48 h, 91% 72 h (1 μg/mL) | ||||
| 3: 64% 24 h, 82% 48 h, 90.5% 72 h (1 μg/mL) | ||||
| 13: 62% 24 h, 78% 48 h, 88.4% 72 h (1 μg/mL) | ||||
| 1: 60% 24 h, 71% 48 h, 85.4% 72 h (1 μg/mL) | ||||
| 14: 55.6% 24 h, 67.5% 48 h, 76.4% 72 h (1 μg/mL) | ||||
| 4: 51% 24 h, 64.4% 48 h, 75% 72 h (1 μg/mL) | ||||
| 8 | Gelidium corneum Aqueous/Ethanol Extract Fractions (F1–F5) | HaCaT | (-) Control: 30% 12 h | [29] |
| F2: 76.76% ± 10.02% 12 h (600 μg/mL) | ||||
| F5: 61.83% ± 7.25% 12 h (1000 μg/mL) | ||||
| F1, F3, F4: no effect | ||||
| 9 | Juglans regia leaf Aqueous/Ethanol Extract | HaCaT | Control: 26.24% ± 2.44% 24 h | [34] |
| Extract: 27.86% ± 3.68% 24 h (200 μg/mL) | ||||
| 10 | Lavandula austroapennina Alcoholic Extracts | HaCaT | Corolla: 20.55% 6 h (1 μg/mL) | [36] |
| Corolla: 100% 24 h (1 μg/mL) | ||||
| Leaf: 33.76% 6 h (1 μg/mL) | ||||
| Leaf: 100% 24 h (1 μg/mL) | ||||
| Stem: 36.79% 6 h (1 μg/mL) | ||||
| Stem: 100% 24 h (1 μg/mL) | ||||
| 11 | Limonium pruinosum extract | GES-1 | (-) Control: 68.3637% ± 2.32% 48 h | [37] |
| EtOAc Extract: 79.9343% ± 1.98% 48 h | ||||
| 12 | Malva sylvestris Extracts (Malva) | HaCaT | (-) Control: ~30% 48 h | [39] |
| 50:50: ~55% 48 h | ||||
| 70:30: ~50% 48 h | ||||
| Malva 50:50: ~85% 48 h (1 mg/mL) | ||||
| Malva 50:50: ~85% 48 h (2 mg/mL) | ||||
| Malva 50:50: ~95% 48 h (4 mg/mL) | ||||
| Malva 70:30: ~85% 48 h (1 mg/mL) | ||||
| Malva 70:30: ~98% 48 h (2 mg/mL) | ||||
| Malva 70:30: 100% 48 h (4 mg/mL) | ||||
| 13 | Olive Mill Wastewater Biopolymer Pectin/Ethanolic Extract | BJ, HaCaT | BJ (-) Control: ~23% 24 h | [41] |
| BJ Pectin (25 μg/mL): ~35% 24 h | ||||
| BJ Pectin (100 μg/mL): ~35% 24 h | ||||
| BJ PELAVF (25 μg/mL): ~30% 24 h | ||||
| BJ PELAVF (100 μg/mL): ~75% 24 h | ||||
| HaCaT (-) Control: ~47% 24 h | ||||
| HaCaT Pectin (25 μg/mL): ~40% 24 h | ||||
| HaCaT Pectin (100 μg/mL): ~50% 24 h | ||||
| HaCaT PELAVF (25 μg/mL): ~70% 24 h | ||||
| HaCaT PELAVF (100 μg/mL): ~80% 24 h | ||||
| 14 | Onosma dichroantha Boiss. Cyclohexane Extract fractions (CE) | HMEC-1 | (-) Control: 34% 16 h | [43] |
| (-) Control: 42% 24 h | ||||
| FR.F: 76% 16 h (250 μg/mL) | ||||
| FR.F: 76% 24 h (250 μg/mL) | ||||
| 15 | Onosma dichroantha Boiss. Root Cyclohexane Extract (CE), Ethyl Acetate (EtOAc), Methanol (MeOH) Extracts | HMEC-1 | (-) Control: 30% 16 h | [42] |
| (-) Control: 45% 24 h | ||||
| CE: 70% 16 h (125 μg/mL) | ||||
| CE: 92% 24 h (125 μg/mL) | ||||
| 16 | Opuntia ficus-indica seed oils Soxhlet extract (SE) and Ultrasound-Assisted Extract (UAE) | BJ.1 | (-) Control: ~20% 24 h | [44] |
| SE: 73% 24 h (100 μg/mL) | ||||
| UAE: 85% 24 h (100 μg/mL) | ||||
| 17 | Origanum vulgare L. Essential Oil (OEO) | NCTC2544 | (-) Control: ~25% 48 h | [45] |
| OEO: ~63% 48 h (25 μg/mL) | ||||
| (-) Control: ~75% 72 h | ||||
| OEO: ~95% 48 h (25 μg/mL) | ||||
| 18 | Phlomis rigida Labill. MeOH Extract | L929 NCTC | (-) Control: 42% 8 h | [48] |
| M.E.: 45.72% 8 h (0.125 mg/mL) | ||||
| M.E.: 49.71% 8 h (0.25 mg/mL) | ||||
| M.E.: 50.1% 8 h (0.5 mg/mL) | ||||
| 19 | Pistacia vera Anacardic Acid (13:0) From Hull Extract (AA 13:0) | NIH 3T3 | (+) Control-Alantoin(50 μg/mL): +33.67% compared to negative control 48 h | [60] |
| (AA 13:0): +31.92% compared to negative control 48 h (1.25 μg/mL) | ||||
| (AA 13:0): +20.06% compared to negative control 48 h (2.5 μg/mL) | ||||
| (AA 13:0): +29.12% compared to negative control 48 h (5 μg/mL) | ||||
| 20 | Pistacia vera L. Hull MeOH Extracts (Hexane-CHCl3, CHCl3-EtOAc, n-BuOH) | NIH 3T3 | (+) Control-Alantoin: 48 h (50 μg/mL) | [57] |
| CHCl3: +49.37% more than (+) Control 48 h (0.02 μg/mL) | ||||
| CHCl3 (fr2): +61.6% more than (+) Control 48 h (169 μg/mL) | ||||
| CHCl3 (fr4): +68.2% more than (+) Control 48 h (0.02 μg/mL) | ||||
| CHCl3 (fr4.III): +76.64% more than (+) Control 48 h (0.01 μg/mL) | ||||
| EtOAc: +15.9% more than (+) Control 48 h (0.04 μg/mL) | ||||
| N-BuOH: +34.9% more than (+) Control 48 h (0.2 μg/mL) | ||||
| 21 | Rosmarinus officinalis Enriched Ghee (RG) | NHF-A12 | (+) Control-EGF(10 nM): 33.23% 12 h | [62] |
| Non-enriched ghee: 29.6% 12 h | ||||
| RG: 45.27% 12 h (50 μg/mL) | ||||
| (-) Control: ~21.9% 12 h | ||||
| (+) Control-EGF(10 nM): 91.57% 24 h | ||||
| Non-enriched ghee: 100% 24 h | ||||
| RG: 100% 24 h (50 μg/mL) | ||||
| (-) Control: 81.84% 24 h | ||||
| 22 | Salvia officinalis Aqueous Extract | NIH 3T3 | (+) Control-FBS 15%: 80% 24 h | [63] |
| (-) Control: 37% 24 h | ||||
| Salvianolic Acid K: 70% 24 h (1 μg/mL) | ||||
| 23 | Santolina rosmarinifolia Essential Oil (EO) | NIH 3T3 | (-) Control: 81% 18 h | [64] |
| EO: 91% 18 h (0.58 mg/mL) | ||||
| 24 | Sarcopoterium spinosum Fruit Ethanolic Extract (SEE) | HECV | (-) Control: 54% 24 h | [65] |
| H2 O2: 25% 24 h (30μM) | ||||
| Counteraction: SEE: 60% 24 h (10 μg/mL) | ||||
| Counteraction: Corilagin (Cg): 62% 24 h (10 μg/mL) | ||||
| Counteraction: Quercentin (Qu): ~30% 24 h (10 μg/mL) | ||||
| Protection: SEE: 64% 24 h (10 μg/mL) | ||||
| Protection: Cg: 68% 24 h (10 μg/mL) | ||||
| Protection: Qu: ~25% 24 h (10 μg/mL) | ||||
| 25 | Syzygium aromaticum Enriched Ghee (CG) | NHF-A12 | (+) Control-EGF (10 nM): 33.23% 12 h | [62] |
| Non-enriched ghee: 29.6% 12 h | ||||
| CG: 44.54% 12 h (50 μg/mL) | ||||
| (-) Control: 21.9% 12 h | ||||
| (+) Control-EGF (10 nM): 91.57% 24 h | ||||
| Non-enriched ghee: 100% 24 h | ||||
| CG: 100% 24 h (50 μg/mL) | ||||
| (-) Control: 81.84% 24 h | ||||
| 26 | Thymbra spicata L. Aqueous Extract (T.W.) | FaO, HECV | (-) Control (HECV): 60% 24 h | [68] |
| T.W. (HECV): 77% 24 h (1.5 μg/mL) | ||||
| Thymbra spicata L. Ethanol Extract (T.E.) | (-) Control (HECV): 60% 24 h | |||
| T.W. (HECV): 68% 24 h (1.5 μg/mL) | ||||
| 27 | Thymus mastichina (L.) L. Essential Oil (TMEO), Hydrolate (TMH) Extracts | L929 NCTC | (+) Control-Alantoin (1 μg/mL): +49.2% than (-) control 12 h | [71] |
| TMEO: ~+10% than (-) control 12 h (0.002% v/v) | ||||
| TMH: +25.1% than (-) control 12 h (2% v/v) | ||||
| DMSO (0.002% v/v):~−10% than (-) control 12 h | ||||
| Cistus ladanifer L. Essential Oil (CLEO), Hydrolate (CLH) Extracts | L929 NCTC | (+) Control-Alantoin (1 μg/mL): +51.8% than (-) control 12 h | ||
| CLEO: +55.7% than (-) control 12 h (0.002% v/v) | ||||
| CLH: +48.4% than (-) control 12 h (2% v/v) | ||||
| DMSO (0.002%v/v):~−10% than (-) control 12 h | ||||
| 28 | Thymus Sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas Soxhlet Ethanol (SE), Soxhlet n-Hexane (SN), Soxhlet n-Hexane/Ethanol (SNE), Soxhlet Ethanol/n-Hexane (SEN), Maceration Ethanol (ME), Maceration n-Hexane (MN), Maceration n-Hexane/Ethanol (MNE), Maceration Ethanol/n-Hexane (MEN), Decoction (D), and Infusion (I) TS Extracts | NIH 3T3 | (+) Control-FGF: 100% | [72] |
| (-) Control: 19.4% | ||||
| TS Extracts: 35.74% −82.96% (D~3.6 x (-) Control, I~4.2 x (-) Control) |
| No. | Natural Complex Mixtures | Antioxidant Assay | Antioxidant Activity Results | Ref. |
|---|---|---|---|---|
| 1 | Calendula arvensis L. (C) Helichrysum italicum (Roth) Don subsp. Microphyllum (Willd.) Nym. (H) Lavandula stoechas L. Post-distillation Waste Extract (L) | ABTS | ABTS: LH IC50 = 8.54 ± 0.82 μg/mL | [16] |
| 2 | Carpobrotus edulis L. N.E.Br. Extract (CAE) | FRAP, ORAC, TEAC, DPPH | FRAP: IC50 = 30.37 μg/mL ORAC: IC50 = 4.41 μg/mL TEAC: IC50 = 36.52 μg/mL DPPH: IC50 = 112.89 μg/mL | [17] |
| 3 | Centaurium spicatum (L.) Fritch Extracts | FRAP, ABTS, DPPH, -OH Scavencging (DEPMPO/OH) | Cs1: ABTS = 23.46 ± 0.45 mmol GAE/100 mg FRAP = 45.40 ± 1.07 mmol GAE/100 mg DPPH = ~40% DEMPO/OH = ~50% | [18] |
| Cs2: ABTS = 37.74 ± 0.74 mmol GAE/100 mg FRAP = 55.13 ± 0.62 mmol GAE/100 mg DPPH = ~43% DEMPO/OH = ~55% | ||||
| Cs3: ABTS = 36.76 ± 0.55 mmol GAE/100 mg FRAP = 53.84 ± 1.29 mmol GAE/100 mg DPPH = ~43% DEMPO/OH = ~55% | ||||
| Cs4: ABTS = 34.47 ± 0.63 mmol GAE/100 mg FRAP = 50.99 ± 0.38 mmol GAE/100 mg DPPH = ~35% DEMPO/OH = ~60% | ||||
| Cs5: ABTS = 21.39 ± 0.23 mmol GAE/100 mg FRAP = 2.70 ± 0.85 mmol GAE/100 mg, DPPH = ~35% DEMPO/OH = ~55% | ||||
| Cs6: ABTS = 29.75 ± 0.55 mmol GAE/100 mg FRAP = 62.41 ± 1.30 mmol GAE/100 mg DPPH = ~35% DEMPO/OH = ~58% | ||||
| Cs7: ABTS = 27.42 ± 0.77 mmol GAE/100 mg FRAP = 54.20 ± 1.34 mmol GAE/100 mg DPPH = ~35% DEMPO/OH = ~55% | ||||
| Cs8: ABTS = 27.18 ± 0.74 mmol GAE/100 mg FRAP = 47.90 ± 1.43 mmol GAE/100 mg DPPH = ~23% DEMPO/OH = ~55% | ||||
| Cs9: ABTS = 24.62 ± 0.69 mmol GAE/100 mg FRAP = 48.51 ± 1.11 mmol GAE/100 mg DPPH = ~25% DEMPO/OH = ~40% | ||||
| 4 | Cistus ladanifer L. Essential Oil (CLEO), Hydrolate (CLH) Extracts | DPPH | Poor Antioxidant Capacity | |
| 5 | Ephedra foeminea Forssk fruits Ethanol (EE), Methanol/Water (EMW), Hexane (Ehex), Ethyl Acetate/Water (Epoly) Extracts | DPPH, ABTS, FRAP, DCFDA (HECV), TBARS (HECV) | DPPH IC50: Epoly= 0.99 ± 0.059 mg/mL EMW= 3.2 ± 0.069 mg/mL EE= 4.88 ± 0.11 mg/mL Ehex > 10 mg/mL | [23] |
| ABTS IC50: Epoly = 0.12 ± 0.03 mg/mL EMW = 0.23 ± 0.005 mg/mL EE = 5.2 ± 0.012 mg/mL Ehex > 10 mg/mL | ||||
| FRAP TEAC: Epoly = 0.37 ± 0.018 mmol TE EMW = 0.15 ± 0.014 mmol TE EE = 0.13 ± 0.01 mmol TE Ehex = N.D. mmol TE | ||||
| DCFDA: +Control (H2O2) = +44% 30 μM 24 h Epoly = −53% 25 μg/mL & −47% 50 μg/mL 24 h EMW = no effect 24 h EE = −45% 50 μg/mL 24 h | ||||
| TBARS: +control (MDA) = +56% 30 μM Epoly = −39% 25 μg/mL and 50 μg/mL EMW = no effect EE = no effect | ||||
| 6 | Gelidium corneum Aqueous/Ethanol Extract Fractions (F1-F5) | DPPH, FRAP, ORAC | DPPH EC50: F1 > 1000 μg/mL F2 = 991.6 μg/mL F3 = 399.6 μg/mL F4 = 973.1 μg/mL F5 > 1000 μg/mL BHT (+Control) = 184.7 μg/mL FRAP: F1 = 31.13 ± 1.31 μM FeSO4/g F2 = 19.22 ± 2.46 μM FeSO4/g F3 = 49.02 ± 5.27 μM FeSO4/g F4 = 19.11 ± 2.54 μM FeSO4/g F5 = 27.96 ± 3.1 μM FeSO4/g BHT (+Control) = 1948 ± 239.1 μM FeSO4/g ORAC: F1 = 46.8 ± 1.17 μmol Trolox/g F2 = 2868 ± 72.29 μmol Trolox/g F3 = 2916 ± 132.8 μmol Trolox/g F4 = 3060 ± 222.2 μmol Trolox/g F5 = 57.58 ± 4.26 μmol Trolox/g BHT (+Control) = 136.4 ± 9.09 μmol Trolox/g | [29] |
| 7 | Juglans regia leaf Aqueous-Ethanol Extract | DPPH, Ferric Reducing Power (FRAP), OxHLIA, TBARS (HaCaT) | DPPH: EC50 = 137 ± 10 μg/mL RP: EC50 = 27.6 ± 0.02 μg/mL TBARS: EC50 = 11.83 ± 1.06 μg/mL OxHLIA: EC50 (60 min) = 10.8 ± 0.5 μg/mL EC50 (120 mins) = 51 ± 1 μg/mL | [34] |
| 8 | Lavandula austroapennina Alcoholic Extract | ABTS, DPPH, FRAP | - | [36] |
| 9 | Limonium pruinosum Extract | DPPH, Hydroxyl Radical Scavenging assay (HRSA) | DPPH: IC50 = 35.88 ± 2.2 μg/mL HRSA: IC50 = 65.87 ± 1.19 μg/mL | [37] |
| 10 | Malva sylvestris Extracts (Malva) | ABTS | ABTS: RSA(70:30) = 87% RSA(50:50) = 96% | [39] |
| 11 | Olive Mill Wastewater Biopolymer Pectin/Ethanolic Extract | DPPH, ABTS | DPPH IC50: LAVF = 0.095 ± 0.003 mg/mL ELAVF1S = 0.152 ± 0.005 mg/mL ELAVF1M = 0.245 ± 0.010 mg/mL ELAVF2S = 0.485 ± 0.021 mg/mL ELAVF2M = 0.599 ± 0.022 mg/mL ELAVF3S = 3.935 ± 0.174 mg/mL ELAVF3M = 1.725 ± 0.074 mg/mL ELAVF4S = 2.052 ± 0.095 mg/mL ELAVF4M = 4.351 ± 0.141 mg/mL ELAVF5S = 0.620 ± 0.017 mg/mL ELAVF5M = 1.028 ± 0.025 mg/mL | [41] |
| ABTS IC50: LAVF = 0.0185 ± 0.0007 mg/mL ELAVF1S = 0.0371 ± 0.0012 mg/mL ELAVF1M = 0.0574 ± 0.0021 mg/mL ELAVF2S = 0.1202 ± 0.0043 mg/mL ELAVF2M = 0.1458 ± 0.051 mg/mL ELAVF3S = 2.048 ± 0.0845 mg/mL ELAVF3M = 1.389 ± 0.0428 mg/mL ELAVF4S = 1.789 ± 0.0528 mg/mL ELAVF4M = 3.4862 ± 0.1415 mg/mL ELAVF5S = 0.2581 ± 0.0098 mg/mL ELAVF5M = 0.733 ± 0.0254 mg/mL | ||||
| 12 | Origanum vulgare L. Essential Oil (OEO) | DPPH, DCFDA (NCTC2544) | DPPH SC50: OEO = 114 ± 6 μg/mL BHT (+Control) = 8.6 ± 0.3 μg/mL DCFDA: INF-γ/Histamine = 0% reduction in ROS INF-γ/Histamine/Indomethacin: ~74% (10 μM) INF-γ/Histamine/OEO: ~41% (25 μg/mL) Control: ~25% | [45] |
| 13 | Phlomis rigida Labill. MeOH Extract | DPPH, ABTS, LOX inhibition | DPPH IC50: 0.89 mg/mL ABTS IC50: 0.99 mg/mL LOX inhibition IC50: 19.5 ± 2.8 μg/mL | [48] |
| 14 | Salvia officinalis Aqueous Extract | DCFDA (NIH 3T3) | DCFDA statistically significant effects: SOW_C (0.01 and 1 μg/mL) SOW_F (1 and 10 μg/mL) SOW_H (0.01 & 1 μg/mL) | [63] |
| 15 | Sarcopoterium spinosum Fruits Ethanolic Extract (SEE) | DCFDA (HECV), TBARS (HECV), GSH/GSSG ratio (HECV) | Counteraction: DCFDA: H2O2: +20% (30 μM) SEE: −22% (10 μg/mL) Cg: −33% (10 μg/mL) Qu: −22% (10 μg/mL) TBARS: H2O2: +97% (30 μM) SEE: −110% (10 μg/mL) Cg: −109% (10 μg/mL) Qu: −111% (10 μg/mL) GSH/GSSG: (-) Control: 9.5 ± 0.3 H2O2:6.3 ± 0.7 (30 μM) SEE:10.5 ± 2 (10 μg/mL) Cg:11.1 ± 1.1 (10 μg/mL) Qu: no effect (10 μg/mL). Protection: DCFDA: H2O2:+20% (30 μM) SEE: −24% (10 μg/mL) Cg: −24% (10 μg/mL) Qu: no effect (10 μg/mL) TBARS: H2O2: +63% (30 μM) SEE: −70% (10 μg/mL) Cg: −72% (10 μg/mL) Qu: −64% (10 μg/mL) GSH/GSSG: (-) Control: 9.5 ± 0.3 H2O2:6.6 ± 0.6 (30 μM) SEE:11.3 ± 0.5 (10 μg/mL) Cg:10.7 ± 1.3 (10 μg/mL) Qu:12.3 ± 1.6 (10 μg/mL) | [65] |
| 16 | Thymbra spicata L. Aqueous Extract (T.W.) | DPPH, DCFDA (HECV cells), TBARS (FaO) | DPPH IC50: 25.8 μg/mL ± 1.28 DCFDA 24 h (H2O2-insulted HECV cells): DCF = −58% (1.5 μg/mL) TBARS MDA level 24 h (+oleate/palmitate): FaO = −70% (0.15 μg/mL), −68% (1.5 μg/mL), −75% (15 μg/mL) HECV = −37% (1.5 μg/mL) | [68] |
| Thymbra spicata L. Ethanol Extract (T.E.) | DPPH, DCFDA (HECV cells), TBARS (FaO) | DPPH IC50: 24.5 μg/mL ± 0.9 DCFDA 24 h (H2O2-insulted HECV cells): DCF = −62%(1.5 μg/mL) TBARS MDA level 24 h (+oleate/palmitate): FaO = −110% (0.15 μg/mL), −110% (1.5 μg/mL), −102% (15 μg/mL) HECV = −58% (1.5 μg/mL) | ||
| 17 | Thymus mastichina (L.) L. Essential Oil (TMEO), Hydrolate (TMH) Extracts | DPPH | Poor Antioxidant Capacity | [71] |
| 18 | Thymus sipyleus Boiss. Subsp. rosulans (Borbas) jalas Soxhlet Ethanol (SE), Soxhlet n-Hexane (SN), Soxhlet n-Hexane/Ethanol (SNE), Soxhlet Ethanol/n-Hexane (SEN), Maceration Ethanol (ME), Maceration n-Hexane (MN), Maceration n-Hexane/Ethanol (MNE), Maceration Ethanol/n-Hexane (MEN), Decoction (D), and Infusion (I) TS Extracts | DPPH | DPPH IC50: SE = 121.63 ± 1.67 μg/mL, SNE = 100.21 ± 1.19 μg/mL, ME = 104.91 ± 1.04 μg/mL, MNE = 95.19 ± 1.62 μg/mL, D = 43.5 ± 1.02 μg/mL I = 87.38 ± 1.73 μg/mL Ascorbic Acid = 27.63 ± 1.12 μg/mL SN, SEN, MN, MEN = ND | [72] |
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
Glossary
References
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Shah, J.B. The History of Wound Care. J. Am. Col. Certif. Wound Spec. 2012, 3, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, S.; Farahpour, M.R. Topical Application of Salvia officinalis Hydroethanolic Leaf Extract Improves Wound Healing Process. Indian J. Exp. Biol. 2017, 55, 98–106. [Google Scholar] [PubMed]
- Alves-Silva, J.M.; Cocco, E.; Piras, A.; Gonçalves, M.J.; Silva, A.; Falconieri, D.; Porcedda, S.; Cruz, M.T.; Maxia, A.; Salgueiro, L. Unveiling the Chemical Composition and Biological Properties of Salvia cacaliifolia Benth. Essential Oil. Plants 2023, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Ongarora, B. Recent Technological Advances in the Management of Chronic Wounds: A Literature Review. Health Sci. Rep. 2022, 5, e641. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2015, 14, 89–96. [Google Scholar] [CrossRef]
- Johnson, J.B.; Broszczak, D.A.; Mani, J.S.; Anesi, J.; Naiker, M. A Cut above the Rest: Oxidative Stress in Chronic Wounds and the Potential Role of Polyphenols as Therapeutics. J. Pharm. Pharmacol. 2022, 74, 485–502. [Google Scholar] [CrossRef]
- PRISMA Statement. Available online: https://www.prisma-statement.org (accessed on 14 January 2025).
- Ruiz-Crespo, S.; Trejo-Gabriel-Galan, J.M.; Cavia-Saiz, M.; Muñiz, P. Coffee Component 3-Caffeoylquinic Acid Increases Antioxidant Capacity but Not Polyphenol Content in Experimental Cerebral Infarction. Neurochem. Res. 2012, 37, 1085–1090. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Mhimed, O.; Al- Alsadoon, L.; Taqa, G. Evaluating the Effect of Topical Flavonoid Luteolin on Skin Wound Healing in Rabbits. Egypt. J. Vet. Sci. 2023. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cheng, H.-L.; Kuan, Y.-H.; Liang, T.-J.; Chao, Y.-Y.; Lin, H.-C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Aldoghachi, F.E.H.; Noor Al-Mousawi, U.M.; Shari, F.H. Antioxidant Activity of Rosmarinic Acid Extracted and Purified from Mentha piperita. Arch. Razi Inst. 2021, 76, 1279–1287. [Google Scholar] [CrossRef]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast Proliferation and Migration in Wound Healing by Phytochemicals: Evidence for a Novel Synergic Outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Bazzicalupo, M.; Cornara, L.; Burlando, B.; Cascini, A.; Denaro, M.; Smeriglio, A.; Trombetta, D. Carpobrotus edulis (L.) N.E.Br. Extract as a Skin Preserving Agent: From Traditional Medicine to Scientific Validation. J. Integr. Med. 2021, 19, 526–536. [Google Scholar] [CrossRef]
- Božunović, J.; Ivanov, M.; Petrović, J.; Gašić, U.; Nakarada, Đ.; Milutinović, M.; Aničić, N.; Giba, Z.; Mišić, D.; Stojković, D. Solvent System-Guided Extraction of Centaurium spicatum (L.) Fritch Provides Optimized Conditions for the Biological and Chemical Characteristics of the Herbal Extracts. Pharmaceuticals 2023, 16, 245. [Google Scholar] [CrossRef]
- Brás, T.; Rosa, D.; Gonçalves, A.C.; Gomes, A.C.; Brazinha, C.; Neves, L.A.; Duarte, M.F.; Crespo, J.G. Fractionation of Cynara cardunculus Ethanolic Extracts Using Diananofiltration. Sep. Purif. Technol. 2021, 256, 117856. [Google Scholar] [CrossRef]
- Brás, T.; Rosa, D.; Gonçalves, A.C.; Gomes, A.C.; Alves, V.D.; Crespo, J.G.; Duarte, M.F.; Neves, L.A. Development of Bioactive Films Based on Chitosan and Cynara cardunculus Leaves Extracts for Wound Dressings. Int. J. Biol. Macromol. 2020, 163, 1707–1718. [Google Scholar] [CrossRef]
- Aoki, S.K.; Pamma, R.; Hernday, A.D.; Bickham, J.E.; Braaten, B.A.; Low, D.A. Contact-Dependent Inhibition of Growth in Escherichia Coli. Science 2005, 309, 1245–1248. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, M.; Liu, X.; Luo, L.; Yuan, Z.; Xia, Y.; Zeng, Y. Torsional Mode versus Conventional Ultrasound Mode Phacoemulsification: Randomized Comparative Clinical Study. J. Cataract Refract. Surg. 2007, 33, 287–292. [Google Scholar] [CrossRef]
- Khalil, M.; Khalifeh, H.; Saad, F.; Serale, N.; Salis, A.; Damonte, G.; Lupidi, G.; Daher, A.; Vergani, L. Protective Effects of Extracts From Ephedra Foeminea Forssk Fruits against Oxidative Injury in Human Endothelial Cells. J. Ethnopharmacol. 2020, 260, 112976. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Afonso, J.; Rodrigues, F.; Teixeira, J. Experimental Results and Characteristics of A Solar Concentrator with Tracking System. Supplement to: Barbosa, F.; Afonso, J.L.; Rodrigues, F.B.; Teixeira, J.C. Development of a solar concentrator with tracking System. Mech. Sci. 2016, 7, 233–245. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical Composition and Effect against Skin Alterations of Bioactive Extracts Obtained by the Hydrodistillation of Eucalyptus globulus Leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef]
- Ayyad, M.; Fakhry, A.; Moustafa, A. Plant Biodiversity in the Saint Catherine Area of the Sinai Peninsula, Egypt. Biodivers. Conserv. 2000, 9, 265–281. [Google Scholar] [CrossRef]
- Elsaid, M.B.; Elnaggar, D.M.; Owis, A.I.; AbouZid, S.F.; Eldahmy, S. Production of Isoquinoline Alkaloids from the In Vitro Conserved Fumaria Parviflora and Their In Vitro Wound Healing Activity. Nat. Prod. Res. 2022, 36, 2575–2579. [Google Scholar] [CrossRef]
- Matias, M.; Martins, A.; Alves, C.; Silva, J.; Pinteus, S.; Fitas, M.; Pinto, P.; Marto, J.; Ribeiro, H.; Murray, P.; et al. New Insights into the Dermocosmetic Potential of the Red Seaweed Gelidium Corneum. Antioxidants 2023, 12, 1684. [Google Scholar] [CrossRef]
- Matias, M.; Pinteus, S.; Martins, A.; Silva, J.; Alves, C.; Mouga, T.; Gaspar, H.; Pedrosa, R. Gelidiales Are Not Just Agar—Revealing the Antimicrobial Potential of Gelidium corneum for Skin Disorders. Antibiotics 2022, 11, 481. [Google Scholar] [CrossRef]
- Serra, D.; Cruciani, S.; Garroni, G.; Sarais, G.; Kavak, F.F.; Satta, R.; Montesu, M.A.; Floris, M.; Ventura, C.; Maioli, M. Effect of Helichrysum italicum in Promoting Collagen Deposition and Skin Regeneration in a New Dynamic Model of Skin Wound Healing. Int. J. Mol. Sci. 2024, 25, 4736. [Google Scholar] [CrossRef]
- Serra, D.; Bellu, E.; Garroni, G.; Cruciani, S.; Sarais, G.; Dessì, D.; Pashchenko, A.; Satta, R.; Montesu, M.A.; Nečas, A.; et al. Hydrolate of Helichrysum italicum Promotes Tissue Regeneration During Wound Healing. Physiol. Res. 2023, 72, 809–818. [Google Scholar] [CrossRef]
- Kiselova-Kaneva, Y.; Galunska, B.; Nikolova, M.; Dincheva, I.; Badjakov, I. High Resolution LC-MS/MS Characterization of Polyphenolic Composition and Evaluation Of Antioxidant Activity of Sambucus ebulus Fruit Tea Traditionally Used in Bulgaria as a Functional Food. Food Chem. 2022, 367, 130759. [Google Scholar] [CrossRef] [PubMed]
- Besrour, N.; Oludemi, T.; Mandim, F.; Pereira, C.; Dias, M.I.; Soković, M.; Stojković, D.; Ferreira, O.; Ferreira, I.C.F.R.; Barros, L. Article Valorization of Juglans regia Leaves as Cosmeceutical Ingredients: Bioactivity Evaluation and Final Formulation Development. Antioxidants 2022, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and Aromatic Lamiaceae Plants in Greece: Linking Diversity and Distribution Patterns with Ecosystem Services. Forests 2020, 11, 661. [Google Scholar] [CrossRef]
- Gravina, C.; Formato, M.; Piccolella, S.; Fiorentino, M.; Stinca, A.; Pacifico, S.; Esposito, A. Lavandula austroapennina (Lamiaceae): Getting Insights into Bioactive Polyphenols of a Rare Italian Endemic Vascular Plant. Int. J. Mol. Sci. 2023, 24, 8038. [Google Scholar] [CrossRef]
- Sultan, M.H.; Bedair, R.; Ragab, O.G.; Abd-ELShafy, E.; Mahfouz, A.Y.; Daigham, G.E. Biological Activities And Ecological Aspects of Limonium pruinosum (L.) Collected from Wadi Hof Eastern Desert, Egypt, as a Promising Attempt for Potential Medical Applications. Biomass Convers. Biorefinery 2023, 14, 23887–23907. [Google Scholar] [CrossRef]
- Barros, E.M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera Frugiperda on Different Hosts and Damage to Reproductive Structures in Cotton. Entomol. Exp. Appl. 2010, 137, 237–245. [Google Scholar] [CrossRef]
- Contardi, M.; Ayyoub, A.M.M.; Summa, M.; Kossyvaki, D.; Fadda, M.; Liessi, N.; Armirotti, A.; Fragouli, D.; Bertorelli, R.; Athanassiou, A. Self-Adhesive and Antioxidant Poly(Vinylpyrrolidone)/Alginate-Based Bilayer Films Loaded with Malva Sylvestris Extracts as Potential Skin Dressings. ACS Appl. Bio Mater. 2022, 5, 2880–2893. [Google Scholar] [CrossRef]
- Kowalska-Baron, A. Theoretical Insight into Antioxidant Mechanism of Caffeic Acid Against Hydroperoxyl Radicals in Aqueous Medium at Different pH-Thermodynamic and Kinetic Aspects. Int. J. Mol. Sci. 2024, 25, 12753. [Google Scholar] [CrossRef]
- Aiello, F.; Malivindi, R.; Motta, M.F.; Crupi, P.; Nicoletti, R.; Benincasa, C.; Clodoveo, M.L.; Rago, V.; Spizzirri, U.G.; Restuccia, D. Synthesis and Characterization of a Biopolymer Pectin/Ethanolic Extract from Olive Mill Wastewater: In Vitro Safety and Efficacy Tests on Skin Wound Healing. Int. J. Mol. Sci. 2023, 24, 5075. [Google Scholar] [CrossRef]
- Safavi, F.; Farimani, M.M.; Golalipour, M.; Leung, P.-C.; Lau, K.-M.; Kwok, H.-F.; Wong, C.-W.; Bayat, H.; Lau, C.B.-S. Investigations on the Wound Healing Properties of Onosma dichroantha Boiss Root Extracts. S. Afr. J. Bot. 2019, 125, 344–352. [Google Scholar] [CrossRef]
- Safavi, F.; Moridi Farimani, M.; Golalipour, M.; Bayat, H. In Vitro Wound Healing Potential of Cyclohexane Extract of Onosma Dichroantha Boiss. Based On Bioassay-Guided Fractionation. Sci. Rep. 2023, 13, 5018. [Google Scholar] [CrossRef]
- Badawy, E.; Saleh, I.A.; Shams, K.A.; Abel-Azim, N.S.; Abdur-Rahman, M.; Mahmoud, K.; Abd-Rabou, A.A.; Fahmi, A.A. Chemical Composition and Bioactivities of Egyptian Opuntia Ficus-Indica Seeds Oils Obtained by Conventional and Ultrasound-Assisted Extraction Techniques. Egypt. J. Chem. 2023, 66, 91–101. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Akaydin, H.D.; Elvin, N.; Andreopoulos, Y. The Performance of a Self-Excited Fluidic Energy Harvester. Smart Mater. Struct. 2012, 21, 025007. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in Neurodegenerative Diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Okur, M.E.; Karadağ, A.E.; Özhan, Y.; Sipahi, H.; Daylan, B.; Demirci, B.; Demirci, F. Anti-Inflammatory, Analgesic and In Vivo-In Vitro Wound Healing Potential of the Phlomis Rigida Labill. Extract. J. Ethnopharmacol. 2021, 266, 113408. [Google Scholar] [CrossRef]
- Süntar, I.; Küpeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the Wound Healing Potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of Apigenin as an Active Component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef]
- Mathai, R.V.; Sar, S.K.; Mitra, J.C.; Jindal, M.K.; Wang, F. Ethanolic Extraction and GC-MS Analysis Of Antioxidant and Anticancer Bioactive Compounds from Mentha Arvensis and Aegle marmelos. Nat. Prod. Res. 2024, 1–7. [Google Scholar] [CrossRef]
- Baishya, T.; Das, P.; Ashraf, G.J.; Dua, T.K.; Paul, P.; Nandi, G.; Jajo, H.; Dutta, A.; Kumar, A.; Bhattacharya, M.; et al. Antioxidant Activity, Cytotoxicity Assay, and Evaluation Of Bioactive Compounds Using GC-MS and HPTLC-Based Bioassay in the Extracts of Osbeckia stellata var. crinita (Benth. ex Naudin) Grown in Manipur, India. Kuwait J. Sci. 2024, 51, 100229. [Google Scholar] [CrossRef]
- López, P.L.; Guerberoff, G.K.; Grosso, N.R.; Olmedo, R.H. Antioxidant-Efficient Indicator Determinate by the Relationship Between β-Myrcene/Caryophyllene (α, β) on Hop (Humulus lupulus) Essential Oils under an Accelerated Oxidation Test. Ind. Crops Prod. 2023, 205, 117399. [Google Scholar] [CrossRef]
- Ebrahimzadeh Mousavi, H.; Taheri Mirghaed, A.; Hoseini, S.M.; Ghelichpour, M.; Aghaei Moghaddam, A.; Gharavi, B.; Aydın, B. Myrcene as Water Conditioner, Stress-Reducing and Antioxidant Agent in Transportation of Common Carp, Cyprinus carpio, with Plastic Bags. Aquac. Rep. 2023, 28, 101458. [Google Scholar] [CrossRef]
- Alma, M.H.; Mavi, A.; Yildirim, A.; Digrak, M.; Hirata, T. Screening Chemical Composition and In Vitro Antioxidant and Antimicrobial Activities of the Essential Oils from Origanum syriacum L. Growing in Turkey. Biol. Pharm. Bull. 2003, 26, 1725–1729. [Google Scholar] [CrossRef]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A Review of Their Traditional Uses, Phytochemistry, and Pharmacology. ScientificWorldJournal 2013, 2013, 219815. [Google Scholar] [CrossRef]
- Tsokou, A.; Georgopoulou, K.; Melliou, E.; Magiatis, P.; Tsitsa, E. Composition and Enantiomeric Analysis of the Essential Oil of the Fruits and the Leaves of Pistacia vera from Greece. Molecules 2007, 12, 1233–1239. [Google Scholar] [CrossRef]
- Sarkhail, P.; Navidpour, L.; Rahimifard, M.; Hosseini, N.M.; Souri, E. Bioassay-Guided Fractionation and Identification of Wound Healing Active Compound from Pistacia vera L. hull Extract. J. Ethnopharmacol. 2020, 248, 112335. [Google Scholar] [CrossRef]
- Happi, G.M.; Ntabo, V.K.; Tcho, A.T.; Wansi, J.D. Naturally Occurring Dimeric Triterpenoids: Occurrence, Chemistry and Bioactivities. Phytochemistry 2022, 200, 113242. [Google Scholar] [CrossRef]
- Gomes Júnior, A.L.; Islam, M.T.; Nicolau, L.A.D.; de Souza, L.K.M.; de SouzaLopes Araújo, T.; Lopes de Oliveira, G.A.; de Melo Nogueira, K.; da Silva Lopes, L.; Medeiros, J.-V.R.; Mubarak, M.S.; et al. Anti-Inflammatory, Antinociceptive, and Antioxidant Properties of Anacardic Acid in Experimental Models. ACS Omega 2020, 5, 19506–19515. [Google Scholar] [CrossRef]
- Sarkhail, P.; Hashemi, G.; Souri, E. Evaluation of Wound Healing and Cytotoxic Activities of Anacardic Acid (13:0) Isolated from Pistacia vera Hull Extract. Res. J. Pharmacogn. 2024, 11, 19–25. [Google Scholar] [CrossRef]
- Lodh, J.; Khamrui, K.; Prasad, W.G. Optimization of Heat Treatment and Curcumin Level for the Preparation of Anti-Oxidant Rich Ghee from Fermented Buffalo Cream by Central Composite Rotatable Design. J. Food Sci. Technol. 2018, 55, 1832–1839. [Google Scholar] [CrossRef]
- Maiza, A.; Kamgang Nzekoue, F.; Ghazouani, T.; Afif, M.; Caprioli, G.; Fiorini, D.; Vittori, S.; Maggi, F.; Buccioni, M.; Martì Navia, A.; et al. Butter Oil (Ghee) Enrichment with Aromatic Plants: Chemical Characterization and Effects on Fibroblast Migration in an In-Vitro Wound Healing Model. Arab. J. Chem. 2020, 13, 8909–8919. [Google Scholar] [CrossRef]
- Tsitsigianni, E.; Tomou, E.-M.; Almpani, C.; Rallis, M.C.; Skaltsa, H. Biological Activities of Lamiaceae Species: Bio-Guided Isolation of Active Metabolites from Salvia officinalis L. Agronomy 2023, 13, 1224. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Gonçalves, M.J.; Silva, A.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical Profile, Anti-Microbial and Anti-Inflammaging Activities of Santolina rosmarinifolia L. Essential Oil from Portugal. Antibiotics 2023, 12, 179. [Google Scholar] [CrossRef]
- Zbeeb, H.; Baldini, F.; Zeaiter, L.; Vergani, L. The Anti-Inflammatory Potential of an Ethanolic Extract from Sarcopoterium spinosum Fruits for Protection and/or Counteraction against Oxidative Stress in Dysfunctional Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 1601. [Google Scholar] [CrossRef]
- Akkol, E.K.; Güvenç, A.; Yesilada, E. A Comparative Study on the Antinociceptive and Anti-Inflammatory Activities of Five Juniperus taxa. J. Ethnopharmacol. 2009, 125, 330–336. [Google Scholar] [CrossRef]
- Avci, R.; Kaderli, B.; Akalp, F.D. Intravitreal Triamcinolone Injection for Chronic Diffuse Diabetic Macular Oedema. Clin. Exp. Ophthalmol. 2006, 34, 27–32. [Google Scholar] [CrossRef]
- Khalil, M.; Khalifeh, H.; Baldini, F.; Salis, A.; Damonte, G.; Daher, A.; Voci, A.; Vergani, L. Antisteatotic and Antioxidant Activities of Thymbra spicata L. Extracts in Hepatic and Endothelial Cells as In Vitro Models of Non-Alcoholic Fatty Liver Disease. J. Ethnopharmacol. 2019, 239, 111919. [Google Scholar] [CrossRef]
- Chroho, M.; Rouphael, Y.; Petropoulos, S.A.; Bouissane, L. Carvacrol and Thymol Content Affects the Antioxidant and Antibacterial Activity of Origanum compactum and Thymus zygis Essential Oils. Antibiotics 2024, 13, 139. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxidative Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Ramos, L.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Thymus mastichina (L.) L. and Cistus ladanifer L. for Skin Application: Chemical Characterization and In Vitro Bioactivity Assessment. J. Ethnopharmacol. 2023, 302. [Google Scholar] [CrossRef]
- Ustuner, O.; Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Sigirci, B.D.; Ak, S.; Akpulat, H.A.; Donmez, C.; Koca-Caliskan, U. In Vitro Evaluation of Antioxidant, Anti-Inflammatory, Antimicrobial and Wound Healing Potential of Thymus Sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas. Molecules 2019, 24, 3353. [Google Scholar] [CrossRef]
- Elshafay, A.; Omran, E.S.; Abdelkhalek, M.; El-Badry, M.O.; Eisa, H.G.; Fala, S.Y.; Dang, T.; Ghanem, M.A.T.; Elbadawy, M.; Elhady, M.T.; et al. Reporting Quality in Systematic Reviews of In Vitro Studies: A Systematic Review. Curr. Med. Res. Opin. 2019, 35, 1631–1641. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Ahmad, N. In Vitro and In Vivo Characterization Methods for Evaluation of Modern Wound Dressings. Pharmaceutics 2022, 15, 42. [Google Scholar] [CrossRef]
- Miles, R.A.; Price, R. Using Altmetric Data Responsibly: A Guide to Interpretation and Good Practice. 2023. Available online: http://hdl.handle.net/10919/116448 (accessed on 12 June 2024).
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Sellem, I.; Kaaniche, F.; Chakchouk, A.M.; Mellouli, L. Anti-Oxidant, Antimicrobial and Anti-Acetylcholinesterase Activities of Organic Extracts from Aerial Parts of Three Tunisian Plants and Correlation with Polyphenols and Flavonoids Contents. Bangladesh J. Pharmacol. 2016, 11, 531–544. [Google Scholar] [CrossRef]
- Belabbes, R.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Tabti, B.; Costa, J.; Muselli, A. Chemical Variability, Antioxidant and Antifungal Activities of Essential Oils and Hydrosol Extract of Calendula arvensis L. from Western Algeria. Chem. Biodivers. 2017, 14, e1600482. [Google Scholar] [CrossRef]
- Ercetin, T.; Senol, F.S.; Erdogan Orhan, I.; Toker, G. Comparative Assessment of Antioxidant and Cholinesterase Inhibitory Properties of the Marigold Extracts from Calendula arvensis L. and Calendula officinalis L. Ind. Crops Prod. 2012, 36, 203–208. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Antioxidant Properties of Marigold Extracts. Food Res. Int. 2004, 37, 643–650. [Google Scholar] [CrossRef]
- Khouchlaa, A.; El Baaboua, A.; El Moudden, H.; Lakhdar, F.; Bakrim, S.; El Menyiy, N.; Belmehdi, O.; Harhar, H.; El Omari, N.; Balahbib, A.; et al. Traditional Uses, Bioactive Compounds, and Pharmacological Investigations of Calendula arvensis L.: A Comprehensive Review. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 2482544. [Google Scholar] [CrossRef]
- Abudunia, A.-M.; Marmouzi, I.; Faouzi, M.E.A.; Ramli, Y.; Taoufik, J.; El Madani, N.; Essassi, E.M.; Salama, A.; Khedid, K.; Ansar, M.; et al. Anticandidal, Antibacterial, Cytotoxic and Antioxidant Activities of Calendula arvensis Flowers. J. Mycol. Med. 2017, 27, 90–97. [Google Scholar] [CrossRef]
- Meddeb, E.; Charni, M.; Ghazouani, T.; Cozzolino, A.; Fratianni, F.; Raboudi, F.; Nazzaro, F.; Fattouch, S. Biochemical and Molecular Study of Carpobrotus edulis Bioactive Properties and Their Effects on Dugesia sicula (Turbellaria, Tricladida) Regeneration. Appl. Biochem. Biotechnol. 2017, 182, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.; Antunes, M.; Fernandes, W.; Campos, M.J.; Azevedo, Z.M.; Freitas, V.; Rocha, J.M.; Tecelão, C. Physicochemical and Nutritional Profile of Leaves, Flowers, and Fruits of the Edible Halophyte Chorão-Da-Praia (Carpobrotus edulis) on Portuguese West Shores. Food Biosci. 2021, 43, 101288. [Google Scholar] [CrossRef]
- Erhabor, R.C.; Erhabor, J.O.; Nkadimeng, S.M.; McGaw, L.J. In Vitro Antimicrobial, Antibiofilm and Antioxidant Activities of Six South African Plants with Efficacy Against Selected Foodborne Pathogens. S. Afr. J. Bot. 2022, 146, 643–652. [Google Scholar] [CrossRef]
- Bouftira, I.; Abdelly, C.; Sfar, S. Antioxidant Properties of Mesembryanthemum Crystallinum and Carpobrotus edulis Extracts. Asian J. Chem. 2009, 21, 549–559. [Google Scholar]
- Zarrouk, A.; Smach, M.A.; Hafsa, J.; Sghaier, R.; Majdoub, H.; Hammami, M.; Charfeddine, B. Effects of Carpobrotus edulis Extract on Oxidative Stress and 158N Oligodendrocyte Death. Biomed. Environ. Sci. 2019, 32, 291–299. [Google Scholar] [CrossRef]
- Ondua, M.; Njoya, E.M.; Abdalla, M.A.; McGaw, L.J. Anti-Inflammatory and Antioxidant Properties of Leaf Extracts of Eleven South African Medicinal Plants Used Traditionally to Treat Inflammation. J. Ethnopharmacol. 2019, 234, 27–35. [Google Scholar] [CrossRef]
- Shahat, A.A.; Cos, P.; Hermans, N.; Apers, S.; De Bruyne, T.; Pieters, L.; Berghe, D.V.; Vlietinck, A.J. Anticomplement and Antioxidant Activities of New Acetylated Flavonoid Glycosides from Centaurium spicatum. Planta Medica 2003, 69, 1153–1156. [Google Scholar] [CrossRef]
- Allam, A.E.; El-Shanawany, M.A.; Backheet, E.Y.; Nafady, A.M. Phytochemical Investigation of the Aerial Parts of Centaurium Spicatum with Hepatoprotective and mRNA Enzymatic Inhibition Activities. Bull. Pharm. Sciences. Assiut 2014, 37, 65–76. [Google Scholar] [CrossRef]
- Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol Diversity and Antioxidant Activity of European Cistus creticus L. (cistaceae) Compared to Six Further, Partly Sympatric Cistus Species. Plants 2021, 10, 615. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; Mozos-Pascual, M.D.L.; Tarantilis, P.; Herraiz-Peñalver, D.; Santana-Méridas, O. Polyphenol Composition and Antioxidant and Metal Chelating Activities of the Solid Residues from the Essential Oil Industry. Ind. Crops Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Soldado, D.; Guerreiro, O.; Fialho, L.; Cachucho, L.; Francisco, A.; Santos-Silva, J.; Bessa, R.J.B.; Jerónimo, E. Inclusion of the Cistus ladanifer L. Plant and Its Condensed Tannin Extract in Lamb Diets-Effects on Animal Antioxidant Status and Oxidative Stability of Meat. Anim. Feed Sci. Technol. 2024, 316, 116070. [Google Scholar] [CrossRef]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential Oils from Residual Foliage of Forest Tree and Shrub Species: Yield and Antioxidant Capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef] [PubMed]
- Chaloupková, V.; Mediavilla, I.; Bados, R.; Houdková, M.; Rondevaldová, J.; Esteban, L.S. Annual Variation in Yield, Chemical Composition, Antioxidant and Antibacterial Activity Of Rockrose (Cistus ladanifer L.) Essential Oils. Biocatal. Agric. Biotechnol. 2024, 60, 103279. [Google Scholar] [CrossRef]
- Benamari, O.; Labhar, A.; Ahidar, N.; Salhi, A.; Ahari, M.; Elyoussfi, A.; Benyoub, B.; Amhamdi, H. Investigation of the Antioxidant and Anti-Inflammatory Capacities of Different Extracts from Cistus ladanifer L. Leaves in the Ait Ammart Region (Northern Morocco). Ecol. Eng. Environ. Technol. 2024, 25, 178–184. [Google Scholar] [CrossRef]
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crops Prod. 2009, 30, 165–167. [Google Scholar] [CrossRef]
- Adadi, I.; Ayadi, R.E.; Bentayeb, A.; Aaziz, H.; Bouymajane, A.; Altemimi, A.B.; Cacciola, F.; Ibaoui, H.E. Phytochemical Profile, In Vivo Anti-Inflammatory and Wound Healing Activities of the Aqueous Extract from Aerial Parts of Cistus ladanifer L. J. Pharm. Biomed. Anal. 2022, 219, 114960. [Google Scholar] [CrossRef]
- Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.B.S.; Díaz, T.S.; Silva, A.M. Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities. Plants 2022, 11, 1477. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × Incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Buzzanca, C.; Di Stefano, V.; D’Amico, A.; Gallina, A.; Melilli, M.G. A systematic review on Cynara cardunculus L.: Bioactive compounds, nutritional properties and food-industry applications of a sustainable food. Nat. Prod. Res. 2024, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ucar Turker, A.; Basay, S.; Cimen, A.; Baba, Y.; Birinci Yildirim, A. Evaluation of the Phenolic Content and the Nutraceutical Potential of Ancestor and Cultivated Artichokes. Chem. Biodivers. 2024, 21, e202400203. [Google Scholar] [CrossRef] [PubMed]
- Nechchadi, H.; Kacimi, F.E.; McDonald, A.; Boulbaroud, S.; Berrougui, H.; Ramchoun, M. Phytochemical Content, Antioxidant, and Anti-Inflammatory Activities of Morrocan Cynara cardunculus L. var. Ferocissima Leaf Methanolic Extract. Plant Foods Hum. Nutr. 2024, 79, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Malfa, G.A.; Santangelo, R.; Bianchi, S.; Pappalardo, F.; Taviano, M.F.; Miceli, N.; Di Giacomo, C.; Tomasello, B. Wild Artichoke (Cynara cardunculus subsp. sylvestris, Asteraceae) Leaf Extract: Phenolic Profile and Oxidative Stress Inhibitory Effects on HepG2 Cells. Molecules 2023, 28, 2475. [Google Scholar] [CrossRef] [PubMed]
- Baali, N.; Belloum, Z.; Benayache, F.; Benayache, S. Cynara Cardunculus Flavonoids-rich Fraction Alleviates Liver Injury in Mice Overconsumed Fructose Model. Recent Adv. Food Nutr. Agric. 2024, 15, 74–82. [Google Scholar] [CrossRef]
- Laghezza Masci, V.; Mezzani, I.; Alicandri, E.; Tomassi, W.; Paolacci, A.R.; Covino, S.; Vinciguerra, V.; Catalani, E.; Cervia, D.; Ciaffi, M.; et al. The Role of Extracts of Edible Parts and Production Wastes of Globe Artichoke (Cynara cardunculus L. var. scolymus (L.)) in Counteracting Oxidative Stress. Antioxidants 2025, 14, 116. [Google Scholar] [CrossRef]
- Tajini, F.; Jelassi, A.; Hamdani, A.; Salem, A.; Abdelhedi, O.; Ouerghui, A.; Sebai, H. Antioxidant Activities and Laxative Effect of Bioactive Compounds from Cynara cardunculus var. sylvestris. Sains Malays. 2024, 53, 1617–1630. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Duarte, M.P.; Andrade, M.A.; Mateus, A.R.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Exploring Cynara cardunculus L. by-products potential: Antioxidant and antimicrobial properties. Ind. Crops Prod. 2024, 222, 119559. [Google Scholar] [CrossRef]
- Masala, V.; Jokić, S.; Aladić, K.; Molnar, M.; Casula, M.; Tuberoso, C.I.G. Chemical Profiling and Evaluation of Antioxidant Activity of Artichoke (Cynara cardunculus var. scolymus) Leaf By-Products’ Extracts Obtained with Green Extraction Techniques. Molecules 2024, 29, 4816. [Google Scholar] [CrossRef]
- Cerulli, A.; Cuozzo, R.; Melis, M.P.; Serreli, G.; Deiana, M.; Masullo, M.; Piacente, S. In-Depth LC-ESI/HRMS-Guided Phytochemical Analysis and Antioxidant Activity Analysis of Eco-Sustainable Extracts of Cynara cardunculus (Carciofo Di Paestum PGI) Leaves. Plants 2024, 13, 3591. [Google Scholar] [CrossRef]
- Lanzoni, D.; Grassi Scalvini, F.; Petrosillo, E.; Nonnis, S.; Tedeschi, G.; Savoini, G.; Buccioni, A.; Invernizzi, G.; Baldi, A.; Giromini, C. Antioxidant Capacity and Peptidomic Analysis of In Vitro Digested Camelina sativa L. Crantz and Cynara cardunculus Co-Products. Sci. Rep. 2024, 14, 14456. [Google Scholar] [CrossRef]
- Gonçalves, A.; Sampaio, C.I.; Ševčovičová, A.; Dias, A.M.; Oliveira, R. Cynaropicrin- and Chlorogenic Acid-Rich Extracts Easily Prepared from Cynara cardunculus var. Scolymus: Antioxidant and Antigenotoxic Properties. Biocatal. Agric. Biotechnol. 2023, 52, 102808. [Google Scholar] [CrossRef]
- Meliani, N.; Achiri, R.; El Amine Dib, M.; Muselli, A. Assessment of Chemical Composition and Investigation into the Antioxidant, Anti-inflammatory, and Hemolytic Properties of Hexane Extracts from Cynara cardunculus subsp. Cardunculus and Cynara cardunculus subsp. sylvestris. Curr. Chem. Biol. 2024, 18, 46–52. [Google Scholar] [CrossRef]
- Osmic, N.; Culum, D.; Ibragic, S. Catechins and Other Phenolic Compounds in Herb of Eight Ephedra Species in Comparison to Camellia Sinensis. Nat. Prod. Res. 2024, 38, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.K.; Mohamed, A.A.; Ali, S.I.; Alghamdi, N.M.; Abdel-Rahman, A.M.; Al-Sobeai, S. Chemical Ingredients and Antioxidant Activities of Underutilized Wild Fruits. Heliyon 2019, 5, e01874. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; Khleifat, K.M.; Alqaraleh, M.; Al-Hraishat, E.; Al-Limoun, M.O.; Qaralleh, H.; Al-Dujaili, E.A.S. Antioxidant and Antihyperglycemic Effects of Ephedra foeminea Aqueous Extract in Streptozotocin-Induced Diabetic Rats. Nutrients 2022, 14, 2338. [Google Scholar] [CrossRef]
- Al-Nemi, R.; Makki, A.A.; Sawalha, K.; Hajjar, D.; Jaremko, M. Untargeted Metabolomic Profiling and Antioxidant Capacities of Different Solvent Crude Extracts of Ephedra foeminea. Metabolites 2022, 12, 451. [Google Scholar] [CrossRef]
- Sharma, N.; Shakya, A.K.; Shrivastava, S.; Shukla, S. Antioxidant and Ameliorative Effect of Indian Fumaria Parviflora Aqueous Extract against CCl4 induced Hepatotoxicity. J. Ayurveda Holist. Med. (JAHM) 2023, 11. [Google Scholar]
- Tripathi, M.; Singh, B.K.; Raisuddin, S.; Kakkar, P. Abrogation of Nimesulide Induced Oxidative Stress and Mitochondria Mediated Apoptosis by Fumaria parviflora Lam. Extract. J. Ethnopharmacol. 2011, 136, 94–102. [Google Scholar] [CrossRef]
- Dousti, B.; Habibi, A.; Nabipor, F. Biosynthesis of Zinc Oxide Nanoparticles Using Fumaria Parviflora Extract and Evaluation of Their Antibacterial and Antioxidant Activities. BioTechnologia (Pozn) 2021, 102, 65–73. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Casas Arrojo, V.; Arrojo Agudo, M.A.; Cárdenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Basharat, Z.; Afzaal, M.; Saeed, F.; Islam, F.; Hussain, M.; Ikram, A.; Pervaiz, M.U.; Awuchi, C.G. Nutritional and Functional Profile of Carob Bean (Ceratonia siliqua): A Comprehensive Review. Int. J. Food Prop. 2023, 26, 389–413. [Google Scholar] [CrossRef]
- Quintano, E.; Ganzedo, U.; Díez, I.; Figueroa, F.L.; Gorostiaga, J.M. Solar Radiation (PAR and UVA) and Water Temperature in Relation to Biochemical Performance of Gelidium Corneum (Gelidiales, Rhodophyta) in Subtidal Bottoms off the Basque Coast. J. Sea Res. 2013, 83, 47–55. [Google Scholar] [CrossRef]
- Santos, J.M.; Jesus, B.C.; Ribeiro, H.; Martins, A.; Marto, J.; Fitas, M.; Pinto, P.; Alves, C.; Silva, J.; Pedrosa, R.; et al. Extraction of Macroalgae Phenolic Compounds for Cosmetic Application Using Eutectic Solvents. Algal Res. 2024, 79, 103438. [Google Scholar] [CrossRef]
- Cebollada, P.; Gomes, N.G.M.; Andrade, P.B.; López, V. An Integrated In Vitro Approach on the Enzymatic and Antioxidant Mechanisms of Four Commercially Available Essential Oils (Copaifera officinalis, Gaultheria fragrantissima, Helichrysum italicum, and Syzygium aromaticum) Traditionally Used Topically for Their Anti-Inflammatory Effects. Front. Pharmacol. 2024, 14, 1310439. [Google Scholar] [CrossRef]
- Dzamic, A.M.; Mileski, K.S.; Ciric, A.D.; Ristic, M.S.; Sokovic, M.D.; Marin, P.D. Essential Oil Composition, Antioxidant and Antimicrobial Properties of Essential Oil and Deodorized Extracts of Helichrysum italicum (Roth) G. Don. J. Essent. Oil-Bear. Plant. 2019, 22, 493–503. [Google Scholar] [CrossRef]
- Glumac, M.; Jažo, Z.; Paštar, V.; Golemac, A.; Čikeš Čulić, V.; Bektić, S.; Radan, M.; Carev, I. Chemical Profiling and Bioactivity Assessment of Helichrysum italicum (Roth) G. Don. Essential Oil: Exploring Pure Compounds and Synergistic Combinations. Molecules 2023, 28, 5299. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavčič Višnjevec, A.; Jenko Pražnikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef]
- Piras, F.; Sogos, V.; Pollastro, F.; Appendino, G.; Rosa, A. Arzanol, a Natural Phloroglucinol α-Pyrone, Protects HaCaT Keratinocytes Against H2O2-Induced Oxidative Stress, Counteracting Cytotoxicity, Reactive Oxygen Species Generation, Apoptosis, and Mitochondrial Depolarization. J. Appl. Toxicol. 2024, 44, 720–732. [Google Scholar] [CrossRef]
- Kenig, S.; Kramberger, K.; Novak, K.Š.; Karnjuš, I.; Bandelj, D.; Petelin, A.; Pražnikar, Z.J. Helichrysum Italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusions In Reversing The Traits Of Metabolic Syndrome: A Double-Blind Randomized Comparative Trial. Food Funct. 2022, 13, 7697–7706. [Google Scholar] [CrossRef]
- Adebayo, O.R.; Adegoke, B.M.; Oladele, J.O.; Afolabi, F.; Efunwole, O.O.; Shittu, S.A.; Oyetade, O.A.; Adedokun, A.A.; Adedayo, O.A.; Arogundade, L.A. Anti-Inflammatory, Antioxidant Activity, and Acetylcholinesterase Inhibitory Activity of Leaf Extract of Juglans Regia: Insight into the Treatment of Neurodegenerative Diseases. Not. Sci. Biol. 2024, 16, 11872. [Google Scholar] [CrossRef]
- Mateș, L.; Banc, R.; Zaharie, F.A.; Rusu, M.E.; Popa, D.-S. Mechanistic Insights into the Biological Effects and Antioxidant Activity of Walnut (Juglans regia L.) Ellagitannins: A Systematic Review. Antioxidants 2024, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.A.; Nawaz, M.F.; Tariq, T.; Khan, M.R.; Saeed, H.; Abdi, G.; Aadil, R.M. Astounding the Synergistic Interplay of Walnuts in Combating Inflammation and Oxidative Damage. J. Funct. Foods 2024, 119, 106292. [Google Scholar] [CrossRef]
- Francis, M.; Padmaja, S.; Radhakrishnan, A.; Paulraj, C.S.; Shankar, S.; Isaac, J.B.; Kalaichelvan, S.; Selvaraj, S. Evaluation of Antioxidant, Antibacterial and Antidiabetic Activity of Juglans Regia Root Extract: In Vitro and In Vivo Studies. J. Nat. Remedies 2024, 24, 2263–2275. [Google Scholar] [CrossRef]
- Al-Nadaf, A.H.; Thiab, S.; Obidat, R.; AL-Arman, S.; Shahin, N.A. Pharmacological Evaluation of the Protective Effect of Hydroalcoholic Extract of Walnut (Juglans regia L.) Leaf on 6-Mercaptopurine-Induced Hepatotoxicity in Rats. Phytomedicine Plus 2024, 4, 100617. [Google Scholar] [CrossRef]
- Adamovic, M.; Adamovic, A.; Andjic, M.; Dimitrijevic, J.; Zdravkovic, N.; Kostic, O.; Pecarski, D.; Pecarski, T.; Obradovic, D.; Tomovic, M. The Botany, Phytochemistry and the Effects of the Juglans regia on Healthy and Diseased Skin. Cosmetics 2024, 11, 163. [Google Scholar] [CrossRef]
- Conforti, F.; Perri, M.R.; Guerrini, A.; Sacchetti, G.; Statti, G. Lavandula austroapennina and Lavandula angustifolia Essential Oils and Bioactive Components: In Vitro Anti-Denaturation Effect of Lavender from the Pollino Massif (Southern Italy). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2023, 157, 339–345. [Google Scholar] [CrossRef]
- Marrelli, M.; De Luca, M.; Toma, C.-C.; Grande, F.; Occhiuzzi, M.A.; Caruso, R.; Conforti, F.; Statti, G. Enhancing the Nitric Oxide Inhibitory Activity Using a Combination of Plant Essential Oils and Mixture Design Approach. Heliyon 2024, 10, e31080. [Google Scholar] [CrossRef]
- Chianese, A.; Gravina, C.; Morone, M.V.; Ambrosino, A.; Formato, M.; Palma, F.; Foglia, F.; Nastri, B.M.; Zannella, C.; Esposito, A.; et al. Lavandula Austroapennina: Assessment of the Antiviral Activity of Lipophilic Extracts from Its Organs. Viruses 2023, 15, 1648. [Google Scholar] [CrossRef]
- Chaouchi, O.; Farida, F. Antimicrobial and Antioxidant Activities of Algerian Lavandula Stoechas Essential Oil. EPSTEM 2024, 29, 16–21. [Google Scholar] [CrossRef]
- Radi, M.; Eddardar, Z.; Drioiche, A.; Remok, F.; Hosen, M.E.; Zibouh, K.; Ed-Damsyry, B.; Bouatkiout, A.; Amine, S.; Touijer, H.; et al. Comparative Study of the Chemical Composition, Antioxidant, and Antimicrobial Activity of the Essential Oils Extracted from Lavandula Abrialis and Lavandula Stoechas: In Vitro and In Silico Analysis. Front. Chem. 2024, 12, 3385. [Google Scholar] [CrossRef] [PubMed]
- Ndhlala, A.R.; Işık, M.; Kavaz Yüksel, A.; Dikici, E. Phenolic Content Analysis of Two Species Belonging to the Lamiaceae Family: Antioxidant, Anticholinergic, and Antibacterial Activities. Molecules 2024, 29, 480. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, F.; Hamdouch, A.; Zine, S.; Chebli, B.; Hadek, M.E.; Idrissi, L.M.H. Essential Oil Content, Chemical Composition, Antioxidant Activity and Antiviral Potential against Covid-19 of Four Aromatic and Medicinal Plants from South of Morocco. Arab. J. Med. Aromat. Plants 2022, 8, 77–93. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, P.; Bharti, C.; Shanmugam, S.K. Evaluation of Hepatoprotective Efficacy of a Polyherbal Unani Formulation Majoon-Najah against Cadmium Chloride-induced Liver Damage. Curr. Drug Res. Rev. 2023, 15, 188–198. [Google Scholar] [CrossRef]
- Ayhan, S.; Bayazeid, O.; Baran, M.Y.; Yusufoglu, H.S.; Yalçın, F.N.; Kuruüzüm-Uz, A. The Neurologic Effects of Lavandula stoechas L. subsp. stoechas. Phytochem. Lett. 2025, 65, 145–151. [Google Scholar] [CrossRef]
- Al-Joufi, F.A.; Shaukat, S.; Hussain, L.; Khan, K.R.; Hussain, N.; Al Haddad, A.H.I.; Alqahtani, A.; Alqahtani, T.; Momenah, M.A.; Ibrahim, S.A.; et al. Lavandula stoechas Significantly Alleviates Cigarette Smoke-Induced Acute Lung Injury via Modulation of Oxidative Stress and the NF-κB Pathway. Food Biosci. 2024, 59, 103834. [Google Scholar] [CrossRef]
- Boudermine, S.; Malafronte, N.; Mencherini, T.; Esposito, T.; Aquino, R.P.; Beghidja, N.; Benayache, S.; D’Ambola, M.; Vassallo, A. Phenolic Compounds from Limonium pruinosum. Nat. Prod. Commun. 2015, 10, 1934578X1501000228. [Google Scholar] [CrossRef]
- Kenouche, S.; Latreche, A.; Bicha, S.; Bentamene, A.; Lassed, S.; Boucheham, R.; Boubekri, N.; Creche, J.; Zama, D.; Benayache, S.; et al. Phytochemical and Antioxidant Activity of Limonium pruinosum (L.). Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1855–1859. [Google Scholar]
- Rahmani, R.; Debouba, M.; Aydi, S.S.; Aydi, S.; Bouajila, J. Comparative Analysis of Organic Extracts Bioactivity from Two Limonium. Mill Species Growing Wild in Tunisian Salty Marshes. Chem. Biodivers. 2023, 20, e202301177. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Behbudi, G.; Mazraedoost, S.; Omidifar, N.; Gholami, A.; Chiang, W.-H.; Babapoor, A.; Pynadathu Rumjit, N. A Review on Health Benefits of Malva sylvestris L. Nutritional Compounds for Metabolites, Antioxidants, and Anti-Inflammatory, Anticancer, and Antimicrobial Applications. Evid.-Based Complement. Altern. Med. 2021, 2021, 5548404. [Google Scholar] [CrossRef]
- Infantino, I.R.; Cubisino, S.A.M.; Nibali, S.C.; Foti, P.; Tomasello, M.F.; Boninelli, S.; Battiato, G.; Magrì, A.; Messina, A.; Romeo, F.V.; et al. Phenolic Extract from Olive Mill Wastewater Sustains Mitochondrial Bioenergetics upon Oxidative Insult. Food Chem. Mol. Sci. 2025, 10, 100234. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Yıldız, R.; Korkmaz, A.; Mert, B.D.; Calıskan, C.G.; Oner, E. Evaluation of Extra Virgin Olive Oil Compounds Using Computational Methods: In Vitro, ADMET, DFT, Molecular Docking and Human Gene Network Analysis Study. BMC Chem. 2025, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Nezamdoost, T.; Bagherieh-Najjar, M.B.; Aghdasi, M. Biogenic Synthesis of Stable Bioactive Silver Chloride Nanoparticles Using Onosma dichroantha Boiss. Root Extract. Mater. Lett. 2014, 137, 225–228. [Google Scholar] [CrossRef]
- Mohammed, M.A.; El-Gengaihi, S.E.; Maklad, Y.A.; Shabana, M.E.; Naeim Attia, H. Role of Rich Phenolics and Betanin Profiles from Opuntia Ficus-Indica Fruits in the Prevention of Diabetic Complications Using Metabolomics Study. Sci. Rep. 2025, 15, 5780. [Google Scholar] [CrossRef]
- Parralejo-Sanz, S.; Antunes-Ricardo, M.; Gloria Lobo, M.; Requena, T.; Cano, M.P. Assessment of the Immunomodulatory Potential of Betalain- and Phenolic-Rich Extracts from Opuntia Cactus Fruits. Food Biosci. 2025, 65, 106093. [Google Scholar] [CrossRef]
- Peña-Vázquez, G.I.; Serrano-Sandoval, S.N.; Rodríguez-Rodríguez, J.; Antunes-Ricardo, M.; Guajardo-Flores, D. Anti-Inflammatory and Antioxidant Activity of Functional Lipids Extracted Through Sustainable Technologies from Mexican Opuntia ficus-indica Seeds. Food Chem. 2025, 467, 142258. [Google Scholar] [CrossRef]
- Panagiotidou, C.; Bouloumpasi, E.; Irakli, M.; Chatzopoulou, P. Characterization of Natural Bioactive Compounds from Greek Oregano Accessions Subjected to Advanced Extraction Techniques. Plants 2024, 13, 3087. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Walasek-Janusz, M. Chemical Composition, Biological Activity, and Potential Uses of Oregano (Origanum vulgare L.) and Oregano Essential Oil. Pharmaceuticals 2025, 18, 267. [Google Scholar] [CrossRef]
- Mrabet, A.; Annaz, H.; Abdelfattah, B.; Ouabou, M.; Kounnoun, A.; Cacciola, F.; Simou, A.; Bouayad, N.; Rharrabe, K.; Khaddor, M. Antioxidant, Insecticidal, Antifeedant, and Repellent Activities of Oregano (Origanum vulgare). Int. J. Environ. Health Res. 2025, 35, 382–397. [Google Scholar] [CrossRef]
- Heydari, F.; Department of Organic and Phytochemistry, Faculty of Chemistry, Kharazmi University, Tehran, Iran; Ghafarzadegan, R. Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Karaj, Iran; Mofasseri, M.; School of Chemistry, University College of Science, University of Tehran, Tehran, Iran; Ghasemi, S.V.; Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Karaj, Iran; Kashefi, M.; Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Karaj, Iran; et al. Phytochemical Analysis and Biological Activities of Essential Oil and Extract of Phlomis rigida Labill. J. Med. Plants 2021, 20, 13–22. [Google Scholar] [CrossRef]
- Alkhathami, A.G.; El-Fakharany, E.M.; El-Sayed, M.H.; Atwa, A.; Ali, F.K.; Hamad, N.; Askar, H.; Ashry, M. Chemopreventive Effect of Pistacia vera Leaf Extract Against Mammary Carcinoma Induced by Dimethyl-Benz(a)anthracene In Vivo and In Vitro: Potential Role of Antioxidant, Antiinflammatory and Immune Mechanisms. Food Chem. Toxicol. 2025, 196, 115229. [Google Scholar] [CrossRef] [PubMed]
- Seker, G.; Akbas, M.Y. Evaluation of Bioactivities of Pistacia vera L. hull Extracts as a Potential Antimicrobial and Antioxidant Natural Source. Food Sci. Technol. Int. 2024, 30, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Khadivi, A.; Nikoogoftar-Sedghi, M.; Tunç, Y. Agronomic Characteristics, Mineral Nutrient Content, Antioxidant Capacity, Biochemical Composition, and Fatty Acid Profile of Iranian Pistachio (Pistacia vera L.) Cultivars. BMC Plant Biol. 2025, 25, 68. [Google Scholar] [CrossRef]
- Irshad, S.; Iftikhar, S.; Riaz, M.; Mahmood, A.; Mushtaq, A.; Saleem, Y.; Shamim, R.; Akter, Q.S. Chemical Fingerprinting, Antimicrobial, Antioxidant, Anti-Inflammatory, and Anticancer Potential of Greenly Synthesized Silver Nanoparticles from Pistachio (Pistacia vera) Nuts and Senna (Cassia angustifolia Vahl.) Leaves. Food Sci. Nutr. 2024, 12, 4989–5006. [Google Scholar] [CrossRef]
- Hashempur, M.H.; Zareshahrabadi, Z.; Shenavari, S.; Zomorodian, K.; Rastegari, B.; Karami, F. Deep Eutectic Solvent-Based Extraction of Rosemary Leaves: Optimization Using Central Composite Design and Evaluation of Antioxidant and Antimicrobial Activities. New J. Chem. 2025, 49, 4495–4505. [Google Scholar] [CrossRef]
- Darra, R.; Majdalawieh, A.F.; Mahasneh, A.; Rah, B.; Hamad, M.; Kanan, S.M. Anti-Proliferative Effects of Rosmarinus officinalis L. (Rosemary) Against Human Breast and Liver Carcinoma cells. Food Biosci. 2025, 65, 106164. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Zhang, R.; Yu, H.; Zhang, L. Network Pharmacology Mechanism of Rosmarinus officinalis L.(Rosemary) to Improve Cell Viability and Reduces Apoptosis in Treating Alzheimer’s Disease. BMC Complement. Med. Ther. 2025, 25, 94. [Google Scholar] [CrossRef]
- Bendaas, R.; Messaadia, L.; Bekkar, Y.; Bourougaa, L.; Messaoudi, A. Comprehensive In Vitro and Molecular Docking Analysis of Antioxidant and Antimicrobial Properties of Salvia officinalis L. Extracts and Essential Oils. J. Mol. Struct. 2025, 1331, 141621. [Google Scholar] [CrossRef]
- Schmidt, J.; Juhasz, K.; Bona, A. Exploring the Chemical Profile, In Vitro Antioxidant and Anti-Inflammatory Activities of Santolina rosmarinifolia Extracts. Molecules 2024, 29, 1515. [Google Scholar] [CrossRef]
- Chibani, S.; Bensouici, C.; Kabouche, A.; Kabouche, Z.; Al-Dabbas, M.M.; Aburjai, T. Flavonoids and Antioxidant Activity of Santolina rosmarinifolia from Algeria. Chem. Nat. Compd. 2012, 48, 877–878. [Google Scholar] [CrossRef]
- Alfarrayeh, I.; Tarawneh, K.; Almajali, D.; Al-Awaida, W. Evaluation of the Antibacterial and Antioxidant properties of the Methanolic extracts of four Medicinal plants selected from Wadi Al-Karak, Jordan related to their Phenolic contents. Res. J. Pharm. Technol. 2022, 15, 2110–2116. [Google Scholar] [CrossRef]
- Kaçar, D.; Bayraktar, O.; Erdem, C.; Alamri, A.S.; Galanakis, C.M. Antioxidant and Antimicrobial Activities of Plants Grown in the Mediterranean Region. JSFA Rep. 2022, 2, 452–461. [Google Scholar] [CrossRef]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; da Calabrese, K.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid.-Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef]
- Pajouhi, A.; Pajouhi, N.; Karimi Rouzbahani, A.; Assaei, R. Pretreatment Administration of Hydro-Ethanolic Extract of Syzygium Aromaticum Prevents Thioacetamide-Induced Liver Injury in Rat. Egypt. Liver J. 2024, 14, 90. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus spp. Plants-Food Applications and Phytopharmacy Properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Modulation of Cell Death Pathways for Cellular Protection and Anti-Tumoral Activity: The Role of Thymus spp. Extracts and Their Bioactive Molecules. Int. J. Mol. Sci. 2023, 24, 1691. [Google Scholar] [CrossRef]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.A.; Ferreira, S.; Duarte, A.P. Chemical Composition, Antioxidant, and Antimicrobial Activities of Six Commercial Essential Oils. Lett. Appl. Microbiol. 2023, 76, ovac042. [Google Scholar] [CrossRef]
- Mateus, A.R.S.; Serrano, C.; Almeida, C.; Soares, A.; Rolim Lopes, V.; Sanches-Silva, A. Beyond Thymol and Carvacrol: Characterizing the Phenolic Profiles and Antioxidant Capacity of Portuguese Oregano and Thyme for Food Applications. Appl. Sci. 2024, 14, 8924. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ruiz-Medina, A.; Zengin, G.; Ak, G.; Jugreet, S.; Mahomoodally, M.F.; Emre, G.; Orlando, G.; Libero, M.L.; Acquaviva, A.; et al. New Biological and Chemical Evidences of Two Lamiaceae Species (Thymbra capitata and Thymus sipyleus subsp. rosulans): In Vitro, In Silico and Ex Vivo Approaches. Molecules 2022, 27, 9029. [Google Scholar] [CrossRef]
- Boga, M.; Ozkan, E.E.; Ersoy, E.; Tuncay, E.; Canturk, Y.Y.; Cinar, E.; Kara, E.M.; Zengin, G. Identification and Quantification of Phenolic and Volatile Constituents in Five Different Anatolian Thyme Species Using LC–MS/MS and GC-MS, with Biological Activities. Food Biosci. 2021, 43, 101141. [Google Scholar] [CrossRef]
- Altememy, D.; Patra, I.; Hussam, F.; Jabr, H.S.; Alwan, N.H.; Dawood, A.H.; Abdelamir, D.F.; Hamad, D.A.; Shokri, S.; Darvishi, M. Determination and Evaluation of Total Antioxidant Capacity of Methanolic Extracts of Quercus Brantii, Thymbra Spicata, Citrullus Colocynthis. Adv. Life Sci. 2022, 9, 372–379. [Google Scholar]
- Zou, M.; Zhong, Z.; Wen, C. Thymbra Spicata Leaf Extract Driven Biogenic Synthesis of Au/Fe3O4 Nanocomposite and Its Bio-application in the Treatment of Different Types of Leukemia. Open Chem. 2023, 21, 20220353. [Google Scholar] [CrossRef]
- Yuca, H.; Civaş, A.; Tekman, E.; Nadiroğlu, R.; Karakaş, Ş.; Dürüster, Ş.; Keçeci, E.B.; Bona, M.; Nobarirezaeyeh, M.; Aydin, B.; et al. Qualitative and Quantitative Phytochemicals of Essential Oils and Extracts of Thymbra spicata subsp. spicata L. as a Spice for Diabetes Mellitus. Flavour Fragr. J. 2023, 38, 378–391. [Google Scholar] [CrossRef]
- Rosselli, D. The Language of Biomedical Sciences. Lancet 2016, 387, 1720–1721. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M.; et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 707479. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Criterion | Inclusion | Exclusion |
|---|---|---|
| Study Design |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Language |
|
|
| Timeframe |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chorti-Tripsa, E.; Galanis, V.-Z.; Constantinides, T.C.; Kontogiorgis, C. Natural Products from the Mediterranean Area as Wound Healing Agents—In Vitro Studies: A Systematic Review. Antioxidants 2025, 14, 484. https://doi.org/10.3390/antiox14040484
Chorti-Tripsa E, Galanis V-Z, Constantinides TC, Kontogiorgis C. Natural Products from the Mediterranean Area as Wound Healing Agents—In Vitro Studies: A Systematic Review. Antioxidants. 2025; 14(4):484. https://doi.org/10.3390/antiox14040484
Chicago/Turabian StyleChorti-Tripsa, Eleftheria, Vasilis-Zois Galanis, Theodoros C. Constantinides, and Christos Kontogiorgis. 2025. "Natural Products from the Mediterranean Area as Wound Healing Agents—In Vitro Studies: A Systematic Review" Antioxidants 14, no. 4: 484. https://doi.org/10.3390/antiox14040484
APA StyleChorti-Tripsa, E., Galanis, V.-Z., Constantinides, T. C., & Kontogiorgis, C. (2025). Natural Products from the Mediterranean Area as Wound Healing Agents—In Vitro Studies: A Systematic Review. Antioxidants, 14(4), 484. https://doi.org/10.3390/antiox14040484











