Sirtuin 3 Protects Lung Adenocarcinoma from Ferroptosis by Deacetylating and Stabilizing Mitochondrial Glutamate Transporter Solute Carrier Family 25 Member A22
Abstract
1. Introduction
2. Methods
2.1. Bioinformatics Analysis
2.2. Reagents and Antibodies
2.3. Tissue Microarray
2.4. Immunohistochemistry (IHC)
2.5. Cell Lines and Culture Conditions
2.6. RSL3 Treatment
2.7. DFO and Fer-1 Treatment
2.8. Cell Viability Assay
2.9. Western Blotting
2.10. Quantitative Real-Time PCR (RT-qPCR)
2.11. Construction of the SLC25A22K83R Point Mutation Plasmid
2.12. shRNA and Stable Cell Lines
2.13. Mitochondrial Acetylation Assay
2.14. Lipid Peroxidation (LPO) Assay
2.15. Mitochondrial Fe2+
2.16. Mitochondrial ROS
2.17. Transmission Electron Microscopy
2.18. Animal Experiments
2.19. Co-Immunoprecipitation (Co-IP)
2.20. GSH
2.21. Statistical Analysis
3. Results
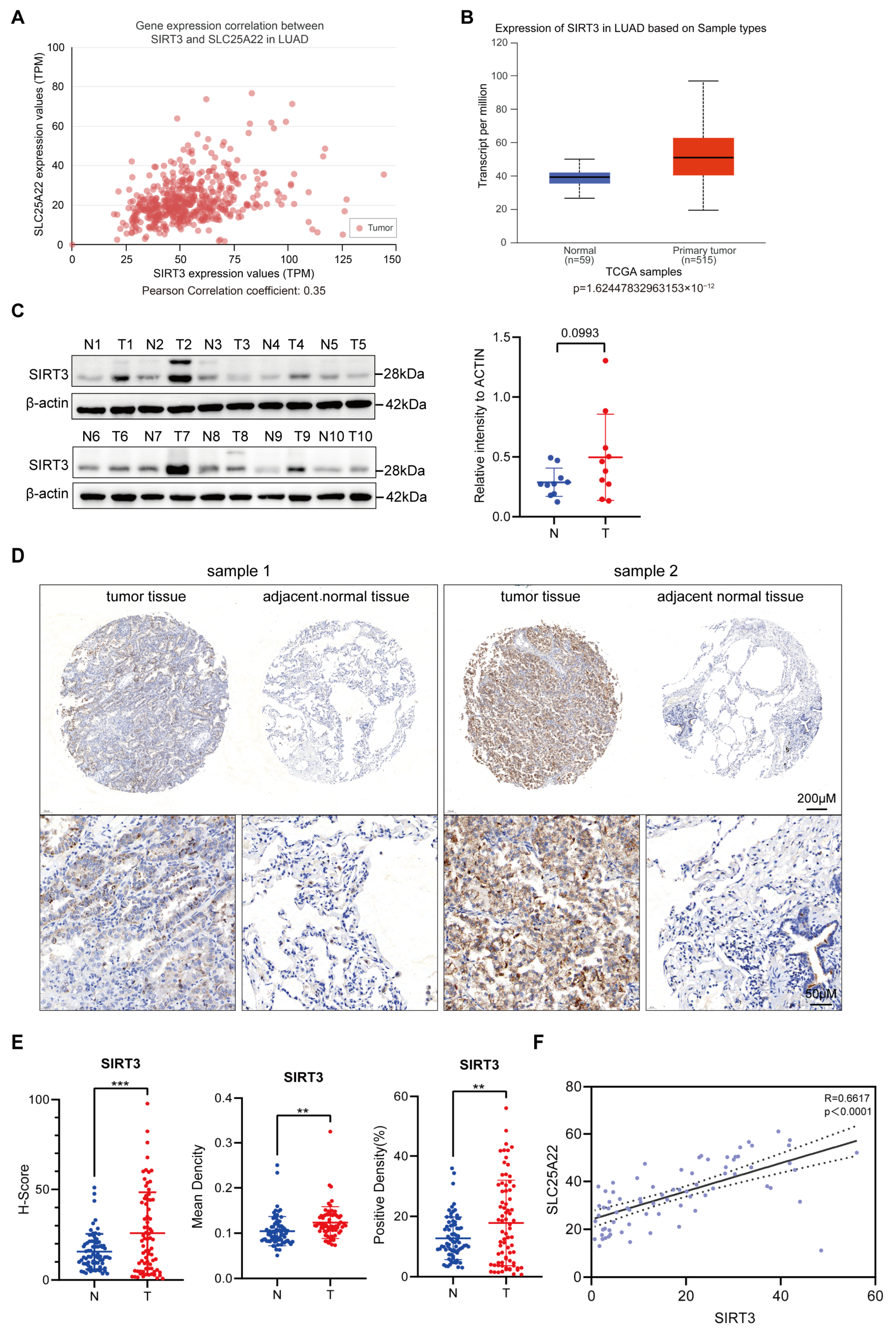
3.1. High Expression of SLC25A22 in LUAD Predicts Poor Prognosis
3.2. SIRT3 Expression Is Positively Correlated with SLC25A22 Expression in LUAD
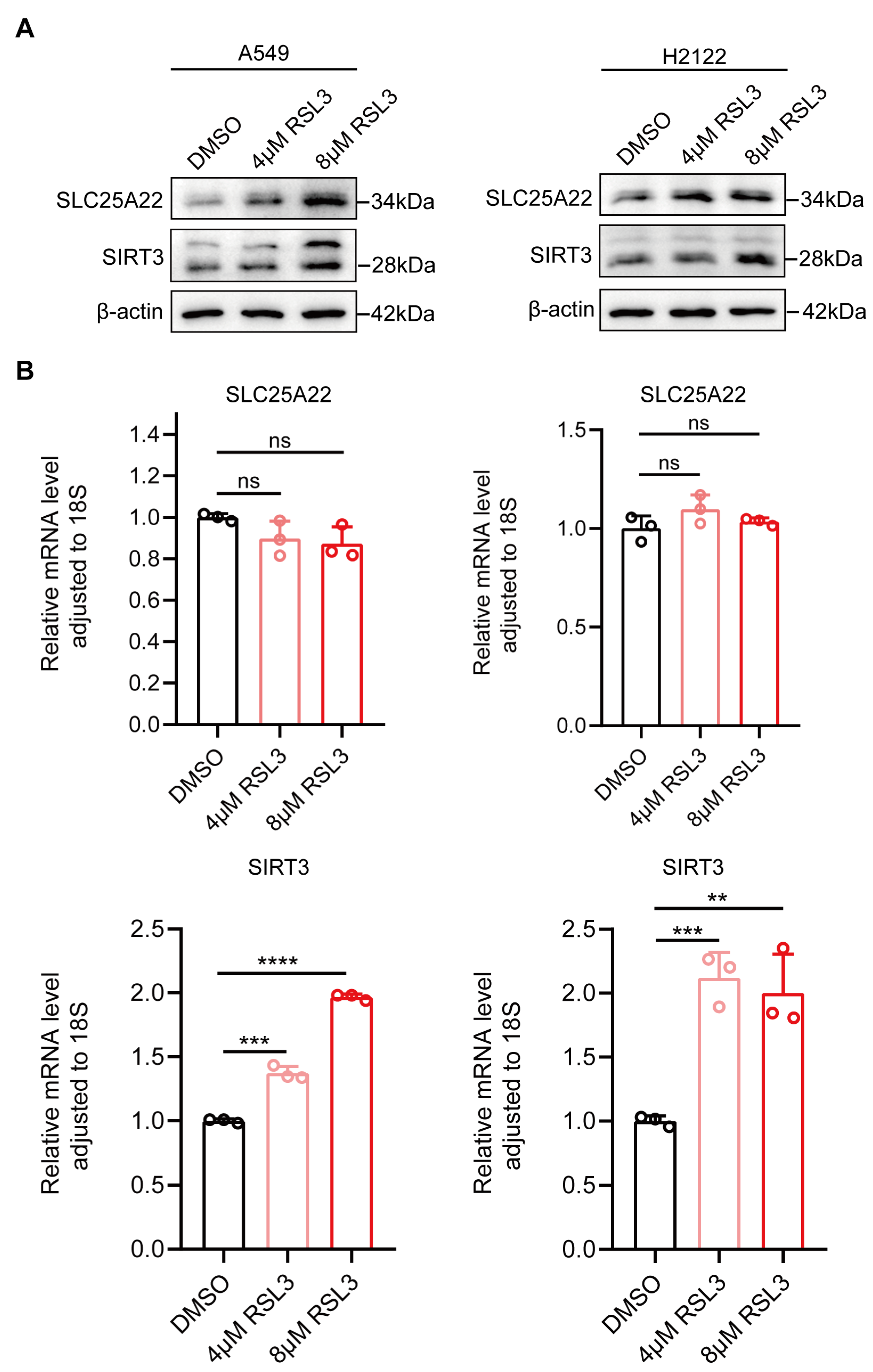
3.3. SLC25A22 and SIRT3 Are Both Upregulated During RSL3-Induced Ferroptosis of LUAD Cells
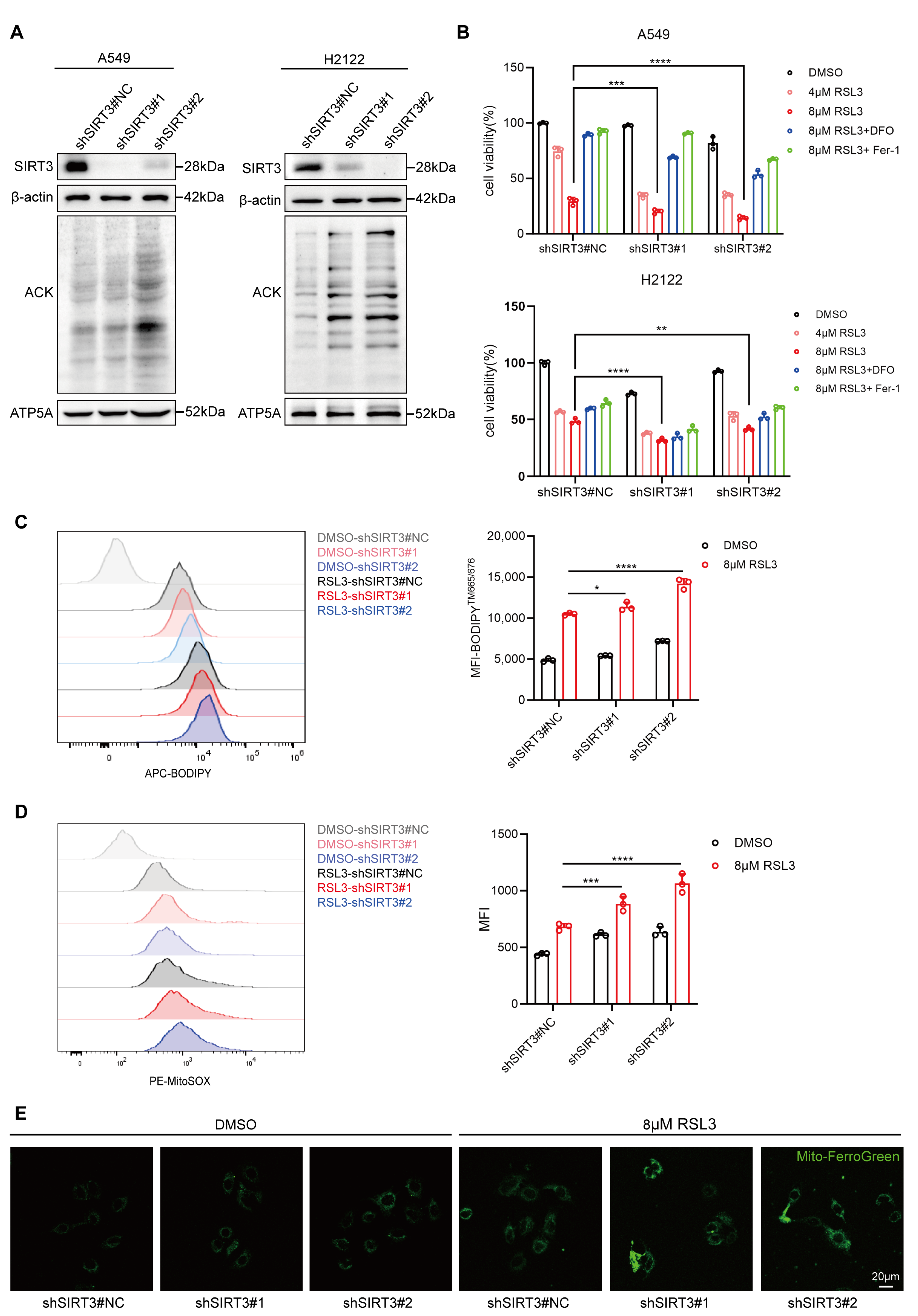
3.4. SIRT3 Protects LUAD Cells from RSL3-Induced Ferroptosis In Vitro
3.5. SIRT3 Protects LUAD from RSL3-Induced Ferroptosis In Vivo
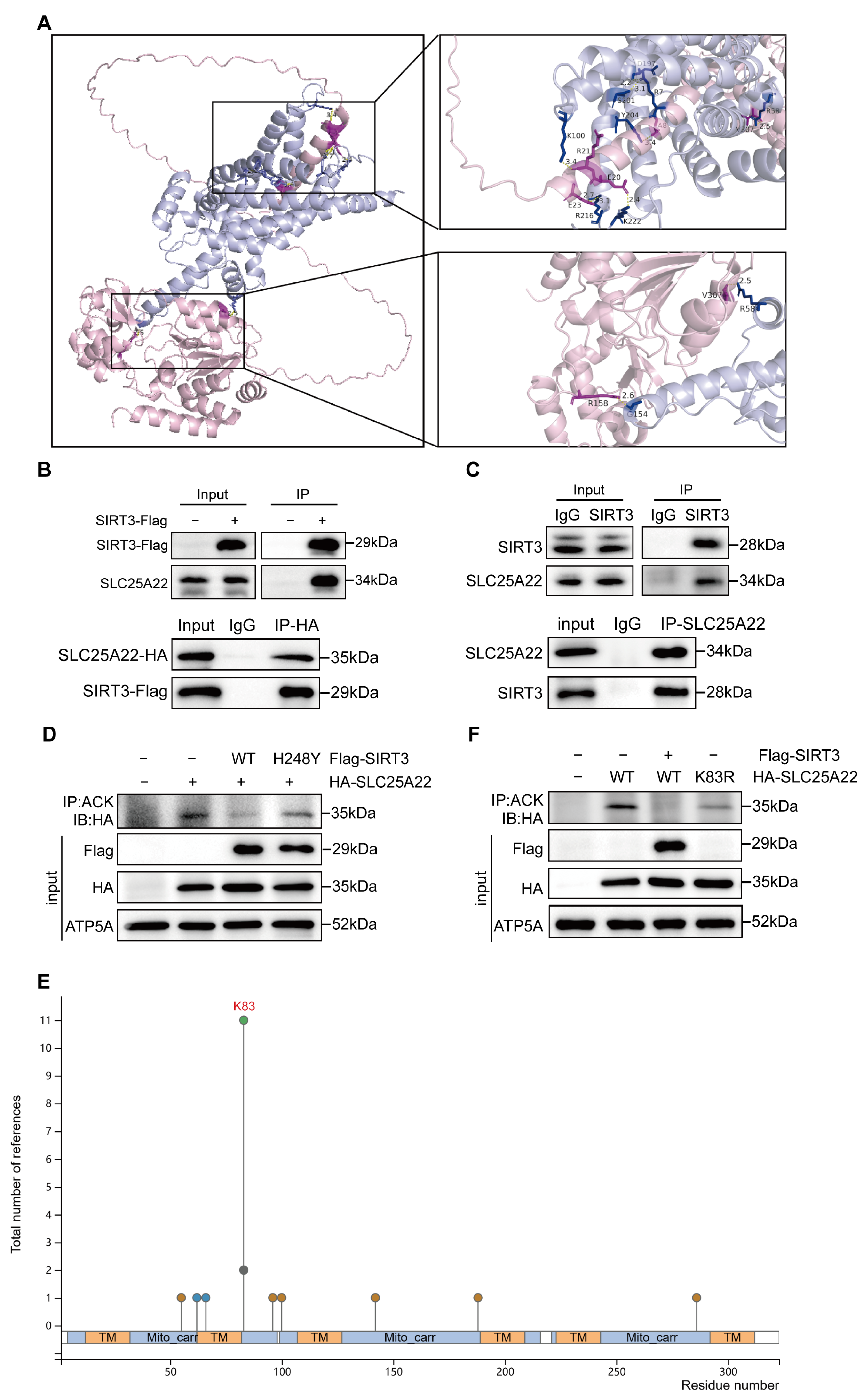
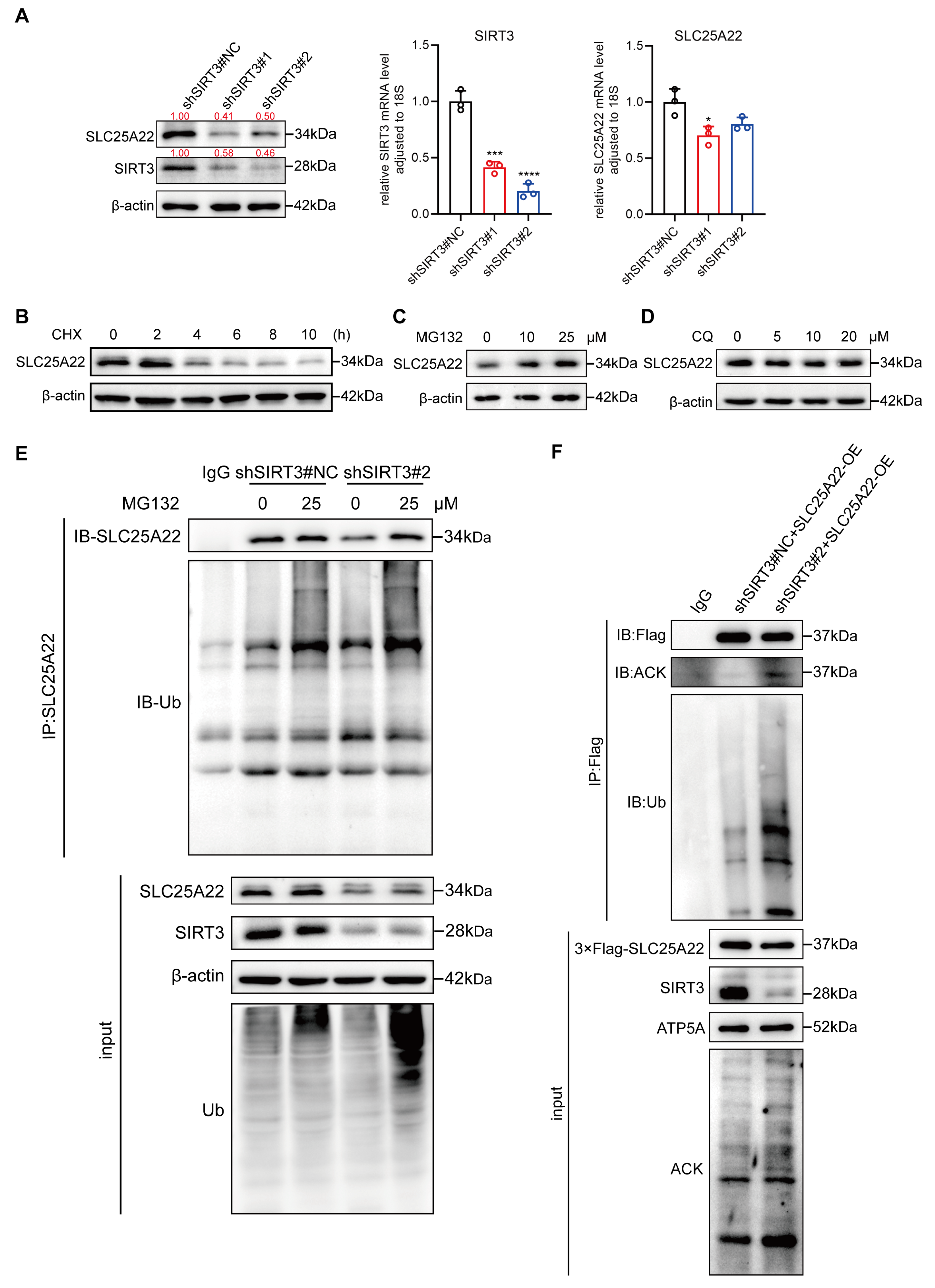
3.6. SIRT3 Binds to and Deacetylates SLC25A22
3.7. SIRT3 Decreases Ubiquitination and Increases Stability of SLC25A22
3.8. SIRT3 Protects LUAD Cells from RSL3-Induced Ferroptosis at Least Partially Due to Deacetylation of SLC25A22
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar]
- Lei, G.; Zhuang, L.; Gan, B.Y. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B.Y. The roles of ferroptosis in cancer: Tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell 2024, 42, 513–534. [Google Scholar]
- Nakamura, T.; Conrad, M. Exploiting ferroptosis vulnerabilities in cancer. Nat. Cell Biol. 2024, 26, 1407–1419. [Google Scholar] [PubMed]
- Liu, Y.E.; Lu, S.P.; Wu, L.L.; Yang, L.; Yang, L.X.; Wang, J.H. The diversified role of mitochondria in ferroptosis in cancer. Cell Death Dis. 2023, 14, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.H.; Yi, J.M.; Zhu, J.J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X.J. Role of mitochondria in ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar]
- Ruprecht, J.J.; Kunji, E.R.S. The SLC25 mitochondrial carrier family: Structure and Mechanism. Trends Biochem. Sci. 2020, 45, 244–258. [Google Scholar] [CrossRef]
- Yang, Y.; He, J.X.; Zhang, B.; Zhang, Z.S.; Jia, G.Z.; Liu, S.Q.; Wu, T.; He, X.L.; Wang, N. SLC25A1 promotes tumor growth and survival by reprogramming energy metabolism in colorectal cancer. Cell Death Dis. 2021, 12, 1108–1117. [Google Scholar]
- Zhang, R.W.; Peng, X.G.; Du, J.X.; Boohaker, R.; Estevao, I.L.; Grajeda, B.I.; Cox, M.B.; Almeida, I.C.; Lu, W.Q. Oncogenic KRAS reprograms lipid metabolism by upregulating SLC25A1 to frive sancreatic tumorigenesis. Cancer Res. 2023, 83, 3739–3752. [Google Scholar]
- Yuan, P.; Mu, J.; Wang, Z.J.; Ma, S.J.; Da, X.W.; Song, J.; Zhang, H.X.; Yang, L.; Li, J.B.; Yang, J.Y. Down-regulation of SLC25A20 promotes hepatocellular carcinoma growth and metastasis through suppression of fatty-acid oxidation. Cell Death Dis. 2021, 12, 361–373. [Google Scholar]
- Cheng, L.L.; Deepak, R.N.V.K.; Wang, G.Q.; Meng, Z.Y.; Tao, L.; Xie, M.Q.; Chi, W.N.; Zhang, Y.M.; Yang, M.M.; Liao, Y.L.; et al. Hepatic mitochondrial NAD transporter SLC25A47 activates AMPKa mediating lipid metabolism and tumorigenesis. Hepatology 2023, 78, 1828–1842. [Google Scholar]
- Lu, M.J.; Busquets, J.; Impedovo, V.; Wilson, C.N.; Chan, H.R.; Chang, Y.T.; Matsui, W.; Tiziani, S.; Cambronne, X.A. SLC25A51 decouples the mitochondrial NAD plus /NADH ratio to control proliferation of AML cells. Cell Metab. 2024, 36, 808–821.e6. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Qian, Y.; Li, X.N.; Xu, J.Y.; Kang, W.; Tong, J.H.; To, K.F.; Jin, Y.; Li, W.L.; Chen, H.R.; et al. SLC25A22 promotes proliferation and survival of colorectal cancer cells with KRAS mutations and xenograft tumor progression in mice via intracellular synthesis of aspartate. Gastroenterology 2016, 151, 945–960.e6. [Google Scholar]
- Wong, C.C.; Xu, J.Y.; Bian, X.Q.; Wu, J.L.; Kang, W.; Qian, Y.; Li, W.L.; Chen, H.R.; Gou, H.Y.; Liu, D.B.; et al. In colorectal cancer cells with mutant KRAS, SLC25A22-mediated glutaminolysis reduces DNA demethylation to increase WNT dignaling, dtemness, and frug tesistance. Gastroenterology 2020, 159, 2163–2180. [Google Scholar]
- Zhou, Q.M.; Peng, Y.; Ji, F.F.; Chen, H.R.; Kang, W.; Chan, L.S.; Gou, H.Y.; Lin, Y.F.; Huang, P.M.; Chen, D.Y.; et al. Targeting of SLC25A22 boosts the immunotherapeutic response in KRAS-mutant colorectal cancer. Nat. Commun. 2023, 14, 4677–4694. [Google Scholar] [PubMed]
- Du, P.C.; Liang, H.B.; Fu, X.W.; Wu, P.; Wang, C.; Chen, H.M.; Zheng, B.B.; Zhang, J.; Hu, S.H.; Zeng, R.G.; et al. SLC25A22 promotes proliferation and metastasis by activating MAPK/ERK pathway in gallbladder cancer. Cancer Cell Int. 2019, 19, 33–43. [Google Scholar] [PubMed]
- Shin, E.; Kim, B.; Kang, H.; Lee, H.; Park, J.; Kang, J.; Park, E.; Jo, S.; Kim, H.Y.; Lee, J.S.; et al. Mitochondrial glutamate transporter SLC25A22 uni-directionally export glutamate for metabolic rewiring in radioresistant glioblastoma. Int. J. Biol. Macromol. 2023, 253, 127511–127527. [Google Scholar]
- Onyango, P.; Celic, I.; McCaffery, J.M.; Boeke, J.D.; Feinberg, A.P. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc. Natl. Acad. Sci. USA 2002, 99, 13653–13658. [Google Scholar]
- Zhang, J.; Ye, J.; Zhu, S.; Han, B.; Liu, B. Context-dependent role of SIRT3 in cancer. Trends Pharmacol. Sci. 2024, 45, 173–190. [Google Scholar]
- Jeong, S.M.; Lee, J.; Finley, L.W.S.; Schmidt, P.J.; Fleming, M.D.; Haigis, M.C. SIRT3 regulates cellular iron metabolism and cancer growth by repressing iron regulatory protein 1. Oncogene 2015, 34, 2115–2124. [Google Scholar]
- Liu, L.G.; Li, Y.; Cao, D.Y.; Qiu, S.M.; Li, Y.S.; Jiang, C.K.; Bian, R.; Yang, Y.; Li, L.; Li, X.C.A.; et al. SIRT3 inhibits gallbladder cancer by induction of AKT-dependent ferroptosis and blockade of epithelial-mesenchymal transition. Cancer Lett. 2021, 510, 93–104. [Google Scholar] [PubMed]
- Jin, Y.; Gu, W.; Chen, W.C. Sirt3 is critical for p53-mediated ferroptosis upon ROS-induced stress. J. Mol. Cell Biol. 2021, 13, 151–154. [Google Scholar]
- Li, X.H.; Zhang, W.L.; Xing, Z.C.; Hu, S.M.; Zhang, G.Q.; Wang, T.E.; Wang, T.S.; Fan, Q.J.; Chen, G.Q.; Cheng, J.K.; et al. Targeting SIRT3 sensitizes glioblastoma to ferroptosis by promoting mitophagy and inhibiting SLC7A11. Cell Death Dis. 2024, 15, 168–182. [Google Scholar] [PubMed]
- Liu, Y.; Wang, Y.; Lin, Z.; Kang, R.; Tang, D.L.; Liu, J. SLC25A22 as a key mitochondrial transporter against ferroptosis by producing glutathione and monounsaturated fatty acids. Antioxid. Redox Signal. 2023, 39, 166–185. [Google Scholar] [PubMed]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.Z.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010, 143, 802–812. [Google Scholar]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–137. [Google Scholar]
- Tao, R.D.; Coleman, M.C.; Pennington, J.D.; Ozden, O.; Park, S.H.; Jiang, H.Y.; Kim, H.S.; Flynn, C.R.; Hill, S.; McDonald, W.H.; et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 2010, 40, 893–904. [Google Scholar]
- Shimazu, T.; Hirschey, M.D.; Hua, L.; Dittenhafer-Reed, K.E.; Schwer, B.; Lombard, D.B.; Li, Y.; Bunkenborg, J.; Alt, F.W.; Denu, J.M.; et al. SIRT3 deacetylates mitochondrial 3-Hydroxy-3-Methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010, 12, 654–661. [Google Scholar]
- Hallows, W.C.; Yu, W.; Smith, B.C.; Devries, M.K.; Ellinger, J.J.; Someya, S.; Shortreed, M.R.; Prolla, T.; Markley, J.L.; Smith, L.M.; et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell 2011, 41, 493. [Google Scholar]
- Jang, S.; Chapa-Dubocq, X.R.; Tyurina, Y.Y.; St Croix, C.M.; Kapralov, A.A.; Tyurin, V.A.; Bayir, H.; Kagan, V.E.; Javadov, S. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 2021, 45, 102021–102032. [Google Scholar] [PubMed]
- Ta, N.; Qu, C.; Wu, H.; Zhang, D.; Sun, T.T.; Li, Y.J.; Wang, J.; Wang, X.H.; Tang, T.S.; Chen, Q.; et al. Mitochondrial outer membrane protein FUNDC2 promotes ferroptosis and contributes to doxorubicin-induced cardiomyopathy. Proc. Natl. Acad. Sci. USA 2022, 119, 2117396119. [Google Scholar]
- Zhang, Z.L.; Guo, M.; Shen, M.; Kong, D.S.; Zhang, F.; Shao, J.J.; Tan, S.Z.; Wang, S.J.; Chen, A.P.; Cao, P.; et al. The BRD7-P53-SLC25A28 axis regulates ferroptosis in hepatic stellate cells. Redox Biol. 2020, 36, 101619. [Google Scholar]
- Zhao, S.M.; Xu, W.; Jiang, W.Q.; Yu, W.; Lin, Y.; Zhang, T.F.; Yao, J.; Zhou, L.; Zeng, Y.X.; Li, H.; et al. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar]
- Xing, Z.C.; Jiang, X.G.; Chen, Y.L.; Wang, T.E.; Li, X.H.; Wei, X.Y.; Fan, Q.J.; Yang, J.; Wu, H.M.; Cheng, J.K.; et al. Glutamine deprivation in glioblastoma stem cells triggers autophagic SIRT3 degradation to epigenetically restrict CD133 expression and stemness. Apoptosis 2024, 29, 1619–1631. [Google Scholar]
- Gong, Y.B.; Tang, N.; Liu, P.R.; Sun, Y.J.; Lu, S.X.; Liu, W.W.; Tan, L.; Song, C.P.; Qiu, X.S.; Liao, Y.; et al. Newcastle disease virus degrades SIRT3 via PINK1-PRKN-dependent mitophagy to reprogram energy metabolism in infected cells. Autophagy 2022, 18, 1503–1521. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Wang, T.; Xing, Z.; Shi, Q.; Gu, J.; Fan, Q.; Wang, H.; Chen, B.; Cheng, J.; Cai, R. Sirtuin 3 Protects Lung Adenocarcinoma from Ferroptosis by Deacetylating and Stabilizing Mitochondrial Glutamate Transporter Solute Carrier Family 25 Member A22. Antioxidants 2025, 14, 403. https://doi.org/10.3390/antiox14040403
Wei X, Wang T, Xing Z, Shi Q, Gu J, Fan Q, Wang H, Chen B, Cheng J, Cai R. Sirtuin 3 Protects Lung Adenocarcinoma from Ferroptosis by Deacetylating and Stabilizing Mitochondrial Glutamate Transporter Solute Carrier Family 25 Member A22. Antioxidants. 2025; 14(4):403. https://doi.org/10.3390/antiox14040403
Chicago/Turabian StyleWei, Xiangyun, Tiange Wang, Zhengcao Xing, Qinyun Shi, Jianmin Gu, Qiuju Fan, Hao Wang, Bin Chen, Jinke Cheng, and Rong Cai. 2025. "Sirtuin 3 Protects Lung Adenocarcinoma from Ferroptosis by Deacetylating and Stabilizing Mitochondrial Glutamate Transporter Solute Carrier Family 25 Member A22" Antioxidants 14, no. 4: 403. https://doi.org/10.3390/antiox14040403
APA StyleWei, X., Wang, T., Xing, Z., Shi, Q., Gu, J., Fan, Q., Wang, H., Chen, B., Cheng, J., & Cai, R. (2025). Sirtuin 3 Protects Lung Adenocarcinoma from Ferroptosis by Deacetylating and Stabilizing Mitochondrial Glutamate Transporter Solute Carrier Family 25 Member A22. Antioxidants, 14(4), 403. https://doi.org/10.3390/antiox14040403






