GSH-Responsive Nano-Photosensitizer for Potentiating Photodynamic Therapy Through Multi-Pronged Synergistic Upregulation of Ferroptosis Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
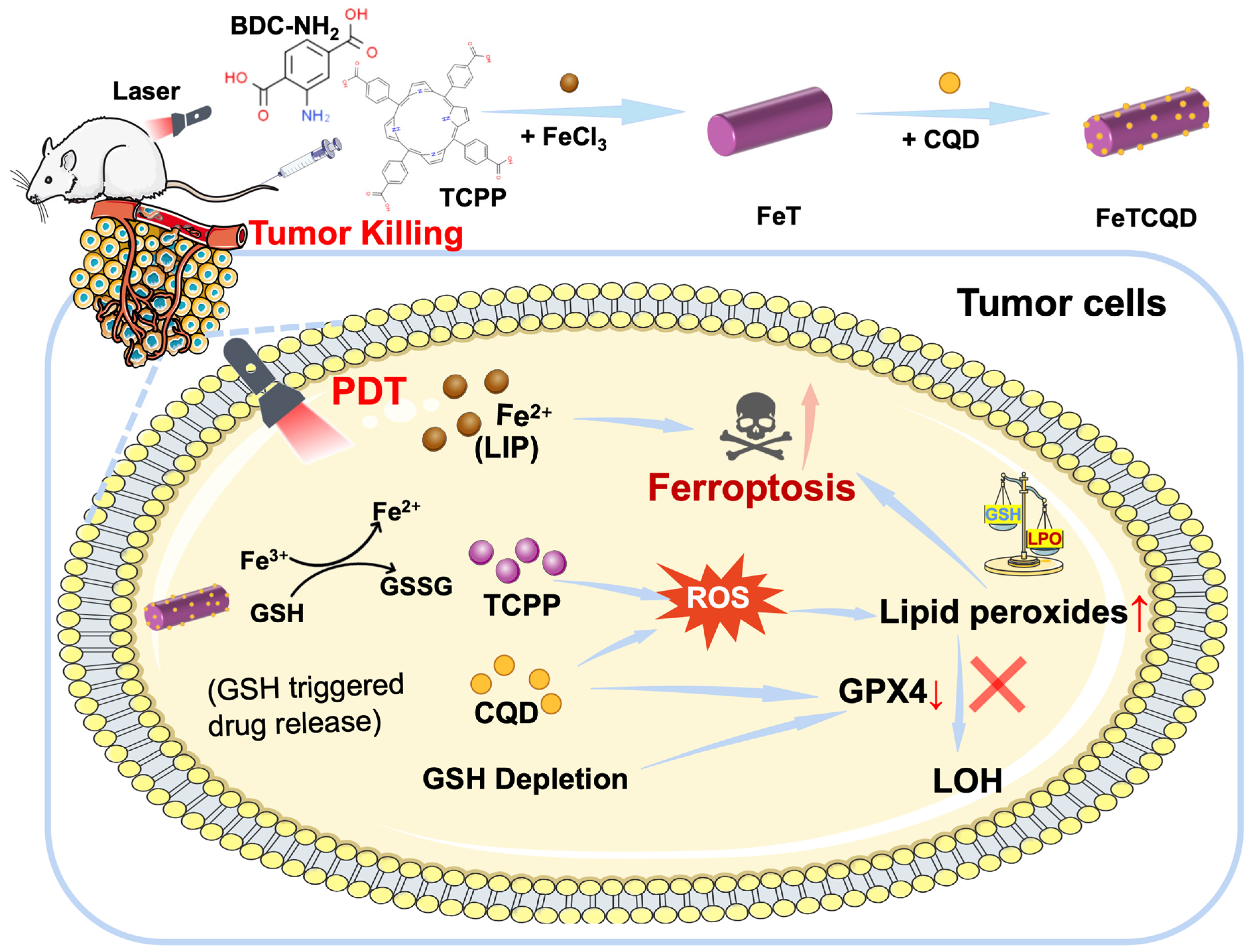
2.2. Synthesis of FeTCQD
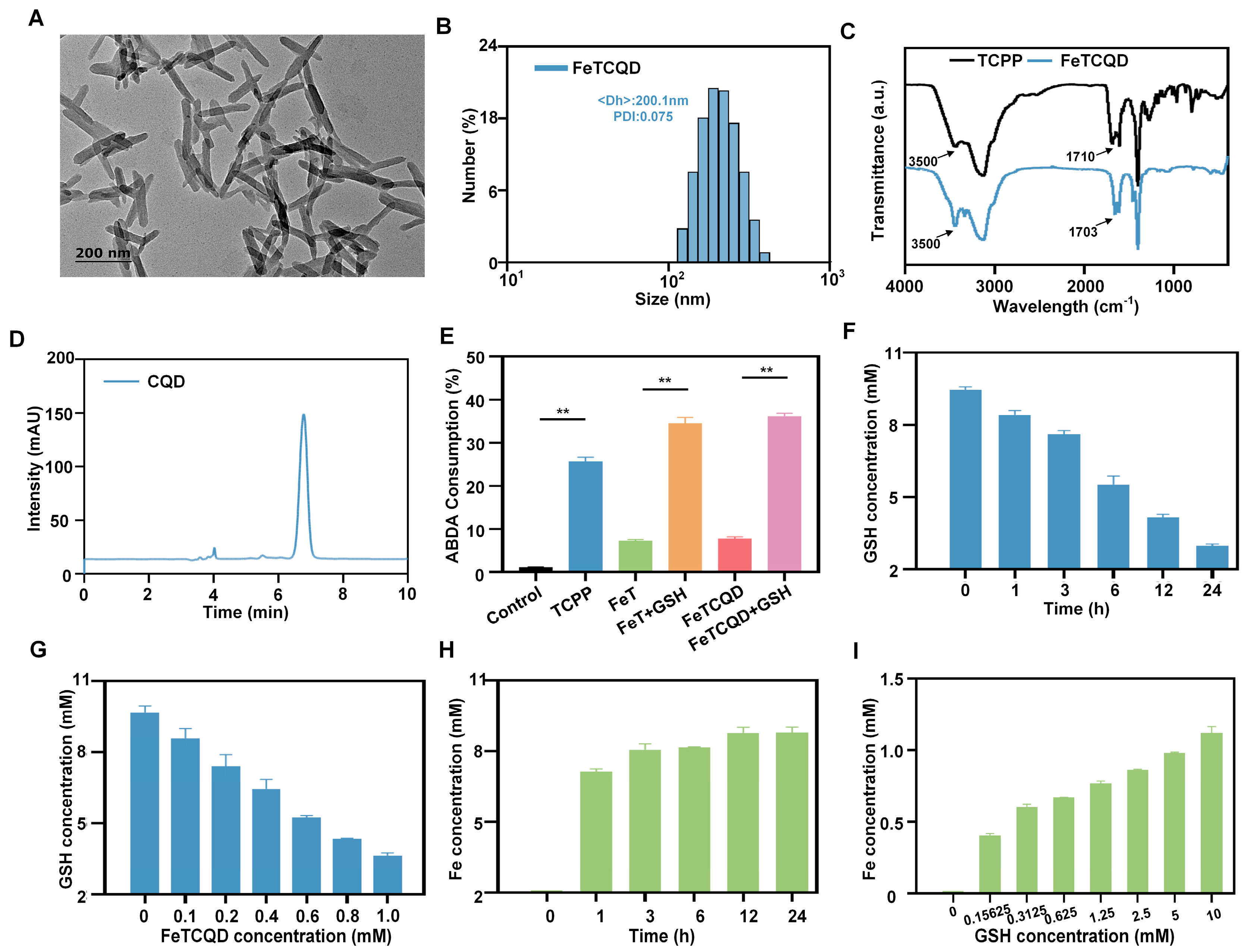
2.3. Characterizations
2.4. GSH-Mediated Detection of FeTCQD in Solutions
2.5. Release of Iron Ions from Nanorods FeTCQD
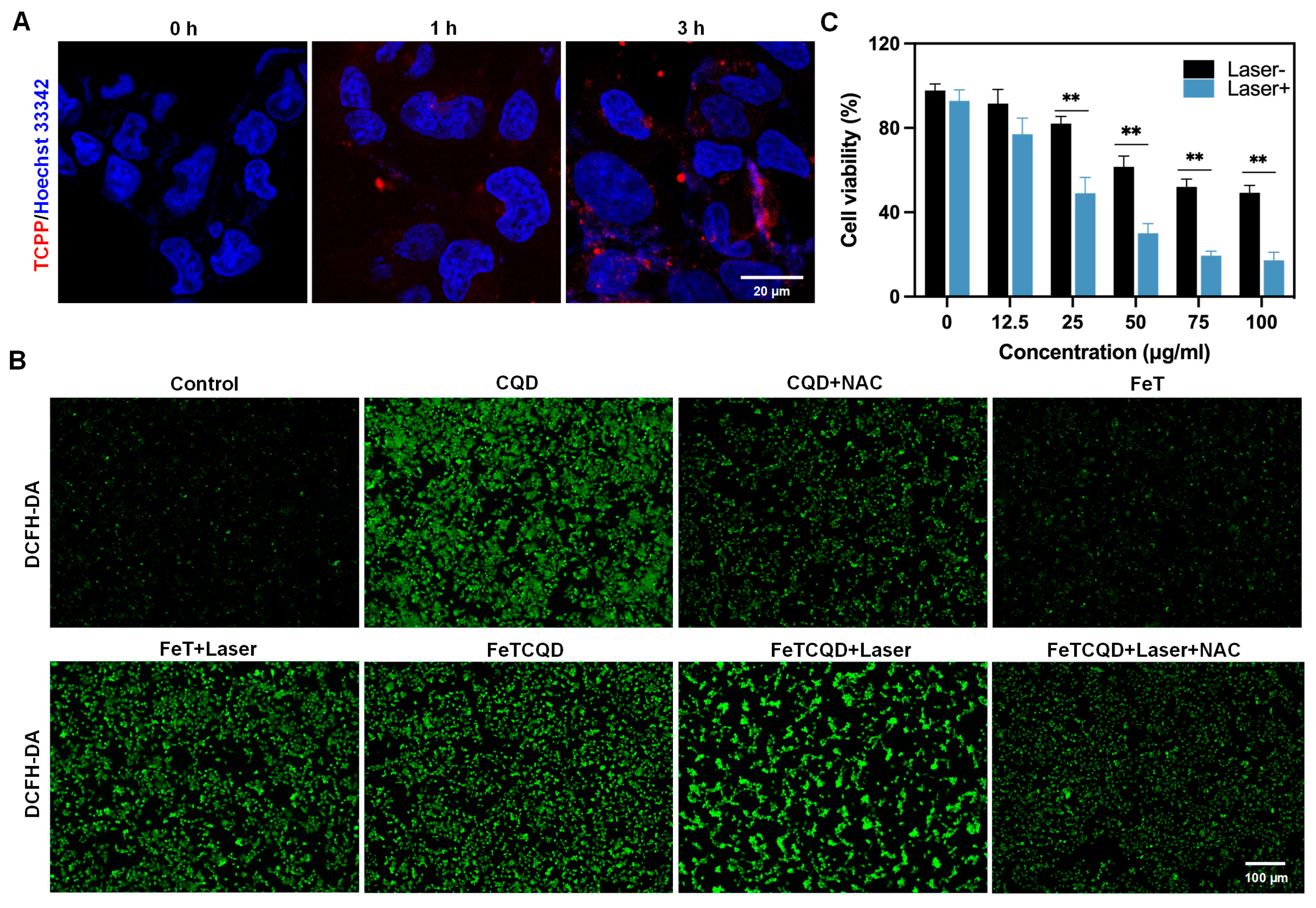
2.6. Cell Culture and Cellular Uptake
2.7. Cytotoxicity Evaluation
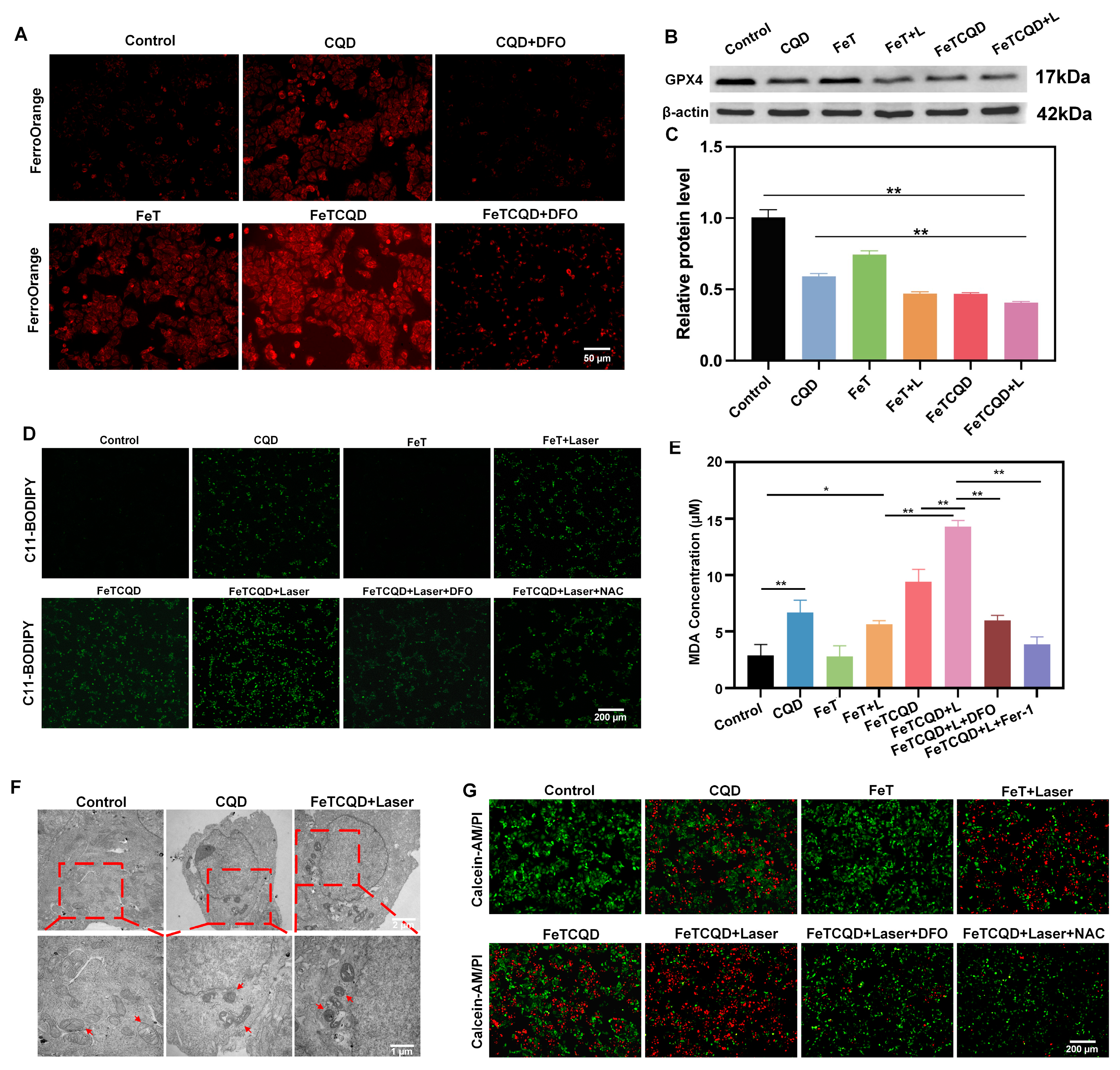
2.8. Intracellular Iron Ion Release Measurement
2.9. GPX4 Analysis
2.10. Intracellular MDA Measurement
2.11. Tumor Models
3. Results and Discussion
3.1. Synthesis and Characterization of FeTCQD
3.2. Cellular Uptake and Antitumor Effects of FeTCQD In Vitro
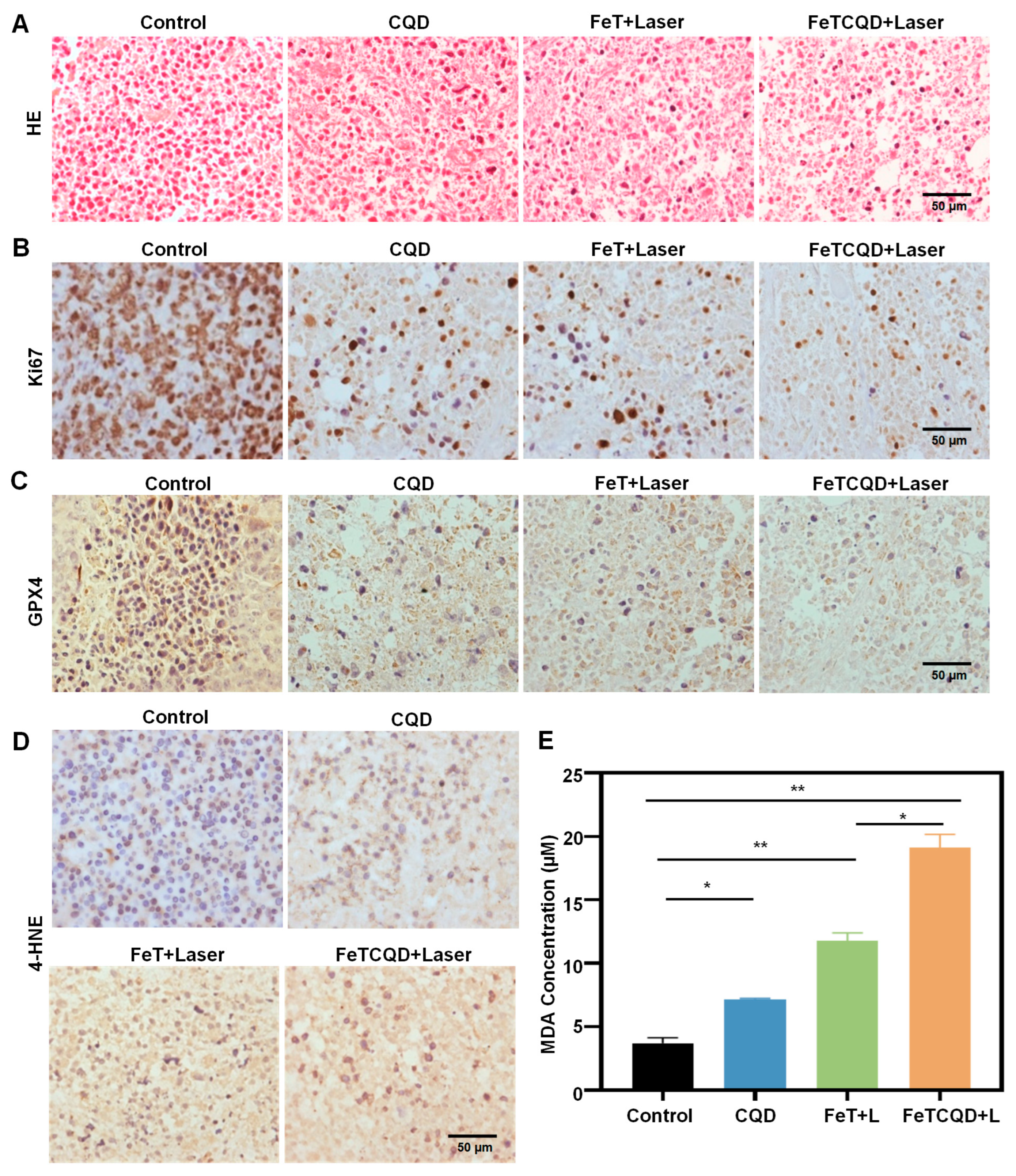
3.3. Mechanism of FeTCQD-Induced Cell Death In Vitro
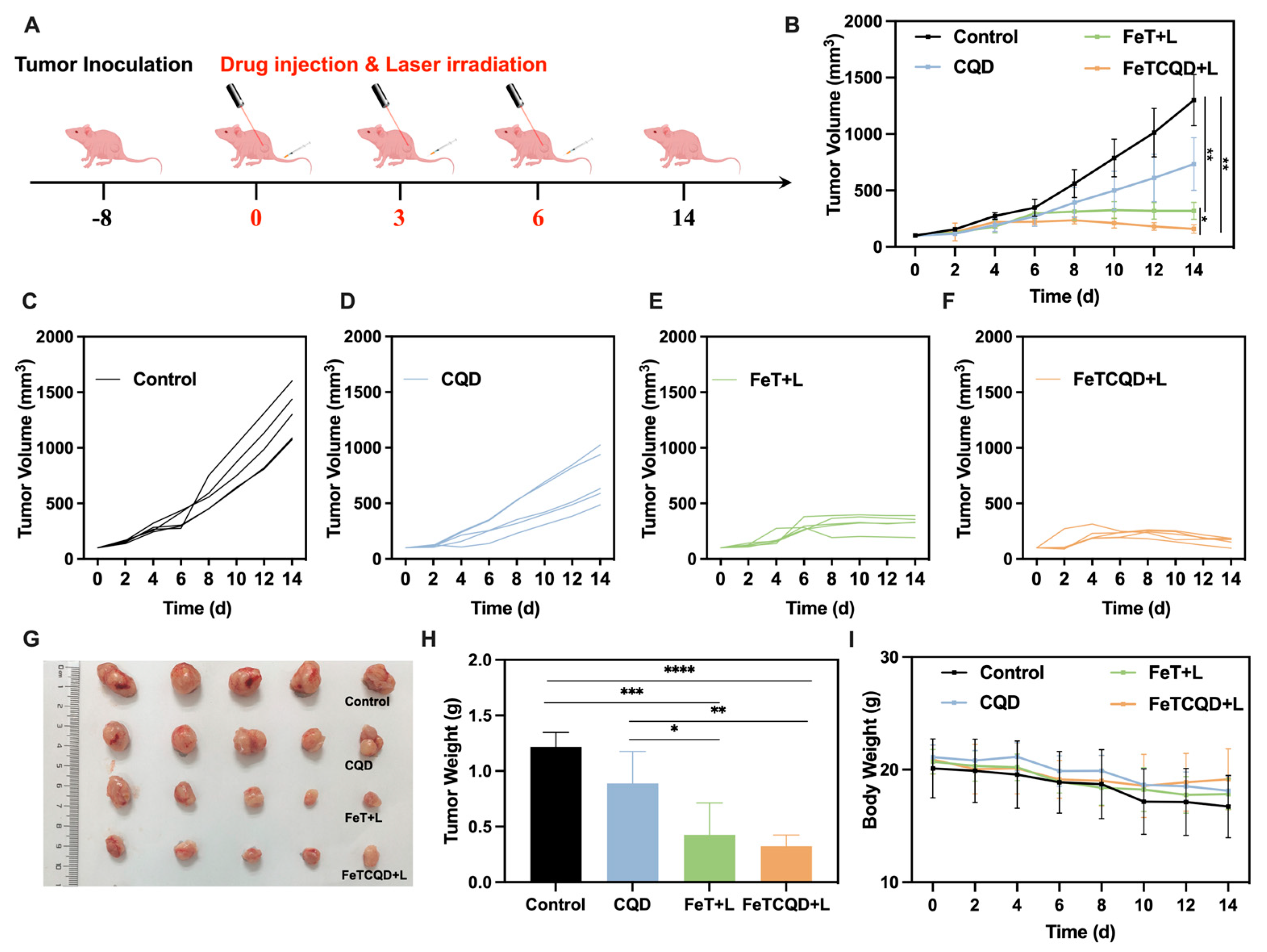
3.4. In Vivo Antitumor Effect of FeTCQD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDT | Photodynamic therapy |
| CQD | Chlorquinaldol |
| GPX4 | Glutathione peroxidase 4 |
| LIP | Liable iron pool |
| TCPP | Meso-tetra (4-carboxyphenyl) porphyrin |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Verkuijl, S.J.; Furnée, E.J.B.; Kelder, W.; Hoff, C.; Hess, D.A.; Wit, F.; Zijlstra, R.J.; Trzpis, M.; Broens, P.M.A. Long-term Bowel Dysfunction and Decline in Quality of Life Following Surgery for Colon Cancer: Call for Personalized Screening and Treatment. Dis. Colon Rectum 2022, 65, 1531. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Wang, B.; Li, X.; Feng, Z.; Ma, C.; Gao, L.; Yu, Y.; Zhang, J.; Zheng, P.; Wang, Y.; et al. Photodynamic therapy improves the clinical efficacy of advanced colorectal cancer and recruits immune cells into the tumor immune microenvironment. Front. Immunol. 2022, 13, 1050421. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, X.; Tian, P.; Xu, K.; Luo, J.; Li, H.; Yuan, M.; Liu, X.; Zhong, Y.; Wei, P.; et al. Laser-Ignited Lipid Peroxidation Nanoamplifiers for Strengthening Tumor Photodynamic Therapy Through Aggravating Ferroptotic Propagation and Sustainable High Immunogenicity. Small 2023, 20, 2306402. [Google Scholar] [CrossRef]
- Li, J.; Yi, H.; Fu, Y.; Zhuang, J.; Zhan, Z.; Guo, L.; Zheng, J.; Yu, X.; Zhang, D.-Y. Biodegradable iridium coordinated nanodrugs potentiate photodynamic therapy and immunotherapy of lung cancer. J. Colloid Interface Sci. 2025, 680, 9–24. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zang, D.; Chang, Y.; Su, W.; Li, G.; Zhang, J.; Yang, P.; Ma, X.; Guo, Y. pH-responsive oxygen self-sufficient smart nanoplatform for enhanced tumor chemotherapy and photodynamic therapy. J. Colloid Interface Sci. 2024, 675, 1080–1090. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Ma, Y.; Xiao, F.; Lu, C.; Wen, L. Multifunctional Nanosystems Powered Photodynamic Immunotherapy. Front. Pharmacol. 2022, 13, 905078. [Google Scholar] [CrossRef]
- Liang, L.; Wen, L.; Weng, Y.; Song, J.; Li, H.; Zhang, Y.; He, X.; Zhao, W.; Zhan, M.; Li, Y.; et al. Homologous-targeted and tumor microenvironment-activated hydroxyl radical nanogenerator for enhanced chemoimmunotherapy of non-small cell lung cancer. Chem. Eng. J. 2021, 425, 131451. [Google Scholar] [CrossRef]
- Ao, M.; Yu, F.; Li, Y.; Zhong, M.; Tang, Y.; Yang, H.; Wu, X.; Zhuang, Y.; Wang, H.; Sun, X.; et al. Carrier-free nanoparticles of camptothecin prodrug for chemo-photothermal therapy: The making, in vitro and in vivo testing. J. Nanobiotechnology 2021, 19, 350. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, C.; Guan, Q.; Wei, M.; Li, Z. Nanoparticle-mediated synergistic anticancer effect of ferroptosis and photodynamic therapy: Novel insights and perspectives. Asian J. Pharm. Sci. 2023, 18, 100829. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-H.; Gan, L.; Tian, L.-Y.; Huang, B.-X.; Xiao, Q.; Zhang, Y.-J.; Xiao, M.-T.; Zheng, B.-D.; Ye, J. Mutually reinforced cancer treatment based on phototherapy combined with ferroptosis. Chem. Eng. J. 2024, 493, 152397. [Google Scholar] [CrossRef]
- Shui, S.; Zhao, Z.; Wang, H.; Conrad, M.; Liu, G. Non-enzymatic lipid peroxidation initiated by photodynamic therapy drives a distinct ferroptosis-like cell death pathway. Redox Biol. 2021, 45, 102056. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Berghe, T.V. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, X.; Xie, F.; Zhang, L.; Yan, H.; Huang, J.; Zhang, C.; Zhou, F.; Chen, J.; Zhang, L. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 2022, 42, 88–116. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Yan, H.; Talty, R.; Aladelokun, O.; Bosenberg, M.; Johnson, C.H. Ferroptosis in colorectal cancer: A future target? Br. J. Cancer 2023, 128, 1439–1451. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sui, S.; Wang, L.; Li, H.; Zhang, L.; Xu, S.; Zheng, X. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J. Cell. Physiol. 2020, 235, 3425–3437. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Leng, J.; Tan, J.; Zhao, Y.; Xie, S.; Zhao, S.; Yan, X.; Zhu, L.; Luo, J.; Kong, L.; et al. Discovery of Novel Potent Covalent Glutathione Peroxidase 4 Inhibitors as Highly Selective Ferroptosis Inducers for the Treatment of Triple-Negative Breast Cancer. J. Med. Chem. 2023, 66, 10036–10059. [Google Scholar] [CrossRef]
- Shimada, K.; Skouta, R.; Kaplan, A.; Yang, W.S.; Hayano, M.; Dixon, S.J.; Brown, L.M.; Valenzuela, C.A.; Wolpaw, A.J.; Stockwell, B.R. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016, 12, 497–503. [Google Scholar] [CrossRef]
- Allimuthu, D.; Adams, D.J. 2-Chloropropionamide As a Low-Reactivity Electrophile for Irreversible Small-Molecule Probe Identification. ACS Chem. Biol. 2017, 12, 2124–2131. [Google Scholar] [CrossRef]
- Eaton, J.K.; Furst, L.; Ruberto, R.A.; Moosmayer, D.; Hilpmann, A.; Ryan, M.J.; Zimmermann, K.; Cai, L.L.; Niehues, M.; Badock, V.; et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat. Chem. Biol. 2020, 16, 497–506. [Google Scholar] [CrossRef]
- Bidossi, A.; Bottagisio, M.; De Grandi, R.; Drago, L.; De Vecchi, E. Chlorquinaldol, a topical agent for skin and wound infections: Anti-biofilm activity and biofilm-related antimicrobial cross-resistance. Infect. Drug Resist. 2019, 12, 2177–2189. [Google Scholar] [CrossRef]
- Bortolin, M.; Bidossi, A.; De Vecchi, E.; Avveniente, M.; Drago, L. In vitro Antimicrobial Activity of Chlorquinaldol against Microorganisms Responsible for Skin and Soft Tissue Infections: Comparative Evaluation with Gentamicin and Fusidic Acid. Front. Microbiol. 2017, 8, 1039. [Google Scholar] [CrossRef]
- Wang, L.; Deng, K.; Gong, L.; Zhou, L.; Sayed, S.; Li, H.; Sun, Q.; Su, Z.; Wang, Z.; Liu, S.; et al. Chlorquinaldol targets the β-catenin and T-cell factor 4 complex and exerts anti-colorectal cancer activity. Pharmacol. Res. 2020, 159, 104955. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.-Q.; Qin, Q.-P.; Bai, Y.-X.; Cao, Q.-Q.; Zhang, Y.; Liu, Y.-C.; Chen, Z.-F.; Liang, H. Synthesis and antitumor mechanism of a new iron(III) complex with 5,7-dichloro-2-methyl-8-quinolinol as ligands. Med. Chem. Commun. 2017, 8, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.; Mudarmah, K.; Pant, B.D.; Krause, J.A.; Zheng, Y.-R.; Huang, S.D. Transferrin-inspired iron delivery across the cell membrane using [(L2Fe)2(μ-O)] (L = chlorquinaldol) to harness anticancer activity of ferroptosis. Dalton Trans. 2024, 53, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, K.; Wang, Y.; Liu, Y.; Wang, T.; Ding, S.; Zhang, X.; Xiong, W.; Zheng, F.; Yang, H.; et al. One-Pot Synthesis of Tumor-Microenvironment Responsive Degradable Nanoflower-Medicine for Multimodal Cancer Therapy with Reinvigorating Antitumor Immunity. Adv. Healthc. Mater. 2023, 12, 2302016. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Xu, K.; Feng, J.; Zhao, X.; Tian, P.; Luo, J.; Xu, L.; Song, J.; Lu, C. GSH-Responsive Nano-Photosensitizer for Potentiating Photodynamic Therapy Through Multi-Pronged Synergistic Upregulation of Ferroptosis Sensitivity. Antioxidants 2025, 14, 407. https://doi.org/10.3390/antiox14040407
Ma Y, Xu K, Feng J, Zhao X, Tian P, Luo J, Xu L, Song J, Lu C. GSH-Responsive Nano-Photosensitizer for Potentiating Photodynamic Therapy Through Multi-Pronged Synergistic Upregulation of Ferroptosis Sensitivity. Antioxidants. 2025; 14(4):407. https://doi.org/10.3390/antiox14040407
Chicago/Turabian StyleMa, Yunong, Kexin Xu, Jing Feng, Xi Zhao, Peilin Tian, Jiayang Luo, Luyao Xu, Jiaxing Song, and Cuixia Lu. 2025. "GSH-Responsive Nano-Photosensitizer for Potentiating Photodynamic Therapy Through Multi-Pronged Synergistic Upregulation of Ferroptosis Sensitivity" Antioxidants 14, no. 4: 407. https://doi.org/10.3390/antiox14040407
APA StyleMa, Y., Xu, K., Feng, J., Zhao, X., Tian, P., Luo, J., Xu, L., Song, J., & Lu, C. (2025). GSH-Responsive Nano-Photosensitizer for Potentiating Photodynamic Therapy Through Multi-Pronged Synergistic Upregulation of Ferroptosis Sensitivity. Antioxidants, 14(4), 407. https://doi.org/10.3390/antiox14040407





