Brain-Targeted Reactive Oxygen Species in Hypertension: Unveiling Subcellular Dynamics, Immune Cross-Talk, and Novel Therapeutic Pathways
Abstract
1. Introduction
1.1. Hypertension as a Multi-Factorial Disease: Beyond Traditional Perspectives
1.2. The Role of Brain ROS in Understanding the Pathogenesis of Hypertension
1.3. Scope of the Review
2. Advancing the Understanding of ROS
2.1. Novel Sources and Contexts of ROS Production in Hypertension
2.1.1. Underexplored Sources of ROS: ER Stress and Microglia-Derived ROS
2.1.2. Role of Aldosterone-Induced ROS in Hypertension
2.1.3. ROS Generation in Normotensive vs. Hypertensive States
2.2. Dynamic Role of ROS in Brain Plasticity and Hypertension Progression
3. Emerging Insights into Subcellular ROS Localization in Hypertension
3.1. Compartmentalized ROS Production in Subcellular Organelles
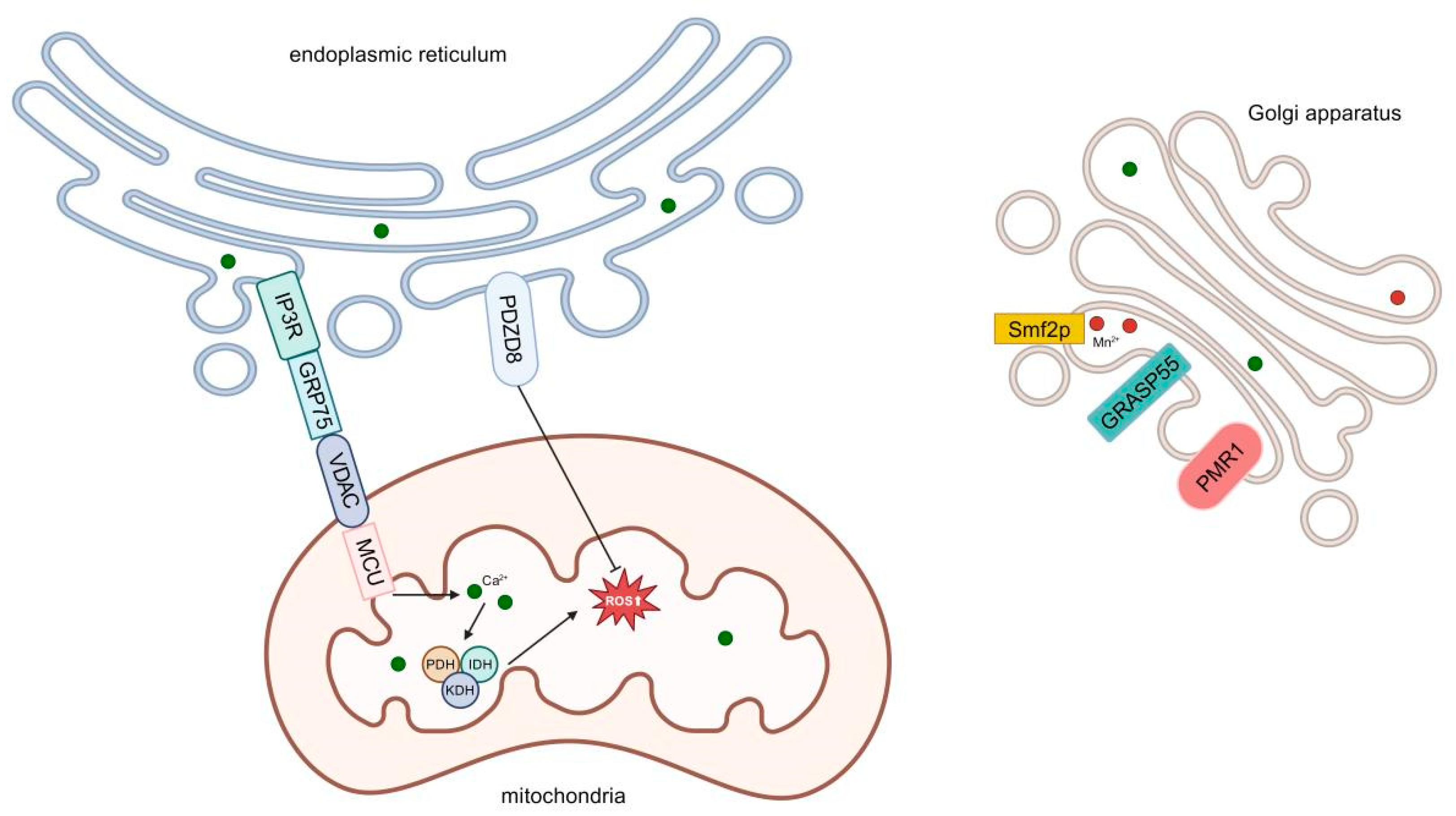
3.1.1. The Role of Endoplasmic Reticulum–Mitochondrial Contact Sites in ROS Generation
3.1.2. ROS Communication Between Organelles and Its Impact on Neuronal Function
3.2. Role of Microdomains in Neuronal ROS Signaling
4. Cross-Talk Between ROS and Brain–Immune Interactions
4.1. ROS-Driven Neuroinflammation in Autonomic Regions of Brain
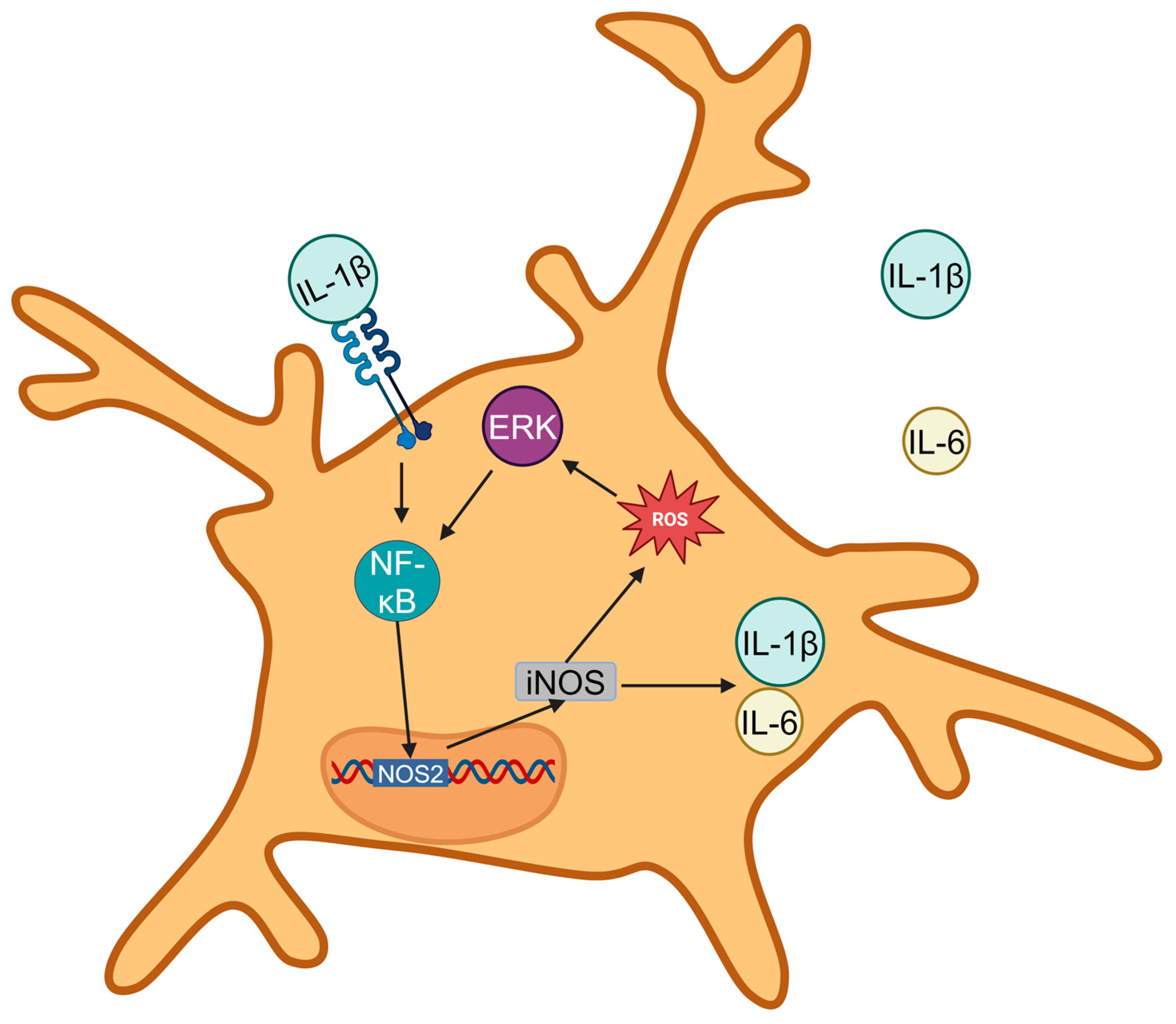
4.1.1. Mechanisms Linking ROS with Microglial Activation and Cytokine Release in PVN, RVLM, and Nucleus Tractus Solitarii (NTS)
4.1.2. Feedback Loops Sustaining ROS and Inflammatory Signaling in Hypertension
4.2. Astrocytic ROS: A Novel Contributor to Autonomic Dysregulation
4.2.1. Impact of Astrocytic ROS on Neuronal Excitability and Neurotransmitter Balance
4.2.2. Role of Astrocytes in Amplifying the Hypertensive Response
5. ROS and Epigenetic Regulation in Brain Hypertension
5.1. Histone Modifications and DNA Methylation Regulated by ROS in Hypertensive States
5.2. Non-Coding RNAs as Mediators of ROS Signaling in Hypertension
6. Precision Antioxidant Strategies: Subcellular Modulation and Immune Integration in Hypertension Therapy
6.1. Brain-Targeted Delivery Systems for Antioxidant Therapy
6.2. Modulating ROS at Subcellular Levels
6.3. Targeting ROS and Inflammation in Hypertension: Bridging Subcellular Dynamics and Immune Cross-Talk
7. Future Perspectives: Expanding the Research Horizon
7.1. Exploring ROS and Brain Connectivity in Hypertension
7.2. Translating ROS Research into Human Hypertension Study
8. Precision Medicine for ROS-Driven Hypertension
8.1. Translating ROS Research into Human Hypertension
8.2. Integrating Environmental and Lifestyle Factors
8.3. Enhancing Precision with Advanced Tools
9. Conclusions
9.1. The Pivotal Role of ROS in HTN
9.2. Emerging Therapeutic Strategies
9.3. Challenges in Translational Research
9.4. Future Directions and Collaborative Efforts
9.5. A Holistic Approach to HTN Management
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Z.; Lan, Y.; Li, J.; Wang, P.; Xiong, X. The role of chinese herbal medicine in the regulation of oxidative stress in treating hypertension: From therapeutics to mechanisms. Chin. Med. 2024, 19, 150. [Google Scholar] [PubMed]
- Schäfer, K. Do not lose your nerves: Autonomic neuromodulation in pulmonary arterial hypertension. JACC Basic Transl. Sci. 2024, 9, 493–495. [Google Scholar] [PubMed]
- Caetano, E.S.P.; Mattioli, S.V.; da Silva, M.L.S.; Martins, L.Z.; Almeida, A.A.; da Rocha, A.L.V.; Nunes, P.R.; Grandini, N.A.; Correa, C.R.; Zochio, G.P.; et al. Sildenafil attenuates oxidative stress and endothelial dysfunction in lead-induced hypertension. Basic Clin. Pharmacol. Toxicol. 2023, 133, 142–155. [Google Scholar]
- Gao, H.L.; Yang, Y.; Tian, H.; Xu, S.L.; Li, B.W.; Fu, L.Y.; Liu, K.L.; Shi, X.L.; Kang, Y.M.; Yu, X.J. Puerarin alleviates blood pressure via inhibition of ros/tlr4/nlrp3 inflammasome signaling pathway in the hypothalamic paraventricular nucleus of salt-induced prehypertensive rats. Nutrients 2024, 16, 2580. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E.; Strunecka, A.; Blaylock, R.L.; Patocka, J.; Strunecky, O. Immunoexcitotoxicity as the central mechanism of etiopathology and treatment of autism spectrum disorders: A possible role of fluoride and aluminum. Surg. Neurol. Int. 2018, 9, 74. [Google Scholar]
- Chen, X.; Yan, X.; Yu, C.; Chen, Q.H.; Bi, L.; Shan, Z. Ptsd increases risk for hypertension development through pvn activation and vascular dysfunction in sprague dawley rats. Antioxidants 2024, 13, 1423. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Chen, G.; Tong, L.; Ye, X.; Yang, H.; Liu, H.; Zhang, H.; Lu, W.; Zhang, S.; et al. Pdzd8-mediated endoplasmic reticulum-mitochondria associations regulate sympathetic drive and blood pressure through the intervention of neuronal mitochondrial homeostasis in stress-induced hypertension. Neurobiol. Dis. 2023, 183, 106173. [Google Scholar]
- Duan, W.; Ye, P.; Leng, Y.Q.; Liu, D.H.; Sun, J.C.; Tan, X.; Wang, W.Z. Oxidative stress in the rvlm mediates sympathetic hyperactivity induced by circadian disruption. Neurosci. Lett. 2022, 791, 136917. [Google Scholar]
- Xi, H.; Li, X.; Zhou, Y.; Sun, Y. The regulatory effect of the paraventricular nucleus on hypertension. Neuroendocrinology 2024, 114, 1–13. [Google Scholar]
- Li, Y.; Xie, Y.; Liu, R.; Wang, Z.; Chen, P.; Wang, M.; Yu, Z.; Wang, W.; Luo, X. Knockout of microglial hv1 proton channel reduces neurotoxic a1 astrocytes and neuronal damage via the ros/stat3 pathway after spinal cord injury. Glia 2023, 71, 2418–2436. [Google Scholar]
- Camargo, L.L.; Wang, Y.; Rios, F.J.; McBride, M.; Montezano, A.C.; Touyz, R.M. Oxidative stress and endoplasmic reticular stress interplay in the vasculopathy of hypertension. Can. J. Cardiol. 2023, 39, 1874–1887. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Pan, W.; Xu, Y.; Zhang, J.; Wan, J.; Jiang, H. Microglia-mediated neuroinflammation: A potential target for the treatment of cardiovascular diseases. J. Inflamm. Res. 2022, 15, 3083–3094. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yu, Y.; Kang, Y.M.; Wei, S.G.; Felder, R.B. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1067–H1074. [Google Scholar] [CrossRef]
- Chen, M.; Lee, J.; Malvin, R.L. Central administration of aldosterone increases blood pressure in rats. Clin. Exp. Hypertens. A 1989, 11, 459–472. [Google Scholar] [CrossRef]
- Rodrigues, D.; Costa, T.J.; Silva, J.F.; Neto, J.T.O.; Alves, J.V.; Fedoce, A.G.; Costa, R.M.; Tostes, R.C. Aldosterone negatively regulates nrf2 activity: An additional mechanism contributing to oxidative stress and vascular dysfunction by aldosterone. Int. J. Mol. Sci. 2021, 22, 6154. [Google Scholar] [CrossRef] [PubMed]
- Prevatto, J.P.; Torres, R.C.; Diaz, B.L.; Silva, P.; Martins, M.A.; Carvalho, V.F. Antioxidant treatment induces hyperactivation of the hpa axis by upregulating acth receptor in the adrenal and downregulating glucocorticoid receptors in the pituitary. Oxid. Med. Cell. Longev. 2017, 2017, 4156361. [Google Scholar] [CrossRef]
- Lv, Q.; Yang, Q.; Cui, Y.; Yang, J.; Wu, G.; Liu, M.; Ning, Z.; Cao, S.; Dong, G.; Hu, J. Effects of taurine on ace, ace2 and hsp70 expression of hypothalamic-pituitary-adrenal axis in stress-induced hypertensive rats. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 871–886. [Google Scholar]
- Daimon, M.; Kamba, A.; Murakami, H.; Takahashi, K.; Otaka, H.; Makita, K.; Yanagimachi, M.; Terui, K.; Kageyama, K.; Nigawara, T.; et al. Association between pituitary-adrenal axis dominance over the renin-angiotensin-aldosterone system and hypertension. J. Clin. Endocrinol. Metab. 2016, 101, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Greene, P.; Ryan, T.; Cerimele, B.; Schwertschlag, U.; Weinberger, M.; Voelker, J. The renin angiotensin aldosterone system and frusemide response in congestive heart failure. Br. J. Clin. Pharmacol. 1995, 39, 51–57. [Google Scholar] [CrossRef]
- Yan, S.; Sheak, J.R.; Walker, B.R.; Jernigan, N.L.; Resta, T.C. Contribution of mitochondrial reactive oxygen species to chronic hypoxia-induced pulmonary hypertension. Antioxidants 2023, 12, 2060. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [PubMed]
- Hirooka, Y. How can endocan be used as a specific biomarker of endothelial dysfunction in hypertension? Hypertens. Res. 2024, 47, 794–795. [Google Scholar] [CrossRef]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar]
- Bernátová, I.; Bališ, P.; Goga, R.; Behuliak, M.; Zicha, J.; Sekaj, I. Lack of reactive oxygen species deteriorates blood pressure regulation in acute stress. Physiol. Res. 2016, 65, S381–S390. [Google Scholar] [PubMed]
- Thomas, P.; Dasgupta, I. The role of the kidney and the sympathetic nervous system in hypertension. Pediatr. Nephrol. 2015, 30, 549–560. [Google Scholar] [PubMed]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative stress and hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [PubMed]
- Li, X.; Liu, H.; Li, D.; Lei, H.; Wei, X.; Schlenk, D.; Mu, J.; Chen, H.; Yan, B.; Xie, L. Dietary seleno-l-methionine causes alterations in neurotransmitters, ultrastructure of the brain, and behaviors in zebrafish (danio rerio). Environ. Sci. Technol. 2021, 55, 11894–11905. [Google Scholar] [CrossRef]
- Sah, R.; Galeffi, F.; Ahrens, R.; Jordan, G.; Schwartz-Bloom, R.D. Modulation of the gaba(a)-gated chloride channel by reactive oxygen species. J. Neurochem. 2002, 80, 383–391. [Google Scholar]
- Knapp, L.T.; Klann, E. Potentiation of hippocampal synaptic transmission by superoxide requires the oxidative activation of protein kinase c. J. Neurosci. 2002, 22, 674–683. [Google Scholar]
- Dvoriantchikova, G.; Grant, J.; Santos, A.R.; Hernandez, E.; Ivanov, D. Neuronal nad(p)h oxidases contribute to ros production and mediate rgc death after ischemia. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2823–2830. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome p450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [PubMed]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity. J. Exp. Neurosci. 2016, 10, 23–48. [Google Scholar] [CrossRef]
- Liu, H.; Xing, R.; Ou, Z.; Zhao, J.; Hong, G.; Zhao, T.J.; Han, Y.; Chen, Y. G-protein-coupled receptor gpr17 inhibits glioma development by increasing polycomb repressive complex 1-mediated ros production. Cell Death Dis. 2021, 12, 610. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ros govern the lps-induced pro-inflammatory response in microglia cells by regulating mapk and nf-κb pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar]
- Huber, M.J.; Basu, R.; Cecchettini, C.; Cuadra, A.E.; Chen, Q.H.; Shan, Z. Activation of the (pro)renin receptor in the paraventricular nucleus increases sympathetic outflow in anesthetized rats. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H880–H887. [Google Scholar] [PubMed]
- Cho, D.Y.; Han, J.H.; Kim, I.S.; Lim, J.H.; Ko, H.M.; Kim, B.; Choi, D.K. The acetyltransferase gcn5 contributes to neuroinflammation in mice by acetylating and activating the nf-κb subunit p65 in microglia. Sci. Signal. 2025, 18, eadp8973. [Google Scholar] [CrossRef]
- Onasanwo, S.A.; Velagapudi, R.; El-Bakoush, A.; Olajide, O.A. Inhibition of neuroinflammation in bv2 microglia by the biflavonoid kolaviron is dependent on the nrf2/are antioxidant protective mechanism. Mol. Cell. Biochem. 2016, 414, 23–36. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Wang, X.; Tong, L.; Chen, G.; Zhou, S.; Zhang, H.; Liu, H.; Lu, W.; Wang, G.; et al. Microglia-derived tnf-α contributes to rvlm neuronal mitochondrial dysfunction via blocking the ampk-sirt3 pathway in stress-induced hypertension. J. Neuroinflamm. 2023, 20, 137. [Google Scholar]
- Takagishi, M.; Waki, H.; Bhuiyan, M.E.; Gouraud, S.S.; Kohsaka, A.; Cui, H.; Yamazaki, T.; Paton, J.F.; Maeda, M. Il-6 microinjected in the nucleus tractus solitarii attenuates cardiac baroreceptor reflex function in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R183–R190. [Google Scholar] [CrossRef]
- Chen, X.; Yan, X.; Gingerich, L.; Chen, Q.H.; Bi, L.; Shan, Z. Induction of neuroinflammation and brain oxidative stress by brain-derived extracellular vesicles from hypertensive rats. Antioxidants 2024, 13, 328. [Google Scholar] [CrossRef]
- Woods, C.; Marques-Lopes, J.; Contoreggi, N.H.; Milner, T.A.; Pickel, V.M.; Wang, G.; Glass, M.J. Tumor necrosis factor α receptor type 1 activation in the hypothalamic paraventricular nucleus contributes to glutamate signaling and angiotensin ii-dependent hypertension. J. Neurosci. 2021, 41, 1349–1362. [Google Scholar] [PubMed]
- Haspula, D.; Clark, M.A. Neuroinflammation and sympathetic overactivity: Mechanisms and implications in hypertension. Auton. Neurosci. 2018, 210, 10–17. [Google Scholar] [PubMed]
- Verhoog, Q.P.; Holtman, L.; Aronica, E.; van Vliet, E.A. Astrocytes as guardians of neuronal excitability: Mechanisms underlying epileptogenesis. Front. Neurol. 2020, 11, 591690. [Google Scholar] [CrossRef]
- Garnier, A.; Vidal, A.; Benali, H. A theoretical study on the role of astrocytic activity in neuronal hyperexcitability by a novel neuron-glia mass model. J. Math. Neurosci. 2016, 6, 10. [Google Scholar]
- Cojocaru, K.A.; Luchian, I.; Goriuc, A.; Antoci, L.M.; Ciobanu, C.G.; Popescu, R.; Vlad, C.E.; Blaj, M.; Foia, L.G. Mitochondrial dysfunction, oxidative stress, and therapeutic strategies in diabetes, obesity, and cardiovascular disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef]
- Gruber, T.; Pan, C.; Contreras, R.E.; Wiedemann, T.; Morgan, D.A.; Skowronski, A.A.; Lefort, S.; De Bernardis Murat, C.; Le Thuc, O.; Legutko, B.; et al. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 2021, 33, 1155–1170.e1110. [Google Scholar] [PubMed]
- Koehler, R.C.; Gebremedhin, D.; Harder, D.R. Role of astrocytes in cerebrovascular regulation. J. Appl. Physiol. 2006, 100, 307–317. [Google Scholar] [CrossRef][Green Version]
- Stern, J.E.; Son, S.; Biancardi, V.C.; Zheng, H.; Sharma, N.; Patel, K.P. Astrocytes contribute to angiotensin ii stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 2016, 68, 1483–1493. [Google Scholar]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ros in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar]
- Dabertrand, F.; Hannah, R.M.; Pearson, J.M.; Hill-Eubanks, D.C.; Brayden, J.E.; Nelson, M.T. Prostaglandin e2, a postulated astrocyte-derived neurovascular coupling agent, constricts rather than dilates parenchymal arterioles. J. Cereb. Blood Flow Metab. 2013, 33, 479–482. [Google Scholar]
- Usui, T.; Okada, M.; Mizuno, W.; Oda, M.; Ide, N.; Morita, T.; Hara, Y.; Yamawaki, H. Hdac4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1894–H1904. [Google Scholar]
- Tian, Y.; Shi, H.; Zhang, D.; Wang, C.; Zhao, F.; Li, L.; Xu, Z.; Jiang, J.; Li, J. Nebulized inhalation of lpae-hdac10 inhibits acetylation-mediated ros/nf-κb pathway for silicosis treatment. J Control. Release 2023, 364, 618–631. [Google Scholar] [PubMed]
- Kee, H.J.; Kim, G.R.; Lin, M.Q.; Choi, S.Y.; Ryu, Y.; Jin, L.; Piao, Z.H.; Jeong, M.H. Expression of class i and class ii a/b histone deacetylase is dysregulated in hypertensive animal models. Korean Circ. J. 2017, 47, 392–400. [Google Scholar] [PubMed]
- Wu, Q.; Ni, X. Ros-mediated DNA methylation pattern alterations in carcinogenesis. Curr. Drug Targets 2015, 16, 13–19. [Google Scholar]
- Uysal, F.; Sukur, G.; Bozdemir, N.; Cinar, O. DNA methyltransferase (dnmt) silencing causes increased cdx2 and nanog levels in surviving embryos. Int. J. Dev. Biol. 2023, 67, 1–8. [Google Scholar] [PubMed]
- Joáo Job, P.M.; Dos Reis Lívero, F.A.; Junior, A.G. Epigenetic control of hypertension by DNA methylation: A real possibility. Curr. Pharm. Des. 2021, 27, 3722–3728. [Google Scholar]
- Wu, X.N.; Li, J.Y.; He, Q.; Li, B.Q.; He, Y.H.; Pan, X.; Wang, M.Y.; Sang, R.; Ding, J.C.; Gao, X.; et al. Targeting the phf8/yy1 axis suppresses cancer cell growth through modulation of ros. Proc. Natl. Acad. Sci. USA 2024, 121, e2219352120. [Google Scholar]
- Li, H.; Yu, K.; Hu, H.; Zhang, X.; Zeng, S.; Li, J.; Dong, X.; Deng, X.; Zhang, J.; Zhang, Y. Mettl17 coordinates ferroptosis and tumorigenesis by regulating mitochondrial translation in colorectal cancer. Redox Biol. 2024, 71, 103087. [Google Scholar]
- Odroniec, A.; Olszewska, M.; Kurpisz, M. Epigenetic markers in the embryonal germ cell development and spermatogenesis. Basic Clin. Androl. 2023, 33, 6. [Google Scholar] [CrossRef]
- Julian, C.G.; Pedersen, B.S.; Salmon, C.S.; Yang, I.V.; Gonzales, M.; Vargas, E.; Moore, L.G.; Schwartz, D.A. Unique DNA methylation patterns in offspring of hypertensive pregnancy. Clin. Transl. Sci. 2015, 8, 740–745. [Google Scholar] [CrossRef]
- Climent, M.; Viggiani, G.; Chen, Y.W.; Coulis, G.; Castaldi, A. Microrna and ros crosstalk in cardiac and pulmonary diseases. Int. J. Mol. Sci. 2020, 21, 4370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, P.; Hu, J.; Huang, Y.; Zhang, F.; Li, L.; Wang, E.; Guo, Q.; Ye, Z. Lncrna meg3 alleviates diabetic cognitive impairments by reducing mitochondrial-derived apoptosis through promotion of fundc1-related mitophagy via rac1-ros axis. ACS Chem. Neurosci. 2021, 12, 2280–2307. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Jiang, C. Recent advances in nanomedicines for the treatment of ischemic stroke. Acta Pharm. Sin. B 2021, 11, 1767–1788. [Google Scholar] [CrossRef] [PubMed]
- Landowski, L.M.; Niego, B.; Sutherland, B.A.; Hagemeyer, C.E.; Howells, D.W. Applications of nanotechnology in the diagnosis and therapy of stroke. Semin. Thromb. Hemost. 2020, 46, 592–605. [Google Scholar] [CrossRef]
- Sim, T.M.; Tarini, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-based technology approaches to the management of neurological disorders. Int. J. Mol. Sci. 2020, 21, 6070. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, V.N.; Nguyen, D.T.; Kodibagkar, V.D.; Stabenfeldt, S.E. Nanoparticle-based therapeutics for brain injury. Adv. Healthc. Mater. 2018, 7, 1700668. [Google Scholar] [CrossRef]
- Mohammed, F.S.; Omay, S.B.; Sheth, K.N.; Zhou, J. Nanoparticle-based drug delivery for the treatment of traumatic brain injury. Expert Opin. Drug Deliv. 2023, 20, 55–73. [Google Scholar] [CrossRef]
- Khalin, I.; Adarsh, N.; Schifferer, M.; Wehn, A.; Groschup, B.; Misgeld, T.; Klymchenko, A.; Plesnila, N. Size-selective transfer of lipid nanoparticle-based drug carriers across the blood brain barrier via vascular occlusions following traumatic brain injury. Small 2022, 18, e2200302. [Google Scholar] [CrossRef]
- Copeland, C.; Stabenfeldt, S.E. Leveraging the dynamic blood-brain barrier for central nervous system nanoparticle-based drug delivery applications. Curr. Opin. Biomed. Eng. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant therapy in oxidative stress-induced neurodegenerative diseases: Role of nanoparticle-based drug delivery systems in clinical translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J. Recent progress in autocatalytic ceria nanoparticles-based translational research on brain diseases. ACS Appl. Nano Mater. 2020, 3, 1043–1062. [Google Scholar] [CrossRef]
- Mandalapu, S.R.; Hou, S.; Jockusch, S.; Shan, Z.; Bi, L. A novel class of indole derivatives: Enhanced bioavailability, permeability, and antioxidant efficacy for thromboembolic disease therapy. Med. Chem. Res. 2024, 33, 1368–1373. [Google Scholar]
- Liu, W.; Liu, L.; Li, H.; Xie, Y.; Bai, J.; Guan, J.; Qi, H.; Sun, J. Targeted pathophysiological treatment of ischemic stroke using nanoparticle-based drug delivery system. J. Nanobiotechnol. 2024, 22, 499. [Google Scholar]
- Szeto, H.H. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006, 8, E277–E283. [Google Scholar] [CrossRef] [PubMed]
- Shinn, L.J.; Lagalwar, S. Treating neurodegenerative disease with antioxidants: Efficacy of the bioactive phenol resveratrol and mitochondrial-targeted mitoq and skq. Antioxidants 2021, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Schnug, E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Aldosari, S.; Awad, M.; Harrington, E.O.; Sellke, F.W.; Abid, M.R. Subcellular reactive oxygen species (ros) in cardiovascular pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef]
- Mason, S.A.; Wadley, G.D.; Keske, M.A.; Parker, L. Effect of mitochondrial-targeted antioxidants on glycaemic control, cardiovascular health, and oxidative stress in humans: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2022, 24, 1047–1060. [Google Scholar]
- Pant, T.; Uche, N.; Juric, M.; Bosnjak, Z.J. Clinical relevance of lncrna and mitochondrial targeted antioxidants as therapeutic options in regulating oxidative stress and mitochondrial function in vascular complications of diabetes. Antioxidants 2023, 12, 898. [Google Scholar] [CrossRef]
- Hou, S.; Yan, X.; Gao, X.; Jockusch, S.; Gibson, K.M.; Shan, Z.; Bi, L. Enhancing cardiomyocyte resilience to ischemia-reperfusion injury: The therapeutic potential of an indole-peptide-tempo conjugate (iptc). ACS Omega 2024, 9, 39401–39418. [Google Scholar]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.K.; Mohandas, S.; Ramkumar, K.M. Role of er stress inhibitors in the management of diabetes. Eur. J. Pharmacol. 2022, 922, 174893. [Google Scholar] [CrossRef]
- Deka, D.; D’Incà, R.; Sturniolo, G.C.; Das, A.; Pathak, S.; Banerjee, A. Role of er stress mediated unfolded protein responses and er stress inhibitors in the pathogenesis of inflammatory bowel disease. Dig. Dis. Sci. 2022, 67, 5392–5406. [Google Scholar] [CrossRef]
- Haas, M.J.; Warda, F.; Bikkina, P.; Landicho, M.A.; Kapadia, P.; Parekh, S.; Mooradian, A.D. Differential effects of cyclooxygenase-2 (cox-2) inhibitors on endoplasmic reticulum (er) stress in human coronary artery endothelial cells. Vasc. Pharmacol. 2022, 142, 106948. [Google Scholar] [CrossRef]
- Barman, S.A.; Bordan, Z.; Batori, R.; Haigh, S.; Fulton, D.J.R. Galectin-3 promotes ros, inflammation, and vascular fibrosis in pulmonary arterial hypertension. Adv. Exp. Med. Biol. 2021, 1303, 13–32. [Google Scholar]
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial hypertension-oxidative stress and inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Peng, B.; Huang, G.; Diao, C.; Qin, Y.; Hong, Y.; Lin, J.; Lin, Y.; Jiang, L.; Tang, N.; et al. Inhibition of nox4 with glx351322 alleviates acute ocular hypertension-induced retinal inflammation and injury by suppressing ros mediated redox-sensitive factors activation. Biomed. Pharmacother. 2023, 165, 115052. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, X.; Zhao, Y.; Chen, B.; Li, X.; Qi, R. Ginkgolide b reduces lox-1 expression by inhibiting akt phosphorylation and increasing sirt1 expression in oxidized ldl-stimulated human umbilical vein endothelial cells. PLoS ONE 2013, 8, e74769. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, M.; Smith, D.A.; Williams, I.M.; Borja, M.C.; Neville, D.C.; Butters, T.D.; Dwek, R.A.; Platt, F.M. Nsaids increase survival in the sandhoff disease mouse: Synergy with n-butyldeoxynojirimycin. Ann. Neurol. 2004, 56, 642–649. [Google Scholar] [CrossRef]
- Xu, S.; Hu, B.; Dong, T.; Chen, B.Y.; Xiong, X.J.; Du, L.J.; Li, Y.L.; Chen, Y.L.; Tian, G.C.; Bai, X.B.; et al. Alleviate periodontitis and its comorbidity hypertension using a nanoparticle-embedded functional hydrogel system. Adv. Healthc. Mater. 2023, 12, e2203337. [Google Scholar] [CrossRef]
- Moradifar, N.; Kiani, A.A.; Veiskaramian, A.; Karami, K. Role of organic and inorganic nanoparticles in the drug delivery system for hypertension treatment: A systematic review. Curr. Cardiol. Rev. 2022, 18, e110621194025. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ren, J.; Yang, L. Nanoparticles in the new era of cardiovascular therapeutics: Challenges and opportunities. Int. J. Mol. Sci. 2023, 24, 5205. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the akt/nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Oxidative stress and vascular stiffness in hypertension: A renewed interest for antioxidant therapies? Vasc. Pharmacol. 2019, 116, 45–50. [Google Scholar]
- Choi, C.H.; Iordanishvili, E.; Shah, N.J.; Binkofski, F. Magnetic resonance spectroscopy with transcranial direct current stimulation to explore the underlying biochemical and physiological mechanism of the human brain: A systematic review. Hum. Brain Mapp. 2021, 42, 2642–2671. [Google Scholar]
- Hsieh, C.J.; Hou, C.; Zhu, Y.; Lee, J.Y.; Kohli, N.; Gallagher, E.; Xu, K.; Lee, H.; Li, S.; McManus, M.J.; et al. [(18)f]rostrace detects oxidative stress in vivo and predicts progression of alzheimer’s disease pathology in app/ps1 mice. EJNMMI Res. 2022, 12, 43. [Google Scholar]
- Cuevas, S.; Villar, V.A.M.; Jose, P.A. Genetic polymorphisms associated with reactive oxygen species and blood pressure regulation. Pharmacogen. J. 2019, 19, 315–336. [Google Scholar]
- Decharatchakul, N.; Settasatian, C.; Settasatian, N.; Komanasin, N.; Kukongviriyapan, U.; Intharaphet, P.; Senthong, V. Association of genetic polymorphisms in sod2, sod3, gpx3, and gstt1 with hypertriglyceridemia and low hdl-c level in subjects with high risk of coronary artery disease. PeerJ 2019, 7, e7407. [Google Scholar]
- Chen, A.; Wang, R.; Kang, Y.; Liu, J.; Wu, J.; Zhang, Y.; Zhang, Y.; Shao, L. Tongue-brain-transported zno nanoparticles induce abnormal taste perception. Adv. Healthc. Mater. 2023, 12, e2203316. [Google Scholar]
- Parvez, S.; Kaushik, M.; Ali, M.; Alam, M.M.; Ali, J.; Tabassum, H.; Kaushik, P. Dodging blood brain barrier with “nano” warriors: Novel strategy against ischemic stroke. Theranostics 2022, 12, 689–719. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, M.; Du, D.; Shan, Z.; Bi, L.; Chen, Q.-H. Brain-Targeted Reactive Oxygen Species in Hypertension: Unveiling Subcellular Dynamics, Immune Cross-Talk, and Novel Therapeutic Pathways. Antioxidants 2025, 14, 408. https://doi.org/10.3390/antiox14040408
Wang R, Wang M, Du D, Shan Z, Bi L, Chen Q-H. Brain-Targeted Reactive Oxygen Species in Hypertension: Unveiling Subcellular Dynamics, Immune Cross-Talk, and Novel Therapeutic Pathways. Antioxidants. 2025; 14(4):408. https://doi.org/10.3390/antiox14040408
Chicago/Turabian StyleWang, Renjun, Min Wang, Dongshu Du, Zhiying Shan, Lanrong Bi, and Qing-Hui Chen. 2025. "Brain-Targeted Reactive Oxygen Species in Hypertension: Unveiling Subcellular Dynamics, Immune Cross-Talk, and Novel Therapeutic Pathways" Antioxidants 14, no. 4: 408. https://doi.org/10.3390/antiox14040408
APA StyleWang, R., Wang, M., Du, D., Shan, Z., Bi, L., & Chen, Q.-H. (2025). Brain-Targeted Reactive Oxygen Species in Hypertension: Unveiling Subcellular Dynamics, Immune Cross-Talk, and Novel Therapeutic Pathways. Antioxidants, 14(4), 408. https://doi.org/10.3390/antiox14040408







