Oxo-Hydrazyl as a Substitute for the Stable Free Radicals Employed in Measuring Total Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
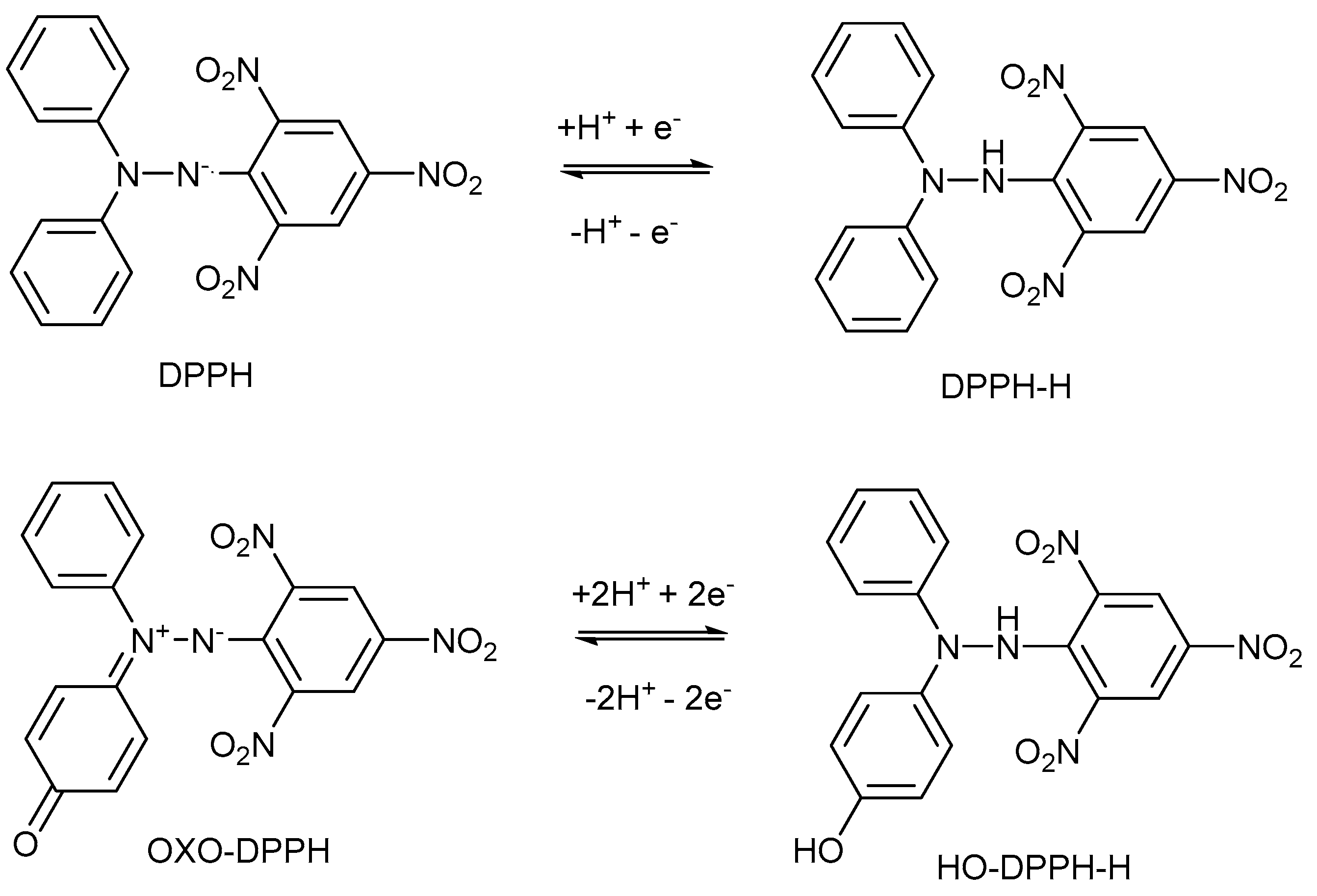
2.1. General Characteristics of DPPH·, ABTS·+, and Oxo-DPPH Derivative, and Their Mechanism of Action
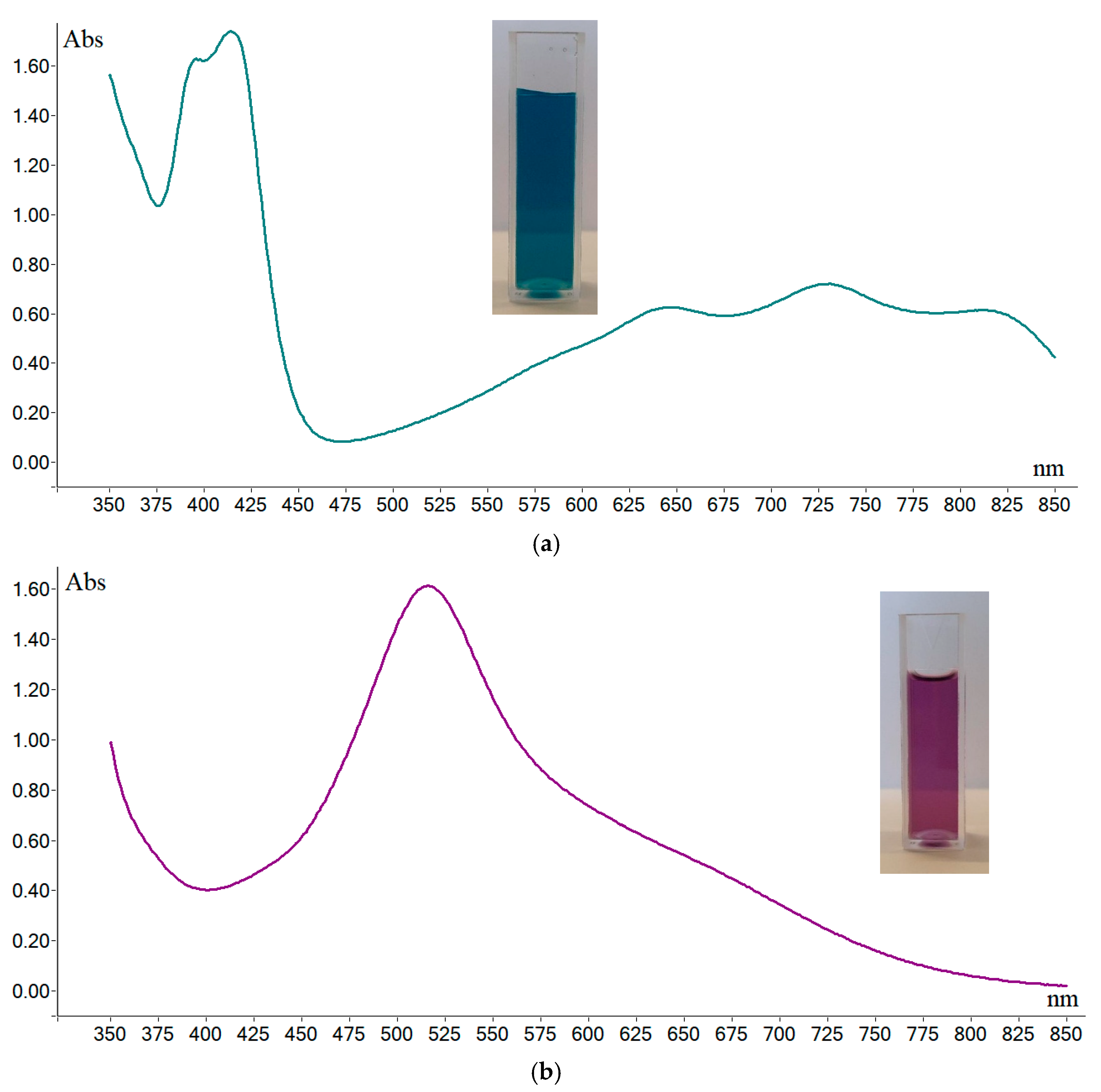
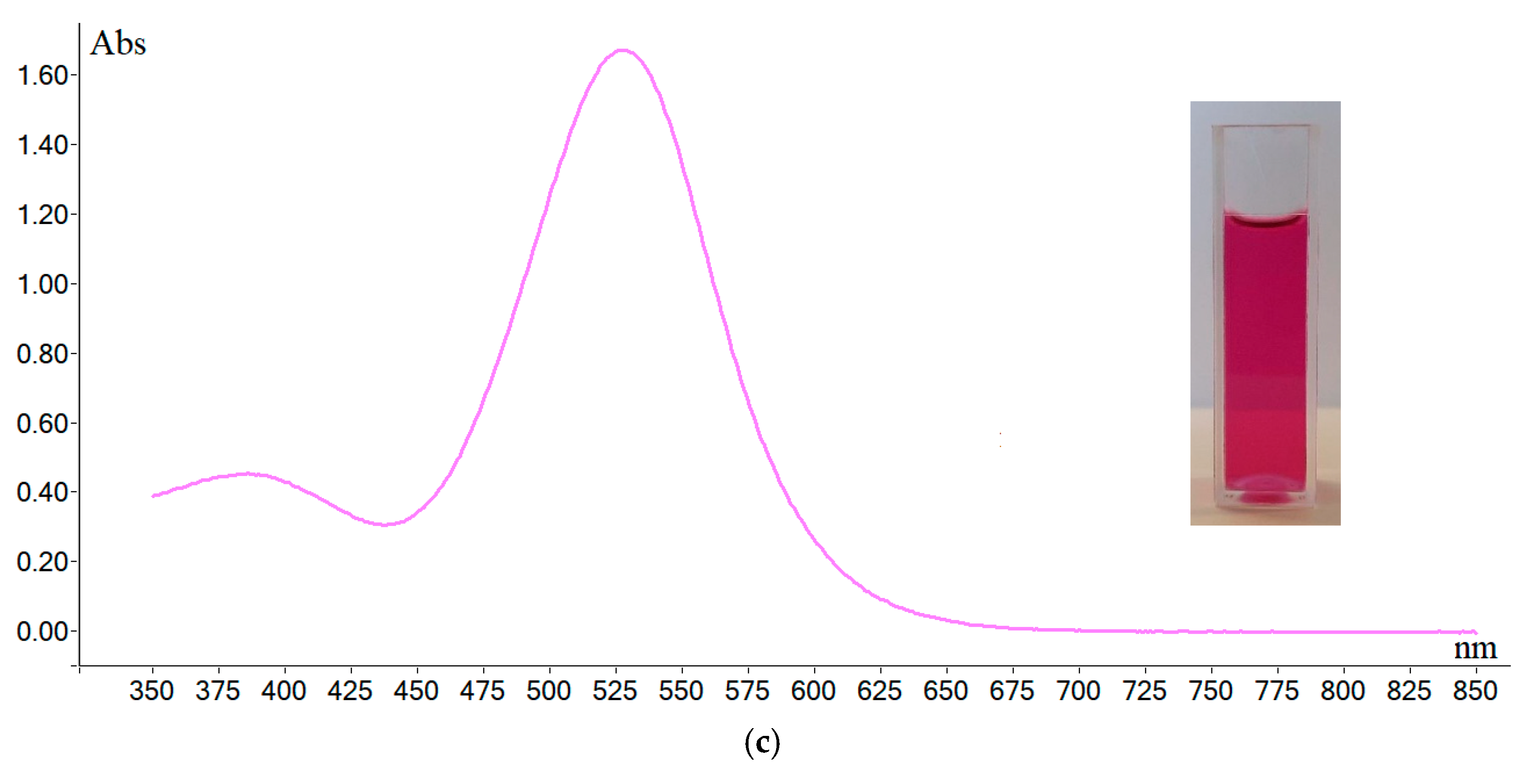
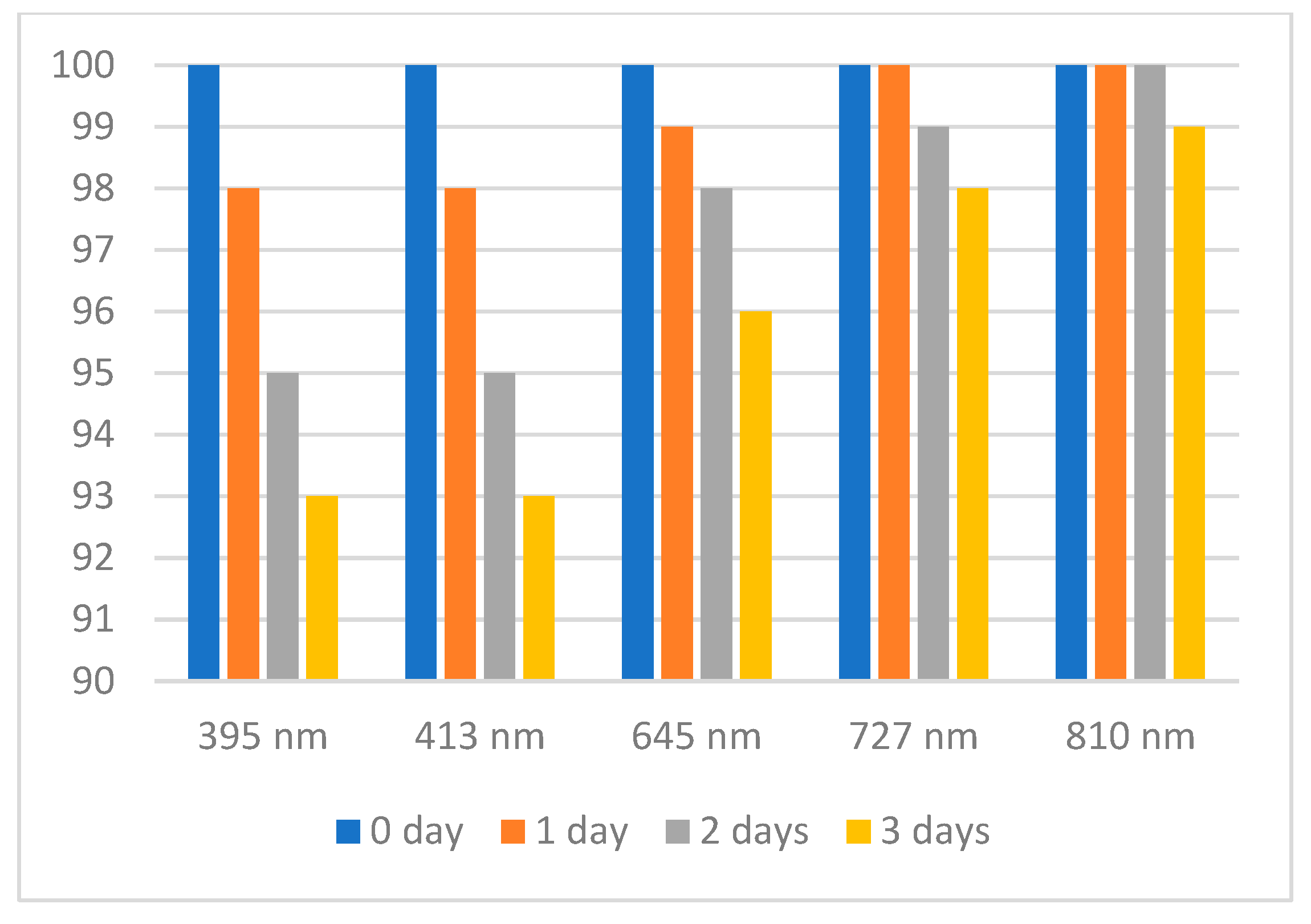
2.2. Stability Tests of the Solution of DPPH·, ABTS·+, and Oxo-DPPH
2.3. TAC Measurements
2.4. Mechanism of Action
3. Materials and Methods
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. U.-Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pryor, R.L.; Wu, X.; Snaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121. [Google Scholar] [CrossRef]
- Stoian, M.; Kuncser, A.; Neatu, F.; Florea, M.; Popa, M.; Voicu, S.; Chifiriuc, M.; Hanganu, A.; Anghel, M.; Tudose, M. Green synthesis of aminated hyaluronic acid-based silver nanoparticles on modified titanium dioxide surface: Influence of size and chemical composition on their biological properties. Int. J. Biol. Macromol. 2023, 253, 127445. [Google Scholar] [CrossRef]
- Tudose, M.; Culita, D.; Musuc, A.; Somacescu, S.; Ghica, C.; Chifiriuc, M.; Bleotu, C. Lipoic acid functionalized SiO2@Ag nanoparticles. Synthesis, characterization and evaluation of biological activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Tudose, M.; Culita, D.C.; Baratoiu-Carpen, R.D.; Mitran, R.-A.; Kuncser, A.; Romanitan, C.; Popescu, R.C.; Savu, D.I. Novel antitumor agents based on fluorescent benzofurazan derivatives and mesoporous silica. Int. J. Mol. Sci. 2022, 23, 15663. [Google Scholar] [CrossRef]
- Bujor, A.; Hanganu, A.; Tecuceanu, V.; Madalan, A.M.; Tudose, M.; Marutescu, L.; Popa, M.; Chifiriuc, C.M.; Zarafu, I.; Ionita, P. Biological evaluation and structural analysis of some aminodiphenylamine derivatives. Antioxidants 2023, 12, 713. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidant characterization: Methodology and mechanism. Biochem. Pharmacol. 1995, 49, 1341–1348. [Google Scholar] [CrossRef]
- Rodríguez, J.; Olea-Azara, C.; Cavieres, C.; Norambuena, E.; Delgado-Castro, T.; Soto- Delgado, J.; Araya-Maturana, R. Antioxidant properties and free radical- scavenging reactivity of a family of hydroxynaphthalenones and dihydroxyanthracenones. Bioorganic Med.Chem. 2007, 15, 7058–7065. [Google Scholar] [CrossRef]
- Sawai, Y.; Moon, J. NMR Analytical approach to clarify the molecular mechanisms of the antioxidative and radical-scavenging activities of antioxidants in tea using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 6247–6253. [Google Scholar] [CrossRef]
- Takatsuka, M.; Goto, S.; Kobayashi, K.; Otsuka, Y.; Shimada, Y. Evaluation of pure antioxidative capacity of antioxidants: ESR spectroscopy of stable radicals by DPPH and ABTS assays with singular value decomposition. Food Biosci. 2022, 48, 101714. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cheregi, M.; Danet, A. Total antioxidant capacity of some commercial fruit juices: Electrochemical and spectrophotometrical approaches. Molecules 2009, 14, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Tian, S.; Jiang, J.; Han, D.; Yu, X.; Wang, K.; Li, D.; Lu, D.; Yu, A.; Zhang, Z. Determination of antioxidant capacity of diverse fruits by electron spin resonance (ESR) and UV–vis spectrometries. Food Chem. 2017, 221, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Kozmelj, T.R.; Voinov, M.A.; Grilc, M.; Smirnov, A.I.; Jasiukaitytė-Grojzdek, E.; Lucia, L.; Likozar, B. Lignin structural characterization and its antioxidant potential: A comparative evaluation by EPR, UV-Vis spectroscopy, and DPPH assays. Int. J. Mol. Sci. 2024, 25, 9044. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G.; De Caro, V.; Cardamone, C.; Oliveri, G.; D’Oca, M.C. EPR spectroscopy coupled with spin trapping as an alternative tool to assess and compare the oxidative stability of vegetable oils for cosmetics. Appl. Sci. 2024, 14, 10766. [Google Scholar] [CrossRef]
- de Gaulejac, N.; Provost, C.; Vivas, N. Comparative study of polyphenol scavenging activities assessed by different methods. J. Agric. Food Chem. 1999, 47, 425–431. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.; Dommesa, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Susannah, L.; Scott, W.J.; Andreja, C.; James, H. Spectroscopic parameters, electrode potentials, acid ionization constants, and electron exchange rates of the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) radicals and ions. J. Phys. Chem. 1993, 97, 6710–6714. [Google Scholar] [CrossRef]
- Matsumoto, K.; Taniarashi, M.; Tsutaho, Y.; Yamada, A.; Yosho, A.; Osakai, T.; Hotta, H. Redox reactions between ABTS.+ and dihydrobenzenes as studied by cyclic voltammetry. Analytic. Sci. 2022, 38, 227–230. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta 1998, 1379, 381. [Google Scholar] [CrossRef]
- Nakanishi, I.; Shoji, Y. Electrochemical redox behavior of 2,2-diphenyl-1-picrylhydrazyl radical solubilized by β-cyclodextrin in water. Electrochem. Commun. 2022, 134, 107190. [Google Scholar] [CrossRef]
- Dobre, A.F.; Madalan, A.M.; Ionescu, S.; Hanganu, A.; Lete, C.; Popescu, C.; Paun, A.; Matache, M.; Ionita, P. Zwitterion or diradicaloid? The case of diazenium betaines derived from DPPH. J. Molec. Struct. 2023, 1275, 134703. [Google Scholar] [CrossRef]
- Cano, A.; Maestre, A.B.; Hernández-Ruiz, J.; Arnao, M.B. ABTS/TAC methodology: Main milestones and recent applications. Processes 2023, 11, 185. [Google Scholar] [CrossRef]
- Ionita, P.; Tuna, F.; Andruh, M.; Constantinescu, T.; Balaban, A.T. Synthesis and characterization of some novel homo- and hetero-diradicals of hydrazyl and nitroxide type. Aust. J. Chem. 2007, 60, 173–179. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of the antioxidant capacity of food products: Methods, applications and limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Öztürk, H.İ.; Özçoban, A. Analysis of the chemical composition, antimicrobial, and antioxidant qualities of microwave and supercritical CO2-extracted lavender essential oils cultivated in a hyperarid region of Türkiye. Molecules 2024, 29, 5605. [Google Scholar] [CrossRef]
- Martinotti, S.; Bonsignore, G.; Ranzato, E. Propolis: A natural substance with multifaceted properties and activities. Int. J. Mol. Sci. 2025, 26, 1519. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Ivanov, I.; Todorova, M.; Apostolova, S.; Tzoneva, R.; Nikolova, K. Phenolic content, antioxidant activity and in vitro anti-inflammatory and antitumor potential of selected bulgarian propolis samples. Biomedicines 2025, 13, 334. [Google Scholar] [CrossRef]
- Motawi, T.K.; Ahmed, S.A.; Hamed, M.A.; El-Maraghy, S.A.; Aziz, W.M. Melatonin and/or rowatinex attenuate streptozotocin-induced diabetic renal injury in rats. J. Biomed. Res. 2019, 33, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Paulis, G. Inflammatory mechanisms and oxidative stress in prostatitis: The possible role of antioxidant therapy. Res. Rep. Urol. 2018, 10, 75–87. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. A Review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Angeli, L.; Imperiale, S.; Ding, Y.; Scampicchio, M.; Morozova, K. A novel stoichio-kinetic model for the dpph• assay: The importance of the side reaction and application to complex mixtures. Antioxidants 2021, 10, 1019. [Google Scholar] [CrossRef]
- Aouadi, K.; Hajlaoui, H.; Arraouadi, S.; Ghannay, S.; Snoussi, M.; Kadri, A. HPLC/MS Phytochemical profiling with antioxidant activities of echium humile desf. extracts: Admet prediction and computational study targeting human peroxiredoxin 5 receptor. Agronomy 2021, 11, 2165. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 223, 123470. [Google Scholar] [CrossRef] [PubMed]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the conditions for determination of antioxidant activity by ABTS and DPPH assays—A practical approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Is it possible to use the DPPH and ABTS methods for reliable estimation of antioxidant power of colored compounds? Chem. Pap. 2018, 72, 393–400. [Google Scholar] [CrossRef]

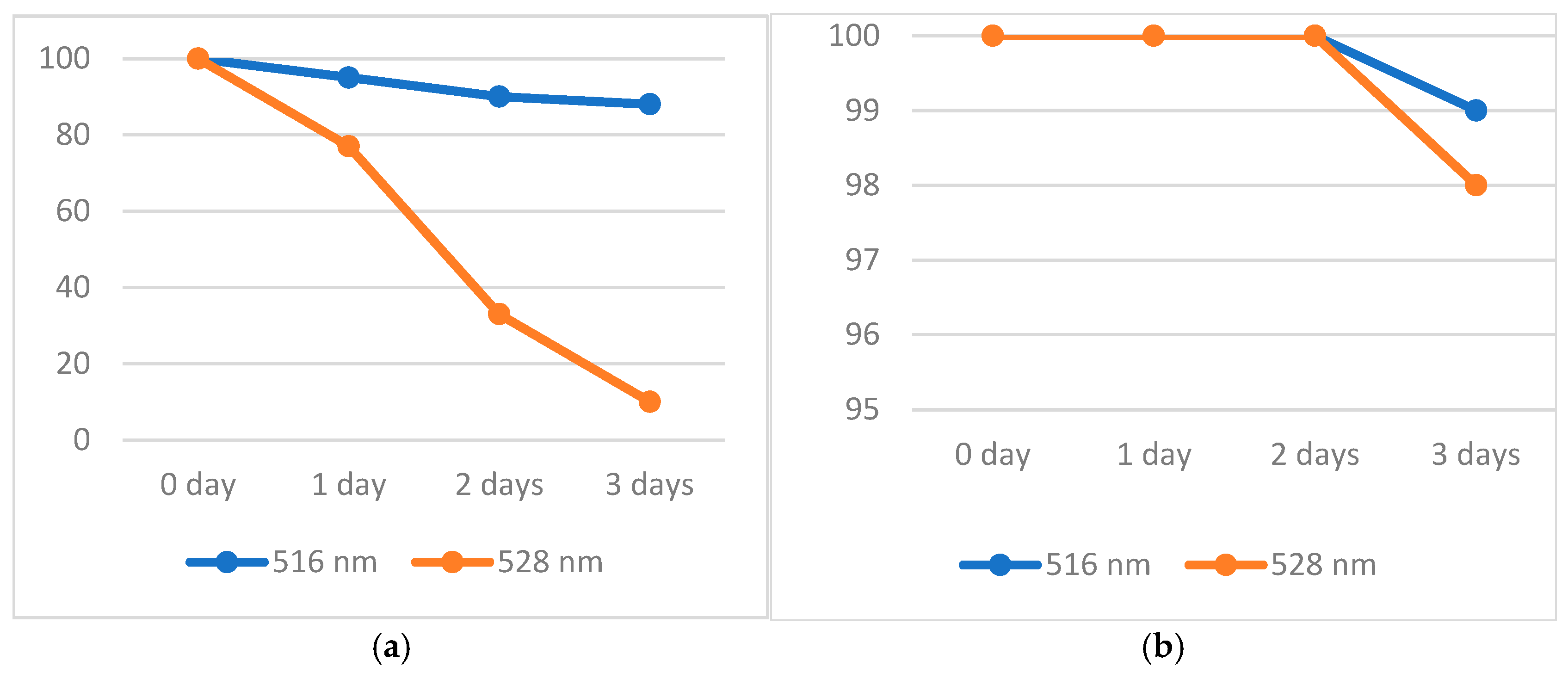
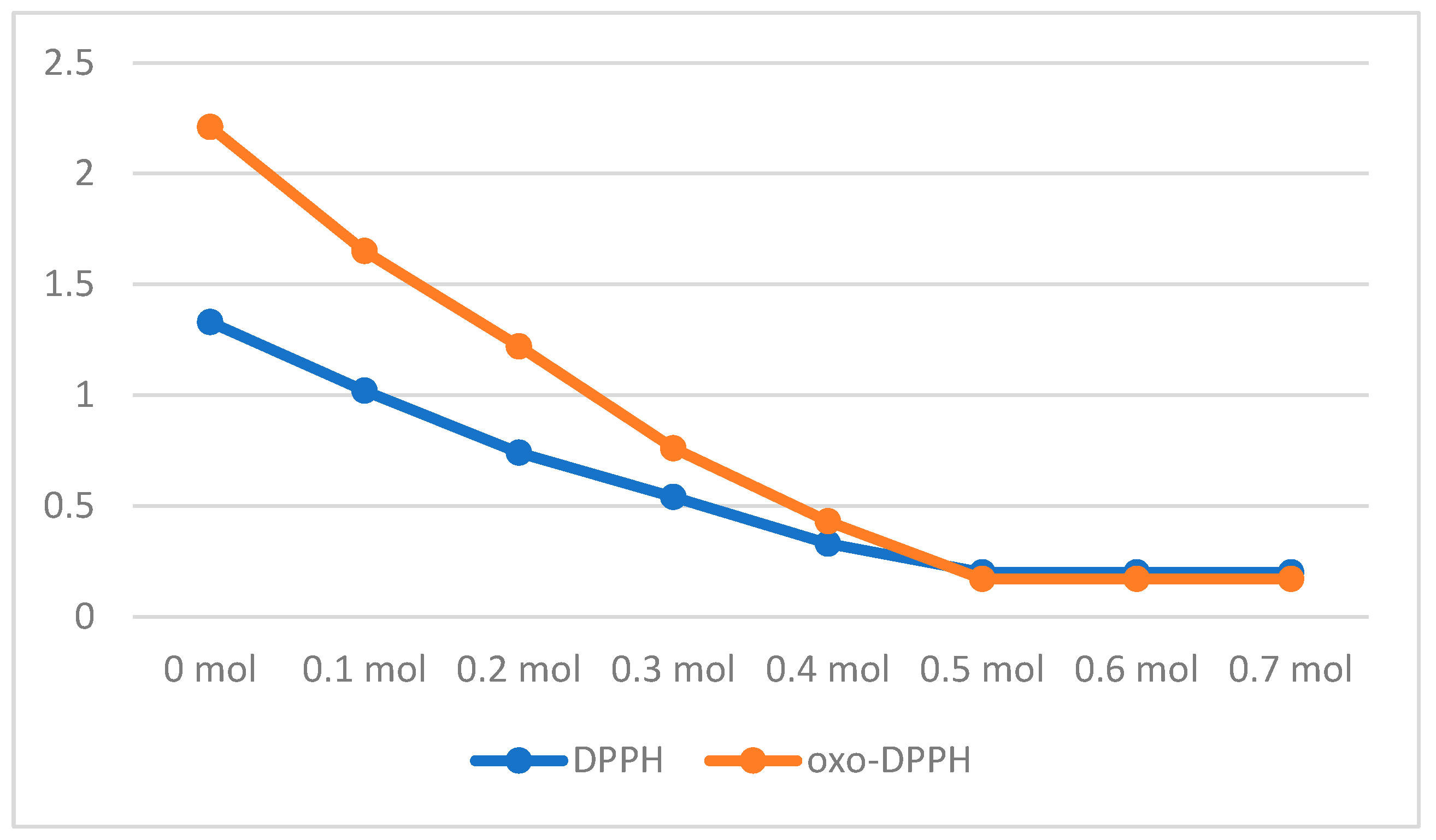
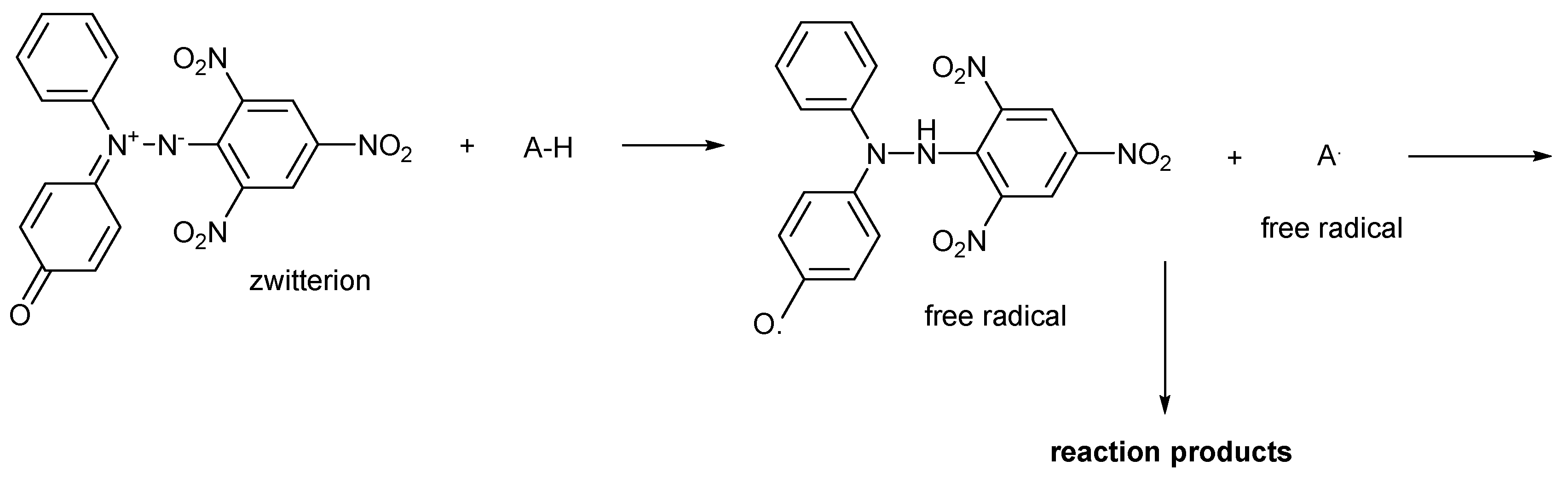
| Property | ABTS·+ | DPPH· | oxo-DPPH |
|---|---|---|---|
| Availability | Simple preparation from ABTS | Commercially available | Single step synthesis from DPPH-H or DPPH· |
| Solubility | water | organic solvents | organic solvents, water (slightly) |
| Life-time in solution | weeks | weeks | days-weeks |
| Life-time in solid | - | stable | stable |
| Wavelength absorbance (nm) and molar extinction coefficient (e) | 395 (35,000) ^ 414 (36,000) 645 (13,000) 728 (16,000) 811 (13,000) | 516 (11,000) ^^ | 385 (6000) ^^ 528 (25,000) |
| E ox | 0.50 V | 0.34 V | 0.29 V |
| Reduced counterpart | ABTS | DPPH-H | HO-DPPH-H |
| No. of e− involved | 1 | 1 | 2 |
| No. of H+ involved | 1 | 1 | 2 |
| Theoretical equivalent ascorbic acid (mol) | 1/2 | 1/2 | 1 |
| Theoretical equivalent Trolox (mol) | 1 | 1 | 2 |
| Natural Antioxidants | ABTS·+ (Various Wavelengths) | DPPH· (516 nm) | oxo-DPPH (528 nm) |
|---|---|---|---|
| Lavender extract | - | 18.64 | 27.48 |
| Propolis extract | 27.77 (811 nm) 30.03 (727 nm) 30.93 (645 nm) 36.82 (413 nm) 37.25 (395 nm) | 30.55 | 27.55 |
| Terpenes mixture | 18.80 (811 nm) 20.42 (727 nm) 21.60 (645 nm) 26.07 (413 nm) 28.08 (395 nm) | 23.31 | 15.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionita, P. Oxo-Hydrazyl as a Substitute for the Stable Free Radicals Employed in Measuring Total Antioxidant Activity. Antioxidants 2025, 14, 463. https://doi.org/10.3390/antiox14040463
Ionita P. Oxo-Hydrazyl as a Substitute for the Stable Free Radicals Employed in Measuring Total Antioxidant Activity. Antioxidants. 2025; 14(4):463. https://doi.org/10.3390/antiox14040463
Chicago/Turabian StyleIonita, Petre. 2025. "Oxo-Hydrazyl as a Substitute for the Stable Free Radicals Employed in Measuring Total Antioxidant Activity" Antioxidants 14, no. 4: 463. https://doi.org/10.3390/antiox14040463
APA StyleIonita, P. (2025). Oxo-Hydrazyl as a Substitute for the Stable Free Radicals Employed in Measuring Total Antioxidant Activity. Antioxidants, 14(4), 463. https://doi.org/10.3390/antiox14040463






