Seed Halopriming as an Effective Strategy to Enhance Salt Tolerance in Cakile maritima: Activation of Antioxidant and Genetic Responses
Abstract
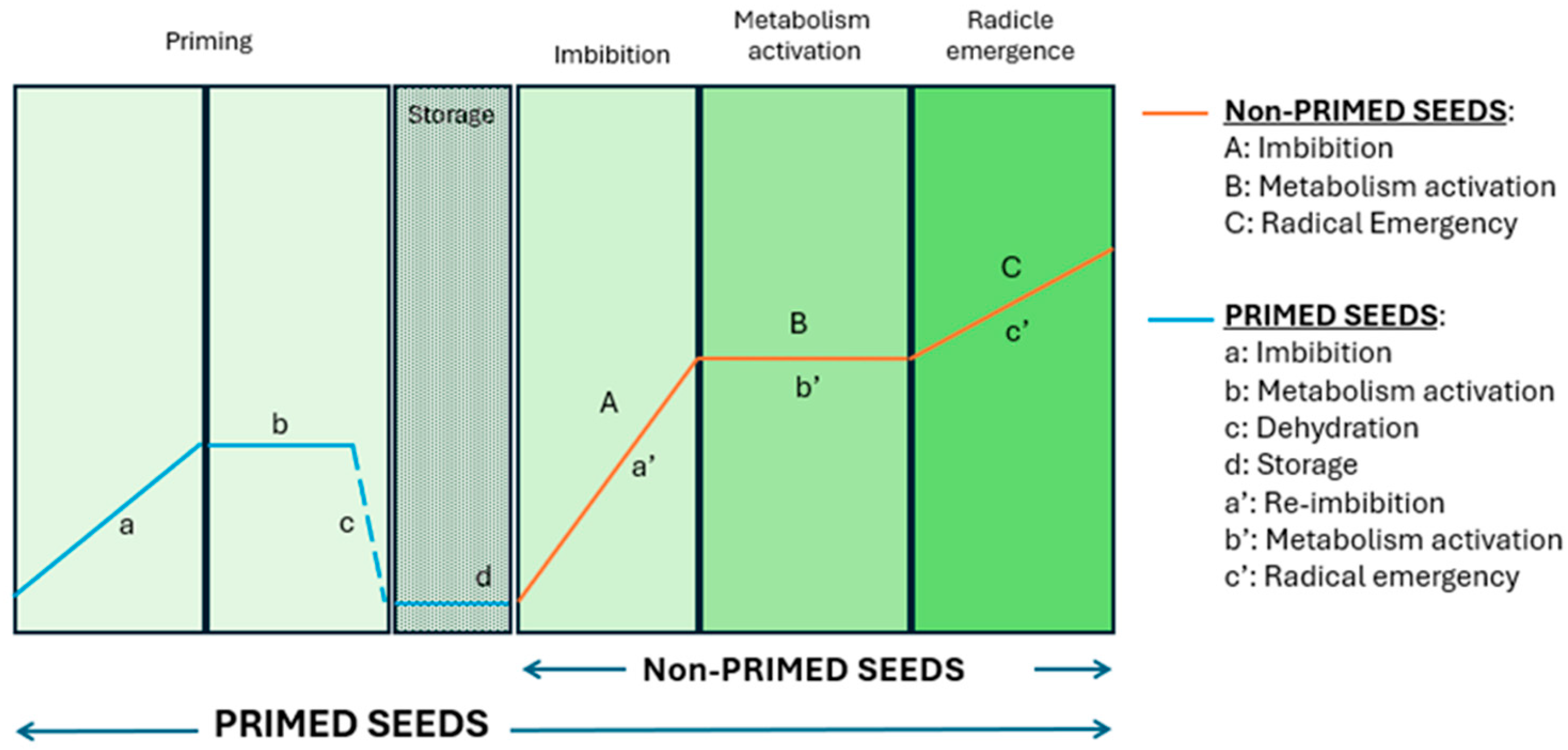
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
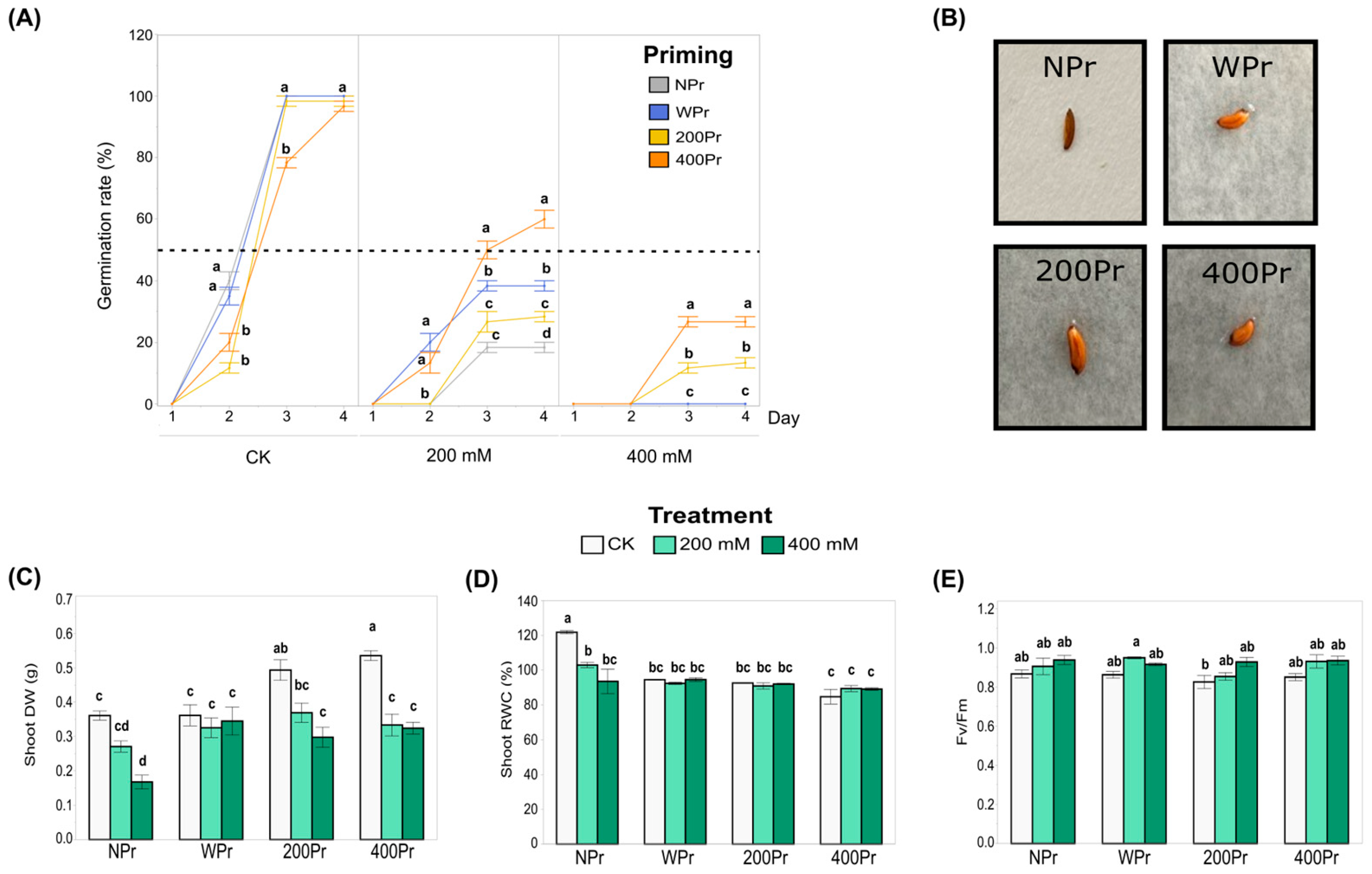
2.3. Biomass Production and Relative Water Content
2.4. Photochemical Efficiency and Stomatal Density
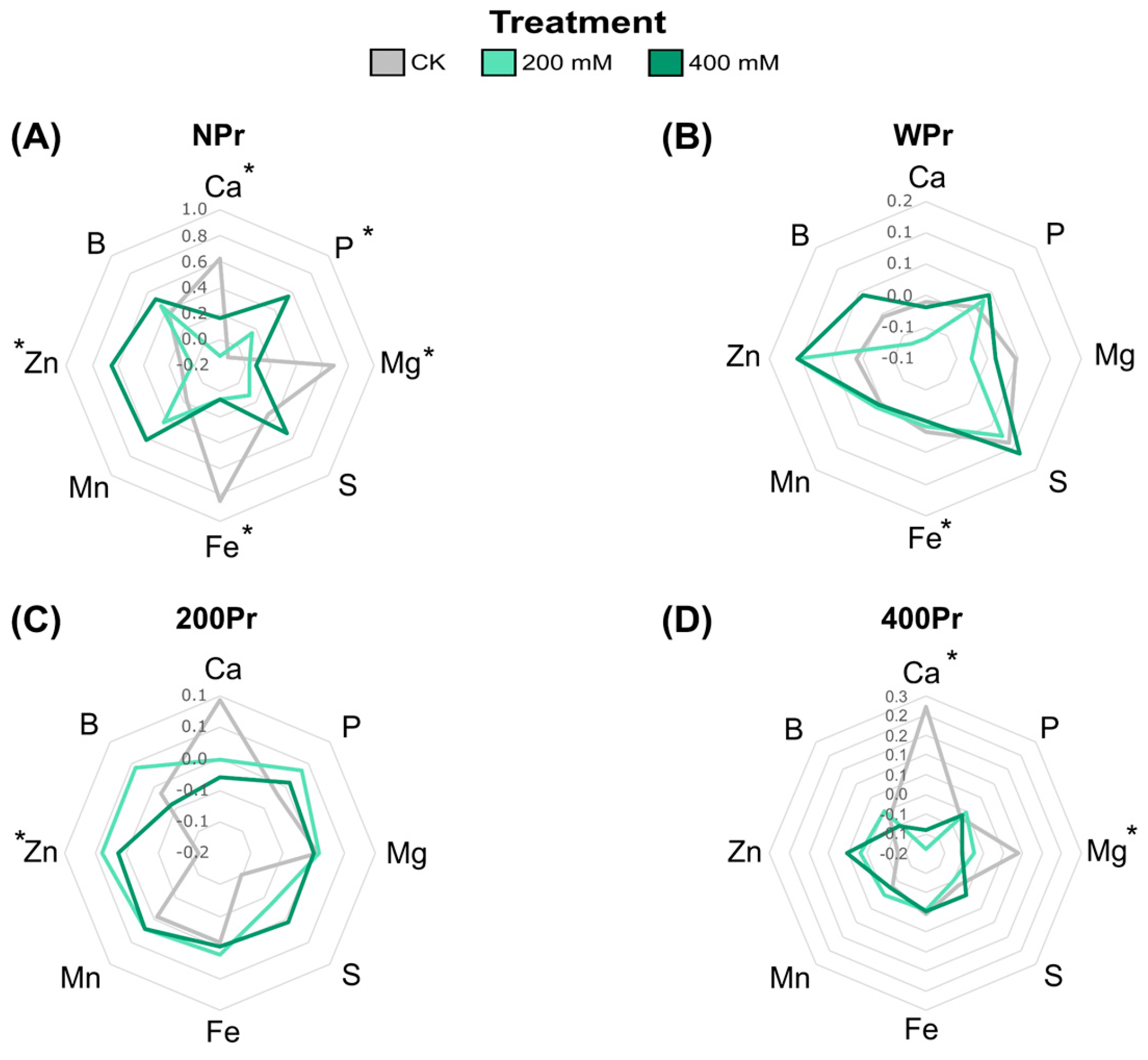
2.5. Determination of Mineral Elements
2.6. Oxidative Stress Markers
2.7. Total Antioxidant Capacity and Antioxidant Enzyme Activity
2.8. RT-qPCR Analysis of Genes Involved in Ion Homeostasis and Antioxidant Defence
2.9. Statistical Analysis
3. Results
3.1. Effect of Seed Priming on Physiological Parameters
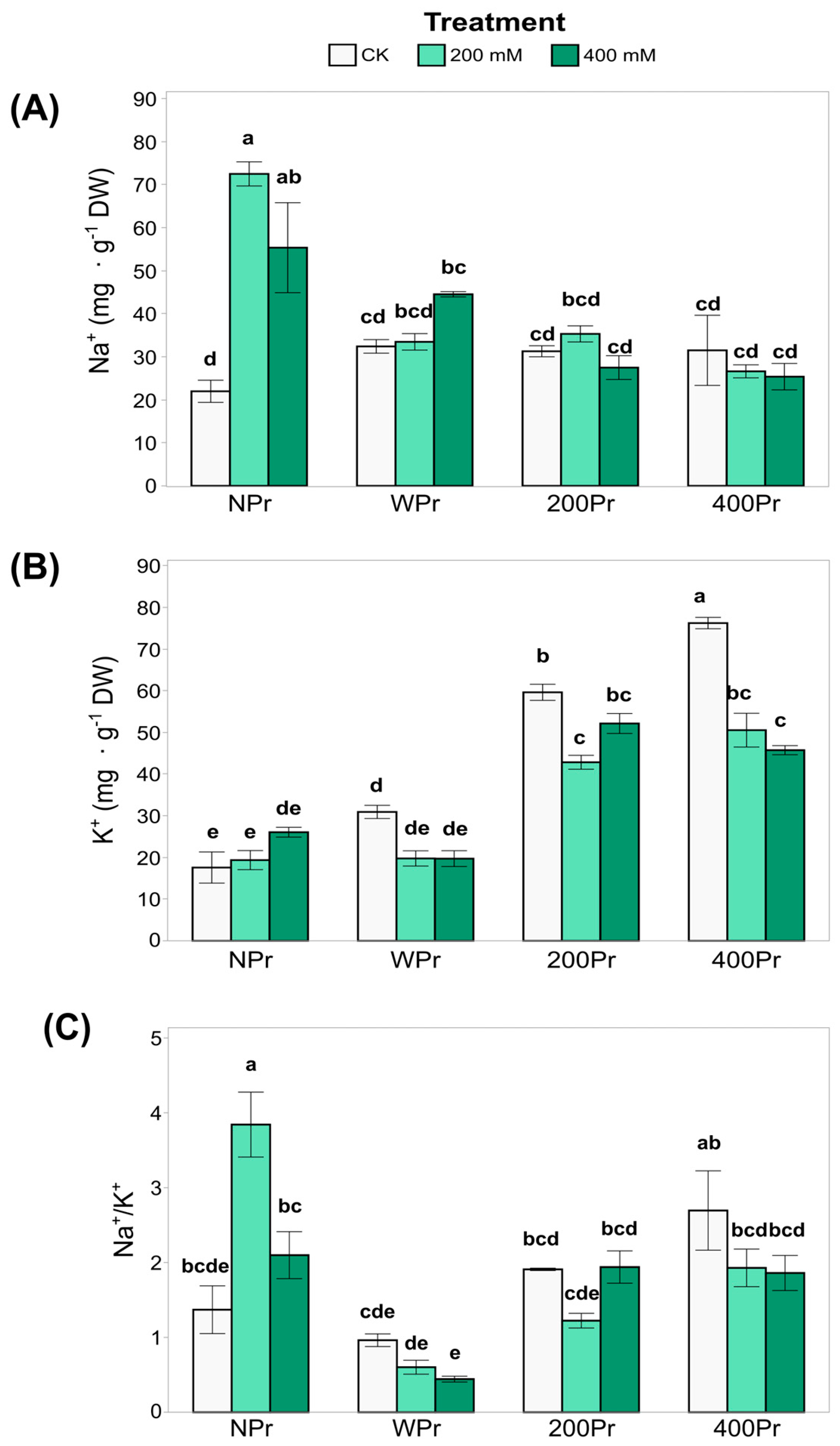
3.2. Effect of Seed Priming on Na+ and K+ Accumulation and Mineral Nutrition
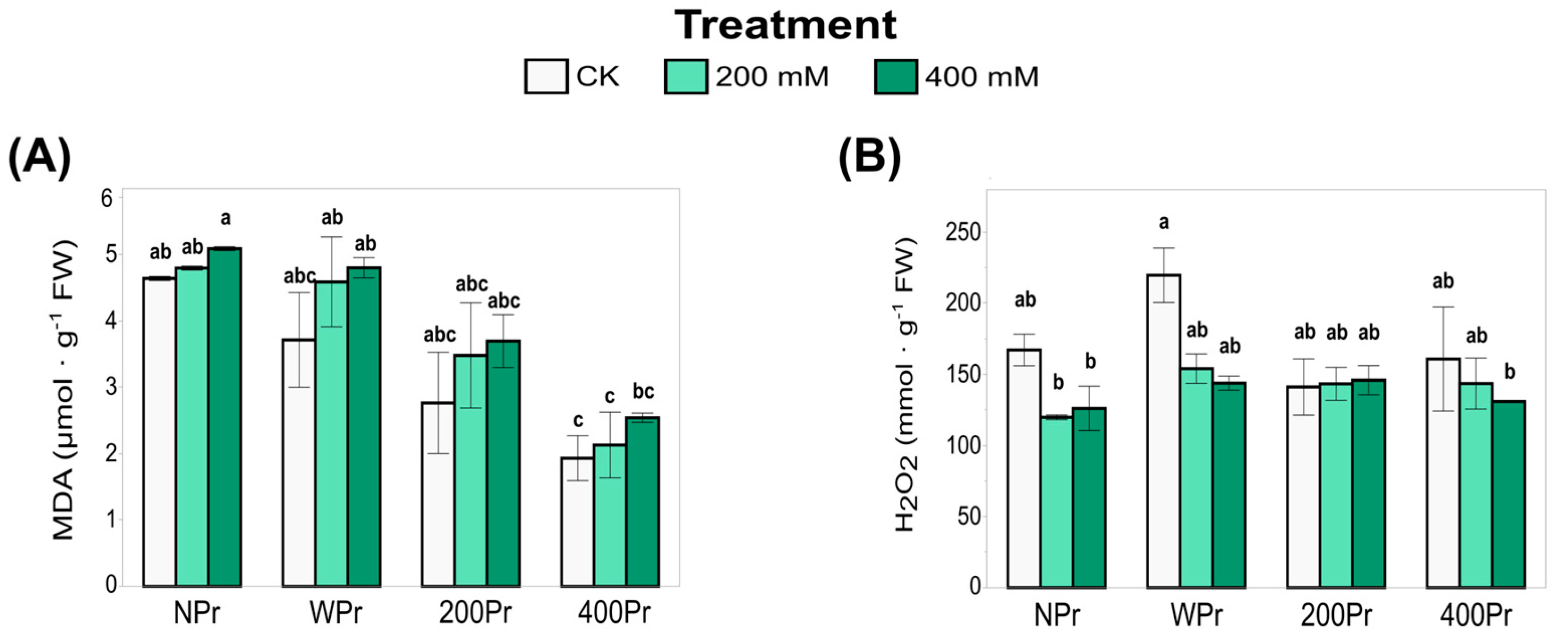
3.3. Oxidative Status
3.4. Total Antioxidant Capacity and Antioxidant Enzyme Activity
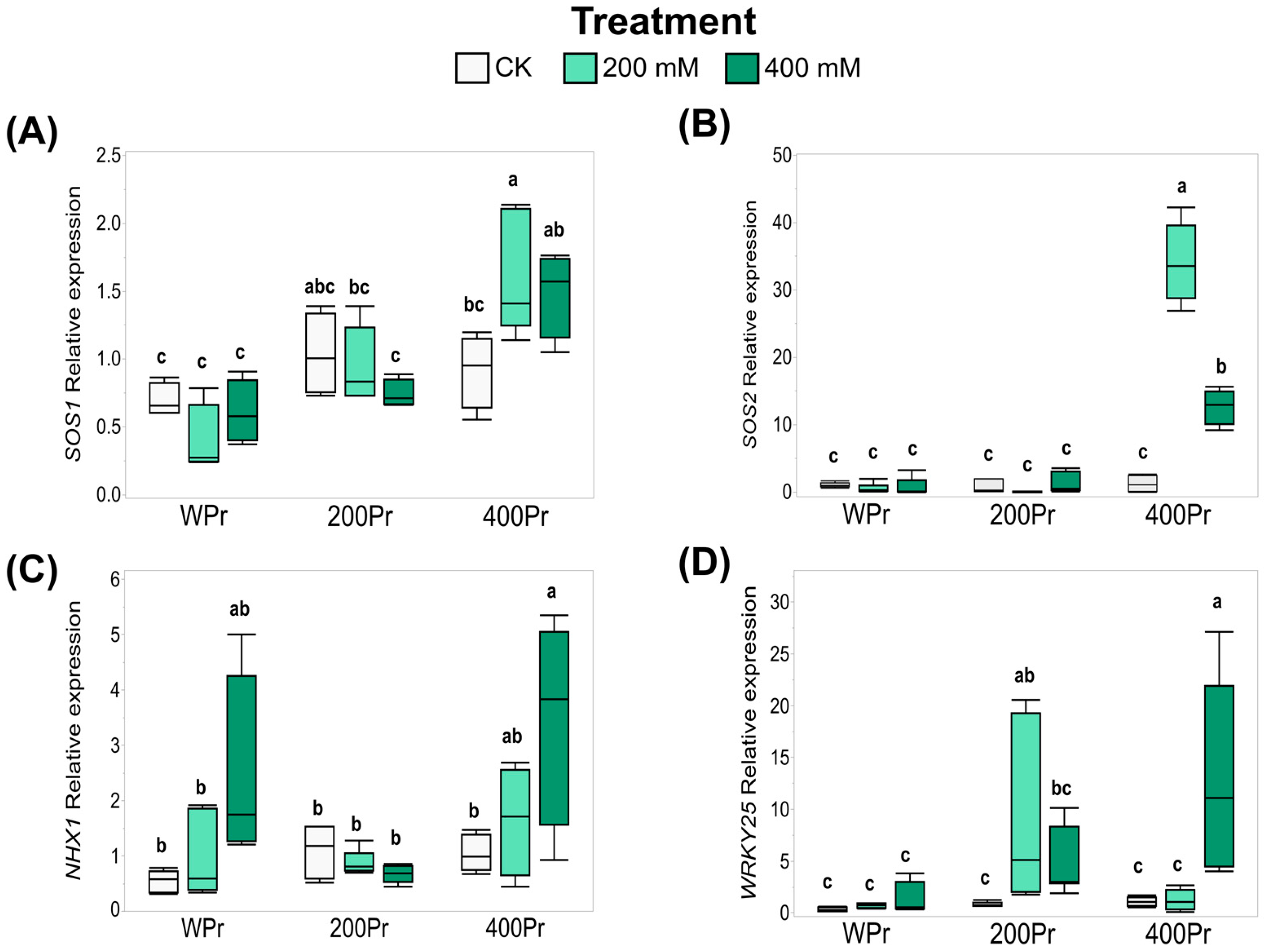
3.5. Effects of Seed Priming on Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| CC | Climate change |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GP | Germination percentage |
| MDA | Malondialdehyde |
| NPr | Non-primed seeds/plants |
| Pr | Primed seeds/plants |
| ROS | Reactive oxygen species |
| RWC | Relative water content |
| SD | Stomatal density |
| SOD | Superoxide dismutase |
| TCA | Trichloroacetic acid |
| TEAC | Total antioxidant capacity |
| WPr | Water-primed seeds/plants |
References
- Singh, V.K.; Singh, R.; Tripathi, S.; Devi, R.S.; Srivastava, P.; Singh, P.; Kumar, A.; Bhadouria, R. Chapter 6—Seed Priming: State of the Art and New Perspectives in the Era of Climate Change. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 143–170. ISBN 978-0-12-818032-7. [Google Scholar]
- Bhadouria, R.; Singh, R.; Singh, V.K.; Borthakur, A.; Ahamad, A.; Kumar, G.; Singh, P. Chapter 1—Agriculture in the Era of Climate Change: Consequences and Effects. In Climate Change and Agricultural Ecosystems; Choudhary, K.K., Kumar, A., Singh, A.K., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–23. ISBN 978-0-12-816483-9. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Chapter 11—Germination Ecology of Plants with Specialized Life Cycles and/or Habitats. In Seeds, 2nd ed.; Baskin, C.C., Baskin, J.M., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 869–1004. ISBN 978-0-12-416677-6. [Google Scholar]
- Omuto, C.T. Mapeo de Suelos Afectados por Salinidad—Manual Técnico; FAO: Rome, Italy, 2021; ISBN 978-92-5-133840-7. [Google Scholar]
- Paul, S.; Dey, S.; Kundu, R. Seed Priming: An Emerging Tool towards Sustainable Agriculture. Plant Growth Regul. 2022, 97, 215–234. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.U.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed Priming in Field Crops: Potential Benefits, Adoption and Challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Parera, C.A.; Cantliffe, D.J. Chapter 4—Presowing Seed Priming. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994; Volume 16, pp. 109–141. ISBN 978-0-470-65056-1. [Google Scholar]
- Shelar, A.; Singh, A.V.; Maharjan, R.S.; Laux, P.; Luch, A.; Gemmati, D.; Tisato, V.; Singh, S.P.; Santilli, M.F.; Shelar, A.; et al. Sustainable Agriculture through Multidisciplinary Seed Nanopriming: Prospects of Opportunities and Challenges. Cells 2021, 10, 2428. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Molecular Processes Induced in Primed Seeds—Increasing the Potential to Stabilize Crop Yields under Drought Conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the Concept and Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 11–41. ISBN 978-981-13-8625-1. [Google Scholar]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 1–10. ISBN 978-981-13-8625-1. [Google Scholar]
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘Primeomics’: Plants Memorize Their Germination under Stress. Biol. Rev. 2021, 96, 1723–1743. [Google Scholar] [CrossRef]
- Levitt, J. Chapter 2—The Nature of Stress Injury and Resistance. In Chilling, Freezing, and High Temperature Stresses, 2nd ed.; Levitt, J., Ed.; Academic Press: Cambridge, MA, USA, 1980; pp. 10–19. ISBN 978-0-12-445501-6. [Google Scholar]
- Souguir, M.; Hassiba, F.; Hannachi, C. Effect of NaCl Priming on Seed Germination of Tunisian Fenugreek (Trigonella foenum-graecum L.) Under Salinity Conditions. J. Stress Physiol. Biochem. 2013, 9, 86–96. [Google Scholar]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium Chloride Toxicity and the Cellular Basis of Salt Tolerance in Halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Ellouzi, H.; Ben Hamed, K.; Cela, J.; Munné-Bosch, S.; Abdelly, C. Early Effects of Salt Stress on the Physiological and Oxidative Status of Cakile maritima (Halophyte) and Arabidopsis thaliana (Glycophyte). Physiol. Plant. 2011, 142, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Popova, O.V.; Yang, O.; Dietz, K.-J.; Golldack, D. Differential Transcript Regulation in Arabidopsis thaliana and the Halotolerant Lobularia maritima Indicates Genes with Potential Function in Plant Salt Adaptation. Gene 2008, 423, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Mircea, D.M.; Ruiz-González, M.X.; Brocal-Rubio, P.; Boscaiu, M.; Vicente, O. Cakile maritima: A Halophyte Model to Study Salt Tolerance Mechanisms and Potential Useful Crop for Sustainable Saline Agriculture in the Context of Climate Change. Plants 2024, 13, 2880. [Google Scholar] [CrossRef]
- Houmani, H.; Rodríguez-Ruiz, M.; Palma, J.M.; Abdelly, C.; Corpas, F.J. Modulation of Superoxide Dismutase (SOD) Isozymes by Organ Development and High Long-Term Salinity in the Halophyte Cakile maritima. Protoplasma 2016, 253, 885–894. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Schwab, A.P. A New Soil Test for Simultaneous Extraction of Macro- and Micro-nutrients in Alkaline Soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A Comparative Evaluation of Thiobarbituric Acid Methods for the Determination of Malondialdehyde in Biological Materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Beauchamp, C.O.; Fridovich, I. Isozymes of Superoxide Dismutase from Wheat Germ. Biochim. Biophys. Acta BBA-Protein Struct. 1973, 317, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase In Vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Phytozome. Available online: https://phytozome-next.jgi.doe.gov/ (accessed on 6 March 2025).
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Ma, J.F.; Rengel, Z.; Zhao, F. Chapter 8—Beneficial Elements. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 249–269. ISBN 978-0-12-384905-2. [Google Scholar]
- Abbas, G.; Saqib, M.; Akhtar, J.; ul Haq, M.A. Interactive Effects of Salinity and Iron Deficiency on Different Rice Genotypes. J. Plant Nutr. Soil Sci. 2015, 178, 306–311. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A Role for Zinc in Plant Defense Against Pathogens and Herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Debez, A.; Belghith, I.; Pich, A.; Taamalli, W.; Abdelly, C.; Braun, H.-P. High Salinity Impacts Germination of the Halophyte but Primes Seeds for Rapid Germination upon Stress Release. Physiol. Plant. 2018, 164, 134–144. [Google Scholar] [CrossRef]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A. Seed Priming and Transgenerational Drought Memory Improves Tolerance against Salt Stress in Bread Wheat. Plant Physiol. Biochem. 2017, 118, 362–369. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential Response of Wheat Genotypes to Long Term Salinity Stress in Relation to Oxidative Stress, Antioxidant Activity and Osmolyte Concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Percival, G.C.; Fraser, G.A.; Oxenham, G. Foliar Salt Tolerance of Acer Genotypes Using Chlorophyll Fluorescence. Arboric. Urban For. AUF 2003, 29, 61–65. [Google Scholar] [CrossRef]
- Shabala, S. Learning from Halophytes: Physiological Basis and Strategies to Improve Abiotic Stress Tolerance in Crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Omamt, E.N.; Hammes, P.S.; Robbertse, P.J. Differences in Salinity Tolerance for Growth and Water-use Efficiency in Some Amaranth (Amaranthus spp.) Genotypes. N. Z. J. Crop Hortic. Sci. 2006, 34, 11–22. [Google Scholar] [CrossRef]
- Orsini, F.; Accorsi, M.; Gianquinto, G.; Dinelli, G.; Antognoni, F.; Carrasco, K.B.R.; Martinez, E.A.; Alnayef, M.; Marotti, I.; Bosi, S.; et al. Beyond the Ionic and Osmotic Response to Salinity in Chenopodium quinoa: Functional Elements of Successful Halophytism. Funct. Plant Biol. 2011, 38, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Habashi, F. Zinc, Physical and Chemical Properties. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 2537–2538. ISBN 978-1-4614-1533-6. [Google Scholar]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc Absorption in Plants: Uptake, Transport, Translocation and Accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Aktaş, H.; Abak, K.; Öztürk, L.; Çakmak, İ. The Effect of Zinc on Growth and Shoot Concentrations of Sodium and Potassium in Pepper Plants under Salinity Stress. Turk. J. Agric. For. 2006, 30, 407–412. [Google Scholar]
- Al-Zahrani, H.S.; Alharby, H.F.; Hakeem, K.R.; Rehman, R.U. Exogenous Application of Zinc to Mitigate the Salt Stress in Vigna radiata (L.) Wilczek—Evaluation of Physiological and Biochemical Processes. Plants 2021, 10, 1005. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Keeping Active Oxygen Under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and Activity of Superoxide Dismutase, Peroxidase and Glutathione Reductase in Cotton under Salt Stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Assaha, D.; Liu, L.; Mekawy, A.; Ueda, A.; Nagaoka, T.; Saneoka, H. Effect of Salt Stress on Na Accumulation, Antioxidant Enzyme Activities and Activity of Cell Wall Peroxidase of Huckleberry (Solanum scabrum) and Eggplant (Solanum melongena). Int. J. Agric. Biol. 2015, 17, 1149–1156. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring Control: The Evolution of ROS-Induced Oxidative Stress and Redox Signaling Pathways in Plant Stress Responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Al-Shehbaz, I.A.; Beilstein, M.A.; Kellogg, E.A. Systematics and Phylogeny of the Brassicaceae (Cruciferae): An Overview. Plant Syst. Evol. 2006, 259, 89–120. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami-Rad, S.; Zeinalzade, N.; Atazadeh, E.; Akhani, H.; Poschenrieder, C. Differential Functional Traits Underlying the Contrasting Salt Tolerance in Lepidium Species. Plant Soil 2020, 448, 315–334. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS Homeostasis in Halophytes in the Context of Salinity Stress Tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, J.D.; Zhang, J.; Zeng, F.; Živanović, B.D.; Shabala, L.; Zhou, M.; Shabala, S. Linking Oxidative and Salinity Stress Tolerance in Barley: Can Root Antioxidant Enzyme Activity Be Used as a Measure of Stress Tolerance? Plant Soil 2013, 365, 141–155. [Google Scholar] [CrossRef]
- Houmani, H.; Debez, A.; de Freitas-Silva, L.; Abdelly, C.; Palma, J.M.; Corpas, F.J. Potassium (K+) Starvation-Induced Oxidative Stress Triggers a General Boost of Antioxidant and NADPH-Generating Systems in the Halophyte Cakile maritima. Antioxidants 2022, 11, 401. [Google Scholar] [CrossRef]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Z.-Z.; Zhou, X.-F.; Yin, H.-B.; Li, X.; Xin, X.-F.; Hong, X.-H.; Zhu, J.-K.; Gong, Z. Overexpression of SOS (Salt Overly Sensitive) Genes Increases Salt Tolerance in Transgenic Arabidopsis. Mol. Plant 2009, 2, 22–31. [Google Scholar] [CrossRef]
- Shabala, S.; Mackay, A. Chapter 5—Ion Transport in Halophytes. In Advances in Botanical Research; Turkan, I., Ed.; Plant Responses to Drought and Salinity Stress; Academic Press: Cambridge, MA, USA, 2011; Volume 57, pp. 151–199. [Google Scholar]
- Maathuis, F.J.M. Sodium in Plants: Perception, Signalling, and Regulation of Sodium Fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef]
- Zhu, J.-K. Genetic Analysis of Plant Salt Tolerance Using Arabidopsis. Plant Physiol. 2000, 124, 941–948. [Google Scholar] [CrossRef]
- Qiu, Q.-S.; Guo, Y.; Quintero, F.J.; Pardo, J.M.; Schumaker, K.S.; Zhu, J.-K. Regulation of Vacuolar Na+/H+ Exchange in Arabidopsis thaliana by the Salt-Overly-Sensitive (SOS) Pathway*. J. Biol. Chem. 2004, 279, 207–215. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling Salt Stress Signaling in Plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luo, D.; Rehman, M.; Li, X.; Wang, C.; Cao, S.; Xu, G.; Wang, M.; Chen, C.; Nie, J.; et al. Seed Priming Using Different Agents Can Alleviate Salt Stress in Kenaf (Hibiscus cannabinus L.) by Activating Antioxidant System and Related Genes Expression. Physiol. Mol. Biol. Plants 2024, 30, 1741–1757. [Google Scholar] [CrossRef] [PubMed]
- Patade, V.Y.; Bhargava, S.; Suprasanna, P. Halopriming Mediated Salt and Iso-Osmotic PEG Stress Tolerance and, Gene Expression Profiling in Sugarcane (Saccharum officinarum L.). Mol. Biol. Rep. 2012, 39, 9563–9572. [Google Scholar] [CrossRef]
- Doll, J.; Muth, M.; Riester, L.; Nebel, S.; Bresson, J.; Lee, H.-C.; Zentgraf, U. Arabidopsis thaliana WRKY25 Transcription Factor Mediates Oxidative Stress Tolerance and Regulates Senescence in a Redox-Dependent Manner. Front. Plant Sci. 2020, 10, 1734. [Google Scholar] [CrossRef]
- Jiang, Y.; Deyholos, M.K. Functional Characterization of Arabidopsis NaCl-Inducible WRKY25 and WRKY33 Transcription Factors in Abiotic Stresses. Plant Mol. Biol. 2009, 69, 91–105. [Google Scholar] [CrossRef]
- Hussain, M.; Waqas-Ul-Haq, M.; Farooq, S.; Jabran, K.; Farroq, M. The Impact of Seed Priming and Row Spacing on the Productivity of Different Cultivars of Irrigated Wheat Under Early Season Drought—Corrigendum. Exp. Agric. 2016, 52, 491. [Google Scholar] [CrossRef]
- The State of Nutrition: Progress Towards Global Nutrition Targets. Available online: https://www.fao.org/3/cc0639en/online/sofi-2022/global-nutrition-targets-trends.html (accessed on 5 February 2025).
- Nadeem, F.; Azhar, M.; Anwar-ul-Haq, M.; Sabir, M.; Samreen, T.; Tufail, A.; Awan, H.U.M.; Juan, W. Comparative Response of Two Rice (Oryza sativa L.) Cultivars to Applied Zinc and Manganese for Mitigation of Salt Stress. J. Soil Sci. Plant Nutr. 2020, 20, 2059–2072. [Google Scholar] [CrossRef]

| Name | Gene Accession Number | Forward Primer (5′->3′) | Reverse Primer (5′->3′) |
|---|---|---|---|
| NHX1 | Camar.0770s0019 | GCT ACT GGT CTG ATA AGT GC | GCC AGG TGT AAT GGG ACA TC |
| SOS1 | Camar.0267s0003 | TCT GAA CGA GCA AGG CAA CT | GCT TTC TGA TTT CGC TGC GT |
| SOS2 | Camar.3181s0008 | ACG TTA GAA AGG CTG CTG GT | TGA CCT GCC TGA ATC AAA ACG |
| WRKY25 | Camar.2001s0011 | TCG CCT TCT CCG ATT TGC TT | CGG TTG CGT TTG TAG ATG CC |
| UBQ10 | Camar.1635s0021 | AAC CTC TTC TCC TTC ACA AC | CGT TGT CGA TGG TGT CAG AG |
| Priming | Treatment (NaCl) | Basal Leaves | Mid-Height Leaves |
|---|---|---|---|
| WPr | CK | 100.0% | 100.0% |
| WPr | 200 mM | 85.2% | 63.0% |
| WPr | 400 mM | 110.5% | 66.6% |
| 200Pr | CK | 118.7% | 56.3% |
| 200Pr | 200 mM | 115.3% | 54.0% |
| 200Pr | 400 mM | 75.2% | 55.5% |
| 400Pr | CK | 83.3% | 51.7% |
| 400Pr | 200 mM | 84.7% | 65.4% |
| 400Pr | 400 mM | 131.0% | 82.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolrà, R.; González-Cobo, C.; Corrales, I.; Padilla, R.; Llugany, M. Seed Halopriming as an Effective Strategy to Enhance Salt Tolerance in Cakile maritima: Activation of Antioxidant and Genetic Responses. Antioxidants 2025, 14, 353. https://doi.org/10.3390/antiox14030353
Tolrà R, González-Cobo C, Corrales I, Padilla R, Llugany M. Seed Halopriming as an Effective Strategy to Enhance Salt Tolerance in Cakile maritima: Activation of Antioxidant and Genetic Responses. Antioxidants. 2025; 14(3):353. https://doi.org/10.3390/antiox14030353
Chicago/Turabian StyleTolrà, Roser, Carlos González-Cobo, Isabel Corrales, Rosa Padilla, and Mercè Llugany. 2025. "Seed Halopriming as an Effective Strategy to Enhance Salt Tolerance in Cakile maritima: Activation of Antioxidant and Genetic Responses" Antioxidants 14, no. 3: 353. https://doi.org/10.3390/antiox14030353
APA StyleTolrà, R., González-Cobo, C., Corrales, I., Padilla, R., & Llugany, M. (2025). Seed Halopriming as an Effective Strategy to Enhance Salt Tolerance in Cakile maritima: Activation of Antioxidant and Genetic Responses. Antioxidants, 14(3), 353. https://doi.org/10.3390/antiox14030353










