Long-Term Administration of Antioxidant N-Acetyl-L-Cysteine Impacts Beta Cell Oxidative Stress, Insulin Secretion, and Intracellular Signaling Pathways in Aging Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Model of Aging Study with NAC Administration
2.2. Metabolic Studies in Experimental Mouse Models
2.3. Immunohistological Staining and Morphometric Analyses
2.4. Protein Quantification and Western Blot Analysis
2.5. Statistical Analysis
3. Results
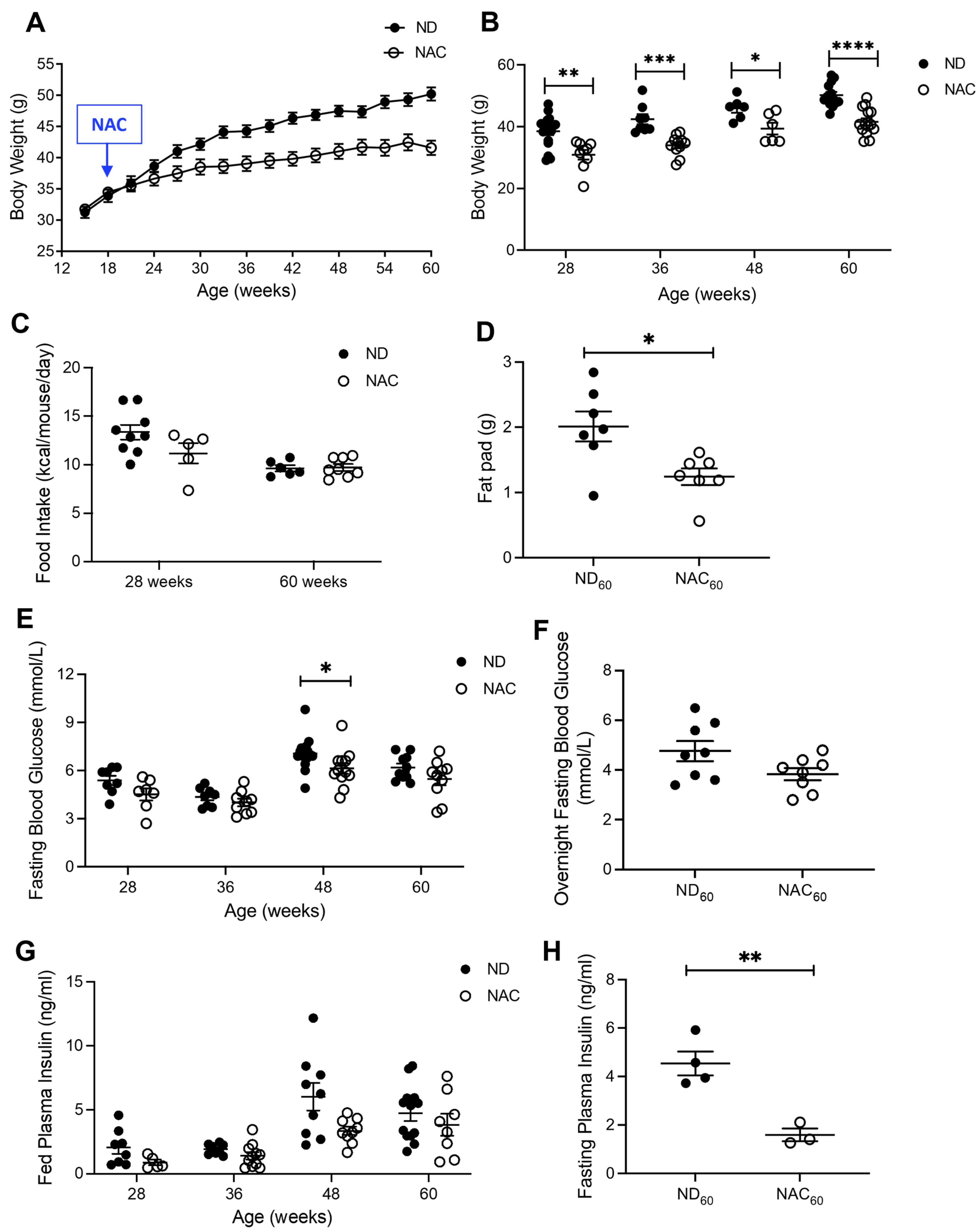
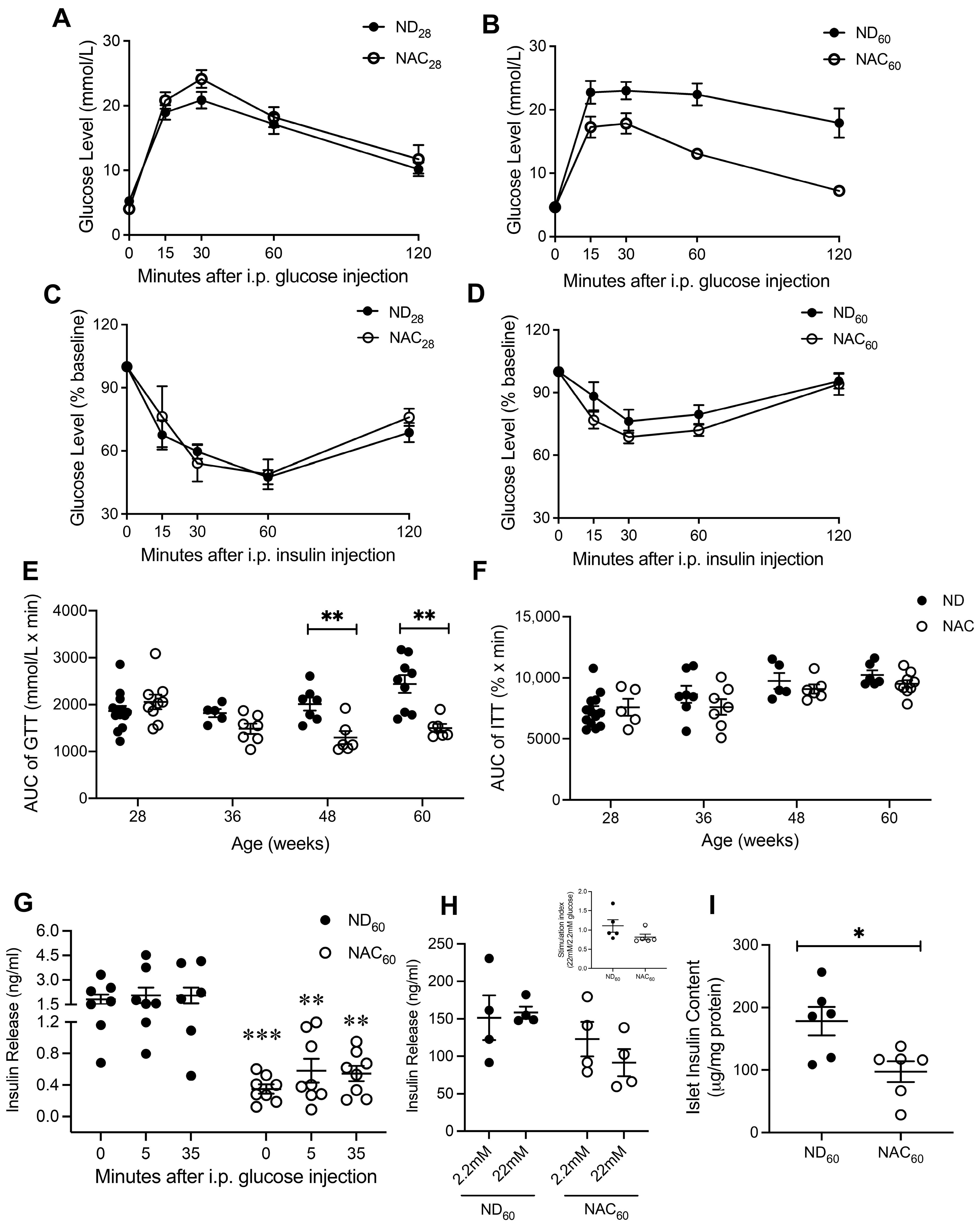
3.1. Long-Term NAC Treatment Improves Glucose Tolerance in Aging Mice
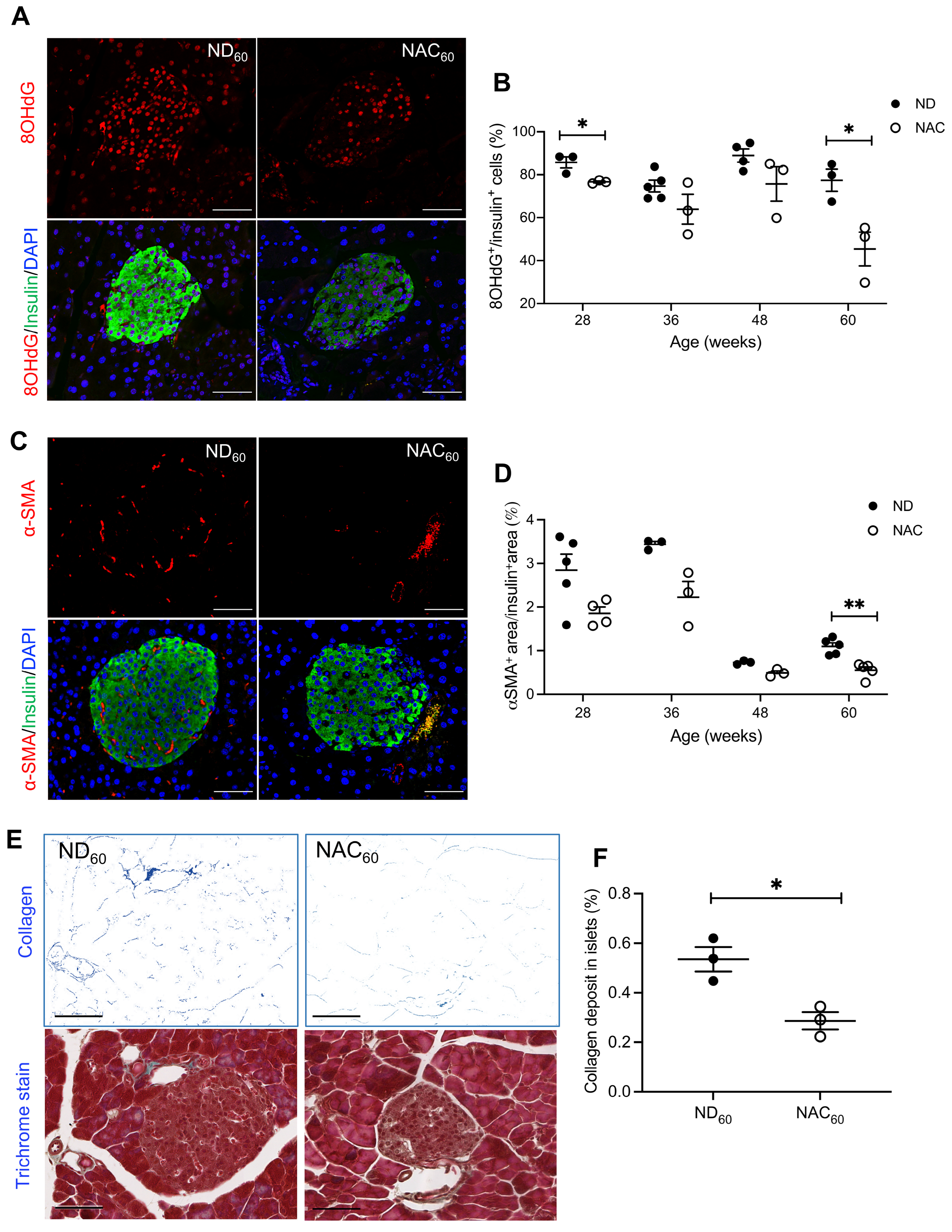
3.2. Long-Term NAC Treatment Reduces Beta Cell Oxidative Stress and Activated Intra-Islet PaSCs in Aging Mice
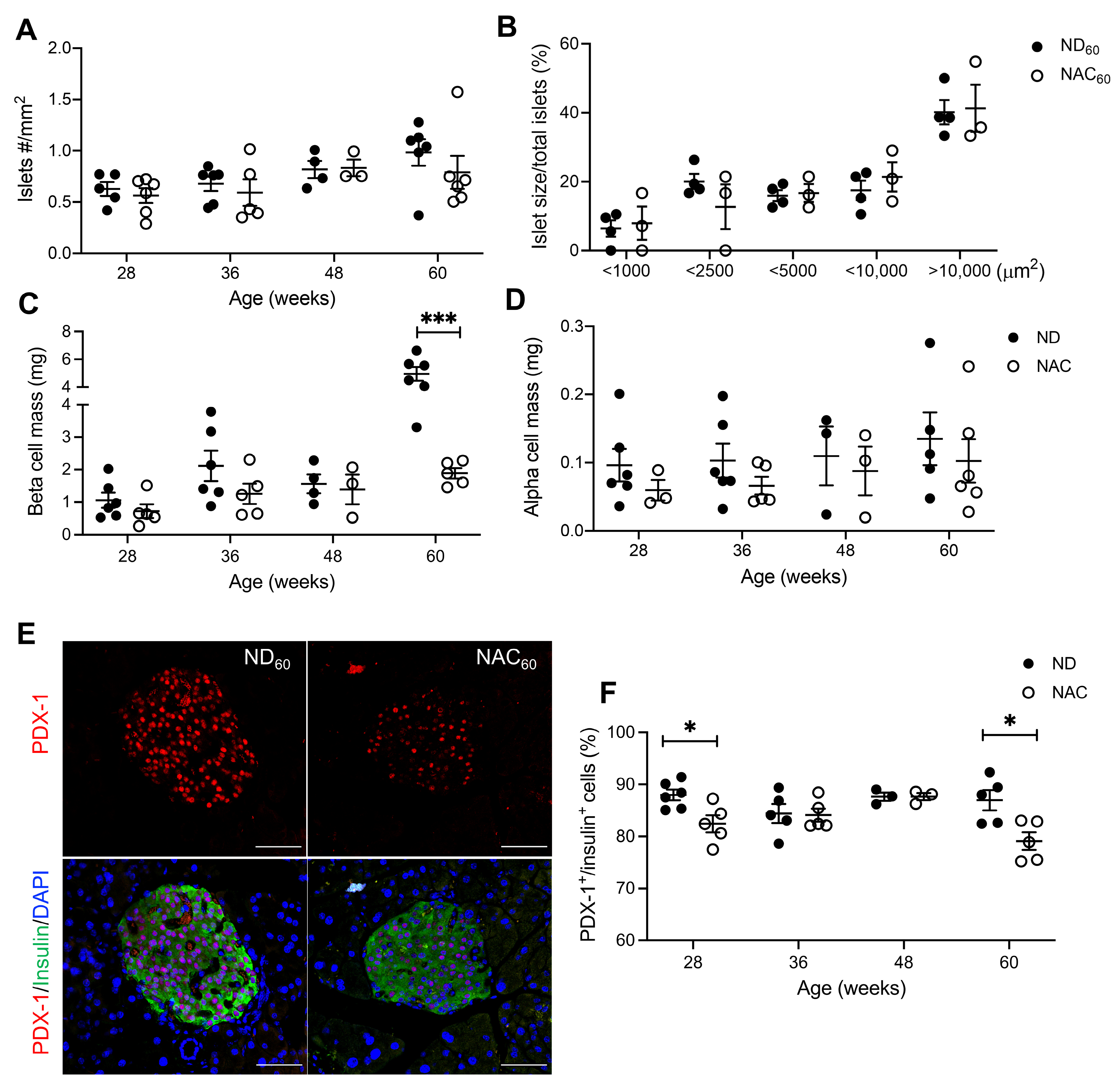
3.3. Long-Term NAC Treatment Preserves Beta Cell Mass but Lowers Nuclear PDX-1 Expression in Aging Mice
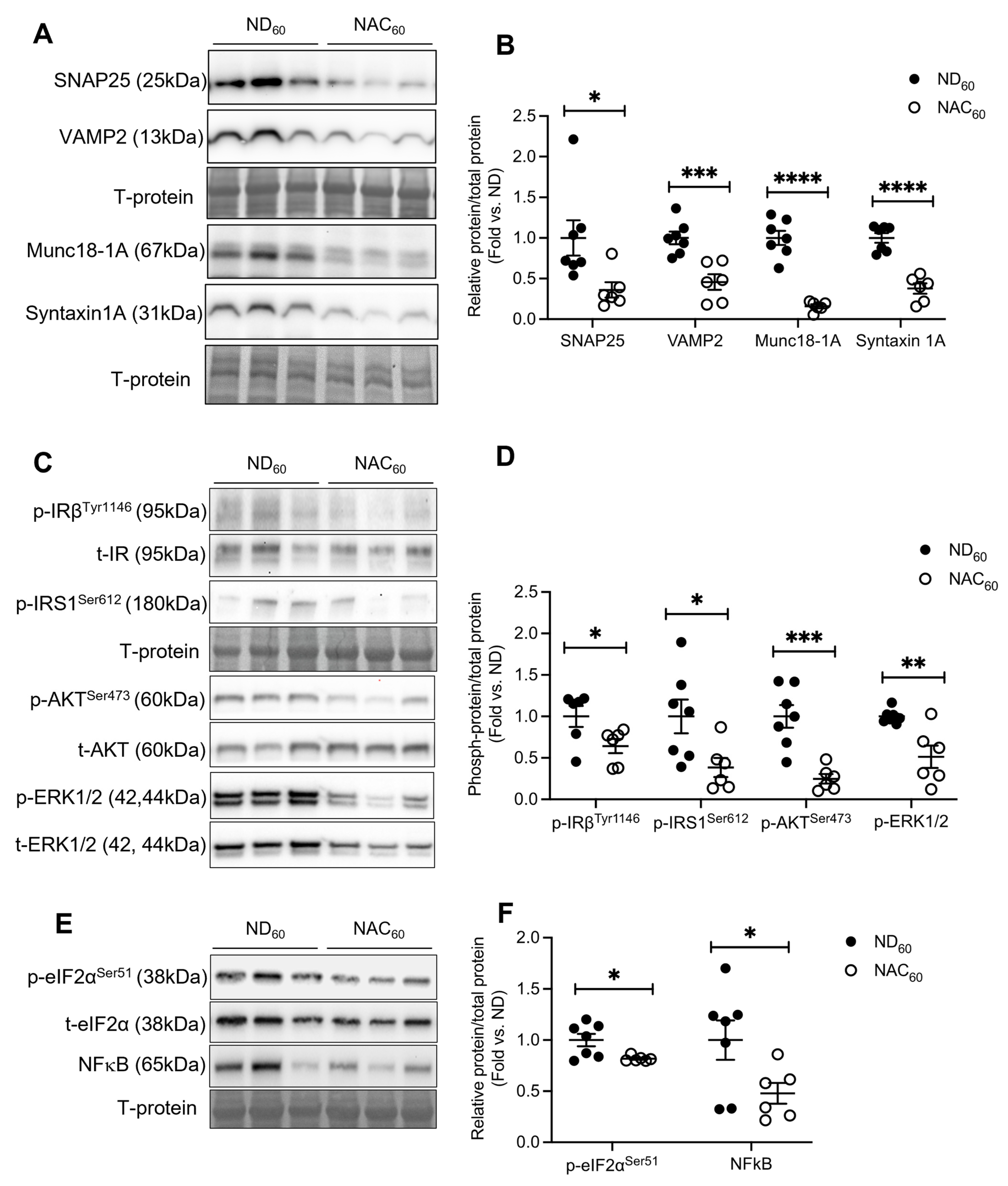
3.4. Long-Term NAC Treatment Alters Beta Cell Insulin Exocytosis Protein Levels and Intracellular Signaling Pathways in Aging Mice
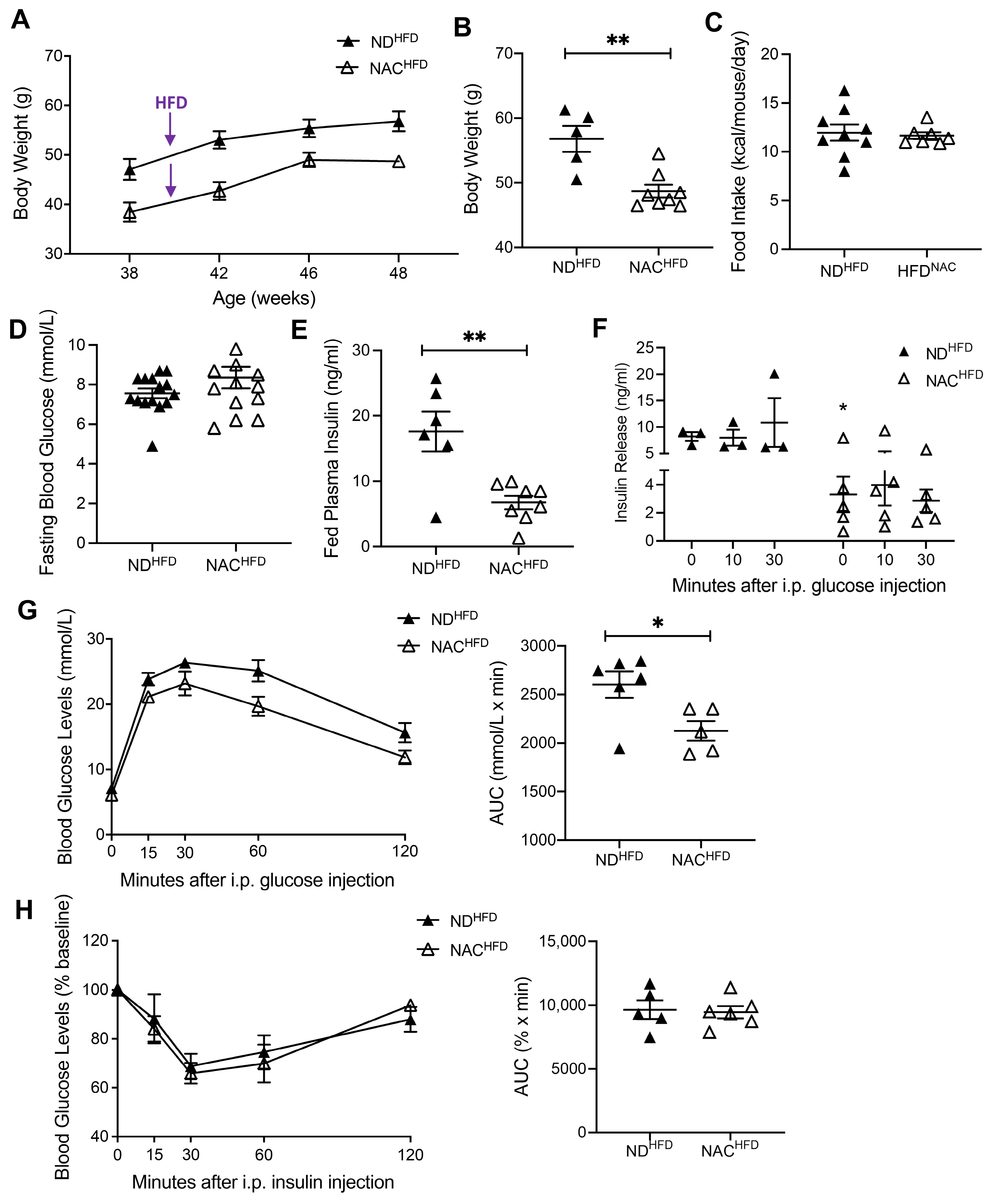
3.5. NAC Preserves Glucose Tolerance in Aging Mice Undergoing HFD Challenge
3.6. NAC Preserved Beta Cell Mass, Nuclear PDX-1, and Reduced Oxidative Stress in Aging Mice Undergoing HFD Challenge
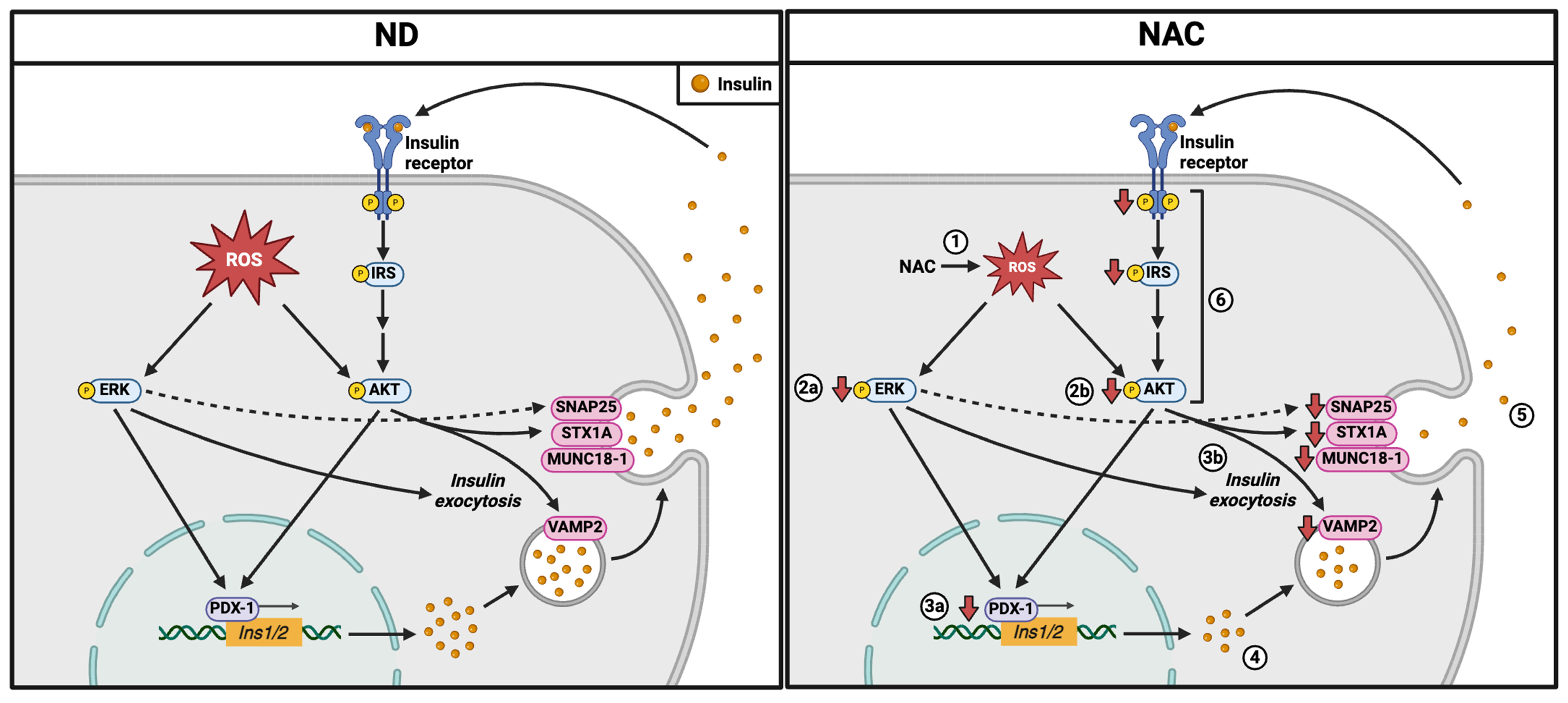
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8OHdG | 8-Oxo-2′-deoxyguanosine |
| α-SMA | Alpha smooth muscle actin |
| GLUT2 | Glucose transporter 2 |
| GSH | Glutathione |
| GSIS | Glucose-stimulated insulin secretion |
| HFD | High-fat diet |
| IPGTT | Intraperitoneal glucose tolerance test |
| IPITT | Intraperitoneal insulin tolerance test |
| Munc18-1A | Mammalian homologue of Unc18-1A |
| NAC | N-acetyl-L-cysteine |
| ND | Normal chow diet |
| PaSC | Pancreatic stellate cell |
| PDX-1 | Pancreatic and duodenal homeobox 1 |
| ROS | Reactive oxygen species |
| SNAP25 | Synaptosome-associated protein of 25 kDa |
| SOD | Superoxide dismutase |
| T2D | Type 2 diabetes mellitus |
| VAMP2 | Vesicle-associated membrane protein 2 |
References
- Abdelhafiz, A.H.; Sinclair, A.J. Diabetes in the Elderly. Medicine 2020, 50, 737–740. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Carpena-Ruiz, M.; Montero-Errasquín, B.; Sánchez-Castellano, C.; Sánchez-García, E. Exclusion of older adults from ongoing clinical trials about type 2 diabetes mellitus. J. Am. Geriatr. Soc. 2013, 61, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Núñez, V.M.; Rosado-Pérez, J.; Santiago-Osorio, E.; Ortiz, R.; Sánchez-Rodríguez, M.A.; Galván-Duarte, R.E. Aging linked to type 2 diabetes increases oxidative stress and chronic inflammation. Rejuvenation Res 2011, 14, 25–31. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Liu, W.; Lu, Y.; Cheng, J.; Chen, Y. β cell aging and age-related diabetes. Aging 2021, 13, 7691–7706. [Google Scholar] [CrossRef]
- Perfetti, R.; Rafizadeh, C.M.; Liotta, A.S.; Egan, J.M. Age-dependent reduction in insulin secretion and insulin mRNA in isolated islets from rats. Am. J. Physiol. Endocrinol. Metab. 1995, 269, E983–E990. [Google Scholar] [CrossRef]
- Apte, M.V.; Haber, P.S.; Applegate, T.L.; Norton, I.D.; McCaughan, G.W.; Korsten, M.A.; Pirola, R.C.; Wilson, J.S. Periacinar stellate shaped cells in rat pancreas: Identification, isolation, and culture. Gut 1998, 43, 128–133. [Google Scholar] [CrossRef]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef]
- Yoon, K.-H.; Lee, J.-H.; Kim, J.-W.; Cho, J.H.; Choi, Y.-H.; Ko, S.-H.; Zimmet, P.; Son, H.-Y. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006, 368, 1681–1688. [Google Scholar] [CrossRef]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative stress and beta-cell dysfunction. Pflügers Arch. Eur. J. Physiol. 2010, 460, 703–718. [Google Scholar] [CrossRef]
- Acharya, J.D.; Ghaskadbi, S.S. Islets and their antioxidant defense. Islets 2010, 2, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, H.; Mizukami, H.; Yagihashi, N.; Wada, R.; Hanyu, C.; Yagihashi, S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002, 45, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Šalamon, Š.; Kramar, B.; Marolt, T.P.; Poljšak, B.; Milisav, I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; McFarlane-Anderson, N.; Gordon-Strachan, G.M.; Wright-Pascoe, R.A.; Jahoor, F.; Boyne, M.S. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, Y.; Ke, L.; Yu, Y. Elevated circulating free fatty acids levels causing pancreatic islet cell dysfunction through oxidative stress. J. Endocrinol. Investig. 2010, 33, 388–394. [Google Scholar] [CrossRef]
- Schuurman, M.; Wallace, M.; Sahi, G.; Barillaro, M.; Zhang, S.; Rahman, M.; Sawyez, C.; Borradaile, N.; Wang, R. N-acetyl-L-cysteine treatment reduces beta-cell oxidative stress and pancreatic stellate cell activity in a high fat diet-induced diabetic mouse model. Front. Endocrinol. 2022, 13, 938680. [Google Scholar] [CrossRef]
- Falach-Malik, A.; Rozenfeld, H.; Chetboun, M.; Rozenberg, K.; Elyasiyan, U.; Sampson, S.R.; Rosenzweig, T. N-Acetyl-L-Cysteine inhibits the development of glucose intolerance and hepatic steatosis in diabetes-prone mice. Am. J. Transl. Res. 2016, 8, 3744–3756. [Google Scholar]
- Al Rijjal, D.; Wheeler, M.B. A protocol for studying glucose homeostasis and islet function in mice. STAR Protoc. 2022, 3, 101171. [Google Scholar] [CrossRef]
- Yashpal, N.K.; Li, J.; Wang, R. Characterization of c-Kit and nestin expression during islet cell development in the prenatal and postnatal rat pancreas. Dev. Dyn. 2004, 229, 813–825. [Google Scholar] [CrossRef]
- Feng, Z.-C.; Riopel, M.; Popell, A.; Wang, R. A survival Kit for pancreatic beta cells: Stem cell factor and c-Kit receptor tyrosine kinase. Diabetologia 2015, 58, 654–665. [Google Scholar] [CrossRef]
- Peters, K.M.; Zhang, R.; Park, C.; Nong, Z.; Yin, H.; Wilson, R.B.; Sutherland, B.G.; Sawyez, C.G.; Pickering, J.G.; Borradaile, N.M. Vitamin D intervention does not improve vascular regeneration in diet-induced obese male mice with peripheral ischemia. J. Nutr. Biochem. 2019, 70, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Oakie, A.; Feng, Z.-C.; Li, J.; Silverstein, J.; Yee, S.-P.; Wang, R. Long-term c-Kit overexpression in beta cells compromises their function in ageing mice. Diabetologia 2019, 62, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B.; Kozlov, A.M.; Tehrani, H.H.; Twumasi-Ankrah, J.S.; Chen, Y.J.; Borrelli, M.J.; Sawyez, C.G.; Maini, S.; Shepherd, T.G.; Cumming, R.C.; et al. Elongation factor 1A1 regulates metabolic substrate preference in mammalian cells. J. Biol. Chem. 2024, 300, 105684. [Google Scholar] [CrossRef]
- Kapadia, R.; Yi, J.-H.; Vemuganti, R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front. Biosci. 2008, 13, 1813. [Google Scholar] [CrossRef]
- Croasdell, A.; Duffney, P.F.; Kim, N.; Lacy, S.H.; Sime, P.J.; Phipps, R.P. PPARγ and the Innate Immune System Mediate the Resolution of Inflammation. PPAR Res. 2015, 2015, 549691. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, S.; Zhou, W. Pancreatic stellate cells: Key players in pancreatic health and diseases (Review). Mol. Med. Rep. 2024, 30, 109. [Google Scholar] [CrossRef]
- Kim, J.-R.; Ryu, H.-H.; Chung, H.J.; Lee, J.H.; Kim, S.W.; Kwun, W.H.; Baek, S.-H.; Kim, J.H. Association of anti-obesity activity of N-acetylcysteine with metallothionein-II down-regulation. Exp. Mol. Med. 2006, 38, 162–172. [Google Scholar] [CrossRef]
- Colman, E.; Katzel, L.I.; Rogus, E.; Coon, P.; Muller, D.; Goldberg, A.P. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism 1995, 44, 1502–1508. [Google Scholar] [CrossRef]
- Feng, H.; Moriyama, T.; Ohuchida, K.; Sheng, N.; Iwamoto, C.; Shindo, K.; Shirahane, K.; Ikenaga, N.; Nagai, S.; Nakata, K.; et al. N-acetyl cysteine induces quiescent-like pancreatic stellate cells from an active state and attenuates cancer-stroma interactions. J. Exp. Clin. Cancer Res. 2021, 40, 133. [Google Scholar] [CrossRef]
- Asaumi, H.; Watanabe, S.; Taguchi, M.; Tashiro, M.; Otsuki, M. Externally applied pressure activates pancreatic stellate cells through the generation of intracellular reactive oxygen species. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 972–978. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.S.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-K.; Zhang, X.; Wu, H.-L.; Gan, Y.; Ye, L.; Zheng, H.; Zhu, Z.; Liu, W.J.; Liu, H.-F. ROS-ERK Pathway as Dual Mediators of Cellular Injury and Autophagy-Associated Adaptive Response in Urinary Protein-Irritated Renal Tubular Epithelial Cells. J. Diabetes Res. 2021, 2021, 6614848. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal. Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Han, F.; Li, C.-F.; Cai, Z.; Zhang, X.; Jin, G.; Zhang, W.-N.; Xu, C.; Wang, C.-Y.; Morrow, J.; Zhang, S.; et al. The critical role of AMPK in driving Akt activation under stress, tumorigenesis and drug resistance. Nat. Commun. 2018, 9, 4728. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018, 8, 373138. [Google Scholar] [CrossRef]
- Cao, J.; Schulte, J.; Knight, A.; Leslie, N.R.; Zagozdzon, A.; Bronson, R.; Neumann, C.A. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009, 28, 1505–1517. [Google Scholar] [CrossRef]
- Cong, Z.-X.; Wang, H.-D.; Wang, J.-W.; Zhou, Y.; Pan, H.; Zhang, D.-D.; Zhu, L. ERK and PI3K signaling cascades induce Nrf2 activation and regulate cell viability partly through Nrf2 in human glioblastoma cells. Oncol. Rep. 2013, 30, 715–722. [Google Scholar] [CrossRef]
- Zipper, L.M.; Mulcahy, R.T. Erk Activation Is Required for Nrf2 Nuclear Localization during Pyrrolidine Dithiocarbamate Induction of Glutamate Cysteine Ligase Modulatory Gene Expression in HepG2 Cells. Toxicol. Sci. 2003, 73, 124–134. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, X.; Qian, J.; Tang, Z.; Zhang, B. Nrf2: Therapeutic target of islet function protection in diabetes and islet transplantation. Biomed. Pharmacother. 2023, 167, 115463. [Google Scholar] [CrossRef]
- Baumel-Alterzon, S.; Scott, D.K. Regulation of Pdx1 by oxidative stress and Nrf2 in pancreatic beta-cells. Front. Endocrinol. 2022, 13, 1011187. [Google Scholar] [CrossRef]
- Baumel-Alterzon, S.; Katz, L.S.; Brill, G.; Jean-Pierre, C.; Li, Y.; Tse, I.; Biswal, S.; Garcia-Ocaña, A.; Scott, D.K. Nrf2 Regulates β-Cell Mass by Suppressing β-Cell Death and Promoting β-Cell Proliferation. Diabetes 2022, 71, 989–1011. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.; Griffen, S.C.; Xia, Y.; Baer, R.J.; German, M.S.; Cobb, M.H. Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic β cells. J. Biol. Chem. 2003, 278, 32969–32977. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.A.; Werneck-De-Castro, J.P.; Lubaczeuski, C.; Johnson, J.D.; Bernal-Mizrachi, E. Pancreatic and duodenal homeobox-1 (PDX1) contributes to β-cell mass expansion and proliferation induced by Akt/PKB pathway. Islets 2020, 12, 32–40. [Google Scholar] [CrossRef]
- Hayes, H.L.; Moss, L.G.; Schisler, J.C.; Haldeman, J.M.; Zhang, Z.; Rosenberg, P.B.; Newgard, C.B.; Hohmeier, H.E. Pdx-1 activates islet α- and β-cell proliferation via a mechanism regulated by transient receptor potential cation channels 3 and 6 and extracellular signal-regulated kinases 1 and 2. Mol. Cell. Biol. 2013, 33, 4017–4029. [Google Scholar] [CrossRef]
- Ikushima, Y.M.; Awazawa, M.; Kobayashi, N.; Osonoi, S.; Takemiya, S.; Kobayashi, H.; Suwanai, H.; Morimoto, Y.; Soeda, K.; Adachi, J.; et al. MEK/ERK Signaling in β-Cells Bifunctionally Regulates β-Cell Mass and Glucose-Stimulated Insulin Secretion Response to Maintain Glucose Homeostasis. Diabetes 2021, 70, 1519–1535. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Plecitá-Hlavatá, L.; Jabůrek, M.; Holendová, B.; Tauber, J.; Pavluch, V.; Berková, Z.; Cahová, M.; Schröder, K.; Brandes, R.P.; Siemen, D.; et al. Glucose-Stimulated Insulin Secretion Fundamentally Requires H2O2 Signaling by NADPH Oxidase 4. Diabetes 2020, 69, 1341–1354. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Haataja, L.; Manickam, N.; Soliman, A.; Tsai, B.; Liu, M.; Arvan, P. Disulfide Mispairing During Proinsulin Folding in the Endoplasmic Reticulum. Diabetes 2016, 65, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Weiss, M.A.; Arunagiri, A.; Yong, J.; Rege, N.; Sun, J.; Haataja, L.; Kaufman, R.J.; Arvan, P. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes. Metab. 2018, 20, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, M.; Minakata, S.; Nishitsuji, K.; Tabata, S.; Matsui, I.-S.L.; Takatani, M.; Usukura, J.; Ito, Y.; Ihara, Y. Calreticulin protects insulin against reductive stress in vitro and in MIN6 cells. Biochimie 2020, 171–172, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, S.; Misu, H.; Takeda, T.; Sugimori, M.; Matsugo, S.; Kaneko, S.; Takamura, T. Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS ONE 2011, 6, e27401. [Google Scholar] [CrossRef]
- Argaev-Frenkel, L.; Rosenzweig, T. Complexity of NAC Action as an Antidiabetic Agent: Opposing Effects of Oxidative and Reductive Stress on Insulin Secretion and Insulin Signaling. Int. J. Mol. Sci. 2022, 23, 2965. [Google Scholar] [CrossRef]
- Cheng, K.K.Y.; Lam, K.S.L.; Wu, D.; Wang, Y.; Sweeney, G.; Hoo, R.L.C.; Zhang, J.; Xu, A. APPL1 potentiates insulin secretion in pancreatic β cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 8919–8924. [Google Scholar] [CrossRef]
- Kaneko, K.; Ueki, K.; Takahashi, N.; Hashimoto, S.; Okamoto, M.; Awazawa, M.; Okazaki, Y.; Ohsugi, M.; Inabe, K.; Umehara, T.; et al. Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metab. 2010, 12, 619–632. [Google Scholar] [CrossRef]
- Spaeth, J.M.; Gupte, M.; Perelis, M.; Yang, Y.-P.; Cyphert, H.; Guo, S.; Liu, J.-H.; Guo, M.; Bass, J.; Magnuson, M.A.; et al. Defining a Novel Role for the Pdx1 Transcription Factor in Islet β-Cell Maturation and Proliferation During Weaning. Diabetes 2017, 66, 2830–2839. [Google Scholar] [CrossRef]
- Reynolds, T.H.; Dalton, A.; Calzini, L.; Tuluca, A.; Hoyte, D.; Ives, S.J. The impact of age and sex on body composition and glucose sensitivity in C57BL/6J mice. Physiol. Rep. 2019, 7, e13995. [Google Scholar] [CrossRef]
- Ehrhardt, N.; Cui, J.; Dagdeviren, S.; Saengnipanthkul, S.; Goodridge, H.S.; Kim, J.K.; Lantier, L.; Guo, X.; Chen, Y.I.; Raffel, L.J.; et al. Adiposity-Independent Effects of Aging on Insulin Sensitivity and Clearance in Mice and Humans. Obesity 2019, 27, 434. [Google Scholar] [CrossRef]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuurman, M.; Nguyen, J.; Wilson, R.B.; Barillaro, M.; Wallace, M.; Borradaile, N.; Wang, R. Long-Term Administration of Antioxidant N-Acetyl-L-Cysteine Impacts Beta Cell Oxidative Stress, Insulin Secretion, and Intracellular Signaling Pathways in Aging Mice. Antioxidants 2025, 14, 417. https://doi.org/10.3390/antiox14040417
Schuurman M, Nguyen J, Wilson RB, Barillaro M, Wallace M, Borradaile N, Wang R. Long-Term Administration of Antioxidant N-Acetyl-L-Cysteine Impacts Beta Cell Oxidative Stress, Insulin Secretion, and Intracellular Signaling Pathways in Aging Mice. Antioxidants. 2025; 14(4):417. https://doi.org/10.3390/antiox14040417
Chicago/Turabian StyleSchuurman, Meg, Jonathan Nguyen, Rachel B. Wilson, Malina Barillaro, Madison Wallace, Nica Borradaile, and Rennian Wang. 2025. "Long-Term Administration of Antioxidant N-Acetyl-L-Cysteine Impacts Beta Cell Oxidative Stress, Insulin Secretion, and Intracellular Signaling Pathways in Aging Mice" Antioxidants 14, no. 4: 417. https://doi.org/10.3390/antiox14040417
APA StyleSchuurman, M., Nguyen, J., Wilson, R. B., Barillaro, M., Wallace, M., Borradaile, N., & Wang, R. (2025). Long-Term Administration of Antioxidant N-Acetyl-L-Cysteine Impacts Beta Cell Oxidative Stress, Insulin Secretion, and Intracellular Signaling Pathways in Aging Mice. Antioxidants, 14(4), 417. https://doi.org/10.3390/antiox14040417




