Abstract
In regenerative medicine, mesenchymal stem cells (MSCs) have shown their importance and potential in tissue reconstruction and immune system modification. However, such cells’ potential is often diminished by factors such as oxidative stress, immune rejection, and inadequate engraftment. This review highlights the role of molecular hydrogen (H2) and cold atmospheric plasma (CAP) as adjunct therapies to improve the effectiveness of MSC therapy. H2 has strong antioxidative and anti-inflammatory actions as it quenches reactive oxygen species and positively stimulates the Nrf2 pathway that promotes MSC survival and life. CAP, being a modulated source of ROS and RNS, also assists MSCs by altering the cellular redox balance, thus facilitating cellular adaptation, migration, and differentiation. H2 and CAP in conjunction with each other assist in establishing an ambience favorable for promoting MSCs’ survival and growth abilities, and reduce the healing time in various pathways such as wound, neuroprotection, and ischemia. Besides these concerns, this review also covers the best administration routes and doses of H2 and CAP together with MSCs in therapy. This study informs on a novel dual method aimed at improving the outcome of MSC therapy while adding several molecular targets and relevant clinical uses concerning these therapies. Research of the future has to deal with bettering these protocols so that the therapeutic benefits can be maximized without long-term implications for clinical applications.
1. Introduction
Mesenchymal stromal cells (MSCs) can treat several disorders due to their immunomodulatory and regenerative properties. However, many difficulties hinder their clinical implementation and efficacy. MSC treatments seldom meet the main efficacy criteria in clinical trials because they are less effective in people than in preclinical studies. This is mostly owing to cell-based therapy translation variability. The lack of uniform MSC identity criteria across trials causes treatment discrepancies. MSC-based therapy is difficult to standardize and replicate due to this inconsistency [1].
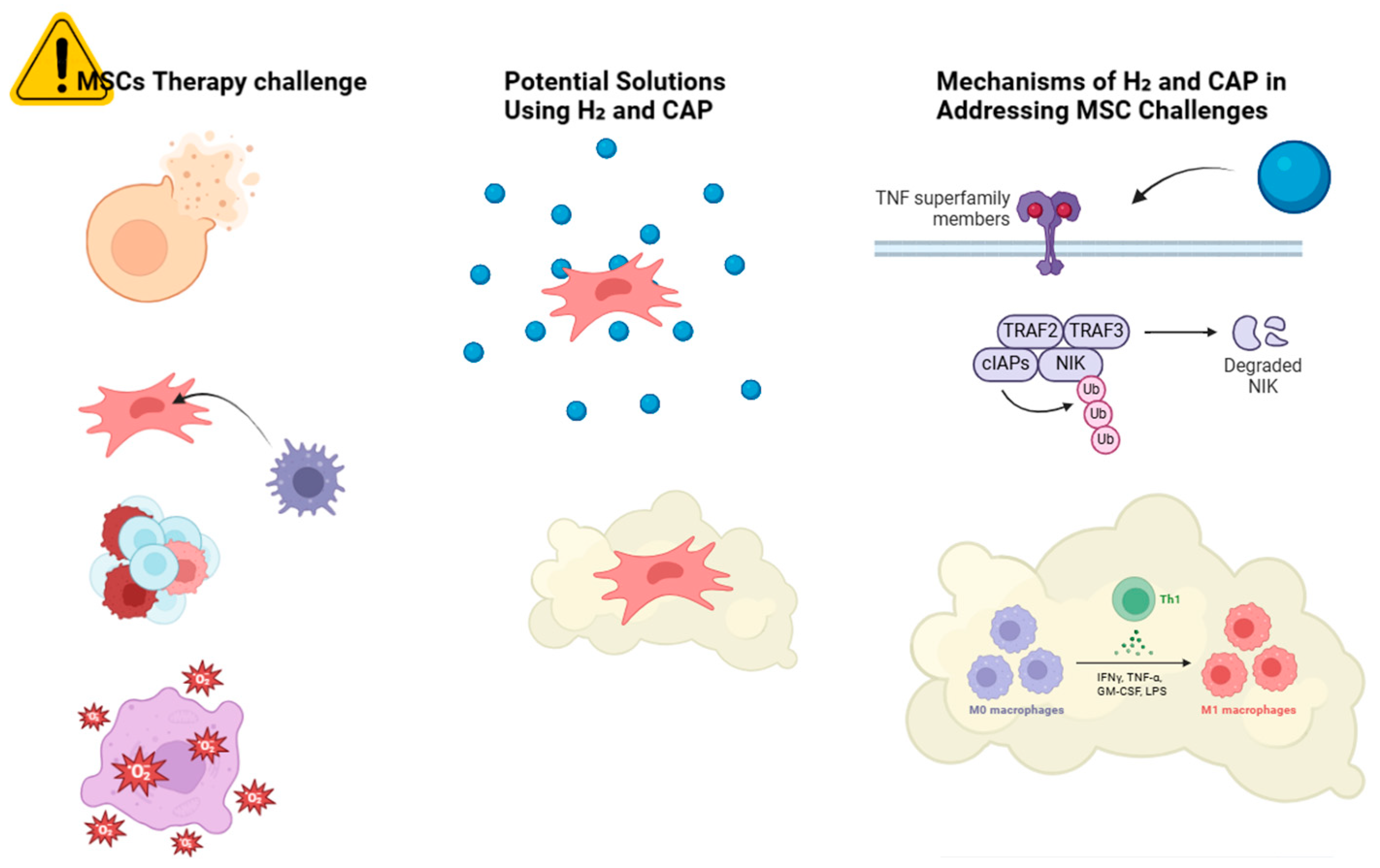
Allogeneic MSCs may lose their benefits due to immune rejection. Senescence may reduce the therapeutic efficacy of autologous MSCs from elderly people [2]. Delivering and targeting MSCs to specific tissues remains difficult. Cell surface adhesion receptor deficiency often reduces MSC engraftment efficiency and therapeutic efficacy [3]. MSC therapy can be compromised by in vitro and in vivo microenvironments. These factors affect MSC migration, viability, and function [4]. Mesenchymal stem cells (MSCs) can start and advance tumors, making their therapeutic usage risky [5], as shown in Figure 1.
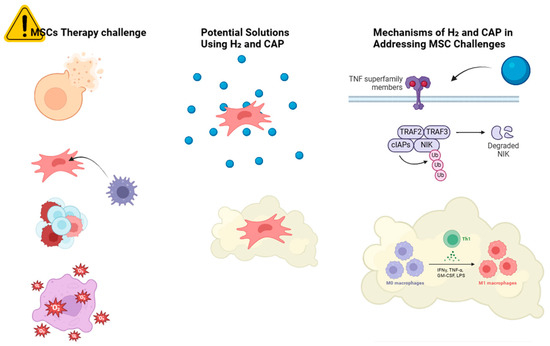
Figure 1.
Schematic overview of the challenges in MSC therapy and potential solutions using H2 and CAP. The challenges include a low survival rate, immunogenicity, tumorigenicity, oxidative stress, and inflammation. followed by H2 solution, they have improved cell survival, antioxidant protection, and reduced cellular mutations. Similarly, CAP treatment includes immunomodulation.
Oxidative stress—an imbalance between ROS production and antioxidant defense—affects stem cell survival, differentiation, and function. This synthesis uses multiple studies to analyze how oxidative stress impacts stem cells. Increased ROS levels inhibit MSC proliferation, promote senescence, decrease osteogenic differentiation, and increase adipogenic differentiation [6]. Damage to cellular macromolecules by oxidative stress causes senescence and apoptosis in stem cells [7]. Oxidative stress inhibits stem cell self-renewal and promotes neural lineage differentiation in human embryonic stem cells. MAPK/ERK1/2 signaling promotes spontaneous neuronal differentiation via reactive oxygen species-induced oxidative stress [8]. Hypoxia preconditioning (HPC) maintains oxidant status and reduces ROS to protect MSCs from oxidative stress. Human adipose-derived stem cells (hASCs) live longer after preconditioning with low H2O2 concentrations due to increased antioxidant defenses and metabolic changes [8]. The HSP90/NF-κB signaling pathway is essential for neural stem cell (NSC) survival during oxidative stress. Regulating HSP90 can protect against oxidative damage. Oxidative stress triggers stem cell autophagy via the ERK1/2 signaling pathway, which can kill cells if not handled [9].
Antioxidants neutralize free radicals to reduce oxidative stress illnesses. New antioxidant delivery methods and technology have been the focus of recent studies. Cell-based tests, notably those using Caco-2 cells, are becoming more popular for assessing antioxidant activity and bioavailability than in vitro and in vivo methods [10]. Nanoparticles, liposomes, and gel-based formulations are being studied to improve antioxidant bioavailability and efficacy. These techniques overcome deficiencies including dietary antioxidant solubility and instability. Metal-containing catalytic antioxidants, including manganese-based compounds, have shown promise in scavenging a wide range of reactive oxygen species. These are being investigated for their potential in treating cardiovascular, neurodegenerative, and inflammatory diseases [11]. New strategies are being developed to target specific subcellular regions where redox dysregulation occurs, such as mitochondria and caveolae.
These techniques include gene and miRNA therapies, nanoparticle technology, and micro-peptide targeting. Synthesis of antioxidant polymers from sustainable and natural monomers is improving. These polymers are used in food packaging, medicine delivery, and synthetic polymer biodegradation [11]. Endophytes from medicinal plants are being explored as sources of novel antioxidants. These microorganisms produce unique metabolites with antioxidant properties, offering potential for new natural antioxidant drugs.
This study aims to investigate the impact of molecular hydrogen and cold atmospheric plasma on the viability and therapeutic efficacy of mesenchymal stem cells (MSCs). This involves examining the impact of these factors on the antioxidant responses of cells, their biological functions, and their ability to migrate to regenerative areas. This research aims to determine the optimal conditions for maximizing synergistic effects to improve the outcomes of MSC therapy, particularly in the contexts of wound healing and tissue regeneration. Understanding the mechanisms underlying these effects may provide insights for developing more effective treatments for various disorders, including the potential for targeted stimulation of apoptosis.
The research gap specifically pertains to the detailed mechanisms explaining how molecular hydrogen and cold atmospheric plasma (CAP) synergistically enhance antioxidant effects in mesenchymal stem cells (MSCs). This study examines the long-term impacts of combined molecular hydrogen and cold atmospheric plasma treatment on mesenchymal stem cells, with a focus on the potential cytotoxicity and genetic stability, and moreover, evaluates the long-term sustainability of the antioxidant effects. The optimal dosage and administration protocols for combined treatment aim to maximize therapeutic benefits and minimize adverse effects.
2. Molecular Hydrogen and Cold Atmospheric Plasma: Fundamental Concepts
Molecular hydrogen (H2) and cold atmospheric plasma (CAP) are two separate entities characterized by unique chemical and physical properties. CAP is an ionized gas near room temperature, consisting of neutral particles, charged particles, reactive species, and electrons. It can be produced in ambient air and generates energetic species including electrons, metastables, reactive oxygen species (ROS), reactive nitrogen species (RNS), ultraviolet radiation, and localized electric fields [12]. It is devoid of color, scentless, non-toxic, and extremely combustible. Similarly, CAP can interact with liquids, such as tap water, altering their chemical composition by generating reactive species like hydrogen peroxide, hydroxide ions, and nitrate ions. While these reactive species can be transient, their effects on the liquid’s chemistry can be measured and analyzed using various analytical methods [13].
Hydrogen is the lightest and most prevalent element in the universe. It exists as a gas at standard temperature and pressure, with a boiling point of −252.87 °C. Nonetheless, CAP therapy can elicit substantial biological responses, such as the production of hydrogen peroxide in cancer cells, resulting in DNA and mitochondrial damage, elevated intracellular reactive oxygen species, and the initiation of apoptotic processes. Its effects are being investigated for potential applications in cancer therapy [14]. Although H2 is comparatively stable, it can combine with oxygen to produce water, thus releasing energy. It can also engage in many chemical reactions, including hydrogenation and reduction activities.
2.1. Generation Methods and Delivery Systems
CAP is an ionized gas generated at near-ambient temperatures, abundant in reactive oxygen and nitrogen species (RONS) including hydrogen peroxide and nitrites [14]. Helium, air, and argon are commonly utilized gases for CAP generation, with plasma jets being the predominant production technique. The most prevalent way of applying CAP to cells or tissues involves the direct use of plasma jets or plasma-treated media [15]. CAP may alter the stem cell niche or directly irradiate stem cells to affect their fate, encompassing adhesion, proliferation, differentiation, and death. CAP-treated hydrogels have been engineered to localize and administer RONS, assuring prolonged release while reducing systemic diffusion [16]. CAP elicits cellular reactions including apoptosis, diminished cell viability, and mitochondrial impairment via the production of reactive oxygen and nitrogen species (RONS). Additionally, CAP can augment drug delivery by enhancing cell membrane permeability, typically necessitating a synergy of plasma-induced electric fields and plasma chemistry.
Delivery mechanisms for molecular hydrogen in MSC therapy generally encompass inhalation, hydrogen-enriched water, or direct injection into the circulatory system. Numerous studies have investigated these strategies to guarantee safe and effective administration. Molecular hydrogen can be delivered via inhalation, consumption of hydrogen-rich water, or intravenous injection of hydrogen-rich saline [17]. These methods have demonstrated an enhancement of the hydrogen concentration in the blood and tissues, which is essential for its therapeutic benefits. Moreover, the inhalation of hydrogen gas has been advocated in cli [18] nical environments for the management of COVID-19 pneumonia, owing to its antioxidative, anti-inflammatory, and anti-apoptotic characteristics [19].
2.2. Biological Interactions
The potential biomedical uses of cold atmospheric plasma (CAP) are being investigated more and more, especially because of its capacity to produce reactive oxygen and nitrogen species (RONS). This synthesis examines the connections between CAP and mesenchymal cells, which are essential for numerous therapeutic applications. By combining CAP exposure with certain biomaterials, like those that include silica nanoparticles laden with iron oxide, mesenchymal stem cells (MSCs) can proliferate more quickly and improve osteogenic differentiation [20].
Even cancer cells that have undergone the epithelial-to-mesenchymal transition (EMT) are susceptible to the selective lethal effects of CAP and plasma-activated medium (PAM). This selectivity is ascribed to elevated amounts of reactive oxygen species (ROS) in mesenchymal-like cancer cells [21]. Human skin fibroblasts and adipose-derived stromal cells (ASC) may exhibit a senescence phenotype following brief exposure to CAP. This phenotype is marked by DNA damage, proliferation inhibition, and the release of pro-inflammatory cytokines. Notwithstanding this, the cells preserve certain functional features. CAP-induced reactive oxygen and nitrogen species (RONS) can engage with cellular components to activate signaling pathways, such as the Trk/Ras/ERK pathway, resulting in distinct physiological consequences, including brain development. This underscores the intricate interaction between CAP-generated species and cellular signaling pathways [22]. CAP may alter the microenvironment, resulting in indirect effects on cellular behavior. Modifications in the redox status of the microenvironment can affect cellular viability and apoptotic pathways.
Molecular hydrogen has demonstrated considerable molecular and cellular effects that position it as a potentially beneficial agent in regenerative medicine, particularly regarding its influence on stem cells [23]. A similar gaseous molecule called hydrogen sulfide (H2S) can affect the mesenchymal–epithelial transition (MET) in cancer cells and participate in cellular signaling. This could have consequences for interpreting hydrogen’s broader function in cellular processes as summarized in Table 1.

Table 1.
Comparison of the properties and applications H2 and CAP.
3. Antioxidant Mechanisms
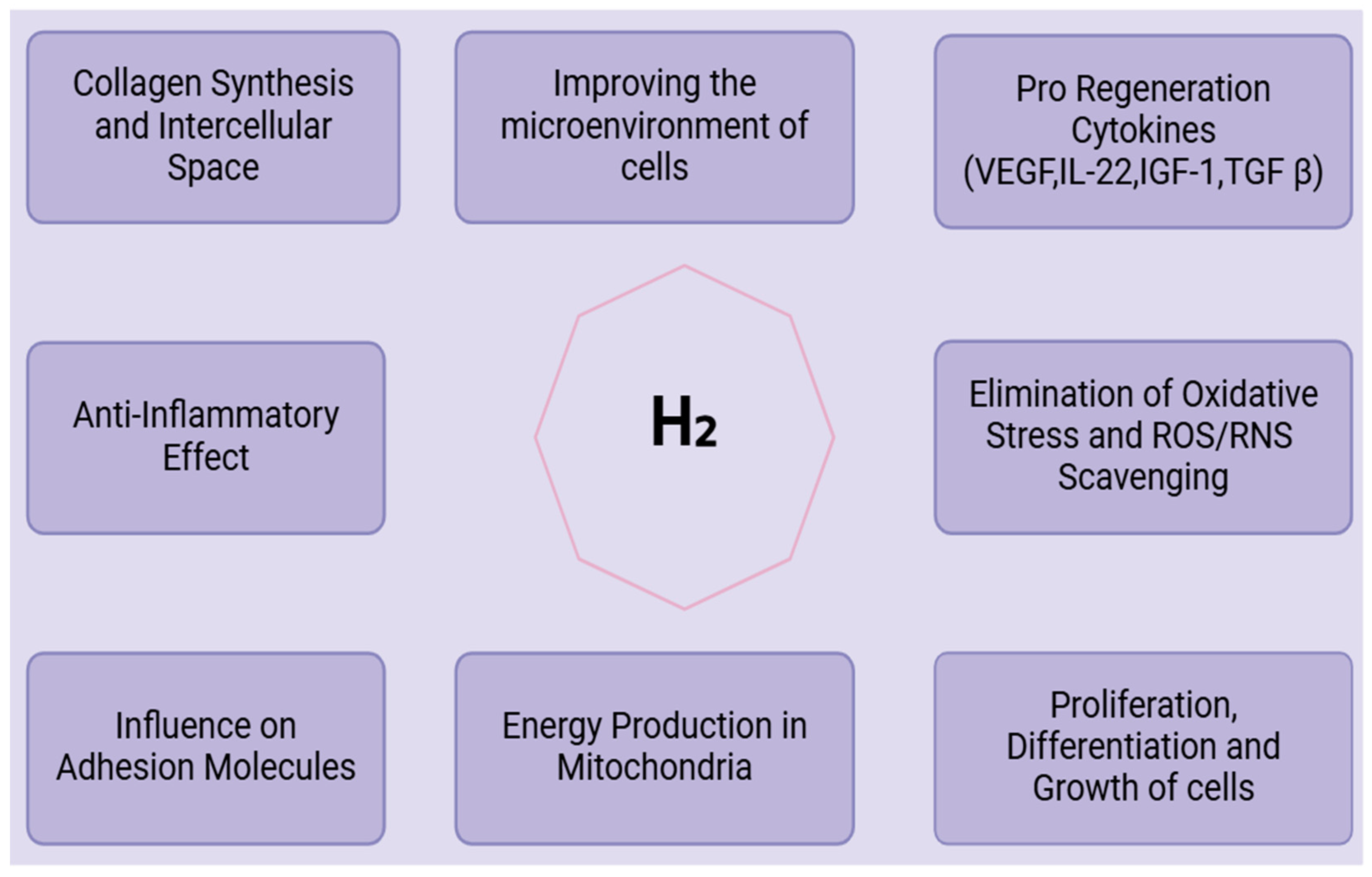
Molecular hydrogen (H2) demonstrates substantial antioxidant properties, chiefly via direct scavenging actions, activation of the Nrf2 pathway, and regulation of mitochondrial function. These methods enhance its therapeutic efficacy in addressing disorders associated with oxidative stress. Molecular hydrogen (H2) serves as a scavenger for free radicals and reactive oxygen species (ROS), mitigating the detrimental effects of hydroxyl radicals and peroxynitrite while preserving functionally significant ROS [24]. They serve as anti-inflammatory and anti-apoptotic agents by interacting with potent oxidants, such as hydroxyl and nitrosyl radicals, within cells, and mitigating oxidative stress. H2 promotes the Nrf2 pathway, an essential regulator of the antioxidant response, by preventing its degradation through KEAP1, further facilitating Nrf2’s translocation to the nucleus to commence the production of antioxidant genes, as found by Liam Baird and Masayuki Yamamoto (2020). By promoting mitophagy, increasing the number of mitochondria, and lowering reactive oxygen species (ROS) levels in the mitochondria, molecular hydrogen (H2) improves the survival and myogenic differentiation of adipose-derived stem cells [18].
4. Synergistic Effects on MSC Biology
4.1. Cell Survival and Proliferation
Mesenchymal stem cells differentiate into many cell types, including osteocytes, adipocytes, chondrocytes, neurons, cardiomyocytes, and endothelial cells, making them promising for application in regenerative treatment [25]. Multiple signals from the cellular and non-cellular environment of the cells control self-renewal in the stem cell-like state, including proliferation, differentiation, and migration [26]. Obtaining enough cells to start cell therapy is a limiting factor in therapeutic applications. Long-term in vitro cell culture to achieve appropriate cell numbers may affect the gene regulation and differentiation capacity of these cells as a result of long-term cell culture-induced stress. Furthermore, cell death following in vivo injection of MSCs is a limiting factor, since the majority of donor MSCs are eliminated after injection and do not engraft in substantial numbers in the recipient system [27]. This demands a more efficient cell expansion mechanism that encourages high cell proliferation, survival, and differentiation. The non-cellular microenvironment can be modified to drive cell proliferation, survival, or differentiation. Several investigations on the non-cellular microenvironment have found that cell shape is an important factor [28]. Other studies have shown that matrix rigidity [29], combined with the mechanical feedback provided by the extracellular matrix proteins, guided MSC differentiation [30]. According to a recent study, MSCs received biochemical signals for differentiation through early extracellular matrix proteins before they were changed by the cells during differentiation [31]. All of these studies strongly show that the non-cellular physical microenvironment affects MSC differentiation, which can be altered for targeted cell differentiation during tissue engineering.
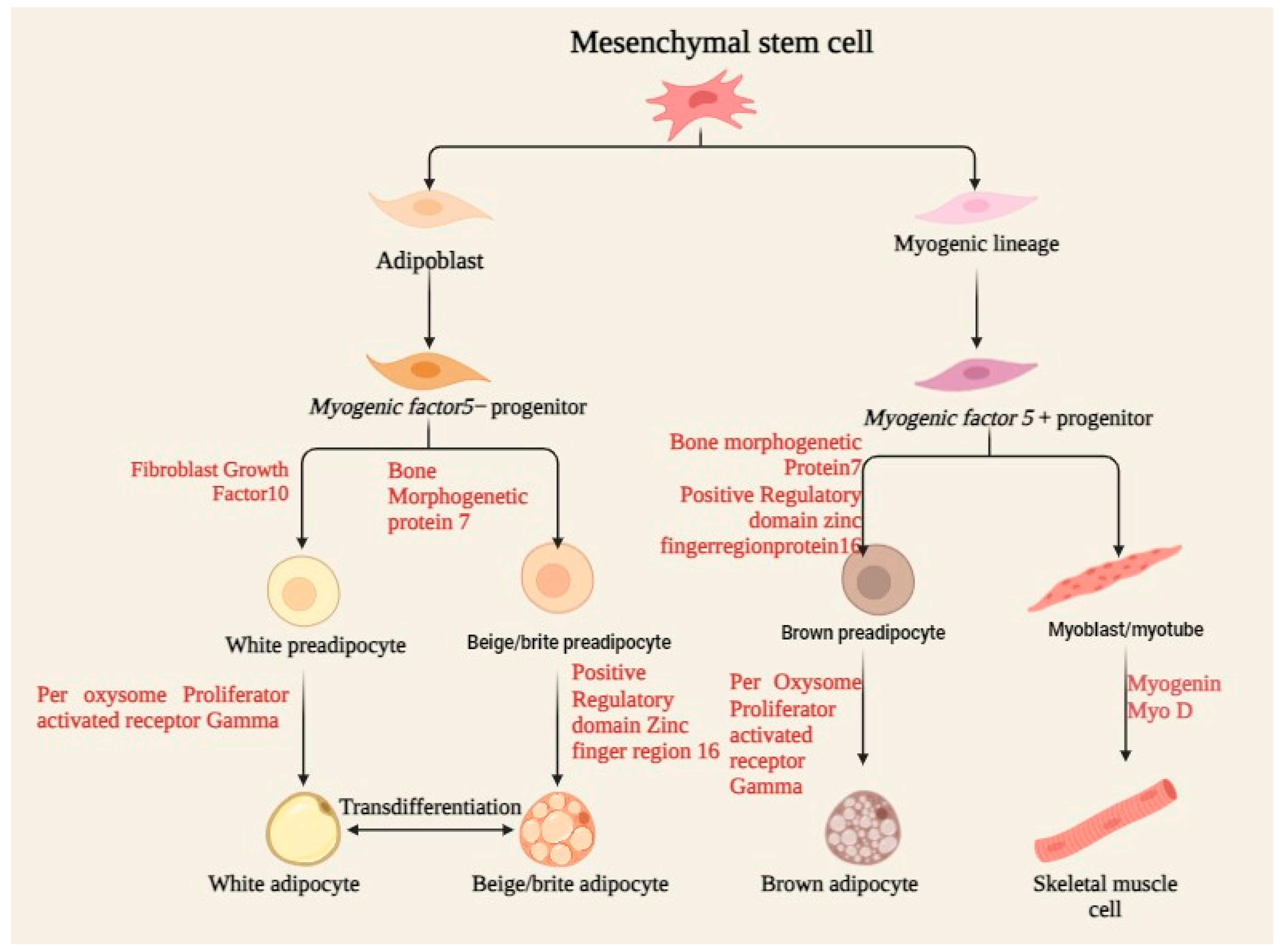
4.2. Differentiation Capacity
Mesenchymal stem cells (MSCs) are multipotent stromal cells that differentiate into a variety of cell types including osteoblasts, chondrocytes, and neurons, as demonstrated in Figure 2. The differentiation capacity of MSCs is regulated by several biochemical and biophysical parameters, often acting in concert to guide cell fate. This review discusses how several environmental factors interact with each other to modulate MSCs’ differentiation capacity. The surface shape at the nanoscale, together with immobilized growth factors, can also synergistically influence MSC differentiation. TiO2 nanotubes of varying diameters, when coated with bone morphogenetic protein-2 (BMP-2), have differential impacts on MSC differentiation. A BMP-2 coating on 100 nm nanotubes promotes chondrogenic differentiation, whereas 15 nm nanotubes stimulate osteogenic differentiation. This implies that the lateral nanoscale spacing of BMP-2 provides environmental cues modulating lineage-specific differentiation and cell survival [32]. The combination of biochemical and structural cues in a 3D-printed matrix can direct MSC development for targeted tissue regeneration.
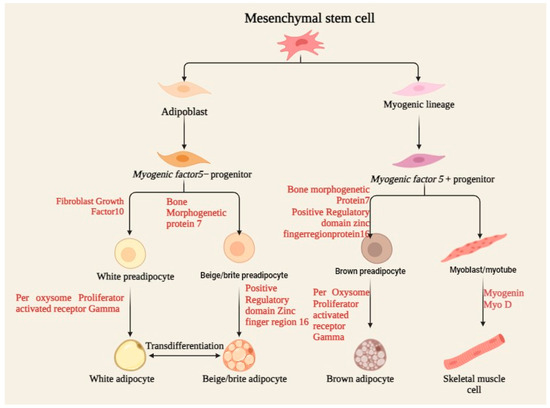
Figure 2.
Process of MSC differentiation, including osteoblasts, chondrocytes, and adipocytes, under specific biochemical and mechanical cues, supporting tissue regeneration and repair.
4.3. Migration and Homing
MSC homing is a multistep process involving numerous molecular interactions. The interaction between MSCs and the endothelium involves, first, the use of selectins, followed by an activation event with cytokines, arresting by integrins, and finally passing across the endothelial barrier through the action of matrix remodelers, after which the cells extra vascularly migrate into chemokine gradients [33]. Key signaling pathways implicated in MSC homing include the PI3K-Akt, MAPK, and Jak/Stat pathways, which are activated in response to chemokines such as stromal cell-derived factor-1 (SDF-1) [34]. Several ways have been tested to improve MSC homing efficiency. Genetic manipulation of MSCs to overexpress particular factors, such as fibroblast growth factor 21 (FGF21), has been found to improve their migratory potential and homing to injured regions [35]. Cell surface modification and the use of biomaterial carriers are other important factors in regulating MSC migration.
4.4. Paracrine Effect
Mesenchymal stem cells are well known for their strong regeneration capabilities, mainly attributed to the effects of paracrine activities. These are widely reported in the production of a wide spectrum of bioactive chemicals affecting the behavior of cells and tissues around them towards healing and regeneration. Recently, mounting interest can also be seen in the literature on the role of the microenvironment and biomaterials in modifying these paracrine effects through the improvement of MSCs’ therapeutic potential.
It has been shown that the enhancement of cell–cell contact by biomaterials improves the paracrine activity of MSCs. Thus, inhibition of N-cadherin significantly reduces the paracrine effects of MSCs, apparently showing that cell–cell contacts could modify the activity of MSCs [36]. Similarly, research on low-temperature-printed hierarchical porous sponges found that these scaffolds improve MSC adherence, retention, and survival, enhancing cell–material interactions. The synergetic effects of MSCs are described in Table 2. MSCs cultivated on these sponges demonstrated dramatically better paracrine activities, including increased production of immunomodulatory, angiogenic, and osteogenic components. MSCs’ paracrine actions play an important role in a variety of regeneration processes. MSC paracrine effects are regulated through complicated interactions with the extracellular matrix, adjacent cells, and soluble substances [37].

Table 2.
Demonstration of synergetic effects on MSC biology.
5. Synergistic Effects on H2O2 and CAP
5.1. Molecular Mechanism of Interaction
The synergistic effects of hydrogen peroxide (H2O2) and cold atmospheric plasma (CAP) have received a lot of attention because of their potential uses in medical and microbiological disinfection. This review seeks to explain the molecular mechanisms underlying the interaction of H2O2 and CAP, with an emphasis on their combined effects on tumor cells and microbial disinfection. The combination of H2O2 and nitrite, both long-lived species in CAP, is critical in causing selective apoptosis in tumor cells. The reaction between H2O2 and nitrite produces peroxynitrite, which is the first stage of the process. This peroxynitrite subsequently reacts with leftover H2O2 to produce singlet oxygen, which deactivates catalase molecules on the surface of tumor cells. The inactivation of catalase is significant because it allows H2O2 and peroxynitrite, which are normally destroyed by catalase, to remain at the inactivation site. This persistence causes the creation of secondary singlet oxygen, which further inactivates catalase and creates a self-sustaining cycle of singlet oxygen generation and catalase inactivation.
Catalase inactivation promotes the entry of H2O2 through aquaporins, depleting intracellular glutathione and sensitizing cells to death by lipid peroxidation. Furthermore, this mechanism creates intercellular apoptosis-inducing HOCl signaling, which is initiated by active NOX1 and completed by lipid peroxidation via hydroxyl radicals, activating the mitochondrial pathway of apoptosis. This model describes the selective impact of CAP and plasma-activated medium (PAM) towards tumor cells, contrary to prior theories that stated ROS/RNS from CAP or PAM were sufficient to directly cause cell death in tumor cells [44].
5.2. Complementary Antioxidant Pathways
Several studies have shown that when multiple chemicals are mixed, they provide synergistic antioxidant effects. For example, combining quercetin and catechin has been found to greatly increase antioxidant activity in H2O2-stimulated HepG2 cells. This synergism is mediated by the Keap1–Nrf2 signaling pathway, with BACH1 acting as a negative regulator. The presence of quercetin and catechin stimulates the upregulation of let-7a-5p and miR-25-3p, which in turn downregulate BACH1, resulting in reduced reactive oxygen species (ROS) formation and increased cell proliferation [45]. Similarly, phenolic acids and carotenes have been shown to have synergistic antioxidant effects in H2O2-induced H9c2 cells. Phenolic acids promote carotenoid absorption and membrane transporter expression, boosting the antioxidant response. A combination of β-carotene and caffeic acid reduced intracellular ROS levels and boosted nuclear Nrf2, indicating a synergistic impact through the activation of carotene membrane transporters by phenolic acids [46]. Cold atmospheric plasma (CAP) and plasma-activated media (PAM) have been found to have synergistic effects on tumor cells. The interaction of nitrite and H2O2, two long-lived molecules in CAP, results in the synthesis of peroxynitrite and then singlet oxygen generation. This process disables catalase on the surface of tumor cells, allowing H2O2 and peroxynitrite to accumulate and cause death via lipid peroxidation and the mitochondrial route. This concept emphasizes the specific impact of CAP and PAM on tumor cells, mediated by their own ROS and reactive nitrogen species (RNS) [44].
5.3. Enhancement of Cell Survival
Preconditioning cells with low levels of H2O2 has been found to improve their resistance to oxidative stress. H2O2 preconditioning improves survival in human adipose-derived stem cells (hASCs) by lowering intracellular ROS levels by upregulating the transcription factor Nrf2 and its associated antioxidant enzymes. Furthermore, it decreases the release of pro-inflammatory chemicals and adjusts the cellular metabolism to meet the metabolic needs required for survival under oxidative circumstances [47].
5.4. Optimization and Treatment Parameters
The maximal intracellular H2O2 concentration can be used to quantify cell susceptibility toward exogenous H2O2 in cold atmospheric plasma and plasma-treated liquids for cancer treatment [48]. Combining cold atmospheric pressure plasma jet (CAP) with hydrogen peroxide (H2O2) provides dramatic synergistic effects in bacterial disinfection through enhanced membrane transportation of reactive species and oxidation of intracellular molecules [49]. Singlet oxygen from cold atmospheric plasma (CAP) or plasma-activated medium (PAM) triggers tumor cells to generate high concentrations of secondary singlet oxygen, inactivating their protective catalase and reactivating ROS/RNS-dependent apoptosis-inducing signaling [50]. H2O2 surface treatment improves the surface properties and biological performance of calcium phosphates, enhancing ROS generation and cytocompatibility [51]. A combination of cisplatin and cold atmospheric plasma treatment shows a synergistic anticancer effect with low cytotoxicity against normal cells [52]. A H2 self-generation nano-platform strengthens the curative effect of chemodynamic therapy and enhances the Fenton reaction rate, enhancing multimodal synergetic therapy [53].
6. Technical Considerations and Optimization
One of the most promising things in regenerative medicine is MSCs, which are capable of differentiation into a variety of tissue types, and which play immune-modulatory roles. The technical aspects of optimizing MSC therapy involve multiple facets, such as the selection of sources, from bone marrow, adipose tissue, and umbilical cord, and using efficient isolation techniques like flow cytometry or MACS to ensure that the population obtained is pure [41]. For quality control, appropriate characterization, including surface markers such as CD73, CD90, and CD105, as well as functional assays to evaluate differentiation capacity, must be considered [54]. Delivery modes—whether intravenous, intramuscular, or localized—must also allow for the retention of MSCs at the targeted site; this can be enhanced using biomaterials or genetic modification.
The survival and function of MSCs are dependent on the local microenvironment, particularly through factors such as hypoxia and inflammation. Rather, therapeutic efficacy is achieved through the paracrine mechanism whereby MSCs produce growth factors that trigger tissue repair [55]. The scope of genetic engineering alone enhances the potential for MSCs to target specific diseases or deliver therapeutic agents, while encapsulation in biomaterials protects and controls the delivery [56]. Further emerging technologies are nanotechnology and 3D printing, which are rapidly advancing in the field, primarily through the improvement of MSC delivery methods and tissue engineering. Other promising approaches include personalized medicine, where MSC therapy is matched to the genetic profile and particular characteristics of a disease for a patient.
Molecular hydrogen (H2) is gaining more and more interest as a therapeutic agent due to its strong antioxidant and anti-inflammatory effects, thus making it a potentially useful additive in MSC therapy. To enhance the therapeutic efficacy of molecular hydrogen in MSC applications, the delivery of molecular hydrogen needs to be optimized carefully. There are various ways through which molecular hydrogen can be delivered [57]. Each of them has its advantages and disadvantages. Hydrogen-rich water is another handy, non-invasive delivery system; however, hydrogen loss is one of the major challenges for the storage and consumption of water. The studies [58] validate its protective role against oxidative stress-mediated damage. In contrast, injection methods, while providing site-specific delivery, involve risks of tissue damage and embolism, as reported by studies like [59].
In addition to that, enhancements in hydrogen generation and storage systems in terms of electrolysis and metal–acid reactions are included, with the problems noted regarding both efficiency and purity issues revealed in past studies. The design of hydrogen delivery devices should emphasize portability, ease of use, and compatibility with various delivery methods, with biocompatibility serving as a crucial factor, as evidenced by studies such as [60]. Through better development of delivery systems, researchers may further the therapeutic benefits of MSC therapy, especially when applied in diseases where molecular hydrogen holds beneficial pro-anti-inflammatory and regenerative actions.
Cold atmospheric plasma (CAP) is a kind of non-thermal plasma that is generated at atmospheric pressure, characterized by the presence of reactive oxygen and nitrogen species (ROS and RNS), charged particles, and ultraviolet radiation. Such properties might make CAP a potential therapeutic tool for biomedical applications specifically for MSCs. The possible application of CAP for MSC therapy becomes meaningful only in the case of adjustment and optimization of several technical conditions. Among these CAP devices are atmospheric pressure plasma jets (APPJs), in which dielectric barrier discharges can generate homogeneous plasma and work at high values of output power while being very sensitive to arcing, and which possess a complicated design [61,62]. Gliding arcs provide a deep penetration compared with plasma density but are dangerous and susceptible to thermal damage [63].
Other important factors in CAP optimization for MSC therapy are treatment parameters. These include plasma exposure time, power, and gas composition. Studies assert that optimal plasma exposure times depend on the biological effects intended and the type of MSCs [64]. The influence of plasma power on MSC viability, proliferation, and differentiation is significant, with research indicating that lower power levels are typically optimal for maintaining cell function [65]. Furthermore, the composition of gases can be customized to provoke particular responses in MSCs, thereby enhancing processes such as wound healing [66]. Combination therapies, for instance, can also be utilized to further enhance the potential of CAP by leveraging synergistic effects with growth factors for tissue regeneration. In conclusion, optimizing the configurations of CAP devices, treatment parameters, and cell culture conditions can empower CAP as an effective tool for augmenting MSC therapy and improving regenerative outcomes.
Timing and Duration of Molecular Hydrogen and Cold Atmospheric Plasma in Enhancing Mesenchymal Stem Cell Therapy
The timing and duration of treatments utilizing molecular hydrogen (H2) and cold atmospheric plasma (CAP) are highly critical and, in themselves, can significantly impact the effectiveness of mesenchymal stem cell therapy. Preconditioning MSCs with H2 before injection has been established to enhance their survival, migration, and differentiation capacity. Simultaneous administration of H2 during MSC therapy enhanced engraftment and therapeutic outcomes. The period of H2 treatment depends on the disease condition to be treated: short-term H2 treatment might be enough for acute diseases, whereas long-term H2 treatment is necessary for chronic diseases. However, the duration depends on the disease severity, response from the patient, and potential side effects [67]. In the same way, optimal timing of CAP treatment could prepare tissues before MSC application, enhance engraftment during treatment, and facilitate tissue regeneration afterwards through MSC-based therapies. The treatment period with CAP shares a similar process: temporary therapy with H2 is successful in treating acute diseases, while chronic diseases require treatment over a longer time. In the case of H2 as well as CAP treatment, it is crucial to have quality control. In H2, gas purity, testing the concentration of hydrogen, and stability are of utmost importance [68]. CAP quality control overlaps plasma parameters like power and gas composition together with uniformity, with safety measures directed toward ensuring proper functioning of the device as well as the efficiency of the biological effects produced [69]. Optimization of the timing, duration, and quality control of both H2 and CAP treatments can significantly improve the efficacy of MSC therapies for several clinical applications.
In short, MSC therapy optimization indeed requires careful choices among the sources of MSCs, delivery modalities, and advanced technologies such as genetic engineering, nanotechnology, and personalized medicine.
7. Clinical Applications and Future Perspectives
7.1. Current Clinical Status
The contemporary clinical status of molecular hydrogen and cold atmospheric plasma in enhancing MSC therapy is optimistic yet remains in its infancy. The antioxidant and anti-inflammatory properties of H2 were recently highlighted by studies undertaken in [70], thereby adding power to MSCs’ survival, migration, and differentiation capability. Thus, they may unlock new treatment therapies for ischemic heart disease, organ failure, or diseases stemming from inflammation. Nonetheless, there remains a necessity for large-scale randomized trials, particularly due to challenges related to standardized delivery methods and dosimetry. A review [71] highlighted the growing interest in H2 for stem cell therapies; however, it underscored the importance of obtaining more substantial clinical evidence to verify its efficacy and safety.
The therapeutic application of H2 has been tested in numerous clinical trials, but its administration has been evaluated for multiple diseases, such as stroke, diabetes, and inflammatory bowel disease. Still, a very limited number of reports exist showing specific data on the combination of H2 and MSC treatment. Large-scale randomized controlled trials are required to determine the clinical efficacy of this combination. In some experiments, it has been demonstrated that molecular hydrogen may help with oxidative stress and inflammation, which are different indicators of MSCs’ therapeutic potential. Studies such as [72] present an increasing interest in H2 due to its use as a medical gas. Still, there remain many challenges, such as the variability in delivery methods, like inhalation and hydrogen-rich water, and dosage, making it complex to standardize H2 therapy across various clinical settings. There is also the lack of a standardized procedure for the accurate quantification of hydrogen concentration in tissues, limiting the ability to tailor treatments.
Table 3 represents the many different clinical trials for CAP and molecular hydrogen indicating favorable effects, especially within applications related to wound healing and other dermatological issues. When applying CAP with MSCs, the latter’s ability to produce ROS/RNS properties has been advantageous, although there are relatively few clinical trials that focus solely on the issue of MSC therapy. Some of those studies demonstrated that CAP treatment increased the regenerative capacity of MSCs, which means that it increased their effectiveness in tissue repair [73,74]. Along with clinical applications, safety issues for patients have posed challenges regarding long-term exposure. On the other hand, the vast variability of configurations and dosimetry in CAP devices and the lack of uniform treatment parameters have hindered the standardization of therapy. More studies need to be conducted to strengthen these technical aspects further.

Table 3.
Clinical trials and outcomes of molecular hydrogen and cold atmospheric plasma.
7.2. Potential Therapeutic Applications
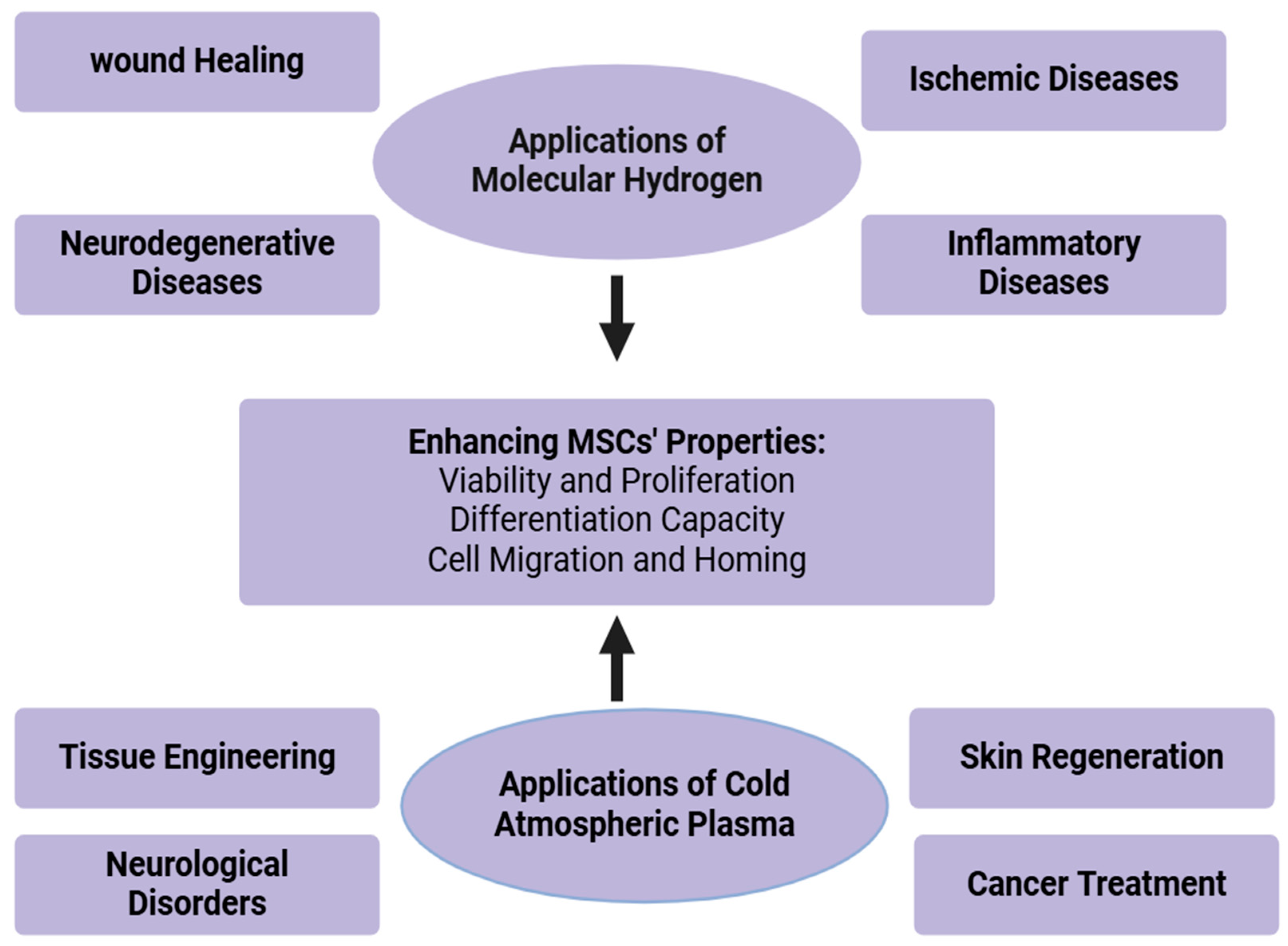
Molecular hydrogen (H2) and cold atmospheric plasma (CAP) now represent advanced agents that show much promise for improving MSC therapy. H2 and CAP enable cell and tissue biochemical features to be selectively modulated by a unique combination of the potent antioxidant and anti-inflammatory effects of H2 and by changes in the behavior of cells modified by CAP with improved tissue regeneration. This has been shown to improve the therapeutic potential of MSCs through their antioxidant and anti-inflammatory properties. Oxidative stress and modulated immune responses that will contribute to the survival, migration, and differentiation of MSCs are reduced by molecular hydrogen and form a favorable microenvironment for effective stem cell therapy.
Molecule hydrogen has been considered an agent for enhancing results in ischemic conditions, in which tissue damage arises due to a low supply of blood. Figure 3 represents the therapeutic applications of molecular hydrogen. The antioxidant activity of H2 helps reverse oxidative injury due to reperfusion injury following ischemia. In stroke, H2 enhanced the therapeutic effectiveness of MSCs in ischemic stroke by improving the survival and repair of cells and neurons [77]. In the case of myocardial infarction, H2 can protect heart tissue from ischemia–reperfusion injury, and thus has the potential to improve cardiac function in patients after a heart attack. In peripheral artery disease and other similar diseases, H2 protects tissues from ischemic injury through its modulative action on oxidative stress and inflammation. Hydrogen molecules have neuroprotective effects and seem, thus, promising adjuncts in MSC therapy against neurodegenerative diseases, being capable of diminishing oxidative stress and inflammation in the brain and improving survival, as well as the differentiation capacity of MSCs into neural cells.
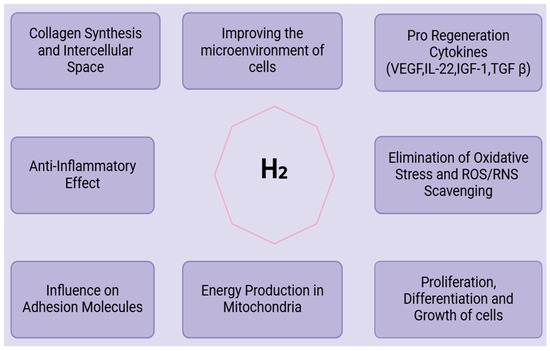
Figure 3.
Clinical applications of molecular hydrogen.
In the case of Alzheimer’s disease, there is evidence that H2 can prevent the oxidative stress associated with the disease and improve the preservation of cognitive functions. Studies on preclinical research have shown that H2 inhalation has provided neuroprotection to dopaminergic neurons and has delayed the worsening of Parkinson’s disease. H2, for its anti-inflammatory properties, is beneficial in inflammatory diseases [79]. By reducing inflammation and oxidative damage, H2 enhances the ability of MSCs to repair and regenerate tissues in conditions characterized by chronic inflammation. H2 could diminish the symptoms of rheumatoid arthritis as it suppresses pro-inflammatory immune responses, thus enhancing the ability of MSCs to repair and regenerate damaged cartilage. H2, in a preclinical model of colitis, was shown to exert positive effects through the reduction of intestinal inflammation [80]. The survival and migration of MSCs have shown great promise in improving molecular hydrogen’s healing of wounds. It can improve the healing of challenging wounds such as diabetic ulcers, burns, and pressure ulcers [80], as illustrated in Figure 3.
Cold atmospheric plasma is a partially ionized gas, producing reactive oxygen and nitrogen species (RONS) that are known to become involved in cell functions such as proliferation, migration, and differentiation. CAP has great potential in the use of MSC therapy to combat treatments for wound healing, cancer, and other clinical applications. CAP has been extensively studied in enhancing tissue regeneration and promoting chronic wound healing. The synergistic effects of CAP in combination with MSC therapy seem to have superiority in cellular proliferation and tissue repair. CAP, in combination with MSCs, would accelerate the healing of diabetic ulcers by accelerating angiogenesis and having lower risks of infection [76]. CAP also has potent activity on skin conditions such as dermatitis, psoriasis, and acne. By its microbicidal action, it can also suppress inflammation and facilitate the regeneration of skin, so it is one useful reagent in many dermatologic treatments [81]. CAP has been shown to possess potential as an adjunct in the treatment of cancers because it could mediate the selective killing of cancerous cells and can also enhance the effects of chemotherapy and radiotherapy [82]. The combination of molecular hydrogen and cold atmospheric plasma in MSC therapy presents synergistic effects, enhancing the overall therapeutic potential of MSCs. This duality may improve cell proliferation, migration, differentiation, and survival altogether, leading to better outcomes in all conceivable therapeutic applications [78]. However, future research should be dedicated to standardizing treatment protocols, optimizing dosages, and ensuring long-term safety in clinical settings. Applications are illustrated in Figure 4.
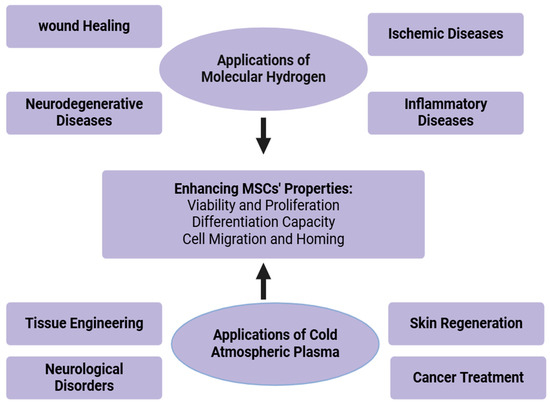
Figure 4.
Therapeutic applications of molecular hydrogen and cold atmospheric plasma.
7.3. Potential Challenges with Solutions for Combining Molecular Hydrogen and Cold Atmospheric Plasma in Mesenchymal Stem Cell Therapy
The combination of molecular hydrogen, H2, with cold atmospheric plasma, CAP, in MSC therapy is intricate and throws up many challenges that need due contemplation. Some challenges include the necessity of optimizing dosages to the regulatory issues regarding using such therapies. The optimal dose for the pre-treatment of MSCs using molecular hydrogen is not clear; an extended exposure might be harmful due to side effects. Optimal dosing with molecular hydrogen should be established through extensive preclinical and clinical studies [83]. Long-term risks should be evaluated using longer follow-up studies. Marks of oxidative stress and inflammation should be continuously monitored for safety. The failure to standardize protocols in applying molecular hydrogen and CAP across studies and clinical settings brings about variable outcomes. This would involve coordination among research institutions, industry, and bodies of regulation to formulate uniform procedures for applying H2 and CAP [84]. The production and application of molecular hydrogen and CAP are expensive. This shall set a cost to patients wanting to undergo such therapies. Investment in research to improve the cost-efficiency of producing hydrogen and CAP devices should involve partnership with industries to help scale up their production and distribution [84]. The issue with MSCs is that they can induce a perverse immune response leading to rejection. Molecular hydrogen and CAP need to be optimized regarding their immunomodulatory properties to decrease this chance.
Combination therapies with immunosuppressive agents or genetic modification of MSCs to reduce their immunogenicity would counter rejection. Preclinical studies should evaluate whether H2 and CAP can drive MSC immune evasion [85]. The technical challenge lies in the fact that it is hard to manufacture and install CAP to such high precision. Inconsistency in the outcome arises due to heterogeneous devices that have been developed and treatment parameters [84]. The proper orientation of healthcare workers about using devices appropriately will ensure consistent and reproducible treatments. The radicals produced by CAP are also good but, if not controlled, may cause oxidative stress and cellular injury [44]. CAP can easily manipulate proliferation and differentiation cell fates, which in the wrong sites could lead to undesirable therapeutic outcomes [65]. The interaction between molecular hydrogen and CAP might fail to result in an optimal effect if it is not orchestrated. The synergy effects of H2 and CAP should be preclinically examined. Studies on the time, sequence, and dose–response will indicate the best possible time when treatment is given, so that maximum benefit may be achieved [44]. The use of molecular hydrogen as part of MSC treatment with CAP brings additional challenges in the ethical and regulatory senses. The regulatory bodies and the ethical issues will be concerned about the consent and safety of the patient, before the administration of treatment [67]. Addressing these challenges with collaborative research, technological breakthroughs, and careful regulatory planning will eventually lead to the successful integration of molecular hydrogen and CAP in mesenchymal stem cell therapy.
8. Conclusions
In conjunction with the introduction of molecular hydrogen (H2) and cold atmospheric plasma into MSC therapy, several promising research directions can be drawn forth that may considerably enhance the therapeutic efficacy of MSCs. For starters, it is deemed crucial to understand the mechanisms at the molecular basis of the effects of H2 and CAP on MSCs. Further investigation of specific molecular pathways will elaborate on how such agents may alter cellular function in the context of proliferation, differentiation, and tissue regeneration. The research has shown that this improves cell survival and differentiation in MSC therapy. Moreover, the simultaneous use of H2 with growth factors can likely prove to optimize MSC therapy for various purposes, as shown by [46]. This may pave the way for personalized medicine, tailoring treatments based on the characteristics and responses of patients, such as the example shown in [25]. Follow-up studies for long periods are essential to evaluate the longevity and sustainability of these therapeutic effects. Research work such as [66] will help confirm and establish the efficacy and safety of MSC therapies based on their H2 and CAP enhancement, which is fundamental for clinical translation. Lastly, the advanced technology associated with CAP devices can further improve the precision and effectiveness of the treatment by doing so through discussions presented as in [68]. Similarly, the nanoparticle-based delivery systems targeted to H2 therapy studies, such as [86], may potentially promote advancing the therapeutic response with minimal adverse effects.
Author Contributions
Conceptualization, Literature search and analysis, Manuscript drafting M.Y.A.: Conceptualization, Critical revisions and editing F.A.P.; Final approval of the manuscript I.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal stem cell senescence and rejuvenation: Current status and challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef]
- Sarkar, D.; Spencer, J.A.; Phillips, J.A.; Zhao, W.; Schafer, S.; Spelke, D.P.; Mortensen, L.J.; Ruiz, J.P.; Vemula, P.K.; Sridharan, R. Engineered cell homing. Blood J. Am. Soc. Hematol. 2011, 118, e184–e191. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Pei, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Improved therapeutics of modified mesenchymal stem cells: An update. J. Transl. Med. 2020, 18, 42. [Google Scholar] [CrossRef]
- Todosenko, N.; Khlusov, I.; Yurova, K.; Khaziakhmatova, O.; Litvinova, L. Signal pathways and microRNAs in osteosarcoma growth and the dual role of mesenchymal stem cells in oncogenesis. Int. J. Mol. Sci. 2023, 24, 8993. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Wong, N.-K.; Xiao, J.; So, K.-F. Oxidative stress in stem cell aging. Cell Transplant. 2017, 26, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Hematti, P. Effects of oxidative stress on mesenchymal stem cell biology. Oxidative Med. Cell. Longev. 2016, 2016, 2989076. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Khanna, P.; Wong, B.S.E.; Heng, Z.S.L.; Subhramanyam, C.S.; Thanga, L.Z.; Tan, S.W.S.; Baeg, G.H. Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget 2018, 9, 4223. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Fauzia, E.; Siddiqui, A.J.; Yadav, S.K.; Kumari, N.; Singhai, A.; Khan, M.A.; Janowski, M.; Bhutia, S.K.; Raza, S.S. Oxidative stress enhances autophagy-mediated death of stem cells through Erk1/2 signaling pathway–implications for neurotransplantations. Stem Cell Rev. Rep. 2021, 17, 2347–2358. [Google Scholar] [CrossRef]
- Martinelli, E.; Granato, D.; Azevedo, L.; Gonçalves, J.E.; Lorenzo, J.M.; Munekata, P.E.; Simal-Gandara, J.; Barba, F.J.; Carrillo, C.; Rajoka, M.S.R. Current perspectives in cell-based approaches towards the definition of the antioxidant activity in food. Trends Food Sci. Technol. 2021, 116, 232–243. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Cui, H.; Zhu, W.; Talbot, A.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The strong cell-based hydrogen peroxide generation triggered by cold atmospheric plasma. Sci. Rep. 2017, 7, 10831. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef]
- Boehm, D.; Heslin, C.; Cullen, P.J.; Bourke, P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci. Rep. 2016, 6, 21464. [Google Scholar] [CrossRef]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Controlling stem cell fate using cold atmospheric plasma. Stem Cell Res. Ther. 2020, 11, 368. [Google Scholar] [CrossRef]
- Hamouda, I.; Tampieri, F.; Canal, C.; Labay, C.; Ginebra, M. Production of reactive species in alginate hydrogels for cold atmospheric plasma-based therapies. Sci. Rep. 2019, 9, 16160. [Google Scholar]
- Kurokawa, R.; Seo, T.; Sato, B.; Hirano, S.-i.; Sato, F. Convenient methods for ingestion of molecular hydrogen: Drinking, injection, and inhalation. Med. Gas Res. 2015, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Fei, W.-Y.; Liu, M.-S.; Zhang, Y.-C.; Gao, R.-S.; Hu, Y.-Y.; Pang, E.-K.; Hou, L. Molecular hydrogen promotes adipose-derived stem cell myogenic differentiation via regulation of mitochondria. Curr. Stem Cell Res. Ther. 2023, 18, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a novel therapeutic molecule, regulates oxidative stress, inflammation, and apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Canal, C.; Labay, C. Positive effect of cold atmospheric nitrogen plasma on the behavior of mesenchymal stem cells cultured on a bone scaffold containing iron oxide-loaded silica nanoparticles catalyst. Int. J. Mol. Sci. 2020, 21, 4738. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, R.; Thomas, P.; Zhao, L.; Zhou, R.; Mandal, S.; Jolly, M.K.; Richard, D.J.; Rehm, B.H.; Ostrikov, K. Epithelial-to-mesenchymal transition enhances cancer cell sensitivity to cytotoxic effects of cold atmospheric plasmas in breast and bladder cancer systems. Cancers 2021, 13, 2889. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-Y.; Hong, Y.J.; Lim, J.; Choi, J.S.; Choi, E.H.; Kang, S.; Rhim, H. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials 2018, 156, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Artamonov, M.Y.; Martusevich, A.K.; Pyatakovich, F.A.; Minenko, I.A.; Dlin, S.V.; LeBaron, T.W. Molecular hydrogen: From molecular effects to stem cells management and tissue regeneration. Antioxidants 2023, 12, 636. [Google Scholar] [CrossRef]
- Radyuk, S.N. Mechanisms underlying the biological effects of molecular hydrogen. Curr. Pharm. Des. 2021, 27, 626–735. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef]
- Jaganathan, B.; Tisato, V.; Vulliamy, T.; Dokal, I.; Marsh, J.; Dazzi, F.; Bonnet, D. Effects of MSC co-injection on the reconstitution of aplastic anemia patient following hematopoietic stem cell transplantation. Leukemia 2010, 24, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Trappmann, B.; Chen, C.S. How cells sense extracellular matrix stiffness: A material’s perspective. Curr. Opin. Biotechnol. 2013, 24, 948–953. [Google Scholar] [CrossRef]
- Li, B.; Moshfegh, C.; Lin, Z.; Albuschies, J.; Vogel, V. Mesenchymal stem cells exploit extracellular matrix as mechanotransducer. Sci. Rep. 2013, 3, 2425. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Pittrof, A.; Killian, M.S.; Schmuki, P.; von der Mark, K. Synergistic control of mesenchymal stem cell differentiation by nanoscale surface geometry and immobilized growth factors on TiO2 nanotubes. Small 2012, 8, 98–107. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal stromal cell homing: Mechanisms and strategies for improvement. Iscience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Popielarczyk, T.L.; Huckle, W.R.; Barrett, J.G. Human bone marrow-derived mesenchymal stem cells home via the PI3K-Akt, MAPK, and Jak/Stat signaling pathways in response to platelet-derived growth factor. Stem Cells Dev. 2019, 28, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Shahror, R.A.; Ali, A.A.A.; Wu, C.-C.; Chiang, Y.-H.; Chen, K.-Y. Enhanced homing of mesenchymal stem cells overexpressing fibroblast growth factor 21 to injury site in a mouse model of traumatic brain injury. Int. J. Mol. Sci. 2019, 20, 2624. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials 2017, 140, 103–114. [Google Scholar] [CrossRef]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Niche-mimicking interactions in peptide-functionalized 3D hydrogels amplify mesenchymal stromal cell paracrine effects. Biomaterials 2020, 230, 119639. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Linsley, C.; Wu, B.; Tawil, B. The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts. Tissue Eng. Part A 2013, 19, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Xu, J.; Zhao, Y.; Ye, C.; Sun, Q.; Liu, C.; Huo, B. Synergistic effects of fluid shear stress and adhesion morphology on the apoptosis and osteogenesis of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2022, 110, 1636–1644. [Google Scholar] [CrossRef]
- Wagner, J.; Kean, T.; Young, R.; Dennis, J.E.; Caplan, A.I. Optimizing mesenchymal stem cell-based therapeutics. Curr. Opin. Biotechnol. 2009, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Planell, J.A.; Mateos-Timoneda, M.A.; Engel, E. Role of ECM/peptide coatings on SDF-1α triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomater. 2015, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-R.; Suh, H.; Yu, J.H.; Kim, H.; Seo, J.H.; Seo, C.H. Astroglial activation by an enriched environment after transplantation of mesenchymal stem cells enhances angiogenesis after hypoxic-ischemic brain injury. Int. J. Mol. Sci. 2016, 17, 1550. [Google Scholar] [CrossRef]
- Bauer, G. The synergistic effect between hydrogen peroxide and nitrite, two long-lived molecular species from cold atmospheric plasma, triggers tumor cells to induce their own cell death. Redox Biol. 2019, 26, 101291. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, Y.; Sun-Waterhouse, D.-X.; Zhai, H.; Guan, H.; Rong, X.; Li, F.; Yu, J.-C.; Li, D.-P. MicroRNA-based regulatory mechanisms underlying the synergistic antioxidant action of quercetin and catechin in H2O2-stimulated HepG2 cells: Roles of BACH1 in Nrf2-dependent pathways. Free Radic. Biol. Med. 2020, 153, 122–131. [Google Scholar] [CrossRef]
- Pan, Y.; Deng, Z.-Y.; Chen, X.; Zhang, B.; Fan, Y.; Li, H. Synergistic antioxidant effects of phenolic acids and carotenes on H2O2-induced H9c2 cells: Role of cell membrane transporters. Food Chem. 2021, 341, 128000. [Google Scholar] [CrossRef]
- Garrido-Pascual, P.; Alonso-Varona, A.; Castro, B.; Burón, M.; Palomares, T. H2O2-preconditioned human adipose-derived stem cells (HC016) increase their resistance to oxidative stress by overexpressing Nrf2 and bioenergetic adaptation. Stem Cell Res. Ther. 2020, 11, 335. [Google Scholar] [CrossRef]
- Bengtson, C.; Bogaerts, A. The quest to quantify selective and synergistic effects of plasma for cancer treatment: Insights from mathematical modeling. Int. J. Mol. Sci. 2021, 22, 5033. [Google Scholar] [CrossRef] [PubMed]
- Baik, K.Y.; Jo, H.; Ki, S.H.; Kwon, G.-C.; Cho, G. Synergistic effect of hydrogen peroxide and cold atmospheric pressure plasma-jet for microbial disinfection. Appl. Sci. 2023, 13, 3324. [Google Scholar] [CrossRef]
- Bauer, G. Signal amplification by tumor cells: Clue to the understanding of the antitumor effects of cold atmospheric plasma and plasma-activated medium. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 2, 87–98. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh, S.; Kargozar, S.; Khakhi, J.V. Improved osteogenesis and angiogenesis of theranostic ions doped calcium phosphates (CaPs) by a simple surface treatment process: A state-of-the-art study. Mater. Sci. Eng. C 2021, 124, 112082. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M.; Jeong, Y.-I.; Kook, M.-S.; Kim, B.-H. Combinatorial effect of cold atmosphere plasma (Cap) and the anticancer drug cisplatin on oral squamous cell cancer therapy. Int. J. Mol. Sci. 2020, 21, 7646. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, Y.; Shi, J.; Wang, L. NIR-driven water splitting H2 production nanoplatform for H2-mediated cascade-amplifying synergetic cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 23677–23688. [Google Scholar] [CrossRef]
- Park, J.S.; Suryaprakash, S.; Lao, Y.-H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16. [Google Scholar] [CrossRef]
- Najar, M.; Melki, R.; Khalife, F.; Lagneaux, L.; Bouhtit, F.; Moussa Agha, D.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Merimi, M. Therapeutic mesenchymal stem/stromal cells: Value, challenges and optimization. Front. Cell Dev. Biol. 2022, 9, 716853. [Google Scholar] [CrossRef]
- Pawitan, J.A.; Bui, T.A.; Mubarok, W.; Antarianto, R.D.; Nurhayati, R.W.; Dilogo, I.H.; Oceandy, D. Enhancement of the therapeutic capacity of mesenchymal stem cells by genetic modification: A systematic review. Front. Cell Dev. Biol. 2020, 8, 587776. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Peng, Y.; Qin, C.; Fan, F.; Liu, J.; Long, J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free Radic. Res. 2018, 52, 1311–1322. [Google Scholar] [CrossRef]
- Jeong, E.-S.; Bajgai, J.; You, I.-S.; Rahman, M.H.; Fadriquela, A.; Sharma, S.; Kwon, H.-U.; Lee, S.-Y.; Kim, C.-S.; Lee, K.-J. Therapeutic effects of hydrogen gas inhalation on trimethyltin-induced neurotoxicity and cognitive impairment in the C57BL/6 mice model. Int. J. Mol. Sci. 2021, 22, 13313. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.-N.; Yin, X.-X.; Song, W.-G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Malyavko, A.; Wang, Q.; Lin, L.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma cancer treatment, a critical review. Appl. Sci. 2021, 11, 7757. [Google Scholar] [CrossRef]
- Cheng, F.; Yan, D.; Chen, J.; Wang, Z.; Horkowitz, A.; Keidar, M.; Sotomayor, E.M. Enhancing innate and adaptive immune systems by cold atmospheric plasma (CAP) and its antitumor immunity. arXiv 2022, arXiv:2201.12737. [Google Scholar]
- He, R.; Li, Q.; Shen, W.; Wang, T.; Lu, H.; Lu, J.; Lu, F.; Luo, M.; Zhang, J.; Gao, H. The efficacy and safety of cold atmospheric plasma as a novel therapy for diabetic wound in vitro and in vivo. Int. Wound J. 2020, 17, 851–863. [Google Scholar] [CrossRef]
- Fernández-Santos, M.E.; Garcia-Arranz, M.; Andreu, E.J.; García-Hernández, A.M.; López-Parra, M.; Villarón, E.; Sepúlveda, P.; Fernández-Avilés, F.; García-Olmo, D.; Prosper, F. Optimization of mesenchymal stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Front. Immunol. 2022, 13, 918565. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Bezbaruah, R.; Rynjah, D.; Newar, A.; Sengupta, S.; Pegu, P.; Dey, N.; Bora, S.C.; Barman, D. Cold Atmospheric Plasma: A Noteworthy Approach in Medical Science. Sci. Pharm. 2023, 2, 79–103. [Google Scholar] [CrossRef]
- Stańczyk, B.; Wiśniewski, M. The Promising Potential of Cold Atmospheric Plasma Therapies. Plasma 2024, 7, 465–497. [Google Scholar] [CrossRef]
- Hirano, S.-i.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Clinical Use and Treatment Mechanism of Molecular Hydrogen in the Treatment of Various Kidney Diseases including Diabetic Kidney Disease. Biomedicines 2023, 11, 2817. [Google Scholar] [CrossRef]
- Sim, M.; Kim, C.-S.; Shon, W.-J.; Lee, Y.-K.; Choi, E.Y.; Shin, D.-M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Joo, S.Y.; Cho, Y.S.; Park, J.H.; Kim, J.-B.; Seo, C.H. Effect of combining low temperature plasma, negative pressure wound therapy, and bone marrow mesenchymal stem cells on an acute skin wound healing mouse model. Int. J. Mol. Sci. 2020, 21, 3675. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.; Pavani, K.; Ramaraghavulu, R.; Shim, J. Microwave synthesis of NiMn2O4/Ni-foam: Efficient bifunctional electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2024, 54, 691–699. [Google Scholar] [CrossRef]
- Artamonov, M.Y.; LeBaron, T.W.; Sokov, E.L.; Kornilova, L.E.; Pyatakovich, F.A.; Minenko, I.A. Intraosseous Administration of Molecular Hydrogen: A Novel Technique—From Molecular Effects to Tissue Regeneration. Mol. Hydrog. Health Dis. 2024, 27, 417–433. [Google Scholar]
- Russell, G. A Study into the Biological Activity and Therapeutic Potential of Molecular Hydrogen and Oxyhydrogen Gases. School of Applied Sciences. Ph.D. Thesis, University of the West of England, Bristol, UK, 2024. [Google Scholar]
- Liu, Z.; Du, X.; Xu, L.; Shi, Q.; Tang, X.; Cao, Y.; Song, K. The therapeutic perspective of cold atmospheric plasma in periodontal disease. Oral Dis. 2024, 30, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Evangelina, R.; Samuel, J.P.; Singh, P.; Saha, S.; Singhal, M.; Gandhirajan, R.K. Cold atmospheric plasma (CAP) in wound healing: Harnessing a dual-edged sword. Redox Exp. Med. 2024, 2024, e230026. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. Intraarticular injections of mesenchymal stem cells in knee osteoarthritis: A review of their current molecular mechanisms of action and their efficacy. Int. J. Mol. Sci. 2022, 23, 14953. [Google Scholar] [CrossRef] [PubMed]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical applications of cold atmospheric plasma: State of the science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef]
- Kura, B.; Slezak, J. The Protective Role of Molecular Hydrogen in Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2024, 25, 7884. [Google Scholar] [CrossRef] [PubMed]
- von Woedtke, T.; Emmert, S.; Metelmann, H.-R.; Rupf, S.; Weltmann, K.-D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Phys. Plasmas 2020, 27, 70601. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-i.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold atmospheric plasma therapy in wound healing. Process Biochem. 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.-i.; Ichikawa, Y.; Sato, B.; Satoh, F.; Takefuji, Y. Hydrogen is promising for medical applications. Clean Technol. 2020, 2, 529–541. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Bhattacharya, S. Hydrogen gas sensing methods, materials, and approach to achieve parts per billion level detection: A review. Int. J. Hydrog. Energy 2019, 44, 26076–26099. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Mu, W.; Ma, Q.; Zhai, X.; Jin, B.; Liu, Y.; Zhang, N. Delivery Strategy to Enhance the Therapeutic Efficacy of Liver Fibrosis via Nanoparticle Drug Delivery Systems. ACS Nano 2024, 18, 20861–20885. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).