Oxidative Stress Regulates CDH3 Expression in Lung Cancer Cells via OGG1-Mediated SP1 Binding
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Antibodies
2.3. Transcriptome Sequencing
2.4. Cell Transfection
2.5. Overall Survival or Disease-Free Survival Analysis
2.6. Transwell Assay
2.7. Clone Formation Assay
2.8. Growth Curve
2.9. Western Blotting
2.10. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.11. Immunofluorescence (IF) Assay
2.12. Chromatin Immunoprecipitation (ChIP) Assay
2.13. Statistical Analysis
3. Results
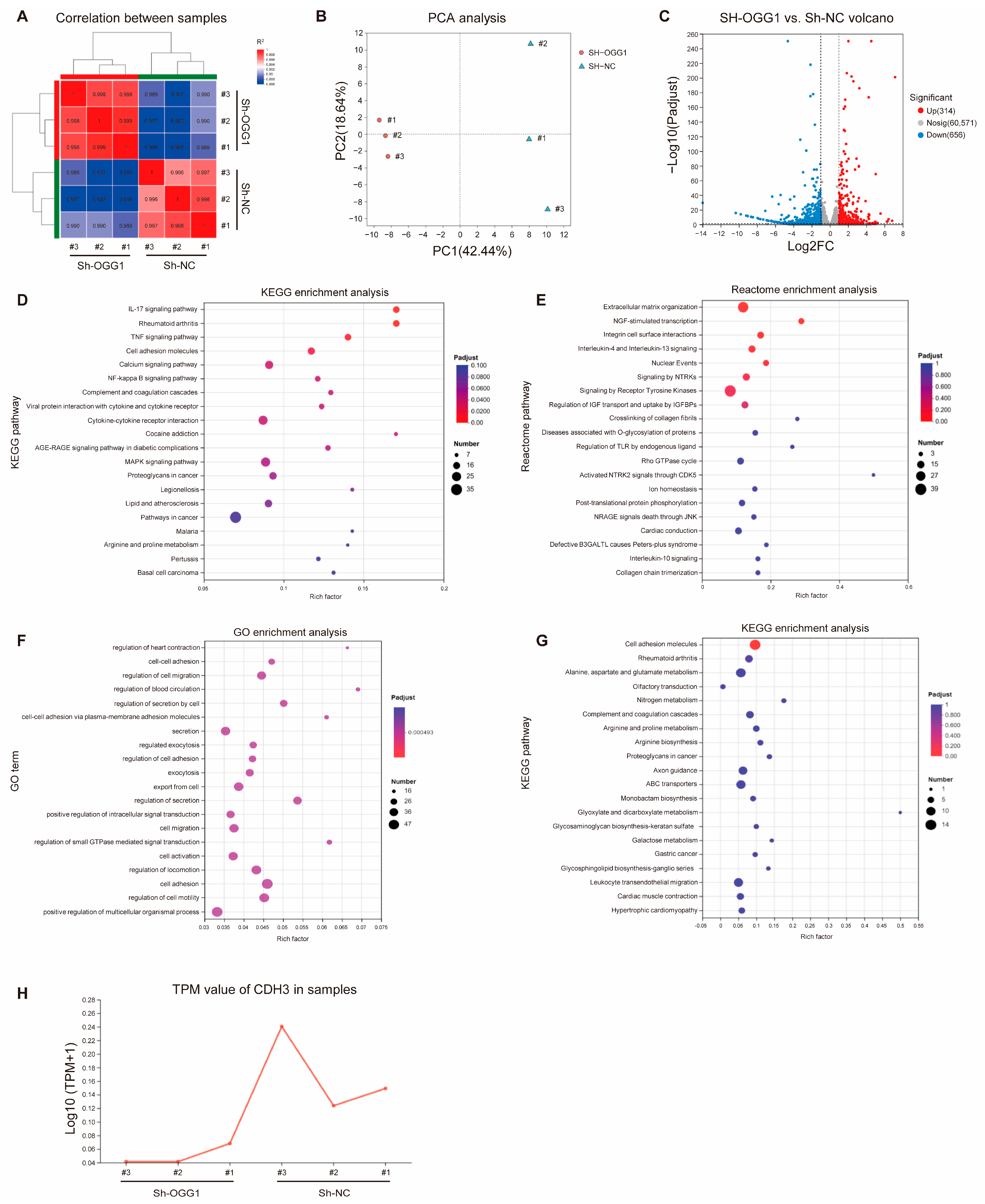
3.1. Identification of Downstream Molecules of OGG1 Through RNA Sequencing Analysis
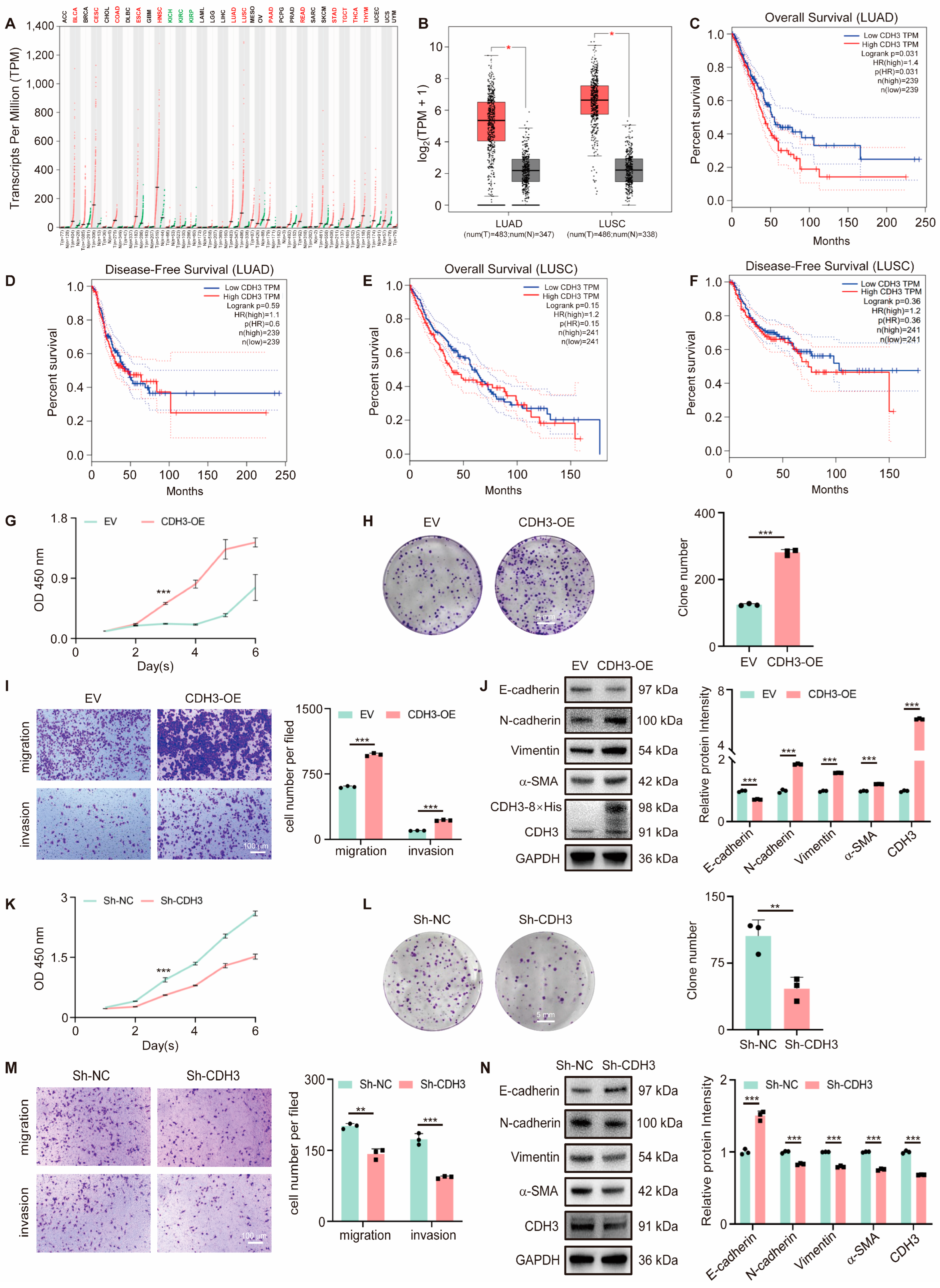
3.2. CDH3 Promotes Tumor Cell Proliferation and Migration
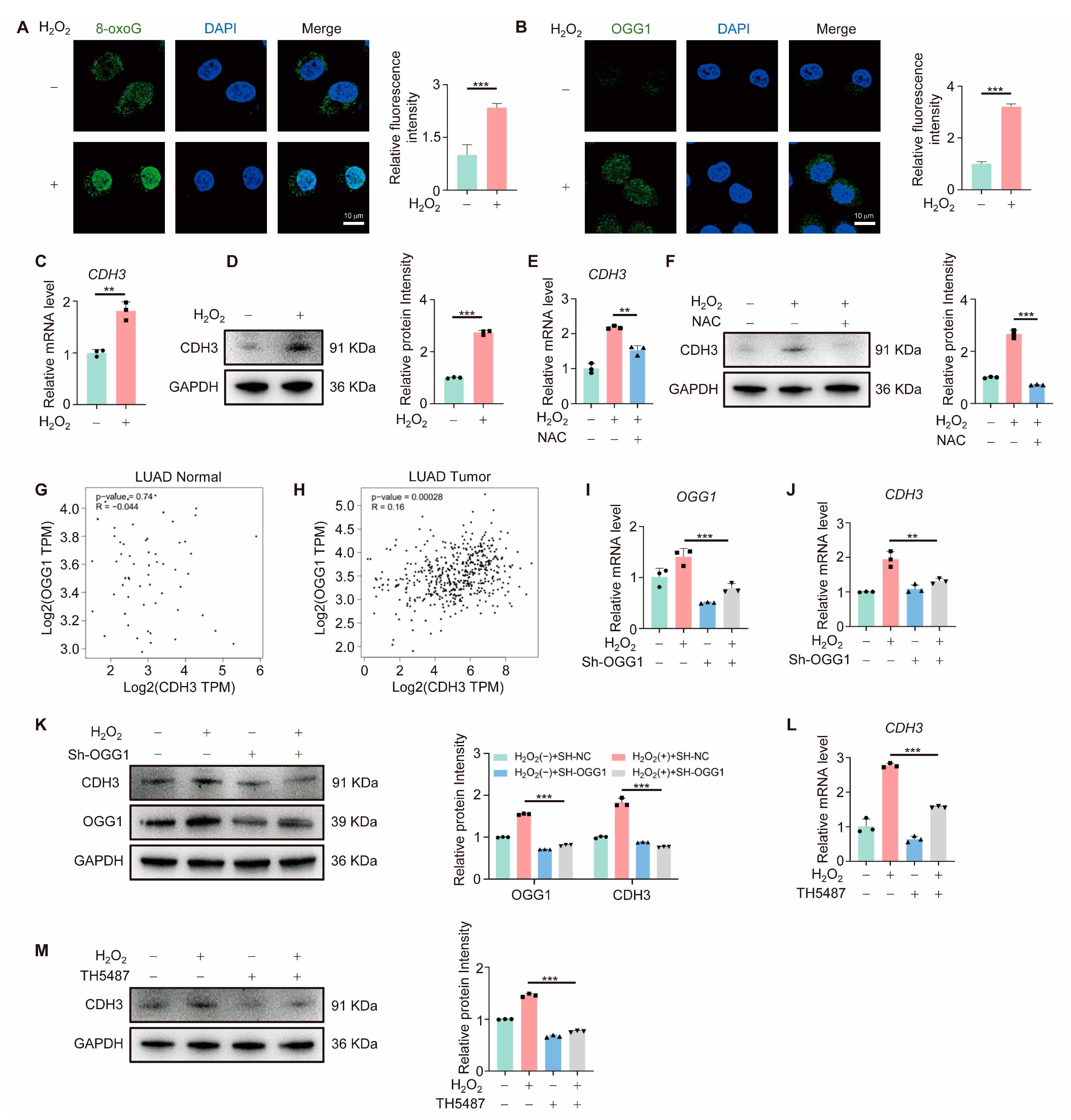
3.3. OGG1 Regulates CDH3 Expression
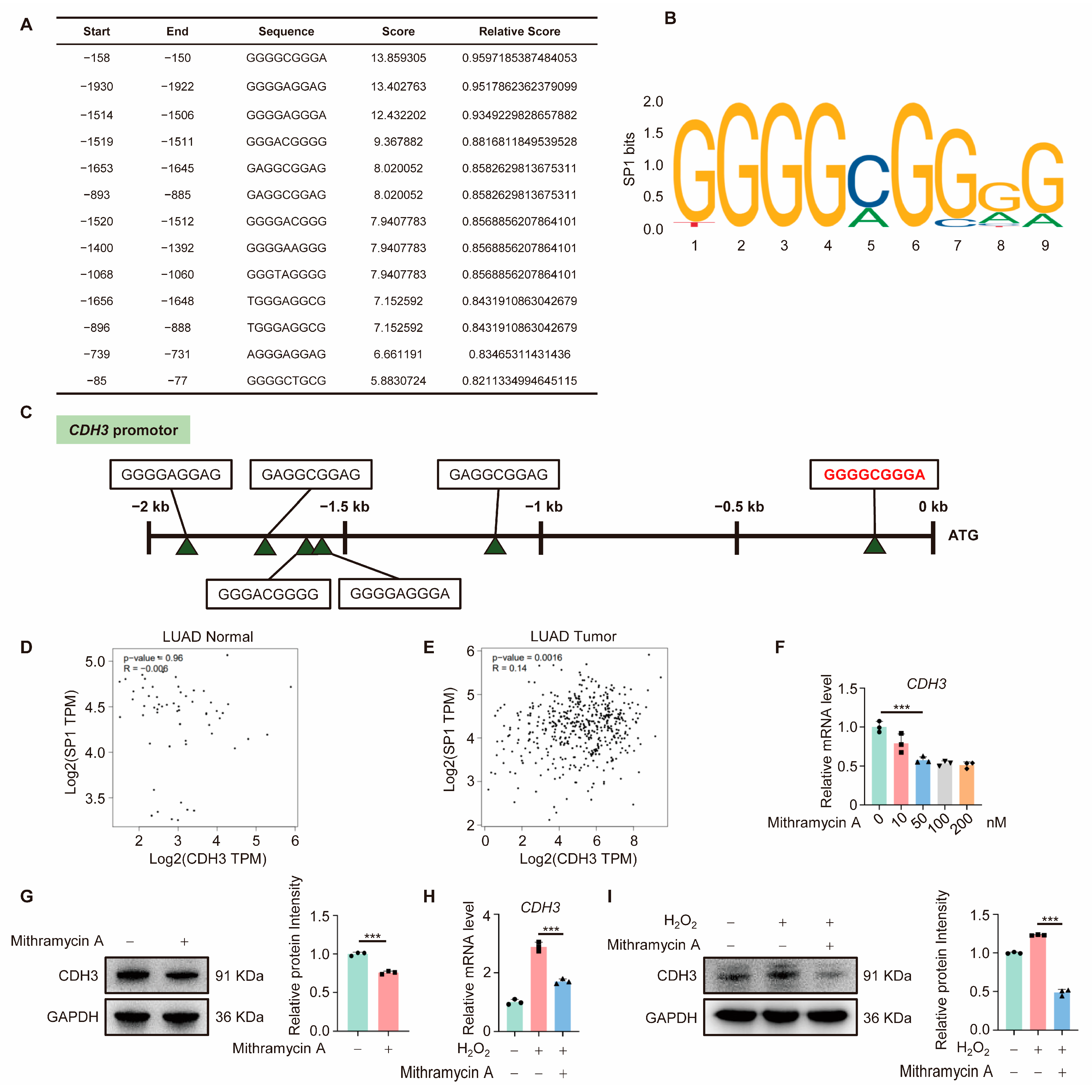
3.4. CDH3 Is Downstream of SP1
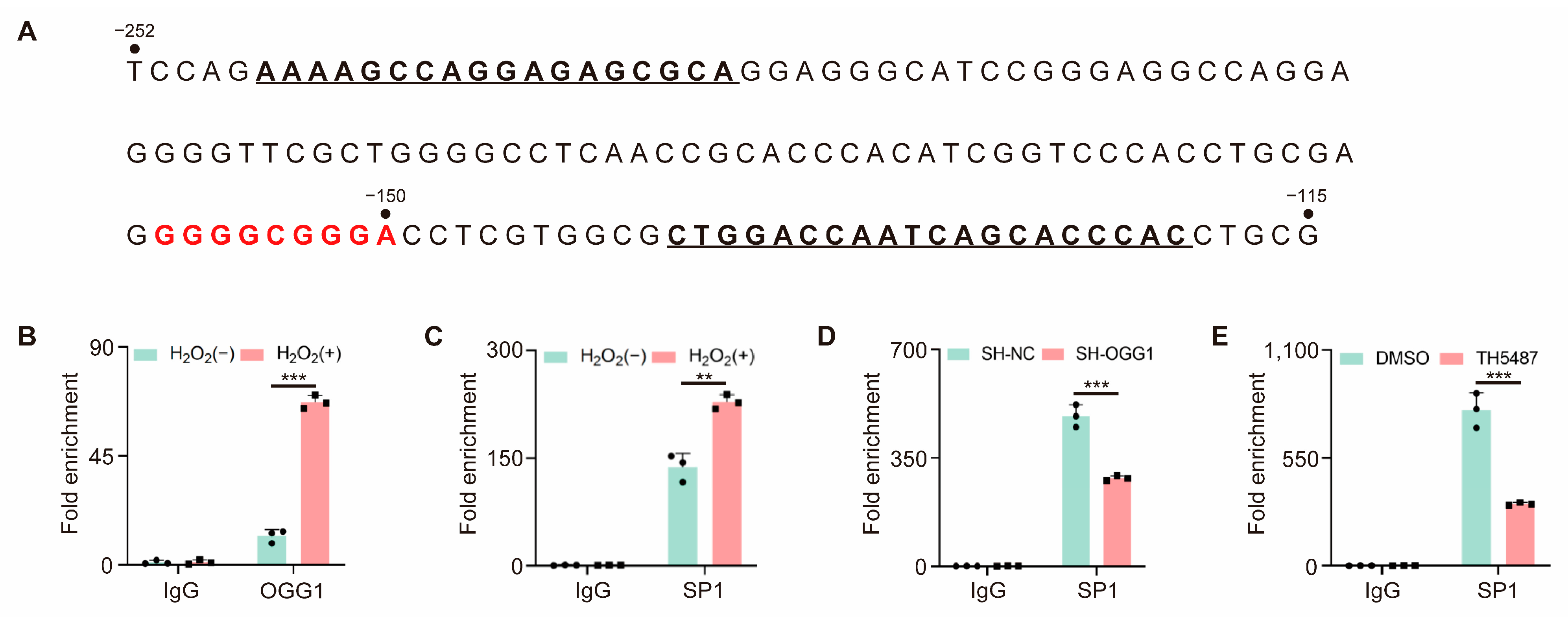
3.5. OGG1 Is Involved in SP1 Binding in the CDH3 Promoter Region
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Liu, Y.; Zhao, S.; Xu, W.; Li, Y.; Zhao, P.; Wang, D.; Cheng, H.; Ke, Y.; Zhang, X. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat. Commun. 2021, 12, 7094. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, G.; Zheng, Y.; Yang, Y.; Chen, C.; Xia, J.; Liang, L.; Lei, C.; Hu, Y.; Cai, X.; et al. SLC27A5 deficiency activates NRF2/TXNRD1 pathway by increased lipid peroxidation in HCC. Cell Death Differ. 2020, 27, 1086–1104. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.C.; Board, P.G.; Blackburn, A.C. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Mol. Cancer 2011, 10, 142. [Google Scholar] [CrossRef]
- Chen, F.; Su, R.; Ni, S.; Liu, Y.; Huang, J.; Li, G.; Wang, Q.; Zhang, X.; Yang, Y. Context-dependent responses of Drosophila intestinal stem cells to intracellular reactive oxygen species. Redox Biol. 2021, 39, 101835. [Google Scholar] [CrossRef] [PubMed]
- Burkovics, P.; Hajdu, I.; Szukacsov, V.; Unk, I.; Haracska, L. Role of PCNA-dependent stimulation of 3′-phosphodiesterase and 3′-5′ exonuclease activities of human Ape2 in repair of oxidative DNA damage. Nucleic Acids Res. 2009, 37, 4247–4255. [Google Scholar] [CrossRef]
- Park, J.W.; Han, Y.I.; Kim, S.W.; Kim, T.M.; Yeom, S.C.; Kang, J.; Park, J. 8-OxoG in GC-rich Sp1 binding sites enhances gene transcription in adipose tissue of juvenile mice. Sci. Rep. 2019, 9, 15618. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Qin, X.; Jiang, X.; Zhang, J.; Mao, L.; Jiang, Z.; Jiang, Y.; Liu, G.; Qiu, J.; et al. Recombinant ACE2 protein protects against acute lung injury induced by SARS-CoV-2 spike RBD protein. Crit. Care 2022, 26, 171. [Google Scholar] [CrossRef]
- Duan, S.; Han, X.; Akbari, M.; Croteau, D.L.; Rasmussen, L.J.; Bohr, V.A. Interaction between RECQL4 and OGG1 promotes repair of oxidative base lesion 8-oxoG and is regulated by SIRT1 deacetylase. Nucleic Acids Res. 2020, 48, 6530–6546. [Google Scholar] [CrossRef]
- Ba, X.; Bacsi, A.; Luo, J.; Aguilera-Aguirre, L.; Zeng, X.; Radak, Z.; Brasier, A.R.; Boldogh, I. 8-oxoguanine DNA glycosylase-1 augments proinflammatory gene expression by facilitating the recruitment of site-specific transcription factors. J. Immunol. 2014, 192, 2384–2394. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Y.; Huang, M.; Liang, W.; Zhao, X.; Li, Q.; Wang, S.; Hu, Z.; He, L.; Gao, T.; et al. Asymmetrical arginine dimethylation of histone H4 by 8-oxog/OGG1/PRMT1 is essential for oxidative stress-induced transcription activation. Free Radic. Biol. Med. 2021, 164, 175–186. [Google Scholar] [CrossRef]
- Pan, L.; Zhu, B.; Hao, W.; Zeng, X.; Vlahopoulos, S.A.; Hazra, T.K.; Hegde, M.L.; Radak, Z.; Bacsi, A.; Brasier, A.R.; et al. Oxidized Guanine Base Lesions Function in 8-Oxoguanine DNA Glycosylase-1-mediated Epigenetic Regulation of Nuclear Factor kappaB-driven Gene Expression. J. Biol. Chem. 2016, 291, 25553–25566. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Hao, W.; Xue, Y.; Wang, K.; Zheng, X.; Luo, J.; Ba, X.; Xiang, Y.; Qin, X.; Bergwik, J.; et al. 8-Oxoguanine targeted by 8-oxoguanine DNA glycosylase 1 (OGG1) is central to fibrogenic gene activation upon lung injury. Nucleic Acids Res. 2023, 51, 1087–1102. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, J.; Rao, H.; Xin, J.; Song, X.; Liu, R.; Shao, S.; Hou, J.; Kong, L.; Hu, Z.; et al. The 8-oxoguanine DNA glycosylase-synaptotagmin 7 pathway increases extracellular vesicle release and promotes tumour metastasis during oxidative stress. J. Extracell. Vesicles 2024, 13, e12505. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Lin, C.; Krill, L.S.; Eskander, R.; Guo, Y.; Zi, X.; Hoang, B.H. Flavokawain B, a kava chalcone, inhibits growth of human osteosarcoma cells through G2/M cell cycle arrest and apoptosis. Mol. Cancer 2013, 12, 55. [Google Scholar] [CrossRef]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Naydenov, N.G.; Lechuga, S.; Zalavadia, A.; Mukherjee, P.K.; Gordon, I.O.; Skvasik, D.; Vidovic, P.; Huang, E.; Rieder, F.; Ivanov, A.I. P-Cadherin Regulates Intestinal Epithelial Cell Migration and Mucosal Repair, but Is Dispensable for Colitis Associated Colon Cancer. Cells 2022, 11, 1467. [Google Scholar] [CrossRef]
- Yi, S.; Yang, Z.L.; Miao, X.; Zou, Q.; Li, J.; Liang, L.; Zeng, G.; Chen, S. N-cadherin and P-cadherin are biomarkers for invasion, metastasis, and poor prognosis of gallbladder carcinomas. Pathol. Res. Pract. 2014, 210, 363–368. [Google Scholar] [CrossRef]
- Sridhar, S.; Rajesh, C.; Jishnu, P.V.; Jayaram, P.; Kabekkodu, S.P. Increased expression of P-cadherin is an indicator of poor prognosis in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2020, 179, 301–313. [Google Scholar] [CrossRef]
- Kumara, H.; Bellini, G.A.; Caballero, O.L.; Herath, S.A.C.; Su, T.; Ahmed, A.; Njoh, L.; Cekic, V.; Whelan, R.L. P-Cadherin (CDH3) is overexpressed in colorectal tumors and has potential as a serum marker for colorectal cancer monitoring. Oncoscience 2017, 4, 139–147. [Google Scholar] [CrossRef]
- Jang, N.R.; Choi, J.H.; Gu, M.J. Aberrant Expression of E-cadherin, N-cadherin, and P-cadherin in Clear Cell Renal Cell Carcinoma: Association With Adverse Clinicopathologic Factors and Poor Prognosis. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 223–230. [Google Scholar] [CrossRef]
- Ma, W.; Hu, J. Downregulated CDH3 is correlated with a better prognosis for LUAD and decreases proliferation and migration of lung cancer cells. Genes. Genomics 2024, 46, 713–731. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, S.; Wu, Q.; Dou, N.; Li, Y.; Gao, Y. KLF4-Mediated CDH3 Upregulation Suppresses Human Hepatoma Cell Growth and Migration via GSK-3beta Signaling. Int. J. Biol. Sci. 2019, 15, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, A.; Ribeiro, A.S.; Pinho, S.; Milanezi, F.; Carneiro, V.; Sousa, B.; Sousa, S.; Oliveira, C.; Machado, J.C.; Seruca, R.; et al. ICI 182,780 induces P-cadherin overexpression in breast cancer cells through chromatin remodelling at the promoter level: A role for C/EBPbeta in CDH3 gene activation. Hum. Mol. Genet. 2010, 19, 2554–2566. [Google Scholar] [CrossRef]
- Albergaria, A.; Resende, C.; Nobre, A.R.; Ribeiro, A.S.; Sousa, B.; Machado, J.C.; Seruca, R.; Paredes, J.; Schmitt, F. CCAAT/enhancer binding protein beta (C/EBPbeta) isoforms as transcriptional regulators of the pro-invasive CDH3/P-cadherin gene in human breast cancer cells. PLoS ONE 2013, 8, e55749. [Google Scholar] [CrossRef]
- Wang, R.; Hao, W.; Pan, L.; Boldogh, I.; Ba, X. The roles of base excision repair enzyme OGG1 in gene expression. Cell Mol. Life Sci. 2018, 75, 3741–3750. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Topalovski, M.; Toombs, J.E.; Wright, C.M.; Moore, Z.R.; Boothman, D.A.; Yanagisawa, H.; Wang, H.; Witkiewicz, A.; Castrillon, D.H.; et al. Fibulin-5 Blocks Microenvironmental ROS in Pancreatic Cancer. Cancer Res. 2015, 75, 5058–5069. [Google Scholar] [CrossRef]
- Tumurkhuu, G.; Shimada, K.; Dagvadorj, J.; Crother, T.R.; Zhang, W.; Luthringer, D.; Gottlieb, R.A.; Chen, S.; Arditi, M. Ogg1-Dependent DNA Repair Regulates NLRP3 Inflammasome and Prevents Atherosclerosis. Circ. Res. 2016, 119, e76-90. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Thomsen, H.; da Silva Filho, M.I.; Weinhold, N.; Hoffmann, P.; Nothen, M.M.; Marina, A.; Jockel, K.H.; Schmidt, B.; Pechlivanis, S.; et al. Enrichment of B cell receptor signaling and epidermal growth factor receptor pathways in monoclonal gammopathy of undetermined significance: A genome-wide genetic interaction study. Mol. Med. 2018, 24, 30. [Google Scholar] [CrossRef]
- Xing, L.; Yang, R.; Wang, X.; Zheng, X.; Yang, X.; Zhang, L.; Jiang, R.; Ren, G.; Chen, J. The circRNA circIFI30 promotes progression of triple-negative breast cancer and correlates with prognosis. Aging 2020, 12, 10983–11003. [Google Scholar] [CrossRef]
- Huang, W.Y.; Lai, C.H.; Peng, S.L.; Hsu, C.Y.; Hsu, P.H.; Chu, P.Y.; Feng, C.L.; Lin, Y.H. Targeting Tumor Cells with Nanoparticles for Enhanced Co-Drug Delivery in Cancer Treatment. Pharmaceutics 2021, 13, 1327. [Google Scholar] [CrossRef]
| Name | Sequence (5′-3′) |
|---|---|
| qPCR-CDH3-F | ATCATCGTGACCGACCAGAAT |
| qPCR-CDH3-R | GACTCCCTCTAAGACACTCCC |
| qPCR-OGG1-F | CACACTGGAGTGGTGTACTAGC |
| qPCR-OGG1-R | CCAGGGTAACATCTAGCTGGAA |
| qPCR-β-actin-F | CATGTACGTTGCTATCCAGGC |
| qPCR-β-actin-R | CTCCTTAATGTCACGCACGAT |
| Name | Sequence (5′-3′) |
|---|---|
| ChIP-CDH3-F | AAAAGCCAGGAGAGCGCA |
| ChIP-CDH3-R | GTGGGTGCTGATTGGTCCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Guo, J.; Xu, S.; Hou, Y.; Pan, F.; Guo, Z. Oxidative Stress Regulates CDH3 Expression in Lung Cancer Cells via OGG1-Mediated SP1 Binding. Antioxidants 2025, 14, 332. https://doi.org/10.3390/antiox14030332
Ma Y, Guo J, Xu S, Hou Y, Pan F, Guo Z. Oxidative Stress Regulates CDH3 Expression in Lung Cancer Cells via OGG1-Mediated SP1 Binding. Antioxidants. 2025; 14(3):332. https://doi.org/10.3390/antiox14030332
Chicago/Turabian StyleMa, Ying, Jiarong Guo, Shichu Xu, Yanjun Hou, Feiyan Pan, and Zhigang Guo. 2025. "Oxidative Stress Regulates CDH3 Expression in Lung Cancer Cells via OGG1-Mediated SP1 Binding" Antioxidants 14, no. 3: 332. https://doi.org/10.3390/antiox14030332
APA StyleMa, Y., Guo, J., Xu, S., Hou, Y., Pan, F., & Guo, Z. (2025). Oxidative Stress Regulates CDH3 Expression in Lung Cancer Cells via OGG1-Mediated SP1 Binding. Antioxidants, 14(3), 332. https://doi.org/10.3390/antiox14030332






