Selenium Yeast Attenuated Lipopolysaccharide-Induced Inflammation in Porcine Mammary Epithelial Cells by Modulating MAPK and NF-κB Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of SeY
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Real-Time PCR
2.5. Measurement of Inflammatory Factor Levels
2.6. Antioxidant Enzymes Assay
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Viability of PMECs
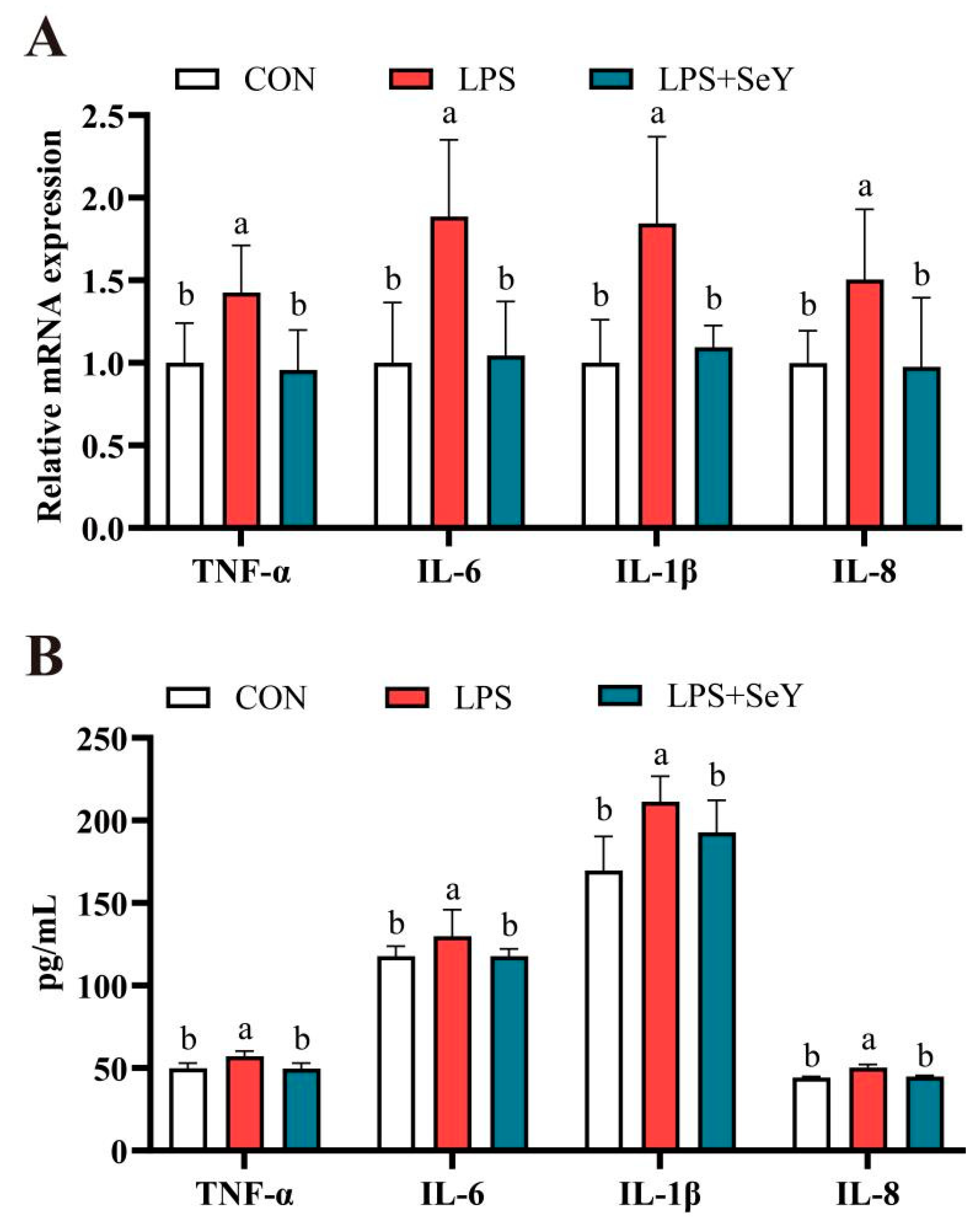
3.2. Inflammatory Factors
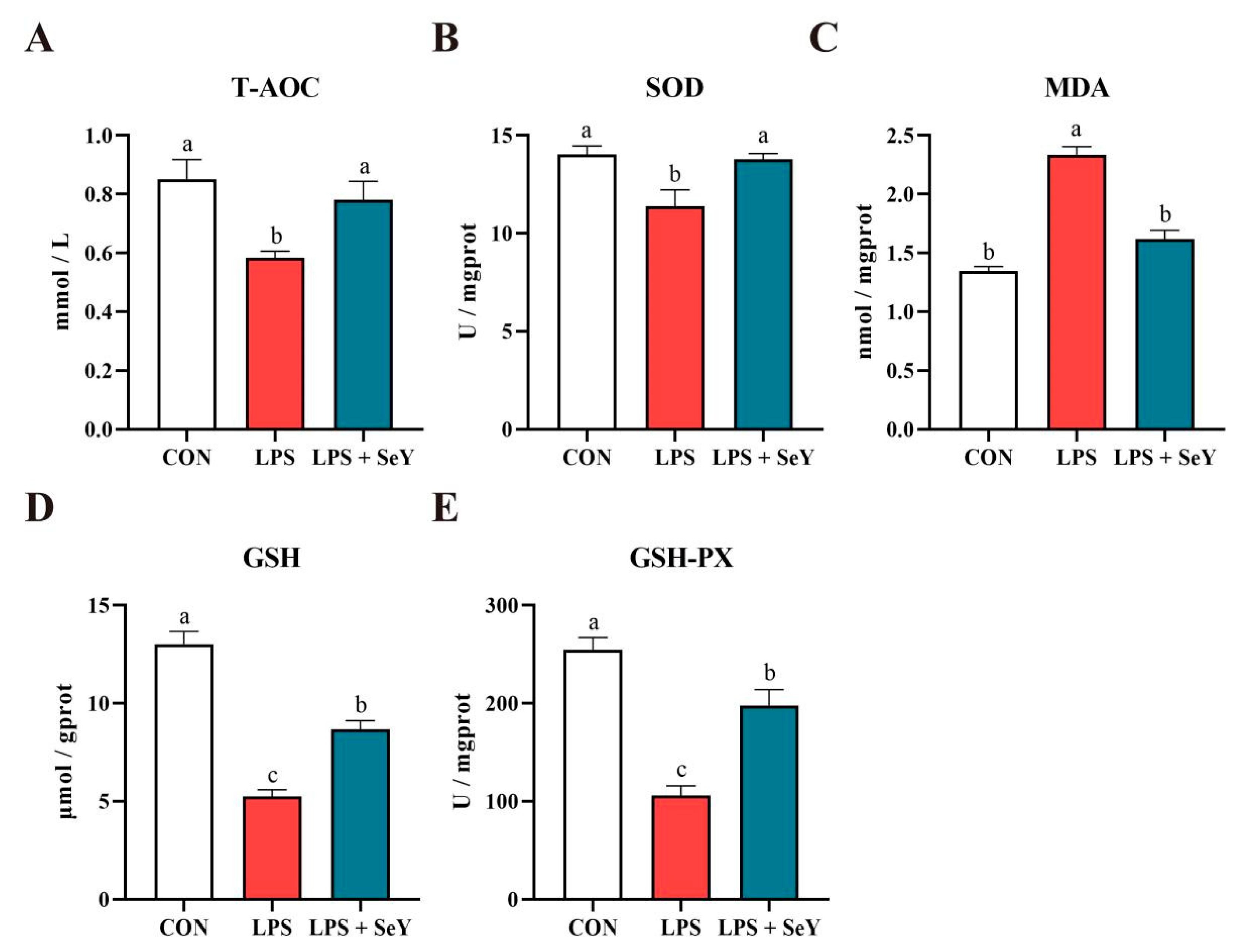
3.3. Antioxidant Levels
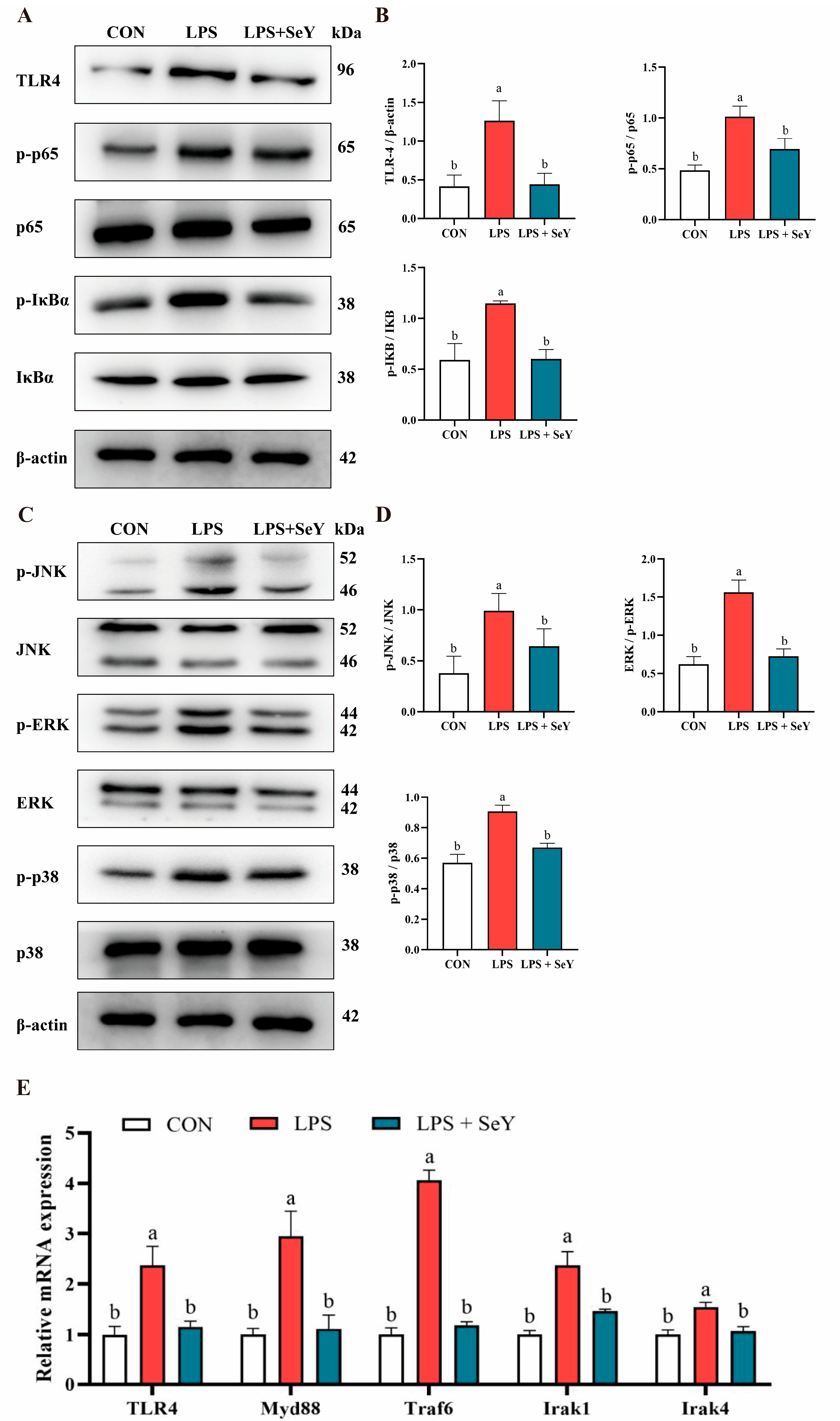
3.4. NF-κB and MAPK Signaling Pathways
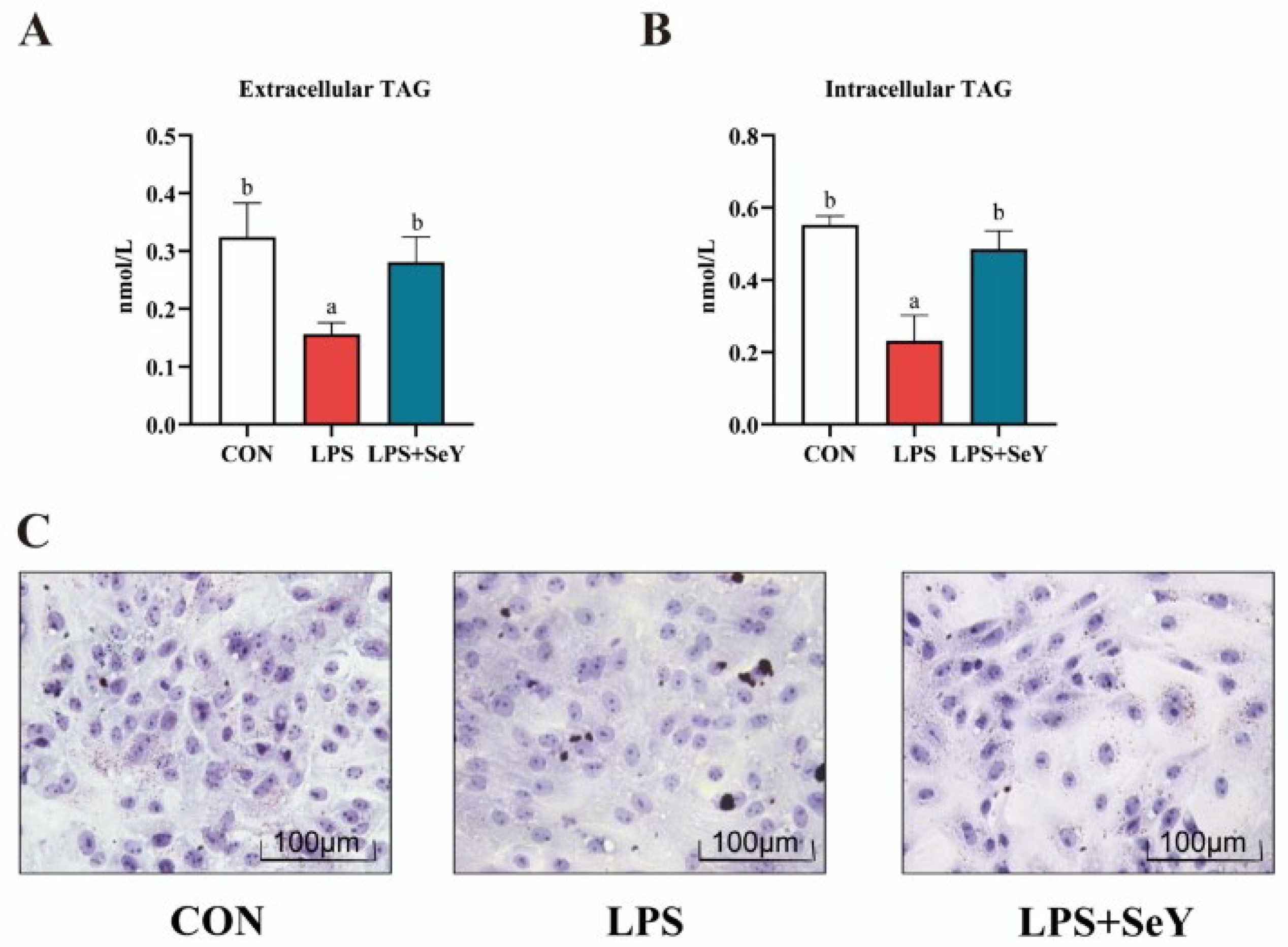
3.5. Intracellular and Extracellular Triglyceride Levels in PMECs
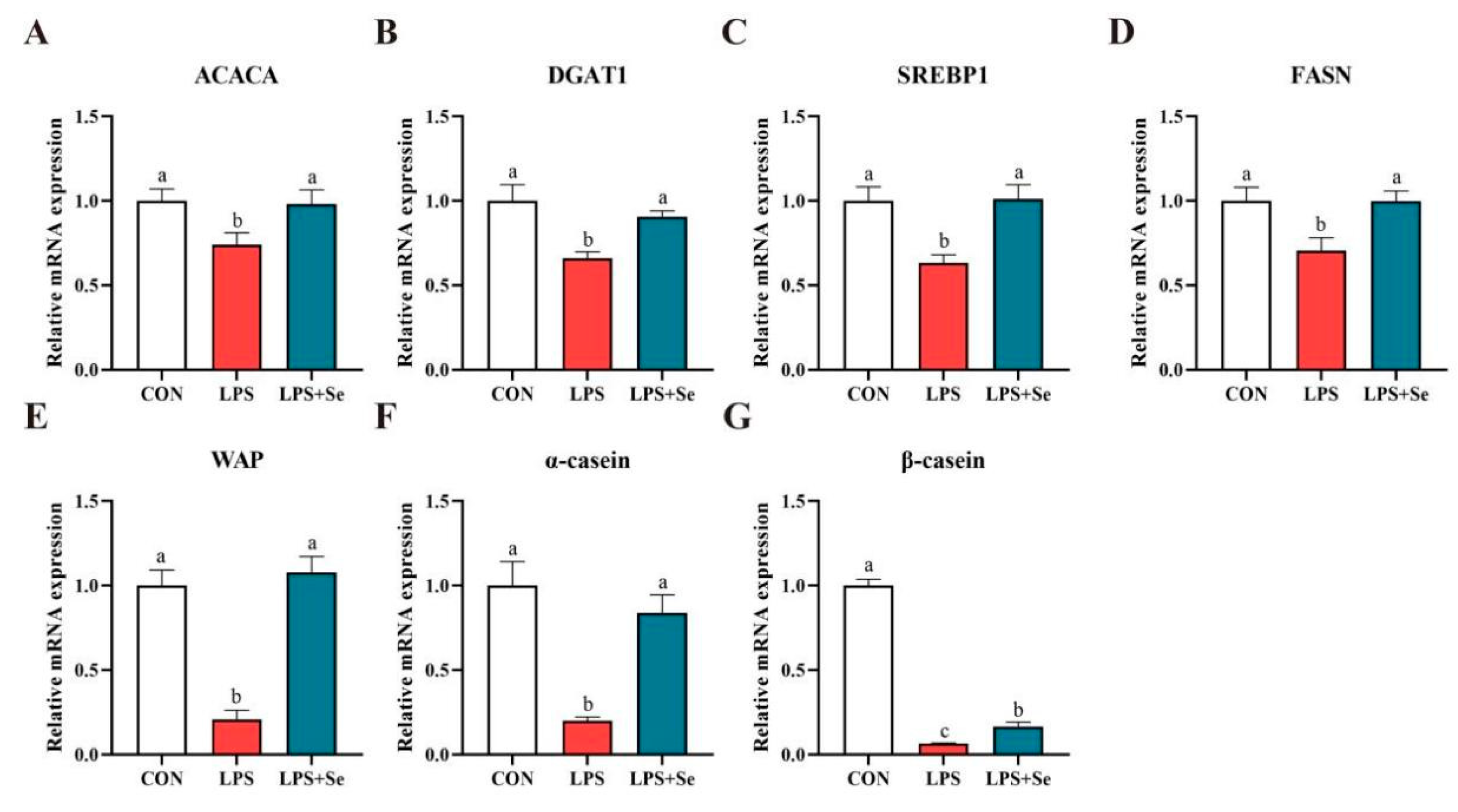
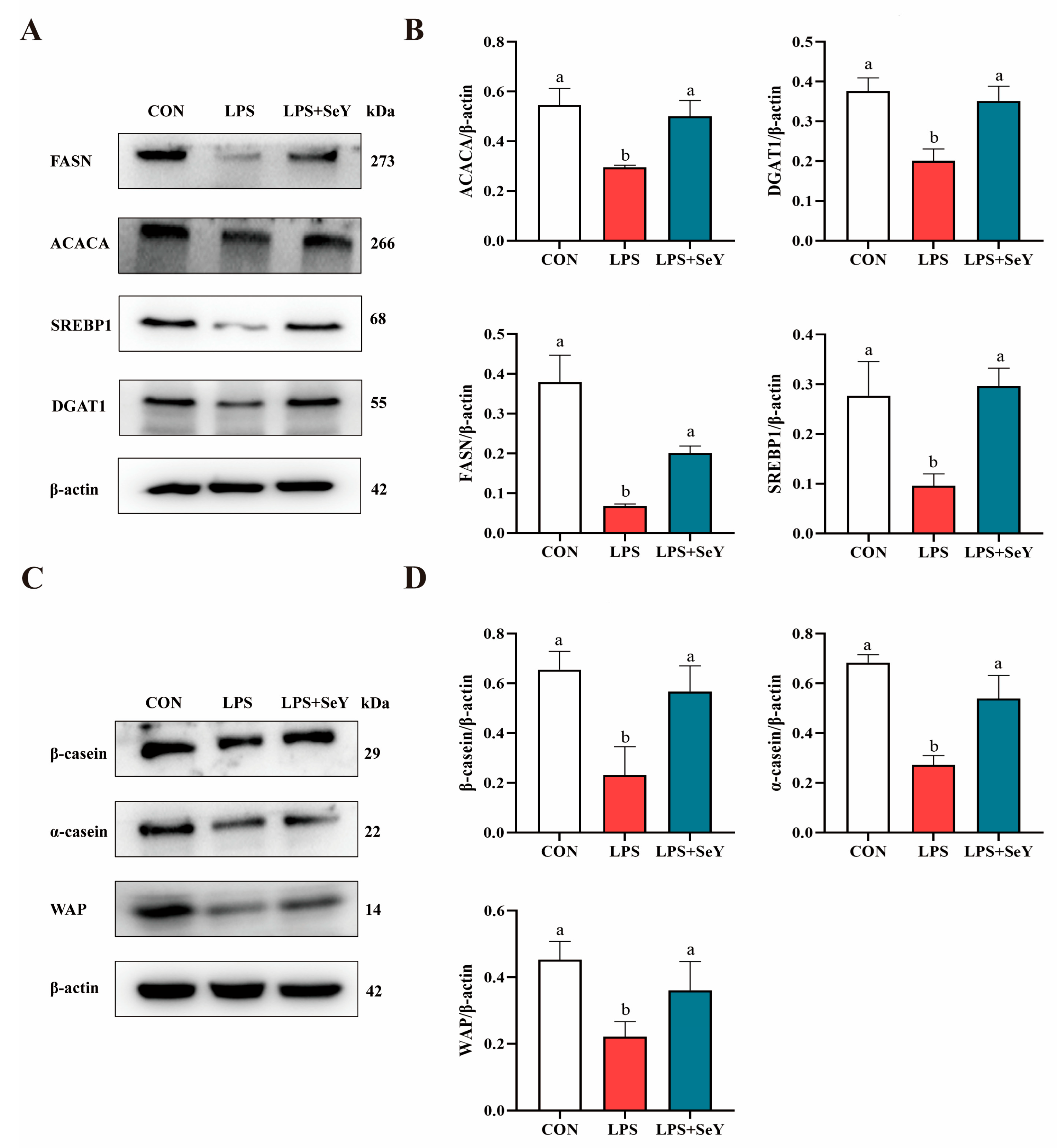
3.6. mRNA and Protein Expression Related to Milk Fat and Protein Synthesis
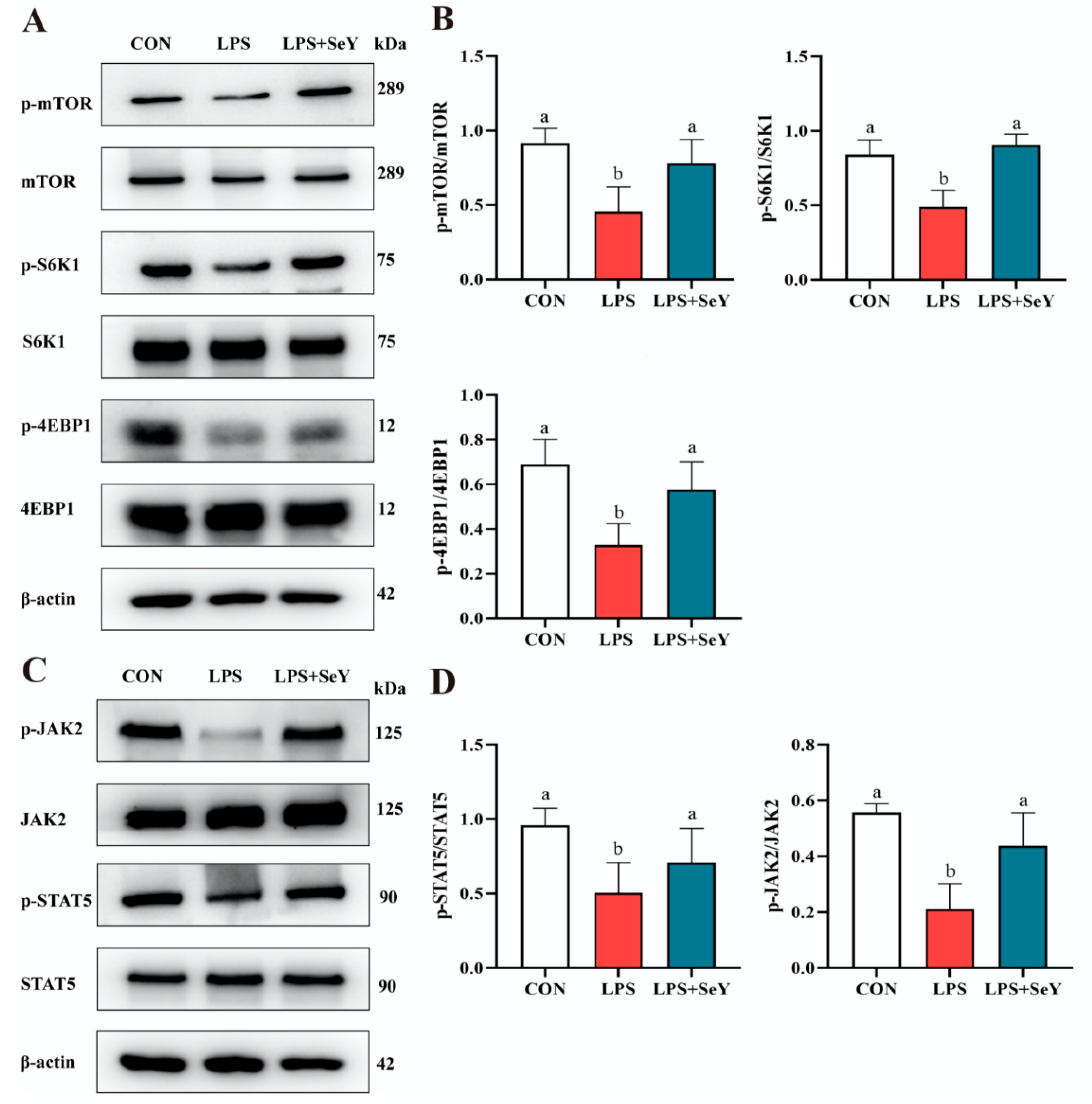
3.7. Pathways Related to Milk Fat and Protein Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.T.; Dong, G.Z.; Ao, C.; Zhang, D.G.; Erdene, K.; Zhang, F.Q.; Wen, J.; Zhang, T.L. Effects of continuous low dose infusion of lipopolysaccharide on inflammatory responses, milk production and milk quality in dairy cows. J. Anim. Physiol. Anim. Nutr. 2018, 102, e262-e269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chang, G.; Xu, T.; Xu, L.; Guo, J.; Jin, D.; Shen, X. Lipopolysaccharide derived from the digestive tract activates inflammatory gene expression and inhibits casein synthesis in the mammary glands of lactating dairy cows. Oncotarget 2016, 7, 9652–9665. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.I.; Lin, Y.E.; Bian, Y.; Gao, X.; Qu, B.O.; Li, Q. 14-3-3gamma regulates cell viability and milk fat synthesis in lipopolysaccharide-induced dairy cow mammary epithelial cells. Exp. Ther. Med. 2016, 11, 1279–1287. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Wenz, J.R.; Barrington, G.M.; Garry, F.B.; Ellis, R.P.; Magnuson, R.J. Escherichia coli isolates’ serotypes, genotypes, and virulence genes and clinical coliform mastitis severity. J. Dairy Sci. 2006, 89, 3408–3412. [Google Scholar] [CrossRef]
- Halasa, T.; Huijps, K.; Osteras, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, F.; Zhang, Y.; Lv, Y.; Heng, J.; Min, T.; Li, L.; Guan, W. Recent progress of porcine milk components and mammary gland function. J. Anim. Sci. Biotechnol. 2018, 9, 77. [Google Scholar] [CrossRef]
- Wu, Z.; Heng, J.; Tian, M.; Song, H.; Chen, F.; Guan, W.; Zhang, S. Amino acid transportation, sensing and signal transduction in the mammary gland: Key molecular signalling pathways in the regulation of milk synthesis. Nutr. Res. Rev. 2020, 33, 287–297. [Google Scholar] [CrossRef]
- Aitken, S.L.; Corl, C.M.; Sordillo, L.M. Immunopathology of mastitis: Insights into disease recognition and resolution. J. Mammary Gland Biol. Neoplasia 2011, 16, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, N.; Soucy, G.; Rivest, S. Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J. Neurochem. 2001, 79, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. Endotoxins: Lipopolysaccharides of gram-negative bacteria. In Endotoxins: Structure, Function and Recognition; Subcellular Biochemistry Series; Springer Dordrecht: Dordrecht, The Netherlands, 2010; Volume 53, pp. 3–25. [Google Scholar] [CrossRef]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [PubMed]
- Bandow, K.; Kusuyama, J.; Shamoto, M.; Kakimoto, K.; Ohnishi, T.; Matsuguchi, T. LPS-induced chemokine expression in both MyD88-dependent and -independent manners is regulated by Cot/Tpl2-ERK axis in macrophages. FEBS Lett. 2012, 586, 1540–1546. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Milan Manani, S.; Virzi, G.M.; Giuliani, A.; Baretta, M.; Corradi, V.; De Cal, M.; Biasi, C.; Crepaldi, C.; Ronco, C. Lipopolysaccharide Evaluation in Peritoneal Dialysis Patients with Peritonitis. Blood Purif. 2020, 49, 434–439. [Google Scholar] [CrossRef]
- Hung, Y.-L.; Suzuki, K. The pattern recognition receptors and lipopolysaccharides (LPS)-induced systemic inflammation. Int. J. Res. Stud. Med. Health Sci. 2017, 2, 1–7. [Google Scholar]
- Burk, R.F. Selenium, an antioxidant nutrient. Nutr. Clin. Care 2002, 5, 75–79. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, selenoproteins, and immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Lv, C.H.; Wang, T.; Regmi, N.; Chen, X.; Huang, K.; Liao, S.F. Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.; Fisinin, V. Selenium in sow nutrition. Anim. Feed Sci. Technol. 2016, 211, 18–30. [Google Scholar] [CrossRef]
- Chen, J.; Han, J.; Guan, W.; Chen, F.; Wang, C.; Zhang, Y.; Lv, Y.; Lin, G. Selenium and vitamin E in sow diets: I. Effect on antioxidant status and reproductive performance in multiparous sows. Anim. Feed Sci. Technol. 2016, 221, 111–123. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, F.; Guan, W.; Song, H.; Tian, M.; Cheng, L.; Shi, K.; Song, J.; Chen, F.; Zhang, S. Increasing selenium supply for heat-stressed or actively cooled sows improves piglet preweaning survival, colostrum and milk composition, as well as maternal selenium, antioxidant status and immunoglobulin transfer. J. Trace Elem. Med. Biol. 2019, 52, 89–99. [Google Scholar] [CrossRef]
- Xiong, L.; Lin, T.; Yue, X.; Zhang, S.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal Selenium-Enriched Yeast Supplementation in Sows Enhances Offspring Growth and Antioxidant Status through the Nrf2/Keap1 Pathway. Antioxidants 2023, 12, 2064. [Google Scholar] [CrossRef]
- Chen, J.; Tian, M.; Guan, W.; Wen, T.; Yang, F.; Chen, F.; Zhang, S.; Song, J.; Ren, C.; Zhang, Y.; et al. Increasing selenium supplementation to a moderately-reduced energy and protein diet improves antioxidant status and meat quality without affecting growth performance in finishing pigs. J. Trace Elem. Med. Biol. 2019, 56, 38–45. [Google Scholar] [CrossRef]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and selenoproteins: An overview on different biological systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef]
- Calamari, L.; Petrera, F.; Bertin, G. Effects of either sodium selenite or Se yeast (Sc CNCM I-3060) supplementation on selenium status and milk characteristics in dairy cows. Livest. Sci. 2010, 128, 154–165. [Google Scholar] [CrossRef]
- Juniper, D.T.; Phipps, R.H.; Givens, D.I.; Jones, A.K.; Green, C.; Bertin, G. Tolerance of ruminant animals to high dose in-feed administration of a selenium-enriched yeast. J. Anim. Sci. 2008, 86, 197–204. [Google Scholar] [CrossRef]
- Mahan, D. Effect of organic and inorganic selenium sources and levels on sow colostrum and milk selenium content. J. Anim. Sci. 2000, 78, 100–105. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Heng, J.; Song, H.; Tian, M.; Chen, F.; Guan, W. Combined yeast culture and organic selenium supplementation during late gestation and lactation improve preweaning piglet performance by enhancing the antioxidant capacity and milk content in nutrient-restricted sows. Anim. Nutr. 2020, 6, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Chamani, M.; Amanlou, H.; Nikkhah, A.; Sadeghi, A.A.; Dehkordi, F.K.; Rafiei, M.; Shirani, V. The effect of feeding inorganic and organic selenium sources on the hematological blood parameters, reproduction and health of dairy cows in the transition period. Acta Scientiarum Anim. Sci. 2019, 42, e45371. [Google Scholar] [CrossRef]
- Al-Waeli, A.; Zoidis, E.; Pappas, A.; Demiris, N.; Zervas, G.; Fegeros, K. The role of organic selenium in cadmium toxicity: Effects on broiler performance and health status. Animal 2013, 7, 386–393. [Google Scholar] [CrossRef]
- Falk, M.; Bernhoft, A.; Framstad, T.; Salbu, B.; Wisløff, H.; Kortner, T.M.; Kristoffersen, A.B.; Oropeza-Moe, M. Effects of dietary sodium selenite and organic selenium sources on immune and inflammatory responses and selenium deposition in growing pigs. J. Trace Elem. Med. Biol. 2018, 50, 527–536. [Google Scholar] [CrossRef]
- Zavodnik, L.; Shimkus, A.; Belyavsky, V.; Voronov, D.; Shimkiene, A.; Voloshin, D.B. Effects of organic selenium yeast administration on perinatal performance, growth efficiency and health status in pigs. Arch. Zootech. 2011, 14, 5. [Google Scholar]
- Zheng, Y.M.; He, X.Y. Characteristics and EGFP expression of porcine mammary gland epithelial cells. Res. Vet. Sci. 2010, 89, 383–390. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, L.; Chen, J.; Tian, Z.; Liu, J.; Chen, F.; Ren, M.; Guan, W.; Zhang, S. Artemisinin Protects Porcine Mammary Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Injury by Regulating the NF-kappaB and MAPK Signaling Pathways. Animals 2021, 11, 1528. [Google Scholar] [CrossRef]
- Asfaw, M.; Negash, A. Review on impact of bovine mastitis in dairy production. Adv. Biol. Res. 2017, 11, 126–131. [Google Scholar]
- Khan, M.Z.; Ma, Y.; Xiao, J.; Chen, T.; Ma, J.; Liu, S.; Wang, Y.; Khan, A.; Alugongo, G.M.; Cao, Z. Role of Selenium and Vitamins E and B9 in the Alleviation of Bovine Mastitis during the Periparturient Period. Antioxidants 2022, 11, 657. [Google Scholar] [CrossRef]
- Friendship, R.; O’sullivan, T. Sow health. In The Gestating and Lactating Sow; Wageningen Academic: Wageningen, The Netherlands, 2015; pp. 409–421. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Watson, A.D.; Kerr, D.E. Genome-wide expression analysis of lipopolysaccharide-induced mastitis in a mouse model. Infect. Immun. 2006, 74, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Johnzon, C.-F.; Dahlberg, J.; Gustafson, A.-M.; Waern, I.; Moazzami, A.A.; Östensson, K.; Pejler, G. The effect of lipopolysaccharide-induced experimental bovine mastitis on clinical parameters, inflammatory markers, and the metabolome: A kinetic approach. Front. Immunol. 2018, 9, 1487. [Google Scholar] [CrossRef]
- Zhang, G.; Ghosh, S. Molecular mechanisms of NF-κB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin Res. 2000, 6, 453–457. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, R.; Wang, T.; Jiang, H.; Guo, M.; Zhou, E.; Sun, Y.; Yang, Z.; Xu, S.; Cao, Y. Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation 2014, 37, 478–485. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 4. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid hormones, oxidative stress, and inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Aboul-Soud, M.A.; Al-Othman, A.M.; El-Desoky, G.E.; Al-Othman, Z.A.; Yusuf, K.; Ahmad, J.; Al-Khedhairy, A.A. Hepatoprotective effects of vitamin E/selenium against malathion-induced injuries on the antioxidant status and apoptosis-related gene expression in rats. J. Toxicol. Sci. 2011, 36, 285–296. [Google Scholar] [CrossRef]
- Wu, C.; Cui, C.; Zheng, X.; Wang, J.; Ma, Z.; Zhu, P.; Lin, G.; Zhang, S.; Guan, W.; Chen, F. The selenium yeast vs selenium methionine on cell viability, selenoprotein profile and redox status via JNK/P38 pathway in porcine mammary epithelial cells. Front. Vet. Sci. 2022, 9, 850935. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, Y.; Ren, K.; Wang, W.; Li, S. Hydroxy-selenomethionine helps cows to overcome heat stress by enhancing antioxidant capacity and alleviating blood-milk barrier damage. Anim. Nutr. 2025, 20, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bi, C.; Wang, Y.; Sun, J.; Meng, X.; Li, J. Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways. BMC Vet. Res. 2018, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xv, S.; Bi, C. Selenium Counteracts Tight Junction Disruption and Attenuates the NF-kappaB-Mediated Inflammatory Response in Staphylococcus aureus-Infected Mouse Mammary Glands. Biol. Trace Elem. Res. 2025, 203, 963–972. [Google Scholar] [CrossRef]
- Purba, F.Y.; Ueda, J.; Nii, T.; Yoshimura, Y.; Isobe, N. Effects of intrauterine infusion of bacterial lipopolysaccharides on the mammary gland inflammatory response in goats. Vet. Immunol. Immunopathol. 2020, 219, 109972. [Google Scholar] [CrossRef]
- Tong, M.; Li, S.; Hui, F.; Meng, F.; Li, L.; Shi, B.; Zhao, Y.; Guo, X.; Guo, Y.; Yan, S. Effects of Dietary Selenium Yeast Supplementation on Lactation Performance, Antioxidant Status, and Immune Responses in Lactating Donkeys. Antioxidants 2024, 13, 275. [Google Scholar] [CrossRef]
- Song, Y.; Weng, Y.; Liu, S.; Usman, M.; Loor, J.J.; Lin, G.; Hu, Q.; Luo, J.; Wang, P. Effects of reduced levels of organic trace minerals in proteinate forms and selenium yeast in the mineral mix on lactation performance, milk fatty acid composition, nutrient digestibility, and antioxidant status in dairy goats. J. Anim. Sci. 2024, 102, skae187. [Google Scholar] [CrossRef]
- Liu, L.; Chen, D.; Yu, B.; Luo, Y.; Huang, Z.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Yan, H. Influences of selenium-enriched yeast on growth performance, immune function, and antioxidant capacity in weaned pigs exposure to oxidative stress. BioMed. Res. Int. 2021, 2021, 5533210. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, X.; Liu, Y.; Jiang, X.; Che, L.; Lin, Y.; Zhuo, Y.; Feng, B.; Fang, Z.; Hua, L. Silibinin Alleviates Lipopolysaccharide Induced Inflammation in Porcine Mammary Epithelial Cells via mTOR/NF-κB Signaling Pathway. Mol. Nutr. Food Res. 2023, 67, 2200715. [Google Scholar] [CrossRef]
- Reczynska, D.; Witek, B.; Jarczak, J.; Czopowicz, M.; Mickiewicz, M.; Kaba, J.; Zwierzchowski, L.; Bagnicka, E. The impact of organic vs. inorganic selenium on dairy goat productivity and expression of selected genes in milk somatic cells. J. Dairy Res. 2019, 86, 48–54. [Google Scholar] [CrossRef]

| Genes | Accession | Sequence Primers (5′–3′) | Size (bp) |
|---|---|---|---|
| TNF-α | NM_214022.1 | F-ATGGGCTGTACCTCATCTACTC | 141 |
| R-GGCTCTTGATGGCAGAGAGG | |||
| IL-6 | NM_214399.1 | F-TGGCTACTGCCTTCCCTACC | 132 |
| R-CAGAGATTTTGCCGAGGATG | |||
| IL-1β | XM_021081828.1 | F-CCGAAGAGGGACATGGAGAA | 88 |
| R-AGTTGGGGTACAGGGCAGAC | |||
| IL-8 | NM_213867.1 | F-AGGACCAGAGCCAGGAAGAGAC | 108 |
| R-CACAGAGAGCTGCAGAAAGCAG | |||
| TLR4 | NM_001113039.2 | F-CCTGGATGATGTTAGCAGCGATGG | 124 |
| R-GACGAAGACTGGGTGAGGAATGAAC | |||
| Myd88 | NM_001099923.1 | F-TTCTGATGGGCACCTGGAGAGAG | 141 |
| R-CGTCTGGTCCATTGCTAGTGAACTC | |||
| Irak1 | XM_003135490.4 | F-GGTGGAAGAGGAGGCTGAGGAG | 113 |
| R-CGACGATCTGGGCAGCAATGG | |||
| Irak4 | NM_001112693.1 | F-CAGCCCTGTTGTTCACGTAGCC | 90 |
| R-TTGATGAGCGACCCGTTTCTATTGG | |||
| Traf6 | XM_005652801.2 | F-CAACAGCCAGAGGAAATCCGAGAC | 148 |
| R-CACGCCACCTGCAAGAGAATACC | |||
| β-actin | XM_021086047.1 | F-TGCGGGACATCAAGGAGAAG | 176 |
| R-AGTTGAAGGTGGTCTCGTGG |
| Antibody | Company | Code No. | Dilution |
|---|---|---|---|
| TLR4 | Proteintech (Rosemont, IL, USA) | 66350-1 | 1:1000 |
| NF-κB | CST (Hong Kong, China) | 4764S | 1:1000 |
| p-NF-κB | CST | 3033S | 1:1000 |
| IκB | Abcam (Cambridge, UK) | ab32518 | 1:1000 |
| p-IκB | Abcam | ab133462 | 1:1000 |
| p38 | Proteintech | 66234-1 | 1:1000 |
| p-P38 | CST | 4092S | 1:1000 |
| JNK | CST | 9252S | 1:1000 |
| p-JNK | CST | 4668S | 1:1000 |
| ERK | CST | 9102S | 1:1000 |
| p-ERK | CST | 9101S | 1:1000 |
| β-actin | Abcam | ab8226 | 1:3000 |
| Goat Anti-Rabbit IgG | Zenbio (Durham, NC, USA) | 511203 | 1:5000 |
| Goat Anti-Mous IgG | Zenbio | 511103 | 1:5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Su, S.; Zhang, B.; Chen, D.; Yuan, S.; Guan, W.; Zhang, S. Selenium Yeast Attenuated Lipopolysaccharide-Induced Inflammation in Porcine Mammary Epithelial Cells by Modulating MAPK and NF-κB Signaling Pathways. Antioxidants 2025, 14, 334. https://doi.org/10.3390/antiox14030334
He Z, Su S, Zhang B, Chen D, Yuan S, Guan W, Zhang S. Selenium Yeast Attenuated Lipopolysaccharide-Induced Inflammation in Porcine Mammary Epithelial Cells by Modulating MAPK and NF-κB Signaling Pathways. Antioxidants. 2025; 14(3):334. https://doi.org/10.3390/antiox14030334
Chicago/Turabian StyleHe, Zhenting, Senlin Su, Bing Zhang, Dongpang Chen, Siyu Yuan, Wutai Guan, and Shihai Zhang. 2025. "Selenium Yeast Attenuated Lipopolysaccharide-Induced Inflammation in Porcine Mammary Epithelial Cells by Modulating MAPK and NF-κB Signaling Pathways" Antioxidants 14, no. 3: 334. https://doi.org/10.3390/antiox14030334
APA StyleHe, Z., Su, S., Zhang, B., Chen, D., Yuan, S., Guan, W., & Zhang, S. (2025). Selenium Yeast Attenuated Lipopolysaccharide-Induced Inflammation in Porcine Mammary Epithelial Cells by Modulating MAPK and NF-κB Signaling Pathways. Antioxidants, 14(3), 334. https://doi.org/10.3390/antiox14030334








